Abstract
Background
Alzheimer's disease (AD) is growing in frequency and new therapies are urgently needed.
Methods
We assessed clinicaltrials.gov (accessed 1-4-2016) to determine the number and characteristics of trials in phase I, phase II, and phase III for treatment of AD.
Results
There are currently 24 agents in 36 trials in phase III of AD drug development. Seven of these 24 agents are symptomatic cognitive-enhancing compounds, and 17 are disease-modifying treatments (DMTs). Most DMTs address amyloid-related targets (76%). There are 45 agents in phase II being assessed in 52 clinical trials. Phase II trials include 30 DMTs, with 26 small molecules and 4 immunotherapies. There are 24 agents in the first phase of AD drug development.
Discussion
Amyloid is the principal target of late-stage development programs. There are relatively few agents in clinical trials for AD suggesting a need to amplify the drug discovery ecosystem.
Keywords: Alzheimer's disease, Drug development, Phase I, Phase II, Phase III, Biomarkers, Amyloid, Tau, Cognitive enhancement
Alzheimer's disease (AD) is rapidly becoming a major public health threat with increasing numbers of affected individuals as the world's population ages. There are currently 5.3 million Americans and 35 million people worldwide with AD dementia, and the number will increase to nearly 15 million in the United States and over 100 million globally by 2050 if treatments are not found [1], [2].
New therapies are needed for this burgeoning population of affected and at-risk persons that improve the symptoms of patients with memory and cognitive decline, prevent or delay the onset of AD in individuals who are at-risk for the disease, or slow progression in those with declining cognition. New therapies are being assessed in clinical trials but the success rate of AD drug development has been low with the last new novel agent approved in 2003 [3].
To gain insight into the current AD treatment pipeline, we reviewed all trials registered in clinicaltrials.gov (accessed 1/4/2016), the US government website that lists all US and most global clinical trials. Registration of new trials on the site is required for trials approved by the US Food and Drug Administration (FDA) since 2007 [4]. We reviewed this comprehensive website for all agents in clinical trials for AD dividing them into those in phase I, phase II, and phase III. The purpose of the study was to understand the landscape of AD drug development and determine evolutions occurring in AD drug development from historical practices. The goal is to assess the state of AD drug development, anticipate the emergence of new therapies, review emerging pharmacologic mechanisms and clinical trial approaches, and derive lessons possibly helpful in the drug development process.
1. Methods
We interrogated clinicaltrials.gov with the information summarized here accessed on January 4, 2016. We used the search features of the site to capture all agents listed for AD in phase I, II, and III. We captured the trial title, beginning date, anticipated ending date, anticipated duration, number of subjects to be enrolled, number of arms of the study (usually a placebo arm and one or more treatment arms with different doses of the test agent), whether a biomarker was described, and whether the sponsor was a biopharma company, the National Institutes of Health (NIH), a combination of biopharma and NIH, or “other.” We included trials that were recruiting, active but not recruiting—trials that have completed recruiting and are continuing as the efficacy or safety of the agent is being determined—and enrolling by invitation. We did not include trials listed as not yet recruiting, completed, terminated, suspended, or withdrawn. These exclusions were based on our interest in the currently active pipeline and what agents could evolve in the near term. Reasons for terminating, suspending, or withdrawing trials are often not provided, and we could not draw conclusions about these trials or the agents involved. The agents and trials reviewed comprise a comprehensive list of agents currently in trials. The list is not exhaustive because not all non-US trials are registered on clinicaltrials.gov, and there is sometimes a delay in registering trials. The mechanism of action of each agent was determined from the information on clinicaltrials.gov (e.g., the mechanism is often noted in the title of the trial or in a description of the trial) or from a comprehensive search of the literature if the mechanism was not provided on the federal website. In a few cases, the mechanism is undisclosed. We grouped the mechanisms into symptomatic or disease modifying. We further divided the symptomatic agents into those that were putative cognitive-enhancing agents or those that addressed neuropsychiatric symptoms. Disease-modifying therapies (DMT) were divided into those that targeted amyloid-related targets, those that aimed at modifying tau-related mechanisms and those with “other” mechanisms such as neuroprotection or metabolic effects [5]. The definitions of disease-modification and neuroprotection are controversial and evolving [6], [7]; the terminology is used here to conveniently classify the types of mechanisms for agents in current AD drug-development programs. We did not include nonpharmacologic therapeutic approaches such as devices, cognitive therapies, and medical foods.
2. Results
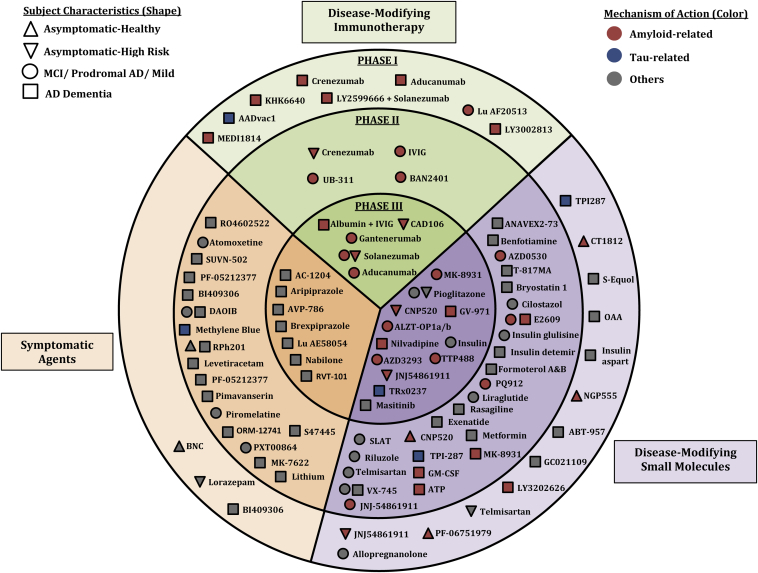
There are currently 93 agents in some phase of drug development for AD. Fig. 1 provides a comprehensive overview of the agents currently in clinical trials for AD.
