Abstract
Introduction
Amylin receptor serves as a portal for the expression of deleterious effects of amyloid β-protein (Aβ), a key pathologic hallmark of Alzheimer's disease. Previously, we showed that AC253, an amylin receptor antagonist, is neuroprotective against Aβ toxicity in vitro and abrogates Aβ-induced impairment of hippocampal long-term potentiation.
Methods
Amyloid precursor protein–overexpressing TgCRND8 mice received intracerebroventricularly AC253 for 5 months. New cyclized peptide cAC253 was synthesized and administered intraperitoneally three times a week for 10 weeks in the same mouse model. Cognitive functions were monitored, and pathologic changes were quantified biochemically and immunohistochemically.
Results
AC253, when administered intracerebroventricularly, improves spatial memory and learning, increases synaptic integrity, reduces microglial activation without discernible adverse effects in TgCRND8 mice. cAC253 demonstrates superior brain permeability, better proteolytic stability, and enhanced binding affinity to brain amylin receptors after a single intraperitoneal injection. Furthermore, cAC253 administered intraperitoneally also demonstrates improvement in spatial memory in TgCRND8 mice.
Discussion
Amylin receptor is a therapeutic target for Alzheimer's disease and represents a disease-modifying therapy for this condition.
Keywords: Amylin receptor, AC253 peptide, Alzheimer's disease, Amyloid β, In vivo imaging, Brain penetration
1. Introduction
Alzheimer's disease (AD) is the most common form of dementia [1], which is characterized by deposition of amyloid β-protein (Aβ) intracellularly and extracellularly within cortical and limbic brain structures that are critical for memory and cognitive functions [2], [3], [4]. A central question in AD research is the amyloid protein is a cause or a consequence of the disease, and at present, it appears that the likely answer is both [5]. Several lines of evidence strongly support a role for Aβ in the pathogenesis of AD: (1) AD is associated with inherited amyloid precursor protein (APP) mutations, (2) neurotoxicity of soluble oligomeric Aβ when applied to neurons, and (3) APP-overexpressing mice that recapitulate certain neuropathologic and behavioral features of AD [6], [7], [8], [9]. On the other hand, adverse events in clinical trials for AD using Aβ vaccine-based therapy and the subsequent failure of monoclonal antibody therapies and inhibitors of the Aβ-generating gamma-secretase enzyme in improving cognitive functions in patients [6] have forced a rethink of these approaches as disease-modifying treatment strategies in AD. Nonetheless, it is hard to imagine a definitive treatment for AD that will not serve to ameliorate in some form the neurotoxic effects of Aβ, because this is a key “upstream” event in AD pathogenesis (as established by alterations in cerebrospinal fluid (CSF) Aβ levels decades before clinical onset) [7].
Multiple receptors have been implicated in mediating Aβ disruption of neuronal and synaptic processes in AD and thus identified as potential targets for developing anti-Aβ therapies, although none have fulfilled this goal yet [8], [9]. Importantly, the amylin receptor, comprising heterodimers of the calcitonin receptor (CTR) with receptor activity-modifying proteins (RAMPs), serves as a portal for the expression of deleterious effects of Aβ and human amylin (hAmylin) [10]. Amylin is a 37-amino acid peptide hormone that is cosecreted with insulin by beta cells of the pancreas that control glucose levels in blood; however, amylin has the propensity to aggregate and form amyloid oligomers and fibrils in the pancreas in type 2 diabetes [11] and in AD brains [12]. Our prior studies show that hAmylin, like Aβ, causes dysfunction and death of neurons preferentially affected in AD [13], [14]. Furthermore, amylin receptor antagonists, such as AC253 (a 24-amino acid peptide), are neuroprotective against Aβ-induced toxicity [13], [15] and can reverse impairment of Aβ- or hAmylin-induced depression of hippocampal long-term potentiation (LTP), a cellular surrogate of memory [16]. Recently, two other groups have reported similar cognitive therapeutic benefits with pramlintide [17], [18], a synthetic nonamyloidogenic analog of amylin, and our recent findings have further confirmed that pramlintide acts in a manner similar to AC253 by attenuating Aβ- and hAmylin-induced depression of LTP [19].
Herein, we first tested the in vivo therapeutic efficacy of AC253 in an AD mouse model and show that chronic intracerebroventricular (icv) administration of the amylin receptor antagonist, AC253, improves learning and memory deficits in transgenic APP-overexpressing (TgCRND8) mice. These behavioral improvements coincided with an increase in synaptic-related proteins, including synapsin 1 and synaptophysin, along with a decrease in the microglia marker, Iba1. We further developed and tested a new disulfide head-to-tail cyclized version of the AC253 peptide (cAC253). cAC253 shows enhanced brain penetration after a single intraperitoneal (ip) injection and demonstrates improved proteolytic stability and pharmacokinetic behavior compared with its linear counterpart AC253. Importantly, using a panel of genetically engineered mice with different levels of amylin receptor expression, we show that systemically administered cAC253 is strongly localized to the hippocampal region, which coincides with the distribution of amylin receptors in the brain, and its concentration in the brain correlates with the expression levels of the amylin receptor in the brain. Finally, ip administration of cAC253 for 10 weeks attenuates cognitive decline and improves learning and memory impairments in TgCRND8 mice, which occurs in parallel with a reduction in cerebral Aβ deposits. Taken together, our findings identify the amylin receptor as a viable target for disease-modifying therapies in AD and that the amylin receptor antagonist, cAC253, is a new promising therapeutic candidate in AD patients.
2. Methods
2.1. In vivo mouse models and in vitro cell models
All in vivo experiments were carried out in accordance with the relevant laws and guidelines set by the Canadian Council for Animal Care and with the approval of the Animal Care Use Committee (Health Sciences) at the University of Alberta. For behavioral experiments, APP overexpressing transgenic (TgCRND8) and wild-type (Wt) littermate mice (male and female) were implanted with Alzet minipumps (Durect Co, Cupertino, CA) through which icv artificial CSF or AC253 (2.4 μg/day) was continuously administered for 5 months beginning at the age of 3 months. Morris Water Maze (MWM) and T-maze testing for spatial memory were performed at the age of 3 and 8 months. For in vivo behavioral experiments involving peripheral (ip) administration of cAC253, TgCRND8 and Wt mice (male and female) were equally and randomly distributed into four groups, Tg-NS (n = 10; NS [normal saline]), Tg-cAC253 (n = 10), Wt-NS (n = 10), and Wt-cAC253 (n = 10). Mice received ip injections of either NS or cAC253 (200 μg/kg) three times a week starting at the age of 3.5 months for 10 weeks. All behavioral experiments were carried out double-blinded, that is, the individual testing behavior was blinded to drug treatments. For the MWM, mice learn to locate a hidden platform using spatial cues outside the maze, and all animals underwent 7 days of hidden platform testing. After completion of training, a probe trial was performed in which the platform was removed and putative indices of spatial memory were measured. T-maze test is a simple reward base test for spatial memory, where animals are required to correctly identify the food arm of the maze and scored on latency and correct responses. Heterozygous CTR (het CTR) mice (C57BL/6J background) with a 50% deletion of CTR [20] were obtained from Drs R. A. Davey and J. D. Zajac (Department of Medicine, Austin Health, University of Melbourne, Heidelberg, Victoria). Primary human fetal neurons (HFNs), mouse neuronal cell line (N2a cells), HEK293 cells stable expressing amylin 3 (AMY3) subtype of amylin receptors were used for in vitro characterized amylin receptor antagonism effects.
