Abstract
Introduction
Alzheimer's disease is associated with early synaptic loss. Specific nutrients are known to be rate limiting for synapse formation. Studies have shown that administering specific nutrients may improve memory function, possibly by increasing synapse formation. This Dutch study explores the Effect of a specific Nutritional Intervention on cerebral Glucose Metabolism in early Alzheimer's disease (NL-ENIGMA, Dutch Trial Register NTR4718, http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=4718). The NL-ENIGMA study is designed to test whether the specific multinutrient combination Fortasyn Connect present in the medical food Souvenaid influences cerebral glucose metabolism as a marker for improved synapse function.
Methods
This study is a double-blind, randomized controlled parallel-group single-center trial. Forty drug-naive patients with mild cognitive impairment or mild dementia with evidence of amyloid deposition are 1:1 randomized to receive either the multinutrient combination or placebo once daily. Main exploratory outcome parameters include absolute quantitative positron emission tomography with 18F-fluorodeoxyglucose (including arterial sampling) and standard uptake value ratios normalized for the cerebellum or pons after 24 weeks.
Discussion
We expect the NL-ENIGMA study to provide further insight in the potential of this multinutrient combination to improve synapse function.
Keywords: Alzheimer's disease, Nutritional intervention, Diet, Randomized clinical trial, Synapse formation, Synaptic activity, [18F]FDG-PET
Highlights
-
•
This study explores the Effect of a specific Nutritional Intervention on cerebral Glucose Metabolism in early Alzheimer's disease (AD).
-
•
Forty drug-naive patients with mild cognitive impairment or mild dementia because of AD will be analyzed.
-
•
Synapse function is explored using positron emission tomography with 18F-fluorodeoxyglucose including arterial sampling.
1. Introduction
The complex pathophysiology of Alzheimer's disease (AD) has not yet been unravelled. Pathological changes associated with cognitive decline, hallmarking this progressive neurodegenerative disease, include accumulation of amyloid-β (Aβ) plaques and neurofibrillary tangles. Synaptic loss is already seen before these events occur, associated with degeneration of the synaptic membrane leading to reduced synaptic activity [1]. To date, there is no satisfactory treatment for AD. Current pharmaceutical treatments, such as acetylcholinesterase inhibitors donepezil, rivastigmine, galantamine, and the N-methyl-D-aspartic acid receptor antagonist memantine, only provide a temporary reduction of symptoms and are not without side effects, illustrating the strong unmet need for effective therapeutic interventions with fewer side effects.
Specific dietary intake, particularly the Mediterranean diet, has been shown to be associated with a reduced risk of cognitive decline and developing AD [2], [3], [4], [5], [6], [7], [8], [9], [10], [11]. Based on observations that the protective effects of diet cannot easily be attributed to the actions of individual nutrients and that the simultaneous enrichment of specific nutrients can act synergistically in simulating membrane phospholipid synthesis and increasing dendritic spine density, Souvenaid—containing the multinutrient combination Fortasyn Connect—has been developed to support synapse formation and function in AD [12], [13], [14]. This multinutrient combination comprises nutrients that act as precursors and cofactors in the synthesis pathway of phosphatides, that is, docosahexaenoic acid, eicosapentaenoic acid, uridine monophosphate, choline, phospholipids, vitamins B6, B12, C, and E, folate, and selenium. In AD patients, lower plasma and brain levels of these nutrients have been observed compared with controls [15]. In animal models, including transgenic AD mice, dietary intervention with this multinutrient combination has been shown to enhance phospholipid synthesis, to maintain white and gray matter integrity, to reduce the impact of amyloid-induced neurodegeneration and loss of functional connectivity, to increase numbers of hippocampal cholinergic synapses, and to improve cholinergic neurotransmission and hippocampus-dependent cognitive performance [13], [16], [17], [18], [19], [20].
Previous studies investigated the effect of Souvenaid in patients with AD. Small but significant positive effects have been observed on primary outcome memory function in patients with mild AD in two separate randomized controlled trials [21], [22]. No effects were observed on coprimary outcome Alzheimer's Disease Assessment Scale—Cognitive Subscale (ADAS-cog) [21]. In patients with more advanced AD, who were on stable AD medication, no significant add-on effect of the multinutritional intervention on primary outcome ADAS-cog was observed [23]. Results of a large European Union–funded study in prodromal AD patients are pending [24]. Probing the mode of action of Souvenaid, a previous study in patients with mild AD found effects on functional connectivity and brain network organization based on secondary outcome electroencephalography (EEG), suggesting that this multinutrient combination affects synapse function [22], [25]. Using magnetoencephalography (MEG) as exploratory outcome parameter, this effect could not be replicated in a smaller sample (Van Straaten et al., 2016, in preparation).
