Abstract
Background
Poststroke depression (PSD) is a common form of stroke patients. Whether the risk of PSD is influenced by the stroke lesion location and sex remains a matter of debate. The objective of this study was to examine the association between the risk of PSD and the stroke lesion location and sex by performing a systematic meta‐analysis.
Methods
Subgroup analyses were performed according to the time interval after stroke onset to assessment for PSD. A total of 31 reports involving 5,309 subjects (for lesion location analysis) and 5,489 subjects (for sex analysis) suffering from stroke were included in this meta‐analysis.
Results
The pooled odds ratio (OR) of PSD after a left‐hemisphere stroke, compared with a right‐hemisphere stroke was 1.11 (95% confidence interval [CI] 0.82–1.49) and OR of PSD after a male stroke, compared with a female stroke was 0.68 (95% CI 0.58–0.81). Subacute poststroke subgroup (1–6 months) significantly favored PSD occurring after a left hemisphere stroke (OR = 1.50, 95% CI 1.21–1.87). Furthermore, there was a statistically significant association between PSD and female stroke for studies with acute poststroke group (OR = 0.73, 95% CI 0.62–0.86) and subacute poststroke stroke phase (OR = 0.69, 95% CI 0.56–0.86).
Conclusions
This systematic review suggests that patients with left hemisphere stroke may be more susceptible to PSD during subacute phase of stroke and female stroke may be more susceptible to PSD during acute and subacute phase of stroke.
Keywords: meta‐analysis, poststroke depression, sex, systematic review, the stroke lesion location
1. INTRODUCTION
Poststroke depression (PSD) has been recognized as a common sequela of stroke, with an increased morbidity and mortality (Williams, Ghose, & Swindle, 2004). PSD are approximately 85% of patients with strokes (Robinson & Jorge, 2016), and are associated with more serious functional dysfunction (Cully et al., 2005), delayed rehabilitation outcomes (Gillen, Tennen, McKee, Gernert‐Dott, & Affleck, 2001), as well as social withdrawal after stroke (Feibel & Springer, 1982; Robinson, Starr, Kubos, & Price, 1983). Furthermore, after depression remission over a long period of time, PSD have often a high risk of relapse (Ayerbe, Ayis, Rudd, Heuschmann, & Wolfe, 2011). In the last 40 years, there is a wide dissemination of the idea that the brain lesion location can influence the risk of depression after a stroke.
Although a large number of studies have focused on the association between the presence or absence of PSD and the stroke lesion location, the clinical association remains unclear (Robinson & Jorge, 2016). Robinson and colleagues at Johns Hopkins University first reported the hypothesis that the risk of becoming depressed after stroke onset is associated with the location of brain lesion and originates the concept of PSD (Robinson, Shoemaker, Schlumpf, Valk, & Bloom, 1975), and later left‐hemisphere strokes, especially, lesions of the left frontal region (Mayberg et al., 1988; Parikh et al., 1990; Robinson, Kubos, Starr, Rao, & Price, 1983). Subsequently, a large number of studies attempt to confirm these findings. Some studies have replicated the relationship between PSD and left‐hemisphere brain lesions. In contrast, some have even indicated the opposite effect—that PSD is related to right‐hemisphere brain lesions (MacHale, O'Rourke, Wardlaw, & Dennis, 1998). Recent systematically review studies have also not identified the association between the presence or absence of PSD and the stroke lesion location. Two systematical review and meta‐analyses suggest that there is no relationship between risk of PSD and a specific location of stroke (Carson et al., 2000; Hadidi, Treat‐Jacobson, & Lindquist, 2009). Bhogal and colleagues conduct a systematic review and maintain that PSD is associated with the left brain hemispheric lesion after stroke onset (Bhogal, Teasell, Foley, & Speechley, 2004), while Yu and colleagues report a weak association between PSD and right brain hemispheric lesion after stroke onset (Yu et al., 2004). A recent systematic review suggests the association between PSD and lesion location is affected by stratification of time between stroke and the assessment of PSD, and finds a strong relationship between PSD and right brain hemispheric lesion within subacute poststroke phase (Wei et al., 2015). These variances in the association between PSD and lesion location can be explained by the mixed stroke type (Esparrago Llorca, Castilla‐Guerra, Fernandez Moreno, Ruiz Doblado, & Jimenez Hernandez, 2015; Tsai, Anderson, Thomas, & Sudlow, 2016, 2015) and stratification of gender (Poynter et al., 2009; Mackay & Mensah, 2004). More especially, it is reported that there is a sex‐specific prevalence of PSD and these discrepancies in medical care and rehabilitation are considered to be reflected in a difference in the prevalence of PSD between the sexes (Poynter et al., 2009).Therefore, it is plausible that these variances in the association between PSD and stroke lesion location are reflected in gender effect on the association.
This systematic review extends the related and existed literatures and further explores the potential reasons of the heterogeneity that might exist among results. The principal aim of this study was to investigate the specific association between the presence or absence of PSD and stroke lesion location and sex more precisely and completely.
2. METHODS
2.1. Searching strategy
This systematic review included all studies in stroke patients with an assessment of depression, which examined the association between the presence or absence of PSD and the stroke lesion location and gender were initially eligible for inclusion.
We first performed a comprehensive searching strategy updated to 19 August 2016, via PubMed, PsycINFO, ISI Web of Science, EMBASE, and CINAHL. These keywords in our searching strategy were as follows: “stroke or cerebrovascular or poststroke or poststroke” and “depression or mood disorder or affective disorder or depressive disorder”. The on‐line abstracts were reviewed, and reprints of all potentially eligible studies were obtained.
In addition, we supplemented forward citation searches of key relevant reviews and perused the reference lists of included primary articles and relevant reviews by hand to identify additional citations not identified by the databases.
2.2. Study selection
The inclusion criteria used to choose subjects as follows: (1) patients in the studies experienced at least one stroke (over age 15) with men and/or women; (2) the relationship between PSD and stroke lesion location must have been investigated; (3) information sufficient for the computation of effect sizes must have been provided; (4) the studies defining depressive disorder as a diagnosis made using an operationalized definition (Diagnostic and Statistical Manual III, IIIR, or IV, or International Classification of Diseases, ninth or tenth revision); (5) imaging using either computed tomography (CT) or magnetic resonance imaging (MRI) scanning; (6) the studies must have been published in English and involved human subjects.
