Abstract
Introduction
There are currently no effective treatments preventing conversion from mild cognitive impairment (MCI) to Alzheimer's disease. Cilostazol is a selective type-3 phosphodiesterase inhibitor that ameliorates accumulation of amyloid-β and has prevented cognitive decline in rodent models. Furthermore, cilostazol is known to suppress platelet aggregation, protect vascular endothelia, dilate vessels, and increase cerebral blood flow. Beneficial effects have also been shown in observational cohort studies, demonstrating the need for a prospective clinical trial.
Methods
The Cilostazol for prevention of COnversion from MCI to Dementia (COMCID) study is a double-blind, randomized phase II study of patients with MCI. Participants will receive cilostazol or placebo for 96 weeks. The primary objective is to evaluate whether cilostazol slows down cognitive decline measured by the Mini-Mental State Examination. Secondary objectives are assessing time to conversion from MCI to dementia and assessing incremental changes in several psychological assessment scales.
Discussion
The COMCID trial will identify the therapeutic potential of cilostazol. This trial, which is based on a drug repositioning strategy, may aid the development of a neurovascular treatment for neurocognitive disorders.
Keywords: Alzheimer's disease, Cilostazol, Clearance, Clinical trial, Drug repositioning
1. Introduction
Epidemiologic investigations have proposed that strict control of vascular risk factors is an effective preventative strategy for dementia because of the close relationship between Alzheimer's disease (AD) and cerebrovascular disease (CVD) [1]. AD is thought to result from an imbalance between production and clearance of amyloid-β (Aβ), and CVD is related to excessive Aβ production and elimination failure [2]. Decreased cerebral blood flow is closely related to CVD and seems to modulate amyloid precursor protein cleavage enzymes, such as β- and γ-secretase, leading to increased Aβ production [3]. A large proportion of Aβ clearance takes place through vascular-mediated systems, via active transport across the blood-brain barrier, perivascular lymphatic and paravascular glymphatic drainage networks, all of which can be disturbed by CVD [4]. Familial cases of AD may be, at least in part, attributable to excess Aβ production. However, insufficient Aβ clearance is more crucial in cases of sporadic AD [5]. Therefore, researchers have increasingly sought to address the problem of Aβ elimination in AD therapy [6].
The vasoactive drug cilostazol inhibits type-3 phosphodiesterase and is expected to promote Aβ clearance. In rodent models exhibiting Aβ accumulation, administering cilostazol prevented Aβ deposition and improved cognitive function by increasing hemodynamic reserve and facilitating perivascular drainage of Aβ [7]. The brain parenchyma is devoid of a conventional lymphatic system, although the lymphatic vessels were recently found to exist along meningeal vessels [8]. Interstitial fluid and solutes, including Aβ, are cleared through a perivascular drainage route, which is formed by two basement membranes in the walls of cerebral arteries [9]. Cilostazol was found to facilitate this clearance system in vivo [7]. Furthermore, experimental studies indicate that cilostazol reduces Aβ production and suppresses tau phosphorylation by inhibiting glycogen synthase kinase 3β, via enhancement of casein kinase 2α/silent information regulator 1 phosphorylation, in vitro [7], [10], [11]. Whether such effects are viable in AD and mild cognitive impairment (MCI) patients is not yet clear as only a minor fraction of cilostazol passes through the blood-brain barrier [12].
Cilostazol is currently prescribed in Asia, Europe, and the United States as an antiplatelet drug for symptomatic treatment of peripheral arterial disease. It is also used in Asia for the secondary prevention of ischemic stroke. The second Cilostazol Stroke Prevention Study for patients with cerebral infarction showed that hemorrhagic stroke was significantly less frequent with cilostazol treatment than with aspirin [13], [14]. The prevention of cerebral hemorrhage may be explained by its protective effects on vascular endothelial cells [15]. Furthermore, cilostazol is known to dilate blood vessels, leading to increased cerebral blood flow [16]. These results suggest that cilostazol could be suitable for patients with both AD neurodegeneration and CVD.
Favorable effects have been reported in observational clinical studies, which demonstrated efficacy of cilostazol in patients with MCI [17], donepezil-treated patients with clinically probable AD [18], [19], and AD with CVD [20]. These results have thus highlighted the need for a comprehensive prospective cohort study to determine whether cilostazol helps preserve cognitive function in patients with MCI.
