Abstract
Introduction
There is an urgent need to develop new treatments for Alzheimer's disease (AD) and to understand the drug development process for new AD therapies.
Methods
We assessed the agents in the AD pipeline as documented in clinicaltrials.gov for phase I, phase II, and phase III, accessed 1/5/2017.
Results
There are 105 agents in the AD treatment development pipeline, of which 25 agents are in 29 trials in phase I, 52 agents are in 68 trials in phase II, and 28 agents are in 42 trials in phase III. Seventy percent of drugs in the AD pipeline are disease-modifying therapies (DMTs). Fourteen percent are symptomatic cognitive enhancers, and 13% are symptomatic agents addressing neuropsychiatric and behavioral changes (2% have undisclosed mechanisms). Most trials are sponsored by the biopharmaceutical industry. Trials include patients with preclinical AD (cognitively normal with biomarker evidence of AD), prodromal AD (mild cognitive symptoms and biomarker evidence of AD), and AD dementia. Biomarkers are included in many drug development programs particularly those for DMTs. Thirteen of 46 phase II DMT trials have amyloid imaging as an entry criterion, and 10 of 28 phase III trials incorporate amyloid imaging for diagnosis and entry. A large number of participants are needed for AD clinical trials; in total, 54,073 participants are required for trials spanning preclinical AD to AD dementia. When compared with the 2016 pipeline, there are eight new agents in phase I, 16 in phase II, and five in phase III.
Discussion
The AD drug development pipeline has 105 agents divided among phase I, phase II, and phase III. The trials include a wide range of clinical trial populations, many mechanisms of action, and require a substantial number of clinical trial participants. Biomarkers are increasingly used in patient identification and as outcome measures, particularly in trials of DMTs.
Keywords: Alzheimer's disease, Phase I, Phase II, Phase III, Biomarkers, Preclinical AD, Prodromal AD, AD dementia
Alzheimer's disease (AD) is increasing rapidly in frequency as the world's population ages and more people enter the major risk period for this age-related disorder. From the 5.3 million US citizens affected now, the number of victims will increase to 13 million or more by 2050; worldwide the total number of affected individuals will increase to a staggering 100 million [1]. The cost of care in the US, currently more than $200 billion annually, will grow to an unsupportable $1 trillion annually by 2050 [1].
New therapies are urgently needed to treat affected patients and to prevent, defer, slow the decline, or improve the symptoms of AD. It has been estimated that the overall frequency of the disease would be decreased by nearly 50% if the onset of the disease could be delayed by 5 years [2]. Symptomatic treatments are drugs aimed at cognitive enhancement or control of neuropsychiatric symptoms and typically work through neurotransmitter mechanisms; disease-modifying therapies or treatments (DMTs) are agents that prevent, delay, or slow progression and target the underlying pathophysiologic mechanisms of AD [3].
To understand the progress of drug development, describe the timelines regarding when drugs could become available, and interrogate the current drug development approaches for AD treatments, we examined the AD drug development pipeline as currently revealed in clinicaltrials.gov. We present our findings as a means of understanding and ultimately improving AD drug development. This paper continues the themes developed in our 2016 pipeline report [4] and impacts the understanding of the likelihood of reaching the national goal of having meaningful therapy for AD by 2025 [5].
1. Methods
Clinicaltrials.gov includes a comprehensive list of all clinical trials of AD and describes the trial features in text form. The “Common Rule” governing clinicaltrials.gov was updated in 2016 [6], [7]. Registration is mandated for all trials from sponsors with an Investigational New Drug or Investigational New Device. Trials must be submitted to the site within 21 days of the enrollment of the first trial participant. Results must be submitted to clincaltrials.gov within 12 months of completion of final data collection for the prespecified primary outcome measures; clinicaltrials.gov can be regarded as a comprehensive resource for the study of clinical trials governed by the US Food and Drug Administration (FDA) or the National Institutes of Health (NIH). Not all non-US trials are registered on clinicaltrials.gov—especially phase I trials. We also cannot attest to compliance with the rule governing clinicaltrials.gov, and some agents may not be registered or sponsors may not adhere to required timelines.
We examined clinicaltrials.gov as of January 5, 2017. We captured all trials of all agents in phases I, II, and III. In a comprehensive database, we entered the trial title, beginning date, projected end date, calculated duration, number of subjects planned to be enrolled, number of arms of the study (usually a placebo arm and one or more treatment arms with different doses of the experimental agent), whether a biomarker was described, and sponsorship by a biopharma company, NIH, academic medical center, “other” entity such as a consortium or a philanthropic organization, or a combination of the aforementioned sponsors. We included trials that were recruiting, active but not recruiting (e.g., trials that have completed recruiting and are continuing as the efficacy or safety of the agent is being determined), enrolling by invitation, and not yet recruiting. We did not include trials listed as completed, terminated, suspended, or withdrawn. Information on these trials or the reasons for suspension or termination is often incomplete. The choice of types of trials included was informed by our intention of understanding the currently active pipeline and to know what agents could evolve in the near term. We did not include trials of nonpharmacologic therapeutic approaches such as devices, cognitive therapies, and medical food. We did not include trials of biomarkers.
The mechanism of action (MOA) of each agent was determined from the information on clinicaltrials.gov (e.g., the mechanism is often noted in the title of the trial or in a description of the trial) or from a comprehensive search of the literature if the mechanism was not provided on the federal website. In a few cases, the mechanism is undisclosed and could not be identified in the literature. We grouped the mechanisms into symptomatic agents or DMTs. We divided the symptomatic agents into those that are putative cognitive enhancing agents or those that address neuropsychiatric and behavioral symptoms. DMTs were divided into those that target amyloid-related mechanisms, those that have tau-related MOAs, and those with “other” mechanisms such as anti-inflammatory MOAs, growth factors, or metabolic effects. Stem cell therapies were included in the “other” category.
2. Results
2.1. Overview
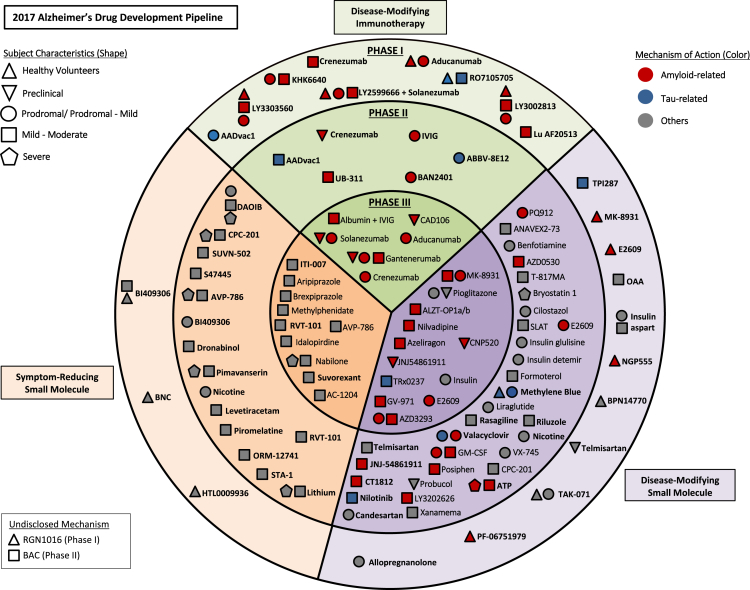
Fig. 1 provides an overview of all agents currently in the AD pipeline. The circles reveal the stages of development (I, II, and III), the colors pertain to the MOA of the agent, and the shape denotes the population in which the agent is being tested (normal volunteers, cognitive normal at-risk individuals, prodromal AD, and AD dementia).
Fig. 1.
Agents in clinical trials for the treatment of Alzheimer's disease in 2017 (from clinicaltrials.gov accessed 1/5/2017). Abbreviations: ATP, adenosine triphosphate; BNC, bisnorcymserine; GM-CSF, granulocyte-macrophage colony-stimulating factor; OAA, oxaloacetate; IVIG, intravenous immunoglobulin; SLAT, simvastatin + L-arginine + tetrahydrobiopterin.
In all, there are 105 agents in the pipeline as shown on clinicaltrials.gov, of which 25 are agents in 29 trials in phase I, 52 agents are in 68 trials in phase II, and 28 agents are in 42 trials in phase III. Across all stages, 70% are DMTs, 14% are symptomatic cognitive enhancers, 13% are symptomatic agents addressing neuropsychiatric and behavioral changes, and 2% have undisclosed MOAs.
Of all trials, 65.5% are sponsored by the biopharma industry, 16.6% by Academic Medical Centers, 3.6% by Academic Medical Center-NIH collaborations, and 10.8% by the collaborations between consortiums/philanthropic organizations and one or more of the following: biopharma, NIH, and Academic Medical Centers. One trial is sponsored by NIH, one trial by biopharma-NIH collaboration, and one trial by a biopharma-NIH-Academic Medical Center collaboration.
2.2. Phase I
Phase I first-in-human trials are generally conducted in healthy volunteers unless they are assessing immunotherapies where the potential long-term modification of the immune system makes participation of normal controls impermissible. These trials generally progress from single ascending dose trials where increasing doses are administered once in supervised settings to assess tolerability and establish a maximum tolerated dose to multiple ascending dose trials where individuals receive doses for 14 to 28 days [8], [9], [10]. Single and multiple ascending dose studies typically include cohorts of 6 to 12 individuals assigned asymmetrically to drug or placebo (e.g., four on placebo and eight on drug in a 12-person cohort). The goal of phase I trials is to assess the safety and tolerability of the agent, identify the doses to be advanced to phase II, and document the pharmacokinetics (PK) of the drug (e.g., half-life, time to maximum serum concentration [T-max], maximum serum concentration for each dose [C-max], bioavailability, dose-blood level linearity, etc). PK studies are conducted in animals before phase I but must be repeated in the first-in-human setting to establish the PK characteristics specific to men and women. Food effects on drug absorption and drug-drug interactions are also assessed in phase I studies. A cohort of healthy elderly is often included to assess the effect of age on PK parameters. In phase I/II studies, a cohort of individuals with AD may be included.
