Abstract
Introduction
In two solanezumab trials for mild-to-moderate Alzheimer's disease (AD) dementia, 27% of patients had biomarker confirmation of amyloid status. Of these, approximately 25% of mild patients and approximately 10% of moderate patients were amyloid negative and, as a group, did not exhibit clinical progression typical of AD. This post-hoc analysis describes a statistical surrogate for amyloid status.
Methods
Quantile regression was used to examine solanezumab treatment effects at fixed percentiles of varying degrees of clinical progression, with lowest percentiles (minimal progression atypical of AD) and higher percentiles acting as surrogates for amyloid negativity or positivity, respectively.
Results
In mild patients, solanezumab treatment effect was greater in higher percentiles of progression and less in lowest percentiles (AD-atypical). In moderate patients, solanezumab did not show effects across most percentiles.
Discussion
Results are compatible with design of the ongoing solanezumab EXPEDITION 3 trial that limits patients to those with mild AD dementia and evidence of amyloid pathology.
Keywords: Alzheimer's disease, Progression, Amyloid, Solanezumab, Quantile regression
1. Introduction
The amyloid cascade hypothesis proposes that Alzheimer's disease (AD) is caused by an imbalance between amyloid beta (Aβ) production and clearance, ultimately giving rise to amyloid plaques in brain tissue [1], [2]. Subjects with mild cognitive impairment who are amyloid positive are more likely to progress to dementia than those subjects who are amyloid negative [3], [4], [5], [6].
Solanezumab is a humanized monoclonal antibody developed for the treatment of AD. It strongly and selectively binds to a mid-domain of soluble Aβ peptides in the periphery and central nervous system [7], [8]. The EXPEDITION and EXPEDITION2 (EXP&EXP2—NCT00905372 and NCT00904683) trials were identically designed phase 3, multicenter, randomized, double-blind, placebo-controlled studies of solanezumab. The primary objective of the studies was to determine whether solanezumab treatment would slow clinical decline versus placebo in patients with mild-to-moderate AD dementia over 80 weeks of treatment. Cognitive decline was measured using the Alzheimer's Disease Assessment Scale Cognitive subscale (ADAS-Cog) [9], and functional decline was measured using the Alzheimer's Disease Cooperative Study-Activities of Daily Living (ADCS-ADL) [10].
Using these measures, the results of the two separate studies showed solanezumab did not produce significant slowing of cognitive or functional decline relative to placebo in patients with mild-to-moderate AD dementia [11]. However, in a prespecified pooled analyses of the mild AD subgroup, solanezumab produced a slowing of cognitive decline (ADAS-Cog14; least squares [LS] mean treatment difference of 2.13 points [34%]; P = .001) and a slowing of functional decline (ADCS-ADL instrumental subset; LS mean treatment difference of 1.21 points [18%]; P = .045) relative to placebo at 80 weeks. In the patients with moderate AD dementia, solanezumab did not provide a slowing of cognitive or functional decline [12].
All patients in EXP&EXP2 met clinical diagnostic criteria for probable AD based on National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer's Disease and Related Disorders Association criteria [13]. Under optional study addenda, baseline amyloid status was assessed in a subset of patients (27%), using either cerebrospinal fluid Aβ1–42 or florbetapir (18F) imaging. Baseline amyloid status was found to be negative in a substantial fraction of patients with clinically defined mild-to-moderate AD dementia: approximately 25% of patients with mild dementia [12] and approximately 10% of patients with moderate dementia. Notably, patients lacking evidence of amyloid pathology did not exhibit progressive clinical decline typical of AD [14], [15].
Because baseline amyloid status was unknown in most patients (73%), a comparison of solanezumab and placebo treatment effects on clinical progression in amyloid positive versus negative subjects in the full study cohort was not possible. Accordingly, we developed a novel statistical strategy using quantile regression to analyze treatment effect on fixed percentiles of clinical progression (minimal clinical progression considered atypical of amyloid-positive AD patients to a continuum of clinical progression considered more typical of amyloid-positive AD patients).