2. Methods
2.1. Study design
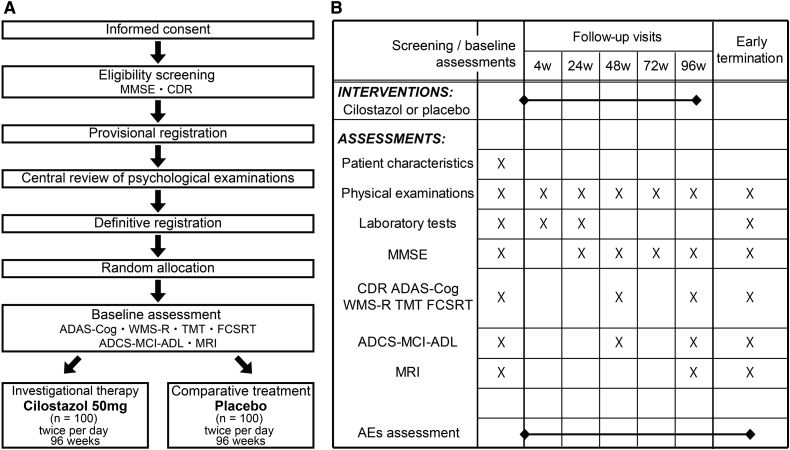
The investigator-initiated Cilostazol for prevention of COnversion from MCI to Dementia (COMCID) trial is a double-blind, placebo-controlled, randomized early phase II study aimed at evaluating the efficacy and safety of cilostazol in patients with MCI (Fig. 1). Two hundred MCI patients will be randomly assigned to cilostazol or placebo control arms. The allocation ratio is 1 to 1.
Fig. 1.
Study design for the COMCID trial (ClinicalTrials.govNCT02491268, UMIN Clinical Trials Registry UMIN000017764). (A) Schematic flow diagram of the enrollment process. (B) The schedule of interventions and assessments. MMSE, Mini-Mental State Examination; CDR, Clinical Dementia Rating; ADAS-Cog, Alzheimer's Disease Assessment Scale–cognitive subscale; WMS-R, Wechsler Memory Scale-Revised; TMT, Trail making test; FCSRT, Free and Cued Selective Reminding Test; ADCS-MCI-ADL, Alzheimer's Disease Cooperative Study–Mild Cognitive Impairment–Activities of Daily Living; MRI, magnetic resonance imaging; AEs, adverse events.
2.2. Study objectives
The primary objective of the study is to evaluate the efficacy of cilostazol in patients with MCI in preserving cognitive function measured by the Mini-Mental State Examination (MMSE). The secondary objectives are to evaluate the efficacy of cilostazol in assessing time to conversion from MCI to “all-cause dementia,” as well as changes in the Clinical Dementia Rating–Sum of Boxes (CDR-SB), Alzheimer's Disease Assessment Scale–cognitive subscale (ADAS-cog) 14, Wechsler Memory Scale-Revised (WMS-R) Logical Memory part II, and Alzheimer's Disease Cooperative Study–Mild Cognitive Impairment–Activities of Daily Living (ADCS-MCI-ADL). Another secondary objective is to explore the efficacy of cilostazol on the hippocampal atrophy, measured by brain magnetic resonance imaging (MRI).