To further investigate the presumed effect of this multinutrient combination on synaptic function, we designed the Dutch double-blind randomized controlled parallel-group single-center study exploring the Effect of this specific Nutritional Intervention on cerebral Glucose Metabolism in early Alzheimer's disease (NL-ENIGMA, Dutch Trial Register NTR4718, http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=4718). Synaptic function is assessed using positron emission tomography with 18F-fluorodeoxyglucose ([18F]FDG-PET). [18F]FDG is a direct index for synapse function and density because the uptake of [18F]FDG is driven by synaptic terminals generating ATP for synthesis, release, and recycling of neurotransmitters, the maintenance of the normal resting potential, and the recovery from action potentials [26], [27], [28].
In the present study, the mode of action of the specific multinutrient combination is further explored using structural magnetic resonance imaging (MRI), resting-state functional MRI (rs-fMRI) networks, diffusion tensor imaging (DTI), structural brain networks, derived using graph theory based on DTI data, arterial spin labelling (ASL), and cerebrospinal fluid (CSF) and blood markers. We hypothesize that the intake of the multinutrient combination positively affects glucose metabolism after 24-week intervention compared with placebo.
2. Methods and design
2.1. Patients
We include 40 patients from the VU University Alzheimer Center outpatient memory clinic diagnosed with MCI or mild dementia because of AD (randomly distributed based on availability). Diagnoses are made in a multidisciplinary consensus team, including at least a neurologist, neuropsychologist, and neuroradiologist, and according to the core clinical criteria of the National Institute on Aging and Alzheimer's Association [29], [30], [31]. For diagnosis of MCI, impairment in one or more cognitive domains has to be present, based on clinical interpretation of performances on an extensive neuropsychological test battery, whereas independency of functional abilities is preserved [30]. Presence of AD pathology is evidenced by the presence of abnormal AD biomarkers: CSF tau/Aβ1–42 ratio > 0.52, positive [11C] Pittsburgh compound B (PiB), or [18F]Florbetaben PET scan [32]. PET scans are visually assessed by an experienced nuclear medicine physician (BNMvB). [11C]PiB PET scans (∼370 MBq, 60–90 minutes post injection) are considered positive when abnormal binding is seen in more than one cortical brain region of interest (ROI) (i.e., frontal, parietal, temporal, or occipital). [18F]Florbetaben PET scans (∼300 MBq, 90–110 minutes post injection) are considered positive when abnormal binding is seen in at least one cortical ROI (i.e., lateral temporal, frontal, posterior cingulate, precuneus, and parietal). Further inclusion criteria include 1) age between 50–85 (inclusive) years old, 2) MMSE ≥ 20, and 3) availability of a study partner. Exclusion criteria include 1) the presence of any other significant neurological or psychiatric disorder; 2) diabetes; 3) stroke or severe white matter hyperintensities (defined as Fazekas scale [33] score 3 on MRI); 4) use of donepezil, rivastigmine, galantamine, memantine, or Souvenaid within 3 months before baseline; 5) contraindications to PET, arterial sampling, or MRI; 6) use of omega-3 fatty acid–containing supplements or oily fish consumption more than twice a week within 2 months before baseline; 7) use of atropine, scopolamine, tolterodine, hyoscyamine, biperiden, benztropine, trihexyphenidyl, oxybutynin, antipsychotics, vitamins B, C, and/or E (>200% recommended daily intake), consumption of high-energy and/or protein nutritional supplements, a change in dose of lipid-lowering medication, antidepressants, and antihypertensive medication, or the use of other investigational products within 1 month before baseline; and 8) investigator's uncertainty regarding the willingness or ability of patient to comply with the protocol requirements.
2.2. Randomization
Patients eligible for inclusion are 1:1 randomized to receive the active product or the placebo product. To enable equal distribution of the active product over different stages of the disease, we stratify randomization based on MMSE score at screening (group 1: MMSE 20–24, group 2: MMSE 25–30). The details of the randomization are unknown to the investigators, site staff, and study staff from Nutricia Research.
2.3. Study product
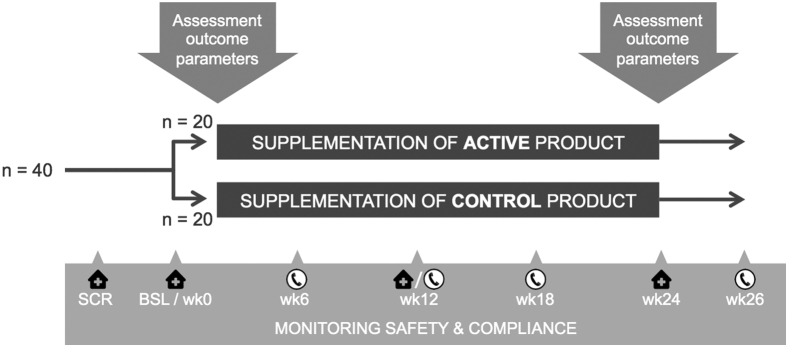
The active product contains the specific multinutrient combination Fortasyn Connect (for the nutritional composition, see Table 1). The placebo product is an isocaloric control drink lacking the specific multinutrient combination, but otherwise identical to the active product. Participants consume the products (125 mL) once daily at breakfast for a period of 24 weeks. To check product compliance, we ask participants and study partners to keep a diary for daily registration of product adherence. We check compliance with participant and partner at every visit and telephone call (Fig. 1).