2. Methods
Details of the EXP&EXP2 trials can be found in previous publications [11], [12]. Briefly, 1012 (EXP) and 1040 (EXP2) patients, aged ≥55 years with clinically diagnosed mild or moderate AD dementia (baseline Mini-Mental State Examination (MMSE) scores of 20–26 or 16–19, respectively), were randomly assigned to receive placebo or solanezumab (400 mg IV) every 4 weeks for 80 weeks. Existing standard of care (SOC) medications for AD were permitted (cholinesterase inhibitors and/or memantine) provided the patients had been treated for a minimum of 4 months and on a stable dose for at least 2 months before randomization.
2.1. Statistical analysis
Unlike in a traditional data analysis where conventional least squares regression is used to model the relationship between covariates and the conditional mean of the response, quantile regression [16] models the relationship between a set of predictor variables (e.g., treatment groups) and specific quantiles (or percentiles) of the response variable. In a least squares regression class such as the mixed model repeated measures (MMRM) approach used in EXP&EXP2, mean treatment effect is merely estimated, whereas quantile regression allows interrogation of the treatment effect on any given quantile of response. Owing to heterogeneity of treatment response, quantile regression is especially useful for applications like EXP&EXP2, where the mean itself might not be an adequate summary measure of treatment response; quantile regression provides the advantage of capturing different treatment effects at different regions of the response distribution. This approach provides a broader comparison of treatment effects across the observed spectrum of disease progression than is possible when comparisons are based on means or another single measure of central tendency. At each visit, patient data in the higher percentiles represented a clinical course more typical of AD and thus were given the surrogate biomarker-identifier of hypothesized positive amyloid/AD status (“AD”). In contrast, data in the lowest percentiles represented an atypical clinical course and were identified with the surrogate biomarker status of hypothesized negative amyloid/non-AD (“non-AD”). Thus, surrogate status of “AD” or “non-AD” allowed a theoretical analysis of the full study cohort.
Within the pooled mild subgroup (baseline MMSE score of 20–26) and the pooled moderate subgroup (baseline MMSE score of 16–19) of patients from the EXP&EXP2 data set, five fixed percentiles (quantiles: 20th, 30th, 40th, 60th, and 80th) of clinical progression were examined in this post-hoc quantile regression analysis. Five percentiles were chosen based on knowledge that in the subset of patients with known amyloid status, approximately 20% were amyloid negative. Quantile regression was applied to compare the treatment difference on the change from baseline ADAS-Cog14 and ADCS-iADL scores at each visit time point within each fixed percentile and compared to results from the MMRM methodology used to analyze the full intent-to-treat (ITT) patient data set. Included in the model, using least absolute deviations with covariates of study, were baseline score, treatment, AD dementia severity at baseline (mild or moderate), baseline concomitant SOC medication use, and age.
3. Results
Baseline characteristics for the mild and moderate AD study population are provided in Table 1. For the mild AD study population, on average, patients were 74 years of age, 54% were female, and 58% were APOE4 carriers, with the following mean scores on cognitive and functional tests: MMSE: 23; ADAS-Cog14: 30; ADCS-iADL: 43. For the moderate AD study population, on average, patients were 74 years, 61% were female, and 61% were APOE4 carriers, with the following mean scores on cognitive and functional tests: MMSE, 18; ADAS-Cog14, 42; and ADCS-iADL: 35.
Table 1.
Baseline characteristics for the mild and moderate AD study population
| Baseline Characteristics | Mild AD study population |
Moderate AD study population |
||||
|---|---|---|---|---|---|---|
| Placebo (n = 663)∗ | Solanezumab (n = 659)∗ | Total (N = 1322) | Placebo (n = 359)∗ | Solanezumab (n = 364)∗ | Total (N = 723) | |
| Age, mean (SD), years | 73.3 (7.9) | 73.9 (8.1) | 73.6 (8.0) | 73.7 (8.2) | 73.9 (8.2) | 73.8 (8.1) |
| Female, n (%) | 362 (54.6) | 346 (52.5) | 708 (53.6) | 210 (58.5) | 234 (64.3) | 444 (61.4) |
| APOE4 carriers, n (%)† | 367 (59.8) | 329 (55.3) | 696 (57.6) | 201 (61.8) | 199 (61.0) | 400 (61.4) |
| SOC medication at baseline, n (%) | 587 (88.5) | 574 (87.1) | 1161 (87.8) | 333 (92.8) | 337 (92.6) | 670 (92.7) |
| Baseline efficacy measures | ||||||
| MMSE | 22.5 (2.8) | 22.5 (2.8) | 22.5 (2.8) | 17.8 (2.5) | 17.9 (2.6) | 17.8 (2.6) |
| ADAS-Cog14 | 29.6 (8.8) | 30.1 (8.6) | 29.9 (8.7) | 42.6 (10.8) | 42.1 (9.6) | 42.3 (10.2) |
| ADCS-iADL | 42.9 (9.5) | 42.4 (10.0) | 42.7 (9.7) | 34.4 (11.3) | 34.8 (10.8) | 34.6 (11.0) |
Abbreviations: ADAS-Cog, Alzheimer's Disease Assessment Scale—Cognitive subscale (14 item); ADCS-iADL, Alzheimer's Disease Cooperative Study Instrumental Activities of Daily Living Inventory; MMSE, mini mental state examination; SOC, standard of care.
