Abstract
Background
Adversity during early development has been shown to have enduring negative physiological consequences. In turn, atypical physiological functioning has been associated with maladaptive processing of negative affect, including its regulation. The present study therefore explored whether exposure to adverse life events in childhood predicted maladaptive (less flexible) parasympathetic nervous system functioning during the processing of negative affect among adolescents with depression histories.
Methods
An initially clinic-referred, pediatric sample (N = 189) was assessed at two time points. At Time 1, when subjects were 10.17 years old (SD = 1.42), on average, and were depressed, parents reported on adverse life events the offspring experienced up to that point. At Time 2, when subjects were 17.18 years old (SD = 1.28), and were remitted from depression, parents again reported on adverse life events in their offspring’s lives for the interim period. At time 2, subjects’ parasympathetic nervous system functioning (quantified as respiratory sinus arrhythmia) also was assessed at rest, during sad mood induction, and during instructed mood repair.
Results
Extent of adverse life events experienced by T1 (but not events occurring between T1 and T2) predicted less flexible RSA functioning 7 years later during the processing of negative affect. Adolescents with more extensive early life adversities exhibited less vagal withdrawal following negative mood induction and tended to show less physiological recovery following mood repair.
Conclusions
Early adversities appear to be associated with less flexible physiological regulatory control during negative affect experience, when measured later in development. Stress-related autonomic dysfunction in vulnerable youths may contribute to the unfavorable clinical prognosis associated with juvenile-onset depression.
Keywords: early adversity, physiological flexibility, respiratory sinus arrhythmia, negative affect processing, adolescents, depression
1. Introduction
An extensive body of research has documented that stressful events, particularly developmentally early adversities, can have lasting negative effects on neurobiological systems involved in the regulation of affect and response to stress (for reviews, see Kaffman and Meaney, 2007; Lupien et al., 2009; Heim and Binder, 2012). This work has included both non-human and human subjects and has mostly targeted neuroendocrine functioning via the hypothalamic-pituitary-adrenal (HPA) axis and its neural substrates (Chen et al., 2010; Pesonen et al., 2010; Rao et al., 2010; Bush et al., 2011; Hanson et al., 2015).
Autonomic regulation, often indexed as respiratory sinus arrhythmia (RSA; Porges, 2007), is another neurobiological system potentially vulnerable to the negative effects of early adversities. Associations between early-life adversity and RSA impairment have been documented despite considerable heterogeneity in the types of early-life adversities examined and in how impaired RSA was defined (for a review, see Propper and Holochwost, 2013). Namely, negative events as diverse as parental depression (Field et al., 1995; Pickens and Field, 1995), domestic violence (Rigterink et al., 2010), marital conflict (Porter et al., 2003; Moore, 2010; El-Sheikh and Hinnant, 2011), neglect and disordered attachment (Oosterman et al., 2010), and institutional care (McLaughlin et al., 2015) have been associated with altered RSA, either in the form of lower baseline RSA (Field et al., 1995; Pickens and Field, 1995; Porter et al., 2003; Moore, 2010; Rigterink et al., 2010; El-Sheikh and Hinnant, 2011), more blunted RSA in response to an experimental stressor (Oosterman et al., 2010; McLaughlin et al., 2015), or both (Conradt et al., 2014; but see Gottman and Katz, 1989; Katz, 2007; Skowron et al., 2014 for contradictory findings).
In turn, in separate literatures, exposure to adverse life events and altered RSA each has been linked to depression risk (Hammen, 2005; Williamson et al., 2005; Stroud et al., 2008; Kemp et al., 2010; Heim and Binder, 2012; Hamilton and Alloy, 2016; Infurna et al., 2016). The association between stressful, adverse life events and depressive disorders and symptoms has been shown across the life span (e.g., Chapman et al., 2004; Hankin, 2015), both in prospective and retrospective studies (Tram and Cole, 2000; Ge et al., 2001; Pine et al., 2002; Southall and Roberts, 2002; Franko et al., 2004). Notably, the reported associations are robust regardless of how stressful events are defined (e.g., specific traumatic events, total negative event counts, weighted stressful event counts). For example, isolated adverse childhood experiences such as separation from or loss of a parent, as well as cumulative stressful adverse events, predict elevated depression symptoms and increased risk of a depressive disorder (e.g., Agid et al., 1999; Pine et al., 2002). Using total event counts, we found that clinically referred children with depressive disorders experienced about twice as many lifetime stressful events than did a school-based control group, and had accumulated those events by a younger age than did the controls (Mayer et al., 2009).
The relations of altered RSA and depression have been examined in samples that varied in age and type of depression-proneness (Rottenberg, 2007; Kemp et al., 2010; Hamilton and Alloy, 2016). For example, among adults, atypical RSA reactivity (compared to controls) is most consistently found when subjects are currently depressed (Hamilton and Alloy, 2016). Among children, atypical development of resting RSA has been reported for those at high familial risk for depression (Gentzler et al., 2012). Additionally, combinations of high resting RSA and robust RSA reactivity to negative mood induction were found to predict reduced depressive symptoms among depression-prone adults and their children (Yaroslavsky et al., 2013, 2014; Yaroslavsky et al., 2016), and indirectly contributed to lower risk of recurrent depression among adolescents (Kovacs et al., 2016).
While the contribution of early adversities to physiological dysfunction, and the role of stressful or adverse events in depression risk, have been documented in mostly separate literatures (for a review see Anacker et al., 2014), an emerging body of work has sought to link these areas. For example, specific trauma, namely sexual or physical abuse among depressed adults and youths has been associated with greater HPA activation in response to experimental stress (Kaufman et al., 1997; Heim et al., 2000). Further, among depressed women, those with severe trauma history evidenced blunted RSA reactivity to a stress-inducing task, relative to depressed women without severe trauma history (Cyranowski et al., 2011). Since these studies focused on extreme trauma, it is unclear if cumulative exposure to a variety of adverse life events has similar consequences on physiological response systems.