Of the 24 agents whose MOA was revealed in phase I in 2017 (Fig. 1; Table 1), 12 were directed at amyloid-related targets including eight immunotherapies, three had tau-related MOAs, and nine had other mechanisms including four symptomatic cognitive enhancers. Overall, there were 20 DMTs and four symptomatic agents in phase I. The MOA was not revealed for one agent.
Table 1.
Agents currently in phase I of Alzheimer's disease drug development (as of 1/5/2017)
| Agent | Agent mechanism class | Mechanism of action | Clinicaltrials.gov identifier | Status | Sponsor | Start date | Estimated end date |
|---|---|---|---|---|---|---|---|
| AC-1204 | Metabolic | Ketogenic agent | NCT01741194∗ | Active, not recruiting | Accera | Mar-13 | Oct-17 |
| Aducanumab | Anti-amyloid | Monoclonal antibody | NCT02484547 | Recruiting | Biogen | Sep-15 | Feb-22 |
| NCT02477800 | Recruiting | Biogen | Aug-15 | Feb-22 | |||
| Albumin + immunoglobulin | Anti-amyloid | Polyclonal antibody | NCT01561053∗ | Recruiting | Grifols | Mar-12 | Dec-16 |
| ALZT-OP1a + ALZT-OP1b | Anti-amyloid, anti-inflammatory | Anti-amyloid combination, inhibits neuroinflammatory response | NCT02547818 | Recruiting | AZTherapies | Sep-15 | Mar-18 |
| Aripiprazole | Neurotransmitter based | Atypical anti-psychotic (dopamine partial agonist) | NCT02168920 | Recruiting | Otsuka | Jun-14 | Jul-17 |
| AVP-786 | Neurotransmitter based | Mixed transmitter effect; agitation therapy | NCT02442765 | Recruiting | Avanir | Sep-15 | Jul-18 |
| NCT02446132 | Recruiting | Avanir | Dec-15 | Jul-19 | |||
| AZD3293 (LY3314814) | Anti-amyloid | BACE1 inhibitor | NCT02245737∗ | Recruiting | AstraZeneca, Eli Lilly | Sep-14 | Aug-19 |
| NCT02783573 | Recruiting | Jul-16 | Apr-21 | ||||
| NCT02972658 | Not yet recruiting | Mar-17 | Jul-20 | ||||
| Brexpiprazole (OPC-34712) | Neurotransmitter based | Atypical anti-psychotic (dopamine partial agonist) | NCT01862640 | Recruiting | Otsuka, Lundbeck | Jul-13 | Jun-17 |
| NCT01922258 | Recruiting | Otsuka, Lundbeck | Sep-13 | Jun-17 | |||
| CAD106 | Anti-amyloid | Amyloid vaccine | NCT02565511∗ | Recruiting | Novartis, Amgen, NIA, Alzheimer's Association | Nov-15 | Aug-23 |
| CNP520 | Anti-amyloid | BACE inhibitor | |||||
| Crenezumab | Anti-amyloid | Monoclonal antibody | NCT02670083 | Recruiting | Roche/Genentech | Mar-16 | Jul-21 |
| E2609 | Anti-amyloid | BACE inhibitor | NCT02956486 | Recruiting | Eisai, Biogen | 0ct-16 | Jun-20 |
| Gantenerumab | Anti-amyloid | Monoclonal antibody | NCT02051608 | Active, not recruiting | Roche | Mar-14 | Nov-19 |
| NCT01224106 | Active, not recruiting | Roche | Nov-10 | Dec-19 | |||
| NCT01760005∗ | Active, not recruiting | Washington University, Eli Lilly, Roche, NIA, Alzheimer's Association | Dec-12 | Dec-19 | |||
| Idalopirdine (Lu AE58054) | Neurotransmitter based | 5-HT6 antagonist | NCT02079246 | Recruiting, Extension | Lundbeck | Apr-14 | Oct-17 |
| NCT02006654 | Active, not recruiting | Lundbeck | Mar-14 | Mar-17 | |||
| Insulin (humulin) | Metabolic | Metabolic agent | NCT01767909∗ | Recruiting | University of Southern California, NIA, ATRI, Wake Forest University | Sep-13 | Feb-17 |
| ITI-007 | Neurotransmitter based | 5-HT2A antagonist, dopamine receptor modulator | NCT02817906 | Recruiting | Intra-Cellular Therapies, Inc. | Jun-16 | Aug-18 |
| JNJ-54861911 | Anti-amyloid | BACE inhibitor | NCT02569398∗ | Recruiting | Janssen | Nov-15 | May-23 |
| Methylphenidate | Neurotransmitter based | Dopamine reuptake inhibitor | NCT02346201 | Recruiting | Johns Hopkins, NIA | Oct-15 | Aug-20 |
| MK-8931 (verubecestat) | Anti-amyloid | BACE inhibitor | NCT01953601 | Active, not recruiting | Merck | Nov-13 | Mar-21 |
| NCT01739348∗ | Active, not recruiting | Merck | Nov-12 | Jul-19 | |||
| MK-4305 (suvorexant) | Neurotransmitter based | Dual orexin receptor antagonist | NCT02750306 | Recruiting | Merck | May-16 | Jul-17 |
| Nabilone | Neurotransmitter based | Cannabinoid (receptor agent) | NCT02351882∗ | Recruiting | Sunnybrook Health Sciences Centre | Jan-15 | Dec-17 |
| Nilvadipine | Anti-Amyloid | Calcium channel blocker | NCT02017340 | Active, not recruiting | St. James' Hospital Ireland, Alzheimer Europe, Archer Pharmaceuticals | Oct-12 | Dec-17 |
| Pioglitazone | Metabolic | PPAR-gamma agonist, anti-amyloid effect | NCT02284906 | Recruiting, Extension | Takeda | Feb-15 | Apr-21 |
| NCT01931566 | Active, not recruiting | Takeda | Aug-13 | Jul-19 | |||
| RVT-101 (intepirdine) | Neurotransmitter based | 5-HT6 antagonist | NCT02585934 | Recruiting | Axovant Sciences | Oct-15 | Oct-17 |
| NCT02586909 | Recruiting, Extension | Axovant Sciences | Apr-16 | Jun-18 | |||
| Sodium Oligo-mannurarate (GV-971) | Anti-amyloid | Anti-amyloid agent | NCT02293915 | Recruiting | Shanghai Greenvalley Pharmaceutical | Apr-14 | May-17 |
| Solanezumab | Anti-amyloid | Monoclonal antibody | NCT01760005∗ | Active, not recruiting | Washington University, Eli Lilly, Roche, NIA, Alzheimer's Association | Dec-12 | Dec-19 |
| NCT02008357 | Recruiting | Eli Lilly, ATRI | Feb-14 | Oct-20 | |||
| NCT01127633 | Active, not recruiting, Extension | Eli Lilly | Dec-10 | Feb-17 | |||
| NCT01900665 | Active, not recruiting | Eli Lilly | Jul-13 | Feb-17 | |||
| NCT02760602 | Recruiting | Eli Lilly | Jun-16 | Apr-21 | |||
| TRx0237 | Anti-tau | Tau protein aggregation inhibitor | NCT02245568 | Recruiting, Extension | TauRx Therapeutics | Aug-14 | Sept-17 |
| TTP488 (azeliragon) | Anti-amyloid, anti-inflammatory | Anti-amyloid RAGE antagonist | NCT02080364 | Recruiting | vTv Therapeutics | Apr-15 | Jan-19 |
| NCT02916056 | Not yet recruiting | Dec-16 | Nov-20 |
Abbreviations: ATRI, Alzheimer's Therapeutic Research Institute; BACE, β-site amyloid precursor protein cleaving enzyme; NIA, National Institute on Aging; PPAR, peroxisome proliferator-activated receptor; RAGE, receptor for advanced glycation end products.
NOTE. Twenty-eight agents in 42 phase III clinical trials as of January 5, 2017 according to clinicaltrials.gov.
Phase II/III trials. Bolded = new entries into the 2017 phase III pipeline.
Phase I trials were on average 755 days in duration (recruitment and treatment period) and involved 68 patients in each trial.
2.3. Phase II
Phase II trials advance the agents from phase I to trial populations of patients with AD. The goal of these trials is to establish preliminary efficacy based on a biomarker outcome, a clinical measure, or a combination of clinical and biomarker outcomes [11], [12]. Phase IIa trials concentrate on efficacy, and phase IIb trials further refine dosing decisions about the number of doses to be advanced to phase III. Of the 68 trials in phase II of the AD pipeline, 21 included patients with prodromal or prodromal and mild AD, 26 were trials for mild-moderate AD, one included patients with prodromal or mild-moderate AD, and one trial was for mild-moderate or severe AD. Of the symptomatic trials, 10 were for mild-moderate AD and six were for mild-moderate or severe AD.
On average, phase II trials were 1140 days in duration (recruitment plus exposure period) and involved 151 patients in each trial.
Of the 52 agents in the 68 trials, there were 36 DMTs, eight cognitive enhancing agents, seven drugs for behavioral symptoms, and one agent with an unknown MOA (Fig. 1; Table 2). Among the DMTs, 14 addressed amyloid targets, four involved tau-related targets, one addressed both amyloid and tau-related targets, and 17 had other MOAs (e.g., metabolic, or anti-inflammatory). The DMTs include six immunotherapies (four addressing amyloid and two addressing tau). Of the DMTs, 17 are repurposed agents approved for use in another indication. There are seven trials involving stem cell programs.