Number of randomized subjects. For baseline efficacy measures, the number of subjects included in each analysis varies based on the number of subjects with a baseline value for that measure.
Percentage based on number of subjects with APOE4 status available (mild placebo, 614; mild solanezumab, 595; moderate placebo, 325; and moderate solanezumab, 326).
Results of the quantile regression analyses for patients with mild and moderate AD dementia at baseline are shown in Fig. 1 (ADAS-Cog14, 1A; ADCS-iADL, 1B) and Fig. 2 (ADAS-Cog14, 2A; ADCS-iADL, 2B), respectively. These results are compared to the MMRM analysis of the full ITT cohort.
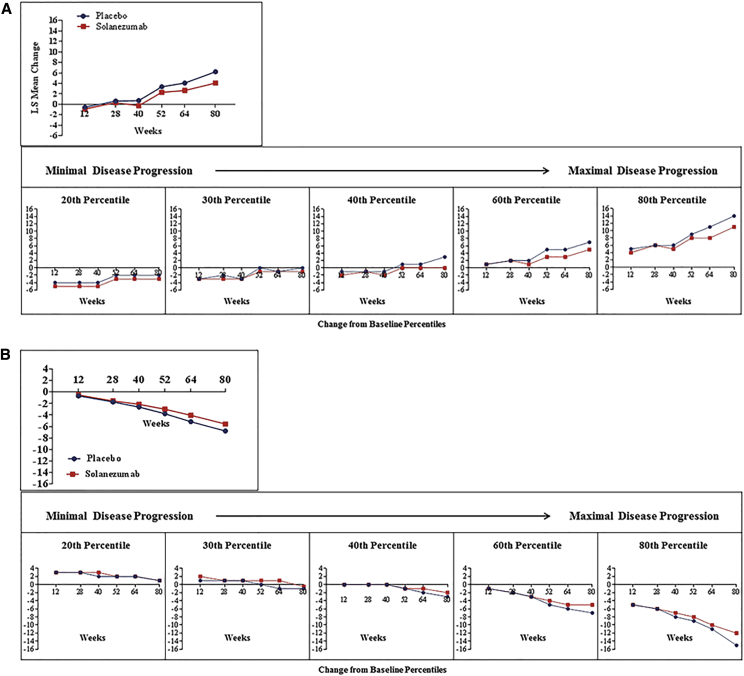
Fig. 1.
Change in ADAS-Cog14 score* by treatment group (A) and change in ADCS-iADL score* by treatment group (B) from baseline to each visit time point: patients with mild AD dementia (MMSE score, 20–26) at baseline.