The exclusion criteria used to choose subjects as follows: (1) studies reported stroke patients in specific locations; (2) studies did reported the results stratified by sex; (3) studies reported stroke patients of a specific age group; (4) studies reported both stroke and head injury unless they reported separate results for stroke patients; (5) We excluded duplicate studies. The duplicate studies were defined by the following criteria: shared sampling frame, similar reported sample characteristics, the same study dates, and the same grant funding numbers. If the study was considered to be duplicate studies we selected the original data on the largest number of subjects. In cases of the same number of subjects, we selected the earliest one; (6) We excluded the only abstracts, case reports, review articles, retrospective recruitment studies or pharmacological intervention studies.
2.3. Data extraction
According to the inclusion and exclusion criteria, two reviewers (Zhang Y and Zhao H) extracted data from the selected studies independently. The primary reviewers made the final decision if the discrepancies were found between all authors. The following information in each included study was extracted, including: (1) first author name, (2) published year, (3) demographic characteristics, (4) method of diagnosis of depression, (5) sample size, (6) the time between stroke and assessment of depression, (7) assessment of lesion localization, (8) history of stroke or depression, (9) the source of patients (acute inpatients, rehabilitation units, community), (10) final conclusions. If a study was the lack of the related information required for our meta‐analysis, we made an attempt to contact the corresponding author to obtain the required information not reported.
2.4. Quality assessment
Two reviewers (Fang Y and Wang SS) evaluated the quality of included studies according to the Newcastle–Ottawa scale (NOS) independently (http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp). Using a “star” rating system, the NOS assesses the quality of the study according to three aspects, including selection, comparability, and exposure (case–control studies) or outcome (cohort studies).Therefore, The scores of each study were classified into the nine grades, ranging from 0 (worst) to 9 stars (best). The quality of each study was further divided into three grades: low (1–3 stars), intermediate (4–5 stars), or high (6–9 stars). Studies with a score equal to or higher than 4 could only be included into this meta‐analysis.
2.5. Statistics analysis
The statistical analysis and forest plots were performed using RevMan 5.1 software (RevMan, 2011). Taking into consideration possible heterogeneity among studies, we first performed a statistical test for heterogeneity using Cochran's Q statistics (p heterogeneity) and I 2 metrics (Gu et al., 2013). The pooled odds ratio (ORs) and 95% confidence interval (CIs) were evaluated using a random‐effects model when if the p heterogeneity < .10 or I 2 > 50%, it was considered to be significant heterogeneity; otherwise, we estimated them by a fixed‐effects model. To evaluate the stability of results, we conducted a sensitivity analysis. That is, by, to evaluate whether our results were affected due to removing one study at a time, we analyzed the rest studies and recalculate the pooled OR. We used the Begg's funnel plot and Egger's test to evaluate whether there was a potential publication bias (Begg & Mazumdar, 1994; Egger, Davey Smith, Schneider, & Minder, 1997). The statistical threshold was set at a p < .05 expect for Cochran's Q test (p heterogeneity < .10).
In addition, we performed subgroups analyses to explore potential sources of the heterogeneity based on each study characteristic. First, we included all studies and calculated the relationships of the presence or absence of PSD and stroke lesion location and sex. The results at the first follow‐up period were just included if several results at different follow‐up periods were reported. Second, we conducted subgroups analyses according to the timing of interview for PSD of the included studies. We named ≤1 month as acute poststroke phase, 1–6 months as subacute poststroke phase, and >6 months as chronic poststroke phase (Wei et al., 2015). According to the source of patients (clinic, rehabilitation center, and community), we also conducted a supplementary analysis.
3. RESULTS
3.1. Characteristics for studies included
We had a initial search and found 4,389 citations. According to the criteria outlined above after screening based on their abstracts, we initially excluded 3,758 citations. After we screened their full texts, we found that 31 original reports included categorical data on both depression and gender and were included in the meta‐analysis (Folstein, Maiberger, & McHugh, 1977; Andersen, Vestergaard, Ingemann‐Nielsen, & Lauritzen, 1995; Herrmann, Bartels, Schumacher, & Wallesch, 1995; Bjerg Bendsen, Bjerg Bendsen, Lauritzen, Vilmar, & Bech, 1997; Kase et al., 1998; Gainotti, Antonucci, Marra, & Paolucci, 2001; Singh et al., 2000; Desmond et al., 2003; Hsieh & Kao, 2005; Nys et al., 2005; Tang et al., 2005; Glodzik‐Sobanska et al., 2006; Caeiro, Ferro, Santos, & Figueira, 2006; Brodaty, Withall, Altendorf, & Sachdev, 2007; Fuentes, Ortiz, Sanjose, Frank, & Diez‐Tejedor, 2009; Nidhinandana et al., 2010; Nishiyama et al., 2010; Tennen et al., 2011; Shi et al., 2015; Choi‐Kwon et al., 2012; Zhang, Pan, Wang, & Zhao, 2013; Rajashekaran, Pai, Thunga, & Unnikrishnan, 2013; Angeleri et al., 1997; Finklestein et al., 1982; Grasso et al., 1994; Ng, Chan, & Straughan, 1995; Sun et al., 2014; Jiang, Lin, & Li, 2014; Saxena & Suman, 2015; Wei et al., 2016; Metoki et al., 2016). The study characteristics and demographics are provided in Table 1.
Table 1.
