Abstract
Introduction
Intermittent hypoxic–hyperoxic training (IHHT) may complement a multimodal training intervention (MTI) for improving cognitive function and exercise tolerance in geriatric patients.
Methods
Thirty-four patients (64–92 years) participated in this randomized controlled trial. Before and after the 5- to 7-week intervention period (MTI + IHHT vs. MTI + ambient air), cognitive function was assessed by the Dementia-Detection Test (DemTect) and the Sunderland Clock-Drawing Test (CDT), and functional exercise capacity by the total distance of the 6-Minute Walk Test (6MWT).
Results
DemTect and CDT indicated significantly larger improvements after MTI + IHHT (+16.7% vs. −0.39%, P < .001) and (+10.7% vs. −8%, P = .031) which was also true for the 6MWT (+24.1% vs. +10.8%, P = .021).
Discussion
IHHT turned out to be easily applicable to and well tolerated by geriatric patients up to 92 years. IHHT contributed significantly to improvements in cognitive function and functional exercise capacity in geriatric patients performing MTI.
Keywords: Intermittent hypoxia, Geriatric patients, Cognitive performance, Dementia, Exercise tolerance, Multimodal training
1. Introduction
As a consequence of the steadily growing life expectancy, the number of people suffering from cognitive dysfunction is increasing steeply. Not surprisingly, more and more anti-dementia drugs, such as memantine and cholinesterase inhibitors, have been developed. However, beneficial effects seem to be rather small in relation to the potential adverse effects of pharmacological therapy [1], [2], [3].
In contrast, several clinical trials report on the positive consequences of short-term interventions with physical activity for cognitive function in old people [4], [5], [6]. The meta-analyses performed by Colcombe and Kramer demonstrated that a multimodal training intervention (MTI), which includes cardiovascular fitness training combined with strength training, showed larger improvements than aerobic training alone [7].
Nevertheless, there is still a great demand for new strategies for the prevention and treatment of dementia. Recently, it would seem that intermittent hypoxic training (IHT) may represent such a strategy. IHT represents a noninvasive, easily applicable method based on repeated resting exposures to an oxygen-deficient gas mixture interspersed by normoxic periods [8]. In contrast to moderate hypoxia, exposure to severe hypoxia, that is, 5% O2, was found to have deleterious consequences in Wistar rats [9]. Also, obstructive sleep apnea with brief and frequently recurrent cycles of hypoxia is well known to be associated with systemic hypertension, stroke, and adverse cardiac events [10]. In addition, chronic hypoxia was shown to have hazardous effects on organ structure and function, may provoke cerebral and myocardial ischemia, and plays a crucial role in regulating tumor growth and metastasis [11]. Short intermittent intervals of moderate hypoxia, however, that is, 12% O2, cause moderate stress followed by beneficial adaptations [12]. IHT may provoke beneficial effects by preconditioning subsequently protecting the heart and/or the brain against deleterious consequences of ischemia reperfusion [13]. Although the excessive production of reactive oxygen species (ROS) and reactive nitrogen species (RNS) represents an important mechanism of cell damage during hypoxia and reoxygenation in mitochondria initiating cellular death pathways, IHT may optimize mitochondrial metabolism, thus preventing adverse consequences of excess mitochondrial ROS generation [14], [15]. In addition, IHT stimulates endothelial nitric oxide (NO) production that leads to vasodilatation, opens reserve capillaries [16], and induces the production of vascular endothelial and fibroblast growth factors to stimulate endothelial proliferation [17]. In clinical research, Burtscher et al. and Katayama et al. showed that hypoxic training improved exercise tolerance in patients suffering from chronic diseases such as cardiovascular disease, chronic obstructive pulmonary disease (COPD) or metabolic diseases [18], [19], [20]. There is also evidence that IHT may protect against neurodegenerative changes and even increase cognitive functions in experimental Alzheimer's disease in rats [21]. To our knowledge, Schega et al. were the first to investigate the positive effects of IHT on cognitive function in elderly subjects aged between 60 and 70 years [22].
Very recently, a modified IHT mode, intermittent hypoxic–hyperoxic training (IHHT), has been proposed as being associated with even more beneficial effects than IHT. The normoxic periods are replaced by moderate hyperoxic periods with 30%–40% oxygen resulting in a faster recovery of oxygen desaturation [23]. The hypoxic–hyperoxic treatment has been claimed to be more effective and produces a faster membrane-stabilizing effect in cells of the heart, liver, and brain compared to IHT in a study with male Wistar rats [24]. A very recent study reported that IHHT improved exercise performance in athletes with overtraining syndrome [25]. The case study of Susta et al. reported a significant improvement of cardiopulmonary efficiency and lactate removal after 3 weeks of daily IHHT [26]. Glazachev showed that IHHT can even improve exercise tolerance, aerobic capacity, and cardiometabolic profile in patients with stages II–III of the New York Heart Association (NYHA II–III) without any additional exercise [27]. None of these studies reported adverse side effects.
Thus, we hypothesized that IHHT performed in parallel with an MTI would produce greater improvements of exercise tolerance and cognitive function in geriatric patients than an MTI alone. The aim of the present study was to evaluate the applicability of IHHT in patients attending in an MTI in a geriatric daily clinic and to investigate its effects on exercise tolerance and cognitive function.