2.2. Immunohistochemistry, protein, and cytotoxicity assays
Immunohistochemistry, enzyme-linked immunosorbent assay (ELISA), and Western blot analysis were carried out on brain sections from all treatment groups in Wt and TgCRND8 mice to examine Aβ burden, amylin receptors (CTR and RAMP3), and synaptic and microglial markers. Peptides AC253, cAC253, and their fluorescent derivatives were synthesized in situ and tested for their amylin receptor antagonist properties using In-cell Western cyclic adenosine monophosphate (cAMP) assay. Neuroprotective properties of cAC253 were confirmed using MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) and live/dead cell assays on cultures of HFNs and N2a cells. Flow cytometry studies were performed to establish specificity of cAC253 binding to the amylin receptor using HEK293 cells expressing AMY3. In vivo and ex vivo imaging of brains was carried out after single ip injection of fluorescently labeled (Cy5) cAC253 to examine its pharmacokinetic profile, proteolytic stability, brain penetrability, and binding to amylin receptors compared with linear AC253. To examine accumulation and specificity of binding for cAC253 to amylin receptors, brain imaging and histologic examination were performed on three genetic strains of mice (TgCRND8, het CTR, and Wt) expressing different levels of amylin receptors after a single ip injection of labeled cAC253.
2.3. Statistical analysis
The statistical data are presented as the mean ± standard error of the mean unless otherwise specified. Significance was determined by one-way analysis of variance, followed by Tukey's post hoc test with Prism software (GraphPad Prism 5; GraphPad Software, San Diego, CA). Differences between groups were considered to be significant at P < .05.
Full detailed descriptions of methods are available in Materials and Methods of the Supplementary Material.
3. Results
3.1. AC253 icv infusion improves cognitive deficits in a transgenic animal model of AD
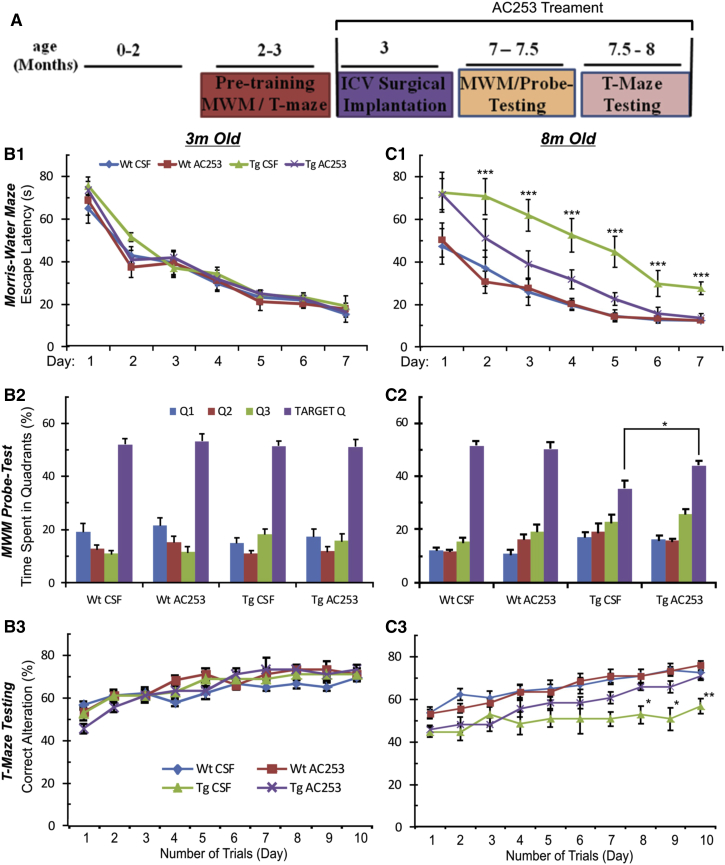
To determine whether amylin receptor antagonists can prevent spatial memory and learning deficits, we performed continuous icv infusions of AC253 or CSF in TgCRND8 or Wt mice, starting at the age of 3 months for 5 months, and measured behavior using MWM and T-maze tests before and after the treatment. At the age of 3 months, no differences in spatial memory and learning were detected in either the MWM or the T-maze between Wt and Tg mice (Fig. 1B1–B3). However, at the age of 8 months, the TgCRND8 mice receiving AC253 showed a marked improvement in latencies to locate the hidden platform over their Tg littermates receiving CSF; Wt control subjects showed no memory deficits with either AC253 of CSF infusions (Fig. 1C1). TgCRND8 mice that were treated with AC253 showed improved memory in probe trial for location of the target quadrant (Fig. 1C2). For the T-maze test, TgCRND8 mice that received AC253 showed an increased percentage of correct arm (food rewarded) choice in T-maze alternation task compared with CSF-treated TgCRND8 mice (Fig. 1B3 and C3). These results indicate that AC253 can prevent cognitive decline in aged APP-expressing mice. None of the mice receiving AC253 show any signs of off-target effects (e.g., sedation, visible signs of motor dysfunction, abnormal feeding or drinking behavior and weight loss, changes in gross appearance such as hair loss, and lack of grooming) throughout the 5 months of treatment, and no significant changes in body weight were observed. These results indicate that AC253 can prevent cognitive decline in aged TgCRND8 mice.
Fig. 1.
Chronic intracerebroventricular (icv) infusions of the amylin receptor antagonist, AC253, improve behavioral performance and spatial memory in TgCRND8 Alzheimer's disease mouse model. (A) Schedule and timeline for behavioral testing and administration of icv AC253. (B1, C1) Data from Morris Water Maze (MWM) testing shows daily escape latencies during platform trials of either wild-type (Wt) or TgCRND8 (Tg) mice receiving icv artificial cerebrospinal fluid (CSF) or AC253 at the age of 3 and 8 months (n = 7 mice in each group; ∗∗∗P < .001 Tg-CSF vs. Tg-AC253). (B2, C2) Probe tests (for retention of platform placement) show comparative analysis of time spent in the four quadrants between treatment groups of Wt and TgCRND8 mice. Target Q, quadrant where the platform is located. (B3, C3) Graph showing the percentage of alteration in T-maze test. (n = 7 mice in each group, ∗P < .05, ∗∗P < .01).
Pathologic examination of mice brains showed no significant difference in the levels of soluble Aβ1–42 or APP protein expression between the two Tg groups (CSF and AC253) as measured by ELISA and Western blot, respectively (Supplementary Fig. 1A-C). There was a trend toward a reduction in Aβ plaque deposition after AC253 icv infusion; however, the difference was found to be statistically nonsignificant (Supplementary Fig. 1D). AC253-treated Tg group showed an increase of 40% and 30% in the expression level of synaptic proteins, synapsin 1 (P < .01) and synaptophysin (P < .05), respectively, compared with Tg-CSF control subjects (Supplementary Fig. 1F). The expression levels of Iba-1, a microglial marker, were significantly reduced (by 50%) in the Tg-AC253 group compared with the Tg-CSF group (P < .05). This finding was further confirmed using immunofluorescence staining (Supplementary Fig. 1F). The amylin receptor (CTR and RAMP3 proteins) expression levels in the brain showed no noticeable difference in the levels of either protein between brains of the two transgenic groups receiving either AC253 or CSF (Supplementary Fig. 1E).