2.3. Enrollment
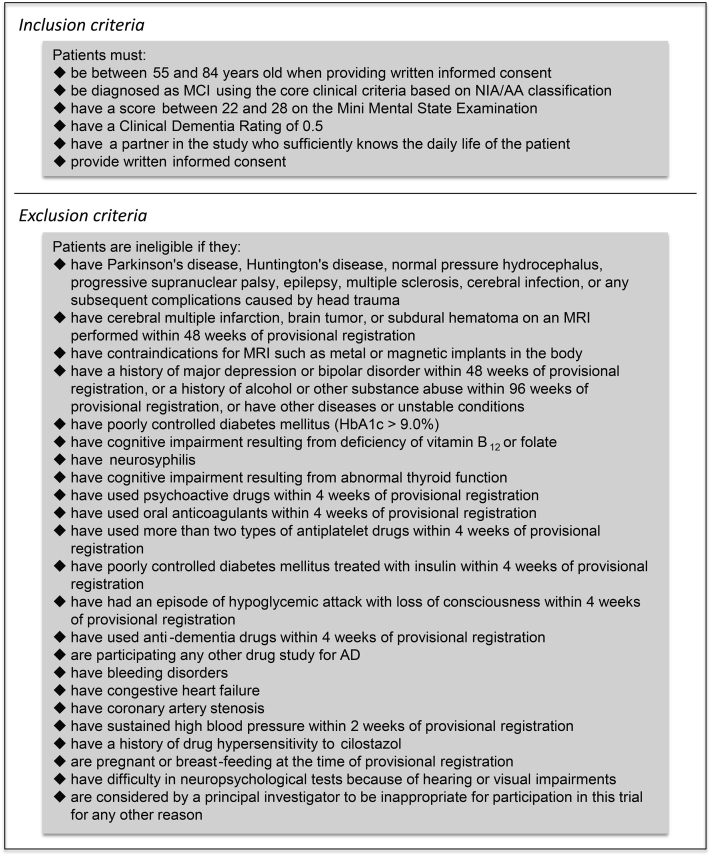
MCI patients are recruited from 15 high-volume centers for patients with dementia in Japan. Potential participants will undergo screening assessment to confirm eligibility (Fig. 2). Blood examinations and MRI investigation for confirming eligibility will not be scheduled in the COMCID trial as only MCI patients who have undergone sufficient laboratory tests for clinical purposes before informed consent about this trial can participate. These laboratory examinations are routinely performed in all trial sites. Pure vascular MCI patients are ineligible, whereas patients with coincidental cerebral infarctions or white matter changes not likely to wholly explain their cognitive dysfunction are eligible. Based on the core clinical criteria stated in the National Institute on Aging-Alzheimer's Association classification [21], MCI is diagnosed when individuals meet the following criteria:
-
•
cognitive concern reflecting a change in cognition reported by the patient, informant, or clinician
-
•
objective evidence of impairment in one or more cognitive domains
-
•
preservation of independence in functional abilities
-
•
not demented
Fig. 2.
Eligibility criteria. MCI, mild cognitive impairment; NIA/AA, National Institute on Aging-Alzheimer's Association; MRI, magnetic resonance imaging; HbA1c, glycated haemoglobin (A1c); AD, Alzheimer's Disease.
In addition, MMSE scores between 22 and 28 (inclusive) and CDR scores of 0.5 are required for provisional registration (Fig. 1). Results from MMSE and CDR will be reviewed by an independent central psychological review board. If the patient is judged to be eligible, the review board will submit the definitive registration, and the enrollment will be completed. The reviewed MMSE and CDR scores will be used for baseline assessment data; baseline CDR scores are 0.5 in all the enrolled participants. Then, ADAS-cog 14, WMS-R Logical Memory part II, and ADCS-MCI-ADL will be performed for baseline assessment. MRI assessments are planned at 4 of the 15 participating institutions.
2.4. Allocation and blinding
Registered patients will be randomly assigned to cilostazol or control arms. The assignment method is minimization, and the stratification factors are age (≥75 years), sex, education (≥12 years), and institution. The randomization will be performed by computer-generated random allocation treatment codes, with patients and physicians blinded to the therapy, and the randomization list will be kept by an independent investigator not involved in the care or assessment of the patients, or data collection and analysis. Emergency unblinding will be performed only when the principal investigators and the COMCID trial coordinating committee admit the necessity to uncover the assigned intervention in order to treat any potential adverse events (AEs) in participants.
2.5. Interventions
Cilostazol and placebo are provided by Otsuka Pharmaceutical Company, Japan. Participants assigned to the cilostazol arm will receive one 50-mg tablet of cilostazol twice a day for 96 weeks. Those assigned to the control arm will receive one placebo tablet twice a day for 96 weeks. If dose reduction resulting from any AEs is needed, the protocol treatment will be discontinued. The study physicians will maintain the drug inventory record of supplied, received, dispensed, destroyed, and returned medication.
2.6. Concomitant medications
The protocol treatment will be stopped when antidementia drugs and anticoagulants are started; when the patient is treated for schizophrenia, major depression, or bipolar disorder; or when major tranquilizers are administered against persistent delirium or behavioral and psychological symptoms of dementia. The use of minor tranquilizers, including benzodiazepines for neurosis or insomnia, is permitted in the trial period. Diet therapy, rehabilitation, and any medications other than prohibited medications will not be restricted.