Table 2.
Agents currently in phase II of Alzheimer's disease drug development (as of 1/5/2017)
| Agent | Agent mechanism class | Mechanism of Action | Clinicaltrials.gov identifier | Status | Sponsor | Start date | Estimated end date |
|---|---|---|---|---|---|---|---|
| AADvac1 | Anti-tau | Monoclonal antibody | NCT02579252 | Recruiting | Axon Neuroscience | Dec-15 | Feb-19 |
| ABBV-8E12 | Anti-tau | Monoclonal antibody | NCT02880956 | Recruiting | AbbVie | Oct-16 | Mar-21 |
| ATP | Anti-amyloid | Inhibits amyloid misfolding and toxicity | NCT02279511 | Active, not recruiting | Fundació Clínic per la Recerca Biomèdica, Spain | Nov-14 | Nov-16 |
| AD-SVF cells | Regenerative | AD-SVF cell infusion | NCT02912169∗ | Recruiting | Ageless Regenerative Institute | Nov-15 | Dec-17 |
| ANAVEX 2-73 | Neuroprotective | Sigma-1 receptor agonist | NCT02244541 | Active, not recruiting | Anavex Life Sciences | Dec-14 | Oct-16 |
| NCT02756858 | Recruiting, extension | Mar-16 | Nov-18 | ||||
| Atomoxetine | Anti-amyloid | Adrenergic uptake inhibitor, SNRI | NCT01522404 | Active, not recruiting | Emory University, NIA | Mar-12 | Dec-17 |
| AVP-786 | Neurotransmitter based | Mixed transmitter effect | NCT02534038 | Recruiting | Avanir | Oct-15 | Mar-18 |
| AZD0530 (saracatinib) | Anti-amyloid | Kinase inhibitor | NCT02167256 | Active, not recruiting | Yale University, ATRI, AstraZeneca | Dec-14 | Dec-17 |
| BAC | Undisclosed | Undisclosed mechanism | NCT02886494 | Not yet recruiting | Charsire Biotechnology | Nov-16 | Nov-19 |
| NCT02467413 | Not yet recruiting | Charsire Biotechnology, A2 Healthcare Taiwan Corporation | Mar-16 | Dec-17 | |||
| BAN2401 | Anti-amyloid | Monoclonal antibody | NCT01767311 | Recruiting | Eisai | Dec-12 | Jul-18 |
| Benfotiamine | Metabolic | Antioxidant | NCT02292238 | Recruiting | Burke Medical Research Institute, Columbia University, NIA, ADDF | Nov-14 | Nov-19 |
| BI409306 | Neuroprotective | Phosphodiesterase 9A inhibitor | NCT02240693 | Recruiting | Boehringer Ingelheim | Jan-15 | Oct-17 |
| NCT02337907 | Recruiting | Boehringer Ingelheim | Jan-15 | Oct-17 | |||
| Bryostatin 1 | Neuroprotective | Protein kinase C modulator | NCT02431468 | Active, not recruiting | Neurotrope Bioscience | Jul-15 | May-17 |
| Candesartan | Neuroprotective, anti-inflammatory | Angiotensin receptor blocker | NCT02646982 | Recruiting | Emory University | Jun-16 | Sep-21 |
| CB-AC-02 (Placenta derived-MSCs) | Regenerative | Stem cell therapy | NCT02899091∗ | Not yet recruiting | CHA Biotech Co. | Sep-16 | Jun-18 |
| Cilostazol | Neuroprotective | Phosphodiesterase 3 antagonist | NCT02491268 | Recruiting | National Cerebral and Cardiovascular Center, Japan | Jul-15 | Jul-18 |
| CPC-201 | Neuroprotective | Cholinesterase inhibitor + peripheral cholinergic antagonist | NCT02549196 | Recruiting | Chase Pharmaceuticals | Oct-15 | Dec-16 |
| NCT02434666 | Active, not recruiting, Extension | Chase Pharmaceuticals | Jan-15 | Dec-16 | |||
| NCT02860065 | Not yet recruiting | Chase Pharmaceuticals | Sep-16 | Jun-17 | |||
| Crenezumab | Anti-amyloid | Monoclonal antibody | NCT01998841 | Recruiting | Genentech, NIA, Banner Alzheimer's Institute | Dec-13 | Sep-20 |
| CT1812 | Anti-amyloid | Sigma-2 receptor modulator | NCT02907567∗ | Recruiting | Cognition Therapeutics | Sep-16 | May-17 |
| DAOIB | Neurotransmitter based | NMDA enhancer | NCT02103673 | Recruiting | Chang Gung Memorial Hospital, Taiwan | Feb-14 | Sep-17 |
| NCT02239003 | Recruiting | Chang Gung Memorial Hospital, Taiwan | Jan-12 | Dec-17 | |||
| Dronabinol | Neurotransmitter based | CB1 and CB2 endocannabinoid receptor partial agonist | NCT02792257 | Not yet recruiting | Mclean Hospital, Johns Hopkins University | Aug-16 | Dec-20 |
| E2609 | Anti-amyloid | BACE inhibitor | NCT02322021 | Recruiting | Eisai, Biogen | Nov-14 | Jan-18 |
| Formoterol | Neuroprotective, anti-inflammatory | β-2 adrenergic receptor agonist | NCT02500784 | Recruiting | Palo Alto Veterans Institute for Research, Mylan, Alzheimer's Association | Jan-15 | Jul-16 |
| hUCB-MSCs | Regenerative | Stem cell therapy | NCT02054208∗ | Recruiting | Medipost | Feb-14 | Feb-18 |
| NCT01547689∗ | Active, not recruiting | Affiliated Hospital to Academy of Military Medical Sciences, China | Mar-12 | Dec-16 | |||
| NCT02513706 | Not yet recruiting | South China Research Center | May-16 | Oct-19 | |||
| NCT02672306∗ | Not yet recruiting | South China Research Center | May-16 | Oct-19 | |||
| NCT02833792 | Recruiting | Stemedica Cell Technologies | Jun-16 | Jun-18 | |||
| Insulin detemir (intranasal) | Metabolic | Increases insulin signaling in the brain | NCT01595646 | Active, not recruiting | Wake Forest School of Medicine, Alzheimer's Association | Nov-11 | Mar-17 |
| Insulin glulisine | Metabolic | Increases insulin signaling in the brain | NCT02503501 | Recruiting | HealthPartners Institute | Aug-15 | Sep-17 |
| JNJ-54861911 | Anti-amyloid | BACE inhibitor | NCT02406027 | Active, not recruiting, Extension | Janssen | Jul-15 | Oct-22 |
| Levetiracetam | Neurotransmitter based | Anticonvulsant | NCT02002819 | Recruiting | University of California, San Francisco | Jun-14 | Dec-17 |
| Liraglutide | Metabolic | Glucagon-like peptide 1 receptor agonist | NCT01843075 | Recruiting | Imperial College London | Jan-14 | Mar-19 |
| Lithium | Neurotransmitter based | Ion channel modulator | NCT02129348 | Recruiting | New York State Psychiatric Institute, NIA | Jun-14 | Apr-19 |
| LY3202626 | Anti-amyloid | BACE Inhibitor | NCT02791191 | Recruiting | Eli Lilly | Jun-16 | Aug-18 |
| Methylene blue | Anti-tau | Tau inhibitor; neuronal stimulant | NCT02380573 | Recruiting | Texas Alzheimer's Research and Care Consortium | Jul-15 | Jul-18 |
| NewGam 10% IVIG | Anti-amyloid | Polyclonal antibody | NCT01300728 | Active, not recruiting | Sutter Health | Jan-11 | Nov-17 |
| Nicotine | Neurotransmitter based | Nicotinic acetylcholine receptor agonist | NCT02720445 | Not yet recruiting | University of Southern California, NIA, ATRI, Vanderbilt University | Dec-16 | Dec-19 |