Table 1.
Nutritional composition of Fortasyn Connect
| Component | Amount per daily dose∗ |
|---|---|
| Eicosapentaenoic acid | 300 mg |
| Docosahexaenoic acid | 1200 mg |
| Phospholipids | 106 mg |
| Choline | 400 mg |
| Uridine monophosphate | 625 mg |
| Vitamin E | 40 mg |
| Selenium | 60 μg |
| Vitamin B12 | 3 μg |
| Vitamin B6 | 1 mg |
| Folic acid | 400 μg |
| Vitamin C | 80 mg |
One bottle (125 mL) a day.
Fig. 1.
Schedule diagram of study design.
2.4. Procedures
Patients eligible for participation are provided with oral and written study information and at least a week for consideration. When interested in participation, participant and partner provide written informed consent at start of first visit. Screening and baseline visit are scheduled on the same day or with a maximum interval of 4 weeks. At baseline visit and after 24 weeks intervention, we collect all study parameters. During intervention period, three contact moments are scheduled to check product compliance, adverse events, and changes in medication use. A schematic diagram of the study design is presented at Fig. 1. We maintain a window for visits and phone calls of ±7 days. Also, we attempt to schedule baseline and follow-up MRI and PET scans on the same time of the day to limit influences of the circadian rhythm on outcome parameters (between and within patients).
During intervention, we request patients 1) not to use any of the products mentioned in the exclusion criteria; 2) not to change in dose or type of lipid lowering medication, antidepressants, and/or antihypertensive medication; and 3) not to use (unless strictly necessary) sedative hypnotics and/or anxiolytics within 3 days before study visit.
2.5. Main exploratory outcome parameters
Main objectives include exploring the effect of 24-week intervention with active product on cerebral glucose metabolism, assessed with [18F]FDG-PET imaging using quantification of regional cerebral metabolism rate for glucose, in patients with MCI or mild dementia because of AD by
-
1.
Absolute quantitative values using arterial sampling and kinetic analysis and
-
2.
Relative semiquantitative standardized uptake value ratios (SUVr) with normalization regions (cerebellum and pons) within a predefined standard uptake time interval of 45–60 minutes post injection.
2.6. Additional exploratory outcome parameters
Additionally, we explore the effect of 24-week intervention with active product on the following:
-
1.
Cerebral glucose metabolism as assessed with [18F]FDG-PET imaging using quantification of [18F]FDG uptake by semiquantitative SUV and SUVr, the latter using normalization regions (cerebellum and pons), within different uptake time intervals post injection;
-
2.
MRI measurements including a) atrophy, b) mean synchronization likelihood of the whole brain, c) mean fractional anisotropy of voxels in white matter skeleton, d) structural brain networks, e) mean cerebral blood flow in AD ROIs, and f) volume of arterial blood flow to the brain;
-
3.
Blood markers, including vitamin E, homocysteine, fatty acid profile in erythrocytes, and possible other nutritional markers (to be determined later);
-
4.
CSF markers, including Aβ1–42, Aβ1–40, total tau, tau phosphorylated at threonine-181 (ptau), and possible markers including nutritional parameters (to be determined later);
-
5.
Cognitive tests assessing memory, attention, and executive functioning; and
-
6.
Intake adherence, tolerance, and safety, including examination of patient medical history, recording of adverse events, and monitoring of vital signs and additional laboratory parameters.
2.7. Demographic and other baseline variables
At baseline, demographic information, that is, age, sex, ethnicity, educational attainment (using the system of Verhage [34], ranging from 1 [low] to 7 [high]), fish consumption, physical activity, smoking habits, alcohol consumption, family history of AD, date of diagnosis MCI or dementia, body height, and apolipoprotein (APOE) genotype, is collected. For APOE genotyping, DNA is isolated from 10 mL blood samples, collected in EDTA tubes. APOE genotype is determined using Light Cycler APOE mutation detection method (Roche Diagnostics GmbH, Mannheim, Germany).
2.8. PET assessment and analyses
A 60-minute dynamic [18F]FDG-PET scan (21 frames) is performed on a Philips TF PET-CT scanner. Patients are in fasting state for at least 8 hours. PET scan is preceded by the placement of an arterial cannula in one of the radial arteries. PET scan starts with a low-dose CT scan for attenuation correction of PET data, and the administration of approximately 180 MBq of [18F]FDG, dissolved in 5 mL of saline.
2.8.1. Quantitative values
Together with tracer administration, dynamic scanning and arterial blood sampling start. Arterial blood is withdrawn continuously at a rate of 5 mL/minute for the first 5 minutes and 2.5 mL/minute thereafter until 60 minutes after injection. At set times (5, 10, 20, 40 and 60 minutes), continuous withdrawal is interrupted briefly for the collection of manual blood samples (5 mL), used to estimate plasma-to-whole-blood ratios of radioactivity.