2.7. Psychological examination
Only psychologists or medical doctors who complete training programs and pass the qualifying examinations will be permitted to carry out the cognitive testing outlined in this study. All records from the psychological examinations, including patients' and partners' answers, as well as examiners' records, will be collected by the independent central psychological review board. The board will comprise one neurologist, one psychiatrist, and three psychologists. CDR is authorized by Washington University, and grading is affirmed by consensus of the board members.
2.8. The imaging study
Hippocampal volume assessment will be performed with a 3.0-T MRI scanner (Skyra, TIM Trio, or Verio; Siemens AG, Germany). Two T1-weighted MRI scans will be collected using a three-dimensional sagittal magnetization-prepared rapid gradient echo sequence, according to the Alzheimer's Disease Neuroimaging Initiative 3.0-T MRI protocol (http://adni.loni.usc.edu/methods/mri-analysis/mri-acquisition/). Imaging data will be sent to the central evaluation committee, comprising four neuroradiologists. Automated hippocampal volumetry will be carried out using FreeSurfer software, version 5.3 [22]. Previous reports showed that these analyzing methods could generate consistent outcome measures for the hippocampal volume with less than 4% of reproducibility errors, even when several MRI scanners from different vendors were used [23], [24]. Fluid-attenuated inversion recovery images for assessing white matter hyperintensities and T2*-weighted images for detecting cerebral microbleeds will be also planned.
2.9. Data collection
Web-based electronic data capture system will be used to collect clinical data obtained from patient medical records. Laboratory data and data from the independent central psychological review board and the central evaluation committee for MRI examinations will be stored separately before being finally integrated. The persons assigned to data management will perform quality control at each step of data handling to ensure the reliability of all data related to the study.
2.10. Statistical analysis
The sample size was determined by feasibility but substantial enough to perform the primary analysis. Our preceding investigation for MCI patients treated with cilostazol showed that the annual changes of total MMSE scores were about ±0 points within approximately 2 years of observation period [17], whereas Petersen et al. reported that the MMSE score changes in MCI patients were about −1.0 point/y [25]. With 90% power, a two-tailed type I error rate of 5%, and an estimate of the population standard deviation of 3.7 for changes in total MMSE scores, this sample size can detect a statistically significant difference if the effect size is more than 1.72 between two treatments. The standard deviation referred to the Alzheimer's Disease Neuroimaging Initiative study and our preceding investigation [17].
We will analyze a full analysis set, defined as data obtained from the registered patients who will receive at least a part of the assigned treatment. However, patients found to be noneligible after the registration should be excluded from the full analysis set.
To evaluate the improvement of the cognitive function by the treatments, the changes in total MMSE scores between the baseline and 96 weeks after starting protocol treatment will be tested by t test or Wilcoxon rank sum test. The COMCID trial will judge the efficacy of cilostazol using this analysis. Furthermore, the scores at baseline, 24, 48, 72, and 96 weeks will be analyzed with a mixed-model repeated measures by considering treatments, gender, and time as fixed effects and patient × time as a random effect.
The time to convert from MCI to “all-cause dementia” will be analyzed using suitable survival analysis approaches, such as Kaplan-Meier with log-rank tests and Cox proportional hazard models. If patients are diagnosed with dementia, the protocol treatment will cease.
Incremental changes in CDR-SB, ADAS-cog 14, WMS-R Logical Memory part II, and ADCS-MCI-ADL will be analyzed with the same procedure as MMSE. Hippocampal volume change on MRI over time will be compared between cilostazol and control arms statistically.
2.11. Exploratory analysis
The followings issues will be evaluated in an exploratory analysis:
-
•
incremental changes in scores from Trail Making Test Part A and Part B
-
•
incremental changes in scores from the Free and Cued Selective Reminding Test
-
•
incremental changes in the serum levels of albumin-Aβ complexes [26]
2.12. Ethical matters
This study has been approved by the National Cerebral and Cardiovascular Center and other institutional review boards. All procedures will comply with the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use “Guideline for Good Clinical Practice” and its revised ministerial ordinances/operational notifications in Japan.
Written informed consent will be obtained from all patients before enrollment. If the written informed consent form is revised, the study physicians will explain the procedure again, revise the written informed consent form, and obtain the subject's voluntary consent for continuation of participation.