Characteristics of eligible studies
| Reference | Year | Source of patients | Prevalence of PSD, % | Onset since stroke | Quality assessment by NOS |
|---|---|---|---|---|---|
| Folstein et al. (1977) | 1977 | Rehabilitation | 45 | 30 days | ********* |
| Finklestein et al. (1982) | 1982 | Rehabilitation | 76 | Within 2 days | ******* |
| Grasso et al. (1994) | 1994 | Clinic | 53.3 | 2.5 months | ******** |
| Andersen et al. (1995) | 1995 | Clinic | 38.3 | Within 7 days | ******** |
| Herrmann et al. (1995) | 1995 | Clinic | 22 | Within 2 months | ****** |
| Ng et al. (1995) | 1995 | Rehabilitation | 55.8 | 21.6 days | ******* |
| Bjerg Bendsen et al. (1997) | 1997 | Rehabilitation | 15.6 | 2 weeks | ******** |
| Angeleri et al. (1997) | 1997 | Clinic | 29.7 | Within 10 days | ******* |
| Kase et al. (1998) | 1998 | Community | 37.9 | 1 week | ********* |
| Singh et al. (2000) | 2000 | Clinic | 36 | 3 months | ****** |
| Gainotti et al. (2001) | 2001 | Rehabilitation | 76.6 | 1 month | ******* |
| Desmond et al. (2003) | 2003 | Clinic | 11.2 | 3 months | ******* |
| Hsieh & Kao (2005) | 2005 | Clinic | 34.3 | Not given | ********* |
| Nys et al. (2005) | 2005 | Clinic | 52 | Within 3 weeks | ******* |
| Tang et al. (2005) gender | 2005 | Clinic | 16.4 | 3 months | ******* |
| Glodzik‐Sobanska et al. (2006) | 2006 | Clinic | 31 | 10 days | ******** |
| Caeiro et al. (2006) | 2006 | Clinic | 46 | <4 days | ******* |
| Brodaty et al. (2007) | 2007 | Clinic | 27.4 | Not given | ******** |
| Fuentes et al. (2009) | 2009 | Clinic | 28.8 | 10 days | ******** |
| Nidhinandana et al. (2010) | 2010 | Clinic | 46.5 | >6 months | ******* |
| Nishiyama et al. (2010) | 2010 | Clinic | 34.3 | 1 months | ******* |
| Tennen et al. (*2011) | 2011 | Clinic + rehabilitation | 37.3 | Within 4 months | ******* |
| Choi‐Kwon et al. (2012) | 2012 | Clinic | 13.7 | 3 months | ******* |
| Rajashekaran et al. (2013) | 2013 | Clinic | 45.2 |
<6 months >2 weeks |
******* |
| Zhang et al. (2013) | 2013 | Clinic | 27.5 | Within 2 weeks | ******* |
| Sun et al. (2014) | 2014 | Clinic | 31.4 | <6 months | ******** |
| Jiang et al. (2014) | 2014 | Clinic | 25 | 2–6 weeks | ******** |
| Saxena & Suman (2015) | 2015 | Clinic | 57 | Not given | ******* |
| Shi et al. (2015) | 2015 | Clinic | 29 | 14 days | ******* |
| Metoki et al. (2016) | 2016 | Rehabilitation | 16.9 | 10 days | ********* |
| Wei et al. (2016) | 2016 | Clinic | 19.3 | 7 days | ********* |
Asterisks indicate number of stars awarded for all items in quality assessment by NOS.
NOS, Newcastle–Ottawa scale; POS, post‐stroke depression.
3.2. Risk effect of assessment
First, all studies were pooled to assess the relationship between the prevalence of PSD and the stroke lesion location. The total 43 studies involved 5,309 stroke patients, including 2,123 left hemisphere stroke and 3,186 right hemisphere stroke. PSD with left hemisphere lesion were 675 patients and PSD with right hemisphere lesion were 963 patients. Because there was a significant heterogeneity (p heterogeneity < .00001, I 2 = 78%, Figure 1), the random‐effects model was used to synthesize the data. The pooled OR with 95% CI was 1.11 (0.82–1.49) for the relationship between the PSD risk and the stroke lesion location (Figure 1).
Figure 1.
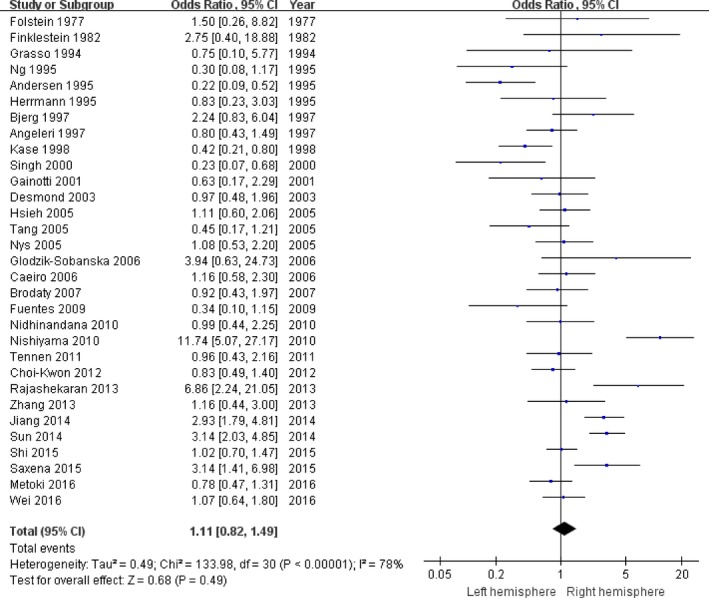
The forest plots of odds ratio with 95% confidence interval for the overall association between stroke lesion location and depression risk
Second, all studies were pooled to assess the relationship between the prevalence of PSD and the gender. The total 43 studies involved 5,489 stroke subjects, including 3,228 male strokes and 2,261 with female strokes. There were 872 PSD with male patients and 771 PSD with female patients. The random‐effects mode was chose l to synthesize the data due to significant heterogeneity (p heterogeneity < .04, I 2 = 33%, Figure 2). The pooled OR with 95% CI was 0.68 (0.58–0.81) for the relationship between PSD risk and gender (Figure 2).
Figure 2.
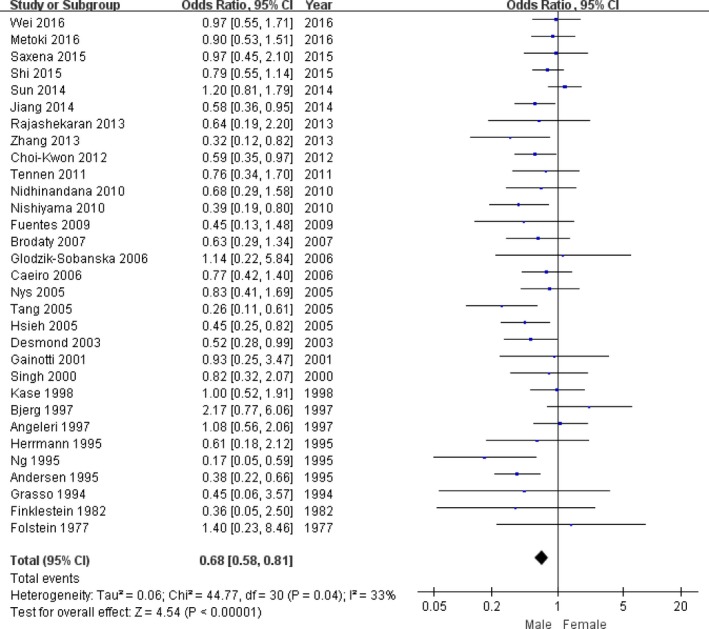
The forest plots of odds ratio with 95% confidence interval for the overall association between gender and depression risk
3.3. Source populations
Subgroups analyses were performed according to population source and are provided in SI results and Figures S1–S3
3.4. Timing of first interview after stroke
Subgroups analyses were performed according to the timing of interview for PSD. In assessing the relationships between the prevalence of PSD and the stroke lesion location, our results showed that significant heterogeneity was found in acute poststroke group (n = 15, p heterogeneity < .00001, I 2 = 72%, Figure 3a), and subacute poststroke group (n = 11, p heterogeneity < .00001, I 2 = 85%, Figure 3b). Therefore, the random‐effects model was performed to synthesize the data. There was only a statistically significant association between PSD and left hemisphere stroke for subacute poststroke group (OR = 1.50, 95% CI 1.21–1.87, Figure 3b). Our results indicated that the stroke patients with left hemisphere lesion may be more susceptible to PSD during subacute phase of stroke. Because the study of chronic poststroke group in this meta‐analysis only included 1 study (Figure 3c), it did not reveal any associations.