2. Methods
2.1. Participants and randomization
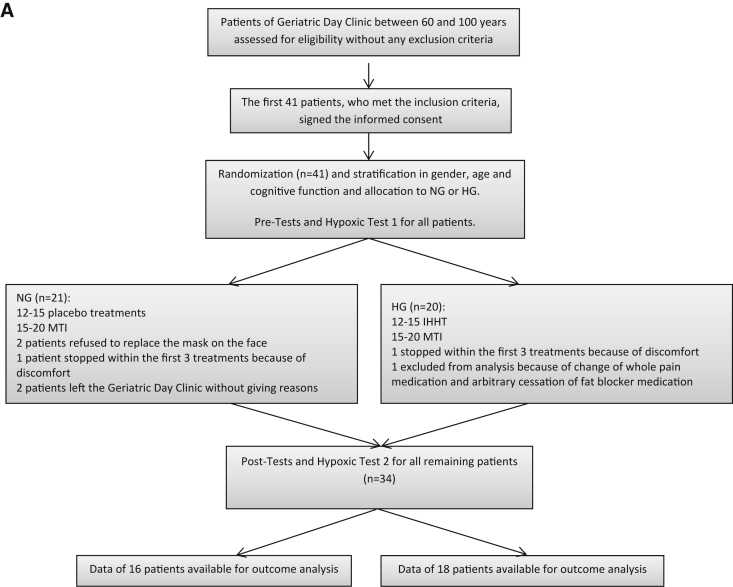
Geriatric patients between 60 and 100 years, who attended in the multimodal training program of the Geriatric Daily Clinic in Klagenfurt (Carinthia, Austria), were invited to participate in this double-blind, randomized, stratified, and placebo-controlled study. Finally, 41 patients between 64 and 92 years participated in the study. The double-blinded study setting was ensured by the fact that neither patients nor therapists were aware about the type of breathing program. All volunteers underwent a routine physical examination. Subjects were not included if they were not able to walk without any staff assistance or suffered from severe dementia with a score of Mini–Mental State Examination (MMSE) less than 12 points, uncontrolled hypertension (systolic blood pressure [BP] > 180 mm Hg and/or diastolic BP > 100 mm Hg), COPD III–IV, decompensated heart failure (NYHA III–IV), or previous intracerebral bleeding. The first 41 patients meeting the inclusion criteria comprised the study population. After obtaining informed and written consent, study participants were assessed by the MMSE which is a practical test for grading the cognitive state of a patient [28]. Thereafter, participants were randomly assigned to either the hypoxia group (HG) or the normoxia group (NG). Subjects were stratified by age (60–80, 81–100 years), gender (male, female), and cognitive function (MMSE 12–21, 22–30 points). Finally, the data of 34 of the 41 included patients who successfully completed the whole study programme were taken for the outcome analysis as shown in Fig. 1.
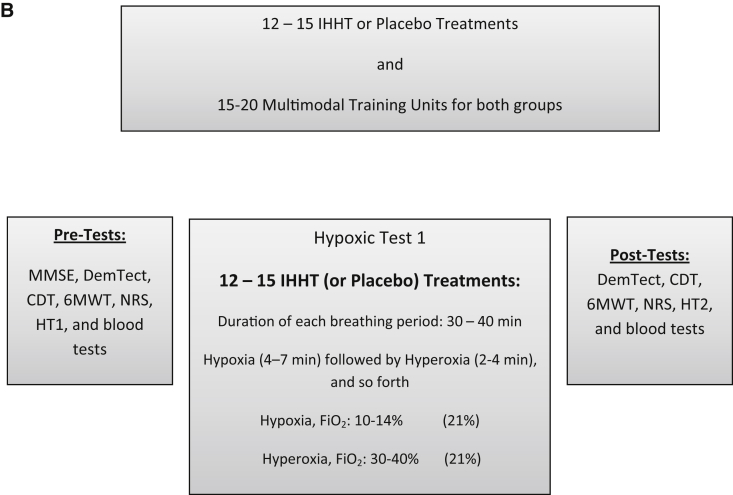
Fig. 1.
(A) Process of inclusion, randomization, stratification, and (B) training program and outcome analysis. Abbreviations: 6MWT, 6-Minute Walk Test; CDT, Clock-Drawing Test; DemTect, Dementia-Detection Test; FiO2, inspiratory oxygen fraction; IHHT, intermittent hypoxic–hyperoxic training; HG, hypoxic group; HT1, hypoxic test 1; HT2, hypoxic test 2; MMSE, Mini–Mental State Examination; NG, normoxic group; NRS, numeric rating scale for pain.
All participants were advised not to change medications, nutrition, and physical activity during the whole study period; only single doses of emergency medication in cases of high blood pressure and pain attacks were accepted, as documented in the medical recordings of the Geriatric Day Clinic.
The study was approved by the local ethics committee and performed in accordance with the ethical standards of the Declaration of Helsinki in 1975.
2.2. Study protocol
2.2.1. Multimodal training intervention
All study participants were included in the same MTI of the Geriatric Day Hospital in Klagenfurt, Austria. The baseline characteristics of both groups are shown in Table 1. The whole therapy unit for all patients includes 15–20 days of therapy, depending on the needs of each patient, with an individual treatment plan of 2–3 days of therapy per week over a period of 5–7 weeks (Fig. 1). All therapy units had been documented in the medical recordings of the hospital. Because of the holistic detection of sick people by a multiprofessional geriatric team, an optimal therapy concept with three focal points was created for each patient. The present study has been integrated into this therapy plan over a period of half a year.
Table 1.
Baseline characteristics of the hypoxic and normoxic groups
| Variable | Hypoxic group (n = 18) | Normoxic group (n = 16) | P values |
|---|---|---|---|
| Gender (m, f) | m 5 (28%)/f 13 (72%) | m 2 (12.5%)/f 14 (87.5%) | .25** |
| Age (years) | 80.9 (7.9) | 83.4 (5.5) | .14 |
| Height (cm) | 163.7 (8.3) | 163.2 (8.5) | .43 |
| Weight (kg) | 72.0 (9.3) | 66.8 (12.3) | .09 |
| BMI (kg/m2) | 27.0 (3.9) | 25.0 (3.6) | .07 |
| MMSE score | 24.9 (3.8) | 24.5 (3.9) | .36 |
| Therapy days (n) | 18.3 (2.4) | 17.5 (2.6) | .16* |
| Systolic blood pressure (mm Hg) | 137.2 (18.9) | 134.6 (15.9) | .38 |
| Diastolic blood pressure (mm Hg) | 77.5 (6.9) | 77.9 (8.7) | .42* |
| Oxygen saturation (%) | 94.2 (6.2) | 93.7 (7.5) | .42 |
| Oxygen saturation, HT (%) | 81.4 (2.8) | 83.9 (5.5) | .27* |
| Total cholesterol (mg/dL) | 231.2 (46.2) | 215.9 (58.1) | .09* |
| Regular medication, n (%) | |||
| Anticoagulants | 9 (50) | 10 (62.5) | .35** |
| β blockers | 7 (38.9) | 8 (50) | .38** |
| ACE inhibitors | 7 (38.9) | 6 (37.5) | .61** |
| AT II inhibitors | 6 (33.3) | 3 (18.8) | .29** |
| Calcium channel blockers | 5 (27.8) | 1 (6.3) | .12** |
| Statins | 1 (5.6) | 4 (25) | .13** |
| Diuretics | 8 (44.4) | 9 (56.3) | .37** |
| Nitrates | 1 (5.6) | 1 (6.3) | .73** |
Abbreviations: BMI, body mass index; MMSE, Mini–Mental State Examination; HT, hypoxic test 1; SD, standard deviation.