The whole-blood radioactivity concentration is continuously measured in a well counter, cross-calibrated against the PET scanner. Then, the plasma input function is derived by multiplying the measured whole-blood curve with the average plasma-to-blood ratios obtained from the discrete samples.
2.8.2. Semiquantitative values
Semiquantitative SUV and SUVr are measured using different uptake time windows after injection. SUVr are normalized to cerebellar gray matter and whole pons uptake values.
2.8.3. Region-of-interest analysis
We will define AD ROIs based on the MetaROI approach described by Landau et al. [35]. Furthermore, we will perform voxel-based analyses using statistical parametric mapping. [18F]FDG influx rate constants (Ki) for the whole brain are calculated with graphical analysis according to the Patlak method [36]. As an input function, we use plasma radioactivity concentration determined by arterial blood sampling. Because net [18F]FDG uptake, described by Ki, is directly proportional to glucose metabolic rate by multiplication with the plasma glucose concentration, the Ki results of the present study are valid for glucose metabolic rates.
2.8.4. Parametric image analysis
Ki images are calculated using the Patlak method, again for each combination of acquisition and reconstruction methods, and with blood-sampling data as the input function [36].
2.9. MRI assessment and analyses
MRI is performed on a 3-T whole-body MR system (Signa HDxt; GE medical Systems, Milwaukee, WI, USA) using an eight-channel head coil. Structural images include a sagittal 3D T1-weighted sequence for measuring atrophy rates in different brain regions using voxel-based morphometry. Mean synchronization likelihood of the brain is assessed using rs-fMRI. Mean fractional anisotropy of voxels in the white matter skeleton is assessed using DTI. Structural brain networks based on DTI data are derived using graph theory. Mean cerebral blood flow in AD ROIs is assessed using ASL, and volume of arterial blood flow to the brain (measured mean blood flow in the carotid arteries and basilar artery times the arterial lumen sizes) is assessed using q-flow.
2.10. Blood sampling and analyses
Venous blood is collected in fasting state. For future analysis, 12 mL clotted blood for serum and 18 mL EDTA blood for plasma are collected, aliquoted into 0.5 mL samples, and stored at −80°C according to the international standards [37]. For safety monitoring, 4 mL EDTA blood and 3 mL heparinized blood are collected and routinely analyzed.
2.11. CSF biomarkers
A total of 12.5 mL CSF is collected in two polypropylene tubes via a lumbar puncture in intervertebral space at level L3/L4, L4/L5, or L5/S1. Part of the CSF is used for routine analyses including number of leucocytes, total protein, and glucose. Within 2 h after collection, the rest is stored at −20°C for analysis of Aβ1–42, total tau, and ptau within 2 months using sandwich enzyme-linked immunosorbent assays (Innotest β-Amyloid1–42, Innotest hTAU-Ag, and Innotest Phosphotau(181P); Innogenetics, Gent, Belgium). The remainder of the CSF is directly transferred to the Alzheimer Center Biobank for storage and future analysis (including Aβ1–40). CSF is aliquoted into 0.5 mL samples and stored at −80°C according to the standard protocols [37].
2.12. Cognitive assessment
Memory is assessed using the Dutch version of the Rey Auditory Verbal Learning Test immediate and delayed recall and recognition test [38]. Attention and executive functioning are assessed using the Trail Making Test (TMT) A and TMT-B, respectively [39].
2.13. Sample size and interim analysis
To the best of our knowledge, there are no other randomized controlled trials that have investigated the effect of the active product using [18F]FDG-PET. Based on the previous [18F]FDG-PET studies, we have chosen a sample size of 40 completers [40], [41], [42], [43], [44], [45], [46], [47], assuming to be sufficient to have 80% power of detecting a difference between the groups of at least 0.91 standard deviation (SD) in an outcome parameter at a significance level of .05 in a two-sided t test.
A study-conduct independent statistician will conduct an interim analysis after 20 patients completed the study to evaluate the estimate used in the sample size calculation (of a difference between the groups of at least 0.91 SD). Interim analysis will be performed on the [18F]FDG-PET outcome parameters, using partially unblinded data. An independent Data Monitoring Committee will make recommendations based on the results of interim analyses. Only upward adaptation of the sample size is allowed.
2.14. Statistical analyses
Statistical analyses will be conducted using linear regression analyses, comparing change in outcome measure between the active and the placebo group, adjusted for the baseline value of the particular outcome and MMSE. Additionally, we will explore whether baseline MMSE modifies the intervention effect. The main outcome parameters will be explored based on per-protocol analyses. Additional intention-to-treat analyses will be carried out.
The significance level for the analysis of outcome variables will be set at <.05 in a two-sided test. Several potential covariates and possible intervention effect moderators were defined: MMSE at screening, diagnosis of dementia, relevant medical events, relevant medication, coexisting diseases, all the demographic and other baseline variables, and product compliance.
