Abstract
Introduction
This randomized, double-blind, placebo-controlled, 90-week study assessed safety, tolerability, and immunogenicity of CAD106 with/without adjuvant in patients with mild Alzheimer's disease.
Methods
One hundred twenty-one patients received up to seven intramuscular injections of CAD106 (150 μg or 450 μg) or placebo ± adjuvant over 60 weeks. An amyloid positron emission tomography (PET) substudy was also conducted.
Results
CAD106 induced strong serological responses (amyloid-beta [Aβ]–Immunoglobuline G[IgG]) in 55.1% (150 μg) and 81.1% (450 μg) of patients (strong serological responders [SSRs]). Serious adverse events (SAEs) were reported in 24.5% (95% confidence interval [CI] 16.7–33.8) of the patients in the active treatment group and in 6.7% (95% CI 0.2–31.9) in the placebo group. Three of the SAEs were classified as possibly related to study drug by the investigators. No evidence of central nervous system inflammation was found. Amyloid-related imaging abnormalities (ARIAs) occurred in six cases, all of them were strong serological responders. None of the ARIAs were symptomatic. Serum Aβ-IgG titer area under the curves correlated negatively with amyloid PET standardized uptake value ratio percentage change from baseline to week 78 within the CAD106-treated patients (r = −0.84, P = .0004). Decrease in cortical gray-matter volume from baseline to week 78 was larger in SSRs than in controls (P = .0077).
Discussion
Repeated CAD106 administration was generally well tolerated. CAD106 450 μg with alum adjuvant demonstrated the best balance between antibody response and tolerability.
Keywords: Alzheimer's disease, CAD106, Active immunotherapy, Amyloid-beta peptides, Safety, Biological biomarkers
1. Introduction
For 15 years, immunotherapy targeting amyloid-β (Aβ)-related proteins or protein fragments have been investigated as a potential disease-modifying treatment for Alzheimer's disease (AD).
AN1792, the first active Aβ immunotherapy tested in a phase 2 clinical trial, demonstrated antibody responses in mild-to-moderate AD [1]. However, this compound was discontinued because 6% of patients experienced meningoencephalitis [2], likely caused by an Aβ-specific T-cell response (TH1-type CD4) [3]. Subsequent active and passive Aβ immunotherapies have avoided this T-cell response by using short or fragmented Aβ peptides or peptide mimetics of the Aβ N-terminus as B-cell epitopes [4], [5], [6]. Recently, two passive immunotherapies (monoclonal antibodies), bapineuzumab and solanezumab, failed to meet their primary end points in cognition and activities of daily living (ADL) in phase-3 trials in patients with mild-to-moderate AD [7], [8]. Unlike passive immunotherapies, active immunotherapies induce a more sustained polyclonal Aβ-specific response [6].
CAD106, a second-generation active Aβ immunotherapy, comprises multiple copies of the Aβ1–6 peptide coupled to a carrier containing 180 copies of bacteriophage Qβ coat protein [9]. CAD106 effectively induced Aβ antibodies in animal models, without activating an Aβ-specific T-cell response [10]. CAD106 doses up to 150 μg without adjuvant induce consistent Aβ-antibody responses in humans with no major safety concerns [9].
This study evaluated the safety and tolerability profile of CAD106 with and without adjuvant and determined the optimal dosage level for future clinical studies. An exploratory substudy used amyloid positron emission tomography (PET) imaging to assess antibody target engagement.
2. Methods
2.1. Study design
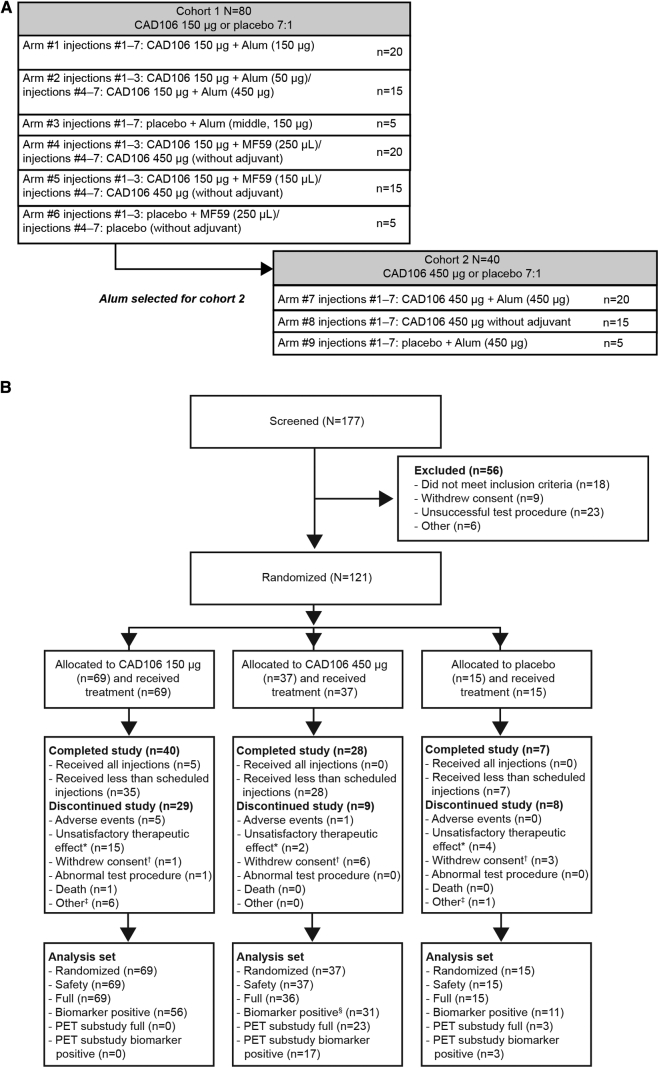
This was a phase 2, 90-week, randomized, double-blind, placebo-controlled, multicenter study (NCT01097096). Patients were centrally randomized into two semi-overlapping cohorts across nine treatment groups with an overall ratio of 7:1 CAD106 versus placebo (Fig. 1A).
Fig. 1.
(A) Study design and planned patient enrollment. (B) Patient disposition and primary reasons for premature withdrawal. *“Unsatisfactory therapeutic effect”: an insufficient immune response meeting nonresponder criteria as prespecified in the protocol and leading to discontinuation based on sponsor decision. Also includes two patients who discontinued treatment based on investigator's decision. †“Withdrawal of consent” attributed mainly to the temporary suspension of immunizations. ‡Other = lost to follow-up, administrative problems, protocol deviation. §Includes the patient with subdural hemorrhage (SDH). Abbreviation: PET, positron emission tomography.
In cohort 1, the effects of two adjuvants (alum [50 μg and 150 μg] and MF59 [squalene-based oil-in-water emulsion, 125 μL and 250 μL]) combined with CAD106 150 μg were investigated. Cohort 2 was initiated after alum was selected as adjuvant, based on cohort 1 data up to week 20. In cohort 2, CAD106 450 μg combined with alum 450 μg or without adjuvant versus placebo (alum 450 μg only) was assessed. Patients received up to seven intramuscular injections of CAD106 (±adjuvant) or placebo (adjuvant only) at weeks 0, 6, 12, 24, 36, 48, and 60. Patients underwent a full evaluation at week 78 and were monitored after treatment for safety up to 90 weeks.
