Abstract
Introduction
Alzheimer's disease (AD) incidence is disproportionately high in African Americans, yet, recruitment of this community to AD clinical trials is challenging.
Methods
We compared 47 African Americans and 78 whites in their willingness to enroll in a hypothetical preclinical AD trial and examined barriers and facilitators in their decision making.
Results
African American race (OR = 0.45; 95% CI, 0.22–0.93) and score on the research attitude questionnaire (OR = 1.12; 95% CI, 1.04–1.22) were independently associated with willingness to participate. African Americans rated study risks, the requirement of a study partner, study procedures, the ratio of drug to placebo, and study location as more important factors in the decision whether to enroll than did whites.
Discussion
These results suggest that researchers will encounter challenges in recruiting African Americans to preclinical AD trials. Future research will be necessary to understand the optimal means to improve recruitment of underrepresented populations.
Keywords: Recruitment, Race, Minority, Prevention, Clinical trial
1. Introduction
Studies show substantial variation in the incidence of Alzheimer's disease (AD) dementia across racial and ethnic groups, and African Americans may be at highest risk [1]. The incidence of AD in African Americans may be twice as high as that of whites [1], [2], [3], [4], [5], [6]. In addition, the rates of dementia diagnosis [7], [8], [9], use of approved AD treatments [10], and survival [11], [12] are all reduced in this community. Research is the key to understand and address these problems. Unfortunately, few African Americans participate in research [13], [14]. Preclinical AD trials, such as the anti-amyloid treatment in asymptomatic AD study (A4 study) [15], are testing interventions in participants before the onset of cognitive impairment or dementia. If these studies do not include adequate representation of African American participants, then it risks perpetuating the disparities in understanding and addressing the burden of AD in this racial group.
Preclinical AD trials, similar to other clinical trials, face challenges in recruitment, especially for participants from diverse racial and ethnic populations. Strategies to enhance minority participation in AD research are well described [16], [17], but a more thorough understanding of the barriers to minority recruitment based on empirical data remains an area of need. In the present study, we compared the willingness to participate in a hypothetical preclinical AD trial between whites and African Americans. We hypothesized that the groups would differ in their approach to deciding whether to participate in AD prevention trials. We examined the influence of specific trial factors in decision making and probed for potential incentives for each racial group by analyzing both quantitative and qualitative data.
2. Methods
2.1. Participants
We performed a post hoc secondary analysis of an interview study that examined the impact of AD biomarker disclosure on AD prevention trial recruitment. The study used a mixed-methods experimental design. One-hundred thirty-two cognitively normal participants were randomly assigned to one of two hypothetical AD prevention trials that either did or did not require disclosure of the results of an amyloid positron emission tomography scan. We probed for responses of participant willingness to enroll and factors that might affect the decision. In the primary analysis [18], we found no difference in participant willingness to enroll, based on whether disclosure of amyloid status was required. In addition, we observed no interaction effects between the disclosure requirement and any other variable, including race. In the current analysis, we compared quantitative and qualitative data between 78 white and 47 African American participants.
All participants were aged 65 years or more and were interviewed in English. They had no previous diagnosis of dementia, mild cognitive impairment, or other neurological or psychiatric disease and no auditory or visual impairments that prevented the conduct of the study interview. They were recruited through a variety of mechanisms, including community education events on AD, and multiple referral sources (Table 1).
Table 1.
Sources of recruitment
| Source | Whites (N = 78) | African Americans (N = 47) |
|---|---|---|
| UCLA ADRC Registry, n (%) | 25 (32.0) | 5 (10.6) |
| Community talk, n (%) | 14 (17.9) | 16 (34.0) |
| Community liaison, n (%) | 3 (3.8) | 17 (36.2) |
| Community referral, n (%) | 6 (7.7) | 4 (8.5) |
| Self-referral, n (%) | 7 (9.0) | 0 (0) |
| UCLA ADRC control subjects, n (%) | 7 (9.0) | 0 (0) |
| Banner Alzheimer's Prevention Initiative Registry, n (%) | 5 (6.4) | 1 (2.1) |
| Caregiver support program, n (%) | 3 (3.8) | 1 (2.1) |
| Clinical referral, n (%) | 2 (2.6) | 1 (2.1) |
| Unknown, n (%) | 6 (7.7) | 2 (4.2) |
Abbreviation: UCLA ADRC, University of California, Los Angeles Alzheimer's Disease Research Center.