| Nilotinib | Anti-tau | Tyrosine kinase inhibitor | NCT02947893 | Not yet recruiting | Georgetown University | Nov-16 | Mar-18 |
| ORM-12741 | Neurotransmitter based | Alpha-2c adrenergic receptor antagonist | NCT02471196 | Recruiting | Orion Corporation, Janssen | Jun-15 | Jul-17 |
| Pimavanserin | Neurotransmitter based | 5-HT2A inverse agonist | NCT02035553 | Active, not recruiting | Acadia | Nov-13 | Nov-16 |
| NCT02992132 | Recruiting | Acadia | Nov-16 | Jun-19 | |||
| Piromelatine | Neurotransmitter based | Melatonin receptor agonist; 5-HT 1A and 1D receptor agonist | NCT02615002 | Recruiting | Neurim Pharmaceuticals | Nov-15 | Mar-18 |
| Posiphen | Anti-amyloid | Selective inhibitor of APP production | NCT02925650∗ | Not yet recruiting | QR Pharma, ADCS | Dec-16 | Dec-18 |
| PQ912 | Anti-amyloid, anti-inflammatory | Glutaminyl-peptide cyclotransferase inhibitor | NCT02389413 | Recruiting | Probiodrug AG, Julius Clinical, VU University Medical Center, Amsterdam | Mar-15 | Mar-17 |
| Probucol | Neuroprotective, anti-inflammatory | Anti-hyperlipidemic | NCT02707458∗ | Not yet recruiting | Douglas Mental Health University Institute, Weston Brain Institute, McGill University | Apr-16 | May-18 |
| Rasagiline | Neuroprotective | Monoamine oxidase B inhibitor | NCT02359552 | Recruiting | The Cleveland Clinic | Feb-15 | May-17 |
| Riluzole | Neuroprotective | Glutamate receptor antagonist; glutamate release inhibitor | NCT01703117 | Recruiting | Rockefeller University | Apr-13 | Nov-18 |
| RVT-101 | Neurotransmitter based | 5-HT6 antagonist | NCT02910102 | Recruiting | Axovant Sciences | Oct-16 | Sep-17 |
| S47445 | Neurotransmitter based | AMPA receptor agonist; nerve growth factor stimulant | NCT02626572 | Active, not recruiting | Servier | Feb-15 | Dec-17 |
| Sargramostim (GM-CSF) | Anti-amyloid | Granulocyte colony stimulator; amyloid removal | NCT01409915 | Recruiting | University of Colorado, Denver, The Dana Foundation | Mar-11 | Jan-17 |
| NCT02667496 | Recruiting | Sanofi, NIA | Nov-16 | Apr-18 | |||
| Simvastatin + L-Arginine + Tetrahydrobiopterin (SLAT) | Neuroprotective | HMG-CoA reductase inhibitor and antioxidant | NCT01439555 | Recruiting | University of Massachusetts, Worcester | Nov-11 | Dec-16 |
| STA-1 | Neuroprotective, anti-inflammatory | Antioxidant properties of echinascoside | NCT01255046 | Not yet recruiting | Sinphar Pharmaceuticals | Dec-15 | Dec-18 |
| SUVN-502 | Neurotransmitter based | 5-HT6 antagonist | NCT02580305 | Recruiting | Suven Life Sciences | Sep-15 | Jun-17 |
| T-817 MA | Neuroprotective | Neurotrophic agent | NCT02079909 | Active, not recruiting | Toyama Chemical, ADCS | Mar-14 | Mar-17 |
| Telmisartan | Neuroprotective, anti-inflammatory | Angiotensin II receptor blocker, PPAR-gamma agonist | NCT02085265 | Recruiting | Sunnybrook Health Sciences Centre, ADDF | Mar-14 | Aug-18 |
| UB-311 | Anti-amyloid | Monoclonal antibody | NCT02551809 | Recruiting | United Neuroscience | Oct-15 | Dec-17 |
| Valacyclovir | Anti-amyloid, Anti-tau | Antiviral agent | NCT02997982 | Recruiting | Umea University | Dec-16 | Dec-17 |
| VX-745 | Neuroprotective, anti-inflammatory | P38 mitogen-activated protein kinase inhibitor | NCT02423200 | Active, not recruiting | EIP Pharma | Apr-15 | Nov-16 |
| NCT02423122 | Active, not recruiting | EIP Pharma | Apr-15 | Sep-16 | |||
| Xanamema | Neuroprotective | Blocks 11-HSD1 enzyme activity, decreasing cortisol in brain | NCT02727699 | Not yet recruiting | Actinogen Medical, ICON Clinical Research | Jun-16 | Aug-18 |
Abbreviations: ADCS, Alzheimer's Disease Cooperative Study; ADDF, Alzheimer's Drug Discovery Foundation; AD-SVF, adipose-derived stromal vascular fraction; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; ATP, adenosine triphosphate; ATRI, Alzheimer's Therapeutic Research Institute; BACE, β-site amyloid precursor protein cleaving enzyme; GM-CSF, granulocyte-macrophage colony-stimulating factor; HMG-CoA, 3-hydroxy-3-methylglutaryl-coenzyme; hUCB-MSCs, human umbilical cord-derived mesenchymal stem cells; IVIG, intravenous immunoglobulin; NIA, National Institute on Aging; NMDA, N-methyl-d-aspartate; PPAR, peroxisome proliferator-activated receptor; SNRI, serotonin-norepinephrine reuptake inhibitors.
NOTE. Fifty-two agents in 68 phase II clinical trials currently ongoing as of January 5, 2017 according to clinicaltrials.gov.
Phase I/II trials. Bolded = new entries into the 2017 phase II pipeline.
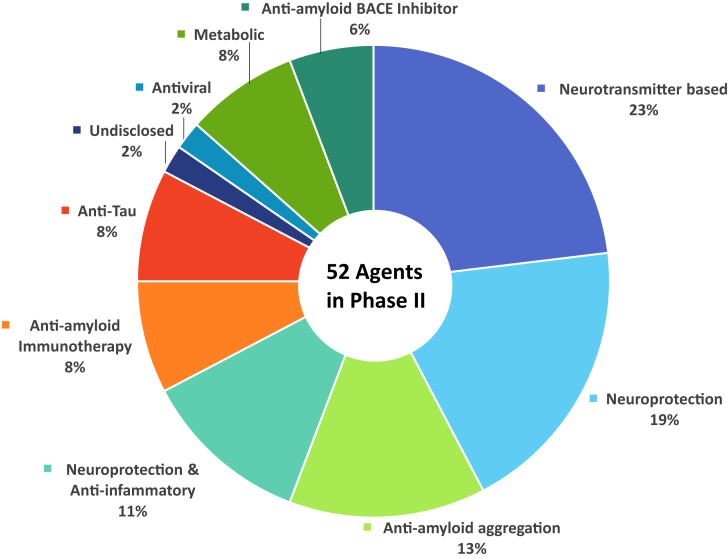
Of the drugs with amyloid targets, there were three β-site amyloid precursor protein cleaving enzyme (BACE) inhibitors, four immunotherapies, and eight anti-aggregation agents. Fig. 2 shows the MOAs of agents in phase II.
Fig. 2.
Mechanisms of action of agents in phase II. Abbreviation: BACE, β-site amyloid precursor protein cleaving enzyme.
2.4. Phase III
Phases II and III trials are often called “learn” and “confirm” trials, respectively, with phase III intended to confirm effects observed in phase II in larger populations treated for longer periods of time [11], [13]. In addition to providing crucial efficacy data, phase III trials also provide exposure data on larger numbers of patient-days essential to establishing the safety and tolerability of the candidate therapy.
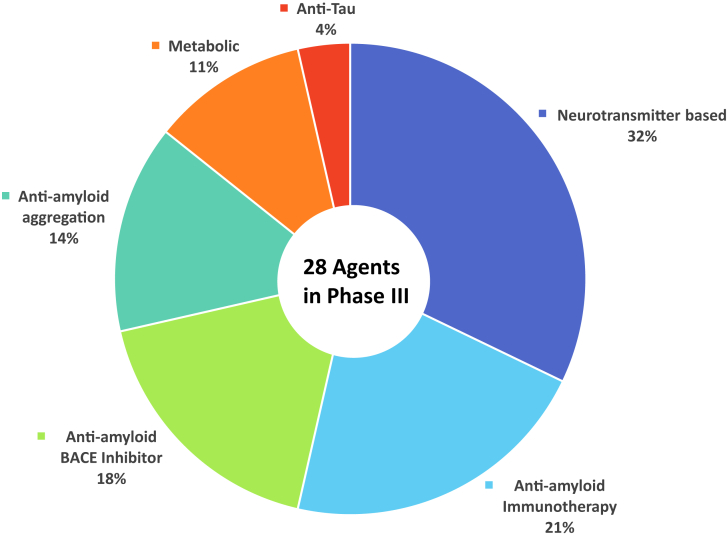
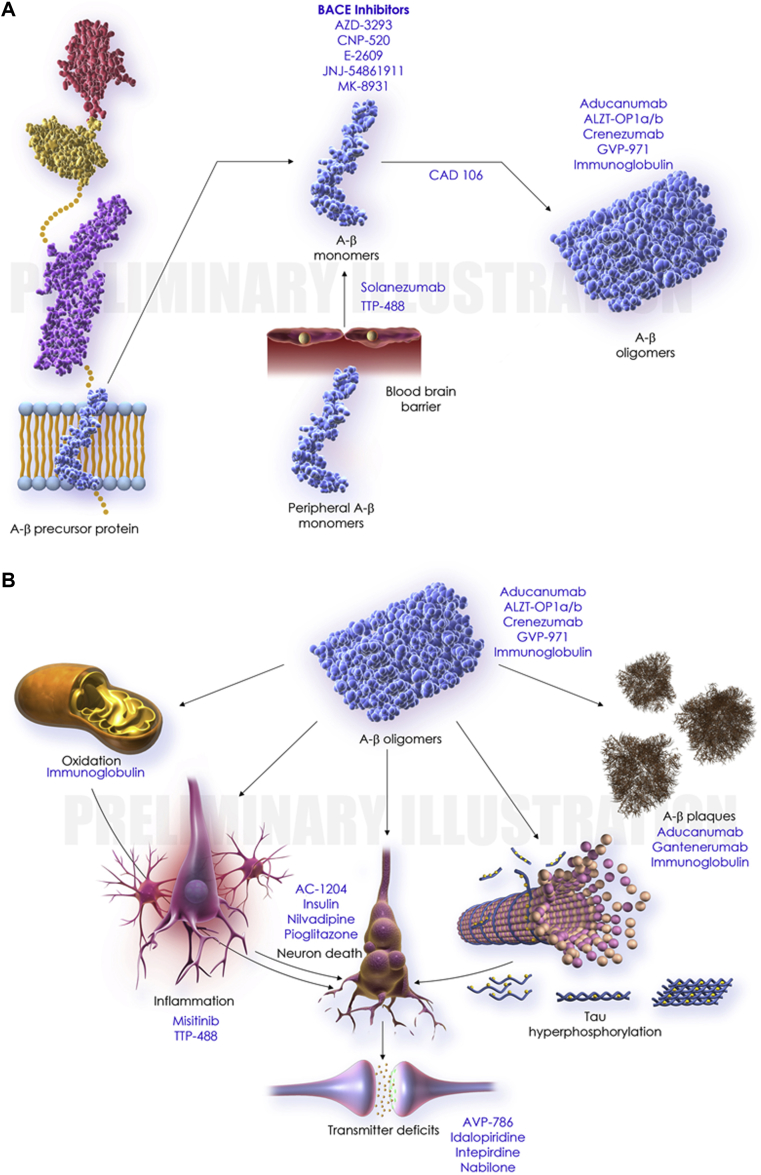
Of the 28 agents in the 42 trials, there were 18 DMTs, three cognitive enhancing agents, and seven drugs for behavioral symptoms. Among the DMTs, 15 addressed amyloid targets, one involved a tau-related target, and two had a metabolic MOA. The DMTs include six immunotherapies (all addressing amyloid). Of the DMTs, four are repurposed agents approved for use in another indication. Of the drugs with amyloid targets, there were five BACE inhibitors, six immunotherapies, and four anti-aggregation agents. Table 3 shows the agents currently in phase III of AD drug development. Fig. 3 shows the MOAs of agents in phase III and Fig. 4 shows an illustration of the drug mechanism of phase III agents and the proposed biology of AD.