The dose of CAD106 150 μg was identical to a dose used without adjuvant in the phase 2a study [11]. The dose of CAD106 450 μg was chosen because tripling of the dose was previously shown to approximately double antibody titers [9]. Alum and MF59 were used as they both increased antibody titers in animals.
Eligible patients aged <85 years with mild AD (Mini–Mental State Examination [MMSE] score: 20–26) diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders version IV and National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association criteria were included. Full inclusion and exclusion criteria are presented in Supplementary Table 1. Exclusion criteria included a history in the past 2 years of central nervous system (CNS) inflammation and on screening, brain magnetic resonance imaging (MRI) of more than one or two microhemorrhages (depending on the field strength). A discontinuation rule was implemented a priori per protocol for patients on CAD106 who did not meet serological responder criteria following the third injection. To maintain blinding, those receiving placebo were discontinued from study treatment in the same 7:1 ratio as the study randomization scheme. All patients provided written informed consent and the study was performed in accordance with the Declaration of Helsinki, Good Clinical Practice standards, and local ethical requirements.
2.2. Objectives
The primary objective was to assess the safety and tolerability of up to seven injections of CAD106 (150 μg or 450 μg) with or without adjuvant (alum or MF59) in patients with mild AD over 90 weeks and to compare immunogenicity of CAD106 across treatment arms.
Secondary objectives included characterization of the antibody response; characterization of the Aβ- and Qβ-specific T-cell response in peripheral blood mononuclear cells (PBMCs); and evaluation of the changes in cerebrospinal fluid (CSF) biomarker levels (Aβ1–40, Aβ1–42, total-tau, phospho-tau) over time.
Exploratory objectives included the effect on AD progression as measured by brain volumetric MRI and clinical assessments. In addition, an amyloid PET substudy with 18F-florbetapir was conducted in cohort 2 to explore the effect of CAD106 on the 18F-florbetapir standardized uptake value ratio (SUVR) over time.
2.3. Assessments of primary outcome measures
2.3.1. Safety and tolerability
An independent unblinded data monitoring committee monitored safety data including adverse events, vital signs, weight, electrocardiograms, and safety laboratory measurements regularly throughout the study.
CNS markers for inflammation (cytosis, IgG, and oligoclonal bands) were assessed at screening, week 38, and optionally at week 78. At screening and weeks 10, 20, 34, 50, and 78, fluid-attenuated inversion recovery, T2, T2* gradient echo, diffusion-weighted imaging, and T1-weighted brain MR sequences were acquired and read centrally, in particular for amyloid-related imaging abnormalities (ARIAs) due to parenchymal vasogenic edema (ARIA-E) or microhemorrhages or subarachnoid hemorrhage (ARIA-H).
Participants were instructed to record details of injection-related reactions (local and systemic) in a diary for at least 7 days after each injection.
2.3.2. Aβ-specific immune response
Aβ- and Qβ–Immunoglobuline G (IgG) titers were measured in serum at screening and scheduled visits using validated enzyme-linked immunosorbent assay (ELISA) methods [9], [11]. Titer levels were expressed in units relative to a reference polyclonal serum. Testing of selected serum samples indicated 10 units of Aβ IgG to roughly correspond to 1:100 in OD50. The lower limit of quantification (LLOQ) was 8.93 units for Aβ IgG and 1.04 units for Qβ IgG [9].
Per protocol, patients treated with CAD106 were classified as serological responders (SRs), strong serological responders (SSRs), or serological nonresponders (NRs) based on serum Aβ-IgG titers based on the following a priori definitions. The threshold for serological response was set to 16 units, based on the LLOQ of serum Aβ-IgG titers plus 3 times the standard deviation (SD). SRs were defined as patients whose serum Aβ-IgG titer was both >16 units between the second and the third injection and >26.8 units (3*LLOQ) after the third injection. SSRs were defined as patients whose Aβ-IgG titer was >35.7 units (4*LLOQ) after ≥2 different injections (not necessarily consecutive) starting from the second injection onwards. The requirement to have titers above the threshold at two distinct time-points enabled responder definition to take into account also the persistence of the antibody response. CAD106-treated patients who did not fulfill the SR criteria were classified as NR.
2.4. Assessments of secondary outcome measures
The IFN-γ ELISPOT assay has been developed to measure the Aβ-specific T-cell response in human clinical trials and to detect low-level responses [12]. T-cell responses to the Aβ1–6 and Aβ1–42 peptides, and the Qβ protein (positive control) were centrally assessed at screening and week 8 in PBMC samples. Every sample was stimulated with phytohemagglutinin as a positive control for cell viability, and in addition, a study-independent Qβ-positive PBMC control sample was used at every run.
Levels of plasma Aβ1–40 were measured using a meso scale discovery (MSD)-based assay to assess the quality of the produced Aβ-specific IgGs [11].
CSF samples were collected in all subjects at screening and week 38, and optionally at week 78. Aβ1–40 and Aβ1–42 concentrations were determined centrally using an MSD-based sandwich assay multiplex protein array platform, and total tau and phospho-tau by ELISA (Innogenetics, Belgium).
2.5. Assessments of exploratory outcome measures
From the T1-weighted volumetric scans, the whole-brain cerebral volume and hippocampal, cortical, and ventricular volumes were determined using NeuroQuant software (CorTechs Labs). For the PET substudy, 18F-florbetapir scans (target injected activity 260 MBq) were performed at screening, and weeks 40 and 78. Image analysis blinded to treatment allocation was performed by Avid Radiopharmaceuticals Inc. using previously described methodology [13]. The global cortical region (average of six regions) using the cerebellum as a reference region was used for interpretation of results.
Changes in clinical status were assessed using Alzheimer's Disease Assessment Scale–Cognitive Subscale (including delayed recall), Alzheimer's disease Cooperative Study–Activities of Daily Living, Clinical Dementia Rating scale, MMSE, and Neuropsychiatric Inventory Questionnaire. In addition, Category Fluency Test–animals, Controlled Oral Word Association Test–letters, and Computerized tests (CogState) were used (data not shown).
2.6. Sample size
A total of 120 patients (cohort 1, n = 80; cohort 2, n = 40) were planned to be enrolled, based on a sample size needed to detect an increased risk of meningoencephalitis. The probability of detecting ≥1 cases of meningoencephalitis in patients was 98.7% across both cohorts, assuming a true meningoencephalitis incidence rate of 6% as observed in the AN1792 study [2].
In addition, the size of each individual treatment arm (n = 20) was chosen to detect differences in the mean area under the curve (AUC) up to week 20 of Aβ-IgG titers across the treatment arms with each of the adjuvants in cohort 1.
2.7. Randomization and masking
Eligible patients were randomized to one treatment arm via an interactive voice response system. CAD106 and adjuvant doses were prepared by an unblinded pharmacist and administered by an unblinded study nurse. Patients, investigators, and the site personnel were masked to the treatment assigned.