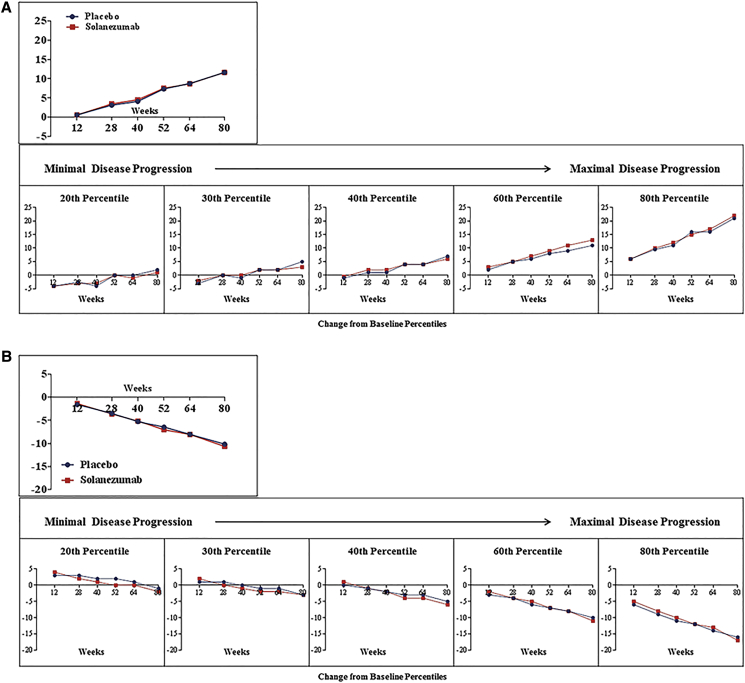
Fig. 2.
Change in ADAS-Cog14 score* by treatment group (A) and change in ADCS-iADL score* by treatment group (B) from baseline to each visit time point: patients with moderate AD dementia (MMSE score 16–19 for moderate AD dementia) at baseline.
Table 2 shows that in patients with mild AD dementia at baseline, the difference in treatment effect on cognition (ADAS-Cog14) was statistically significant for solanezumab in the full ITT cohort (P < .001) and across all five percentiles of clinical progression at week 80. The treatment difference was 2.13 points in the ITT cohort and increased from 1.06 points in the lowest quantile of progression to 2.56 points in the highest percentile of clinical progression.
Table 2.
Treatment (quantile) difference in patients with mild AD dementia at baseline (solanezumab versus placebo, week 80)
| Patients with mild AD dementia at baseline (n = 1322) |
|||||
|---|---|---|---|---|---|
| ADAS-Cog14 |
ADCS-iADL |
||||
| Sola vs PBO treatment difference | P value∗ | Sola vs PBO treatment difference | P value∗ | ||
| All patients | 2.13† | .001 | 1.21† | .045 | |
| Percentile | 20th | 1.06† | .02 | 0.25† | .59 |
| 30th | 1.45† | <.01 | 0.55† | .22 | |
| 40th | 2.10† | <.01 | 1.14† | .04 | |
| 60th | 2.43† | <.01 | 1.72† | <.01 | |
| 80th | 2.56† | <.01 | 2.16† | .05 | |
P values were based on Wald Test.
Values in favor of solanezumab treatment.
Also shown in Table 2, the difference in treatment effect on function (ADCS-iADL) was statistically significant for solanezumab in the ITT cohort (P = .045) and in the highest three percentiles of clinical progression (Table 2). The treatment difference was 1.21 points for the ITT cohort and increased from 0.25 points in the lowest quantile of progression to 2.16 points as the percentile of clinical progression increased.
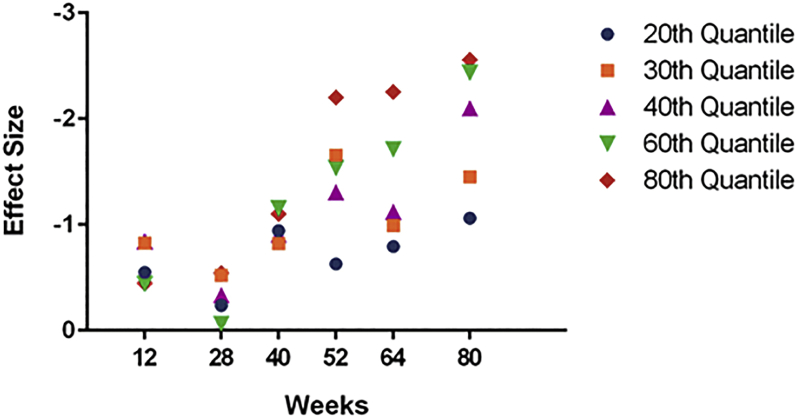
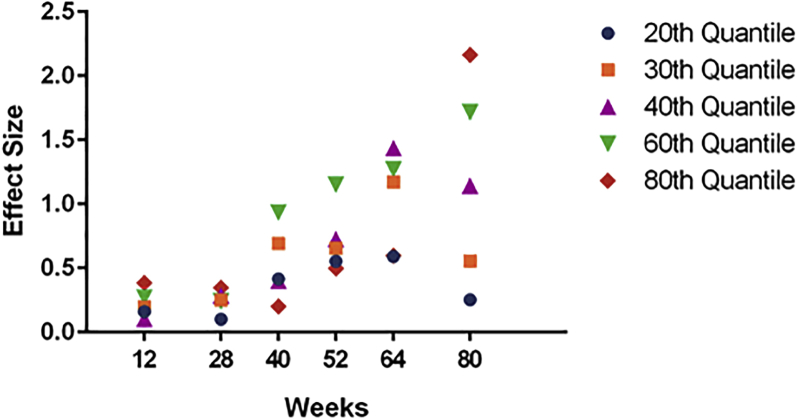
For ADAS-Cog14, the treatment effect in mild patients was increasing over time at higher percentiles (60th and 80th). For ADCS-iADL, the increasing effect was seen at the 60th percentile but was not apparent at the 80th percentile (Fig. 3, Fig. 4).