2.15. Ethical and legal considerations
This study follows the Helsinki Declaration's principles, meaning that all patients sign a written informed consent stating that participation is voluntary and that participation can be withdrawn at any time. The Local Medical Ethics Review Committee approved the study on February 12, 2015.
3. Discussion
The NL-ENIGMA study aims to explore the effect of Souvenaid on cerebral glucose metabolism in mild to very mild patients with biomarker proven AD. The study is a single-center randomized controlled trial with double-blind 24-week intervention of Souvenaid or placebo in 40 drug-naive patients with MCI or mild dementia and presence of amyloid burden. Main exploratory outcome parameters include absolute quantitative [18F]FDG uptake and relative semiquantitative SUVr within a predefined time window. Additionally, [18F]FDG SUV and SUVr using different uptake time intervals, MRI, CSF and blood markers, and cognitive tests are explored. First participant was included in March 2015. We hypothesize to observe a positive effect of the nutritional intervention compared with control product on cerebral [18F]FDG uptake in AD-specific regions.
We use an exploratory approach because the effect of the specific multinutrient combination has never been assessed using [18F]FDG-PET. Previous studies have indicated that this nutritional intervention increases synaptic density, enhances cholinergic neurotransmission, and reduces the impact of amyloid-induced neurodegeneration and loss of functional connectivity [13], [16], [17], [18], [19], [20]. A previous clinical study in patients with mild AD using EEG demonstrated an effect of the multinutrient combination on functional connectivity and brain network organization, suggesting that its mode of action includes alteration of synapse function [22], [25]. A subsequent MEG study failed to replicate this finding, possibly because of lack of power and imbalanced group characterization (Van Straaten et al., 2016, in preparation).
[18F]FDG-PET is a well-known method to study synapse function [26], [27], [28]. As main outcome parameters, we include quantitative [18F]FDG values because the active product could affect the reference region. Sample size and duration of intervention are based on [18F]FDG outcome parameters. Additionally, SUVr, several MRI sequences, blood and CSF markers, and cognitive tests will be explored. Because our approach is mainly biological and mechanical, we will primarily conduct per-protocol analyses.
In conclusion, the NL-ENIGMA study is a randomized controlled trial exploring the effect of Souvenaid on brain glucose metabolism as measurement for synapse function in 40 patients with MCI or early dementia because of AD.
Research in Context.
-
1.
Systematic review: We searched PubMed for publications regarding the effect of nutritional interventions on synapse formation in Alzheimer's disease (AD).
-
2.
Interpretation: Specific dietary intake, particularly the Mediterranean diet, has been shown to be associated with a reduced risk of cognitive decline and developing AD. Several nutrients (e.g., docosahexaenoic acid, eicosapentaenoid acid, uridine monophosphate, choline, phospholipids, certain vitamins, and selenium) together act as precursors and cofactors in the synthesis pathway of phosphatides, essential in formation of synapses. Souvenaid contains the specific combination of these nutrients. In humans, positron emission tomography with 18F-fluporodeoxyglucose ([18F]FDG-PET) is the best technique to investigate synapse function but has not been used yet in combination with Souvenaid.
-
3.
Future directions: In the present Dutch study exploring the Effect of a specific Nutritional Intervention on cerebral Glucose Metabolism in early Alzheimer's disease (NL-ENIGMA), we hypothesize that the active product will have a positive effect compared with the control product on cerebral [18F]FDG uptake in AD-specific regions. We expect the present study to provide further insight into the potential of an intervention to target synaptic function in AD.
Acknowledgments
The NL-ENIGMA study is a collaboration between the VU University Medical Center (VUmc) and Nutricia Research. VUmc is the sponsor and is responsible for all aspects of the clinical study. Nutricia Research is supporting as follows:
-
•
responsible for the production of the study products and the distribution of the study products to VUmc,
-
•
contributes in safety monitoring,
-
•
contributes scientific expertise for the clinical study.
-
•
study monitoring is the responsibility of VUmc, and Nutricia Research will perform comonitoring, and
-
•
VUmc investigators have access to the final dataset, and Nutricia Research will provide additional statistical input.