The present study seeks to extend the literature by integrating information on early adversities and physiological dysfunction measured years later, in the context of high depression risk. Specifically, we explored for the first time if reduced RSA flexibility among adolescents, who had histories of childhood-onset major depressive disorder, is related to developmentally early adversities. We focused on reduced physiological flexibility because context-appropriate RSA modulation is thought to reflect a functionally adaptive response to environmental demands (Porges, 1995; Porges et al., 1996). Early adversities were quantified as event counts, based on parental reports at initial (time 1 or T1) assessment. Several years later (time 2 or T2), we assessed RSA flexibility during the processing of sad affect and focused on individual differences in the context of a repeated-measures design. We hypothesized a dose-response relationship between greater exposure to childhood adversities and less flexible RSA functioning while adolescents were processing dysphoric affect.
Although the literature on the detrimental effects of adversities on later physiological functioning is most compelling for exposure during the early phases of development, we also examined the potential contribution of temporally closer T2 (more recent) adversities. In order to eliminate the confounding effects of current depression on physiological functioning (Salomon et al., 2013), we tested our hypotheses with adolescents who were remitted from their last episode of major depression.
2. Methods and Materials
2.1 Participants
We report on adolescent probands with histories of childhood onset major depressive episodes (MDE) who were assessed at two time points, approximately 7.01 years (SD = 1.15) apart, and whose depressions were in remission at the time of the physiological assessment. The Time 1 (T1) assessments were part of a genetic investigation in a national sample of clinically referred children in Hungary, recruited through 23 child mental health clinical sites. At T1, probands were 7- to 14-years old and met DSM-IV (American Psychiatric Association [APA] 1994) criteria for a depressive disorder, as determined via a stringent series of research diagnostic interviews (for details, see Tamás et al., 2007; Kovacs et al., 2015).
The subsequent study of emotion regulation (Time 2 [T2] assessments) enrolled only a portion of the prior, original sample due to funding constraints. Because the T2 study included a laboratory-based physiological protocol, living within commuting distance of the research laboratories was the primary enrollment criterion. Subjects were aged 14–18 years at enrollment in the T2 study of emotion regulation.
The present sample includes 189 probands (96.3% Caucasian). Average age at T1 was 10.17 years (SD =1.42); there were 122 (64.6%) boys. Age at onset of first MDD episode was 8.97 years (SD = 1.71); the average number of major depressive episodes was 1.5 (SD = 0.73). At T1, average socioeconomic status (SES), using the Hollingshead (2011) index was M = 32.93, SD = 13.18, which corresponds to middle class. At T2, subjects’ average age was 17.18 years (SD = 1.28). By T2, 59.8% of the youths still had only one MDD episode, while 30.2% had 2 episodes, and 10.1% had 3 or more episodes. At T2, only 3 probands were taking psychotropic medication, consistent with the sample’s remitted status. The temporal windows for the data collection in this article included January 2001 to February 2006 for T1, and April 2010 to May 2013 for T2.
2.2 Procedures
At T1, all participants received a psychosocial assessment that included the following: a) 2 separate standardized semi-structured clinical diagnostic interviews, at least one month apart, by different trained interviewers with parents about the subjects and with the subjects about themselves, which used DSM-IV diagnostic criteria (APA, 1994), b) review of the diagnoses by two trained senior child psychiatrists who then had to achieve “best estimate” diagnoses (Maziade et al., 1992), c) ascertainment of psychosocial information from parents, including offspring’s’ history of stressful life events, medication use, and cigarette smoking history, d) completion of self-rated questionnaires, including the Paffenbarger Physical Activity Questionnaire (PPAQ; Paffenbarger et al., 1978), and e) determination of participants’ weight and height. The procedures have been described in detail elsewhere (e.g., Kiss et al., 2007; Tamás et al., 2007). At T2, participants completed again a full psychosocial evaluation and an experimental protocol with psychophysiological recording (also noted in Kovacs et al., 2015, and below).
2.3 Quantification of Adverse, Stressful Life Events
Exposure to major adverse life events was determined twice during fully structured interviews with the parent as to whether or not the offspring had been exposed to the specified events. At T1, the parent reported on life-time events to which the offspring was exposed up to T1. At T2, the parent report on events experienced by the offspring only during the period between T1 and T2. Time elapsed between T1 and T2 was 6.29 years (SD = 1.14), on average. The interview queried about 31 adverse life events, which represent five developmentally meaningful event clusters: a) Parental health: hospitalization, physical illness, or psychiatric illness of biological or stepparents; b) Death of close relatives: parental, or other death in the family; c) Sociodemographic events: financial problem, moving, parental unemployment, natural disaster, loss of home; d) Intrafamilial events: birth, hospitalization, psychiatric illness of sibling, foster care, family arguments, and divorce of biological parents; and e) Physical or sexual abuse. The final score was the arithmetic sum of the events that were endorsed.
We have previously reported that our event list discriminated normal school-based controls and youths with major depressive disorder (Mayer et al., 2009). Furthermore, the rate of negative life events reported for our Hungarian school-based controls (Mayer et al., 2009) was comparable to that reported for normative USA samples in studies with similar lists of life events (Ge et al., 1994; Franko et al., 2004).
2.4 Measurement of RSA
At T2, RSA was measured as part of a multicomponent physiological protocol via an electrocardiogram (ECG), using Mindware BioLab software. Participants were asked to sit as still as possible during the experimental protocol. A research assistant tracked compliance and no collection errors were noted.
The ECG signals were acquired according to published guidelines (Berntson et al., 1997); the electrodes were placed on the participant’s left and right rib cage at heart level. Heart values were sampled online at 1000Hz using the Mindware Bionex system (MindWare Technologies, Ltd., Gahanna, OH). Subject’s respiration was measured only during paced breathing and reactivity periods. Respiration was collected by placing a band around the abdomen, calibrated against a fixed volume bag. Respiration rate was calculated breath by breath by the Mindware HRV software (MindWare Technologies, Ltd., Gahanna, OH).
RSA was calculated using MindWare HRV 3.0.21 software (MindWare Technologies, Ltd., Gahanna, OH). R-wave markers in the ECG signal were processed with the MAD/MED artifact detection algorithm; signals were visually inspected and suspected artifacts were manually corrected (Berntson et al., 1997). The interbeat interval (IBI) series was resampled in equal 250 ms intervals, linearly detrended, and tapered using a Hanning window. Heart rate variability (HRV) was calculated using Fast Fourier transformation analysis of the IBI series and RSA was defined as the log transformed high frequency (HF) power band of HRV (0.15–0.40 Hz range; see Berntson et al., 1997). Hereafter we refer to HF-HRV as RSA.