NOTE. Data represent means (SD) or frequencies (%). P values for differences between groups are based on the use of the Welch test if values are normally distributed or on the use of the Mann–Whitney U test (*) if values are not normally distributed. **P values calculated by the Exact Fisher–Yates Test.
The everyday MTI included physiotherapy, occupational therapy, and cycling. The daily 30-minute physiotherapy program consisted of individually tailored strength training on a leg press (3 × 20 repetitions), functional exercises, and a combination of balance and reaction training. Functional exercises were performed on a stepper and the combined balance and reaction training with the use of a computer-aided multifunction (MFT) Challenge Disc (MFT Bodyteamwork, Austria), Airex Balance-beam, and Airex Balance-pad-plus (AIREX, Switzerland). The occupational therapy focused for 60 minutes on the training of strength, flexibility, and coordination of fingers and arms using various devices complemented by cognitive training carried out on a one hand computer (RehaCom Cognitive Therapy Software; UK). The 20-minute cycling program was performed on the rehabilitation trainer MOTOmed viva 1 (RECK, Germany) in a sitting position with a pedaling rate between 28 and 32 at a very low to low resistance. A more effective cardiovascular fitness training on the cycle ergometer was not possible due to the high age and the multimorbidity of the patients. The heart rate of patients was controlled by a nurse and did not exceed 120 beats/minute during the whole cycling period.
2.2.2. IHHT program
Concurrent with the MTI, the IHHT using the ReOxy breathing therapy device (AI Mediq S.A., Luxembourg) was performed. This device delivers a gas mixture with an oxygen content of 10%–30% in nitrogen and continuously monitors arterial oxygen saturation (SpO2) and pulse rate and stores all recorded data. Blood pressure was measured before and after every procedure. To establish an individually tailored IHHT, as recommended by the instructions of AI Mediq S.A., all patients had to take part in the same hypoxic test (HT1) which lasted for 5–10 minutes: all participants breathed a hypoxic gas mixture with low oxygen (12% O2) (cf. 8, 18–20) through a face mask while sitting in an armchair for 5–10 minutes. SpO2 and pulse rate were measured by a pulse oximeter, which was invisible to the patients. Following this, on each therapy day, HG patients underwent a repeated exposure to hypoxic gas mixtures (10%–14% O2) lasting 4–7 minutes, followed by a 2- to 4-minute exposure to a hyperoxic gas mixture (30%–40%) through the face mask (cf. 24–26). Each session lasted 30–40 minutes and included 4–8 hypoxia–hyperoxia cycles, depending on the individual responses and state of health. The therapy was constantly adjusted by the ReOxy breathing therapy device to the individually measured values of oxygen saturation and pulse rate in HT1. Individual minimal SpO2 and maximal pulse rate data in HT1 were used as preset key parameters to be installed into device software for further therapy sessions. While a patient is receiving the hypoxic gas mixture, SpO2 and pulse rate are constantly monitored and transmitted to a monitoring device which compares the current value of SpO2 with the preset value of the individual's minimum SpO2. When reaching the minimum SpO2, the hyperoxic gas mixture is supplied up to restoring baseline resting SpO2, again followed by administering a hypoxic gas mixture [29].
After performing the same HT1, patients in the NG were exposed to the same breathing program as the HG but only breathing a normoxic gas mixture (placebo). Treatment differences were only visible to the two study nurses who provided the therapy and operated the devices. The study participants and the therapist were not informed about the kind of treatment provided.
During the whole stay in the geriatric daily clinic, 12–15 hypoxic or normoxic treatment procedures were performed for both groups 2–3 times a week over a period of 5–7 weeks, always together with the MTI on the same day (Fig. 1). At the end, both groups performed a second hypoxic test (HT2).
2.2.3. Assessments
For the purpose of stratification, all patients were assessed with the MMSE at the beginning of the study, before all treatments started. All other assessments, including the cognitive testing with the Dementia-Detection Test (DemTect) and the Sunderland Clock-Drawing Test (CDT), the evaluation of the functional exercise capacity with the 6-Minute Walk Test (6MWT), and the measurement of the pain situation with the numeric rating scale (NRS) were held at the beginning and at the end of all treatments and therapy units. The taking of blood samples was held in the same way, at the beginning and at the end (Fig. 1).
2.2.4. Cognitive testing
The DemTect is a highly sensitive screening instrument to identify patients with MCI and patients with dementia in early stages [30]. A cognitive screening by the DemTect is claimed to be more reliable than screening by the MMSE, and in particular, in the area of incipient and slightly more advanced cognitive disorders, the DemTect is far superior to the MMSE [31]. The CDT is also a valid and reliable screening test for dementia and cognitive impairment [32]. In our study, we used the free-drawn method.
2.2.5. Evaluation of functional exercise capacity and measurement of pain
The 6MWT was used to assess the functional exercise capacity of the study participants. It was carried out according to the guidelines for the 6-Minute Walk Test of the American Thoracic Society [33]. We assessed pain with the 11-point NRS. It was shown to the study subjects with 0 representing “no pain” and 10 representing “pain as bad as you can imagine” [34].