2.2. Procedure
In a face-to-face interview with a research assistant, participants were given an informed consent form (ICF) describing a hypothetical AD prevention clinical trial that was 36-months long, double-blind, 1:1 randomized, and required visits at a medical center every 6 months. Two versions of the ICF were used based on experimental assignment (details can be found in the primary article [18]). After checking for participants' comprehension, the research assistant used structured and open-ended questions to assess participant willingness to enroll, as well as which trial factors and potential incentives affected their decision. Participants received a $25 gift card to a national retail store for their participation.
The UCLA Institutional Review Board (IRB) approved the study, and all participants underwent IRB-approved informed consent.
2.3. Measures
2.3.1. Demographics
In addition to race, we collected participant age, gender, ethnicity, education level, employment status, job freedom, family history of AD, caregiver status, perceived health condition, and residential distance to the medical center.
2.3.2. Likelihood to enroll in a prevention trial
The primary outcome was assessed using a single question: “How likely would you be to enroll in the described Alzheimer's disease prevention trial,” with a 6-point response scale from “extremely unlikely” to “extremely likely.”
2.3.3. Importance of trial factors
Participants received structured questions on seven trial factors: frequency of visits, location of visits, length of study, requirement of a study partner, study risks, likelihood of receiving placebo, and required procedures. Participants used a 6-point rating scale from “extremely unimportant” to “extremely important” to rate each factor.
2.3.4. Incentives for participation
Participants were asked an open-ended question “are there any things that would have made you more likely to participate?” Additional structured questions asked about six incentives including receiving overall study results, personal blood test results, personal genetic test results, personal cognitive test rests, financial compensation, and estimated personal risk for getting AD. Participants rated each incentive on a 6-point scale ranging from making them “much less likely to enroll” to “much more likely to enroll.”
2.3.5. Covariates
We also assessed participants' knowledge about AD, general attitudes toward research, perceived risk for AD, and subjective cognitive performance. Knowledge about AD was measured by the AD Knowledge Scale (ADKS) [19], a 30-item true or false questionnaire, with higher scores representing greater knowledge. The Research Attitude Questionnaire [20] is a 7-item, 5-point scale (score range, 7–35), for which higher scores represent a more favorable attitude toward research. Perceived risk for AD was measured by a 5-item, 5-point scale (score range, 5–25) [21], with higher scores reflecting a higher perceived risk for AD. Subjective cognitive performance was measured by the Cognitive Change Index [22], a 20-item, 5-point scale (score range, 20–100) that assesses participants' perceived cognitive decline, relative to their own level of function 5 years prior. Higher scores represent greater subjective decline.
2.4. Data analyses
We compared whites and African Americans on demographics and other characteristics, using unpaired t-tests for continuous variables, chi-square tests or Fisher tests for categorical variables, and Cochran–Armitage trend tests for ordinal variables (e.g., self-rated health, distance to the medical center, and job freedom).
Racial differences on willingness to enroll were examined with ordered logistic regression models. We first used unadjusted univariate models to assess for the effects of race and each covariate. Subsequently, using a multivariable model, we examined the effect of race adjusting for effects of covariates, including those covariates with P values <.2 in univariate models. We also examined potential interaction effects between race and the covariates. Because previous analyses found no effect of the requirement of biomarker status disclosure, this variable was not included in any model.
We examined racial differences on each trial factor and each incentive using Cochran–Armitage trend tests. Within each racial group, we used Friedman tests to examine for an overall difference among the factors and the incentives and Wilcoxon signed rank tests for post hoc pairwise comparisons. For open-ended responses, one investigator separated participant interviews into separate comments and developed preliminary themes in which comments were included. Comments were placed on cards and three investigators engaged in a consensus-forming exercise, examining the developed themes and assigning individual comments to those themes, blinded to participant information [23]. Participants' responses were coded dichotomously, indicating present or absent, for each theme. We compared the racial groups on the frequencies of these defined codes.
All analyses were performed in R, version 3.1.3 [24]. Results of statistical tests are reported with a significance level of 0.05.
3. Results
3.1. Participants
Participants were recruited through multiple sources (Table 1). Community lectures served as an effective recruitment tool for both races (18% of whites and 34% of African Americans). A third of whites were recruited from the UCLA AD Research Center potential participant registry [25]. In contrast, a high proportion (36%) of African Americans were recruited through the work of a community liaison who attended establishments such as senior centers and beauty salons, discussed the study, and distributed flyers.
The two racial groups were similar in age, education, gender, employment status, perceived risk for AD, attitudes toward research, and residential distance to the medical center (Table 2). Similar proportions of each group knew someone with AD, had a family history of AD, or were caregivers for a patient with AD. Whites had higher scores on the ADKS (P = .01) and better self-rated health (P = .02) than African Americans.
Table 2.