The protocol included a paced breathing period, which provided the baseline-resting RSA value. For a 180- second period, subjects listened to a soft tone and were instructed to breathe in when the tone was rising, breathe out when the tone was falling, and pause between breaths when there was no tone. The tone pattern was set to induce a respiratory frequency of 12 cycles per minute with a normal fractional inspiratory ratio of 40%.
RSA reactivity was assessed during negative mood induction (watching a sad film clip or trying to solve an unsolvable puzzle). RSA recovery was assessed during an attention refocusing task designed to help subjects recover from negative mood (described in Kovacs et al., 2015). RSA was calculated for each epoch separately. Mean heart rate during the paced breathing baseline was 72.61 (SD = 10.54), it was 68.31 (SD = 9.08) during the sad film, 73.69 (SD = 9.71) during the unsolvable puzzle, and 72.61 (SD = 9.14) during mood repair task.
2.5 Negative mood induction
Subjects were randomized to either a Sad film or an Unsolvable task mood induction condition. The former was a 164 second clip from the Champ (dubbed in Hungarian), in which a child witnesses the death of his boxer champion father after a match (Rottenberg et al., 2005, 2007). This clip has been extensively used with both pediatric and adult samples (e.g., Gross and Levenson, 1995; Rottenberg et al., 2002), and was also pilot tested with Hungarian youths (see Kovacs et al., 2015). The Unsolvable puzzle was a computerized task wherein subjects had to re-recreate a pattern by moving pieces on a computer screen (horizontally or vertically) containing letters of the alphabet. A solvable practice puzzle was first given (15s), which was followed by two puzzles that were programmed to be unsolvable (mirroring Nolen-Hoeksema et al., 1995; Cole et al., 2007). Subjects had 180s to work on each unsolvable puzzle. Subjects also rated the intensity of feeling ‘sad’ and ‘blue’ on a 0- to 7-point Likert-type scale at baseline and after negative mood induction.
For manipulation checks, we computed mean “sadness” ratings based on the above noted two items and entered them in a repeated measures ANOVA of induction type and time (baseline, mood induction). The results showed a main effect of time, such that sad mood increased from baseline to post- mood induction (MBaseline = 0.42, SDBaseline = 0.92, MMoodinduction = 0.85, SD Moodinduction = 1.18; F(1,187) = 22.44, p < .01, partial η2 = .11). Although both types of mood inductions significantly increased sad mood, the increase in sadness was stronger following the unsolvable puzzle than following the sad film (F(1,187) = 8.62, p < .01, partial η2 = .04). Therefore, type of mood induction was entered as a covariate in all relevant analyses.
2.6 Mood repair task
Focusing attention away from one’s dysphoria is regarded as a key emotion regulatory strategy that can attenuate distress (Erber and Tesser, 1992). The effectiveness of attention refocusing for mood repair, which can be implemented through various means, has been documented across the age span in observational and experimental studies (e.g., Rippere, 1977; Thayer et al., 1994; Eisenberg et al., 2000; Posner and Rothbart, 2000; Joormann et al., 2007; Kovacs et al., 2015). In the present study, attention refocusing was implemented via an “illusion kaleidoscope” task, which was adopted from a study of pediatric pain (Carlson et al., 2000). Subjects were handed a standard kaleidoscope (15 cm in length; 4 cm in diameter) and were asked to keep looking through it for 120s. During the task, the experimenter posed standardized questions such as “What shapes do you see? What are the different colors?” to reinforce task engagement.
2.7 Statistical Analyses
RSA scores, sum of early negative life events, interim negative life events, and all related variables were transformed to z-scores. To test our primary hypothesis, we conducted a repeated measures ANCOVA (rANCOVA) on RSA using epoch (resting, reactive, recovery phases) as a within-subject factor and early adverse life event (T1) score as the predictor (independent) variable, with a relevant set of additional variables treated as covariates to control for their effects. In order to examine whether the chronologically later T1 to T2 interim life events contributed to the prediction of RSA, the rANCOVA was repeated with the addition of interim adverse event counts as a further predictor variable. Significant interactions were probed by a set of linear regression models, adjusting for relevant confounding variables.
3. Results
3.1 Characteristics of the Sample and Measures
Table 1 presents the correlations among key variables. Although extent of early adversities (M = 5.90, SD = 2.99) correlated with extent of T1-T2 adversities (M = 3.85, SD = 2.53), the scores in early childhood were higher (t = 9.04, p < .001). The higher adverse event scores at T1 (compared to T1-T2 scores) may partly reflect that the T1 assessment covered a longer period of children’s lives (M = 10.17 years; SD = 1.42) than did the T1-T2 interim assessment (M = 7.01 years, SD = 1.15). As also shown in Table 1, early childhood adversity positively correlated with age, while interim adversity was higher for males than for females (Mmales = 4.38, SDmales = 2.59, Mfemales = 2.91, SDfemales = 2.09, t = 4.01, p < .001).
Table 1.
Correlations among demographic characteristics and key study variables (N = 189).
| Sex | Age | Early events | Interim events | Smoking | BMI | Physical activity | RSA PB | RSA MI | RSA MR | Onset age of MDD | MDD episodes | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | −.03 | −.09 | −.28** | −.12 | −.03 | −.24** | .17* | .13 | .12 | .03 | .08 | |
| Age | .16* | .08 | .36** | .05 | −.19** | −.01 | −.03 | −.02 | .49** | .10 | ||
| Early events | .37** | .17* | −.10 | −.11 | −.09 | .03 | −.03 | .06 | .11 | |||
| Interim events | .08 | .07 | .07 | −.04 | −.02 | −.03 | −.01 | −.03 | ||||
| Smoking | −.12 | .08 | .07 | .15* | .10 | .32** | −.17* | |||||
| BMI | −.03 | .14 | .10 | .05 | −.13 | −.02 | ||||||
| Physical activity | −.03 | .01 | .03 | −.15* | −.03 | |||||||
| RSA PB | .70** | .70** | .01 | −.05 | ||||||||
| RSA MI | .80** | −.01 | .02 | |||||||||
| RSA MR | −.01 | −.01 | ||||||||||
| Onset age of MDD | −.30** | |||||||||||
| MDD episodes |
Sex = high value represents females, Age = age at T2, Early events = number of childhood adversities, interim events = number of adversities over the T1 to T2 follow up period, smoking = high value represent smokers, BMI = Body Mass Index, Physical activity = Paffenbarger Physical Activity score, RSA = Respiratory Sinus Arrhythmia, PB = paced breathing, MI = mood induction, MR = mood repair, Onset age of MDD = age at onset of first MDD episode, MDD episodes = number of episodes.