Conflicts of interest: The NL-ENIGMA study is an investigator-initiated study funded by the Netherlands Organization for Scientific Research (NWO) within the Food, Cognition, and Behavior (FCB) initiative, project N°057-13-003. N.M.E. Scheltens and I. Kuyper are funded by NWO (project nr 057-13-003). F. Barkhof serves/has served on the advisory boards of Bayer-Schering Pharma, Sanofi-Aventis, Biogen Idec, UCB, Merck-Serono, Novartis, and Roche. He has been a speaker at symposia organized by the Serono Symposia Foundation. For all his activities, he receives no personal compensation. C.E. Teunissen serves on the advisory board of Fujirebio and Roche, received research consumables from Euroimmun, IBL, Fujirebio, Invitrogen, and Mesoscale Discovery, performed contract research for IBL, Shire, Boehringer, Roche and Probiodrug, and also received grants from the European Commission, the Dutch Research Council (ZonMW), Association of Frontotemporal Dementia/Alzheimer's Drug Discovery Foundation, ISAO, and the Alzheimer's Drug Discovery Foundation. Dr. Teunissen received research consumables from Euroimmun, IBL, Fujirebio, Invitrogen, and Mesoscale Discovery and performed contract research for IBL, Shire, Boehringer, Roche, and Probiodrug. L.M. Broersen and M.M. Lansbergen are full-time employees of Nutricia Research. Ph. Scheltens is fully employed by VU University Medical Center, Amsterdam, and has received grant support (for the institution) from GE Healthcare, Nutricia Research, Piramal, and MERCK. In the past 2 years, he has received consultancy/speaker fees (paid to the institution) from Probiodrug, EIP Pharma, Piramal, and GE Healthcare. R. Boellaard, W.M. van der Flier, and B.N.M. van Berckel report no conflicts of interest.
References
- 1.Selkoe D.J. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 2.Barberger-Gateau P., Letenneur L., Deschamps V., Pérès K., Dartigues J.F., Renaud S. Fish, meat, and risk of dementia: cohort study. BMJ. 2002;325:932–933. doi: 10.1136/bmj.325.7370.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morris M.C., Evans D.A., Bienias J.L., Tangney C.C., Bennett D.A., Wilson R.S. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch Neurol. 2003;60:940–946. doi: 10.1001/archneur.60.7.940. [DOI] [PubMed] [Google Scholar]
- 4.Kyle D.J., Schaefer E., Patton G., Beiser A. Low serum docosahexaenoic acid is a significant risk factor for Alzheimer's dementia. Lipids. 1999;34:S245. doi: 10.1007/BF02562306. [DOI] [PubMed] [Google Scholar]
- 5.Kalmijn S., Launer L.J., Ott A., Witteman J.C., Hofman A., Breteler M.M. Dietary fat intake and the risk of incident dementia in the Rotterdam Study. Ann Neurol. 1997;42:776–782. doi: 10.1002/ana.410420514. [DOI] [PubMed] [Google Scholar]
- 6.Zandi P.P., Anthony J.C., Khachaturian A.S., Stone S.V., Gustafson D., Tschanz J.T. Reduced risk of Alzheimer disease in users of antioxidant vitamin supplements: the Cache County Study. Arch Neurol. 2004;61:82–88. doi: 10.1001/archneur.61.1.82. [DOI] [PubMed] [Google Scholar]
- 7.Engelhart M.J., Geerlings M.I., Ruitenberg A., van Swieten J.C., Hofman A., Witteman J.C.M. Dietary intake of antioxidants and risk of Alzheimer disease. JAMA. 2002;287:3223–3229. doi: 10.1001/jama.287.24.3223. [DOI] [PubMed] [Google Scholar]
- 8.van Gelder B.M., Tijhuis M., Kalmijn S., Kromhout D. Fish consumption, n-3 fatty acids, and subsequent 5-y cognitive decline in elderly men: the Zutphen Elderly Study. Am J Clin Nutr. 2007;85:1142–1147. doi: 10.1093/ajcn/85.4.1142. [DOI] [PubMed] [Google Scholar]
- 9.Tsivgoulis G., Judd S., Letter A.J., Alexandrov A.V., Howard G., Nahab F. Adherence to a Mediterranean diet and risk of incident cognitive impairment. Neurology. 2013;80:1684–1692. doi: 10.1212/WNL.0b013e3182904f69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh B., Parsaik A.K., Mielke M.M., Erwin P.J., Knopman D.S., Petersen R.C. Association of Mediterranean diet with mild cognitive impairment and Alzheimer's disease: a systematic review and meta-analysis. J Alzheimers Dis. 2014;39:271–282. doi: 10.3233/JAD-130830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Psaltopoulou T., Sergentanis T.N., Panagiotakos D.B., Sergentanis I.N., Kosti R., Scarmeas N. Mediterranean diet, stroke, cognitive impairment, and depression: a meta-analysis. Ann Neurol. 2013;74:580–591. doi: 10.1002/ana.23944. [DOI] [PubMed] [Google Scholar]
- 12.Wurtman R.J., Cansev M., Sakamoto T., Ulus I.H. Use of phosphatide precursors to promote synaptogenesis. Annu Rev Nutr. 2009;29:59–87. doi: 10.1146/annurev-nutr-080508-141059. [DOI] [PubMed] [Google Scholar]