Description of the sample
| Characteristic | White | African American | P value |
|---|---|---|---|
| n | 78 | 47 | |
| Mean age, years ± SD (range) | 73.9 ± 6.6 (65–89) | 72.0 ± 5.2 (66–83) | .10 |
| Female gender, n (%) | 51 (65.4) | 37 (78.7) | .17 |
| Hispanic ethnicity, n (%) | 5 (6.4) | 1 (2.1) | .41 |
| Mean Education, years ± SD (range) | 16.5 ± 2.7 (11–24) | 16.1 ± 2.4 (12–24) | .51 |
| Retired, n (%) | 61 (79.2) | 42 (89.4) | .22 |
| Job freedom | .75 | ||
| Never, n (%) | 0 | 1 (2.1) | |
| Rarely, n (%) | 3 (3.8) | 1 (2.1) | |
| Frequently, n (%) | 13 (16.7) | 8 (17.0) | |
| Always, n (%) | 62 (79.5) | 37 (78.7) | |
| ADKS score, mean ± SD (range) | 24.0 ± 2.9 (18–29) | 22.5 ± 3.5 (15–29) | .01 |
| RAQ score, mean ± SD (range) | 29.3 ± 4.3 (7–35) | 29.9 ± 3.3 (23–35) | .35 |
| AD Caregivers, n (%) | 6 (7.7) | 4 (8.5) | >.99 |
| Family History of AD, n (%) | 21 (27.3) | 11 (23.9) | .84 |
| Do you know someone with AD? | 64 (82.1) | 38 (80.9) | >.99 |
| Risk for AD score, mean ± SD (range) | 16.6 ± 3.8 (5–24) | 15.8 ± 4.6 (5–24) | .28 |
| Rating of overall health | .02 | ||
| Excellent, n (%) | 23 (29.5) | 4 (8.5) | |
| Very good, n (%) | 33 (42.3) | 25 (53.2) | |
| Good, n (%) | 19 (24.4) | 15 (31.9) | |
| Fair, n (%) | 3 (3.8) | 3 (6.4) | |
| Poor, n (%) | 0 | 0 | |
| Distance to the medical center | .14 | ||
| 0–5 miles, n (%) | 40 (51.3) | 13 (27.7) | |
| 5–15 miles, n (%) | 22 (28.2) | 23 (48.9) | |
| 15–30 miles, n (%) | 10 (12.8) | 8 (17.0) | |
| >30 miles, n (%) | 6 (7.7) | 3 (6.4) |
Abbreviations: ADKS, Alzheimer's Disease Knowledge Scale; RAQ, Research Attitude Questionnaire; AD, Alzheimer's disease.
3.2. Willingness to participate
Seventy-two percent (56 of 78) of whites and 51% (24 of 47) of African Americans reported that they were likely to enroll in the AD prevention trial (Table 3). Univariate-ordered logistic regression showed that African Americans were less likely to enroll than whites (odds ratio = 0.45; 95% confidence interval, 0.23–0.86; Table 4). In additional univariate analyses, RAQ score (higher scores associated with greater willingness), retirement status (not retired more willing than retired), and perceived risk for AD (higher perceived risk associated with greater willingness) were significantly associated with likelihood to enroll (P < .05; Table 4). ADKS score (P = .07), Cognitive Change Index score (P = .05), and distance from the medical center (P = .16) were also included in the subsequent multivariable model.
Table 3.
Frequency of responses to the primary outcome question, based on race
| Group | Extremely unlikely | Very unlikely | Somewhat unlikely | Somewhat likely | Very likely | Extremely likely |
|---|---|---|---|---|---|---|
| White, n (%) | 8 (10.3) | 4 (5.1) | 10 (12.8) | 21 (26.9) | 20 (25.6) | 15 (19.2) |
| African American, n (%) | 5 (10.6) | 9 (19.1) | 9 (19.1) | 13 (27.7) | 5 (10.6) | 6 (12.8) |
| Total, n (%) | 13 (10.4) | 13 (10.4) | 19 (15.2) | 34 (27.2) | 25 (20.0) | 21 (16.8) |
Table 4.