3.2. Cyclic AC253 blocks AMY3 receptor activation and Aβ neuronal cell death in vitro
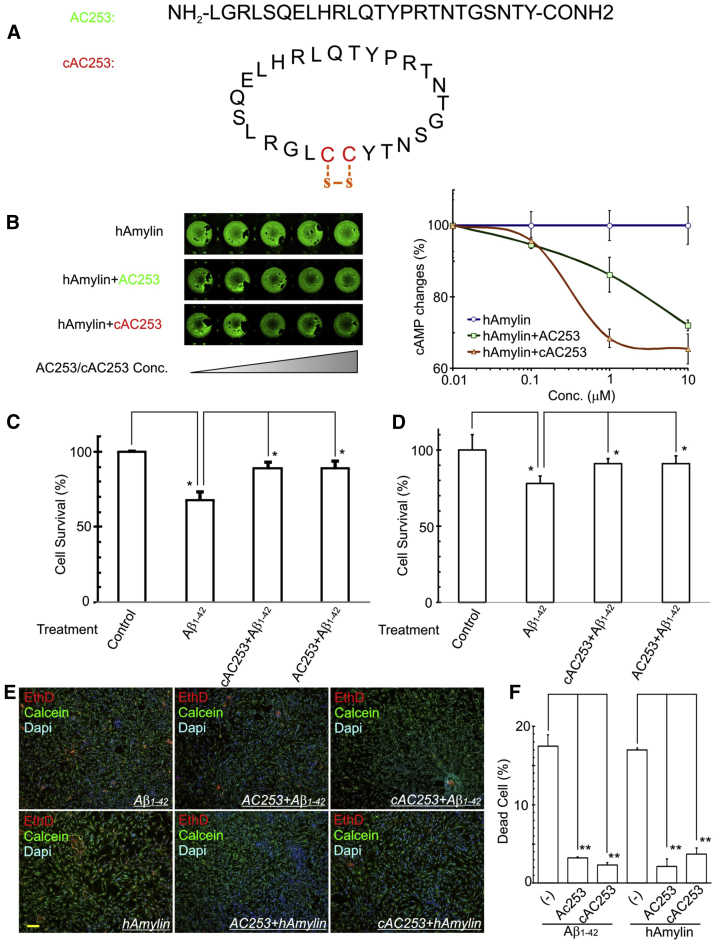
Rapid metabolism by proteolytic enzymes and poor pharmacokinetics are major drawbacks that limit the general use of linear peptides as therapeutic agents. Therefore, on the basis of the promising in vivo efficacy of AC253 peptide on cognitive functions, we decided to develop a new cyclized analog of AC253 (namely, cAC253) for further testing. Peptide cyclization imposes structure rigidity, which enhances binding and selectivity toward the target receptor (agonist/antagonist) and improves enzymatic stability [21], [22]. Restriction of peptide backbone flexibility would prevent liable amino acids from orientating itself in the appropriate position for attack by hydrolytic enzymes. Several cyclic peptides found in nature are used in clinic. The examples are gramicidin and tyrocidine with bactericidal activity, cyclosporin A with immunosuppressive activity, and vancomycin with antibacterial activity, and so on. Accordingly, we imposed a conformational constraint on the cAC253 structure by placing two cysteine residues at both the C and N termini and cyclized it through disulfide bond formation as depicted in Fig. 2A. Synthetic peptides AC253 and cAC253 were obtained in high yields, 40% and 55%, respectively, and purity greater than 97% for both peptides (Supplementary Table 1). Matrix assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry yielded molecular weights in good agreement with the predicted masses of peptides, linear AC253 showed m/z 2805.2 ([M + H]+ calculated 2085.4), and cAC253 m/z 3008.5 ([M + H]+ calculated 3008.8) (Supplementary Fig. 2). Partition coefficient values of peptides proved that both are hydrophilic having log P values of −1.2, and −1.05 for AC253 and cAC253, respectively.
Fig. 2.
cAC253 retains its amylin receptor antagonist and neuroprotective properties against Aβ1–42 cytotoxicity. (A) AC253 and cAC253 amino acid sequences and structure. cAC253 has two additional cysteine amino acids at the C and N termini compared with AC253 and is cyclized using disulfide bond. (B) In-cell Western assay showing both AC253 and cAC253 are capable of inhibiting human amylin (hAmylin) effects on the cellular levels of cyclic adenosine monophosphate (cAMP) via AMY3 receptor activation in HEK293 AMY3-expressing cells. Graph showing changes in cAMP levels in AMY3-expressing HEK293 cells after exposure to different concentrations of AC253 and cAC253 peptides in the presence of 1 μM hAmylin. (C, D) Aβ1–42 (10 μM) applied to primary cultures of human fetal neurons (HFNs) or N2a neuronal cell line induces cell death (measured by MTT assay) that can be attenuated by preapplications of 10 μM either AC253 or cAC253 (n = 5, ∗P < .05) (E) Photomicrographs of live (calcein-green fluorescence)/dead (ethidium-red fluorescence) assay in HFNs, showing the effect of cAC253 or AC253 preincubation on the Aβ1–42- and hAmylin-induced cell death. Scale bar = 100 μm. (F) Histograms showing quantification of live/dead assay. n = 8, ∗P < .05; ∗∗P < .01. Abbreviation: Dapi, 4',6-diamidino-2-phenylindole.
Next, we examined cyclization of the AC253 peptide for its antagonist activity at AMY3 and whether it also retained its neuroprotective properties against Aβ toxicity using two in vitro functional bioassays. Amylin receptors are heterodimers of CTR and one of three RAMPs (RAMP1, 2, and 3), thus generating multiple amylin receptor subtypes, AMY1, AMY2, and AMY3 [23]. Our previous data provide evidence that the AMY3 is the most relevant subtype of amylin receptors in the context of the direct actions of Aβ (and hAmylin) at the level of the cell membrane [10], and we thus targeted this receptor isoform in the present study. Both peptides of AC253 and cAC253 blocked the hAmylin-evoked cAMP increase in a dose-dependent manner; cAC253 was, however, threefold more potent in inhibiting AMY3 receptor activity in AMY3 stable expression HEK293 cells (AMY3-HEK) (Fig. 2B). The AC253 or cAC253 alone had no effect on the cAMP level (Supplementary Fig. 3A). The half-maximal inhibitory concentration for AC253 and cAC253 was approximately 0.85 and 0.3 μM, respectively.
Soluble oligomeric Aβ is known to be toxic in neuronal cell cultures, and we previously demonstrated that AC253 attenuates Aβ1–42- and hAmylin-induced apoptotic cell death in cultured human neurons via its antagonist activity at the AMY3 receptor [13]. Therefore, we examined whether in vitro cAC253 can protect neuronal cells, HFNs and N2a, from Aβ1–42-induced cytotoxicity. Using the MTT and live/dead assays, we observed that both peptides were equally effective in attenuating cell death induced by Aβ1–42 and hAmylin (Fig. 2C–F). Thus, our results confirmed that cAC253 retained its amylin receptor antagonist and neuroprotective properties against Aβ toxicity.