Figure 3.
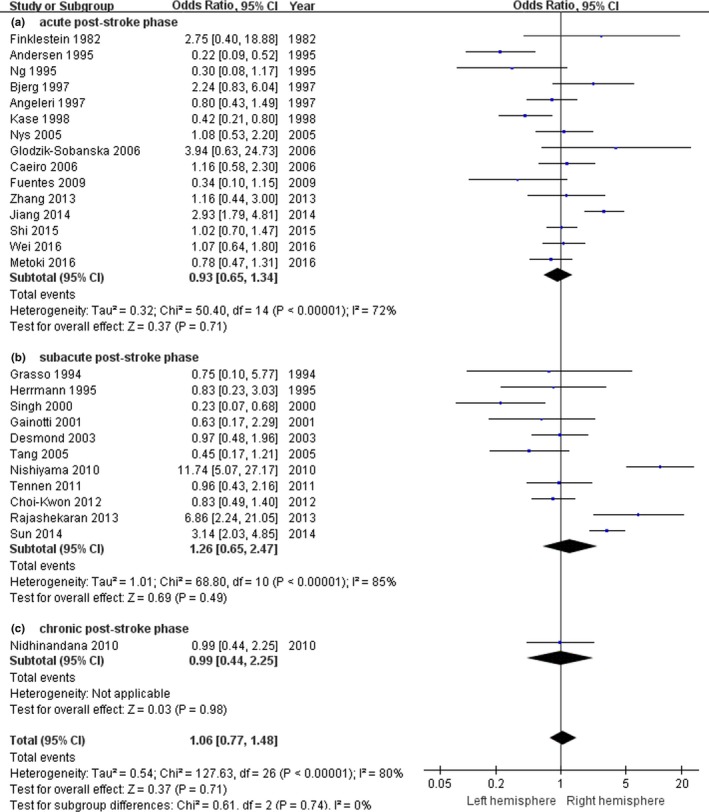
The forest plots of odds ratio with 95% confidence interval for the association between lesion location and depression risk according to time since stroke onset (n = 27)
In assessing the relationship between the sex and the prevalence of PSD, our results showed that there were significant heterogeneity in acute poststroke group (n = 15, p heterogeneity = .04, I 2 = 43%, Figure 4a), and subacute poststroke group (n = 11, p heterogeneity = .07, I 2 = 41%, Figure 4b). Thus, the random‐effects model was performed to synthesize the data. There was a statistically significant association between PSD and female stroke for the acute poststroke group (OR = 0.73, 95% CI 0.62–0.86, Figure 4b) and the subacute poststroke group (OR = 0.69, 95% CI 0.56–0.86, Figure 4b). Our results indicated that female stroke patients may be more susceptible to PSD during acute and subacute phase of stroke. Because the study of chronic poststroke group in this meta‐analysis only included 1 study (Figure 4c), it did not reveal any associations.
Figure 4.
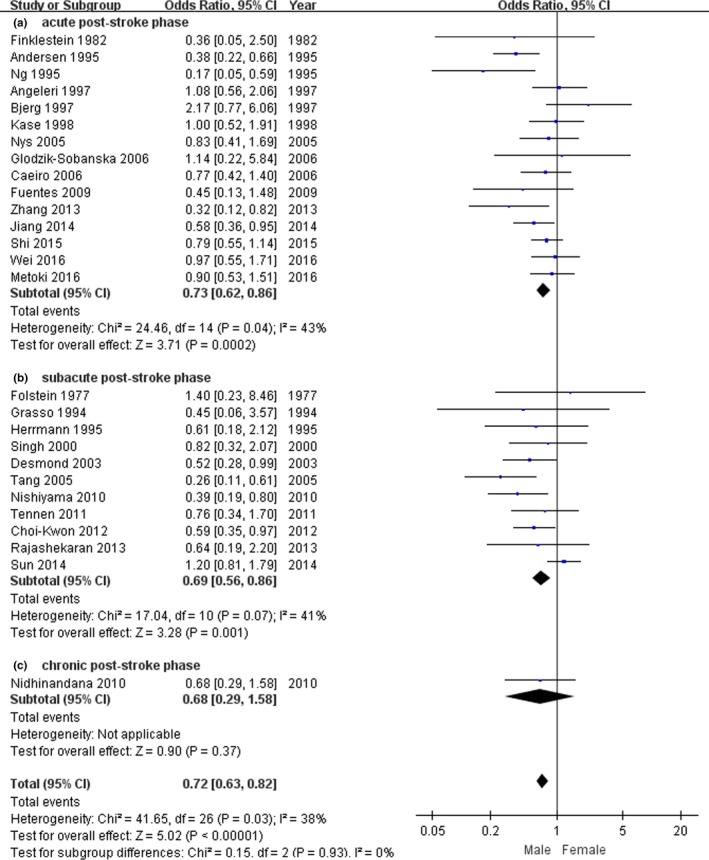
The forest plots of odds ratio with 95% confidence interval for the association between gender and depression risk according to time since stroke onset (n = 27)
3.5. Quality assessment and sensitivity analyses
Quality assessment was provided in Table 1. The sensitive analyses were performed to assess the influence of any single study on the pooled OR. As shown in Table S1 and Table S2, our results showed that the corresponding ORs were not materially changed when any single study was omitted.
3.6. Publication bias
As shown in Figure 5, our results showed that no obvious asymmetry was found in the assessment of the funnel plots. No statistically significant differences were shown in the Egger's test analyses (p > .05).
Figure 5.