Table 3.
Agents currently in phase III of Alzheimer's disease drug development (as of 1/5/2017)
| Agent | Agent mechanism class | Mechanism of Action | Clinicaltrials.gov identifier | Status | Sponsor | Start date | Estimated end date |
|---|---|---|---|---|---|---|---|
| AADvac1 | Anti-tau | Monoclonal antibody directed at Tau epitope | NCT02031198 | Active, not recruiting | Axon Neuroscience | Jan-14 | Dec-16 |
| Aducanumab | Anti-amyloid | Monoclonal antibody | NCT01677572 | Active, not recruiting | Biogen | Oct-12 | Oct-19 |
| NCT02434718 | Active, not recruiting | Biogen | May-15 | Dec-16 | |||
| NCT02782975 | Active, not recruiting | Biogen | May-16 | Nov-16 | |||
| Allopregnanolone injection | Regenerative | GABA receptor modulator | NCT02221622 | Recruiting | University of Southern California, NIA | Aug-14 | Jun-17 |
| BI409306 | Neurotransmitter based | Phosphodiesterase 9A inhibitor | NCT02392468 | Recruiting | Boehringer Ingelheim | Apr-15 | May-17 |
| Bisnorcymserine (BNC) | Neurotransmitter based | Butyrylcholinesterase inhibitor | NCT01747213 | Recruiting | NIA | Nov-12 | Jul-17 |
| BPN14770 | Neuroprotective | Negative allosteric modulator of phosphodiesterase 4D | NCT02840279 | Recruiting | Tetra Discovery Partners | Jun-16 | Dec-16 |
| NCT02648672 | Recruiting | Tetra Discovery Partners | Dec-15 | Apr-16 | |||
| Crenezumab | Anti-amyloid | Monoclonal antibody | NCT02353598 | Recruiting | Genentech | Feb-15 | May-17 |
| E2609 | Anti-amyloid | BACE Inhibitor | NCT02859207 | Not yet recruiting | Eisai, Biogen | Aug-16 | Jan-17 |
| HTL0009936 | Neurotransmitter based | Muscarinic M1 receptor agonist | NCT02546310 | Recruiting | Heptares Therapeutics | Sep-15 | Sep-16 |
| Insulin Aspart Intranasal | Metabolic | Increases insulin signaling in the brain | NCT02462161 | Recruiting | Wake Forest School of Medicine, NIA, General Electric | May-15 | Dec-16 |
| KHK6640 | Anti-amyloid | Amyloid aggregation inhibitor | NCT02127476 | Active, not recruiting | Kyowa Hakko Kirin Pharma | Jul-14 | Apr-17 |
| hMSCs | Regenerative | Stem cell therapy | NCT02600130 | Recruiting | Longeveron LLC | Jan-16 | Oct-19 |
| Lu AF20513 | Anti-amyloid | Polyclonal antibody | NCT02388152 | Recruiting | H. Lundbeck A/S | Mar-15 | May-17 |
| LY2599666 + solanezumab | Anti-amyloid | Monoclonal antibody combination | NCT02614131 | Active, not recruiting | Eli Lilly and Company | Dec-15 | Sep-17 |
| LY3002813 | Anti-amyloid | Monoclonal antibody | NCT01837641 | Active, not recruiting | Eli Lilly and Company | May-13 | Jan-17 |
| NCT02624778 | Recruiting | Eli Lilly and Company | Dec-15 | Jun-20 | |||
| LY3303560 | Anti-amyloid | Monoclonal antibody | NCT02754830 | Recruiting | Eli Lilly and Company | Apr-16 | Apr-17 |
| MK-8931 (verubecestat) | Anti-amyloid | BACE Inhibitor | NCT02910739 | Recruiting | Merck | Oct-16 | Apr-17 |
| NGP 555 | Anti-amyloid | Gamma-secretase modulator | NCT02537938 | Recruiting | NeuroGenetic Pharmaceuticals | Jan-16 | Oct-16 |
| Oxaloacetate (OAA) | Metabolic | Mitochondrial enhancer | NCT02593318 | Recruiting | University of Kansas Medical Center | Oct-15 | Oct-17 |
| PF-06751979 | Anti-amyloid | Undisclosed mechanism | NCT02793232 | Recruiting | Pfizer | Jun-16 | Jan-17 |
| RGN1016 | Undisclosed | Undisclosed mechanism | NCT02820155 | Recruiting | National Taiwan University | Jun-16 | Feb-17 |
| RO7105705 | Anti-tau | Anti-tau antibody | NCT02820896 | Recruiting | Genentech | Jun-16 | May-17 |
| TAK-071 | Neurotransmitter based | Muscarinic M1 receptor modulator | NCT02769065 | Recruiting | Takeda | May-16 | Mar-17 |
| Telmisartan | Neuroprotective, anti-inflammatory | Angiotensin II receptor blocker, PPAR-gamma agonist | NCT02471833 | Recruiting | Emory University | Apr-15 | Mar-18 |
| TPI-287 | Anti-tau | Microtubule protein modulator | NCT01966666 | Active, not recruiting | University of California, San Francisco | May-14 | Nov-17 |
Abbreviations: BACE, β-site amyloid precursor protein cleaving enzyme; GABA, gamma-aminobutyric acid; hMSCs, mesenchymal stem cells; NIA, National Institute on Aging; PPAR, peroxisome proliferator-activated receptor.
NOTE. Twenty-five agents in 29 phase I clinical trials currently ongoing as of January 5, 2017 according to clinicaltrials.gov. Bolded = new entries into the 2017 phase I pipeline.
Fig. 3.
Mechanisms of action of agents in phase III. Abbreviation: BACE, β-site amyloid precursor protein cleaving enzyme.
Fig. 4.
Illustration of drug mechanism (phase III agents) and the proposed biology of Alzheimer's disease. (A) Amyloid cascade. (B) Downstream pathophysiology. Abbreviation: BACE, β-site amyloid precursor protein cleaving enzyme.
Among the DMT trials, there were five prevention trials enrolling cognitively normal participants at high risk for developing AD in the course of the trials; 14 trials of patients with prodromal AD (those with minimal cognitive symptoms and a biomarker indicative of AD-related brain changes) or prodromal/mild AD; and nine trials of patients with mild-moderate AD.
On average, phase III trials involved 1012 patients and were 1677 days in duration (including the recruitment and the treatment period). When divided by MOA, DMT trials were 1948 days long (945.84 treatment days) and included 1212 patients. Cognitive enhancer trials were 1114 days long (215.81 treatment days) and involved 1044 patients. Trials of drugs for behavioral symptoms are 1146 days long (113.61 treatment days) and included 372 patients. For all DMTs, the average duration of therapy is 135 weeks; the average period from trial initiation to primary completion date (final data collection date for primary outcome measure) is 246 weeks. This indicates that 111 weeks (>2 years) is the average anticipated recruitment time. On average, prevention trials recruiting cognitively normal individuals at risk for AD are 363 weeks in duration; trials entering patients with prodromal/prodromal-mild AD are 278 weeks in duration; and trials for patients with mild-moderate AD are 231 weeks in duration. Anticipated recruitment periods for these three types of trials are 134 weeks, 105 weeks, and 107 weeks, respectively.
2.5. Participant numbers
Recruitment is the slowest and most expensive aspect of clinical trials [14]. The period of recruitment may exceed the period of treatment in the clinical trial cycle. The total number of participants needed to populate all clinical trials for AD is large. Table 4 shows the number of participants needed for all current prevention trials, phase I, phase II, and phase III trials.
Table 4.
Number of participants needed for AD clinical trials
| Participant type | Phase I | Phase II | Phase III | Total |
|---|---|---|---|---|
| Healthy volunteers | 864 | 120 | 0 | 984 |
| Preclinical AD | 66 | 323 | 7850 | 8239 |
| Prodromal/prodromal-mild AD | 597 | 3877 | 17,535 | 22,009 |
| Mild-moderate AD | 626 | 4528 | 17,099 | 22,253 |
| Severe AD | 0 | 568 | 20 | 588 |
| Total | 2153 | 9416 | 42,504 | 54,073 |
Abbreviation: AD, Alzheimer's disease.