p < .05,
p < .01
Body mass index (BMI), computed from height and weight (age and sex adjusted), along with cigarette smoking, physical activity index, and respiration rate at mood induction are reported in Table 1 because they are potential confounds of RSA. However, as BMI, physical activity level, and smoking were not significantly correlated with baseline RSA, they were not considered in subsequent statistical models. Although only three probands were on psychotropic medication, which can affect RSA, we nevertheless included this variable in all models (excluding these participants from analysis did not change the significance of the results). While age at onset of first MDD episode did not correlate with RSA (Table 1), it may account for a possible link between adversities and RSA flexibility, thus this variable was also taken into account in our analyses. The influence of respiration rate (M = .26, SD = .03) was entered in the analyses as well, but it did not alter the pattern of results.
3.2 Early Adversities and RSA Flexibility
To test our hypothesis regarding a dose-response relationship between adversity in childhood and less flexible RSA functioning in adolescence, we conducted a rANCOVA of childhood adversity scores on RSA (with sex, T2 age, time elapsed between T1 and T2, medication use, onset age of MDD and type of mood induction as covariates). There were no main effects of early adversities (F(1,181) = .23, p = .63, partial η2 < .01), or experimental epoch (F(2,180) = 0.84, p = .44, partial η2 < .01; Mresting = 7.24, SDresting = 1.15, Mreactivity = 6.60, SDreactivity = 1.03, Mrecovery = 6.81, SDrecovery = .97). Importantly, there was an interaction between early adversity and experimental epoch (F(2,180) = 3.66, p = .03, partial η2 = .04).
To unpack this interaction, we computed two RSA change scores: a) the difference between resting RSA and RSA reactivity values and b) the difference between RSA reactivity and RSA recovery values. The change scores were computed by subtracting the RSA value of the subsequent epoch (reactivity or recovery) from the RSA value of the prior epoch (baseline or reactivity): thus, positive RSA change scores represent RSA Augmentation (higher RSA) and negative values represent RSA Withdrawal (lower RSA). A linear regression predicting RSA change score for resting vs. reactivity (controlling for sex, age at T2, time elapsed between T1 and T2, medication use and type of mood induction) indicated a negative association with early adversities (B = −.05, t = −2.55, p = .01; ΔR2 = .034). In other words, early adversities predicted blunted RSA withdrawal during mood induction.
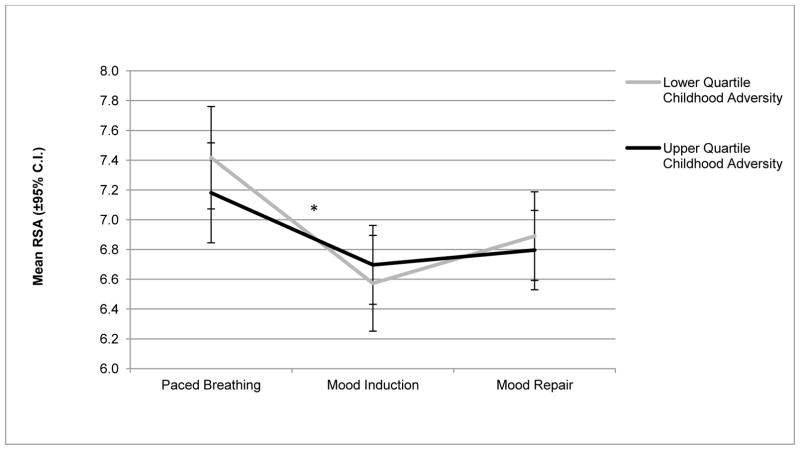
A second linear regression predicting RSA change score for reactivity vs. recovery (controlling for the above noted covariates) indicated a marginal positive association with early adversities (B = .03, t = 1.83, p = .07; ΔR2 = .017). In other words, early adversities predicted a trend toward less RSA augmentation during mood repair. As illustrated in Figure 1, youths who had been exposed to higher rates of early adversities displayed less RSA flexibility during different phases of sadness processing than youths exposed to lower levels of early life adversities.
Figure 1.
RSA values (with 95% confidence intervals) across three assessment points in the experimental protocol (paced breathing baseline; sad mood induction; instructed mood repair) among adolescent probands in the upper and lower quartiles of exposure to childhood adversity.
*p < 0.05, for RSA change score from mood induction to mood repair (B = −0.05, t = −2.55, p = .01; ΔR2 = 0.034).
3.3 Later Adversities and RSA Flexibility
Do negative life events occurring later in development also have adverse effects on autonomic functioning? To examine this question, we repeated the above noted rANCOVA with the addition of the T1 to T2 interim life event scores. There was no main effect for recent/interim life events (F(1,180) = 0.04, p = .85, partial η2 < .01), nor was there a significant epoch by recent stressful life events interaction (F(2, 179) = .45, p = .64, partial η2 < .01). However, the T1 early adversities score by epoch interaction remained significant (F(2,179) = 3.98, p = .02, partial η2 = .04). Thus, the relationship between adverse life events and RSA functioning during the processing of dysphoric affect was explained by earlier adversities experienced during childhood rather than by adversities the youths experienced during their adolescence.
3. Discussion
Using a prospective design, we examined whether childhood adversities have harmful effects on autonomic nervous system functioning later in life during the processing of sad affect among adolescents who had histories of childhood-onset major depressive disorder. We focused on physiological functioning during the processing of dysphoric affect because of its importance in the context of depression risk.