Fig. 3.
Effect size of solanezumab treatment over time on ADAS-Cog14 by quantile in patients with mild AD dementia.
Fig. 4.
Effect size of solanezumab treatment over time on ADCS-iADL by quantile in patients with mild AD dementia.
In patients with moderate AD dementia at baseline, there were no statistically significant treatment group differences within the ITT cohort or any percentile groups on either cognition or function measures at week 80 (Table 3).
Table 3.
Treatment (quantile) differences in patients with moderate AD dementia at baseline (solanezumab versus placebo, week 80)
| Patients with Moderate AD dementia at baseline (n = 723) |
|||||
|---|---|---|---|---|---|
| ADAS-Cog14 |
ADCS-iADL |
||||
| Sola vs PBO treatment difference | P value∗ | Sola vs PBO treatment difference | P value∗ | ||
| All patients | 0.01 | .991 | 0.51 | .55 | |
| Percentile | 20th | 0.13 | .90 | 0.63 | .49 |
| 30th | 0.30 | .70 | 0.74 | .40 | |
| 40th | 0.05 | .95 | 0.73 | .46 | |
| 60th | 0.14† | .91 | 0.97 | .33 | |
| 80th | 2.3 | .21 | 0.79 | .63 | |
P values were based on Wald test.
Values in favor of solanezumab treatment.
4. Discussion
Because amyloid status was not assessed in all patients participating in the EXP&EXP2 solanezumab clinical trials, this post-hoc quantile regression analysis was used to examine treatment effects across percentiles of varying degrees of clinical progression, with higher percentiles (considered more typical disease progression) and lowest percentiles (minimal disease progression) acting as hypothetical surrogates for amyloid positivity (AD) or negativity (non-AD), respectively.
In patients with mild AD dementia, treatment effect of solanezumab was greater when clinical progression was more typical of amyloid-positive AD patients, that is, in percentiles with a theoretically larger homogenous subset of amyloid-positive patients. When patients' clinical progression was minimal, or atypical of amyloid-positive AD patients, solanezumab showed less effect in slowing disease progression, suggesting that in the ITT population, the effect of solanezumab may have been attenuated by a theoretically higher percentage of amyloid-negative patients. In patients with moderate AD dementia, solanezumab did not have a statistically significant treatment effect within any clinical progression percentile. Results reported herein based on quantile analyses are generally consistent with those previously obtained by LS mean analyses [11], [12] regarding treatment effect of solanezumab in mild AD dementia and lack of treatment effect in moderate AD dementia.