Results of univariate ordinal logistic models
| Variable (reference group) | Univariate Models |
Multivariable Model |
||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Race (white) | 0.45 (0.23–0.86)∗ | .02 | 0.45 (0.22–0.93)∗ | .03 |
| Age | 0.98 (0.94–1.04) | .62 | ||
| Education | 1.06 (0.94–1.20) | .37 | ||
| RAQ score | 1.12 (1.04–1.22)∗ | .006 | 1.12 (1.04–1.22)∗ | .005 |
| ADKS score | 1.10 (0.99–1.21) | .07 | 1.06 (0.95–1.18) | .26 |
| Ethnicity (Non-Latino) | 0.74 (0.19–2.90) | .67 | ||
| Retirement status (Non-retired) | 0.43 (0.19–0.95)∗ | .04 | 0.56 (0.24–1.33) | .19 |
| Family history (No family history) | 1.26 (0.61–2.63) | .54 | ||
| Perceived risk for AD | 1.10 (1.02–1.19)∗ | .02 | 1.08 (1.00–1.18) | .07 |
| Cognitive Change Index | 1.03 (1.00–1.06) | .05 | 1.03 (1.00–1.06) | .07 |
| Rating of overall health | 1.15 (0.77–1.72) | .49 | ||
| Distance from the medical center | 1.28 (0.91–1.81) | .16 | 1.25 (0.86–1.81) | .25 |
Abbreviations: ADKS, Alzheimer's Disease Knowledge Scale; RAQ, Research Attitude Questionnaire; AD, Alzheimer's Disease.
P < .05.
The final multivariable model showed that, after adjusting for covariates, African Americans remained significantly less likely to enroll than whites (OR = 0.45; 95% CI, 0.22–0.93). The only other predictor that remained significant was RAQ score (OR = 1.12; 95% CI, 1.04–1.22), with every one point higher score associated with 12% higher likelihood to enroll. There was no interaction effect between race and RAQ total score or between race and any of the individual RAQ items (data not shown).
3.3. Importance of trial factors
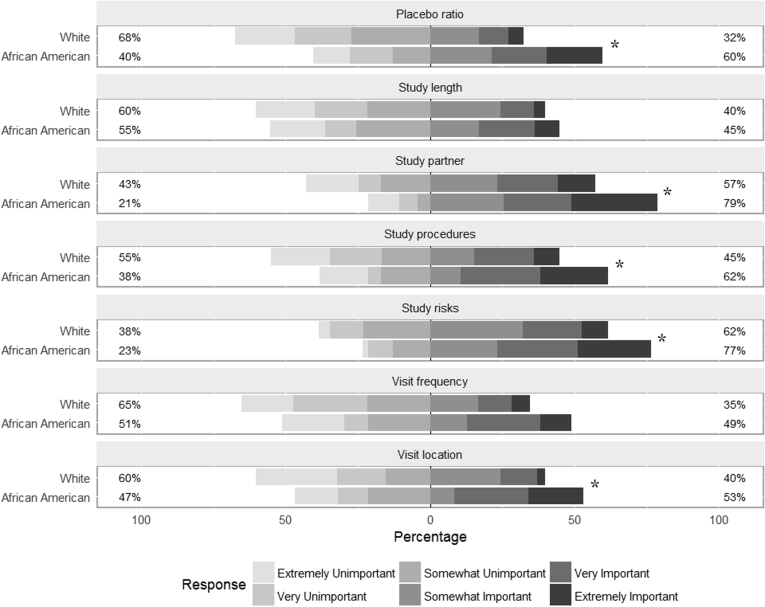
African American participants rated five of seven trial factors as being of greater importance to the decision whether to enroll than did whites (Fig. 1), including study risks, the requirement of a study partner, study procedures, ratio of drug to placebo, and study location (Cochran–Armitage test, P < .05). Frequency of study visits and total study length were rated similarly by the two groups.
Fig. 1.
Participant ratings of the importance of trial factors in the decision whether to enroll for each racial group. * indicates P < .05 for racial differences.
Within each racial group, Friedman tests showed that participants' ratings of importance significantly differed among the seven trial factors (P < .0001). In both groups, study risks and the requirement of a study partner were rated as significantly more important than the remaining five factors (Wilcoxon signed rank test, P < .05 for all pairwise comparisons).
3.4. Incentives for participation
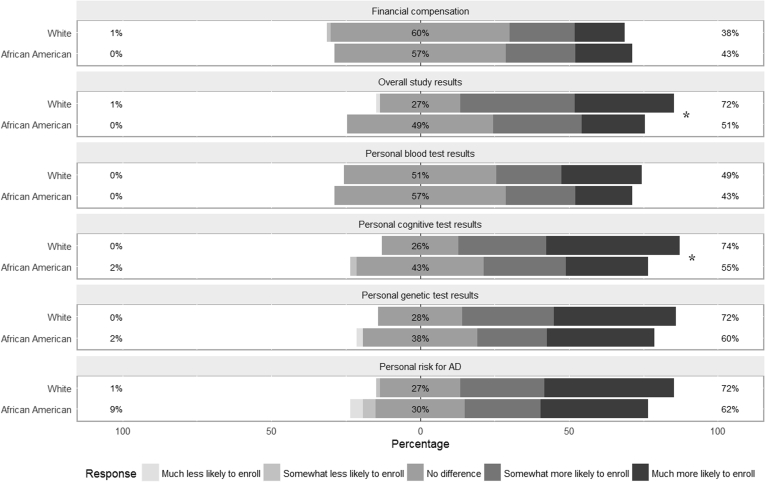
Quantitative and qualitative data showed mixed results for potential incentives for enrollment. In response to open-ended questions, African Americans more frequently mentioned financial compensation (23% vs. 14%) and returning of research results (19% vs. 6%) as potential incentives than did whites. In structured questions that examined six potential incentives (Fig. 2), there was no difference between African Americans and whites in the impact that financial incentives would have on enrollment. More whites than African Americans responded that returning cognitive test results and returning overall study results would make them more likely to enroll (Cochran Armitage test, P < .05). No difference between the groups was found for the remaining incentives.