3.3. cAC253 has enhanced binding to AMY3 receptor in vitro
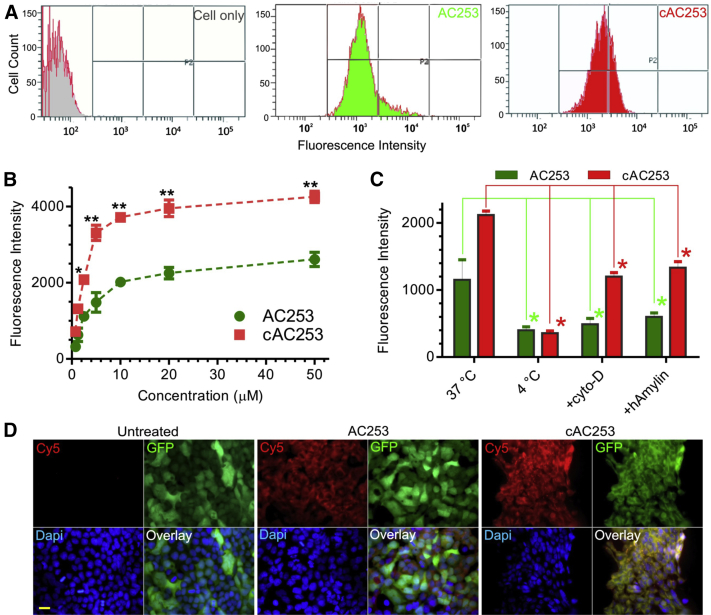
We next examined the in vitro binding efficacy and specificity of cAC253 compared with AC253 in AMY3-HEK cells using flow cytometry and fluorescence microscopy. Cy5 near-infrared (NIR)-fluorescently labeled peptides (AC253 and cAC253) were synthesized and characterized using MALDI-TOF mass spectrometry, which displayed molecular weights in agreement with calculated values (Supplementary Table 1). From flow cytometry assay (Fig. 3A), cAC253 displayed threefold enhanced binding and uptake into AMY3-HEK cells compared with AC253 with mean fluorescence intensity of 3300 and 1200 for cAC253 and AC253, respectively. This enhanced binding explains the observed improvement in the antagonistic activity of cAC253 at the amylin receptors using a cAMP assay in AMY-expressing HEK293 cells. Cell binding to both antagonists increases in a dose-dependent manner with Kd of 1.45 ± 0.5 and 2.6 ± 1.0 μM for cAC253 and AC253, respectively (Fig. 3B). Further increase in peptide concentration beyond 10 μM revealed saturation, suggesting that these peptides bind and interact with the AMY3-HEK cells through a receptor-based mechanism. In Wt HEK293 cells, both antagonists demonstrated a 10-fold decrease in binding and uptake compared with that for AMY3-HEK cells, thus further confirming AMY3 binding specificity (Supplementary Fig. 3B). To determine the intracellular delivery mechanisms, we examined the delivery efficiency of our peptides at different temperatures, 4°C and 37°C, and in the presence of cytochalasin D (CytoD), an inhibitor of endocytosis via clathrin-coated pits. Both peptides behaved similarly and a marked decrease in binding and uptake was observed at 4°C with mean fluorescence intensity of 356.6 ± 35 and 400 ± 75 for cAC253 and AC253, respectively, indicating that peptide cell uptake occurs via an energy-dependent endocytic pathway (Fig. 3C). In the presence of CytoD, the cell uptake of both peptides was significantly decreased (threefold) with mean fluorescence intensity of 1100 ± 100 and 450 ± 50, for cAC253 and AC253, respectively, suggesting that clathrin endocytosis is, at least in part, responsible for the uptake of peptides (Fig. 3C). Binding of the antagonists to AMY3-HEK cells was competitively inhibited when cells were preincubated with unlabeled hAmylin, and peptide cell uptake was partly inhibited with mean fluorescence intensity of 1250 ± 200 and 500 ± 100 for cAC253 and AC253, respectively (Fig. 3C). With fluorescence microscopy, we observed strong binding of both peptides to the cell membrane of AMY3-HEK cells (Fig. 3D).
Fig. 3.
cAC253 has enhanced specific binding to AMY3 receptor in vitro compared with linear AC253. (A) Flow cytometry histograms comparing Cy5-labeled AC253 and cAC253 peptides (5 μM) for HEK293 AMY3 cells binding and uptake after 60 minutes of incubation in serum-free media at 37°C. (B, C) Graphs showing the dose-dependent uptake of Cy5-labeled peptides in HEK293 AMY3 cells and quantification of cell uptake of peptides at 4°C in the presence of cytocholasin D (CytoD, an endocytosis inhibitor) and in the presence of human amylin (a competitive binding inhibitor). Data are from two independent experiments carried out in triplicate (n = 8 for each point, ∗P < .05; ∗∗P < .01). (D) Representative fluorescence microscopy images showing Cy5-labeled peptides binding to GFP-labeled HEK293 AMY3 cells at 37°C for 60 minutes of incubation (scale bar = 10 μm). Abbreviations: DAPI, 4',6-diamidino-2-phenylindole; GFP, green fluorescent protein.
3.4. cAC253 has better brain penetration, pharmacokinetic profile, and enzymatic stability than AC253
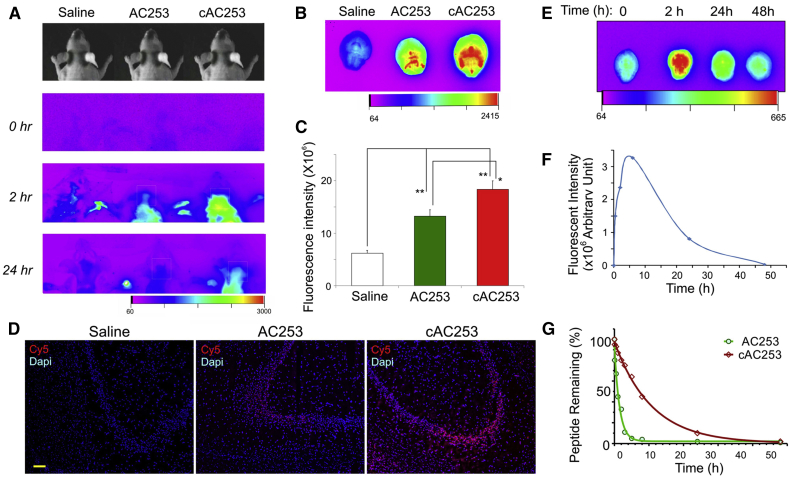
For peptidergic drugs to be an effective therapy for AD, they must achieve an effective concentration in the CNS after peripheral administration. Therefore, using the fluorescently labeled peptides, we examined and compared the ability of cAC253 and AC253 to penetrate the blood–brain barrier (BBB) in Wt mice using NIR fluorescence in vivo, and ex vivo brain imaging. After single ip administration, the fluorescence signal for both peptides was significantly increased in the brain, and it was detectable in brain regions within 10 minutes after injection. Interestingly, cAC253 showed a stronger brain fluorescence signal compared with AC253 at all-time points. The fluorescence signal for cAC253 was slowly washed out, even it was clearly visible at 24 hours time point, indicating its higher brain retention (Fig. 4A). A peak fluorescence signal for both peptides was observed at 2 hours, which was therefore selected as an optimal time point for further ex vivo experiments. To ensure that the fluorescence observed was mainly because of the peptide, Cy5 dye was injected alone and brain imaging showed that it could not on its own cross the BBB. Furthermore, to confirm if cAC253 peptide is indeed in its intact form in the brain, we used liquid chromatography tandem-mass spectrometry (LC-MS/MS) on brain tissue homogenates that received the ip injection. As shown in reversed-phase high-performance liquid chromatography (RP-HPLC) chromatograms, consistent with Cy5-cAC253 control peak that elutes at 37 minutes, a similar peak was identified in the brain homogenate from animals that received cAC253. Mass spectrometry of the eluted peak at the denoted retention time revealed a molecular weight of 730.156 ([M + 5H]+ calculated 730.512), consistent with that of the full length peptide and supporting the conclusion that the cAC253 reaches the brain intact (Supplementary Fig. 4).
Fig. 4.
cAC253 demonstrates superior brain permeability, pharmacokinetic profile, and proteolytic stability compared with AC253. (A) In vivo near infrared fluorescence (NIRF) brain imaging of Cy5 AC253 and cAC253 peptides compared with saline injections administered intraperitoneally in wild-type (Wt) mice at 0, 2, 24 hours of time points using Kodak imager. (B) Ex vivo images of brains from Wt mice receiving intraperitoneal (ip) injections of 0.1 mmol AC253 or cAC253 in 200 μL saline compared with saline-injected mice (control). (C) Quantification of brain fluorescence intensity after 2 hours of ip injection of labeled peptides (n = 5 in each group, ∗P < .05, ∗∗P < .01). (D) Brain sections from ex vivo experiments in (B) showing AC253 and cAC253 fluorescent labeling (red) within the hippocampus and nuclear staining with 4',6-diamidino-2-phenylindole (DAPI) (blue). Scale bar = 100 μm. (E) Ex vivo fluorescence brain images showing time-dependent accumulation of cAC253 after a single ip injection of 0.1 mmol of the peptide in 200 μL saline. (F) Quantification of the data shown in (E) for brain accumulation of labeled cAC253 at different time points (n = 3). (G) In vitro stability of cAC253 peptide in human serum compared with AC253 at 37°C. The amount of intact peptide in human serum at different time points was estimated using RP-HPLC.