2.13. Safety profile
If an AE occurs, the study physicians will treat the patient adequately in terms of safety and report the details of the event. If a severe AE (SAE) occurs, the study physicians and principal investigator must report it to the head of institution and the COMCID trial coordinating committee within 24 hours. The coordinating committee should decide whether the SAE needs 7-day, 15-day (Japan's Pharmaceutical Affairs Law, Enforcement Regulations, Article 273), or no reporting. If submission of those reports is required, the principal investigator and the coordinating committee should complete the prespecified submission form and submit it to the head of institution and Japan's Pharmaceuticals and Medical Devices Agency. The coordinating committee should inform of the safety information to the other site investigators and the pharmaceutical company. The other site investigators should submit the reports to the head of institution as soon as possible.
If the SAE is caused by protocol treatment, follow-up will be continued until recovery. The patient will be compensated for any study-related injuries by insurance.
2.14. Data-monitoring committee
For safety monitoring, an independent data-monitoring committee (DMC) will discuss the safety-related end points, including SAEs. The DMC consists of a clinical physician, neurologist, cardiologist, and an expert statistician. If an SAE occurs, the COMCID trial coordinating committee should promptly report the safety information to the member of DMC. The DMC will be held by the initiative of the committee member in accordance with “Standard Operating Procedure: Operation of a Data Monitoring Committee.” The DMC can request revision of the study protocol or stop the trial. When revision of the study protocol is proposed by the DMC, the principal investigators and the coordinating committee should submit the revised protocol to the head of institution and obtain approval from the institutional review board.
2.15. Auditing
The auditors (A.T., M.A., R.U., K.K., and T.K., Office of Clinical Quality Assurance, Otsuka Pharmaceutical Company, Japan) will evaluate whether the conduct of this study is in compliance with the Guideline for Good Clinical Practice and study protocol. Auditing is distinguished from routine monitoring and quality control of the study, which is performed from the standpoint of an outsider as part of quality assurance activities. Two contract research organizations and 4 of the 15 participating institutions where many patients are expected to be registered will be audited three times: before, during, and after this trial.
3. Discussion
This COMCID trial is based upon a drug repositioning strategy, a process aimed at discovering new therapeutic drugs from existing drugs in a clinical setting [27]. The established safety profile possesses many advantages over de novo therapy, contributing to this phase II trial without any additional pharmacokinetic or toxicologic investigations. Drug repositioning is an attractive and timely drug development strategy [27].
The target patient population in this trial is not limited to MCI due to AD or presumed AD. MCI diagnosis is not based on the research criteria but the core clinical criteria in the National Institute on Aging-Alzheimer's Association classification [21], which is equivalent to what the Diagnostic and Statistical Manual of Mental Disorders, fifth edition, terms “mild neurocognitive disorder” [28]. AD and vascular dementia, the two most common causes of dementia, represent two ends in a spectrum of disorders. Most patients exhibit varying degrees of neurodegenerative and vascular pathologies between these extremes [29], providing a rationale for the use of cilostazol in MCI in the general population [30].
Investigational therapy is 100 mg/d of cilostazol administration for 96 weeks, whereas 200 mg/d of cilostazol is commonly used for secondary prevention of ischemic strokes. Our retrospective cohort study showed that cilostazol significantly improved MMSE scores, compared with control groups [17]. The mean follow-up period was 691 days. Importantly, there were no differences in annual MMSE changes between patients receiving 200 mg/d and those receiving 100 mg/d. The same was true in another study where add-on of cilostazol was effective in suppressing annual MMSE decline in mild dementia patients receiving donepezil [18]. Thus, there were no differences in annual MMSE decrease between patients receiving 200 mg/d of cilostazol and those receiving 100 mg/d. However, many previous reports showed that 100 mg/d of cilostazol was much safer than 200 mg/d because of fewer AEs [31]. These data justified both the dosage and the period of investigational treatment adopted in this trial.
The primary outcome of this trial will be measured by MMSE as no clinical studies have so far demonstrated the efficacy of cilostazol on cognitive function using any other psychological battery. MMSE is the most commonly applied test in the assessment of cognitive function in the elderly, but other psychological tests, including CDR and ADAS, are known to be more sensitive and more widely used in other clinical trials. Therefore, further investigation with late phase II study may be required before phase III trial.
In conclusion, the COMCID study is a double-blind, placebo-controlled, multicenter randomized controlled trial intended to demonstrate the efficacy and safety of cilostazol in patients with MCI. Its pleiotropic effects would not only promote Aβ clearance but also retain cerebrovascular integrity to suppress cognitive decline. The results will assess the therapeutic potential of cilostazol, potentially opening a new era of neurovascular therapeutic approaches for neurocognitive disorders.