2.6. Biomarkers
Biomarkers have many roles in clinical trials including identifying patients for trials and verifying diagnosis, assessing target engagement and providing proof of pharmacology, serving as outcomes for DMT trials, and assessing side effects (e.g., monitoring amyloid-related imaging abnormalities [ARIAs] observed with some immunotherapies) [14], [15], [16], [17]. ARIA has emerged as a concern in several major immunotherapy development programs–bapineuzumab, gantenerumab, and aducanumab. Although largely asymptomatic, these changes can lead to permanent neurologic sequelae and will be a focus of research once the efficacy of the immunotherapies is established. Preliminary evidence suggests that longer dose titration periods may decrease the risk of ARIAs [18].
Not all trial descriptions in clinicalrials.gov note if biomarkers are used. Table 5 shows the biomarkers used as outcome measures in current phase II and phase III AD clinical trials as included in the federal website. Of the 28 phase III DMT trials, 10 trials use amyloid positron emission tomography (PET) as an entry criterion, two use cerebrospinal fluid (CSF) amyloid, and two use either amyloid PET or CSF amyloid. Thirteen out of 46 phase II DMT trials used amyloid-PET as an entry criterion.
Table 5.
Biomarkers as outcome measures in phase II and phase III trials for agents in the Alzheimer's disease drug development pipeline (clinicaltrials.gov; 1/5/2017)
| Biomarker | N of trials (%) |
|
|---|---|---|
| Phase III | Phase II | |
| CSF amyloid | 12 (28.6) | 17 (25.0) |
| CSF tau | 13 (31.0) | 16 (23.5) |
| FDG-PET | 5 (11.9) | 10 (14.7) |
| vMRI | 9 (21.4) | 6 (8.8) |
| Plasma amyloid | 4 (9.5) | 5 (7.4) |
| Plasma tau | 0 | 1 (1.5) |
| Amyloid PET | 13 (31.0) | 6 (8.8) |
| Tau PET | 1 (2.4) | 0 |
Abbreviations: CSF, cerebrospinal fluid; FDG, fluorodeoxyglucose; PET, positron emission tomography; vMRI, volumetric magnetic resonance imaging.
2.7. Comparison to 2016 pipeline
In any 1-year period, there is relatively little movement in the AD pipeline. Compared to 2016, there are eight new agents in phase I, 16 in phase II, and five in phase III. Of the new agents in phase II, three of the 16 were previously present in phase I (AADvac1, CT1812, and LY3202626). Of the new agents in phase III, two of the five were previously noted in phase II (crenezumab and E2609).
Agents can appear in phase II without having been listed in phase I because the phase I study was not done in the US and was not registered on clinicaltrials.gov or because they are repurposed agents that had the PK and dosing established through trials for a non-AD indication [15], [19], [20]. Rarely, a repurposed agent can enter directly into phase III without a phase II trial. In the AD pipeline, there are 18 repurposed agents in phase II and nine in phase III.
Seven agents were listed in phase II in 2016 and are not listed in phase I, II, or III in 2017. These agents have at least temporarily exited the drug development pipeline. Four trials for the following agents were completed in 2016 but are not listed in the 2017 pipeline: exenatide, PXT00864, RO4602522, and RPh201. Two trials were terminated (MK-7622 and PF-05212377), and the trial status for metformin changed to “unknown” because it has not been updated for more than 2 years on clinicaltrials.gov.
One agent (masitinib) was listed in phase III in 2016 and is not listed in phase I, II, or III in 2017. Its trial status changed to “unknown” because it has not been updated on clinicaltrials.gov since 2013. Development of this agent has been at least temporarily interrupted.
Candidate agents that are no longer evident in the pipeline may have failed to demonstrate efficacy in well-conducted trials, they may have failed to show efficacy in trials whose features suggest the trials were not well conducted, or there may be industry reasons for halting the program (e.g., insufficient funding, reprioritization of agents in development, change in company business agenda, and management decision after assessment of the competitive landscape) [21].
3. Discussion
There is a modest pipeline of drugs in development for AD. Most candidate therapies are DMTs (70% across all phases), and the remainder are symptomatic agents directed at cognitive enhancement (14%) or treatment of neuropsychiatric symptoms (13%) and agents with undisclosed MOAs (2%). This closely resembles the pipeline as it appeared in 2016 where 73% were DMTs, 18% cognitive enhancers, and 9% psychotropic agents [4]. There are eight new agents in phase I, 16 in phase II, and five in phase III. From the 2016 pipeline, several agents have exited and not yet reappeared including six in phase I, seven in phase II, and one in phase III. Leuco-methylthioninium (LMTM) (or TRx0237) derived from methylene blue and addressing tau aggregation completed its phase III trials in 2016 and failed to meet prespecified outcomes of the trial [22]; however, its open-label extension study is listed as ongoing.
The shortcomings of clinicaltrials.gov as a database are important to recognize when considering the data presented here. Not all phase I trials, especially those conducted outside the US, may be registered, and our phase I data may underestimate the number of phase I candidates. Trials are required to be registered within 21 days of entering the first patient into the trial [7], but not all sponsors meet this deadline. The data provided may not represent the entire universe of AD drug development [23]. We stopped entering new data into our database at a time that allowed submission, peer review, and publication; the data presented are a few months out-of-date (data collection stopped January 5, 2017). Nevertheless, the FDA Modernization Act requires all trials to be registered and the International Committee of Medical Journal Editors requires trials to be registered to be eligible for publication [24]. The clinicaltrials.gov database is acceptably comprehensive—the most complete of any existing database—and a sound basis for drawing conclusions about AD drug development with the caveats mentioned here.
Phase I is not the only source of compounds for phases II and III. Agents repurposed from other indications may have MOAs worthy of exploration in AD. Antihypertensive agents, statins, anti-inflammatory agents, calcium channel blockers, and agents with many other MOAs have been proposed as drugs with effects possibly useful in treating AD and can enter AD drug development pipelines at phase II or phase III [15], [19], [20]. Psychiatric agents are commonly developed in non-AD populations (e.g., major depressive disorder, schizophrenia) before being assessed in AD patients. Safety, tolerability, PK, and dosing observations of phase I may be available from studies conducted for the initial indication. Thus, the phase I observations provided here may not entirely forecast the compounds available for phase II and phase III. Nevertheless, the intellectual property challenges of repurposed compounds such as short-patent life limit biopharma interest in these agents, and only a modest number of repurposed agents are represented in the AD drug development pipeline (18 in phase II and six in phase III). The small number of phase I compounds is a concern for the overall health of the AD drug development pipeline.
A striking observation derived from these data is the long recruitment period anticipated for phase III DMTs. The anticipated recruitment period is often longer than the treatment period, and in many cases, these planned recruitment goals are not met [25], [26]. Prevention trials require more time to recruit than prodromal trials (133.5 weeks and 105.4 weeks, respectively) and mild-moderate AD trials (106.9 weeks). Slow recruitment is among the greatest challenges to efficiency of AD drug development [14]. This observation underlies recent efforts in the AD drug development arena to advance new methods for patient recruitment including electronic engagement of potential participants, creation of registries of candidate subjects, and providing transportation, neighborhood vans, and insurance-based referral mechanisms to facilitate subject recruitment and shorten drug development timelines [27], [28].
A daunting conclusion from this review of clinicaltrials.gov is the large number of participants required to conduct the trials. Registered preclinical AD trials will require 8239 participants; trials of prodromal or prodromal/mild populations require 22,009 participants; trials of mild to moderate AD dementia are forecasted to require 22,253 participants; and trials in severe AD dementia will require 588 participants. In total, 54,073 participants will be required to complete the current AD trials. Trial recruitment is among the slowest and most expensive of all aspects of clinical trial conduct. The recruitment of such large numbers of participants will represent a substantial challenge to the system, and reforms are necessary to accelerate clinical trials and enhance recruitment [27], [28].
BACE inhibitors are among the most common classes of molecules in the AD pipeline. Currently, there are 10 phase II or phase III trials involving BACE inhibitors (Table 6). The pipeline for BACE inhibitors has emerged only recently largely because of the challenging structure-activity requirements for BACE inhibition; agents need to be large enough to block BACE's large active site, while at the same time, agents need to be small enough to pass through the blood-brain barrier and lipophilic enough to enter endosomes where BACE is active [29]. Advances in small-molecule screening approaches have helped overcome these challenges and facilitated the entry of a number of BACE inhibitors into the pipeline [30]. All the BACE inhibitors currently in phases II and III trials are small molecules that demonstrate favorable PK when administered orally. The published results of phase I testing in both healthy participants and AD populations have demonstrated robust (45%–95%) reductions in CSF amyloid β protein (Aβ) levels [31]. This degree of target engagement has not previously been documented with other anti-amyloid therapies leading some authors to posit that BACE inhibition represents the first true test of the amyloid hypothesis [32]. Based on strong biomarker data, some BACE inhibitors have bypassed phase II testing moving directly from phase I into phase III trials. As a result, little is known about the clinical efficacy or safety of long-term BACE inhibition. The first BACE inhibitor trial to be tested in phase III in an AD dementia population was recently terminated for the lack of efficacy [33]. Data from this and other studies will begin to answer the key questions regarding BACE inhibitors including when in the disease continuum should inhibition be started; what degree of amyloid inhibition is required for clinical benefit; does reduction in amyloid synthesis affect clinical progression; and are there unidentified safety concerns with long-term BACE inhibition?
Table 6.