Fig. 1.
Agents currently in clinical trials for AD (shape indicates stage of disease of patients in the trials; color shows the mechanism of action; location shows phase of development and category of activity—immunotherapy, disease-modifying small molecule, symptom-reducing small molecule).
2.1. Phase III
There are 24 agents in 36 trials in phase III of AD drug development. Eight agents are in two or more clinical trials. Of the agents in trials, seven are symptomatic treatments targeting neurotransmitter pathways with cognitive enhancement (3) or neuropsychiatric (4) effects. Encenicline, a nicotinic cognitive-enhancing agent, was put on clinical hold by the FDA pending the review of gastrointestinal effects seen in some trial participants. Of the 17 DMTs in phase III, 12 are small molecules, and 5 are immunotherapies. All the immunotherapies and 8 of the 12 small molecules are directed at amyloid-related targets. There are four beta-site amyloid precursor protein cleaving enzyme (BACE) inhibitors in phase III trials. Amyloid-targeting agents comprise 76% of the late-stage DMT pipeline. There is one antitau agent in phase III—TRx0237.
The mean duration of trials of symptomatic agents was 23.3 weeks; the mean duration of DMT trials was 114.1 weeks. In these phase III trials, the mean number of subjects per arm for symptomatic trials is 392.2 and for trials of DMT agents is 516.1.
Eighty-eight percent (32 of 36) of trials are sponsored by the biopharma industry, 2 are jointly sponsored by NIH and industry, and 2 are sponsored by “other” entities.
Table 1 shows the agents in phase III with their mechanism of action.
Table 1.
Agents currently in phase III of development and their mechanism of action (as of 1/4/2016)
| Agent | Agent mechanism class | Mechanism of action | Clinicaltrials.gov ID | Sponsor | Start date | Estimated end date |
|---|---|---|---|---|---|---|
| AC-1204 | Metabolic | Ketogenic agent | NCT01741194 | Accera | Mar 13 | Oct 17 |
| Aducanumab | Antiamyloid | Monoclonal antibody | NCT02484547 | Biogen | Sep 15 | Feb 22 |
| NCT02477800 | Biogen | Aug 15 | Feb 22 | |||
| Albumin + Immunoglobulin | Antiamyloid | Polyclonal antibody | NCT01561053 | Instituto Grifols, S.A. | Mar 12 | Dec 16 |
| ALZT-OP1a + ALZT-OP1b | Antiamyloid | Antiamyloid combination (undisclosed target) | NCT02547818 | AZTherapies | Sep 15 | Mar 18 |
| Aripiprazole | Neurotransmitter based | Atypical anti-psychotic | NCT02168920 | Otsuka | Jun 14 | Jul 17 |
| AVP-786 | Neurotransmitter based | Mixed transmitter effect | NCT02442765 | Avanir | Sep 15 | Jul 18 |
| NCT02446132 | Avanir | Dec 15 | Jul 19 | |||
| AZD3293 | Antiamyloid | BACE inhibitor | NCT02245737 | AstraZeneca | Sep 14 | May 19 |
| Brexpiprazole (OPC-34712) | Neurotransmitter based | Atypical anti-psychotic | NCT01862640 | Otsuka | Jul 13 | Jun 17 |
| NCT01922258 | Otsuka | Sep 13 | Jun 17 | |||
| CAD106 | Anti-amyloid | Amyloid vaccine | NCT02565511 | Novartis | Nov 15 | Aug 23 |
| CNP520 | Anti-amyloid | BACE inhibitor | NCT02565511 | Novartis | Nov 15 | Aug 23 |
| Gantenerumab | Anti-amyloid | Monoclonal antibody | NCT02051608 | Hoffmann-La Roche | Mar 14 | Mar 19 |
| NCT01224106 | Hoffmann-La Roche | Nov 10 | Oct 20 | |||
| NCT01760005∗ | Washington University School of Medicine | Dec 12 | Dec 19 | |||
| Idalopirdine (Lu AE58054) | Neurotransmitter based | 5-HT6 antagonist | NCT02079246 | H. Lundbeck A/S | Apr 14 | Oct 17 |
| NCT02006654 | H. Lundbeck A/S | Mar 14 | Mar 17 | |||
| NCT02006641 | H. Lundbeck A/S | Feb 14 | Mar 17 | |||
| NCT01955161 | H. Lundbeck A/S | Oct 13 | Oct 16 | |||
| Insulin (Humulin) | Metabolic | Metabolic agent | NCT01767909 | University of Southern California | Sep 13 | Feb 17 |
| JNJ-54861911 | Anti-amyloid | BACE inhibitor | NCT02569398 | Janssen | Oct 15 | May 23 |
| Masitinib | Anti-inflammatory, neuroprotective | Tyrosine kinase inhibitor | NCT01872598 | AB Science | Jan 12 | Dec 16 |
| MK-8931 (Verubecestat) | Anti-amyloid | BACE inhibitor | NCT01953601 | Merck Sharp & Dohme Corp. | Nov 13 | Mar 21 |
| Nabilone | Neurotransmitter based | Cannabinoid (receptor agent) | NCT02351882 | Sunnybrook Health Sciences Centre | Jan 15 | Dec 17 |
| Nilvadipine | Anti-amyloid | Calcium channel blocker | NCT02017340 | St. James's Hospital, Ireland | Oct 12 | Dec 17 |
| Pioglitazone | Metabolic | PPAR-gamma agonist; anti-amyloid effect | NCT02284906 | Takeda | Feb 15 | Apr 21 |
| NCT01931566 | Takeda | Aug 13 | Jul 19 | |||
| RVT-101 | Neurotransmitter based | 5-HT6 antagonist | NCT02585934 | Axovant Sciences | Oct 15 | Oct 17 |
| Sodium oligo-mannurarate (GV-971) | Anti-amyloid | Anti-amyloid agent | NCT02293915 | Shanghai Greenvalley Pharmaceutical Co., Ltd. | Apr 14 | May 17 |
| Solanezumab | Anti-amyloid | Monoclonal antibody | NCT02008357 | Eli Lilly and Company | Feb 14 | Apr 20 |
| NCT01127633 | Eli Lilly and Company | Dec 10 | Nov 18 | |||
| NCT01900665 | Eli Lilly and Company | Jul 13 | Oct 18 | |||
| NCT01760005∗ | Washington University School of Medicine | Dec 12 | Dec 19 | |||
| TRx0237 | Antitau | Anti-tau agent | NCT02245568 | TauRx Therapeutics | Aug 14 | Jan 17 |
| NCT01689246 | TauRx Therapeutics | Jan 13 | Nov 15 | |||
| NCT01689233 | TauRx Therapeutics | Oct 12 | May 16 | |||
| TTP488 (Azeliragon) | Anti-amyloid, anti-inflammatory | Anti-amyloid RAGE antagonist | NCT02080364 | TransTech Pharma | Apr 15 | Mar 18 |
Abbreviations: BACE, beta-site amyloid precursor protein cleaving enzyme; PPAR, peroxisome proliferator-activated receptor; RAGE, receptor for advanced glycation end products.