Fig. 2.
Participant ratings of potential incentives for participation for each racial group. * indicates P < .05 for racial differences.
Within each racial group, Friedman tests confirmed that the six incentives were rated differently (P < .0001 for whites, P < .05 for African Americans). Whites reported that receiving personal cognitive test results, personal risk estimates for getting AD, personal genetic test results, and overall study results would make them more likely to enroll than receiving personal blood test results (Wilcoxon signed rank test, P < .05 for all pairwise comparisons); and that financial compensation was less effective than any other incentive (P < .05 for all pairwise comparisons). African Americans reported that receiving personal genetic test results would make them more likely to enroll than receiving personal blood test results or receiving financial compensation (P < .05); and that receiving personal cognitive results would make them more likely to enroll than receiving personal blood test results (P < .05).
4. Discussion
In this study, African Americans were less likely to express a willingness to participate in AD prevention trials than were whites, a finding that remained after adjusting for potential confounders such as knowledge about AD, perceived risk for AD, attitudes toward research, perceived cognitive decline, retirement status, and residential distance from the medical center. This sample is representative of community members that researchers will attempt to recruit to preclinical AD trials, as they demonstrated interest and favorable attitudes toward AD research. Therefore, our results suggest that researchers may encounter challenges in recruiting African Americans and are in contrast to some recent studies that suggest that African Americans are just as likely as whites to participate in research when presented with the opportunity [26].
African American reluctance in participating in clinical trials and medical research is well-documented [27], [28], [29], [30]. Past research practices with African American participants that were self-serving and unethical have had profound influences on this community, resulting in distrust in doctors, scientists, and the medical system [28], [31]. The consent process, with the goal of informing patients of study risks and benefits, may be misinterpreted by some African Americans as relinquishing their autonomy and as a legal protection for doctors [28]. Our findings that African Americans more heavily weighted a variety of trial aspects, including study risks and study procedures, in their decision than did whites, may partly reflect these issues. Efforts to instill trust in clinical relationships by involving African American personnel who can explain and perform trial procedures may reduce this skepticism [32]. In this study, we used an African American community liaison to aid in recruitment, and this was the greatest source of African American participants.
Both race and research attitudes were independently associated with willingness to enroll, suggesting that research attitudes alone cannot explain the reluctance of African Americans to participate in AD prevention trials. In fact, African Americans' RAQ scores did not differ from the scores of white participants. Others have reported divergent findings. Neugroschl et al. [33] administered the RAQ to 123 diverse attendees of community talks on cognitive aging in New York City and found that nearly half of participants had a less than positive response for the item “participating in medical research is generally safe.” The authors noted that these scores were lower than previously reported means from predominantly white participants [34].
A relatively high proportion of our African American participants were recruited through community talks. African Americans in our study also had less factual knowledge about AD than did whites. African Americans may have fewer sources of information about AD than whites [35], [36] and may view memory loss as a natural and expected part of aging, instead of as a sign of disease [37]. Thus, community education, potentially partnering with trusted community members, may be a promising intervention to increase minority participation in AD prevention research. Unfortunately, our results do not explicitly instruct which educational topics will be most effective to improving participation rates. Nevertheless, community programs that describe the extensive precautions in place to ensure the voluntary nature of research and the safety of participants; that African Americans are at increased risk for AD as a community; and that diverse participation is needed to reduce health care disparities represent a logical starting point [38].