2.8. Statistical analysis
All analyses supporting the primary and secondary objectives for tolerability, immunogenicity, antibody and T-cell response, and safety were performed on the safety analysis set (SAF), which consisted of patients who received ≥1 injection of study medication and had ≥1 post-injection safety assessment. The full analysis set (FAS) consisted of all patients who received ≥1 injection of study medication and had ≥1 post-injection antibody titer measurement. CSF biomarkers and all exploratory objectives (e.g., volumetric MRI, amyloid PET, and clinical scales) were analyzed based on the biomarker positive analysis set (BPAS), which consisted of patients who had baseline Aβ1–42 in CSF <500 pg/mL and, for patients in the PET substudy, PET SUVR >1.1. One patient was excluded from the FAS and BPAS due to a subdural hemorrhage which interfered with the assessments.
The CAD106 dose groups were pooled across the adjuvant and nonadjuvant treatment arms (CAD106 150 μg, CAD106 450 μg, CAD106 total, placebo) for the reporting of all primary safety assessments and secondary and exploratory outcomes. For Aβ-IgG titers and injection-related reactions, data were summarized by the nine originally assigned treatment arms. Aβ-IgG titers were characterized by the AUC for different time intervals depending on the purpose of the analysis.
As per the a priori statistical plan, the main between-group comparisons of exploratory measures were performed between SSRs versus controls (placebo recipients plus CAD106 NRs) based on the assumption that the response to treatment would be driven by the serological response. Exploratory variables were analyzed in the BPAS contrasting SSRs to controls by a mixed-effects repeated-measures model for the change or the percentage change from baseline. In addition, where applicable, the relation to Aβ-IgG titers (assessed by the AUC) from baseline to week 78 was investigated using the Spearman correlation coefficient for subjects treated with CAD106.
3. Results
3.1. Patients
Between March 2010 and March 2011, 177 patients were screened in 30 centers in 10 countries across Europe, Switzerland, Canada, and the USA; 121 patients were randomized (cohort 1, initiated in March 2010: n = 79; cohort 2, initiated in January 2011: n = 42). In total, 106 patients received CAD106 (150 μg [n = 69]; 450 μg [n = 37]) and 15 patients received placebo (Fig. 1B).
In the CAD106 treatment group, 14 (13 %) patients were actively discontinued from the study by the sponsor for not meeting serological responder criteria. In the placebo group, four patients (27%) were discontinued for the same reason to maintain the blinding (Fig. 1B).
All patients were included in the SAF. One patient randomized to CAD106 450 μg was excluded from the pharmacodynamic exploratory analyses because the imaging end-points could not be evaluated due to subdural hemorrhage (SDH) as a result of head trauma followed by an intracerebral hemorrhage (ICH) after surgical evacuation of the SDH.
Demographic and baseline characteristics were broadly similar across the CAD106 total and placebo groups (Table 1). In patients who underwent pharmacogenetic testing (101/121 patients), the majority in the CAD106 total (69.8%) and placebo group (60.0%) were APOE ε4 carriers.
Table 1.
Patient demographic and baseline characteristics (SAF)
| Characteristic | CAD106 150 μg (n = 69) | CAD106 450 μg (n = 37) | CAD106 total (n = 106) | Placebo (n = 15) |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Male | 37 (53.6) | 13 (35.1) | 50 (47.2) | 7 (46.7) |
| Female | 32 (46.4) | 24 (64.9) | 56 (52.8) | 8 (53.3) |
| Age, years | ||||
| Mean (SD) | 67.7 (9.0) | 66.3 (9.4) | 67.2 (9.1) | 68.0 (8.4) |
| Age group, n (%) | ||||
| <65 | 26 (37.7) | 13 (35.1) | 39 (36.8) | 5 (33.3) |
| 65–75 | 27 (39.1) | 17 (45.9) | 44 (41.5) | 6 (40.0) |
| >75 | 16 (23.2) | 7 (18.9) | 23 (21.7) | 4 (26.7) |
| Race, n (%) | ||||
| Caucasian | 67 (97.1) | 37 (100.0) | 104 (98.1) | 14 (93.3) |
| Asian | 1 (1.4) | 0 | 1 (0.9) | 1 (6.7) |
| Other | 1 (1.4) | 0 | 1 (0.9) | 0 |
| Years of education | ||||
| Mean (SD) | 12.3 (3.9) | 12.4 (5.1) | 12.3 (4.3) | 12.9 (5.4) |
| Baseline MHIS, n (%) | ||||
| 0 | 37 (53.6) | 25 (67.6) | 62 (58.5) | 8 (53.3) |
| 1 | 25 (36.2) | 11 (29.7) | 36 (34.0) | 6 (40.0) |
| 2 | 6 (8.7) | 0 | 6 (5.7) | 1 (6.7) |
| 3 | 1 (1.4) | 1 (2.7) | 2 (1.9) | 0 |
| Baseline MMSE | ||||
| Mean (SD) | 22.8 (2.2) | 23.2 (2.2) | 22.1 (2.2) | 22.9 (1.9) |
| Time since first AD symptom was noticed by patient/caregiver (years) | ||||
| Mean (SD) | 4.1 (2.6) | 3.9 (2.2) | 4.0 (2.5) | 3.8 (3.5) |
| Median (range) | 4 (1–12) | 4 (1–10) | 4 (1–12) | 3 (1–15) |
| Time since first AD symptom was diagnosed by physician (years) | ||||
| Mean (SD) | 1.6 (1.5) | 1.5 (1.3) | 1.6 (1.5) | 1.9 (2.8) |
| Median (range) | 1 (0–8) | 1 (0–5) | 1 (0–8) | 1 (0–11) |
| APOE e4 carrier status, n (%) | ||||
| Missing | 8 | 12 | 20 | 0 |
| Non ε4∗ | 18 (29.5) | 8 (32.0) | 26 (30.2) | 6 (40.0) |
| One ε4 allele∗ | 29 (47.5) | 15 (60.0) | 44 (51.2) | 5 (33.3) |
| Two ε4 alleles∗ | 14 (23.0) | 2 (8.0) | 16 (18.6) | 4 (26.7) |
Abbreviations: SAF, safety analysis set; SD, standard deviation; MHIS, Modified Hachinski Ischemic Score; MMSE, Mini–Mental State Examination; AD, Alzheimer's disease; APOE ε4, apolipoprotein E ε4 allele.
Percentage based on the number of patients genotyped.
3.2. Primary outcome measures
3.2.1. Safety
All patients received one or more injection of CAD106 or placebo, with the majority (79.2% CAD106 and 60.0% placebo) of patients receiving five or more of seven scheduled injections. The study was put on temporary hold, and CAD106 injections were suspended in all patients after a case of SDH with ICH was reported (described previously). After evaluation, it was concluded that this event was not related to CAD106 and injections resumed after 2–6 months, depending on country and site. Another two patients (CAD106 150 μg, n = 1; CAD106 450 μg, n = 1) experienced SDH associated with trauma.
Three deaths (malignant mesothelioma, n = 1; laryngeal cancer, n = 1; pneumonia, n = 1) were reported during the study, all in the CAD106 total group (Table 2); none were assessed as related to CAD106 by investigators.