BACE inhibitors in clinical trials for AD
| BACE inhibitors currently in phase II or III of development | |||||
|---|---|---|---|---|---|
| Agent (sponsor) | Clinicaltrials.gov identifier (trial name) | Phase | Population | Start date | Estimated end date |
| CNP520 (Novartis) | NCT02565511 (GENERATION) | II/III | Asymptomatic (homozygote APOE ε4/ε4) | 11/2015 | 08/2023 |
| E2609 (Eisai) | NCT02322021 | II | MCI to moderate AD | 11/2014 | 01/2018 |
| NCT02956486 (MISSION-AD1) | III | MCI to mild AD | 10/2016 | 06/2020 | |
| JNJ54861911 (Janssen) | NCT02406027 | II | MCI to mild AD | 07/2015 | 10/2022 |
| NCT02569398 | II/III | Preclinical (amyloid positive) | 11/2015 | 05/2023 | |
| LY3202626 (Lilly) | NCT02791191 (NAVIGATE-AD) | II | Mild AD | 06/2016 | 08/2018 |
| LY3314814 (Lilly) | NCT02245737 (AMARANTH) | II/III | MCI to mild AD | 9/2014 | 8/2019 |
| NCT02783573 (DAYBREAK ALZ) | III | Mild AD | 7/2016 | 08/2021 | |
| Verubecestat (Merck) | NCT01739348 (EPOCH) | II/III | Mild to moderate AD | 11/2012 | 06/2017 |
Abbreviations: AD, Alzheimer's disease; BACE, β-site amyloid precursor protein cleaving enzyme; MCI, mild cognitive impairment.
Immunotherapies, especially monoclonal antibodies, are also well represented in the AD pipeline. There are currently 16 immunotherapy agents in 31 trials (Table 7). This includes aducanumab, solanezumab, crenezumab, gantenerumab, and BAN2401. Immunotherapies target a variety of epitopes of Aβ. Solanezumab targets soluble Aβ; this agent recently failed to show a drug-placebo difference in a phase III trial. It was terminated as a candidate therapy for AD dementia [34]. It continues in prevention trials. Aducanumab targets multiple Aβ species, has had an encouraging phase I/II trial, and is continuing in phase III [35].
Table 7.
Immunotherapies in clinical trials for AD (clinicaltrials.gov accessed 1/5/2017)
| Agent | Sponsor | Target | Trial phase | Population |
|---|---|---|---|---|
| AADvac1 | Axon Neuroscience | Anti-tau mAb | 1 | AD |
| AADvac1 | Axon Neuroscience | Anti-tau mAb | 2 | Mild-moderate AD |
| ABBV-8E12 | AbbVie | Anti-tau mAb | 2 | Early AD |
| Aducanumab | Biogen | mAb targeting multiple forms of Aβ | 1 | Healthy volunteers |
| Aducanumab | Biogen | mAb targeting multiple forms of Aβ | 1 | Prodromal-mild AD |
| Aducanumab | Biogen | mAb targeting multiple forms of Aβ | 1 | Mild-moderate AD |
| Aducanumab | Biogen | mAb targeting multiple forms of Aβ | 3 | Early AD |
| Aducanumab | Biogen | mAb targeting multiple forms of Aβ | 3 | Early AD |
| Albumin and immunoglobulin | Grifols | Polyclonal antibody targeting multiple forms of Aβ | 3 | Mild-moderate AD |
| BAN2401 | Eisai | mAb targeting N terminal protofibrils | 2 | Early AD |
| CAD106 | Novartis, NIA | Aβ1–6, active vaccine | 2 | AD, at risk |
| Crenezumab | Genentech | mAb targeting soluble oligomer and fibrillar Aβ | 1 | Mild-moderate AD |
| Crenezumab | Genentech, NIA, Academic | mAb targeting soluble oligomer and fibrillar Aβ | 2 | ADAD |
| Crenezumab | Genentech | mAb targeting soluble oligomer and fibrillar Aβ | 3 | Prodromal-mild AD |
| Gantenerumab | Roche | mAb targeting aggregated Aβ | 3 | Mild AD |
| Gantenerumab | Roche | mAb targeting aggregated Aβ | 3 | Prodromal AD |
| Gantenerumab | Roche, Lilly, Alzheimer's Association | mAb targeting aggregated Aβ | 2/3 | AD, at risk |
| Solanezumab | Lilly, Roche, Alzheimer's Association | mAb targeting monomeric Aβ | 2/3 | AD, at risk |
| KH6640 | Kyowa Hakko Kirin | mAb targeting aggregated Aβ | 1 | AD |
| Lu AF20513 | Lundbeck | 1 | Mild AD | |
| NewGam 10% IVIG | Sutter Health | Polyclonal antibody targeting multiple forms of Aβ | 2 | Amnestic MCI |
| LY2599666 & solanezumab | Lilly | Combination of BACE inhibitor and MAb targeting monomeric Aβ | 1 | MCI due to AD |
| LY3303560 | Lilly | 1 | MCI due to AD-mild AD | |
| LY30032813 | Lilly | 1 | MCI due to AD | |
| LY30032813 | Lilly | 1 | Mild-moderate AD | |
| RO7105705 | Genentech | Anti-tau mAb | 1 | Mild-moderate AD |
| Solanezumab | Lilly | mAb targeting monomeric Aβ | 3 | Prodromal AD |
| Solanezumab | Lilly | mAb targeting monomeric Aβ | 3 | Preclinical AD |
| Solanezumab | Lilly | mAb targeting monomeric Aβ | 3 | AD |
| Solanezumab | Lilly | mAb targeting monomeric Aβ | 3 | Mild AD |
| UB-311 | United Neuroscience | mAb targeting N terminal Aβ1–14 | 2 | Mild AD |
Abbreviations: AD, Alzheimer's disease; ADAD, autosomal dominant Alzheimer's disease; mAb, monoclonal antibody; MCI, mild cognitive impairment Yes, the expansion is correct.; IVIG, intravenous immunoglobulin; NIA, National Institute on Aging.
President Obama articulated a goal of cure or meaningful treatment for AD by the year 2025 [5], [36]. A recent analysis of AD drug development showed that it takes on average 13 years for a candidate treatment to move from laboratory to FDA review and 10 years for an agent to navigate the clinical development period from start of phase I to end of FDA review [37]. This means that under current circumstances, an agent must now be in phase II to possibly be approved by 2025 [5]. Although there are promising agents in the pipeline that could achieve this goal, it is clear that given the high rate of failure of AD drug development [38], the aim of having a repertoire of agents that could respond comprehensively and individually to a patient's clinical circumstances within the 2025 timeframe is in jeopardy.
There are many factors contributing to the currently low rate of success of drug development for AD. The understanding of the biology of AD is incomplete, the emphasis on testing single therapies where combinations may be required, the few candidates entering phase I, the lack of predictive validity of animal models, lack of efficacy of candidate therapies, and the emergence of unacceptable side effects all limit successful treatment development. When clinical trials are considered, the slowness of recruitment, lack, until recently, of tests for diagnostic confirmation, the requirement to globalize trials to achieve sufficient recruitment at the expense of data variability, and the heterogeneity of AD may all contribute to the lack of success in trials.
Biomarkers can contribute substantially to drug development success [39]. In AD, there are few biomarkers given the myriad of affected processes; there are only a small number of target engagement biomarkers capable of giving an early readout on proof of pharmacology; there are no surrogate markers known to predict the clinical outcome; and no validated outcome biomarkers have been shown to correlate with clinical outcomes in a trial in support of disease modification. These circumstances disadvantage AD drug development and increase the failure rates, especially for proposed DMTs.
Symptomatic agents are an important part of the AD drug development pipeline. There are four cognitive enhancing agents and no agents targeting behavioral symptoms in phase I clinical trials. Phase II has eight cognitive enhancing agents and seven behavioral agents in trials, whereas phase III has three cognitive enhancing agents and seven behavioral agents. Together there are 15 cognitive enhancers and 14 agents targeting neuropsychiatric symptoms in the pipeline. This comprises 27% of the AD drug development pipeline. Treatments for neuropsychiatric symptoms are more likely than other classes of drugs to enter the pipeline in phase II or III after they have been assessed in phase I with the intent of treating a primary psychiatric illness.
The solution to the problem of the few agents in the AD drug development pipeline, the many challenges facing candidate therapies, and the slow speed of testing drugs can be partially addressed by increased funding. The NIH is critically involved in addressing AD as one of the major challenges to human health that currently goes unchecked. Investment in basic research will assist in identifying more targets and candidate compounds, whereas investment in translational research can help support clinical trials and improve trial methods. Federal small business loans can help fund enterprising new approaches to drug treatment; academic medical centers can spin off biotech startups around promising tests, biomarkers, and drugs; and venture capital can help support startup through early phases of development until larger biopharma companies are ready to take agents through the final most expensive phases of clinical trials and FDA review. Philanthropy and innovative approaches such as venture philanthropy may help accelerate AD drug discovery and development funding [40]. Biopharma sponsors 65.5% of all clinical trials and is the major economic force for drug development.
All these financial instruments, however, are currently in place and are not sufficient to respond to the urgent need. New investment vehicles are needed, and some have been proposed such as private-federal bonds [41], [42]. Medicare and insurance companies who stand to benefit from improved health of the elderly should be engaged in funding conversations. New types of collaborations (e.g., between NIH and biopharma companies and between two biopharma companies) and consortia (e.g., the Alzheimer's Disease Neuroimaging Initiative) are increasing the financial feasibility of bearing the expense of AD drug development and enhancing the chance of success.
The need is great, the challenges many, the rewards high: this is the condition of AD drug development. Monitoring the AD drug development pipeline provides perspective on the success of the response to these challenges.
Research in Context.
-
1.
Systematic review: Drug development for Alzheimer's disease (AD) proceeds through three phases (I, II, and III). By assessing the number of agents in each phase as recorded on clinicaltrials.gov, one can determine current AD drug development activity to assess how many agents are being studied, the success of the research, and how the number of new drugs can be increased.
-
2.