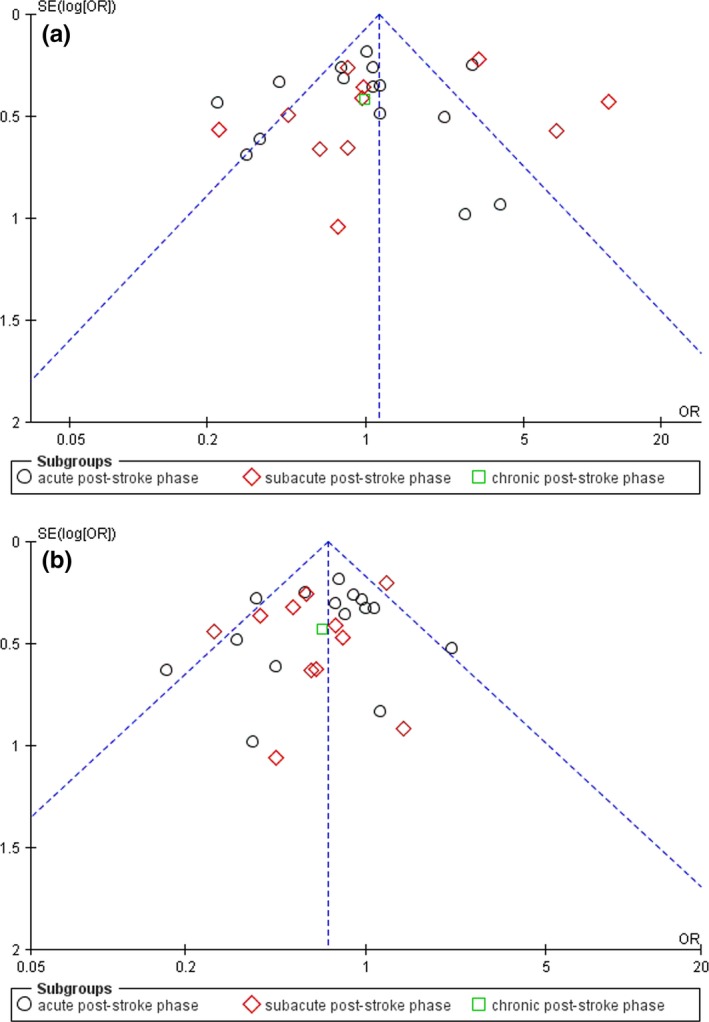
Funnel plot was designed to visualize a potential publication bias according to time since stroke onset (n = 27) (a) representing a potential publication bias for developing post‐stroke depression (PSD) after left hemisphere stroke. (b) representing a potential publication bias for developing PSD after male stroke
4. DISCUSSION
PSD has proved to be a common sequela of stroke. Available clinical data about whether the risk of PSD is influenced by the location of the brain lesion and sex are unclear. Our meta‐analysis revealed that a significant association was found between the presence of PSD and left hemisphere lesions in subacute poststroke patients and PSD appears to be more susceptible to women PSD than men during acute and subacute poststroke phases.
4.1. Limitations of meta‐analysis in this study
There are several potential limitations in this study. Firstly, there was the possibility of information and selection biases and unidentified confounders because all available clinical data were from studies with the full text published in English. Secondly, although the brain stroke lesion and sex were significantly correlated with PSD, it is difficult to determine whether PSD is due to the clinical consequences of stroke. In future studies, we should consider to compare the control group, stroke group with no depression, and PSD to investigate the issue. Finally, it varied widely for the scales for depression assessment across different studies, and it was also not unified in the cut‐off points for different degrees of depressive symptoms.
4.2. Development of an appropriate and standardized measure of depression
It is difficult to measure the depression symptom in stroke patients. The phenomenology of PSD should be considered to be different from the noncomorbid depression. The measurement of PSD would take into account the impairments of function, cognition, and language that often accompany stroke (Bhogal et al., 2004; Wei et al., 2015). For example, stroke was considered to directly lead to the clinical symptoms of psychomotor retardation, fatigue, and sleep and appetite disturbances. These symptoms were classified as depressive symptoms by most conventional scales, which may result in an increased false‐positive depression scores (Wei et al., 2015). Therefore, it needs to develop an appropriate and standardized measure of depression to diagnose PSD.
4.3. Optimal time after stroke onset to assessment for PSD
Growing evidence has indicated that different clinical PSD subtypes are classified according to the different time interval between the depression diagnosis and the stroke onset and may exist different pathological mechanisms (Wei et al., 2015). A large number of studies have reported that the time interval can influence the relationship between the occurrence of PSD and the location of brain stroke lesion. The ≤3 months acute poststroke significantly favored PSD occurring after left brain anterior lesions (Santos et al., 2009; Shimoda & Robinson, 1999). During the 3–6 months poststroke period, the PSD occurring could be influenced by the proximity of the lesion to the frontal pole in both hemispheres (Santos et al., 2009; Shimoda & Robinson, 1999). The 1–2 years chronic stroke patients significantly favored PSD occurring after left hemisphere lesion. Furthermore, the occurrence of PSD with right brain hemisphere lesion is related to the stroke lesion size and closely to the occipital pole (Santos et al., 2009; Shimoda & Robinson, 1999). Carson et al. (2000) have indicated that PSD is not associated with stroke lesion location. Narushima, Kosier, & Robinson (2003) have reported that the occurrence of PSD is associated with left frontal and left basal ganglia lesions when depression was assessed within 2 months after stroke. Yu et al. (2004) have reported that the occurrence of PSD is associated with right hemisphere lesions during 4–9 months after stroke. Two meta‐analysis studies from either acute or chronic stroke patients have reported no significant association between PSD and lesion location (Ayerbe, Ayis, Wolfe, & Rudd, 2013; Kutlubaev & Hackett, 2014). However, a recent meta‐analysis showed that PSD has been associated right hemisphere lesion during 1–6 months after stroke (Wei et al., 2015). Our conclusion revealed that the occurrence of PSD is associated with left hemisphere lesions in subacute poststroke patients. This supported our view that these variances in the association between PSD and lesion location can be explained by the stratification of gender. Therefore, it is plausible that these variances in the association between PSD and stroke lesion location are reflected in gender effect on the association.
In addition, Sinyor et al. (1986) have indicated that the sex differences in the frequency of PSD may be masked by the excluding patients, who had a history of psychiatric disorder. More especially, it is reported that there is a sex‐specific prevalence of PSD. And the sex discrepancy in the prevalence of PSD are considered to reflect these differences in rehabilitation and medical care (Poynter et al., 2009). In our systematic review, the occurrence of PSD appears to be more common among women than men in acute and subacute poststroke patients. Reasons for higher prevalence of women PSD may be explained that the general population have the greater prevalence, such as psychosocial inequities and genetic factors and may also be explained by recovery, differential support, and access to rehabilitation (Grace et al., 2005). Furthermore, women are considered to be more likely than men to be diagnosed with some psychiatric disorders (Bekhbat & Neigh, 2017). For instance, Paradiso & Robert (1998) suggest that the sex discrepancy may owe to the differences of brain functioning and organization and further suggest that the women PSD is related to left‐sided brain lesions, but not men PSD. In the women PSD, the younger age, a history of psychiatric disorder, and impaired cognition are considered to be the psychosocial risk factors (Lavie, Milani, Cassidy, & Gilliland, 1999). Future study is needed to determine which factors may explain the greater rate of the women PSD and assess whether sex‐specific differences should be considered to be include as a factor in response to treatment.