Among the limitations of this study is that it is a retrospective secondary analysis of a study that measured hypothetical behaviors, rather than actual enrollment decisions. The protocol was designed to examine the impact of disclosing amyloid status on recruitment, not to examine differences among racial groups. The questions that were asked about trial barriers and facilitators were developed for a general trial audience and did not address specific racial and cultural differences. Similarly, the RAQ focuses on general attitudes toward research, not race-specific elements. The sources of white and African American participants differed substantially (Table 1). More white than African American participants had previously enrolled in longitudinal research or potential participant registries, creating the possibility that differing levels of previous participation [39], rather than race accounted for the observed findings. In sub-analyses limited to those participants who were de novo recruited for this study; however, we observed similar trends suggesting that race is associated with willingness to participate (data not shown). Even among those enrolled in AD prevention registries, African Americans may be less likely to endorse participation in trials, especially those involving a drug [40]. Thus, novel recruitment methods to not only reach diverse participants but to overcome the barriers to their enrollment will likely be necessary to successfully increase minority participation. These methods are needed not only for African Americans, but also for other racial and ethnic groups that are traditionally underrepresented in AD research, such as Latinos and Asian Americans [13], [17], [41].
In conclusion, our results show a significant racial difference in willingness to participate in preclinical AD trials that is not explained by the other covariates. All of our findings should be viewed as preliminary and will require assessment in future research, which should seek to more fully understand the barriers to minority enrollment and the optimal means to improve recruitment of underrepresented populations. Nevertheless, differences in AD knowledge and well-described barriers such as lack of trust suggest that community education to inform African Americans about AD risk and the need for equitable research participation to overcome health disparities may be one of the keys to improving participation rates in preclinical AD trials in this group.
Research in context.
-
1.
Systematic review: We reviewed the literature using traditional and Internet sources (e.g., PubMed and Google Scholar). Many studies show that the incidence of AD is disproportionately high in African Americans. Challenges and strategies for recruiting this racial group to AD clinical research are well described but are infrequently based on empirical data.
-
2.
Interpretation: Our findings suggest that African Americans have lower willingness to participate in preclinical AD trials than do whites and that improving African American participation rates may require more than simply increasing awareness of research opportunities within this community.
-
3.
Future directions: Our results are based on a secondary analysis, rather than a study specifically designed to examine racial differences. Future studies should examine potential cultural factors underlying the barriers to African American recruitment and should include other minority groups traditionally underrepresented in AD research.
Acknowledgments
We thank the participants and community liaisons for making this study possible. This work was supported by Alzheimer's Association NIRG 12-242511. Y.Z., D.E., S.K., E.T., and J.G. were also supported by NIA AG016570. J.G. is currently supported by NIA AG016573. J.K. was supported by NIA P30-AG01024. E.T., S.K., and J.G. were supported by the Sidell-Kagan Foundation. Y.Z. performed the statistical analyses, drafted the article, and approved the final draft. D.E. and J.K. designed the study, edited the article for content, and approved the final draft. S.K. and E.T. participated in the analyses, edited the article for content, and approved the final draft. J.G. secured the funding, designed and oversaw the study, edited the article for content, and approved the final draft.
References
- 1.Mayeda E.R., Glymour M.M., Quesenberry C.P., Whitmer R.A. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement. 2016;12:216–224. doi: 10.1016/j.jalz.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yaffe K., Falvey C., Harris T.B., Newman A., Satterfield S., Koster A. Effect of socioeconomic disparities on incidence of dementia among biracial older adults: Prospective study. BMJ. 2013;347 doi: 10.1136/bmj.f7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evans D.A., Bennett D.A., Wilson R.S., Bienias J.L., Morris M.C., Scherr P.A. Incidence of Alzheimer disease in a biracial urban community: relation to apolipoprotein E allele status. Arch Neurol. 2003;60:185–189. doi: 10.1001/archneur.60.2.185. [DOI] [PubMed] [Google Scholar]