Table 2.
Summary of adverse events and MRI findings (SAF)
| n (%) | CAD106 150 μg (n = 69) | CAD106 450 μg (n = 37) | CAD106 total (n = 106) | Placebo (n = 15) |
|---|---|---|---|---|
| Summary of adverse events | ||||
| Deaths∗ | 2 (2.9) | 1 (2.7) | 3 (2.8) | 0 |
| SAEs | 18 (26.1) | 8 (21.6) | 26 (24.5) | 1 (6.7) |
| Discontinuations due to SAEs | 3 (4.3) | 2 (5.4) | 5 (4.7)† | 0 |
| Discontinuations due to AEs | 6 (8.7) | 2 (5.4) | 8 (7.5)‡ | 0 |
| Most frequent AEs (>10% of patients in either treatment group) | ||||
| Headache | 10 (14.5) | 7 (18.9) | 17 (16.0) | 1 (6.7) |
| Nasopharyngitis | 10 (14.5) | 6 (16.2) | 16 (15.1) | 2 (13.3) |
| Pyrexia | 7 (10.1) | 4 (10.8) | 11 (10.4) | 0 |
| Hypertension | 7 (10.1) | 4 (10.8) | 11 (10.4) | 0 |
| Back pain | 7 (10.1) | 3 (8.1) | 10 (9.4) | 0 |
| Insomnia | 7 (10.1) | 2 (5.4) | 9 (8.5) | 0 |
| Urinary tract infection | 6 (8.7) | 3 (8.1) | 9 (8.5) | 2 (13.3) |
| Fall | 5 (7.2) | 4 (10.8) | 9 (8.5) | 2 (13.3) |
| Depression | 4 (5.8) | 5 (13.5) | 9 (8.5) | 1 (6.7) |
| Fatigue | 6 (8.7) | 2 (5.4) | 8 (7.5) | 2 (13.3) |
| Osteoarthritis | 7 (10.1) | 0 | 7 (6.6) | 0 |
| Arthralgia | 5 (7.2) | 1 (2.7) | 6 (5.7) | 2 (13.3) |
| Aggression | 4 (5.8) | 1 (2.7) | 5 (4.7) | 2 (13.3) |
| Cough | 3 (4.3) | 2 (5.4) | 5 (4.7) | 2 (13.3) |
| Agitation | 2 (2.9) | 1 (2.7) | 3 (2.8) | 2 (13.3) |
| Anxiety | 1 (1.4) | 1 (2.7) | 2 (1.9) | 3 (20.0) |
| Decreased weight | 1 (1.4) | 0 | 1 (0.9) | 2 (13.3) |
| Summary of MRI findings | ||||
| ARIA-E | 0 | 1 (2.7) | 1 (0.9) | 0 |
| ARIA-H | 5 (7.2) | 0 | 5 (4.7) | 0 |
| ≥2 microhemorrhages | 4 (5.8)§ | 0 | 4 (3.8)§ | 0 |
| Subarachnoid hemorrhage/superficial hemosiderosis | 1 (1.4) | 0 | 1 (0.9) | 0 |
| Intraparenchymal hemorrhage | 0 | 1 (2.7) | 1 (0.9) | 0 |
| Epidural or subdural hemorrhage‖ | 0 | 2 (5.4)¶ | 2 (1.9) | 0 |
| Ischemic stroke | 1 (1.4) | 0 | 1 (0.9) | 0 |
| White-matter disease worsening | 2 (2.9) | 0 | 2 (1.9) | 0 |
Abbreviations: MRI, magnetic resonance imaging; SAF, safety analysis set; SAE, serious adverse event; AE, adverse event; ARIA, amyloid-related imaging abnormalities, with isolated vasogenic edema or sulcal effusions (ARIA-E)/with microhemorrhages or superficial hemosiderosis (ARIA-H).
Two patients died soon after discontinuation due to SAEs (malignant mesothelioma due to chronic asbestosis and laryngeal carcinoma, respectively). In both cases, the PI classified the SAE as unrelated.
One case each of atrial fibrillation (CAD106 150 μg), subdural hemorrhage (CAD106 450 μg), malignant mesothelioma (CAD106 150 μg), laryngeal cancer (CAD106 450 μg), and lobar pneumonia (CAD106 150 μg). The latter three resulted in death.
In addition to the SAEs mentioned previously, the remaining AEs included one case each of ARIA-H, one case of aggression and irritability, and one case with worsening of AD, all occurring in the CAD106 150 μg group.
Three patients were discontinued from the study as per protocol with various reasons recorded (n = 1 due to a microhemorrhage recorded as an AE, n = 1 due to microhemorrhage as part of an abnormal test procedure [MRI], n = 1 withdrew consent). For one patient, the microhemorrhages were detected retrospectively at the end of the study during the data cleaning process.
Hemorrhage included subdural hematoma, epidural hematoma, subarachnoid hematoma, and parenchymal hemorrhage.
Includes an SAE of subdural hemorrhage that resulted in study discontinuation and an SAE of subdural hematoma.
Serious adverse events (SAEs) were reported in 24.5% (95% confidence interval [CI] 16.7–33.8) of patients in the CAD106 total group versus 6.7% (95% CI 0.2–31.9) for placebo (Supplementary Table 2).
Most of the SAEs were reported only in single subjects. Three of the 26 SAEs in the CAD106 total group (allergic dermatitis [immediately after the fourth injection], atrial fibrillation [6 weeks after first injection], and acute psychosis [14 weeks after seventh injection]) were deemed potentially related to CAD106 by investigators.
The incidence of AEs was similar between the CAD106 total and placebo groups (83.0% vs. 80.0%). Most AEs were mild to moderate in severity. The most commonly reported AEs in the CAD106 total group (with large numerical imbalances considering the 7:1 randomization ratio) were headache, hypertension, and pyrexia (Table 2). AEs did not appear to be CAD106 dose dependent.
Among the CAD106-treated patients, 10 discontinued treatment (9.4% [4.6–16.7]) compared with none for placebo (0% [0.0–21.8]). Reasons for withdrawal were AEs (n = 7) or ARIA-H (n = 4). None were assessed as being related to CAD106 by the investigator except for one case of ARIA-H and the case of atrial fibrillation (described previously).
MRI findings compatible with ARIA were detected at scheduled scans in six CAD106-treated patients, with ARIA-H being confirmed in five patients in the CAD106 150 μg group and ARIA-E in a single patient on CAD106 450 μg (Table 2). All ARIA-H cases of more than two new cerebral microhemorrhages and one case of subarachnoid hemorrhage led to treatment discontinuation. ARIA-E was initially observed at the scheduled MRI scan at week 36 in a cortical location in a patient after the first three injections of CAD106 450 μg without adjuvant (Supplementary Fig. 1). Following spontaneous resolution, the patient received the last two CAD106 450 μg injections at weeks 48 and 60 as per protocol, and the MRI finding did not reoccur. All patients with ARIA-H or ARIA-E were asymptomatic and all were SSRs.
There were no symptoms or signs of CNS inflammation as assessed by MRI or CSF parameters and no consistent clinically relevant changes in vital signs, safety laboratory measurements, and electrocardiogram parameters.