NOTE. Twenty-four agents in 36 phase III clinical trials currently ongoing (active, not recruiting, and active, recruiting) as of January 4, 2016 according to clinicaltrials.gov.
Same trial studying gantenerumab and solanezumab independently.
2.2. Phase II
There are 45 agents in phase II of AD drug development being assessed in 52 clinical trials. The pipeline includes 12 symptomatic cognitive-enhancing agents and three agents addressing neuropsychiatric symptoms. There are 30 DMTs being studied in phase II drug development programs; 26 of these are small molecules and four are immunotherapies. Amyloid-related targets comprise the mechanism of action of nine of the 26 small molecules and all four of the immunotherapies. Forty-three percent of phase II DMTs have amyloid-targeting mechanisms of action. Sixteen agents have “other mechanisms” including ten putative neuroprotective agents and six addressing metabolic problems. There is one antitau agent in phase II and one stem cell program (with two trials) in phase II of development.
Phase II trials of symptomatic agents have a mean duration of 19.1 weeks and trials of DMTs in phase II have a mean duration of 49.5 weeks. On average, there are 67.1 subjects per arm in phase II trials of symptomatic treatments and 76.9 subjects per arm in trials of DMT agents.
Of the 52 trials for the 45 agents, 29 are industry-sponsored, four are sponsored by NIH, and 18 are sponsored by “other” entities such as academic medical centers and philanthropic foundations. One trial is jointly sponsored by NIH and industry.
Table 2 shows the agents in phase II with their mechanism of action.
Table 2.
Agents currently in phase II of AD drug development and their mechanism of action (as of 1/4/2016)
| Agent | Agent mechanism class | Mechanism of action | Clinicaltrials.gov ID | Sponsor | Start date | Estimated end date |
|---|---|---|---|---|---|---|
| Adenosine triphosphate | Antiamyloid | Inhibits amyloid misfolding and toxicity | NCT02279511 | Fundació Clínic per la Recerca Biomèdica | Nov 14 | Nov 16 |
| ANAVEX 2-73 | Neuroprotective | Sigma-1 receptor agonist | NCT02244541 | Anavex Life Sciences Corp. | Dec 14 | Oct 16 |
| Atomoxetine | Antiamyloid | Adrenergic uptake inhibitor | NCT01522404 | Emory University | Mar 12 | Dec 17 |
| AZD0530 (saracatinib) | Antiamyloid | Kinase inhibitor | NCT02167256 | Yale University | Dec 14 | Dec 16 |
| BAN2401 | Antiamyloid | Monoclonal antibody | NCT01767311 | Eisai | Dec 12 | Jul 18 |
| Benfotiamine | Metabolic | Antioxidant | NCT02292238 | Burke Medical Research Institute | Nov 14 | Nov 19 |
| BI 409306 | Neuroprotective | PDE9 inhibitor | NCT02240693 | Boehringer Ingelheim | Jan 15 | Jun 17 |
| NCT02337907 | Boehringer Ingelheim | Jan 15 | May 17 | |||
| Byrostatin 1 | Neuroprotective | Protein kinase C inhibitor | NCT02431468 | Neurotrope Bioscience | Jul 15 | Apr 17 |
| Cilostazol | Neuroprotective | PDE3 antagonist | NCT02491268 | National Cerebral and Cardiovascular Center | Jul 15 | Jul 18 |
| CNP520 | Antiamyloid | BACE inhibitor | NCT02576639 | Novartis | Aug 15 | Mar 16 |
| CPC-201 | Neurotransmitter based | Cholinesterase inhibitor + peripheral cholinergic antagonist | NCT02185053 | Chase Pharmaceuticals Corporation | Jul 14 | Mar 16 |
| NCT02434666 | Chase Pharmaceuticals Corporation | Jan 15 | Jul 16 | |||
| Crenezumab | Anti-amyloid | Monoclonal antibody | NCT01998841 | Genentech | Dec 13 | Sep 20 |
| DAOIB | Neurotransmitter based | NMDA enhancer | NCT02103673 | Chang Gung Memorial Hospital | Feb 14 | Sep 16 |
| NCT02239003 | Chang Gung Memorial Hospital | Jan 12 | Jul 16 | |||
| E2609 | Anti-amyloid | BACE inhibitor | NCT02322021 | Eisai | Nov 14 | Jul 19 |
| Exenatide | Metabolic | Glucagon-like peptide 1 receptor agonist | NCT01255163 | National Institute on Aging (NIA) | Nov 10 | Dec 18 |
| Formoterol A&B | Neuroprotective | Beta-2 adrenergic receptor agonist | NCT02500784 | Palo Alto Veterans Institute for Research | Jan 15 | Jul 16 |
| hUCB-MSCs | Neuroprotective | Stem cell therapy | NCT02054208 | Medipost Co | Feb 14 | Feb 18 |
| NCT01547689 | Affiliated Hospital to Academy of Military Medical Sciences, Beijing, China | Mar 12 | Dec 16 | |||