- 4.Plassman B.L., Langa K.M., Fisher G.G., Heeringa S.G., Weir D.R., Ofstedal M.B. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29:125–132. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shadlen M.F., Siscovick D., Fitzpatrick A.L., Dulberg C., Kuller L.H., Jackson S. Education, Cognitive Test Scores, and Black-White Differences in Dementia Risk. J Am Geriatr Soc. 2006;54:898–905. doi: 10.1111/j.1532-5415.2006.00747.x. [DOI] [PubMed] [Google Scholar]
- 6.Tang M.X., Cross P., Andrews H., Jacobs D., Small S., Bell K. Incidence of AD in African-Americans, Caribbean hispanics, and caucasians in northern Manhattan. Neurology. 2001;56:49–56. doi: 10.1212/wnl.56.1.49. [DOI] [PubMed] [Google Scholar]
- 7.Clark P.C., Kutner N.G., Goldstein F.C., Peterson-Hazen S., Garner V., Zhang R. Impediments to timely diagnosis of Alzheimer's disease in African Americans. J Am Geriatr Soc. 2005;53:2012–2017. doi: 10.1111/j.1532-5415.2005.53569.x. [DOI] [PubMed] [Google Scholar]
- 8.Connell C.M., Roberts J.S., McLaughlin S.J., Carpenter B.D. Black and white adult family members' attitudes toward a dementia diagnosis. J Am Geriatr Soc. 2009;57:1562–1568. doi: 10.1111/j.1532-5415.2009.02395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Connell C.M., Roberts J.S., McLaughlin S.J., Akinleye D. Racial differences in knowledge and beliefs about Alzheimer disease. Alzheimer Dis Assoc Disord. 2009;23:110–116. doi: 10.1097/WAD.0b013e318192e94d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehta K.M., Yin M., Resendez C., Yaffe K. Ethnic differences in acetylcholinesterase inhibitor use for Alzheimer disease. Neurology. 2005;65:159–162. doi: 10.1212/01.wnl.0000167545.38161.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helzner E., Scarmeas N., Cosentino S., Tang M., Schupf N., Stern Y. Survival in Alzheimer disease A multiethnic, population-based study of incident cases. Neurology. 2008;71:1489–1495. doi: 10.1212/01.wnl.0000334278.11022.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehta K., Yaffe K., Perez-Stable E., Stewart A., Barnes D., Kurland B. Race/ethnic differences in AD survival in US Alzheimer's Disease Centers. Neurology. 2008;70:1163–1170. doi: 10.1212/01.wnl.0000285287.99923.3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faison W.E., Schultz S.K., Aerssens J., Alvidrez J., Anand R., Farrer L.A. Potential ethnic modifiers in the assessment and treatment of Alzheimer's disease: challenges for the future. Int Psychogeriatr. 2007;19:539–558. doi: 10.1017/S104161020700511X. [DOI] [PubMed] [Google Scholar]
- 14.Watson J.L., Ryan L., Silverberg N., Cahan V., Bernard M.A. Obstacles And Opportunities In Alzheimer's Clinical Trial Recruitment. Health Aff. 2014;33:574–579. doi: 10.1377/hlthaff.2013.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sperling R.A., Rentz D.M., Johnson K.A., Karlawish J., Donohue M., Salmon D.P. The A4 study: stopping AD before symptoms begin? Sci Transl Med. 2014;6:228fs13. doi: 10.1126/scitranslmed.3007941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gauthier M.A., Clarke W.P. Gaining and sustaining minority participation in longitudinal research projects. Alzheimer Dis Assoc Disord. 1999;13:S29–S33. doi: 10.1097/00002093-199904001-00008. [DOI] [PubMed] [Google Scholar]
- 17.Olin J.T., Dagerman K.S., Fox L.S., Bowers B., Schneider L.S. Increasing ethnic minority participation in Alzheimer disease research. Alzheimer Dis Assoc Disord. 2002;16:S82–S85. doi: 10.1097/00002093-200200002-00009. [DOI] [PubMed] [Google Scholar]
- 18.Grill J.D., Zhou Y., Elashoff D., Karlawish J. Disclosure of amyloid status is not a barrier to recruitment in preclinical Alzheimer's disease clinical trials. Neurobiol Aging. 2016;39:147–153. doi: 10.1016/j.neurobiolaging.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carpenter B.D., Balsis S., Otilingam P.G., Hanson P.K., Gatz M. The Alzheimer's Disease Knowledge Scale: development and psychometric properties. Gerontologist. 2009;49:236–247. doi: 10.1093/geront/gnp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rubright J.D., Cary M.S., Karlawish J.H., Kim S.Y. Measuring how people view biomedical research: Reliability and validity analysis of the Research Attitudes Questionnaire. J Empir Res Hum Res Ethics. 2011;6:63–68. doi: 10.1525/jer.2011.6.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts J.S., Connell C.M. Illness representations among first-degree relatives of people with Alzheimer disease. Alzheimer Dis Assoc Disord. 2000;14:129–136. doi: 10.1097/00002093-200007000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Rattanabannakit C., Risacher S.L., Gao S., Lane K., Brown S.A., McDonald B.C. The Cognitive Change Index as a Measure of Self and Informant Perception of Cognitive Decline: Relation to Neuropsychological Tests. J Alzheimers Dis. 2016;51:1145–1155. doi: 10.3233/JAD-150729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryan G.W., Bernard H.R. Techniques to identify themes. Field Methods. 2003;15:85–109. [Google Scholar]