The majority of patients reported, by diary, one or more injection-related reactions (>73%) mostly in the CAD106-treatment group (Supplementary Table 3). The highest incidence of clinically relevant reactions, such as local reactions, chills, myalgia, arthralgia, fatigue, and fever, was observed in the CAD106 450 μg without adjuvant arm. The addition of alum to CAD106 450 μg resulted in an unexpected decrease in frequency of most injection-related systemic reactions compared with CAD106 450 μg without adjuvant (Supplementary Table 3).
3.2.2. Immune response
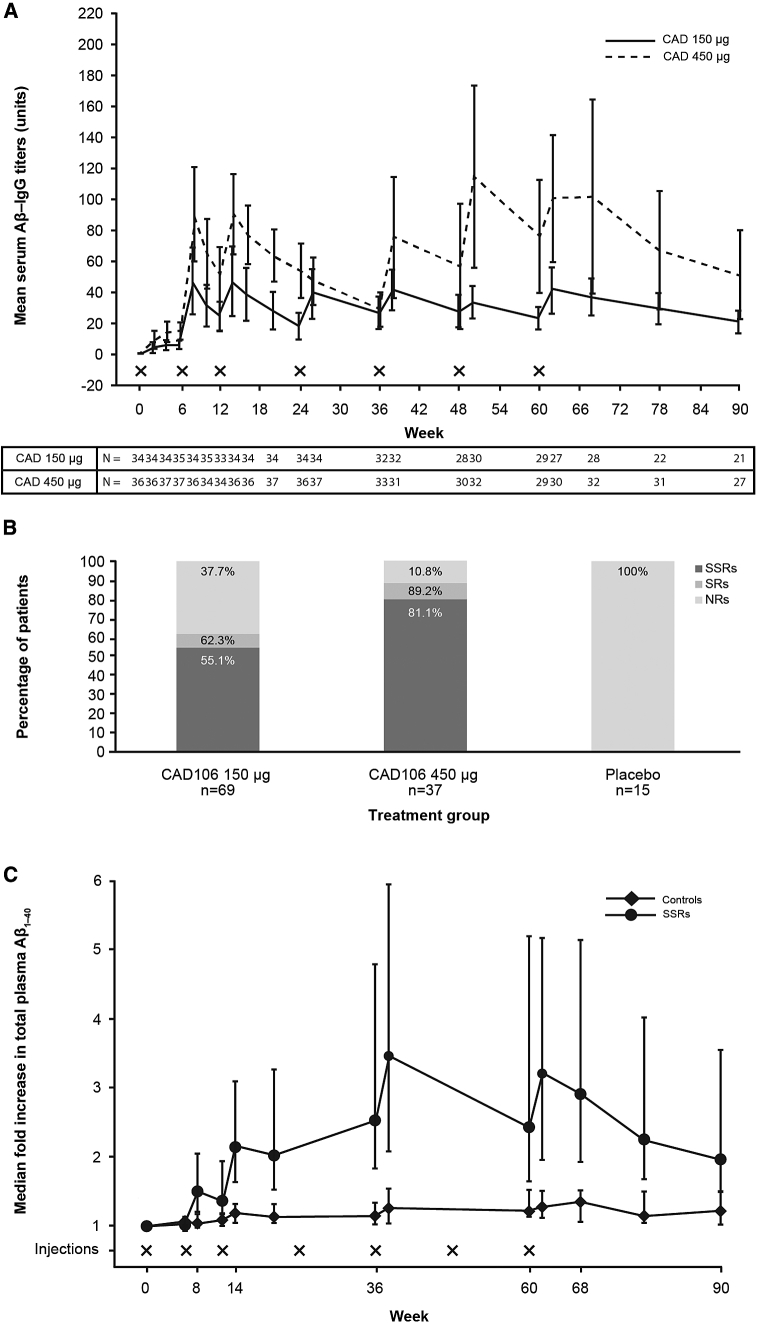
Aβ-IgG was not detected in serum at baseline or in placebo-treated patients. Reproducible and sustained Aβ-IgG responses were observed following the second to the seventh injections (Fig. 2A). Over the initial 20 weeks, the median AUC of Aβ-IgG titers increased by 1.86-fold upon the 3-fold CAD106 dose increase from 150 μg to 450 μg (P = .0010). CAD106 induced a strong Aβ-IgG response (SSR) in 55.1% and 81.1% of the patients on 150 μg and 450 μg doses, respectively, versus none on placebo (Fig. 2B). An increase in mean serum Qβ IgG titers was also observed with the higher dose (Supplementary Fig. 2).
Fig. 2.
(A) Mean serum Aβ-IgG titers (±95% CI) by week (SAF); CAD106 150 μg group only includes patients who received the randomized dose of 150 μg throughout the study and did not switch to the higher dose of 450 μg; X denotes the time of the injection. The apparent decline in titers from weeks 24–36 with CAD106 450 mg was attributed to the temporary suspension of immunizations when the majority of patients missed the fourth injection in cohort 2. (B) Proportion of patients meeting the responder criteria by CAD106 dose group (SAF); includes serological nonresponder (NR), serological responder (SR), and strong serological responder (SSR). (C) Median and upper/lower quartile fold increase in total plasma Aβ1–40, by visit (SAF); patient with subdural hemorrhage (SDH) was excluded; controls include both placebo and NR. Abbreviations: Aβ, amyloid beta; CI, confidence interval; NR, nonresponder; SAF, safety analysis set; SR, serological responder; SSR, strong serological responder.
Neither of the adjuvants used in this study at any of the dosages tested led to a significant increase in antibody titers. Alum was selected for cohort 2 because the dose could be further increased to 450 μg and was then shown, when added to CAD106 450 μg, to improve tolerability (see Supplementary Table 3).