Our subjects constitute a sample at high-risk for future depression because all of them had past episodes of major depression, from which they were in remission. However, because we did not have a never-depressed control group, we cannot tease out the extent to which depression may have moderated the effects of early adversities on later physiological functioning. Our central finding is that depression-prone adolescents with more extensive exposure to childhood adversities exhibited less flexible RSA functioning during the experience and regulation of dysphoric affect than did their peers with lower levels of early stress exposure. Reduced RSA flexibility was manifest as less extensive vagal withdrawal in response to negative mood induction and as a trend toward less extensive vagal augmentation following mood repair. Withdrawal of vagal influences on the heart during stress or external provocation and a rapid return of vagal influences post-stress are considered to be important substrates of flexible and adaptive physiological and behavioral functioning (Porges, 1995, 1997).
The present results add to a large body of literature on the association between early adversities or negative life events and enduring dysfunctional physiological changes in humans (e.g., Pesonen et al., 2010; Rao et al., 2010). Specifically, we found that an additive accumulation of adverse negative life events was associated with physiological impairment. While similar associations have been documented when early-life adversities were defined by exposure to a discrete stressor (Field et al., 1995; Pickens and Field, 1995; Porter et al., 2003; Moore, 2010; Oosterman et al., 2010; Rigterink et al., 2010; El-Sheikh and Hinnant, 2011; Conradt et al., 2014; McLaughlin et al., 2015), our findings suggest that such associations are not event specific but represent a generalized pattern. In other words, early adversities appear to contribute to impaired physiological self-regulation in a dose-response fashion.
Results of our study also extend the literature on RSA and stress. Although we cannot argue for a causal association between early adversity and atypical RSA in response to affective challenge, our study is the first to examine adverse life events that temporally predated RSA assessment. Further, while previous studies have focused on RSA at rest and in response to sad mood induction (e.g., Katz and Gottman, 1997; El-Sheikh et al., 2001; El-Sheikh and Whitson, 2006; McLaughlin et al., 2015), we examined RSA while youths were in the process of repairing their negative mood. Exploring RSA under differing conditions (rest, mood induction, mood repair) provides a more ecologically valid picture of RSA functioning over time (Yaroslavsky et al., 2013). Further, while adversity early in life has been associated with blunted RSA reactivity to experimental stress (Oosterman et al., 2010; Cyranowski, et al., 2011; McLaughlin et al., 2015), the present results suggest that early stress may play a role in the lack of context-appropriate RSA flexibility.
Exposure to adversities during the first decade of life (but not during adolescence) was associated with atypical RSA functioning, underscoring that the consequences of stress appear to vary as a function of the developmental timing of the exposure. There are windows of vulnerability when brain regions and more downstream response systems might be maximally sensitive to environmental influences, the effects of which persist over time (Andersen and Teicher, 2008). Our finding that exposure to more recent negative life events did not contribute to dysfunctional RSA (beyond the prediction provided by early adversities) is in line with reports on the effects of stressors on various other physiological systems, such as cortisol secretion (Pesonen et al., 2010; Bosch et al., 2012) and regional brain volume (Andersen and Teicher, 2008).
Finally, the finding of less flexible RSA functioning during the laboratory experience and regulation of dysphoric affect, as a function of early adversity, also adds to the literature on mood repair. Maladaptive mood repair responding (by self-report) has been associated with atypical, less flexible RSA (Williams et al., 2015; Kovacs et al., 2016). Overall, it appears that physiological regulatory flexibility in the context of affective challenge may be one contributor to individual differences in competent mood repair performance, which, in turn, may be compromised by exposure to adversities during early development.
In spite of its strengths, the present study also had several limitations. Most critically, as already noted, because we lack a control group with parallel T1 and T2 data, we cannot address whether the findings are specific to youths with depression histories. The absence of an experimental manipulation of stress makes it difficult to argue for a causal association between childhood adversities and laboratory-based physiological functioning. Further, our index of life events did not take into account the length of exposure to a given stressor, or multiple exposures to the same stressor across time. Despite these limitations, the association between developmentally early adversities and later physiological dysfunction in the present sample may help to better understand the mood repair difficulties and unfavorable long-term clinical prognosis of youths with pediatric-onset depression (Kovacs et al., 2016).
Highlights.
We explored the link between adversities and PNS functioning in depression prone youths.
Youths with more adversities exhibited less RSA flexibility 7 years later.
Less RSA flexibility was exhibited during experience of negative affect and recovery.
Recent adversities did not contribute to the prediction of RSA flexibility.
Acknowledgments
This study was supported by NIH Grants MH056193, MH084938, and Hungarian Scientific Research Fund Grant NN85285.