In conclusion, converging evidence suggests that in the future, a prospective cohort study will be best suited for assessing the association between lesion location and sex and PSD. The clinical implications of this systematic review include the need for screening and identification of PSD among female stroke patients according to the different time interval between the depression diagnosis and stroke onset, in particular, and then provision of appropriate treatment.
CONFLICT OF INTEREST
The authors declare no competing financial interests.
Supporting information
ACKNOWLEDGMENTS
This study was supported by the Medical Science and Technique Research Projects of Henan Province (No. 2011030040).
Zhang Y, Zhao H, Fang Y, Wang S, Zhou H. The association between lesion location, sex and poststroke depression: Meta‐analysis. Brain Behav. 2017;7:e00788 https://doi.org/10.1002/brb3.788
REFERENCES
- Andersen, G. , Vestergaard, K. , Ingemann‐Nielsen, M. , & Lauritzen, L. (1995). Risk factors for post‐stroke depression. Acta Psychiatrica Scand., 92, 193–198. [DOI] [PubMed] [Google Scholar]
- Angeleri, F. , Angeleri, V. A. , Foschi, N. , Giaquinto, S. , Nolfe, G. , Saginario, A. , & Signorino, M. (1997). Depression after stroke: An investigation through catamnesis. Journal of Clinical Psychiatry, 58, 261–265. [DOI] [PubMed] [Google Scholar]
- Ayerbe, L. , Ayis, S. , Rudd, A. G. , Heuschmann, P. U. , & Wolfe, C. D. (2011). Natural history, predictors, and associations of depression 5 years after stroke: The South London Stroke Register. Stroke, 42, 1907–1911. [DOI] [PubMed] [Google Scholar]
- Ayerbe, L. , Ayis, S. , Wolfe, C. D. , & Rudd, A. G. (2013). Natural history, predictors and outcomes of depression after stroke: Systematic review and meta‐analysis. British Journal of Psychiatry, 202, 14–21. [DOI] [PubMed] [Google Scholar]
- Begg, C. B. , & Mazumdar, M. (1994). Operating characteristics of a rank correlation test for publication bias. Biometrics, 50, 1088–1101. [PubMed] [Google Scholar]
- Bekhbat, M. , & Neigh, G. N. (2017). Sex differences in the neuro‐immune consequences of stress: Focus on depression and anxiety. Brain, Behavior, and Immunity, https://doi.org/10.1016/j.bbi.2017.02.006. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhogal, S. K. , Teasell, R. , Foley, N. , & Speechley, M. (2004). Lesion location and poststroke depression: Systematic review of the methodological limitations in the literature. Stroke, 35, 794–802. [DOI] [PubMed] [Google Scholar]
- Bjerg Bendsen, B. , Bjerg Bendsen, E. , Lauritzen, L. , Vilmar, T. , & Bech, P. (1997). Post‐stroke patients in rehabilitation. The relationship between biological impairment (CT scanning), physical disability and clinical depression. European Psychiatry: The Journal of the Association of European Psychiatrists, 12, 399–404. [DOI] [PubMed] [Google Scholar]
- Brodaty, H. , Withall, A. , Altendorf, A. , & Sachdev, P. S. (2007). Rates of depression at 3 and 15 months poststroke and their relationship with cognitive decline: The Sydney Stroke Study. American Journal of Geriatric Psychiatry, 15, 477–486. [DOI] [PubMed] [Google Scholar]
- Caeiro, L. , Ferro, J. M. , Santos, C. O. , & Figueira, M. L. (2006). Depression in acute stroke. Journal of Psychiatry and Neuroscience, 31, 377–383. [PMC free article] [PubMed] [Google Scholar]
- Carson, A. J. , MacHale, S. , Allen, K. , Lawrie, S. M. , Dennis, M. , House, A. , & Sharpe, M. (2000). Depression after stroke and lesion location: A systematic review. Lancet, 356, 122–126. [DOI] [PubMed] [Google Scholar]
- Choi‐Kwon, S. , Han, K. , Choi, S. , Suh, M. , Kim, Y. J. , Song, H ., … Kim, J. S . (2012). Poststroke depression and emotional incontinence: Factors related to acute and subacute stages. Neurology, 78, 1130–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cully, J. A. , Gfeller, J. D. , Heise, R. A. , Ross, M. J. , Teal, C. R. , & Kunik, M. E. (2005). Geriatric depression, medical diagnosis, and functional recovery during acute rehabilitation. Archives of Physical Medicine and Rehabilitation, 86, 2256–2260. [DOI] [PubMed] [Google Scholar]
- Desmond, D. W. , Remien, R. H. , Moroney, J. T. , Stern, Y. , Sano, M. , & Williams, J. B. (2003). Ischemic stroke and depression. Journal of the International Neuropsychological Society, 9, 429–439. [DOI] [PubMed] [Google Scholar]
- Egger, M. , Davey Smith, G. , Schneider, M. , & Minder, C. (1997). Bias in meta‐analysis detected by a simple, graphical test. BMJ, 315, 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esparrago Llorca, G. , Castilla‐Guerra, L. , Fernandez Moreno, M. C. , Ruiz Doblado, S. , & Jimenez Hernandez, M. D. (2015). Post‐stroke depression: An update. Neurologia, 30, 23–31. [DOI] [PubMed] [Google Scholar]
- Feibel, J. H. , & Springer, C. J. (1982). Depression and failure to resume social activities after stroke. Archives of Physical Medicine and Rehabilitation, 63, 276–277. [PubMed] [Google Scholar]
- Finklestein, S. , Benowitz, L. I. , Baldessarini, R. J. , Arana, G. W. , Levine, D. , Woo, E. , … Stoll, A. L . (1982). Mood, vegetative disturbance, and dexamethasone suppression test after stroke. Annals of Neurology, 12, 463–468. [DOI] [PubMed] [Google Scholar]
- Folstein, M. F. , Maiberger, R. , & McHugh, P. R. (1977). Mood disorder as a specific complication of stroke. Journal of Neurology, Neurosurgery and Psychiatry, 40, 1018–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes, B. , Ortiz, X. , Sanjose, B. , Frank, A. , & Diez‐Tejedor, E. (2009). Post‐stroke depression: Can we predict its development from the acute stroke phase? Acta Neurologica Scandinavica, 120, 150–156. [DOI] [PubMed] [Google Scholar]