3.3. Secondary and exploratory outcome measures
3.3.1. Characterization of the immune response
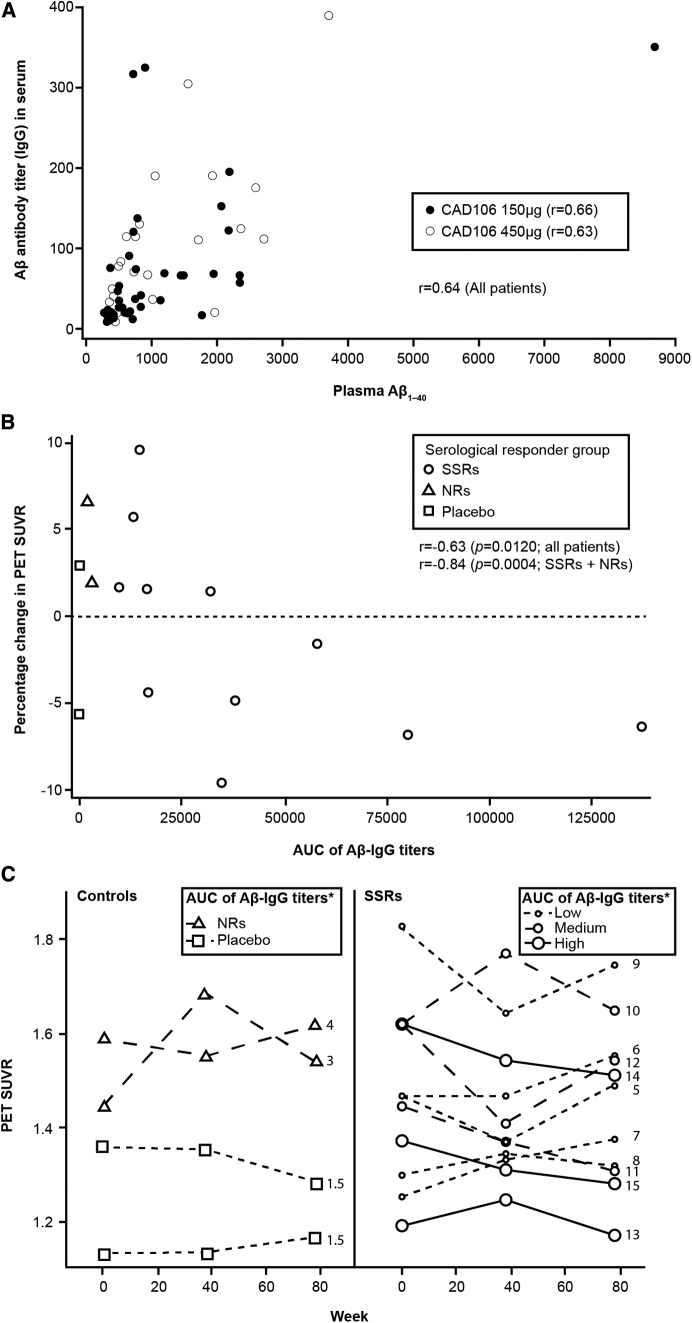
A two- to three-fold increase in median total plasma Aβ1–40 levels was observed in the SSRs over time, whereas no change was observed in controls (Fig. 2C). In the CAD106 total group, a positive linear relationship between plasma Aβ1–40 levels and Aβ-IgG titers was observed, for example, at week 62, 2 weeks after the last injection (r = 0.79) (Fig. 3A).
Fig. 3.
(A) Plasma Aβ1–40 versus Aβ-IgG titers at week 62; r = 0.63 for the total population. (B) Percentage change in PET global cortical SUVR versus AUC of serum Aβ-IgG titers up to week 78 for individual patients (BPAS); the data points show the 15 patients who received a longitudinal amyloid PET. Among those who underwent longitudinal amyloid PET, there were no SR cases. (C) Global cortical SUVR measured by PET from baseline to week 78 for individual patients by serological responder groups (BPAS)*. The number on the plots indicates the rank of the Aβ-IgG titers AUC as shown on part label B. Abbreviations: Aβ, amyloid beta; r, spearman correlation coefficient; AUC, area under the curve; BPAS, biomarker positive analysis set; NR, nonresponder; PET, positron emission tomography; SSR, strong serological responder; SUVR, standardized uptake value ratio.
Similar to previous studies, Aβ-specific T-cell responses to Aβ1–6 or Aβ1–42 peptides were not observed in either treatment group. Using the Qβ carrier peptide as positive control for CAD106 immune response, the expected response against the carrier peptide was observed after 8 weeks in the actively treated group (Supplementary Table 4).
3.3.2. CNS biomarkers
A total of 26 patients from cohort 2 participated in the amyloid PET substudy, of whom 20 (14 SSRs, and six controls [three NRs and three placebo]; no patients were SRs) were included in the BPAS for the PET substudy, with 15 patients (11 SSRs and four controls [two NRs and two placebo]) undergoing PET scans up to week 78.
The baseline mean (SD) SUVR of the composite cortical region did not differ between SSRs and controls (11 SSRs, 1.47 [0.189] and four controls, 1.38 [0.192], Supplementary Table 5).
A significant correlation between percentage change in amyloid PET from baseline to week 78 and the AUC of serum Aβ-IgG titers was observed within the 13 (11 SSRs and 2 NRs) CAD106 treated patients (r = −0.84, P = .0004). When the two placebo patients were included (no serum Aβ-IgG titers, AUC of 0), the correlation remained significant (r = −0.63, P = .0120; Fig. 3B). A longitudinal decrease in amyloid PET SUVR was observed in SSRs but not in controls (mean percentage change [SD] from baseline to week 78 was −1.23% [5.805%] for SSRs and +1.44% [5.129%] for controls [Supplementary Table 5]). Individual PET SUVR measures over time are presented according to the patients' rank in terms of the AUC of serum Aβ-IgG titers (Fig. 3C). A decrease of amyloid load in the 30% of the SSRs with a high AUC was observed compared to those with medium or low AUCs.
The volumetric MRI results indicated a larger percentage decrease in cortical gray-matter volume from baseline to week 78 in SSRs (n = 41) versus controls (n = 22; P = .0077; Supplementary Table 6). The percentage change from baseline did not correlate with the AUC of serum Aβ IgG (r = −0.20; P = .1456) (Supplementary Table 6). No significant between-group differences were observed in the other regions, that is, whole brain, hippocampus, and ventricles.
The longitudinal change in CSF biomarkers (phospho-tau, total tau, Aβ1–40, or Aβ1–42) over the 78-week period did not differ between the SSRs versus controls (Supplementary Table 7).
3.3.3. Clinical assessment
All clinical assessments worsened over time in all groups indicating disease progression. No statistically significant difference was observed between SSRs versus controls for the change from baseline in any clinical scale (uncorrected P > .05; BPAS, Supplementary Table 8). Numerically, the decline in MMSE over 78 weeks was more pronounced in the SSR group (−4.93, n = 39) than in the control group (−2.91, n = 14), with a between-group difference at week 78 of −2.02 (95% CI −4.06, 0.02). Changes in clinical scales did not correlate to AUC of serum Aβ-IgG titers (r < ±0.2). For instance, the correlation coefficient between serum Aβ-IgG titers and the MMSE change from baseline to week 78 was r = −0.11 (P = .4254).
Summary statistics for the FAS by treatment (CAD106 total and placebo) are presented in Supplementary Tables 9–11.
4. Discussion
This phase 2 study in mild AD met both primary objectives. CAD106 demonstrated an acceptable safety and tolerability profile, while evoking a strong serological response in up to 81% of patients who received CAD106 450 μg.
CAD106 avoids activation of Aβ-reactive T-cells by using a small N-terminal Aβ1–6 peptide. No occurrences of meningoencephalitis, autoimmune disease, CNS inflammation, or Aβ-specific T-cell responses were observed. The incidence of SAEs, headache, hypertension, and pyrexia was higher in the CAD106 total group versus the placebo group. SAEs in the placebo group (6.7%, 95% CI 0.2%, 31.9%) were less frequent than expected based on the incidence observed in placebo groups from similar but much larger trials (19.9% and 20.6% [7]). In contrast with previous CAD106 studies, several cases of ARIA occurred in the treated group, and these individuals had all developed a strong serological response. Reports of ARIA may be related to the higher CAD106 dose (450 μg) or adjuvants used in the present study compared to previous studies (CAD106 doses of 50 and 150 μg without adjuvant), or the higher number of subjects in this trial, all undergoing scheduled T2* MRI sequence required to detect ARIA-H. All six cases were detected at scheduled MRI scans and all were asymptomatic. Patients with more than one or two (depending on MRI field strength) microhemorrhages (a known risk factor for ARIA) were excluded from the study, which may have limited new treatment–emergent ARIA.