Although the small number of patients with known amyloid status precludes robust statistical analysis, we did examine the ADAS-Cog14 change from baseline to week 80 data by quantile in patients with known amyloid status. For the amyloid-positive patients (N = 122), the probability of falling into the lowest percentile of clinical progression is 13.1% with patient distribution shifted to the higher quantiles. Conversely, for the amyloid-negative patients (N = 26), the probability of falling into the lowest percentile is 42.3% with patient distribution shifted to the lower quantiles. Thus, results in this small number of known amyloid-positive and amyloid-negative patients are consistent with use of quantile regression to provide a hypothetical surrogacy for amyloid status and support three hypotheses. First, the effect of solanezumab may have been attenuated by the presence of amyloid-negative patients. Second, a small percentage of amyloid-positive patients in the lowest quantile of progression may explain the small treatment effect demonstrated in this group. Finally, solanezumab's treatment effect was not dominated by the lowest percentiles of clinical progression, theoretically composed of the highest percentage of amyloid-negative patients, but by the highest percentiles of clinical progression, theoretically composed of the highest percentage of amyloid-positive patients. Interestingly, 13.1% of amyloid-positive patients fell in the lowest percentiles of clinical progression, demonstrating that on an individual patient level, amyloid positivity does not guarantee clinical progression with other factors, including neurofibrillary tangle formation, neuronal loss, age, and cognitive reserve playing a modulatory role [6], [17].
A potential limitation of this work is the assumption that demonstrating clinical progression typical of AD can be used as a surrogate for a biomarker-based confirmation of AD clinical diagnosis and/or that clinical progression atypical of AD can be used as a surrogate for the absence of amyloid pathology. These assumptions could falsely ascribe AD pathology to clinical progressors and misclassify AD patients with an unusual clinical trajectory as “non-AD”. Another limitation is that solanezumab's less robust effect in patients with atypical disease progression might be due to a floor effect in the measurement tools instead of a decreased effect in patients without true AD pathology. Another limitation of this work is that the choice of five quantiles was based on the assumption that the percentage of patients in the full cohort who were amyloid negative was the same as that in the known cohort. Finally, although quantile regression produced unbiased estimates of percentiles, the model itself had limitations, including (1) its inability to provide robust estimates for extreme percentiles (e.g., <10% or >10%) when the sample size is small and (2) some convergence issues having occurred while applying repeated measure quantile regression fitting to these data.
Application of this statistical methodology is consistent with the design of the ongoing solanezumab EXPEDITION3 (NCT01900665) trial being limited to patients with mild AD dementia who have evidence of amyloid pathology. The findings also illustrate the potential utility of quantile regression for retrospective investigation of completed treatment studies where universal confirmation of AD diagnosis (e.g., positive amyloid status) was not uniformly determined. Future studies are needed to explore the validity of the clinical course assumptions applied in this study.
Research in context.
-
1.
Systematic review: Authors reviewed the literature pertaining to the amyloid cascade hypothesis and results from two solanezumab trials for mild-to-moderate Alzheimer's disease (AD; EXPEDITION and EXPEDITION2). Although diagnosed with AD, a substantial percentage of study patients were found to be amyloid negative.
-
2.
Interpretation: The authors used quantile regression to examine solanezumab treatment effects at fixed percentiles of clinical progression, with lowest percentiles (minimal progression atypical of AD) and higher percentiles acting as surrogates for amyloid negativity or positivity, respectively. In patients with mild AD dementia, solanezumab treatment effect tended to be greater in higher percentiles. In lowest percentiles, solanezumab showed less effect.
-
3.
Future directions: Results are compatible with design of the ongoing solanezumab EXPEDITION3 trial being limited to patients with mild AD dementia and evidence of amyloid pathology. Quantile regression may be a useful tool for retrospective investigation of completed treatment studies where universal confirmation of AD diagnosis was undetermined.
Acknowledgment
This study was supported by Eli Lilly and Company.
Footnotes
Y.-F.C., K.S., K.A., K.S., J.R., and R.A.D. are full-time employees and minor shareholders of Eli Lilly and Company. X.M. was a full-time employee of Eli Lilly and Company when this post-hoc analysis was conducted.