- Gainotti, G. , Antonucci, G. , Marra, C. , & Paolucci, S. (2001). Relation between depression after stroke, antidepressant therapy, and functional recovery. Journal of Neurology, Neurosurgery and Psychiatry, 71, 258–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillen, R. , Tennen, H. , McKee, T. E. , Gernert‐Dott, P. , & Affleck, G. (2001). Depressive symptoms and history of depression predict rehabilitation efficiency in stroke patients. Archives of Physical Medicine and Rehabilitation, 82, 1645–1649. [DOI] [PubMed] [Google Scholar]
- Glodzik‐Sobanska, L. , Slowik, A. , McHugh, P. , Sobiecka, B. , Kozub, J. , Rich, K. E. , … Szczudlik, A . (2006). Single voxel proton magnetic resonance spectroscopy in post‐stroke depression. Psychiatry Research, 148, 111–120. [DOI] [PubMed] [Google Scholar]
- Grace, S. L. , Abbey, S. E. , Pinto, R. , Shnek, Z. M. , Irvine, J. , & Stewart, D. E. (2005). Longitudinal course of depressive symptomatology after a cardiac event: Effects of gender and cardiac rehabilitation. Psychosomatic Medicine, 67, 52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasso, M. G. , Pantano, P. , Ricci, M. , Intiso, D. F. , Pace, A. , Padovani, A ., … Lenzi, G. L . (1994). Mesial temporal cortex hypoperfusion is associated with depression in subcortical stroke. Stroke, 5, 980–985. [DOI] [PubMed] [Google Scholar]
- Gu, L. , Su, L. , Wu, G. , Chen, Q. , Yan, Y. , Xie, J. , … Tang, N . (2013). Association between TNF‐delta 238G/A polymorphisms and the risk of ischemic stroke. International Journal of Neuroscience, 123, 1–6. [DOI] [PubMed] [Google Scholar]
- Hadidi, N. , Treat‐Jacobson, D. J. , & Lindquist, R. (2009). Poststroke depression and functional outcome: A critical review of literature. Heart and Lung, 38, 151–162. [DOI] [PubMed] [Google Scholar]
- Herrmann, M. , Bartels, C. , Schumacher, M. , & Wallesch, C. W. (1995). Poststroke depression. Is there a pathoanatomic correlate for depression in the postacute stage of stroke? Stroke, 26, 850–856. [DOI] [PubMed] [Google Scholar]
- Hsieh, L. P. , & Kao, H. J. (2005). Depressive symptoms following ischemic stroke: A study of 207 patients. Acta Neurologica Taiwanica, 14, 187–190. [PubMed] [Google Scholar]
- Jiang, X. G. , Lin, Y. , & Li, Y. S. (2014). Correlative study on risk factors of depression among acute stroke patients. European Review for Medical and Pharmacological Sciences, 18, 1315–1323. [PubMed] [Google Scholar]
- Kase, C. S. , Wolf, P. A. , Kelly‐Hayes, M. , Kannel, W. B. , Beiser, A. , & D'Agostino, R. B. (1998). Intellectual decline after stroke: The Framingham Study. Stroke, 29, 805–812. [DOI] [PubMed] [Google Scholar]
- Kutlubaev, M. A. , & Hackett, M. L. (2014). Part II: Predictors of depression after stroke and impact of depression on stroke outcome: An updated systematic review of observational studies. International Journal of Stroke, 9, 1026–1036. [DOI] [PubMed] [Google Scholar]
- Lavie, C. J. , Milani, R. V. , Cassidy, M. M. , & Gilliland, Y. E. (1999). Effects of cardiac rehabilitation and exercise training programs in women with depression. American Journal of Cardiology, 3, 1480–1483, A7. [DOI] [PubMed] [Google Scholar]
- MacHale, S. M. , O'Rourke, S. J. , Wardlaw, J. M. , & Dennis, M. S. (1998). Depression and its relation to lesion location after stroke. Journal of Neurology, Neurosurgery and Psychiatry, 64, 371–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay, J. , & Mensah, G. (2004). The atlas of heart disease and stroke. Geneva, Switzerland: World Health Organization. [Google Scholar]
- Mayberg, H. S. , Robinson, R. G. , Wong, D. F. , Parikh, R. , Bolduc, P. , Starkstein, S. E. , … Wilson, A. A. (1988). PET imaging of cortical S2 serotonin receptors after stroke: Lateralized changes and relationship to depression. American Journal of Psychiatry, 145, 937–943. [DOI] [PubMed] [Google Scholar]
- Metoki, N. , Sugawara, N. , Hagii, J. , Saito, S. , Shiroto, H. , Tomita, T ., … Yasui‐Furukori, N . (2016). Relationship between the lesion location of acute ischemic stroke and early depressive symptoms in Japanese patients. Annals of General Psychiatry, 15, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narushima, K. , Kosier, J. T. , & Robinson, R. G. (2003). A reappraisal of poststroke depression, intra‐ and inter‐hemispheric lesion location using meta‐analysis. Journal of Neuropsychiatry and Clinical Neurosciences, 15, 422–430. [DOI] [PubMed] [Google Scholar]
- Ng, K. C. , Chan, K. L. , & Straughan, P. T. (1995). A study of post‐stroke depression in a rehabilitative center. Acta Psychiatrica Scand., 92, 75–79. [DOI] [PubMed] [Google Scholar]
- Nidhinandana, S. , Sithinamsuwan, P. , Chinvarun, Y. , Wongmek, W. , Supakasem, S. , & Suwantamee, J. (2010). Prevalence of poststroke depression in Thai stroke survivors studied in Phramongkutklao Hospital. Journal of the Medical Association of Thailand, 93(Suppl 6), S60–S64. [PubMed] [Google Scholar]
- Nishiyama, Y. , Komaba, Y. , Ueda, M. , Nagayama, H. , Amemiya, S. , & Katayama, Y. (2010). Early depressive symptoms after ischemic stroke are associated with a left lenticulocapsular area lesion. Journal of Stroke and Cerebrovascular Diseases: the Official Journal of National Stroke Association, 19, 184–189. [DOI] [PubMed] [Google Scholar]
- Nys, G. M. , van Zandvoort, M. J. , van der Worp, H. B. , de Haan, E. H. , de Kort, P. L. , & Kappelle, L. J. (2005). Early depressive symptoms after stroke: Neuropsychological correlates and lesion characteristics. Journal of the Neurological Sciences, 228, 27–33. [DOI] [PubMed] [Google Scholar]
- Paradiso, S. , & Robert, R. (1998). Gender differences in poststroke depression. Journal of Neuropsychiatry and Clinical Neurosciences, 10, 41–47. [DOI] [PubMed] [Google Scholar]