| Insulin detemir | Metabolic | Insulin | NCT01595646 | University of Washington | Nov 11 | Sep 15 |
| Insulin glulisine | Metabolic | Insulin | NCT02503501 | HealthPartners Institute for Education and Research | Aug 15 | Sep 17 |
| JNJ-54861911 | Anti-amyloid | BACE inhibitor | NCT02406027 | Janssen | Jul 15 | Jun 24 |
| NCT02260674 | Janssen | Nov 14 | Jun 16 | |||
| Levetiracetam | Neurotransmitter based | Anticonvulsant | NCT02002819 | University of California, San Francisco | Jun 14 | Jun 17 |
| Liraglutide | Metabolic | Glucagon-like peptide 1 receptor agonist | NCT01843075 | Imperial College London | Jan 14 | Jan 17 |
| Lithium | Neurotransmitter based | Ion channel modulator | NCT02129348 | New York State Psychiatric Institute | Jun 14 | Apr 19 |
| Metformin | Metabolic | Insulin sensitizer | NCT01965756 | University of Pennsylvania | Jan 13 | Dec 16 |
| Methylene Blue | Anti-tau | Tau inhibitor; neuronal stimulant | NCT02380573 | University of Texas Health Science Center at San Antonio | Jul 15 | Jul 18 |
| MK-7622 | Neurotransmitter based | Muscarinic agonist | NCT01852110 | Merck | Oct 13 | Apr 20 |
| MK-8931 | Anti-amyloid | BACE inhibitor | NCT01739348 | Merck | Nov 12 | Jul 19 |
| NewGam 10% IVIG | Anti-amyloid | Polyclonal antibody | NCT01300728 | Sutter Health | Jan 11 | Nov 17 |
| ORM-12741 | Neurotransmitter based | Alpha-2c adrenergic receptor antagonist | NCT02471196 | Orion Corporation | Jun 15 | Feb 17 |
| PF-05212377 (SAM 760) | Neurotransmitter based | 5-HT6 receptor antagonist | NCT01712074 | Pfizer | Nov 12 | Dec 15 |
| Pimavanserin tartrate | Neurotransmitter based | 5-HT2A inverse agonist | NCT02035553 | Acadia | Nov 13 | Jun 16 |
| Piromelatine | Neurotransmitter based | Melatonin receptor agonist; 5-HT 1A and 1D receptor agonist | NCT02615002 | Neurim Pharmaceuticals | Nov 15 | Dec 17 |
| PQ912 | Anti-amyloid, anti-inflammatory | Glutaminyl-peptide cyclotransferase inhibitor | NCT02389413 | Probiodrug AG | Mar 15 | Oct 16 |
| PXT00864 | Neurotransmitter based | Combination of acamprosate and baclofen | NCT02361242 | Pharnext, SAS | Jun 13 | Dec 15 |
| Rasagiline | Neuroprotective | Monoamine oxidase B inhibitor | NCT02359552 | The Cleveland Clinic | Feb 15 | Dec 16 |
| Riluzole | Neuroprotective | Glutamate receptor antagonist; glutamate release inhibitor | NCT01703117 | Rockefeller University | Apr 13 | Nov 17 |
| RPh201 | Neuroprotective | G-protein coupled receptor antagonist | NCT01513967 | Regenera Pharma | Jan 12 | Dec 16 |
| S47445 (formerly CX1632) | Neurotransmitter based | AMPA receptor agonist; nerve growth factor stimulant | NCT02626572 | Institut de Recherches Internationales Servier | Feb 15 | Sep 17 |
| Sagramostim (GM-CSF) | Anti-amyloid | Granulocyte colony stimulator; amyloid removal | NCT01409915 | University of Colorado, Denver | Mar 11 | Jul 16 |
| Sembragiline (RO4602522) | Neurotransmitter based | Monoamine oxidase B inhibitor | NCT01677754 | Hoffmann-La Roche | Nov 12 | Jun 15 |
| Simvastatin + L-Arginine + Tetrahydrobiopterin | Neuroprotective | HMG-CoA reductase inhibitor and antioxidant | NCT01439555 | University of Massachusetts, Worcester | Nov 11 | Dec 16 |
| SUVN-502 | Neurotransmitter based | 5-HT6 antagonist | NCT02580305 | Suven Life Sciences | Sep 15 | Jun 17 |
| T-817 MA | Neuroprotective | Neurotrophic agent | NCT02079909 | Toyama | Mar 14 | Mar 17 |
| Telmisartan | Neuroprotective | PPAR-gamma agonist | NCT02085265 | Sunnybrook Health Sciences Centre | Mar 14 | Aug 18 |
| UB-311 | Anti-amyloid | Monoclonal antibody | NCT02551809 | United Neuroscience | Oct 15 | Jan 18 |
| VX-745 | Neuroprotective | P38 mitogen-activated protein kinase inhibitor | NCT02423200 | EIP Pharma | Apr 15 | Jan 16 |
| NCT02423122 | EIP Pharma | Apr 15 | Sep 16 |
Abbreviations: AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; BACE, beta-site amyloid precursor protein cleaving enzyme. GM-CSF, granulocyte-macrophage colony-stimulating factor; HMG-CoA, 3-hydroxy-3-methylglutaryl-coenzyme; hUCB-MSCs, human umbilical cord-derived mesenchymal stem cells; IVIG, intravenous immunoglobulin; NMDA, N-methyl-D-aspartate; PDE, phosphodiesterase; PPAR, peroxisome proliferator-activated receptor.