2.3. Statistical methods
Data are presented as means and standard deviation or frequencies. The Kolmogorov–Smirnov test was used to test normal distribution of data. Correlation between variables and its significance was determined by the nonparametric Spearman rank correlation coefficient rS. Frequencies were compared by the Fisher–Yates test (exact Fisher test). Significance of differences was tested by use of the Wilcoxon–Mann–Whitney (U) test and in the case of normally distributed data by the Welch test. A P value ≤.05 is considered to indicate statistical significance. Statistical evaluations were performed in R (version 2.7.0 resp. 3.2.3, 2015; The R Foundation for Statistical Computing, ISBN 3-900051-07-0, http://cran.r-project.org) and (elementary statistics and some figures) in HP-RPL (Version 2.08, 2006; Hewlett-Packard Company, San Diego, CA 92123, USA).
3. Results
Both the hypoxic and normoxic sessions were well tolerated. Aside from sleepiness and slight dizziness during the hypoxic treatments, absolutely no side effects were reported. Small gastrointestinal infections and minor infections of the upper respiratory tract occurred in both groups, but they all fully recovered within several days and all patients continued with the training interventions within a week. Mean values of minimum arterial oxygen saturation (measured by pulse oximetry) during the HTs are shown in Table 2.
Table 2.
Cognitive function, functional exercise capacity and pain assessment, cardiorespiratory and biochemical data before and after the intervention
| Variable | Hypoxic group (n = 18) |
Normoxic group (n = 16) |
P values | ||
|---|---|---|---|---|---|
| Pre | Post | Pre | Post | ||
| DemTect | 11.2 (3.5) | 14.2 (3.7) | 11.4 (4.1) | 11.3 (3.6) | <.001* |
| CDT | 7.8 (3.0) | 8.4 (3.0) | 7.5 (2.3) | 6.9 (2.6) | .038* |
| 6MWT (m) | 234.3 (94.8) | 290.7 (83.2) | 250.6 (94.3) | 277.7 (96.3) | .045* |
| NRS | 4.3 (2.4) | 2.7 (1.9) | 2.9 (2.7) | 1.9 (2.0) | .061* |
| Pulse rate (bpm) | 66.5 (8.5) | 66.1 (8.5) | 73.5 (8.0) | 67.8 (5.5) | .076* |
| Systolic blood pressure (mm Hg) | 136.4 (17.9) | 132.5 (14.7) | 134.6 (15.9) | 129.5 (16.4) | .41 |
| Diastolic blood pressure (mm Hg) | 80.8 (18.3) | 73.3 (5.9) | 77.9 (8.7) | 72.9 (6.7) | .36* |
| Oxygen saturation (%) | 94.2 (6.2) | 98.3 (1.2) | 93.7 (7.5) | 97.7 (1.8) | .36* |
| Oxygen saturation, HT (%) | 81.4 (2.8) | 87.1 (7.6) | 83.9 (5.5) | 85.8 (7.5) | .27 |
| Total cholesterol (mg/dL) | 231.2 (46.2) | 220.6 (42.4) | 215.9 (58.1) | 213.1 (62.1) | .08 |
| HDL (mg/dL) | 59.2 (14.6) | 56.9 (15.0) | 54.9 (12.1) | 53.8 (11.1) | .24 |
| LDL (mg/dL) | 143.9 (40.9) | 137.3 (35.2) | 130.6 (47.4) | 125.6 (41.8) | .31* |
| Triglycerides (mg/dL) | 140.3 (59.2) | 131.8 (55.8) | 158.6 (100.1) | 159.3 (118.6) | .26 |
| Erythrocytes (×106/μL) | 4.6 (0.5) | 4.5 (0.5) | 4.2 (0.5) | 4.1 (0.5) | .36* |
| Hemoglobin (g/dL) | 13.3 (1.3) | 13.5 (1.2) | 12.4 (1.4) | 12.6 (1.5) | .46 |
| TNF alpha (ng/L) | 11.3 (5.3) | 12.5 (5.6) | 10.3 (3.3) | 10.7 (3.1) | .08* |
| Interleukin-6 (pg/mL) | 8.5 (3.5) | 9.2 (5.9) | 8.2 (2.6) | 8.2 (2.2) | .24 |
Abbreviations: DemTect, Dementia-Detection Test; CDT, Clock-Drawing Test; 6MWT, 6-Minute Walk Test; NRS, numeric rating scale for pain; TNF alpha, tumor necrosis factor alpha; HT, hypoxic test; SD, standard deviation.
NOTE. Data are means (SD). P values for differences between groups are based on the use of the Welch test if values are normally distributed or on the use of the Mann–Whitney U test (*) if values are not normally distributed. Bold text indicates P values <0.1.
3.1. Cognitive function
Testing of cognitive function by DemTect and CDT did not reveal any significant differences between groups before starting the intervention (Table 2). But after the IHHT combined with MTI, the HG showed a notable increase in cognitive function measured with the DemTect and the CDT. In contrast, no positive changes in the NG after the placebo treatments combined with MTI were detected. The score of the DemTect remained almost the same as that at the start and the score of the CDT even decreased slightly. There was a significant difference in cognitive function between the HG and NG groups regarding the CDT and the DemTect (Table 2, Table 3). The cause of missing values in these tests (one person in the DemTect and four persons in the CDT) was the inability of the patients to write and draw.
Table 3.
Changes of test results (DemTect, CDT, 6MWT and NRS) from pre to post for the hypoxic and normoxic groups and correlations between changes of the overall group
| Variable | Delta pre–post |
P value (Mann–Whitney U test) | |
|---|---|---|---|
| Hypoxic group | Normoxic group | ||
| DemTect | +3 (+16.7%) | −0.07 (−0.39%) | <.001 |
| CDT | +1.07 (+10.7%) | −0.8 (−8%) | .03 |
| 6MWT (m) | +56.26 (+24.1%) | +27.13 (+10.8%) | .02 |
| NRS | −1.56 (−15.6%) | −1.0 (−10%) | .07 |
| Correlations between changes of both groups | Spearman rank correlation coefficient (rS) Both groups |
P value |
|---|---|---|
| 6MWT–DemTect | +0.57 | <.001 |
| 6MWT–CDT | +0.42 | .01 |
| NRS–DemTect | −0.35 | .02 |
| NRS–CDT | −0.44 | .007 |
Abbreviations: DemTect, Dementia-Detection Test; CDT, Clock-Drawing Test; 6MWT, 6-Minute Walk Test; NRS, numeric rating scale for pain. Bold text indicates P values <0.1.