Footnotes
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Financial Disclosures
The authors declare that there is no potential or actual conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agid O, Shapira B, Zislin J, Ritsner M, Hanin B, Murad H, … Lerer B. Environment and vulnerability to major psychiatric illness: A case control study of early parental loss in major depression, bipolar disorder and schizophrenia. Mol Psychiatry. 1999;4(2):163–172. doi: 10.1038/sj.mp.4000473. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Anacker C, O’Donnell KJ, Meaney MJ. Early life adversity and the epigenetic programming of hypothalamic-pituitary-adrenal function. Dialogues in Clin Neurosci. 2014;16(3):321–333. doi: 10.31887/DCNS.2014.16.3/canacker. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends in Neurosci. 2008;31(4):183–191. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT, Eckberg DL, Grossman P, Kaufmann PG, Malik M, … Van Der Molen MW. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiol. 1997;34(6):623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Bosch NM, Riese H, Reijneveld SA, Bakker MP, Verhulst FC, Ormel J, Oldehinkel AJ. Timing matters: Long term effects of adversities from prenatal period up to adolescence on adolescents’ cortisol stress response. The TRAILS study Psychoneuroendocr. 2012;37(9):1439–1447. doi: 10.1016/j.psyneuen.2012.01.013. [DOI] [PubMed] [Google Scholar]
- Bush NR, Obradović J, Adler N, Boyce WT. Kindergarten stressors and cumulative adrenocortical activation: The “first straws” of allostatic load? Dev and Psychopathol. 2011;23(04):1089–1106. doi: 10.1017/S0954579411000514. [DOI] [PubMed] [Google Scholar]
- Carlson KL, Broome M, Vessey JA. Using distraction to reduce reported pain, fear, and behavioral distress in children and adolescents: A multisite study. J for Specialists in Pediatr Nurs. 2000;5(2):75–85. doi: 10.1111/j.1744-6155.2000.tb00089.x. [DOI] [PubMed] [Google Scholar]
- Chapman DP, Whitfield CL, Felitti VJ, Dube SR, Edwards VJ, Anda RF. Adverse childhood experiences and the risk of depressive disorders in adulthood. J of Affect Disord. 2004;82(2):217–225. doi: 10.1016/j.jad.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Chen E, Cohen S, Miller GE. How low socioeconomic status affects 2-year hormonal trajectories in children. Psychol Sci. 2010;21(1):31–37. doi: 10.1177/0956797609355566. [DOI] [PubMed] [Google Scholar]
- Cole DA, Warren DE, Dallaire DH, Lagrange B, Travis R, Ciesla JA. Early predictors of helpless thoughts and behaviors in children: Developmental precursors to depressive cognitions. Clin Child Psychol and Psychiatry. 2007;12(2):295–312. doi: 10.1177/1359104507075936. [DOI] [PubMed] [Google Scholar]
- Conradt E, Abar B, Sheinkopf S, Lester B, Lagasse L, Seifer R, … Hinckley M. The role of prenatal substance exposure and early adversity on parasympathetic functioning from 3 to 6 years of age. Dev Psychobiol. 2014;56(4):821–835. doi: 10.1002/dev.21155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyranowski JM, Hofkens TL, Swartz HA, Salomon K, Gianaros PJ. Cardiac vagal control in non-medicated depressed women and non-depressed controls: Impact of depression status, lifetime trauma history and respiratory factors. Psychosom Med. 2011;73(4):336–343. doi: 10.1097/PSY.0b013e318213925d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N, Fabes RA, Guthrie IK, Reiser M. Dispositional emotionality and regulation: Their role in predicting quality of social functioning. J of Personal and Soc Psychol. 2000;78(1):136–157. doi: 10.1037//0022-3514.78.1.136. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Harger J, Whitson SM. Exposure to interparental conflict and children’s adjustment and physical health: The moderating role of vagal tone. Child Dev. 2001:1617–1636. doi: 10.1111/1467-8624.00369. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Hinnant JB. Marital conflict, respiratory sinus arrhythmia, and allostatic load: Interrelations and associations with the development of children’s externalizing behavior. Dev and Psychopathol. 2011;23(03):815–829. doi: 10.1017/S0954579411000320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sheikh M, Whitson SA. Longitudinal relations between marital conflict and child adjustment: Vagal regulation as a protective factor. J of Fam Psychol. 2006;20(1):30–39. doi: 10.1037/0893-3200.20.1.30. [DOI] [PubMed] [Google Scholar]
- Erber R, Tesser A. Task effort and the regulation of mood: The absorption hypothesis. J of Exp Soc Psychol. 1992;28(4):339–359. [Google Scholar]
- Evans GW, Li D, Whipple SS. Cumulative risk and child development. Psychol Bulletin. 2013;139(6):1342. doi: 10.1037/a0031808. [DOI] [PubMed] [Google Scholar]
- Field T, Pickens J, Fox NA, Nawrocki T, Gonzalez J. Vagal tone in infants of depressed mothers. Dev and Psychopathol. 1995;7(02):227–231. [Google Scholar]
- Franko DL, Striegel-Moore RH, Brown KM, Barton BA, McMahon RP, Schreiber GB, … Daniels SR. Expanding our understanding of the relationship between negative life events and depressive symptoms in black and white adolescent girls. Psychol Med. 2004;34(07):1319–1330. doi: 10.1017/s0033291704003186. [DOI] [PubMed] [Google Scholar]
- Ge X, Conger RD, Elder GH., Jr Pubertal transition, stressful life events, and the emergence of gender differences in adolescent depressive symptoms. Dev Psychol. 2001;37(3):404–417. doi: 10.1037//0012-1649.37.3.404. [DOI] [PubMed] [Google Scholar]
- Ge X, Lorenz FO, Conger RD, Elder GH, Simons RL. Trajectories of stressful life events and depressive symptoms during adolescence. Dev Psychol. 1994;30(4):467–483. [Google Scholar]
- Gentzler AL, Rottenberg J, Kovacs M, George CJ, Morey JN. Atypical development of resting respiratory sinus arrhythmia in children at high risk for depression. Dev Psychobiol. 2012;54(5):556–567. doi: 10.1002/dev.20614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottman JM, Katz LF. Effects of marital discord on young children’s peer interaction and health. Dev Psychol. 1989;25(3):373–381. [Google Scholar]
- Gross JJ, Levenson RW. Emotion elicitation using films. Cogn and Emot. 1995;9(1):87–108. [Google Scholar]