Next, we compared the brain concentrations of cAC253 peptide to that of a well-studied peptide, T7 peptide (HAIYPRH). T7 uses the transferrin receptor to penetrate the BBB and achieve therapeutic concentrations in the brain [24], [25]. Ex vivo brain fluorescence signal from cAC253 was comparable to the fluorescence of the T7 peptide-injected group after 2 hours of injection, (Supplementary Fig. 5). The brain cAC253 concentration after injecting 40 μg Cy5-cAC253 was estimated to be in the range of 10 to 100 nM, a level equal to 0.1% of the injected dose (Supplementary Fig. 6). These estimates of brain levels are comparable to the reported values for various well-studied bioactive peptide hormones such as amylin or insulin [26], [27].
Ex vivo brain imaging 2 hours after ip injection was consistent with the in vivo imaging data, and the signals from peptides injected into mice brains were 2.8- and 2-fold higher for cAC253 and AC253, respectively, than saline injection. In addition, the signal from cAC253 was 1.4-fold higher than AC253 (Fig. 4B and C). Interestingly, although the peptides were distributed throughout the entire brain, the fluorescence was strongly localized to the hippocampal region, which also coincides with the localization of amylin receptor expression in the brain [28]. Histologic analysis of ex vivo–imaged brains confirmed that our peptides were mainly accumulated in the hippocampal region (Fig. 4D). To further investigate the role of amylin receptor in peptide brain uptake, an in vivo competition experiment was performed where mice were injected with Cy5-cAC253 along with a fivefold excess of unlabeled cAC253 peptide. The brain fluorescence signal in animals receiving the peptides mixture was significantly reduced (by ∼75%) in comparison to the Cy5-cAC253 only (Supplementary Fig. 7). Overall our results demonstrate, for the first time, the ability of amylin receptor antagonist peptides (cAC253 and AC253) to penetrate the blood-brain barrier when given peripherally and support the notion that the brain uptake of these peptides occurs via an amylin receptor-based mechanism.
Next, we examined the pharmacokinetic profile and the proteolytic stability of cAC253 in vivo and in vitro, respectively. We assessed the time dependence for peptide cAC253 to clear from the Wt mouse brain after injecting 20 mg/kg intraperitoneally (Fig. 4E and F). Cy5-cAC253 fluorescence signal in the brain reached its maximum at 6 hours after which it slowly declined with an estimated half-life in brain of 16 hours. We next analyzed brain fluorescence levels in mice 2 hours after receiving 0.2, 2, or 20 mg/kg of cAC253 as a single ip dose. Cy5-cAC253 brain fluorescence appears to be concentration-dependent and saturable because a dose increment from 2 to 20 mg/kg achieved a steady state level, which suggests a receptor-mediated brain uptake of this peptide (Supplementary Fig. 8).
Biodistribution evaluation cAC253 in different organs (liver, kidney, spleen, lung, heart, brain, stomach, and intestine) was investigated 2 hours after injecting 20 mg/kg peptide. Ex vivo fluorescence signals indicated that peptide was distributed within all organs examined although uptake in lung, spleen, and heart was considerably less compared with the kidney and the liver, which showed a strong NIR fluorescence intensity in keeping with renal and hepatic clearance of the peptide. AC253 biodistribution data show similar pattern to the cAC253 with observed less accumulation in the brain (Supplementary Fig. 8). Also, we did not observe any histopathologic changes in the kidney or liver from icv AC253-treated mice.
Next, we investigated the influence of cyclization on the proteolytic stability of cAC253 compared with AC253. cAC253 was found to be seven times more stable in human serum compared with AC253 with 7- and 1-hour half-lives, respectively. This confirms that cyclization of AC253 enhanced its stability and conferred protection from proteolytic cleavage, thus increasing the amount of peptide reaching the brain through the systemic circulation (Fig. 4G). By assessing the main degradation fragments in both peptides with MALDI-TOF, both peptides were found to be cleaved at the basic arginine amino acids R3, R10, and R16. Overall, cAC253 is an excellent candidate for peripheral administration and behavioral testing.
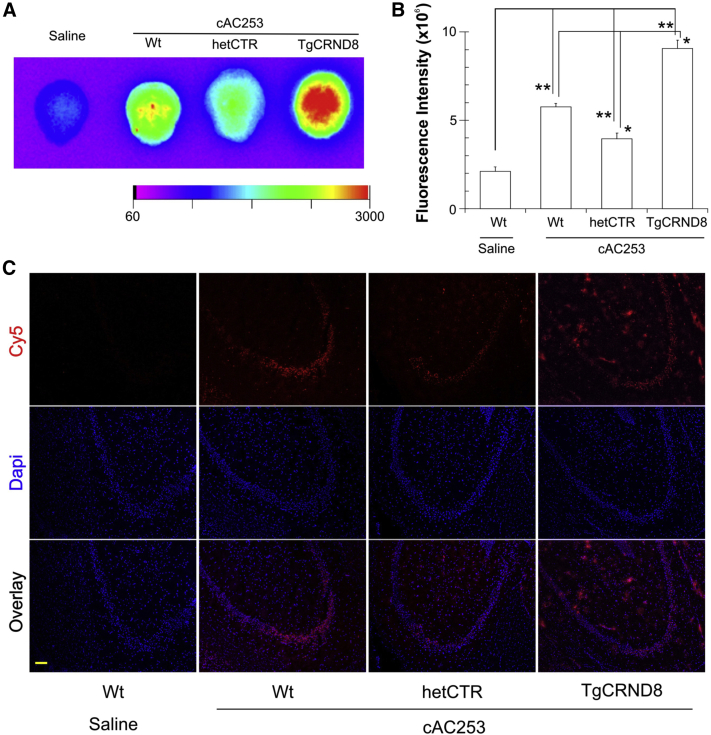
3.5. cAC253 targets the AMY receptor in vivo
To study the connection between the expression level of the amylin receptor and the degree of cAC253 brain permeability, peptide brain fluorescence signal was compared in two genetically engineered mouse models that express differing levels of the CTR receptor. We used two engineered mouse resources: hemizygous CTR mice that exhibit 50% CTR expression (“het CTR”) and hence 50% reduction in the functional amylin receptor [20] and transgenic APP-overexpressing (TgCRND8) mice known to have upregulation of the amylin receptor [13]. After 2 hours of ip injection, ex vivo brain imaging results demonstrated that the fluorescence of cAC253 in brains of TgCRND8 mice increased by 45% compared with Wt age-matched control subjects (Fig. 5A and B). However, for het CTR mice, the brain fluorescence decreased by 30% compared with that of Wt mice (Fig. 5A and B). These data therefore indicate that the amount of cAC253 uptake and accumulation positively correlates with the level of amylin receptor expression in the brain. These observations were further confirmed by immunohistochemical examination of brain sections, which showed that the amount of cAC253-labeled peptide within the hippocampus is correlated to the level of CTR expression (Fig. 5C). In addition, CTR is colocalized with the labeled cAC253. Examination of histologic sections also revealed intense CTR staining along the endothelial cells of the cerebral vessels supporting the notion that the amylin receptors most likely play a role in the brain uptake of the peptide from the vasculature, a hypothesis that is currently under investigation (Supplementary Fig. 9).
Fig. 5.