Reproducible and sustained antibody responses were observed from the second injection in a dose-dependent manner [9]. The antibody response was measured in units in relation to a reference polyclonal rhesus serum, rather than in titer units calculated based on serial dilutions of tested samples as often done for other vaccines (such as AN1792). The approach used in the current study allowed for efficient quantification of a large number of samples in the course of CAD106 development. A direct comparison of the immune response to that evoked by other vaccines with respect to level of antibody titers or number of responders is not warranted as it does not take into account the difference in the quality (e.g., epitopes, affinity, IgG subtypes) of the induced antibodies. For CAD106, the serological responder criteria were set with the aim to identify subjects with persistent and high antibody response. Only SSRs showed an increase in total plasma Aβ1–40, an indicator of peripheral biological activity of the antibodies. This suggests that the criteria for defining the responder groups were set appropriately.
Doubling of antibody titers was observed following an increase of dose of CAD106 from 150 to 450 μg, but no increase was seen following addition of either adjuvants alum or MF59. However, tolerability was improved when alum was added to CAD106 450 μg. Alum is well established as an adjuvant in prophylactic vaccines with no serious safety issues [14]. Based on tolerability and immunogenicity, CAD106 450 μg with alum 450 μg was deemed the best combination to induce optimal Aβ-IgG titers with an acceptable tolerability profile which led to the selection of this CAD106 dose and adjuvant for future studies.
In an exploratory analysis in a subgroup of patients who underwent 18F-florbetapir PET, the percentage change in PET SUVR correlated inversely with Aβ-IgG titers over the 78-week period. However, a direct comparison between the SSR and the NR/placebo groups did not yield a significant difference. The difference between the correlational and the categorical analysis can be explained by the wide spread of antibody titers within the SSR group and the small number of subjects in the NR/placebo group (n = 4).
For the brain volumetric measures, a statistically significant decrease in cortical gray-matter volumes was observed in biomarker-positive SSRs versus controls. An apparent decrease in brain volume has already been reported for two other anti-amyloid immunotherapies, AN1792 [15] and bapineuzumab [7]. The interpretation of the decrease critically depends on whether it results from a volume reduction in the interstitial, neuronal, glial, or any other constituent. Although the antibody response against Aβ correlated with the decrease in amyloid load, there was no such relation with the decrease in cerebral volume. Hence, the absence of correlation makes a direct causal effect of the antibody response unlikely. The changes in cortical volume in CAD106 SSRs could theoretically have affected the longitudinal measure of change in amyloid load (SUVR) but this also requires further investigation.
Numerically, the decline in MMSE was larger in the biomarker-positive SSRs compared to controls. However, this did not reach statistical significance and was not supported by results in any of the other clinical scales or correlations to antibody titers. In addition, the small total sample size, and specifically the small control group (n = 14), and the imbalance between the two groups implicate that the numerical difference is of no significance. When the actively treated group was compared to the placebo group in the full analysis set, the longitudinal change in MMSE (−3.5) was identical between groups (Supplementary Table 11).
This phase 2 study has limitations. The study design was complicated by the fact that the study was carried out in two semi-overlapping cohorts, where CAD106 was administered with or without an adjuvant (alum or MF59). Furthermore, the study was put on a temporary clinical hold for 2–6 months due to the occurrence of one unrelated case of intracerebral hemorrhage. This temporary hold has impacted the dosing regimen for some patients in cohort 2 who did not receive the fourth injection. Finally, patients who did not develop a significant antibody response, as well as matching placebo patients, were withdrawn from the study per protocol. All these factors render the results more difficult to interpret. As a further limitation, amyloid PET SUVR, brain volume, and clinical scale variables were exploratory end points, not powered for significance or adjusted for multiplicity. The small sample size limits the sensitivity for detecting a biological effect and prevents robust conclusions from being made. However, the reduction of amyloid PET SUVR observed in this study is consistent with CNS activity for induced antibodies and the expected mode of action of CAD106.
In conclusion, this phase 2 study met its primary objectives: CAD106 elicited an Aβ-specific immune response with CNS activity and an acceptable safety profile. Amyloid PET provided preliminary evidence suggestive of target engagement. The relationship between CAD106-induced Aβ-IgG antibody titers and clinical efficacy needs to be determined in larger studies, possibly targeting an earlier stage of the disease.
Research in Context.
-
1.
Systematic review: We searched PubMed, with the terms “Alzheimer,” “immunotherapy” or “immunization,” and “clinical trial.” The AN1792 trial was aborted because of meningoencephalitis. The CAD106 phase-1 and phase 2a studies showed favorable safety and immunogenicity. A search of clinicaltrials.gov yielded four other terminated (ACC-001, AFFITOPE AD-02) or ongoing (ACI-24, AF20513) active Aβ immunization trials. Of the passive Aβ immunization trials in AD, none reached primary efficacy end points.
-
2.
Interpretation: The current phase 2b study had a larger sample size and included a higher dose than any of the previous CAD106 studies: the study drug evoked a strong Aβ-specific antibody response in a dose-dependent manner in 55%–81% of patients. Correlation between antibody response and longitudinal change in amyloid load provides preliminary evidence for target engagement.
-
3.
Future directions: A larger-sized phase-3 trial in earlier stages of the disease will evaluate potential clinical benefit and replicability of effect on amyloid load.
Acknowledgments
The authors would like to thank all the patients and investigators who participated in the study, including Dr. N.D. Prins, Dr. S. Cohen, Prof. P. De Deyn, Dr. Z. Nasreddine, Dr. A. Robillard, Dr. D. Bittner, Dr. K.-C. Steinwachs, Prof. C. Caltagirone, Prof. E.A. Scarpini, Prof. M. Tabaton, Prof. S. Sorbi, Prof. G. Bruno, Dr. D. Årsland, Dr. M. Fernandez, Dr. R. Blesa, Dr. E. Balaguer, Dr. N. Andreasen, Dr. M. Jonsson, Prof. R. Kressig, Dr. H. Pihan, Dr. G. Alva, Dr. H. Schwartz, Dr. A. Zacharias, and Dr. J. Ross. In addition, the authors would like to thank Sandrine Kretz, Nathalie Laurent, Igor Vostiar, and Alessandra Vitaliti for performing specific laboratory assessments throughout the study, and Avid Radiopharmaceuticals Inc. for providing control data for the PET imaging analysis. The authors thank Emma Burke, of iMed Comms, an Ashfield Company, part of UDG Healthcare plc, for medical writing assistance with this article, which was funded by Novartis Pharma AG.