3.2. Functional exercise capacity and pain
The functional exercise capacity was measured by the total distance of the 6MWT. After the IHHT or placebo treatments combined with the MTI, both groups showed an increase in the total distance but in the HG, the increase was higher as depicted in Table 2, Table 3. Using the 11-point NRS for the evaluation of the pain situation, there was no statistically significant difference at the beginning between the HG and the NG. After all treatments, the pain situation improved in both groups but just failed to become statistically significant between groups (Table 2, Table 3).
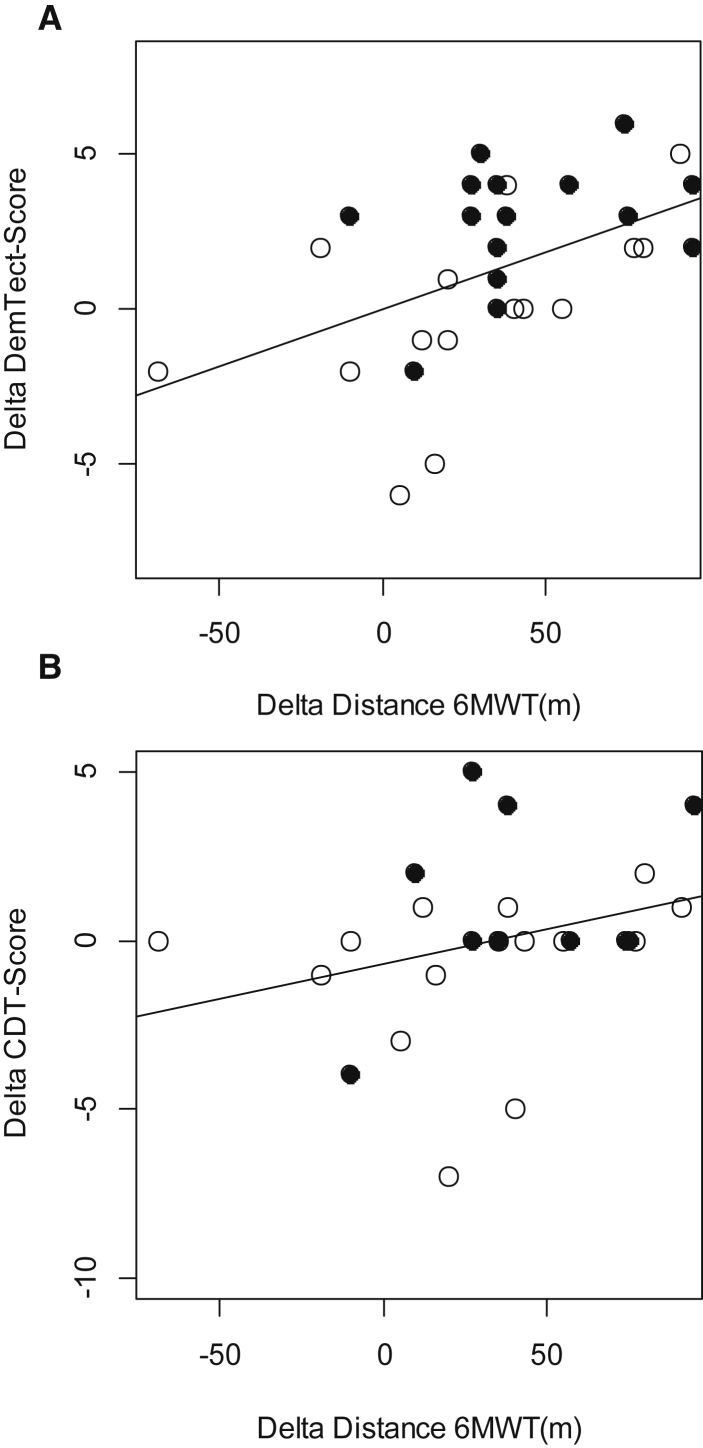
A highly significant correlation was found between the changes of the distance of the 6MWT and the DemTect score and also between the 6MWT and the CDT (Fig. 2, Table 3). We also found a significant negative correlation between the differences of the NRS score and the DemTect score (r = −0.35, P < .05) and also between the NRS score and the CDT score (r = −0.44, P < .05) only for the overall group but not for the subgroups. Cognitive recovery did correlate neither with the subject's age nor with the degree of desaturation or the change of desaturation.
Fig. 2.
Correlation between the changes (A) in the distance of the 6-Minute Walk Test (6MWT) and the Dementia-Detection Test Scores (DemTect) and (B) in the distance of 6MWT and the Clock-Drawing Test (CDT) scores. Hypoxic group: filled circles; normoxic group: open circles. (A) r = 0.57, P < .01 and (B) r = 0.42, P = .01.
3.3. Blood tests and cardiorespiratory parameters
A slight decrease in systolic and diastolic pressure, total cholesterol, HDL, LDL, and triglycerides was observed but with no statistically significant difference between the groups. The amount of erythrocytes, hemoglobin, and interleukin-6 remained pretty much the same with no significant difference. The arterial oxygen saturation and the tumor necrosis factor alpha increased slightly but also with no statistically significant difference between the two groups (Table 2).
4. Discussion
To our knowledge, this is the first study that has investigated the tolerability and the effects on cognitive function of IHHT plus MTI in geriatric patients suffering from mild-to-moderate dementia with a mean age of more than 80 years.
4.1. IHHT applicability
One important aim of the present study was to investigate whether IHHT is tolerable and applicable to geriatric patients performing an MTI. Some patients even desaturated down to 75% SaO2 during the hypoxia periods, but this low oxygen saturation only lasted several seconds because of the subsequently delivered hyperoxia. Neither adverse health problems nor feelings of remarkable discomfort were reported during the whole hypoxic intervention as was true for the normoxic breathing sessions. These findings are in accordance with those reported by Burtscher et al. [18], [20] and Schega et al. [22], who used intermittent hypoxia in healthy elderly and those suffering from COPD.
4.2. IHHT effects on cognitive function and functional exercise capacity
As demonstrated by other authors [35], [36], our short-term MTI in the NG did not result in improved cognitive function. Although the distance in the 6MWT increased by 10.8% in the NG, the increase in functional exercise capacity was not associated with improvement in cognitive performance. Thus, the increase in cognitive function in the HG might mainly be due to the IHHT.