Cyclic AC253 brain uptake correlates with the expression of amylin receptor levels in the brain (A) Brain images from wild-type (Wt), heterozygous CTR knockdown (het CTR, 50% depletion of amylin receptors), or TgCRND8 (that overexpress amylin receptors) mice 2 hours after receiving equimolar intraperitoneal (ip) injections of Cy5-cAC253 (0.1 mmol in 200 μL saline). (B) Quantification of data for brain accumulation of Cy5-cAC253 in the three distinct mouse genotypes. (n = 5 in each group, ∗P < .05, ∗∗P < .01). (C) Brain sections from the three mouse genotypes showing fluorescent labeling through the hippocampus after a single ip injection of Cy5-labeled cAC253 (scale bar = 100 μm). Abbreviation: DAPI, 4',6-diamidino-2-phenylindole.
3.6. Peripheral administration of cAC253 improves cognitive deficits in transgenic mouse model of AD
On the basis of the superior stability, pharmacokinetic profile, and brain penetrability of cAC253 compared with linear AC253, we sought to determine the benefits of cAC253 on spatial memory in TgCRND8 mice when administered systemically. Starting at the age of 3.5 months, groups of TgCRND8 or Wt mice (n = 10 for each group) received either cAC253 (200 μg/kg) or NS intraperitoneally three times a week for 10 weeks. The dose of cAC253 for ip injection was based on the calculated steady state dose of AC253 from the icv experiments, and its frequency of administration was based on the estimated half-life of cAC253 in the brain and serum from our in vivo experiments.
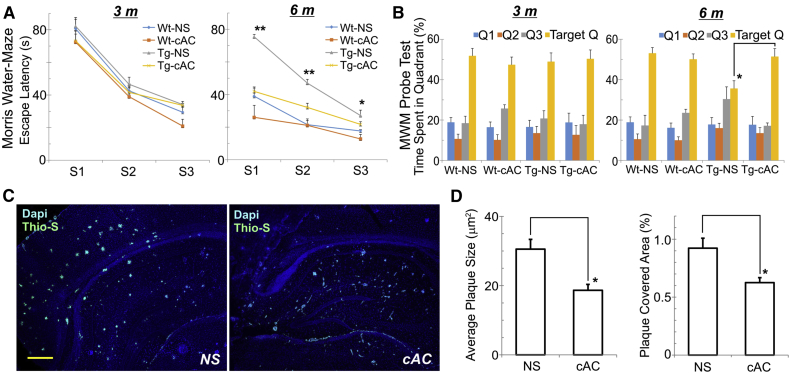
Results showed that at the age of 3 months, no differences in spatial memory and learning deficits were detected in the MWM between Wt and Tg mice (Fig. 6A). However, after 10 weeks of cAC253 ip injections, TgCRND8 mice showed a marked improvement in the MWM test of spatial memory compared with Tg littermates receiving saline. Wt control subjects showed no alterations in spatial memory with either cAC253 or saline injections (Fig. 6A). TgCRND8 mice treated with cAC253 also showed an improved ability to retain location of the platform in the target quadrant after 24 hours (Fig. 6B). TgCRND8 and Wt mice receiving cAC253 did not demonstrate any off-target effects such as sedation, weight loss, signs of abnormal motor dysfunction, feeding/drinking behavior, and hair loss. These animals also did not demonstrate any weight loss over the course of the entire experiment.
Fig. 6.
Peripheral administration of cAC253 improves cognitive function in transgenic AD mice. (A, B) Younger (3 month-old, 3 m) transgenic (Tg) AD mice (TgCRND8) show no significant cognitive impairment compared with their wild-type (Wt) littermates as measured by escape latencies in the Morris Water Maze (MWM) (A) and quadrant preference on probe test (B). At the age of 6 months, TgCRND8 mice began to show cognitive impairment compared with Wt control subjects, whereas intraperitoneal administration of the amylin receptor antagonist, cAC253 (cAC, three times a week for 10 weeks commencing at the age of 3 months) significantly improves MWM and quadrant preference performance compared with Tg mice receiving normal saline (NS). In (A), S1, S2, and S3 refer to the number of the trial session for MWM. (C, D) Immunohistochemical detection of amyloid plaques in brains of saline (NS) or cAC253 (cAC)-treated TgCRND8 mice reveals a significant reduction of the amyloid plaque burden in mice receiving cAC253. ∗P < .05, ∗∗P < .01. Scale bar = 200 μm.
After 10 weeks of cAC253 ip injection, Aβ plaque formation was significantly reduced in both the number of Aβ plaques and total area of Aβ-positive profiles in the cAC253-treated Tg group compared with Tg saline injected mice (Fig. 6C and D).
4. Discussion
The present study shows, for the first time, that chronic central administration of the amylin receptor antagonist, AC253, results in an improvement of the age-dependent spatial memory and learning deficits in transgenic APP-overexpressing (TgCRND8) mice. TgCRND8 mice carry combined APP Swedish (K670M/N671L) and Indiana (V717F) mutations, resulting in an aggressive neuropathology evident by 6 months when the animals also demonstrate large numbers of diffuse and plaque amyloid deposits [29], [30]. These mice exhibit normal behavior at the age of 3 to 4 months, but by 6 months they show a progressive deterioration of cognitive function and spatial memory with increased Aβ burden. In tests of memory retrieval (MWM, T-maze, and probe trial for quadrant preference), 8 month-old TgCRND8 mice, which had received icv infusions of AC253 for more than a period of 5 months, demonstrated a superior performance to age-matched TgCRND8 mice that received CSF infusions. In spite of the duration of drug infusions, we did not observe any off-target effects and mortality within the groups of TgCRND8 mice receiving either CSF or AC253 was identical at 20%, a figure consistent with that reported by other groups that have used this genetic strain of mice [29], [30]. The long-term central administration of amylin receptor antagonist, AC253, therefore appears to be a viable, relatively safe disease-modifying treatment in an AD mouse model.
We have previously shown using several in vitro experimental paradigms that the deleterious effects of Aβ are expressed via its direct actions on amylin receptors and these effects can be blocked with AC253 [13], [15]. In addition, both AC253 and pramlintide restore the Aβ- and hAmylin-evoked depression of hippocampal LTP, a cellular surrogate of memory [16], [19]. We surmised the same mechanism, that is, blocking the brain amylin receptors could account for the in vivo improvement in spatial memory and learning observed in AC253-treated TgCRND8 AD mice. We also examined the brains of Wt and TgCRND8 mice receiving CSF or AC253 to determine whether the antagonism of amylin receptors with AC253 affected markers of AD pathology. Our data revealed that although icv AC253 did not have a statistically significant effect on the total brain levels of APP, Aβ in plaque form, or soluble oligomers of Aβ, there appeared to be a trend toward reduction of the amyloid burden. With systemic administration of cAC253, we did, however, observe a significant reduction in amyloid plaque size and area in treated mice versus control subjects. This would be consistent with prior observations that systemic injections of amylin and pramlintide reduce brain amyloid in transgenic AD mice [27]. Thus, amylin analogs and antagonists both appear to be capable of reducing brain concentrations of amyloid, thereby providing another beneficial mechanism for amylin-based therapies in AD. No differences in the levels of amylin receptor (CTR or RAMP3) expression were observed in Wt or TgCRND8 mice with either CSF or AC253 administration. On the other hand, we did observe a significant increase in synaptic markers (synapsin 1 and synaptophysin) in TgCRND8 mice that received AC253 compared with those receiving CSF. Interestingly, there was also a significant suppression of the microglial marker, Iba1, in the brains of AC253-treated TgCRND8 mice. AC253 may thus improve not only synaptic function as has been observed for in vitro studies of LTP [16], [19] but also attenuate disruption of synaptic integrity in TgCRND8 mice that is attributed to increased Aβ burden. Amylin receptors have also been reported on human microglia and deemed to participate in Aβ-induced activation of inflammasome and cytokine release [31]. Thus, attenuation of this Aβ-driven inflammatory cascade by blockade of amylin receptors with AC253 could in part explain the decreased microglial activation in brains of TgCRND8 mice.