- Hamilton JL, Alloy LB. Atypical reactivity of heart rate variability to stress and depression across development: Systematic review of the literature and directions for future research. Clin Psychol Rev. 2016;50:67–79. doi: 10.1016/j.cpr.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C. Stress and depression. Annu Rev of Clin Psychol. 2005;1:293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. [DOI] [PubMed] [Google Scholar]
- Hankin BL. Depression from childhood through adolescence: Risk mechanisms across multiple systems and levels of analysis. Curr Opin in Psychol. 2015;4:13–20. doi: 10.1016/j.copsyc.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Nacewicz BM, Sutterer MJ, Cayo AA, Schaefer SM, Rudolph KD, … Davidson RJ. Behavioral problems after early life stress: Contributions of the hippocampus and amygdala. Biol Psychiatry. 2015;77(4):314–323. doi: 10.1016/j.biopsych.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Binder EB. Current research trends in early life stress and depression: Review of human studies on sensitive periods, gene–environment interactions, and epigenetics. Exp neurol. 2012;233(1):102–111. doi: 10.1016/j.expneurol.2011.10.032. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, … Nemeroff CB. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. Jama. 2000;284(5):592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. Yale J of Sociol. 2011;8:21–52. [Google Scholar]
- Infurna MR, Reichl C, Parzer P, Schimmenti A, Bifulco A, Kaess M. Associations between depression and specific childhood experiences of abuse and neglect: A meta-analysis. J of Affect Disord. 2016;190:47–55. doi: 10.1016/j.jad.2015.09.006. [DOI] [PubMed] [Google Scholar]
- Joormann J, Siemer M, Gotlib IH. Mood regulation in depression: Differential effects of distraction and recall of happy memories on sad mood. J of Abnorm Psychol. 2007;116(3):484–490. doi: 10.1037/0021-843X.116.3.484. [DOI] [PubMed] [Google Scholar]
- Kaffman A, Meaney MJ. Neurodevelopmental sequelae of postnatal maternal care in rodents: Clinical and research implications of molecular insights. J of Child Psychol and Psychiatry. 2007;48(3–4):224–244. doi: 10.1111/j.1469-7610.2007.01730.x. [DOI] [PubMed] [Google Scholar]
- Katz LF. Domestic violence and vagal reactivity to peer provocation. Biol Psychol. 2007;74(2):154–164. doi: 10.1016/j.biopsycho.2005.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LF, Gottman JM. Buffering children from marital conflict and dissolution. J of Clin Child Psychol. 1997;26(2):157–171. doi: 10.1207/s15374424jccp2602_4. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Perel J, Dahl RE, Moreci P, Nelson B, … Ryan ND. The corticotropin-releasing hormone challenge in depressed abused, depressed nonabused, and normal control children. Biol Psychiatry. 1997;42(8):669–679. doi: 10.1016/s0006-3223(96)00470-2. [DOI] [PubMed] [Google Scholar]
- Kemp AH, Quintana DS, Gray MA, Felmingham KL, Brown K, Gatt JM. Impact of depression and antidepressant treatment on heart rate variability: A review and meta-analysis. Biol Psychiatry. 2010;67(11):1067–1074. doi: 10.1016/j.biopsych.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Kiss E, Gentzler AM, George C, Kapornai K, Tamás Z, Kovacs M, Vetró Á. Factors influencing mother–child reports of depressive symptoms and agreement among clinically referred depressed youngsters in Hungary. J of Affect Disord. 2007;100(1):143–151. doi: 10.1016/j.jad.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M, George CJ, Vetro A, Kapornai K, Kiss E, Baji I, Rottenberg J, Benak I, Yaroslavsky I, Dochnal R, Halas K, Makai A. Maladaptive mood repair, atypical respiratory sinus arrhythmia, and risk of a recurrent major depressive episode among adolescents with prior major depression. Psychol Med. 2016:1–11. doi: 10.1017/S003329171600057X. [DOI] [PubMed] [Google Scholar]
- Kovacs M, Yaroslavsky I, Rottenberg J, George CJ, Baji I, Benák I, … Kapornai K. Mood repair via attention refocusing or recall of positive autobiographical memories by adolescents with pediatric-onset major depression. J of Child Psychol and Psychiatry. 2015;56(10):1108–1117. doi: 10.1111/jcpp.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10(6):434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Mayer L, Lopez-Duran NL, Kovacs M, George CJ, Baji I, Kapornai K, … Vetró Á. Stressful life events in a clinical sample of depressed children in Hungary. J of Affect Disord. 2009;115(1):207–214. doi: 10.1016/j.jad.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maziade MRMA, Roy MA, Fournier JP, Cliche D, Mérette C, Caron C, … Nicole L. Reliability of best-estimate diagnosis in genetic linkage studies of major psychoses: Results from the Quebec pedigree studies. Am J of Psychiatry. 1992;149:1674–1674. doi: 10.1176/ajp.149.12.1674. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Tibu F, Fox NA, Zeanah CH, Nelson CA. Causal effects of the early caregiving environment on development of stress response systems in children. Proc of the Natl Acad of Sci. 2015;112(18):5637–5642. doi: 10.1073/pnas.1423363112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore GA. Parent conflict predicts infants’ vagal regulation in social interaction. Dev and Psychopathol. 2010;22(01):23–33. doi: 10.1017/S095457940999023X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Wolfson A, Mumme D, Guskin K. Helplessness in children of depressed and nondepressed mothers. Dev Psychol. 1995;31(3):377–387. [Google Scholar]
- Oosterman M, De Schipper JC, Fisher P, Dozier M, Schuengel C. Autonomic reactivity in relation to attachment and early adversity among foster children. Dev and Psychopathol. 2010;22(01):109–118. doi: 10.1017/S0954579409990290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paffenbarger RS, Wing AL, Hyde RT. Physical activity as an index of heart attack risk in college alumni. Am J of epidemiol. 1978;108(3):161–175. doi: 10.1093/oxfordjournals.aje.a112608. [DOI] [PubMed] [Google Scholar]
- Pesonen AK, Räikkönen K, Feldt K, Heinonen K, Osmond C, Phillips DI, … Kajantie E. Childhood separation experience predicts HPA axis hormonal responses in late adulthood: A natural experiment of World War II. Psychoneuroendocr. 2010;35(5):758–767. doi: 10.1016/j.psyneuen.2009.10.017. [DOI] [PubMed] [Google Scholar]
- Pickens JN, Field T. Facial expressions and vagal tone of infants of depressed and non-depressed mothers. Early Dev and Parent. 1995;4(2):83–89. [Google Scholar]