- 13.van Wijk N., Broersen L.M., de Wilde M.C., Hageman R.J., Groenendijk M., Sijben J.W. Targeting synaptic dysfunction in Alzheimer's disease by administering a specific nutrient combination. J Alzheimers Dis. 2014;38:459–479. doi: 10.3233/JAD-130998. [DOI] [PubMed] [Google Scholar]
- 14.Mi W., van Wijk N., Cansev M., Sijben J.W., Kamphuis P.J. Nutritional approaches in the risk reduction and management of Alzheimer's disease. Nutrition. 2013;29:1080–1089. doi: 10.1016/j.nut.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 15.Lopes da Silva S., Vellas B., Elemans S., Luchsinger J., Kamphuis P., Yaffe K. Plasma nutrient status of patients with Alzheimer's disease: systematic review and meta-analysis. Alzheimers Dement. 2014;10:485–502. doi: 10.1016/j.jalz.2013.05.1771. [DOI] [PubMed] [Google Scholar]
- 16.Cansev M., van Wijk N., Turkyilmaz M., Orhan F., Sijben J.W., Broersen L.M. Specific multi-nutrient enriched diet enhances hippocampal cholinergic transmission in aged rats. Neurobiol Aging. 2015;36:344–351. doi: 10.1016/j.neurobiolaging.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 17.Koivisto H., Grimm M.O., Rothhaar T.L., Berkecz R., Lütjohann D., Giniatullina R. Special lipid-based diets alleviate cognitive deficits in the APPswe/PS1dE9 transgenic mouse model of Alzheimer's disease independent of brain amyloid deposition. J Nutr Biochem. 2014;25:157–169. doi: 10.1016/j.jnutbio.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 18.Wiesmann M., Jansen D., Zerbi V., Broersen L.M., Garthe A., Kiliaan A.J. Improved spatial learning strategy and memory in aged Alzheimer AβPPswe/PS1dE9 mice on a multi-nutrient diet. J Alzheimers Dis. 2013;37:233–245. doi: 10.3233/JAD-130179. [DOI] [PubMed] [Google Scholar]
- 19.Janickova H., Rudajev V., Dolejsi E., Koivisto H., Jakubik J., Tanila H. Lipid-based diets improve muscarinic neurotransmission in the hippocampus of transgenic APPswe/PS1dE9 mice. Curr Alzheimer Res. 2015;12:923–931. doi: 10.2174/1567205012666151027130350. [DOI] [PubMed] [Google Scholar]
- 20.Zerbi V., Jansen D., Wiesmann M., Fang X., Broersen L.M., Veltien A. Multinutrient diets improve cerebral perfusion and neuroprotection in a murine model of Alzheimer's disease. Neurobiol Aging. 2014;35:600–613. doi: 10.1016/j.neurobiolaging.2013.09.038. [DOI] [PubMed] [Google Scholar]
- 21.Scheltens P., Kamphuis P.J., Verhey F.R., Olde Rikkert M.G., Wurtman R.J., Wilkinson D. Efficacy of a medical food in mild Alzheimer's disease: a randomized, controlled trial. Alzheimers Dement. 2010;6:1–10.e1. doi: 10.1016/j.jalz.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 22.Scheltens P., Twisk J.W., Blesa R., Scarpini E., Arnim von C.A., Bongers A. Efficacy of Souvenaid in mild Alzheimer's disease: results from a randomized, controlled trial. J Alzheimers Dis. 2012;31:225–236. doi: 10.3233/JAD-2012-121189. [DOI] [PubMed] [Google Scholar]
- 23.Shah R.C., Kamphuis P.J., Leurgans S., Swinkels S.H., Sadowsky C.H., Bongers A. The S-Connect study: results from a randomized, controlled trial of Souvenaid in mild-to-moderate Alzheimer's disease. Alzheimers Res Ther. 2013;5:59. doi: 10.1186/alzrt224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soininen H., Visser P., Kivipelto M., Hartmann T. A clinical trial investigating the effects of fortasyn connect (souvenaid) in prodromal Alzheimer's disease: results of the LipiDiDiet study. Neurobiol Aging. 2016;39:S23. [Google Scholar]
- 25.de Waal H., Stam C.J., Lansbergen M.M., Wieggers R.L., Kamphuis P.J., Scheltens P. The effect of souvenaid on functional brain network organisation in patients with mild Alzheimer's disease: a randomised controlled study. PLoS One. 2014;9:e86558. doi: 10.1371/journal.pone.0086558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sperling R.A., Aisen P.S., Beckett L.A., Bennett D.A., Craft S., Fagan A.M. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reiman E.M., Chen K., Alexander G.E., Caselli R.J., Bandy D., Osborne D. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer's dementia. Proc Natl Acad Sci U S A. 2004;101:284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Wilde M.C., Kamphuis P.J., Sijben J.W., Scheltens P. Utility of imaging for nutritional intervention studies in Alzheimer's disease. Eur J Pharmacol. 2011;668:S59–S69. doi: 10.1016/j.ejphar.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 29.van der Flier W.M., Pijnenburg Y.A., Prins N., Lemstra A.W., Bouwman F.H., Teunissen C.E. Optimizing patient care and research: the Amsterdam Dementia Cohort. J Alzheimers Dis. 2014;41:313–327. doi: 10.3233/JAD-132306. [DOI] [PubMed] [Google Scholar]