NOTE. Forty-six agents in 52 phase II clinical trials currently ongoing (active, not recruiting and active, recruiting) as of January 4, 2016 according to clinicaltrials.gov.
2.3. Phase I
There are 24 agents in phase I AD drug-development programs. Of these, three are symptomatic agents, 13 are small molecule DMTs, and eight are DMT immunotherapies. Five of the 13 small molecules and seven of the eight immunotherapies address amyloid-related mechanisms (57% of the DMT mechanisms). One tau-directed antibody and one tau-related small molecule are included in the AD phase I pipeline. Five neuroprotective agents and two metabolic agents are being assessed.
Of the 27 trials of phase I agents, 20 are sponsored by the biopharma industry, two are funded by NIH, one is jointly supported by NIH and industry, and four are funded through other mechanisms.
Table 3 shows the agents in phase I with their mechanism of action.
Table 3.
Agents currently in phase I of development and their mechanism of action (as of 1/4/2016)
| Agent | Agent mechanism class | Mechanism of action | Clinicaltrials.gov ID | Sponsor | Start date | Estimated end date |
|---|---|---|---|---|---|---|
| AADvac1 | Anti-tau | Monoclonal antibody directed at Tau epitope | NCT02031198 | Axon Neuroscience | Jan 14 | Sep 17 |
| ABT-957 | Neuroprotective | Calpain inhibitor | NCT02220738 | AbbVie | Sep 14 | Jun 16 |
| NCT02573740 | AbbVie | Nov 15 | Nov 16 | |||
| Aducanumab | Anti-amyloid | Monoclonal antibody | NCT01677572 | Biogen | Oct 12 | Oct 19 |
| NCT02434718 | Biogen | May 15 | Jul 17 | |||
| Allopregnanolone injection | Neuroprotective | GABA receptor modulator | NCT02221622 | University of Southern California | Aug 14 | Mar 17 |
| BI 409306 | Neurotransmitter based | PDE 9A inhibitor | NCT02392468 | Boehringer Ingelheim | Apr 15 | Oct 16 |
| Bisnorcymserine (BNC) | Neurotransmitter based | Butyrylcholinesterase inhibitor | NCT01747213 | National Institute on Aging (NIA) | Nov 12 | Jul 17 |
| Crenezumab | Anti-amyloid | Monoclonal antibody | NCT02353598 | Genentech | Feb 15 | Sep 17 |
| CT1812 | Anti-amyloid | Sigma-2 receptor modulator; reduces amyloid toxicity | NCT02570997 | Cognition Therapeutics | Sep 15 | Jun 16 |
| GC021109 | Anti-inflammatory, neuroprotective | Anti-inflammatory | NCT02386306 | GliaCure | Feb 15 | Oct 15 |
| Insulin Aspart Intranasal | Metabolic | Insulin | NCT02462161 | Wake Forest School of Medicine | May 15 | Dec 16 |
| JNJ-54861911 | Anti-amyloid | BACE inhibitor | NCT02360657 | Janssen | Feb 15 | Sep 15 |
| KHK6640 | Anti-amyloid | Amyloid aggregation inhibitor | NCT02127476 | Kyowa Hakko Kirin Pharma | Jul 14 | Feb 17 |
| NCT02377713 | Kyowa Hakko Kirin Pharma | Mar 15 | Dec 16 | |||
| Lorazepam | Neurotransmitter based | Benzodiazepam | NCT01780519 | Mayo Clinic | Jan 13 | Sep 16 |
| Lu AF20513 | Anti-amyloid | Monoclonal antibody | NCT02388152 | H. Lundbeck A/S | Mar 15 | Dec 16 |
| LY2599666 + Solanezumab | Anti-amyloid | Monoclonal antibody combination | NCT02614131 | Eli Lilly and Company | Dec 15 | Jul 17 |
| LY3002813 | Anti-amyloid | Monoclonal antibody | NCT01837641 | Eli Lilly and Company | May 13 | Sep 16 |
| LY3202626 | Anti-amyloid | Undisclosed mechanism | NCT02323334 | Eli Lilly and Company | Dec 14 | Feb 16 |
| MEDI1814 | Anti-amyloid | Monoclonal antibody | NCT02036645 | AstraZeneca | Feb 14 | Oct 16 |
| NGP 555 | Anti-amyloid | Gamma-secretase modulator | NCT02534480 | NeuroGenetic Pharmaceuticals | Mar 15 | Nov 15 |
| Oxaloacetate | Metabolic | Mitochondrial enhancer | NCT02593318 | University of Kansas Medical Center | Oct 15 | Oct 17 |
| PF-06751979 | Anti-amyloid | Undisclosed mechanism | NCT02509117 | Pfizer | Jul 15 | Jul 16 |
| S-Equol | Neuroprotective | Estrogen receptor beta agonist | NCT02142777 | University of Kansas Medical Center | Jul 14 | Dec 16 |
| Telmisartan | Neuroprotective | PPAR-gamma agonist | NCT02471833 | Emory University | Apr 15 | Mar 18 |
| TPI-287 | Anti-tau | Microtubule protein modulator | NCT01953705 | University of California, San Francisco | May 14 | Mar 19 |
Abbreviations: BACE = beta-site amyloid precursor protein cleaving enzyme; GABA = gamma-aminobutyric acid; PDE = phosphodiesterase; PPAR = peroxisome proliferator-activated receptor.
NOTE. Twenty-four agents in 27 phase I clinical trials currently ongoing (active, not recruiting, and active, recruiting) as of January 4, 2016, according to clinicaltrials.gov.