- Pine DS, Cohen P, Johnson JG, Brook JS. Adolescent life events as predictors of adult depression. J of Affect Disord. 2002;68(1):49–57. doi: 10.1016/s0165-0327(00)00331-1. [DOI] [PubMed] [Google Scholar]
- Porges SW. Cardiac vagal tone: A physiological index of stress. Neurosci & Biobehav Rev. 1995;19(2):225–233. doi: 10.1016/0149-7634(94)00066-a. [DOI] [PubMed] [Google Scholar]
- Porges SW. Emotion: An Evolutionary By-Product of the Neural Regulation of the Autonomic Nervous Systema. Ann of the New York Acad of Sci. 1997;807(1):62–77. doi: 10.1111/j.1749-6632.1997.tb51913.x. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biol Psychol. 2007;74(2):116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW, Doussard-Roosevelt JA, Portales AL, Greenspan SI. Infant regulation of the vagal “brake” predicts child behavior problems: A psychobiological model of social behavior. Dev Psychobiol. 1996;29(8):697–712. doi: 10.1002/(SICI)1098-2302(199612)29:8<697::AID-DEV5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Porter CL, Wouden-Miller M, Silva SS, Porter AE. Marital harmony and conflict: Links to infants’ emotional regulation and cardiac vagal tone. Infancy. 2003;4(2):297–307. [Google Scholar]
- Posner MI, Rothbart MK. Developing mechanisms of self-regulation. Dev and Psychopathol. 2000;12(03):427–441. doi: 10.1017/s0954579400003096. [DOI] [PubMed] [Google Scholar]
- Propper CB, Holochwost SJ. The influence of proximal risk on the early development of the autonomic nervous system. Dev Rev. 2013;33(3):151–167. [Google Scholar]
- Rao U, Chen LA, Bidesi AS, Shad MU, Thomas MA, Hammen CL. Hippocampal changes associated with early-life adversity and vulnerability to depression. Biol Psychiatry. 2010;67(4):357–364. doi: 10.1016/j.biopsych.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigterink T, Katz LF, Hessler DM. Domestic violence and longitudinal associations with children’s physiological regulation abilities. J of Interpers Violence. 2010;25(9):1669–1683. doi: 10.1177/0886260509354589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippere V. ‘What’s the thing to do when you’re feeling depressed? ’—A pilot study. Behav Res and Ther. 1977;15(2):185–191. doi: 10.1016/0005-7967(77)90104-8. [DOI] [PubMed] [Google Scholar]
- Rottenberg J. Cardiac vagal control in depression: A critical analysis. Biol Psychol. 2007;74(2):200–211. doi: 10.1016/j.biopsycho.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Clift A, Bolden S, Salomon K. RSA fluctuation in major depressive disorder. Psychophysiol. 2007;44(3):450–458. doi: 10.1111/j.1469-8986.2007.00509.x. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Kasch KL, Gross JJ, Gotlib IH. Sadness and amusement reactivity differentially predict concurrent and prospective functioning in major depressive disorder. Emot. 2002;2(2):135–146. doi: 10.1037/1528-3542.2.2.135. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Salomon K, Gross JJ, Gotlib IH. Vagal withdrawal to a sad film predicts subsequent recovery from depression. Psychophysiol. 2005;42(3):277–281. doi: 10.1111/j.1469-8986.2005.00289.x. [DOI] [PubMed] [Google Scholar]
- Salomon K, Bylsma LM, White KE, Panaite V, Rottenberg J. Is blunted cardiovascular reactivity in depression mood-state dependent? A comparison of major depressive disorder remitted depression and healthy controls. Int J of Psychophysiol. 2013;90(1):50–57. doi: 10.1016/j.ijpsycho.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skowron EA, Cipriano-Essel E, Gatzke-Kopp LM, Teti DM, Ammerman RT. Early adversity, RSA, and inhibitory control: Evidence of children’s neurobiological sensitivity to social context. Dev Psychobiol. 2014;56(5):964–978. doi: 10.1002/dev.21175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southall D, Roberts JE. Attributional style and self-esteem in vulnerability to adolescent depressive symptoms following life stress: A 14-week prospective study. Cogn Ther and Res. 2002;26(5):563–579. [Google Scholar]
- Stroud CB, Davila J, Moyer A. The relationship between stress and depression in first onsets versus recurrences: A meta-analytic review. J of Abnorm Psychol. 2008;117(1):206–213. doi: 10.1037/0021-843X.117.1.206. [DOI] [PubMed] [Google Scholar]
- Tamás Z, Kovacs M, Gentzler AL, Tepper P, Gádoros J, Kiss E, … Vetró Á. The relations of temperament and emotion self-regulation with suicidal behaviors in a clinical sample of depressed children in Hungary. J of Abnorm Child Psychol. 2007;35(4):640–652. doi: 10.1007/s10802-007-9119-2. [DOI] [PubMed] [Google Scholar]
- Thayer RE, Newman JR, McClain TM. Self-regulation of mood: Strategies for changing a bad mood, raising energy, and reducing tension. J of Personal and Soc Psychol. 1994;67(5):910–925. doi: 10.1037//0022-3514.67.5.910. [DOI] [PubMed] [Google Scholar]
- Tram JM, Cole DA. Self-perceived competence and the relation between life events and depressive symptoms in adolescence: Mediator or moderator? J of Abnorm Psychol. 2000;109(4):753–760. doi: 10.1037//0021-843x.109.4.753. [DOI] [PubMed] [Google Scholar]
- Williams DP, Cash C, Rankin C, Bernardi A, Koenig J, Thayer JF. Resting heart rate variability predicts self-reported difficulties in emotion regulation: A focus on different facets of emotion regulation. Front in Psychol. 2015;6:1–8. doi: 10.3389/fpsyg.2015.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson DE, Birmaher B, Dahl RE, Ryan ND. Stressful life events in anxious and depressed children. J of Child & Adolesc Psychopharmacol. 2005;15(4):571–580. doi: 10.1089/cap.2005.15.571. [DOI] [PubMed] [Google Scholar]
- Yaroslavsky I, Rottenberg J, Kovacs M. The utility of combining RSA indices in depression prediction. J of Abnorm Psychol. 2013;122(2):314. doi: 10.1037/a0032385. [DOI] [PubMed] [Google Scholar]
- Yaroslavsky I, Rottenberg J, Kovacs M. Atypical patterns of respiratory sinus arrhythmia index an endophenotype for depression. Dev and Psychopathol. 2014;26(4pt2):1337–1352. doi: 10.1017/S0954579414001060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaroslavsky I, Rottenberg J, Bylsma LM, Jennings JR, George C, Baji I, … Kiss E. Parasympathetic nervous system activity predicts mood repair use and its effectiveness among adolescents with and without histories of major depression. J of Abnorm Psychol. 2016;125(3):323–336. doi: 10.1037/abn0000149. [DOI] [PMC free article] [PubMed] [Google Scholar]