- 30.Albert M.S., DeKosky S.T., Dickson D., Dubois B., Feldman H.H., Fox N.C. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKhann G.M., Knopman D.S., Chertkow H., Hyman B.T., Jack C.R., Kawas C.H. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duits F.H., Teunissen C.E., Bouwman F.H., Visser P.J., Mattsson N., Zetterberg H. The cerebrospinal fluid “Alzheimer profile”: easily said, but what does it mean? Alzheimers Dement. 2014;10:713–723.e2. doi: 10.1016/j.jalz.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 33.Fazekas F., Chawluk J.B., Alavi A., Hurtig H.I., Zimmerman R.A. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol. 1987;149:351–356. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- 34.Verhage F. Intelligence and religious persuasion. Ned Tijdschr Psychol. 1964;19:247–254. [PubMed] [Google Scholar]
- 35.Landau S.M., Harvey D., Madison C.M., Koeppe R.A., Reiman E.M., Foster N.L. Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol Aging. 2011;32:1207–1218. doi: 10.1016/j.neurobiolaging.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patlak C.S., Blasberg R.G. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Generalizations. J Cereb Blood Flow Metab. 1985;5:584–590. doi: 10.1038/jcbfm.1985.87. [DOI] [PubMed] [Google Scholar]
- 37.del Campo M., Mollenhauer B., Bertolotto A., Engelborghs S., Hampel H., Simonsen A.H. Recommendations to standardize preanalytical confounding factors in Alzheimer's and Parkinson's disease cerebrospinal fluid biomarkers: an update. Biomark Med. 2012;6:419–430. doi: 10.2217/bmm.12.46. [DOI] [PubMed] [Google Scholar]
- 38.Saan R.J., Deelman B.G. Afd. Neuropsychologie, AZG (interne publicatie); Groningen: 1986. De 15-woordentest A en B (een voorlopige handleiding) [Google Scholar]
- 39.Reitan R.M. Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual Mot Skills. 1958;8:271–276. [Google Scholar]
- 40.Ossenkoppele R., Tolboom N., Foster-Dingley J.C., Adriaanse S.F., Boellaard R., Yaqub M. Longitudinal imaging of Alzheimer pathology using [11C]PIB, [18F]FDDNP and [18F]FDG PET. Eur J Nucl Med Mol Imaging. 2012;39:990–1000. doi: 10.1007/s00259-012-2102-3. [DOI] [PubMed] [Google Scholar]
- 41.Mega M.S., Dinov I.D., Porter V., Chow G., Reback E., Davoodi P. Metabolic patterns associated with the clinical response to galantamine therapy: a fludeoxyglucose f 18 positron emission tomographic study. Arch Neurol. 2005;62:721–728. doi: 10.1001/archneur.62.5.721. [DOI] [PubMed] [Google Scholar]
- 42.Potkin S.G., Anand R., Fleming K., Alva G., Keator D., Carreon D. Brain metabolic and clinical effects of rivastigmine in Alzheimer's disease. Int J Neuropsychopharmacol. 2001;4:223–230. doi: 10.1017/S1461145701002528. [DOI] [PubMed] [Google Scholar]
- 43.Stefanova E., Wall A., Almkvist O., Nilsson A., Forsberg A., Långström B. Longitudinal PET evaluation of cerebral glucose metabolism in rivastigmine treated patients with mild Alzheimer's disease. J Neural Transm (Vienna) 2006;113:205–218. doi: 10.1007/s00702-005-0312-6. [DOI] [PubMed] [Google Scholar]
- 44.Teipel S.J., Drzezga A., Bartenstein P., Möller H.J., Schwaiger M., Hampel H. Effects of donepezil on cortical metabolic response to activation during (18)FDG-PET in Alzheimer's disease: a double-blind cross-over trial. Psychopharmacology (Berl) 2006;187:86–94. doi: 10.1007/s00213-006-0408-1. [DOI] [PubMed] [Google Scholar]
- 45.Tune L., Tiseo P.J., Ieni J., Perdomo C., Pratt R.D., Votaw J.R. Donepezil HCl (E2020) maintains functional brain activity in patients with Alzheimer disease: results of a 24-week, double-blind, placebo-controlled study. Am J Geriatr Psychiatry. 2003;11:169–177. [PubMed] [Google Scholar]
- 46.Wang T., Huang Q., Reiman E.M., Chen K., Li X., Li G. Effects of memantine on clinical ratings, fluorodeoxyglucose positron emission tomography measurements, and cerebrospinal fluid assays in patients with moderate to severe Alzheimer dementia: a 24-week, randomized, clinical trial. J Clin Psychopharmacol. 2013;33:636–642. doi: 10.1097/JCP.0b013e31829a876a. [DOI] [PubMed] [Google Scholar]
- 47.Herholz K., Westwood S., Haense C., Dunn G. Evaluation of a calibrated (18)F-FDG PET score as a biomarker for progression in Alzheimer disease and mild cognitive impairment. J Nucl Med. 2011;52:1218–1226. doi: 10.2967/jnumed.111.090902. [DOI] [PubMed] [Google Scholar]