These findings are similar to those reported by Schega et al. [22]. However, in contrast, Schega et al. found positive effects on cognitive function also related to the physical training alone. An explanation for the lack of improvement in cognitive performance after physical training alone in our study could be the low resilience of very old patients because the patients in Schega et al's study were not older than 70 years and therefore more able to tolerate physical training. This is in concordance to the conclusion of the meta-analysis of Colcombe and Kramer, who found that patients aged between 66 and 71 years seemed to benefit most from exercise [7]. For the study participants who were older than 80 years, it was probably not possible to achieve a training intensity that was high enough to cause such clear cognitive effects. Thus, MTI combined with IHHT seems to be an appropriate method to improve both cognitive function and functional exercise capacity in very old patients. Glazachev, in his study, even reported an increased exercise tolerance and aerobic capacity without any exercising [27]. Just as the baseline data of the LIFE Cognition study revealed that physical performance directly correlates to cognitive performance [37], so too in our study the improvements in cognitive function within the overall group were significantly correlated with the increase in the functional exercise capacity determined by the 6MWT (Fig. 2) confirming the importance of exercise training for improvement of cognitive function.
There are several studies showing positive structural effects of physical training on brain structure and function. Aerobic fitness training can induce a significant increase in brain volume, in gray- and white-matter regions [38] and increases the hippocampal volume in older adults [39]; it also influences the brain-derived neurotrophic factor (BDNF), which seems to play an important role in memory and learning [40], [41].
Similar effects may result from hypoxic treatments. Zhu et al. found that IHT leads to more newborn neurons and enhances the expression of the BDNF in the hippocampus [42], and Satriotomo et al. reported that hypoxic preconditioning increased the BDNF expression in rats after 4 and 10 weeks, especially in the group receiving treatments three times a week [43]. Malyshev et al. suggested that the protective neuronal mechanisms of adaptation to hypoxia may be related to a restriction of oxidative stress in the hippocampus, the limitation of a decrease in NO production induced by β-amyloid, stimulation of antioxidants, heat shock proteins, and increased density of the vascular network in the brain, which are suggested as key pathogenic factors of Alzheimer's disease [44]. Some of these adaptations might be caused by the hypoxia-related erythropoietin (EPO) production in the brain. EPO has been reported to activate signaling cascades initiating improved resistance of the brain to ischemia-reperfusion stress by stabilizing mitochondrial membranes, reduced formation of reactive oxygen and nitrogen species, and likely also by suppression of the production of proinflammatory cytokines and of neutrophil infiltration [45].
As physical activity requires some time to increase cognitive function and studies with a long-term intervention of more than 3 months showed the best effects [7], IHHT seems to already have positive effects after some weeks. Thus, we suggest that our MTI was too short to show measurable increases after several weeks but MTI + IHHT did.
Another explanation for the efficacy of IHHT on cognitive function may be related to the reduction of pain, which was measured by the NRS. Although the pain situation improved in both groups, the pain reduction in the HG tended to be more pronounced. Our findings reveal that beside the increment of exercise capacity, the reduction of pain is also highly associated with an increase in cognitive performance. Schiltenwolf et al. showed that chronic pain impairs cognitive function [46]. Thus, treating pain, for example, by IHHT, also could increase cognitive function.
4.3. Limitations
There are at least five limitations in our study. First, the intervention period may probably have been too short to improve cognitive function by MTI alone. However, this enabled us to demonstrate that IHHT plus MTI can effectively improve cognitive function in old patients even during the relatively short intervention period. Second, the use of IHHT and MTI in this group of patients with a high compliance is probably only possible with much effort on the part of trained supervisors and not easy to translate to a real-life situation. Third, the mechanisms of IHHT remain largely speculative, but its demonstrated efficacy will stimulate further research on this issue. Fourth, due to the small number of male patients, we did not perform sex-specific analyses, and thus, the results may primarily be valid for females. Finally, we did not measure EPO concentrations that might have been related to improvements in cognitive performance.
5. Conclusion
IHHT has proved to be easily applicable to and well tolerated by geriatric patients aged up to 92 years, even when they suffered from moderate dementia. IHHT contributed significantly to improvements in cognitive performance and functional exercise capacity in geriatric patients performing MTI.
Research in Context.
-
1.
Systematic review: Embase and PubMed databases were searched using various combinations of search terms including “hypoxia, hyperoxia, exercise, training, elderly, cognitive impairment, dementia” to identify and evaluate the accumulated knowledge on the applicability and effects of intermittent hypoxic–hyperoxic training (IHHT) in geriatric patients.
-
2.
Interpretation: We demonstrated that IHHT has proved to be easily applicable to and well tolerated by geriatric patients up to 92 years of age and that IHHT contributed significantly to improvements in cognitive performance and functional exercise capacity in these patients. The efficacy of IHHT on cognitive performance in geriatric patients may be related (1) to neuronal mechanisms of adaptation to hypoxia, (2) to improved exercise tolerance, and/or (3) to the reduction of pain.
-
3.
Future directions: Because mechanisms of IHHT remain largely speculative, its demonstrated efficacy will stimulate further research to better understand responsible mechanisms and to substantiate causal links.
Acknowledgments
The authors wish to thank all volunteers who participated in the study. Also, the authors thank AiMediq SA, Luxembourg, for supplying two ReOxy devices and equipment at cost. Additionally, the authors thank the nurses of the Geriatric Day Clinic Draginja Catiz, Rosemarie Mayer, and Dagmar Dornik, who coordinated all appointments and collected blood samples for the outcome analysis and Dr. Evgenia Terziev, Christine Lübke, Gerd Tragner, Sonja Türk, Franz Smolnig, and Waltraud Genser for the assessments and positive cooperation.