References
- 1.Glenner G.G., Wong C.W. Alzheimer's disease and Down syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun. 1984;122:1131–1135. doi: 10.1016/0006-291x(84)91209-9. [DOI] [PubMed] [Google Scholar]
- 2.Hardy J., Selkoe D.J. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 3.Jack C.R., Jr., Wiste H.J., Vemuri P., Weigand S.D., Senjem M.L., Zeng G., Alzheimer's Disease Neuroimaging Initiative Brain beta-amyloid measures and magnetic resonance imaging atrophy both predict time-to-progression from mild cognitive impairment to Alzheimer's disease. Brain. 2010;133:3336–3348. doi: 10.1093/brain/awq277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okello A., Koivunen J., Edison P., Archer H.A., Turkheimer F.E., Nagren K. Conversion of amyloid positive and negative MCI to AD over 3 years: An 11C-PIB PET study. Neurology. 2009;73:754–760. doi: 10.1212/WNL.0b013e3181b23564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Villegmagne V.L., Pike K.E., Chételat G., Ellis K.A., Mulligan R.S., Bourgeat P. Longitudinal assessment of Aβ and cognition in aging and Alzheimer disease. Ann Neurol. 2011;69:181–192. doi: 10.1002/ana.22248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rowe C.C., Bourgeat P., Ellis K.A., Brown B., Lim Y.Y., Mulligan R. Predicting Alzheimer disease with β-amyloid imaging: results from the Australian imaging, biomarkers, and lifestyle study of ageing. Ann Neurol. 2013;74:905–913. doi: 10.1002/ana.24040. [DOI] [PubMed] [Google Scholar]
- 7.DeMattos R.B., Bales K.R., Cummins D.J., Dodart J.C., Paul S.M., Holtzman D.M. Peripheral anti-A beta antibody alters CNS and plasma A beta clearance and decreases brain A beta burden in a mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2001;98:8850–8855. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siemers E.R., Friedrich S., Dean R.A., Gonzales C.R., Farlow M.R., Paul S.M. Safety and changes in plasma and cerebrospinal fluid amyloid β after a single administration of an amyloid β monoclonal antibody in subjects with Alzheimer disease. Clin Neuropharmacol. 2010;33:67–73. doi: 10.1097/WNF.0b013e3181cb577a. [DOI] [PubMed] [Google Scholar]
- 9.Mohs R.C., Knopman D., Petersen R.C., Ferris S.H., Ernesto C., Grundman M. Development of cognitive instruments for use in clinical trials of antidementia drugs: additions to the Alzheimer's Disease Assessment Scale that broaden its scope. Alzheimer Dis Assoc Disord. 1997;11:S13–S21. [PubMed] [Google Scholar]
- 10.Galasko D., Bennett D., Sano M., Ernesto C., Thomas R., Grundman M. An inventory to assess activities of daily living for clinical trials in Alzheimer's disease. Alzheimer Dis Assoc Disord. 1997;11:S33–S39. [PubMed] [Google Scholar]
- 11.Doody R.S., Thomas R.G., Farlow M., Iwatsubo T., Vellas B., Joffe S., Alzheimer's Disease Cooperative Study Steering Committee. Solanezumab Study Group Phase 3 trials of solanezumab for mild-to-moderate Alzheimer's disease. N Engl J Med. 2014;370:311–321. doi: 10.1056/NEJMoa1312889. [DOI] [PubMed] [Google Scholar]
- 12.Siemers E.R., Sundell K.L., Carlson C., Case M., Sethuraman G., Liu-Seifert H. Phase 3 solanezumab trials: secondary outcomes in mild Alzheimer's disease patients. Alzheimers Dement. 2016;12:110–120. doi: 10.1016/j.jalz.2015.06.1893. [DOI] [PubMed] [Google Scholar]
- 13.McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E.M. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 14.Dean R.A., Shaw L.M., Waligorska T., Korecka M., Figurski M., Trojanowski J.Q. Inclusion of patients with Alzheimer's disease pathology in solanezumab EXPEDITION3 using florbetapir (18F) PET imaging or INNO-BIA AlzBio3 CSF Aβ1-42. Alzheimers Dement. 2014;10:P811. [Google Scholar]
- 15.Salloway S., Sperling R., Gregg K., Yu P., Joshi A., Lu M. Incidence and clinical progression of placebo-treated amyloid-negative subjects with mild-to-moderate Alzheimer's disease (AD): results from the phase III PET substudies of bapineuzumab and solanezumab. Alzheimers Dement. 2013;9:P889. (P4–417) [Google Scholar]
- 16.Koenker R., Bassett G., Jr. Regression Quantiles. Econometrica. 1978;46:33–50. [Google Scholar]
- 17.Rowe C.C., Villemagne V.L. Amyloid Imaging with PET in Early Alzheimer Disease Diagnosis. Med Clin North Am. 2013;97:377–398. doi: 10.1016/j.mcna.2012.12.017. [DOI] [PubMed] [Google Scholar]