R.V.'s institution (UZ Leuven) received funding for conducting the present study from Novartis and has clinical trial agreements with Merck, Forum, Biogen, Eli Lilly, and Roche. UZ Leuven has also received consultancy fees from GEHC, grants from Janssen Pharmaceutica NV, and consultancy fees or honoraria from Novartis. M.F. has received grants or research support from Accera, Biogen, Boehringer Ingelheim, Chase Pharmaceuticals, Eisai, Eli Lilly, Genentech, Lundbeck, MedAvante/AstraZeneca, Navidea, and Roche. M.F. has participated in consultancy, advisory boards or DSMB boards for Accera, Alltech, Avanir, Axovant Sciences, Biogen, Eisai, Eli Lilly, EnVivo Pharmaceuticals, FORUM Pharmaceuticals, Genentech, Inc., Grifols, Helicon, Inc. Research, Lundbeck, MedAvante, Medivation Inc., Medtronic, Merck and Co. Inc., Neurotrope Biosciences, Novartis, Pfizer, Prana Biotech, QR Pharma, Riovant Sciences Inc., Roche, Sanofi-Aventis, Schering-Plough, Takeda, Toyama Chemical, and UCB Pharma. M.F. has also participated in speaker's bureaux for Eisai, Pfizer, Forest, Eli Lilly, and Novartis. G.M. has participated in advisory boards for Merck and Eli Lilly. R.S.V.'s institution (Hospital Clinic) received funding for the present study from Novartis. P.S.'s institution (VU University Medical Centre, Amsterdam) received funding for the present study from Novartis, a grant from Piramal Imaging, advisory board fees from Novartis, and principal investigator fees from Probiodrug and EIP Pharma.
J.S. and R.P.M. are employees of Novartis Pharma AG, and M.E.R., A.G., and A.C. are employees of and shareholders in Novartis Pharma AG. J.M.R. is an employee of and shareholder in Novartis Pharmaceuticals. M.E.R. has a US patent application pending covering a pharmaceutical formulation containing CAD106 and an adjuvant and its use in Aβ immunotherapy. A.G. has a US patent covering CAD106 and its use in Aβ immunotherapy.
This research was sponsored by Novartis Pharma AG, Basel, Switzerland.
Authors' contributions: M.E.R., R.P.M., A.C., J.S., P.S., and A.G. made substantial contributions to the conception and design of the study. R.V., M.F., G.M., R.S.V., and P.S. contributed to the acquisition of data. R.V., M.E.R., A.C., J.S., M.F., G.M., P.S., J.M.R., and A.G. contributed to the analysis and interpretation of data. All authors contributed to drafting or critically revising the article for important intellectual content and provided approval of the final article to be published.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.trci.2016.12.003.
Supplementary data
References
- 1.Gilman S., Koller M., Black R.S., Jenkins L., Griffith S.G., Fox N.C. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005;64:1553–1562. doi: 10.1212/01.WNL.0000159740.16984.3C. [DOI] [PubMed] [Google Scholar]
- 2.Orgogozo J.M., Gilman S., Dartigues J.F., Laurent B., Puel M., Kirby L.C. Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology. 2003;61:46–54. doi: 10.1212/01.wnl.0000073623.84147.a8. [DOI] [PubMed] [Google Scholar]
- 3.Cribbs D.H., Ghochikyan A., Vasilevko V., Tran M., Petrushina I., Sadzikava N. Adjuvant-dependent modulation of Th1 and Th2 responses to immunization with beta-amyloid. Int Immunol. 2003;15:505–514. doi: 10.1093/intimm/dxg049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black R.S., Sperling R.A., Safirstein B., Motter R.N., Pallay A., Nichols A. A single ascending dose study of bapineuzumab in patients with Alzheimer disease. Alzheimer Dis Assoc Disord. 2010;24:198–203. doi: 10.1097/WAD.0b013e3181c53b00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siemers E.R., Friedrich S., Dean R.A., Gonzales C.R., Farlow M.R., Paul S.M. Safety and changes in plasma and cerebrospinal fluid amyloid beta after a single administration of an amyloid beta monoclonal antibody in subjects with Alzheimer disease. Clin Neuropharmacol. 2010;33:67–73. doi: 10.1097/WNF.0b013e3181cb577a. [DOI] [PubMed] [Google Scholar]
- 6.Winblad B., Graf A., Riviere M.E., Andreasen N., Ryan J.M. Active immunotherapy options for Alzheimer's disease. Alzheimers Res Ther. 2014;6:7. doi: 10.1186/alzrt237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salloway S., Sperling R., Fox N.C., Blennow K., Klunk W., Raskind M. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer's disease. N Engl J Med. 2014;370:322–333. doi: 10.1056/NEJMoa1304839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doody R.S., Thomas R.G., Farlow M., Iwatsubo T., Vellas B., Joffe S. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer's disease. N Engl J Med. 2014;370:311–321. doi: 10.1056/NEJMoa1312889. [DOI] [PubMed] [Google Scholar]
- 9.Winblad B., Andreasen N., Minthon L., Floesser A., Imbert G., Dumortier T. Safety, tolerability, and antibody response of active Aβ immunotherapy with CAD106 in patients with Alzheimer's disease: randomised, double-blind, placebo-controlled, first-in-human study. Lancet Neurol. 2012;11:597–604. doi: 10.1016/S1474-4422(12)70140-0. [DOI] [PubMed] [Google Scholar]
- 10.Wiessner C., Wiederhold K.H., Tissot A.C., Frey P., Danner S., Jacobson L.H. The second-generation active Abeta immunotherapy CAD106 reduces amyloid accumulation in APP transgenic mice while minimizing potential side effects. J Neurosci. 2011;31:9323–9331. doi: 10.1523/JNEUROSCI.0293-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farlow M.R., Andreasen N., Riviere M.E., Vostiar I., Vitaliti A., Sovago J. Long-term treatment with active Abeta immunotherapy with CAD106 in mild Alzheimer's disease. Alzheimers Res Ther. 2015;7:23. doi: 10.1186/s13195-015-0108-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karlsson A.C., Martin J.N., Younger S.R., Bredt B.M., Epling L., Ronquillo R. Comparison of the ELISPOT and cytokine flow cytometry assays for the enumeration of antigen-specific T cells. J Immunol Methods. 2003;283:141–153. doi: 10.1016/j.jim.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Joshi A.D., Pontecorvo M.J., Clark C.M., Carpenter A.P., Jennings D.L., Sadowsky C.H. Performance characteristics of amyloid PET with florbetapir F 18 in patients with Alzheimer's disease and cognitively normal subjects. J Nucl Med. 2012;53:378–384. doi: 10.2967/jnumed.111.090340. [DOI] [PubMed] [Google Scholar]
- 14.Baylor N.W., Egan W., Richman P. Aluminum salts in vaccines—US perspective. Vaccine. 2002;20:S18–S23. doi: 10.1016/s0264-410x(02)00166-4. [DOI] [PubMed] [Google Scholar]
- 15.Fox N.C., Black R.S., Gilman S., Rossor M.N., Griffith S.G., Jenkins L. Effects of Abeta immunization (AN1792) on MRI measures of cerebral volume in Alzheimer disease. Neurology. 2005;64:1563–1572. doi: 10.1212/01.WNL.0000159743.08996.99. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.