References
- 1.Feldman H.H., Ferris S., Winblad B. Effect of rivastigmine on delay to diagnosis of Alzheimer’s disease from mild cognitive impairment: the InDDEx study. Lancet Neurol. 2007;6:501–512. doi: 10.1016/S1474-4422(07)70109-6. [DOI] [PubMed] [Google Scholar]
- 2.Petersen R.C., Thomas R.G., Grundman M., Bennett D., Doody R., Ferris S. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352:2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 3.Jiang J., Jiang H. Efficacy and adverse effects of memantine treatment for Alzheimer's disease from randomized controlled trials. Neurol Sci. 2015;36:1633–1641. doi: 10.1007/s10072-015-2221-2. [DOI] [PubMed] [Google Scholar]
- 4.Lautenschlager N.T., Cox K.L., Flicker L., Foster J.K., van Bockxmeer F.M., Xiao J. Effect of physical activity on cognitive function in older adults at risk of Alzheimer's disease. JAMA. 2008;300:1027–1037. doi: 10.1001/jama.300.9.1027. [DOI] [PubMed] [Google Scholar]
- 5.Barnes D.E., Santos-Modesitt W., Poelke G., Kramer A.F., Castro C., Middleton L.E. The Mental Activity and eXercise (MAX) Trial. A randomized controlled trial to enhance cognitive function in older adults. JAMA Intern Med. 2013;173:797–804. doi: 10.1001/jamainternmed.2013.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langlois F., Minh Vu T.T., Chasse K., Dupuis G., Kergoat M.J., Bherer L. Benefits of physical exercise training on cognition and quality of life in frail older adults. J Gerontol B Psychol Sci Soc Sci. 2013;68:400–404. doi: 10.1093/geronb/gbs069. [DOI] [PubMed] [Google Scholar]
- 7.Colcombe S., Kramer A.F. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 8.Neubauer J.A. Invited review: physiological and pathophysiological responses to intermittent hypoxia. J Appl Physiol. 2001;90:1593–1599. doi: 10.1152/jappl.2001.90.4.1593. [DOI] [PubMed] [Google Scholar]
- 9.Joyeux-Faure M., Stanke-Labesque F., Lefebvre B., Béguin P., Godin-Ribuot D., Ribuot C. Chronic intermittent hypoxia increases infarction in the isolated rat heart. J Appl Physiol (1985) 2005;98:1691–1696. doi: 10.1152/japplphysiol.01146.2004. [DOI] [PubMed] [Google Scholar]
- 10.Lévy P., Ryan S., Oldenburg O., Parati G. Sleep apnoea and the heart. Eur Respir Rev. 2013;22:333–352. doi: 10.1183/09059180.00004513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michiels C. Physiological and pathological responses to hypoxia. Am J Pathol. 2004;164:1875–1882. doi: 10.1016/S0002-9440(10)63747-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serebrovskaya T.V., Manukhina E.B., Smith M.L., Downey H.F., Mallet R.T. Intermittent hypoxia: cause of or therapy for systemic hypertension? Exp Biol Med. 2008;233:627–650. doi: 10.3181/0710-MR-267. [DOI] [PubMed] [Google Scholar]
- 13.Kolar F., Ostadal B. Molecular mechanisms of cardiac protection by adaptation to chronic hypoxia. Physiol Res. 2004;53:S3–S13. [PubMed] [Google Scholar]
- 14.Thompson J.W., Dave K.R., Young J.I., Perez-Pinzon M.A. Ischemic preconditioning alters the epigenetic profile of the brain from ischemic intolerance to ischemic tolerance. Neurotherapeutics. 2013;10:789–797. doi: 10.1007/s13311-013-0202-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sazontova T.G., Arkhipenko Yu.V. Intermittent hypoxia in resistance of cardiac membrane structures: role of reactive oxygen species and redox signalling. In: Xi L., Serebrovskaya T.V., editors. Intermittent Hypoxia: From Molecular Mechanisms to Clinical Applications. Nova Science Publishers; Hauppauge, NY: 2009. pp. 113–150. Chapter 5. [Google Scholar]
- 16.Manukhina E.B., Vanin A.F., Malyshev I.Yu., Mallet R.T. Intermittent hypoxia-induced cardio- and vasoprotection: Role of NO stores. In: Xi L., Serebrovskaya T.V., editors. Intermittent Hypoxia: From Molecular Mechanisms to Clinical Applications. Nova Science Publishers; Hauppauge, NY: 2009. pp. 79–112. Chapter 4. [Google Scholar]
- 17.El'chaninova S.A., Korenyak N.A., Pavlovskaya L.I., Smagina I.V., Makarenko V.V. The effect of interval hypoxic hypoxia on the vascular endothelial growth factor and basic fibroblast growth factor concentrations in the peripheral blood. Hum Physiol. 2004;30:705–707. [PubMed] [Google Scholar]
- 18.Burtscher M., Haider T., Domej W., Linser T., Gatterer H., Faulhaber M. Intermittent hypoxia increases exercise tolerance in patients at risk or with mild COPD. Respir Physiol Neurobiol. 2009;165:97–103. doi: 10.1016/j.resp.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 19.Katayama K., Matsuo H., Ishida K., Mori S., Miyamura M. Intermittent hypoxia improves endurance performance and submaximal exercise efficiency. High Alt Med Biol. 2003;4:291–304. doi: 10.1089/152702903769192250. [DOI] [PubMed] [Google Scholar]
- 20.Burtscher M., Pachinger O., Ehrenbourg I., Mitterbauer G., Faulhaber M., Pühringer R. Intermittent hypoxia increases exercise tolerance in elderly men with and without coronary artery disease. Int J Cardiol. 2004;96:247–254. doi: 10.1016/j.ijcard.2003.07.021. [DOI] [PubMed] [Google Scholar]
- 21.Manukhina E.B., Goryacheva A.V., Barskov I.V., Viktorov I.V., Guseva A.A., Pshennikova M.G. Prevention of neurodegenerative damage to the brain in rats in experimental Alzheimer's disease by adaptation to hypoxia. Neurosci Behav Physiol. 2010;40:737–743. doi: 10.1007/s11055-010-9320-6. [DOI] [PubMed] [Google Scholar]
- 22.Schega L., Peter B., Törpel A., Mutschler H., Isermann B., Hamacher D. Effects of intermittent hypoxia on cognitive performance and quality of life in elderly adults: a pilot study. Gerontology. 2013;59:316–323. doi: 10.1159/000350927. [DOI] [PubMed] [Google Scholar]
- 23.Glazachev O. Optimization of clinical application of interval hypoxic training. Biomed Eng. 2013;47(3):134–137. [PubMed] [Google Scholar]
- 24.Arkhipenko Y.V., Sazontova T.G., Zhukova A.G. Adaptation to periodic hypoxia and hyperoxia improves resistance of membrane structures in heart, liver, and brain. Bull Exp Biol Med. 2005;140(3):278–281. doi: 10.1007/s10517-005-0466-0. [DOI] [PubMed] [Google Scholar]
- 25.Susta D., Dudnik E., Glazachev O. A program based on repeated hypoxia–hyperoxia exposure and light exercise enhances performance in athletes with overtraining syndrome. Clin Physiol Funct Imaging. 2015 doi: 10.1111/cpf.12296. [DOI] [PubMed] [Google Scholar]
- 26.Susta D, Kellett M, Glazachev O. The effects of intermittent hypoxic-hyperoxic training on high intensity intermittent performance: a case study. Sports Med Res Pract J: ISSN 2223-2524
- 27.Glazachev O. Intermittent hypoxia–hyperoxia exposure improves cardiometabolic profile, exercise tolerance and quality of life: a preliminary study in cardiac patients. Eur J Prev Cardiol EJPC-D-15–00334.