Research in context.
-
1.
Systematic review: The authors reviewed the literature using traditional sources. Several clinical and experimental studies have investigated the effectiveness of cilostazol in treating Alzheimer's disease (AD) and mild cognitive impairment (MCI). These relevant articles are appropriately cited.
-
2.
Interpretation: The COMCID study is a double-blind, placebo-controlled, randomized phase II study that is aimed at evaluating the efficacy and safety of cilostazol in patients with MCI.
-
3.
Future directions: Insufficiency of vascular-mediated amyloid-β (Aβ) elimination seems to be a crucial factor related to sporadic AD. Many researchers are therefore now focusing on facilitating Aβ clearance and have adopted a neurovascular approach to tackle AD and MCI. This trial will not only identify the therapeutic potential of cilostazol for MCI patients but also help open a new era of neurovascular treatments for neurocognitive disorders.
Acknowledgments
The following individuals and institutions participated in the COMCID study: M. Ihara, MD, PhD, K. Nagatsuka, MD, PhD, Department of Stroke and Cerebrovascular Diseases, National Cerebral and Cardiovascular Center, Suita, Japan; K. Kanemaru, MD, PhD, Department of Neurology, Tokyo Metropolitan Geriatric Hospital, Tokyo, Japan; R. Kanki, MD, PhD, Department of Neurology, Osaka City General Hospital, Osaka, Japan; N. Kawabata, MD, PhD, Department of Neurology, Yachiyo Hospital, Anjo, Japan; H. Kida, MD, PhD, M. Satoh, MD, PhD, and H. Tomimoto, MD, PhD, Department of Dementia Prevention and Therapeutics, Graduate School of Medicine, Mie University, Tsu, Japan; H. Kitaguchi, MD, PhD, K. Shindo, MD, PhD, Department of Neurology, Kurashiki Central Hospital, Kurashiki, Japan; H. Kowa, MD, PhD, K. Washida, MD, PhD, Department of Neurology, Graduate School of Medicine, Kobe University, and Y. Yamamoto, MD, PhD, Department of Psychiatry, Graduate School of Medicine, Kobe University, Kobe, Japan; T. Maki, MD, PhD, Department of Neurology, Kyoto University Graduate School of Medicine, Kyoto, Japan; T. Mizuno, MD, PhD, Department of Neurology, Graduate School of Medical Science, Kyoto Prefectural University of Medicine, Kyoto, Japan; R. Ohtani, MD, PhD, Department of Neurology, National Hospital Organization Kyoto Medical Center, Kyoto, Japan; N. Oka, MD, PhD, Department of Neurology, National Hospital Organization Minami Kyoto Hospital, Joyo, Japan; N. Sanjo, MD, PhD, T. Yokota, MD, PhD, Department of Neurology and Neurological Science, Tokyo Medical and Dental University, Tokyo, Japan; H. Shimura, MD, PhD, T. Urabe, MD, PhD, Department of Neurology, Juntendo University Urayasu Hospital, Urayasu, Japan; S. Sudoh, MD, PhD, Clinical Research Center and Department of Neurology, National Hospital of Utano, Kyoto, Japan; and K. Suzuki, MD, PhD, Department of Clinical Research, National Center for Geriatrics and Gerontology, Obu, Japan.
Funding source: The COMCID trial was supported by a subsidization from Otsuka Pharmaceutical Company (#C271) and a grant from the Intramural Research Fund (25-4-5) for Cerebrovascular Diseases of National Cerebral and Cardiovascular Center. Otsuka Pharmaceutical Company had no role in study design, data collection, and analysis. The authors declare no competing financial interests.
Footnotes
This trial (institutional protocol number: TRINEU1321) was registered as ClinicalTrials.govNCT02491268 (https://clinicaltrials.gov/ct2/show/NCT02491268) and UMIN Clinical Trials Registry (UMIN-CTR) UMIN000017764 (https://upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000020389).
Contributor Information
Satoshi Saito, Email: saitosa@ncvc.go.jp.
Masafumi Ihara, Email: ihara@ncvc.go.jp.