2.4. Biomarkers
Biomarkers are playing an increasingly important role in clinical trials of DMTs. Not all trials on clinicaltrials.gov state if biomarkers are included in their trials or discuss the type of biomarkers included, and we discuss the percent of trials that describe which biomarkers are included (Table 4). In current phase III trials, measurement of cerebrospinal fluid (CSF) amyloid beta protein (Aβ) is the most commonly used biomarker (27.7% of trials reporting use of biomarkers), followed by volumetric magnetic resonance imaging (MRI; 25%), CSF tau, and amyloid positron emission tomography (PET; 22.2% each), fluorodeoxyglucose (FDG) PET (19.4%), plasma amyloid (8.3%), and tau PET (2.7%). Phase II biomarkers include CSF amyloid (25%), CSF tau (21.2%), volumetric MRI (15.4%), FDG PET (11.5%), amyloid PET (9.6%), plasma amyloid (5.8%), and plasma tau (3.8%).
Table 4.
Percent of trials with specific biomarkers included (this calculation is based on the number of trials in which the inclusion of biomarkers is described)
| Biomarker | % of trials |
|
|---|---|---|
| Phase III | Phase II | |
| 1. CSF amyloid | 27.7 | 25 |
| 2. CSF tau | 22.2 | 21.1 |
| 3. FDG-PET | 19.4 | 11.5 |
| 4. vMRI | 25 | 15.3 |
| 5. Plasma amyloid | 8.3 | 5.7 |
| 6. Plasma tau | 0 | 3.8 |
| 7. Amyloid PET | 22.2 | 9.6 |
| 8. Tau PET | 2.7 | 0 |
3. Discussion
This analysis of clinicaltrials.gov reveals that there are relatively few agents in AD drug-development programs. The high failure rate in AD drug development and the small number of drugs being assessed suggest that the emergence of a repertoire of AD agents that could be tailored to fit the individual needs of patients is unlikely. The small number of agents in phase I is especially concerning as this phase is the major source of drugs for later stage development. A few repurposed agents can enter at phase II or phase III, but these agents generally have limited patent lives or limited intellectual property opportunities and do not comprise a major source of new candidate compounds [8]. Likewise, immunotherapies often begin in phase I/phase II with patients diagnosed with AD to avoid the risk of permanently altering the immune system of normal volunteers but, as can be seen, there are only a few such agents entering the drug-development pipeline. Overall, the AD ecosystem of AD drug development must be altered to yield more targets and more candidate therapies if a robust pipeline of therapies is to be established.
Other reviews of AD drug development have led to similar conclusions as those presented here. The comprehensive 2010 review by Mangialasche et al. [9] showed that new approaches to cholinergic therapy and many antiamyloid trials were being pursued. There were more agents directed toward tau-related targets in the 2010 pipeline review; most of these have since failed. Fig. 1 is similar to the visualization approach used by Mangialasche et al. [9] and can be used to compare changes over a 6-year period. Similarly, Cummings et al [3] found—using a similar strategy to that used in the current review—that there were relatively few drugs being assessed and that the overall failure rate for AD drug development was a dramatic 99.6%. They also noted that no new novel drugs for AD had been approved since 2003.
We compared AD drug development with oncology drug development to provide a perspective on the observed numbers. In the 2014–2015 period, 135 trials were registered for AD, whereas 4976 trials were registered for cancer (these figures were generated from clinicaltrials.gov using the same search terms as used in the reviewed AD trials and agents). This indicates that the number of agents in trials is much larger for cancer than for AD and the likelihood of finding effective therapies is greater. This disparity likely reflects several influences including the greater success of rate of cancer drug development (19.8% of development programs succeed in cancer vs less than 1 percent of AD drugs [3], [10]). Thirty-one percent of FDA new drug approvals for 2015 were for oncology agents [11]. The low success rate of AD drug development discourages pharmaceutical companies from pursuing research in this area and reduces the enthusiasm of venture capitalists for investing in biotechnology companies whose products address AD-related targets. As a result, fewer targets are identified, and fewer candidate agents discovered and developed. The biological understanding of cancer has identified more putative targets. Greater insight into AD pathophysiology may lead to more target identification and more opportunities to develop mechanistically informed treatments.
The AD drug-development pipeline has amyloid beta-protein production or removal as it major focus. Across all phases, 56% of DMTs have an amyloid-related target. Monoclonal antibodies and BACE inhibitors comprise the two most developed pathways in the current pipeline. Monoclonal antibody approaches have instituted two major changes in drug development based on experiences with the failure of bapineuzumab: (1) patient populations with more mild disease are now the focus of trials [12]; (2) amyloid imaging or CSF Aβ measures are performed at baseline to insure that patients have the target pathology for antiamyloid therapies [13], [14]. BACE inhibitors have included measures of CSF Aβ to demonstrate target engagement and show that the putative goal of reduction is being achieved [15]. Demonstration of target engagement early in the development process makes it more likely—without proving—that clinical benefits may follow long-term therapy [16].
Tau is a relatively unexploited target with only four agents in the pipeline devoted to tau-related pathophysiology. The availability of tau imaging and the consistent relationships shown between tau signals on imaging and the clinical state of the individual indicate that tau is an important target for drug development and that tau imaging may serve as a useful biomarker to help guide drug development [17], [18], [19], [20]. Tau protein is being targeted in trials of experimental therapies for tauopathies including frontotemporal dementia and progressive supranuclear palsy, and learnings from these trials may inform treatment of tau pathology in AD.
Thirty-eight percent of DMTs are small molecule agents that address neuroprotection or metabolic targets such as insulin resistance or PPAR-gamma–related mechanisms. These approaches are more well represented in phase II than phase III and suggest that the repertoire of targets is broadening for agents in the AD pipeline.