- 28.Folstein M.F., Folstein S.E., McHugh P.R. Mini mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 29.Glazachev O, Platonenko A, Spirina G. Vorrichtung Zur Biologisch Regelbaren Auswahl von Individuellen Verlaufen für Eine Intervall-hypoxie-therapie (hypoxietraining): Gebrauchsmusters Nr DE De202012012602, 06.08.2013; Tag der Eintragung 01.06.2012 Gebrauchsmusterinhaber AI MEDIQ S.A., Luxembourg, LU.
- 30.Kalbe E., Kessler J., Calabrese P., Smith R., Passmore A.P., Brand M. DemTect: a new, sensitive cognitive screening test to support the diagnosis of mild cognitive impairment and early dementia. Int J Geriatr Psychiatry. 2004;19:136–143. doi: 10.1002/gps.1042. [DOI] [PubMed] [Google Scholar]
- 31.Kohn N., Kalbe E., Georg H., Kessler J. Vergleich MMST und DemTect: Spezifität und Sensitivität bei primär kognitiven Störungen. Aktuelle Neurologie. 2007;34 (Article in German) [Google Scholar]
- 32.Agrell B., Dehlin O. The clock-drawing test. Age and Ageing. 1998;27:399–403. doi: 10.1093/ageing/afs149. [DOI] [PubMed] [Google Scholar]
- 33.American Thoracic Society ATS Statement: Guidelines for the Six-Minute Walk Test. Official Statement of the American Thoracic Society, approved by the ATS Board Of Directors. 2002. [Google Scholar]
- 34.Hawker G.A., Mian S., Kendzerska T., French M. Measures of adult pain. Arthritis Care Res. 2011;63:S240–S252. doi: 10.1002/acr.20543. [DOI] [PubMed] [Google Scholar]
- 35.Baker L.D., Frank L.L., Foster-Schubert K., Green P.S., Wilkinson C.W., Mc Tiernan A. Effects of aerobic exercise on mild cognitive impairment. A controlled trial. Arch Neurol. 2010;67:71–79. doi: 10.1001/archneurol.2009.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suzuki T., Shimada H., Makizako H., Doi T., Yoshida D., Tsutsumimoto K. Effects of a multicomponent exercise on cognitive function in older adults with amnestic mild cognitive impairment: a randomized trial. BMC Neurol. 2012;12:128. doi: 10.1186/1471-2377-12-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sink K.M., Espeland M.A., Rushing J. The LIFE Cognition Study: design and baseline characteristics. Clin Interv Aging. 2014;9:1425–1436. doi: 10.2147/CIA.S65381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Colcombe S.J., Erickson K.I., Scalf P.E., Kim J.S., Prakash R., McAuley E. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61:1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- 39.Brinke L.F., Bolandzadeh N., Nagamatsu L.S. Aerobic exercise increases hippocampal volume in older women with probable mild cognitive impairment: a 6-month randomised controlled trial. Br J Sports Med. 2015;49:248–254. doi: 10.1136/bjsports-2013-093184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang E.J., Reichardt L.F. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zimmer P., Oberste M., Bloch W. Influence of exercise on the central nervous system—molecular and cellular mechanisms. Dtsch Z Sportmed. 2015;66:42–49. (Article in German) [Google Scholar]
- 42.Zhu X.H., Yan H.C., Zhang J., Qu H.D., Qiu X.S., Chen L. Intermittent hypoxia promotes hippocampal neurogenesis and produces antidepressant-like effects in adult rats. J Neurosci. 2010;30:12653–12663. doi: 10.1523/JNEUROSCI.6414-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Satriotomo I., Vinit S., Flom A.L. Repetitive acute intermittent hypoxia increases bdnf and trkb expression in respiratory motor neurons: dose effects. FASEB J. 2010 www.fasebj.org/cgi/content/meeting_abstract/24/1_MeetingAbstracts/799.16 Available at: Accessed December 22, 2016. [Google Scholar]
- 44.Malyshev I.Y., Wiegant F., Mashina S.Y., Torshin V.I., Goryacheva A.V., Khomenko I.P. Possible use of adaptation to hypoxia in Alzheimer's disease: a hypothesis. Med Sci Monit. 2005;11:HY31–HY38. [PubMed] [Google Scholar]
- 45.Nguyen A.Q., Cherry B.H., Scott G.F., Ryou M.G., Mallet R.T. Erythropoietin: powerful protection of ischemic and post-ischemic brain. Exp Biol Med (maywood) 2014;239:1461–1475. doi: 10.1177/1535370214523703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schiltenwolf M., Akbar M., Hug A., Pfüller U., Gantz S., Neubauer E. Evidence of specific cognitive deficits in patients with chronic low back pain under long-term substitution treatment of opioids. Pain Physician. 2014;17:9–20. [PubMed] [Google Scholar]