- 24.Team RC . R Foundation for Statistical Computing; Vienna: 2015. R: A Language and Environment for Statistical Computer. [Google Scholar]
- 25.Grill J.D., Galvin J.E. Facilitating Alzheimer disease research recruitment. Alzheimer Dis Assoc Disord. 2014;28:1–8. doi: 10.1097/WAD.0000000000000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wendler D., Kington R., Madans J., Van Wye G., Christ-Schmidt H., Pratt L.A. Are racial and ethnic minorities less willing to participate in health research? PLoS Med. 2005;3:e19. doi: 10.1371/journal.pmed.0030019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ballard E.L., Gwyther L.P., Edmonds H.L. Challenges and opportunities: recruitment and retention of African Americans for Alzheimer disease research: lessons learned. Alzheimer Dis Assoc Disord. 2010;24 Suppl:S19–S23. doi: 10.1097/WAD.0b013e3181f12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corbie-Smith G., Thomas S.B., Williams M.V., Moody-Ayers S. Attitudes and beliefs of African Americans toward participation in medical research. J Gen Intern Med. 1999;14:537–546. doi: 10.1046/j.1525-1497.1999.07048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moreno-John G., Gachie A., Fleming C.M., Napoles-Springer A., Mutran E., Manson S.M. Ethnic minority older adults participating in clinical research: developing trust. J Aging Health. 2004;16:93S–123S. doi: 10.1177/0898264304268151. [DOI] [PubMed] [Google Scholar]
- 30.Williams M.M., Scharff D.P., Mathews K.J., Hoffsuemmer J.S., Jackson P., Morris J.C. Barriers and Facilitators of African American Participation in Alzheimer Disease Biomarker Research. Alzheimer Dis Assoc Disord. 2010;24 Suppl:S24–S29. doi: 10.1097/WAD.0b013e3181f14a14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris Y., Gorelick P.B., Samuels P., Bempong I. Why African Americans may not be participating in clinical trials. J Natl Med Assoc. 1996;88:630. [PMC free article] [PubMed] [Google Scholar]
- 32.Areán P.A., Gallagher-Thompson D. Issues and recommendations for the recruitment and retention of older ethnic minority adults into clinical research. J Consult Clin Psychol. 1996;64:875. doi: 10.1037//0022-006x.64.5.875. [DOI] [PubMed] [Google Scholar]
- 33.Neugroschl J., Sewell M., De La Fuente A., Umpierre M., Luo X., Sano M. Attitudes and Perceptions of Research in Aging and Dementia in an Urban Minority Population. J Alzheimers Dis. 2016;53:69–72. doi: 10.3233/JAD-151072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jefferson A.L., Lambe S., Chaisson C., Palmisano J., Horvath K.J., Karlawish J. Clinical research participation among aging adults enrolled in an Alzheimer's Disease Center research registry. J Alzheimers Dis. 2011;23:443–452. doi: 10.3233/JAD-2010-101536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roberts J.S., Connell C.M., Cisewski D., Hipps Y.G., Demissie S., Green R.C. Differences between African Americans and whites in their perceptions of Alzheimer disease. Alzheimer Dis Assoc Disord. 2003;17:19–26. doi: 10.1097/00002093-200301000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Hipps Y.G., Roberts J.S., Farrer L.A., Green R.C. Differences between African Americans and Whites in their attitudes toward genetic testing for Alzheimer's disease. Genet Test. 2003;7:39–44. doi: 10.1089/109065703321560921. [DOI] [PubMed] [Google Scholar]
- 37.Manly J.J., Jacobs D., Mayeux R. Lippincott Williams & Wilkins; Philadelphia: 1999. Alzheimer's Disease Among Different Ethnic and Racial Groups. Alzheimer's Disease, 2nd ed; pp. 117–131. [Google Scholar]
- 38.Satcher D. The Washington Post; Washington, D.C.: 2014. More African Americans Need to Participate in Clinical Trials. [Google Scholar]
- 39.VanEpps E.M., Volpp K.G., Halpern S.D. A nudge toward participation: Improving clinical trial enrollment with behavioral economics. Sci Transl Med. 2016;8:348fs13. doi: 10.1126/scitranslmed.aaf0946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Romero H.R., Welsh-Bohmer K.A., Gwyther L.P., Edmonds H.L., Plassman B.L., Germain C.M. Community engagement in diverse populations for Alzheimer disease prevention trials. Alzheimer Dis Assoc Disord. 2014;28:269–274. doi: 10.1097/WAD.0000000000000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schneider L.S. Drug development, clinical trials, cultural heterogeneity in Alzheimer disease: the need for pro-active recruitment. Alzheimer Dis Assoc Disord. 2005;19:279–283. doi: 10.1097/01.wad.0000190808.97878.b8. [DOI] [PubMed] [Google Scholar]