Symptomatic agents represent an important part of the AD drug-development pipeline. Improvement in cognitive and behavioral symptoms is a major goal of treatment and is achieved only partially by current therapies. There are 25 symptomatic cognitive enhancers or neuropsychiatric agents in the current pipeline comprising 27% of the entire drug-development pipeline. Symptomatic treatments are especially well represented in phase II where they comprise 33% of all agents at that stage of development. These agents enhance cholinergic signaling or capitalize on noncholinergic serotonergic, sigma-1, phosphodiesterase, or N-methyl-D-asparate (NMDA) mechanisms.
There is increasing recognition that combination therapies may be warranted to address the complex biology of AD [21]. Combinations have found success in other complex diseases such as cancer, tuberculosis, and human immunodeficiency virus infections. There are few examples in the AD pipeline of combination of agents in trials; ALZT-OP1a/1b is a combination approach at phase III, simvastatin/l-arginine/tetrahydrobiopterin is being assessed at phase II; and LY2599666 plus solanezumab is being tested in phase I. In addition to these pharmacodynamics combinations, AVP-786 is a pharmacokinetic combination of dextromethorphan and the CYP2D6 inhibitor, quinidine, used to elevate levels of dextromethorphan. Overall, combinations comprise a limited aspect of the AD drug-development pipeline and represent an important future direction of drug development.
Currently, DMTs spend relatively little time in phase II (average 49 weeks) and involve a small number of patients per trial arm (average 67). Given the 100% failure rate of DMTs in phase III, more thorough exploration of these agents in phase II may benefit drug-development programs and the likelihood of phase III success.
Biomarkers play an increasingly important role in AD drug development. The demonstration that approximately 25% of patients included in trials of clinical-diagnosed AD do not have elevated levels of brain amyloid when studied with amyloid imaging indicated that use of biomarkers was critical in identifying a population with the target pathology in trials of antiamyloid agents and that have an accurate diagnosis for inclusion of trials of other agents [13], [14]. Nearly, all current trials of antiamyloid agents require positive amyloid imaging at baseline to insure accurate diagnosis and include amyloid imaging as an outcome to determine the effect of the therapeutic intervention on the brain plaque burden. Target engagement biomarkers are now more commonly used in drug-development programs such as those for BACE inhibitors to show that a biological effect has been achieved and that clinical effects could reasonably be expected. CSF measurements of amyloid and tau, volumetric MRI, and amyloid PET are used approximately equally commonly in DMT programs; no consensus on a single biomarker or combination of biomarkers as optimal to meet regulatory expectations for biomarker data has emerged.
Of trials across all phases of AD drug development, 74% are completely or partially sponsored by the biopharmaceutical industry. Given the prominent role of industry in AD drug development, legislative incentives to attract the pharmaceutical and biotechnology industries to AD may be one of the means of enhancing the number of candidate agents entering the AD pipeline. Increased federal funding to augment the small number of trial sponsored by NIH (9% with total or partial NIH funding) might also enhance the pipeline. Funding for basic science through NIH or venture capital support of biotechnology companies is needed to identify new targets and generate new candidate therapies. Similarly, new strategies in drug development including more emphasis on demonstrating target engagement in early stage development and use of adaptive designs to support clinical trials decision making may accelerate the drug development process and decrease the number of late-stage failures of agents in the pipeline.
This analysis is based on a review of clinicaltrials.gov and is subject to the limitations of that database. While inclusive of all trials in the United States and many non-US countries, it may not include all trials being conducted in other countries and the list of drugs we discuss may not be comprehensive from an international perspective. In addition, not all phase I trials are included on clinicaltrials.gov, especially when they are conducted in non-US phase I units, and we may underestimate the total number of agents being assessed in phase I. There is sometimes a lag in listing trials on clinicaltrials.gov, and the lists included here may not be fully comprehensive for the time window assessed. These limitations will affect some details of the analysis but not the overall view of the landscape of AD drug development.
In summary, the AD drug-development pipeline is modest in size and strikingly smaller than very active areas of experimental therapeutics such as cancer. The phase I candidate pool is particularly small and bodes poorly for a compelling set of agents to be advanced to phase II and III. Amyloid is the most common pharmaceutical target, reflecting the greater understanding of the pathophysiology of this peptide. Symptomatic agents are making progress toward treatment of both cognitive and behavioral symptoms of AD. Biomarkers are being integrated into DMT development programs. Every source of compounds including academic medical centers, NIH, philanthropic funders, biotechnology, and pharmaceutical companies should be attracted to AD drug development to create a larger pipeline and a greater chance of success of AD drug development.
Research in context.
-
1.
Systematic Review: Drug development for Alzheimer's disease (AD) proceeds through three phases (I, II, III). By assessing the number of agents in each phase as recorded on clinicaltrials.gov, one can determine current AD drug development activity to assess how many agents are being studied, the success of the research, and how the number of new drugs can be increased.
-
2.
Interpretation: Our data show that there are 93 drugs in development for treatment of AD. There are more drugs in phase II (45) than in phase III (24) or phase I (24). The small number of phase I compounds suggest that there is insufficient drug discovery activity to supply new agents for testing in clinical trials.
-
3.
Future directions: This review of the AD drug-development pipeline provides insight into the state of AD drug development and encourages review of how best to amplify the drug discovery/development ecosystem.
Acknowledgments
There was no external funding for the study.
Footnotes
Dr. Cummings has received research support from Avid Pharmaceuticals, Teva Pharmaceuticals, and CogState. Dr. Cummings has provided consultation to Abbvie, Acadia, Actinogen, ADAMAS, Alkahest, Alzheon, Anavex, Astellas, Astra Zeneca, Avanir, Axovant, Biogen Idec, Biotie, Boehinger-Ingelheim, Chase, Eisai, Forum, GE Healthcare, Genentech, Grifols, Intracellular Therapies, IRIS, Ionis Pharmaceuticals, Lilly, Lundbeck, MedAvante, Merck, Neurotrope, Novartis, Nutricia, Otsuka, Pfizer, Predemtec, Probiodrug, QR Pharma, Resverlogix, Roche, Servier, Sunovion, Suven, Takeda, Toyoma, Transition Therapeutics, and United Neuroscience, companies. Dr Cummings owns the copyright of the Neuropsychiatric Inventory. Dr Cummings has stock options in Prana, Neurokos, ADAMAS, MedAvante, and QR pharma. Mr. Morstorf and Ms. Lee have no conflicts of interest.