Peptide-based drugs have limited therapeutic utility for CNS disorders chiefly on account of their limited BBB permeability with the failure of beta-secretase 1 (BACE1) inhibitors being a case in point [32]. AC253 is a 24-amino acid peptide whose sequence is extremely hydrophilic in nature, and thus we initially assessed its effects on spatial memory in vivo through an icv route of administration that would deliver the drug directly to the brain. Chronic icv administration of AC253 although beneficial, however, is not practical. We therefore developed a cyclized version of the AC253 peptide (cAC253) through disulfide linkage to improve its physiochemical and biological activity and brain penetrability compared with its linear counterpart. Our design was based on several key factors. First, gaining a fixed geometry through cyclization would enhance peptide-specific and efficient binding to the amylin receptor. Second, because amylin receptors exist as several subtypes (AMY1, AMY2, and AMY3), we hoped that the fixed geometry of cAC253 in comparison to AC253 would also make it more selective for particular receptor subtypes (AMY1 and AMY3) that are more prevalent in the brain [33], [34]. In addition, cyclization also enhances peptide enzymatic stability [35]. We achieved these goals by demonstrating that cyclization in fact enhanced AC253 binding to AMY3-expressing cells and resulted in superior blood-brain barrier permeability after a single ip injection compared with a linear form of AC253 at therapeutically relevant concentrations. Furthermore, cAC253 showed a sevenfold increase in proteolytic stability (t1/2), and better pharmacokinetic profile compared with AC253. In comparison to commercially available pramlintide (Symlin, AstraZeneca), cAC253 offers multiple advantages over pramlintide in the form of better solubility at physiological pH [36], superior brain penetrability when administered systemically (Supplementary Fig. 10), and a shorter peptide sequence that renders it more cost effective therapeutic agent. cAC253 also has a longer duration of action and thus higher bioavailability, which was evident by a lower number of injections per week (three) than required for pramlintide (daily) [18].
Systemic administration of cAC253 for 10 weeks in a mouse AD model recapitulated the improvement in spatial memory evoked by chronic icv infusions of the linear form of the peptide, AC253. TgCRND8 mice receiving cAC253 demonstrated improved escape latencies on MWM testing and also an ability to retain the location of the target quadrant (for the platform). Similar to icv AC253, we did not observe any morbidity or off-target effects of cAC253 administration. The behavioral improvement was also accompanied by a reduction of the amyloid plaque burden in TgCRND8 mice receiving cAC253 compared with saline control subjects. Our data thus identify cAC253 as a novel and potent CNS-permeable peptide that holds promise as a potential therapy for AD. Furthermore, on account of its brain permeability, cAC253 could be used even for wider applications such as serving as a carrier to facilitate delivery of a variety of therapeutic or imaging molecules into the brain.
An important observation in the present study relates to the positive correlation between cAC253 uptake across the blood-brain barrier and the expression levels of the amylin receptor in the brain. In hemizygous CTR mice, which exhibit a 50% knocked down of the CTR (and hence amylin) receptor, we observed a significantly reduced cAC253 fluorescent labeling in the brain including the hippocampus compared with Wt mice that carry a normal complement of amylin receptors. On the contrary, for TgCRND8 mice, in which we have previously reported an upregulation of the amylin receptors [13], cAC253 fluorescence was markedly increased in the same brain regions. Binding of cAC253 to amylin receptors is also supported by the finding that injection of a mixture of Cy5 fluorescently labeled cAC253 and free unlabeled cA253 peptide resulted in a 75% reduction in brain fluorescence levels in comparison to animals injected with the fluorescently labeled cAC253 only. We also observed intense histologic staining for CTR along the endothelial cells of the cerebral vessels in TgCRND8 and Wt mice supporting the possibility that the amylin receptors may be involved in the brain uptake of the amylin peptide from the vasculature. This notion is supported by studies in the cat, where amylin immunoreactive fibers were shown to innervate cerebral vessels and application of this peptide resulted in a relaxation of ring segments of the middle cerebral artery containing endothelium [37].
5. Conclusions
The present study demonstrates that chronic treatment with amylin receptor antagonists improves spatial memory in transgenic model of AD mice, and histopathologic examination shows an increase in markers of synaptic integrity and a reduction in microglial activation. Cyclized form of the amylin receptor antagonist, AC253, demonstrates superior proteolytic stability and brain penetration than the linear form of this peptide and its presence in the brain correlates with the levels of expression of the amylin receptor. Collectively, these observations validate the amylin receptor as a therapeutic target and development of amylin receptor antagonists could serve as effective disease-modifying therapy in AD progression.
Research in Context.
-
1.
Systematic review: At present there are no Food and Drug Administration–approved disease-modifying therapies for Alzheimer's disease. On the basis of our work and that of others, we tested whether administration of the amylin receptor antagonist, AC253, in an Alzheimer's disease (AD) mouse model of mice improves spatial memory and learning.
-
2.
Interpretation: We show that chronic central administration of AC253 improves spatial memory in transgenic AD mice and is well tolerated. We further have developed a cyclized form of AC253 (cAC253) that when administered systemically is proteolytically stable, readily brain penetrant, and binds to hippocampal amylin receptors while retaining its neuroprotectant properties and its ability to improve spatial memory and learning in TgCRND8 mice.
-
3.
Future directions: Amylin antagonists could serve as novel therapeutic compounds for AD. We plan to identify peptide fragment sequences of AC253 that retain the beneficial effects of parent compound AC253 but offer advantages in terms of their synthesis, stability, and administration.
Acknowledgments
This research was supported by grants from the Canadian Institutes of Health Research (CIHR PS 149024), Alberta Bio-Solutions (Alberta Prion Research Institute) and the Alzheimer's Society of Alberta and Northwest Territories (201600006), the University Hospital Foundation, and the Natural Sciences and Engineering Research Council of Canada (NSERC). R.S. is the recipient of Alberta Innovates Heath Sciences (AIHS) clinician postdoctoral fellowship.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.trci.2016.11.005.
Supplementary data
References
- 1.Alzheimer's.net. 2016 Alzheimer's Statistics. Available at: www.alzheimers.net/resources/alzheimers-statistics/. Accessed December 11, 2016.