References
- 1.Larson E.B., Yaffe K., Langa K.M. New insights into the dementia epidemic. N Engl J Med. 2013;369:2275–2277. doi: 10.1056/NEJMp1311405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tarasoff-Conway J.M., Carare R.O., Osorio R.S., Glodzik L., Butler T., Fieremans E. Clearance systems in the brain-implications for Alzheimer disease. Nat Rev Neurol. 2015;11:457–470. doi: 10.1038/nrneurol.2015.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta A., Iadecola C. Impaired Aβ clearance: a potential link between atherosclerosis and Alzheimer's disease. Front Aging Neurosci. 2015;7:115. doi: 10.3389/fnagi.2015.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saito S., Ihara M. Interaction between cerebrovascular disease and Alzheimer pathology. Curr Opin Psychiatry. 2016;29:168–173. doi: 10.1097/YCO.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 5.Mawuenyega K.G., Sigurdson W., Ovod V., Munsell L., Kasten T., Morris J.C. Decreased clearance of CNS β-amyloid in Alzheimer's disease. Science. 2010;330:1774. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saito S., Ihara M. New therapeutic approaches for Alzheimer's disease and cerebral amyloid angiopathy. Front Aging Neurosci. 2014;6:290. doi: 10.3389/fnagi.2014.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maki T., Okamoto Y., Carare R.O., Hase Y., Hattori Y., Hawkes C.A. Phosphodiesterase III inhibitor promotes drainage of cerebrovascular β-amyloid. Ann Clin Transl Neurol. 2014;1:519–533. doi: 10.1002/acn3.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Louveau A., Smirnov I., Keyes T.J., Eccles J.D., Rouhani S.J., Peske J.D. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carare R.O., Hawkes C.A., Jeffrey M., Kalaria R.N., Weller R.O. Review: cerebral amyloid angiopathy, prion angiopathy, CADASIL and the spectrum of protein elimination failure angiopathies (PEFA) in neurodegenerative disease with a focus on therapy. Neuropathol Appl Neurobiol. 2013;39:593–611. doi: 10.1111/nan.12042. [DOI] [PubMed] [Google Scholar]
- 10.Lee H.R., Shin H.K., Park S.Y., Kim H.Y., Lee W.S., Rhim B.Y. Attenuation of β-amyloid-induced tauopathy via activation of CK2α/SIRT1: targeting for cilostazol. J Neurosci Res. 2014;92:206–217. doi: 10.1002/jnr.23310. [DOI] [PubMed] [Google Scholar]
- 11.Park S.H., Kim J.H., Bae S.S., Hong K.W., Lee D.S., Leem J.Y. Protective effect of the phosphodiesterase III inhibitor cilostazol on amyloid β-induced cognitive deficits associated with decreased amyloid β accumulation. Biochem Biophys Res Commun. 2011;408:602–608. doi: 10.1016/j.bbrc.2011.04.068. [DOI] [PubMed] [Google Scholar]
- 12.Akiyama H., Kudo S., Shimizu T. The absorption, distribution and excretion of a new antithrombotic and vasodilating agent, cilostazol, in rat, rabbit, dog and man. Arzneimittelforschung. 1985;35:1124–1132. [PubMed] [Google Scholar]
- 13.Shinohara Y., Katayama Y., Uchiyama S., Yamaguchi T., Handa S., Matsuoka K. Cilostazol for prevention of secondary stroke (CSPS 2): an aspirin-controlled, double-blind, randomised non-inferiority trial. Lancet Neurol. 2010;9:959–968. doi: 10.1016/S1474-4422(10)70198-8. [DOI] [PubMed] [Google Scholar]
- 14.Uchiyama S., Shinohara Y., Katayama Y., Yamaguchi T., Handa S., Matsuoka K. Benefit of cilostazol in patients with high risk of bleeding: subanalysis of cilostazol stroke prevention study 2. Cerebrovasc Dis. 2014;37:296–303. doi: 10.1159/000360811. [DOI] [PubMed] [Google Scholar]
- 15.Hase Y., Okamoto Y., Fujita Y., Kitamura A., Nakabayashi H., Ito H. Cilostazol, a phosphodiesterase inhibitor, prevents no-reflow and hemorrhage in mice with focal cerebral ischemia. Exp Neurol. 2012;233:523–533. doi: 10.1016/j.expneurol.2011.11.038. [DOI] [PubMed] [Google Scholar]