References
- 1.Alzheimer's Association 2015 Alzheimer's disease facts and figures. Alzheimers Dement. 2015;11:332–384. doi: 10.1016/j.jalz.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Sosa-Ortiz A.L., Acosta-Castillo I., Prince M.J. Epidemiology of dementias and Alzheimer's disease. Arch Med Res. 2012;43:600–608. doi: 10.1016/j.arcmed.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Cummings J.L., Morstorf T., Zhong K. Alzheimer's disease drug-development pipeline: Few candidates, frequent failures. Alzheimers Res Ther. 2014;6:37. doi: 10.1186/alzrt269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tasneem A., Aberle L., Ananth H., Chakraborty S., Chiswell K., McCourt B.J. The database for aggregate analysis of ClinicalTrials.gov (AACT) and subsequent regrouping by clinical specialty. PLoS One. 2012;7:e33677. doi: 10.1371/journal.pone.0033677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berk C., Paul G., Sabbagh M. Investigational drugs in Alzheimer's disease: current progress. Expert Opin Investig Drugs. 2014;23:837–846. doi: 10.1517/13543784.2014.905542. [DOI] [PubMed] [Google Scholar]
- 6.Cummings J.L. Controversies in Alzheimer's disease drug development. Int Rev Psychiatry. 2008;20:389–395. doi: 10.1080/09540260802094548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wiendl H., Elger C., Forstl H., Hartung H.P., Oertel W., Reichmann H. Gaps between aims and achievements in therapeutic modification of neuronal damage (“Neuroprotection”) Neurotherapeutics. 2015;12:449–454. doi: 10.1007/s13311-015-0348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Appleby B.S., Nacopoulos D., Milano N., Zhong K., Cummings J.L. A review: treatment of Alzheimer's disease discovered in repurposed agents. Dement Geriatr Cogn Disord. 2013;35:1–22. doi: 10.1159/000345791. [DOI] [PubMed] [Google Scholar]
- 9.Mangialasche F., Solomon A., Winblad B., Mecocci P., Kivipelto M. Alzheimer's disease: clinical trials and drug development. Lancet Neurol. 2010;9:702–716. doi: 10.1016/S1474-4422(10)70119-8. [DOI] [PubMed] [Google Scholar]
- 10.Tufts Center for the Study of Drug Development . Clinical success rates for new cancer drugs double while more enter testing. In: Kenneth I., Kaitin P., editors. Impact Report. Tufts University; Boston, MA: 2013. pp. 1–4. [Google Scholar]
- 11.Mullard A. 2015 FDA drug approvals. Nat Rev Drug Discov. 2016;15:73–76. doi: 10.1038/nrd.2016.15. [DOI] [PubMed] [Google Scholar]
- 12.Doody R.S., Thomas R.G., Farlow M., Iwatsubo T., Vellas B., Joffe S. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer's disease. N Engl J Med. 2014;370:311–321. doi: 10.1056/NEJMoa1312889. [DOI] [PubMed] [Google Scholar]
- 13.Salloway S., Sperling R., Fox N.C., Blennow K., Klunk W., Raskind M. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer's disease. N Engl J Med. 2014;370:322–333. doi: 10.1056/NEJMoa1304839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sevigny J., Suhy J., Chiao P., Chen T., Klein G., Purcell D. Amyloid PET screening for enrichment of early-stage Alzheimer disease clinical trials: Experience in a Phase 1b clinical trial. Alzheimer Dis Assoc Disord. 2016;30:1–7. doi: 10.1097/WAD.0000000000000144. [DOI] [PubMed] [Google Scholar]
- 15.Menting K.W., Claassen J.A. Beta-secretase inhibitor; a promising novel therapeutic drug in Alzheimer's disease. Front Aging Neurosci. 2014;6:165. doi: 10.3389/fnagi.2014.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cook D., Brown D., Alexander R., March R., Morgan P., Satterthwaite G. Lessons learned from the fate of AstraZeneca's drug pipeline: a five-dimensional framework. Nat Rev Drug Discov. 2014;13:419–431. doi: 10.1038/nrd4309. [DOI] [PubMed] [Google Scholar]
- 17.Brier M.R., Gordon B., Friedrichsen K., McCarthy J., Stern A., Christensen J. Tau and Abeta imaging, CSF measures, and cognition in Alzheimer's disease. Sci Transl Med. 2016;8:338ra66. doi: 10.1126/scitranslmed.aaf2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gordon B.A., Friedrichsen K., Brier M., Blazey T., Su Y., Christensen J. The relationship between cerebrospinal fluid markers of Alzheimer pathology and positron emission tomography tau imaging. Brain. 2016;139:2249–2260. doi: 10.1093/brain/aww139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ossenkoppele R., Schonhaut D.R., Scholl M., Lockhart S.N., Ayakta N., Baker S.L. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer's disease. Brain. 2016;139:1551–1567. doi: 10.1093/brain/aww027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scholl M., Lockhart S.N., Schonhaut D.R., O'Neil J.P., Janabi M., Ossenkoppele R. PET Imaging of tau deposition in the aging human brain. Neuron. 2016;89:971–982. doi: 10.1016/j.neuron.2016.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perry D., Sperling R., Katz R., Berry D., Dilts D., Hanna D. Building a roadmap for developing combination therapies for Alzheimer's disease. Expert Rev Neurother. 2015;15:327–333. doi: 10.1586/14737175.2015.996551. [DOI] [PMC free article] [PubMed] [Google Scholar]