- 2.Selkoe D.J. Normal and abnormal biology of the beta-amyloid precursor protein. Annu Rev Neurosci. 1994;17:489–517. doi: 10.1146/annurev.ne.17.030194.002421. [DOI] [PubMed] [Google Scholar]
- 3.Hardy J., Selkoe D.J. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 4.Selkoe D.J. The therapeutics of Alzheimer's disease: where we stand and where we are heading. Ann Neurol. 2013;74:328–336. doi: 10.1002/ana.24001. [DOI] [PubMed] [Google Scholar]
- 5.Hardy J. The amyloid hypothesis for Alzheimer's disease: a critical reappraisal. J Neurochem. 2009;110:1129–1134. doi: 10.1111/j.1471-4159.2009.06181.x. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y.H., Giunta B., Zhou H.D., Tan J., Wang Y.J. Immunotherapy for Alzheimer disease: the challenge of adverse effects. Nat Rev Neurol. 2012;8:465–469. doi: 10.1038/nrneurol.2012.118. [DOI] [PubMed] [Google Scholar]
- 7.Bateman R.J., Xiong C., Benzinger T.L., Fagan A.M., Goate A., Fox N.C. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. N Engl J Med. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel A.N., Jhamandas J.H. Neuronal receptors as targets for the action of amyloid-beta protein (Aβ) in the brain. Expert Rev Mol Med. 2012;14:e2. doi: 10.1017/S1462399411002134. [DOI] [PubMed] [Google Scholar]
- 9.Danysz W., Parsons C.G. Alzheimer's disease, β-amyloid, glutamate, NMDA receptors and memantine—searching for the connections. Br J Pharmacol. 2012;167:324–352. doi: 10.1111/j.1476-5381.2012.02057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu W., Ruangkittisakul A., MacTavish D., Shi J.Y., Ballanyi K., Jhamandas J.H. Amyloid β (Aβ) peptide directly activates amylin-3 receptor subtype by triggering multiple intracellular signaling pathways. J Biol Chem. 2012;287:18820–18830. doi: 10.1074/jbc.M111.331181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Westermark P., Andersson A., Westermark G.T. Islet amyloid polypeptide, islet amyloid, and diabetes mellitus. Physiol Rev. 2011;91:795–826. doi: 10.1152/physrev.00042.2009. [DOI] [PubMed] [Google Scholar]
- 12.Abedini A., Schmidt A.M. Mechanisms of islet amyloidosis toxicity in type 2 diabetes. FEBS Lett. 2013;587:1119–1127. doi: 10.1016/j.febslet.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jhamandas J.H., Li Z., Westaway D., Yang J., Jassar S., MacTavish D. Actions of β-amyloid protein on human neurons are expressed through the amylin receptor. Am J Pathol. 2011;178:140–149. doi: 10.1016/j.ajpath.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jhamandas J.H., MacTavish D. Antagonist of the amylin receptor blocks β-amyloid toxicity in rat cholinergic basal forebrain neurons. J Neurosci. 2004;24:5579–5584. doi: 10.1523/JNEUROSCI.1051-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jhamandas J.H., MacTavish D. β-Amyloid protein (Aβ) and human amylin regulation of apoptotic genes occurs through the amylin receptor. Apoptosis. 2012;17:37–47. doi: 10.1007/s10495-011-0656-3. [DOI] [PubMed] [Google Scholar]
- 16.Kimura R., MacTavish D., Yang J., Westaway D., Jhamandas J.H. Beta amyloid-induced depression of hippocampal long-term potentiation is mediated through the amylin receptor. J Neurosci. 2012;32:17401–17406. doi: 10.1523/JNEUROSCI.3028-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adler B.L., Yarchoan M., Hwang H.M., Louneva N., Blair J.A., Palm R. Neuroprotective effects of the amylin analogue pramlintide on Alzheimer's disease pathogenesis and cognition. Neurobiol Aging. 2014;35:793–801. doi: 10.1016/j.neurobiolaging.2013.10.076. [DOI] [PubMed] [Google Scholar]
- 18.Zhu H., Wang X., Wallack M., Li H., Carreras I., Dedeoglu A. Intraperitoneal injection of the pancreatic peptide amylin potently reduces behavioral impairment and brain amyloid pathology in murine models of Alzheimer's disease. Mol Psychiatry. 2015;20:252–262. doi: 10.1038/mp.2014.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimura R., MacTavish D., Yang J., Westaway D., Jhamandas J.H. Pramlintide antagonizes beta amyloid (Aβ) and human amylin-induced depression of hippocampal long-term potentiation. Mol Neurobiol. 2016 doi: 10.1007/s12035-016-9684-x. [DOI] [PubMed] [Google Scholar]
- 20.Davey R.A., Turner A.G., McManus J.F., Chiu W.S., Tjahyono F., Moore A.J. Calcitonin receptor plays a physiological role to protect against hypercalcemia in mice. J Bone Miner Res. 2008;23:1182–1193. doi: 10.1359/JBMR.080310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bock J.E., Gavenonis J., Kritzer J.A. Getting in shape: controlling peptide bioactivity and bioavailability using conformational constraints. ACS Chem Biol. 2013;8:488–499. doi: 10.1021/cb300515u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gentilucci L., De Marco R., Cerisoli L. Chemical modifications designed to improve peptide stability: incorporation of non-natural amino acids, pseudo-peptide bonds, and cyclization. Curr Pharm Des. 2010;16:3185–3203. doi: 10.2174/138161210793292555. [DOI] [PubMed] [Google Scholar]
- 23.Hay D.L., Christopoulos G., Christopoulos A., Poyner D.R., Sexton P.M. Pharmacological discrimination of calcitonin receptor: receptor activity-modifying protein complexes. Mol Pharmacol. 2005;67:1655–1665. doi: 10.1124/mol.104.008615. [DOI] [PubMed] [Google Scholar]
- 24.Han L., Huang R., Liu S., Huang S., Jiang C. Peptide-conjugated PAMAM for targeted doxorubicin delivery to transferrin receptor overexpressed tumors. Mol Pharm. 2010;7:2156–2165. doi: 10.1021/mp100185f. [DOI] [PubMed] [Google Scholar]
- 25.Wu H., Yao L., Mei J., Li F. Development of synthetic of peptide-functionalized liposome for enhanced targeted ovarian carcinoma therapy. Int J Clin Exp Pathol. 2015;8:207–216. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Banks W.A., Kastin A.J., Maness L.M., Huang W., Jaspan J.B. Permeability of the blood-brain barrier to amylin. Life Sci. 1995;57:1993–2001. doi: 10.1016/0024-3205(95)02197-q. [DOI] [PubMed] [Google Scholar]
- 27.Banks W.A., Kastin A.J. Differential permeability of the blood–brain barrier to two pancreatic peptides: insulin and amylin. Peptides. 1998;19:883–889. doi: 10.1016/s0196-9781(98)00018-7. [DOI] [PubMed] [Google Scholar]
- 28.Roth J.D. Amylin and the regulation of appetite and adiposity: recent advances in receptor signaling, neurobiology and pharmacology. Curr Opin Endocrinol Diabetes Obes. 2013;20:8–13. doi: 10.1097/MED.0b013e32835b896f. [DOI] [PubMed] [Google Scholar]
- 29.Chishti M.A., Yang D.S., Janus C., Phinney A.L., Horne P., Pearson J. Early-onset amyloid deposition and cognitive deficits in transgenic mice expressing a double mutant form of amyloid precursor protein 695. J Biol Chem. 2001;276:21562–21570. doi: 10.1074/jbc.M100710200. [DOI] [PubMed] [Google Scholar]
- 30.Janus C., Pearson J., McLaurin J., Mathews P.M., Jiang Y., Schmidt S.D. A beta peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer's disease. Nature. 2000;408:979–982. doi: 10.1038/35050110. [DOI] [PubMed] [Google Scholar]
- 31.Jhamandas JH, Vukojevic V, MacTavish D, Fu W. Microglial amylin receptors: a novel target for the actions of beta amyloid (Aβ) protein. Society for Neuroscience Meeting Abstracts 3901/B88, Chicago, IL, USA. 2015.
- 32.Vassar R. BACE1 inhibitor drugs in clinical trials for Alzheimer's disease. Alzheimers Res Ther. 2014;6:1–14. doi: 10.1186/s13195-014-0089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Husmann K., Sexton P.M., Fischer J.A., Born W. Mouse receptor-activity-modifying proteins 1, -2 and -3: amino acid sequence, expression and function. Mol Cell Endocrinol. 2000;162:35–43. doi: 10.1016/s0303-7207(00)00212-4. [DOI] [PubMed] [Google Scholar]
- 34.Hay D.L., Poyner D.R., Sexton P.M. GPCR modulation by RAMPs. Pharmacol Ther. 2006;109:173–197. doi: 10.1016/j.pharmthera.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 35.Di L. Strategic approaches to optimizing peptide ADME properties. AAPS J. 2014;17:134–143. doi: 10.1208/s12248-014-9687-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang H., Abedini A., Ruzsicska B., Raleigh D.P. Rationally designed, nontoxic, nonamyloidogenic analogues of human islet amyloid polypeptide with improved solubility. Biochemistry. 2014;53:5876–5884. doi: 10.1021/bi500592p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edvinsson L., Goadsby P.J., Uddman R. Amylin: localization, effects on cerebral arteries and on local cerebral blood flow in the cat. ScientificWorldJournal. 2001;1:168–180. doi: 10.1100/tsw.2001.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.