- Parikh, R. M. , Robinson, R. G. , Lipsey, J. R. , Starkstein, S. E. , Fedoroff, J. P. , & Price, T. R. (1990). The impact of poststroke depression on recovery in activities of daily living over a 2‐year follow‐up. Archives of Neurology, 47, 785–789. [DOI] [PubMed] [Google Scholar]
- Poynter, B. , Shuman, M. , Diaz‐Granados, N. , Kapral, M. , Grace, S. L. , & Stewart, D. E. (2009). Sex differences in the prevalence of post‐stroke depression: A systematic review. Psychosomatics, 50, 563–569. [DOI] [PubMed] [Google Scholar]
- Rajashekaran, P. , Pai, K. , Thunga, R. , & Unnikrishnan, B. (2013). Post‐stroke depression and lesion location: A hospital based cross‐sectional study. Indian Journal of Psychiatry, 55, 343–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RevMan (2011). Review manager (RevMan), version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration. [Google Scholar]
- Robinson, R. G. , & Jorge, R. E. (2016). Post‐stroke depression: A review. American Journal of Psychiatry, 173, 221–231. [DOI] [PubMed] [Google Scholar]
- Robinson, R. G. , Kubos, K. L. , Starr, L. B. , Rao, K. , & Price, T. R. (1983). Mood changes in stroke patients: Relationship to lesion location. Comprehensive Psychiatry, 24, 555–566. [DOI] [PubMed] [Google Scholar]
- Robinson, R. G. , Shoemaker, W. J. , Schlumpf, M. , Valk, T. , & Bloom, F. E. (1975). Effect of experimental cerebral infarction in rat brain on catecholamines and behaviour. Nature, 255, 332–334. [DOI] [PubMed] [Google Scholar]
- Robinson, R. G. , Starr, L. B. , Kubos, K. L. , & Price, T. R. (1983). A two‐year longitudinal study of post‐stroke mood disorders: Findings during the initial evaluation. Stroke, 14, 736–741. [DOI] [PubMed] [Google Scholar]
- Santos, M. , Kovari, E. , Gold, G. , Bozikas, V. P. , Hof, P. R. , Bouras, C. , & Giannakopoulos, P. (2009). The neuroanatomical model of post‐stroke depression: Towards a change of focus? Journal of the Neurological Sciences, 283, 158–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena, A. , & Suman, A. (2015). Magnitude and determinants of depression in acute stroke patients admitted in a rural tertiary care hospital. Journal of Neurosciences in Rural Practice, 6, 202–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, Y. , Xiang, Y. , Yang, Y. , Zhang, N. , Wang, S. , Ungvari, G. S ., … Wang, C . (2015). Depression after minor stroke: Prevalence and predictors. Journal of Psychosomatic Research, 79, 143–147. [DOI] [PubMed] [Google Scholar]
- Shimoda, K. , & Robinson, R. G. (1999). The relationship between poststroke depression and lesion location in long‐term follow‐up. Biological Psychiatry, 45, 187–192. [DOI] [PubMed] [Google Scholar]
- Singh, A. , Black, S. E. , Herrmann, N. , Leibovitch, F. S. , Ebert, P. L. , Lawrence, J. , & Szalai, J. P. (2000). Functional and neuroanatomic correlations in poststroke depression: The Sunnybrook Stroke Study. Stroke, 31, 637–644. [DOI] [PubMed] [Google Scholar]
- Sinyor, D. , Jacques, P. , Kaloupek, D. G. , Becker, R. , Goldenberg, M. , & Coopersmith, H. (1986). Poststroke depression and lesion location. An attempted replication. Brain, 109(Pt 3), 537–546. [DOI] [PubMed] [Google Scholar]
- Sun, N. , Li, Q. J. , Lv, D. M. , Man, J. , Liu, X. S. , & Sun, M. L. (2014). A survey on 465 patients with post‐stroke depression in China. Archives of Psychiatric Nursing, 28, 368–371. [DOI] [PubMed] [Google Scholar]
- Tang, W. K. , Chan, S. S. , Chiu, H. F. , Ungvari, G. S. , Wong, K. S. , Kwok, T. C. , … Ahuja, A. T . (2005). Poststroke depression in Chinese patients: Frequency, psychosocial, clinical, and radiological determinants. Journal of Geriatric Psychiatry and Neurology, 18, 45–51. [DOI] [PubMed] [Google Scholar]
- Tennen, G. , Herrmann, N. , Black, S. E. , Levy, K. S. , Cappell, J. , Li, A. , & Lanctôt, K. L. (2011). Are vascular risk factors associated with post‐stroke depressive symptoms? Journal of Geriatric Psychiatry and Neurology, 24, 215–221. [DOI] [PubMed] [Google Scholar]
- Tsai, C. F. , Anderson, N. , Thomas, B. , & Sudlow, C. L. (2015). Risk factors for ischemic stroke and its subtypes in Chinese vs. Caucasians: Systematic review and meta‐analysis. International Journal of Stroke, 10, 485–493. [DOI] [PubMed] [Google Scholar]
- Tsai, C. F. , Anderson, N. , Thomas, B. , & Sudlow, C. L. (2016). Comparing risk factor profiles between intracerebral hemorrhage and ischemic stroke in Chinese and White populations: Systematic review and meta‐analysis. PLoS ONE, 11, e0151743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, N. , Yong, W. , Li, X. , Zhou, Y. , Deng, M. , Zhu, H. , & Jin, H. (2015). Post‐stroke depression and lesion location: A systematic review. Journal of Neurology, 262, 81–90. [DOI] [PubMed] [Google Scholar]
- Wei, C. , Zhang, F. , Chen, L. , Ma, X. , Zhang, N. , & Hao, J. (2016). Factors associated with post‐stroke depression and fatigue: Lesion location and coping styles. Journal of Neurology, 263, 269–276. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Williams, L. S. , Ghose, S. S. , & Swindle, R. W. (2004). Depression and other mental health diagnoses increase mortality risk after ischemic stroke. American Journal of Psychiatry, 161, 1090–1095. [DOI] [PubMed] [Google Scholar]
- Yu, L. , Liu, C. K. , Chen, J. W. , Wang, S. Y. , Wu, Y. H. , & Yu, S. H. (2004). Relationship between post‐stroke depression and lesion location: A meta‐analysis. Kaohsiung Journal of Medical Sciences, 20, 372–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, W. N. , Pan, Y. H. , Wang, X. Y. , & Zhao, Y. (2013). A prospective study of the incidence and correlated factors of post‐stroke depression in China. PLoS ONE, 8, e78981. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