- 16.Oyama N., Yagita Y., Kawamura M., Sugiyama Y., Terasaki Y., Omura-Matsuoka E. Cilostazol, not aspirin, reduces ischemic brain injury via endothelial protection in spontaneously hypertensive rats. Stroke. 2011;42:2571–2577. doi: 10.1161/STROKEAHA.110.609834. [DOI] [PubMed] [Google Scholar]
- 17.Taguchi A., Takata Y., Ihara M., Kasahara Y., Tsuji M., Nishino M. Cilostazol improves cognitive function in patients with mild cognitive impairment: a retrospective analysis. Psychogeriatrics. 2013;13:164–169. doi: 10.1111/psyg.12021. [DOI] [PubMed] [Google Scholar]
- 18.Ihara M., Nishino M., Taguchi A., Yamamoto Y., Hattori Y., Saito S. Cilostazol add-on therapy in patients with mild dementia receiving donepezil: a retrospective study. PLoS One. 2014;9:e89516. doi: 10.1371/journal.pone.0089516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arai H., Takahashi T. A combination therapy of donepezil and cilostazol for patients with moderate Alzheimer disease: pilot follow-up study. Am J Geriatr Psychiatry. 2009;17:353–354. doi: 10.1097/JGP.0b013e31819431ea. [DOI] [PubMed] [Google Scholar]
- 20.Sakurai H., Hanyu H., Sato T., Kume K., Hirao K., Kanetaka H. Effects of cilostazol on cognition and regional cerebral blood flow in patients with Alzheimer's disease and cerebrovascular disease: a pilot study. Geriatr Gerontol Int. 2013;13:90–97. doi: 10.1111/j.1447-0594.2012.00866.x. [DOI] [PubMed] [Google Scholar]
- 21.Albert M.S., DeKosky S.T., Dickson D., Dubois B., Feldman H.H., Fox N.C. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fischl B., Salat D.H., Busa E., Albert M., Dieterich M., Haselgrove C. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 23.Jovicich J., Czanner S., Han X., Salat D., van der Kouwe A., Quinn B. MRI-derived measurements of human subcortical, ventricular and intracranial brain volumes: reliability effects of scan sessions, acquisition sequences, data analyses, scanner upgrade, scanner vendors and field strengths. Neuroimage. 2009;46:177–192. doi: 10.1016/j.neuroimage.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jovicich J., Marizzoni M., Sala-Llonch R., Bosch B., Bartres-Faz D., Arnold J. Brain morphometry reproducibility in multi-center 3T MRI studies: a comparison of cross-sectional and longitudinal segmentations. Neuroimage. 2013;83:472–484. doi: 10.1016/j.neuroimage.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 25.Petersen R.C., Smith G.E., Waring S.C., Ivnik R.J., Tangalos E.G., Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto K., Shimada H., Koh H., Ataka S., Miki T. Serum levels of albumin-amyloid beta complexes are decreased in Alzheimer's disease. Geriatr Gerontol Int. 2014;14:716–723. doi: 10.1111/ggi.12147. [DOI] [PubMed] [Google Scholar]
- 27.Corbett A., Pickett J., Burns A., Corcoran J., Dunnett S.B., Edison P. Drug repositioning for Alzheimer's disease. Nat Rev Drug Discov. 2012;11:833–846. doi: 10.1038/nrd3869. [DOI] [PubMed] [Google Scholar]
- 28.Saito S., Yamamoto Y., Ihara M. Mild cognitive impairment: at the crossroad of neurodegeneration and vascular dysfunction. Curr Alzheimer Res. 2015;12:507–512. doi: 10.2174/1567205012666150530202508. [DOI] [PubMed] [Google Scholar]
- 29.Kalaria R.N., Akinyemi R., Ihara M. Does vascular pathology contribute to Alzheimer changes? J Neurol Sci. 2012;322:141–147. doi: 10.1016/j.jns.2012.07.032. [DOI] [PubMed] [Google Scholar]
- 30.Kalaria R.N., Ihara M. Dementia: vascular and neurodegenerative pathways—will they meet? Nat Rev Neurol. 2013;9:487–488. doi: 10.1038/nrneurol.2013.164. [DOI] [PubMed] [Google Scholar]
- 31.Thompson P.D., Zimet R., Forbes W.P., Zhang P. Meta-analysis of results from eight randomized, placebo-controlled trials on the effect of cilostazol on patients with intermittent claudication. Am J Cardiol. 2002;90:1314–1319. doi: 10.1016/s0002-9149(02)02869-2. [DOI] [PubMed] [Google Scholar]