Interpretation: Our data show that there are 105 drugs in development for treatment of AD. There are more drugs in phase II (52) than in phase III (28) or phase I (25). The small number of phase I compounds suggests that there is insufficient drug discovery activity to supply new agents for testing in clinical trials.
-
3.
Future directions: This review of the AD drug development pipeline provides insight into the state of AD drug development and can help guide new development programs.
Footnotes
J.C. has provided consultation to AbbVie, Acadia, Actinogen, Alzheon, Anavex, Avanir, Axovant, Bracket, Eisai, Genentech, Lilly, Lundbeck, MedAvante, Merck, Orion, Otsuka, Pfizer, QR, Roche, Suven, and Takeda Pharmaceutical and assessment companies. T.M., G.L., and A.R. have no disclosures. K.Z. is an employee of the Global Alzheimer Platform.
References
- 1.Alzheimer's Association 2015 Alzheimer's disease facts and figures. Alzheimers Dement. 2015;11:332–384. doi: 10.1016/j.jalz.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Alzheimer's Association . Alzheimer's Association; Chicago, IL: 2015. Changing the Trajectory of Alzheimer's Disease: How a Treatment by 2025 Saves Lives and Dollars. [Google Scholar]
- 3.Cummings J.L., Zhong K. Chapter 17-symptomatic cognitive enhancing agents A2-Wolfe. In: Michael S., editor. Developing Therapeutics for Alzheimer's Disease. Academic Press; Boston: 2016. pp. 459–475. [Google Scholar]
- 4.Cummings J., Morstorf T., Lee G. Alzheimer's drug development pipeline: 2016. Alzheimers Dement. 2016;2:222–232. doi: 10.1016/j.trci.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cummings J., Aisen P.S., DuBois B., Frolich L., Jack C.R., Jr., Jones R.W. Drug development in Alzheimer's disease: the path to 2025. Alzheimers Res Ther. 2016;8:39. doi: 10.1186/s13195-016-0207-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hudson K.L., Lauer M.S., Collins F.S. Toward a new era of trust and transparency in clinical trials. JAMA. 2016;316:1353–1354. doi: 10.1001/jama.2016.14668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zarin D.A., Tse T., Williams R.J., Carr S. Trial reporting in clinicaltrials.gov - the final rule. N Engl J Med. 2016;375:1998–2004. doi: 10.1056/NEJMsr1611785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curry S., DeCory H.H., Gabrielsson J. Phase I: the first opportunity for extrapolation from animal data to human exposure. In: Edwards L.D., Fox A.W., Stonier, editors. Principles and Practice of Pharmaceutical Medicine. Wiley-Blackwell; Oxford, UK: 2011. pp. 84–106. [Google Scholar]
- 9.Kelley J. Wiley-Blackwell; Oxford, UK: 2009. Principles of CNS Drug Development: From Test Tube to Patient. [Google Scholar]
- 10.Norfleet E., Gad S.C. Phase I clinical trials. In: Gad C.S., editor. Clinical Trials Handbook. John Wiley & Sons, Inc.; New York, New York: 2009. pp. 245–254. [Google Scholar]
- 11.Gray J.A., Fleet D., Winblad B. The need for thorough phase II studies in medicines development for Alzheimer's disease. Alzheimers Res Ther. 2015;7:67. doi: 10.1186/s13195-015-0153-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan S.B., Machin D. Phase II clinical trials. In: Gad C.S., editor. Clinical Trials Handbook. John Wiley & Sons, Inc.; New York, New York: 2009. pp. 255–277. [Google Scholar]
- 13.Wang S.J., Hung H.M., O'Neill R. Adaptive design clinical trials and trial logistics models in CNS drug development. Eur Neuropsychopharmacol. 2011;21:159–166. doi: 10.1016/j.euroneuro.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Cummings J.L. Biomarkers in Alzheimer's disease drug development. Alzheimers Dement. 2011;7:e13–e44. doi: 10.1016/j.jalz.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Cummings J.L., Zhong K. Repackaging FDA-approved drugs for degenerative diseases: promises and challenges. Expert Rev Clin Pharmacol. 2014;7:161–165. doi: 10.1586/17512433.2014.884923. [DOI] [PubMed] [Google Scholar]
- 16.Mattsson N., Carrillo M.C., Dean R.A., Devous M.D., Sr., Nikolcheva T., Pesini P. Revolutionizing Alzheimer's disease and clinical trials through biomarkers. Alzheimers Dement (Amst) 2015;1:412–419. doi: 10.1016/j.dadm.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sperling R.A., Jack C.R., Jr., Black S.E., Frosch M.P., Greenberg S.M., Hyman B.T. Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: recommendations from the Alzheimer's Association Research Roundtable Workgroup. Alzheimers Dement. 2011;7:367–385. doi: 10.1016/j.jalz.2011.05.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Viglietta V., O'Gorman J., Williams L., Chen T., Enayetallah A., Chiao P. Titration dosing of aducanumab: results of a 12-month interim analysis from a randomized, double-blind, placebo-controlled phase 1b study (PRIME) in patients with prodromal or mild Alzheimer's disease (S7.003) Neurology. 2017;88(S7.003):16. [Google Scholar]
- 19.Appleby B.S., Cummings J.L. Discovering new treatments for Alzheimer's disease by repurposing approved medications. Curr Top Med Chem. 2013;13:2306–2327. doi: 10.2174/15680266113136660162. [DOI] [PubMed] [Google Scholar]
- 20.Appleby B.S., Nacopoulos D., Milano N., Zhong K., Cummings J.L. A review: treatment of Alzheimer's disease discovered in repurposed agents. Dement Geriatr Cogn Disord. 2013;35:1–22. doi: 10.1159/000345791. [DOI] [PubMed] [Google Scholar]
- 21.Jekunen A. Decision-making in product portfolios of pharmaceutical research and development–managing streams of innovation in highly regulated markets. Drug Des Devel Ther. 2014;8:2009–2016. doi: 10.2147/DDDT.S68579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gauthier S., Feldman H.H., Schneider L.S., Wilcock G.K., Frisoni G.B., Hardlund J.H. Efficacy and safety of tau-aggregation inhibitor therapy in patients with mild or moderate Alzheimer's disease: a randomised, controlled, double-blind, parallel-arm, phase 3 trial. Lancet. 2016;388:2873–2884. doi: 10.1016/S0140-6736(16)31275-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zarin D.A., Ide N.C., Tse T., Harlan W.R., West J.C., Lindberg D.A. Issues in the registration of clinical trials. JAMA. 2007;297:2112–2120. doi: 10.1001/jama.297.19.2112. [DOI] [PubMed] [Google Scholar]
- 24.De Angelis C., Drazen J.M., Frizelle F.A., Haug C., Hoey J., Horton R. Clinical trial registration: a statement from the International Committee of Medical Journal Editors. N Engl J Med. 2004;351:1250–1251. doi: 10.1056/NEJMe048225. [DOI] [PubMed] [Google Scholar]
- 25.Babic T., Riordan H.J. Improving screen fail and recruitment rates in Alzheimer's disease clinical trials. J Clin Stud. 2016;8:38–40. [Google Scholar]
- 26.Cummings J., Reynders R., Zhong K. Globalization of Alzheimer's disease clinical trials. Alzheimers Res Ther. 2011;3:24. doi: 10.1186/alzrt86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cummings J.L., Aisen P., Barton R., Bork J., Doody R., Dwyer J. Re-engineering Alzheimer clinical trials: Global Alzheimer Platform Network. J Prev Alzheimers Dis. 2016;3:114–120. doi: 10.14283/jpad.2016.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weiner M.W., Veitch D.P., Aisen P.S., Beckett L.A., Cairns N.J., Green R.C. The Alzheimer's Disease Neuroimaging Initiative 3: continued innovation for clinical trial improvement. Alzheimers Dement. 2017;13:561–571. doi: 10.1016/j.jalz.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menting K.W., Claassen J.A. Beta-secretase inhibitor; a promising novel therapeutic drug in Alzheimer's disease. Frontiers in Aging Neuroscience. 2014;6:165. doi: 10.3389/fnagi.2014.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamada Y., Kiso Y. New directions for protease inhibitors directed drug discovery. Biopolymers. 2016;106:563–579. doi: 10.1002/bip.22780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Evin G. Future therapeutics in Alzheimer's disease: development status of BACE inhibitors. BioDrugs. 2016;30:173–194. doi: 10.1007/s40259-016-0168-3. [DOI] [PubMed] [Google Scholar]
- 32.Vassar R., Kuhn P.H., Haass C., Kennedy M.E., Rajendran L., Wong P.C. Function, therapeutic potential and cell biology of BACE proteases: current status and future prospects. J Neurochem. 2014;130:4–28. doi: 10.1111/jnc.12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hawkes N. Merck ends trial of potential Alzheimer's drug verubecestat. BMJ. 2017;356 doi: 10.1136/bmj.j845. j845. [DOI] [PubMed] [Google Scholar]
- 34.Le Couteur D.G., Hunter S., Brayne C. Solanezumab and the amyloid hypothesis for Alzheimer's disease. BMJ. 2016;355 doi: 10.1136/bmj.i6771. i6771. [DOI] [PubMed] [Google Scholar]
- 35.Sevigny J., Chiao P., Bussière T., Weinreb P.H., Williams L., Maier M. The antibody aducanumab reduces Abeta plaques in Alzheimer's disease. Nature. 2016;537:50–56. doi: 10.1038/nature19323. [DOI] [PubMed] [Google Scholar]
- 36.Snyder H.M., Hendrix J., Bain L.J., Carrillo M.C. Alzheimer's disease research in the context of the national plan to address Alzheimer's disease. Mol Aspects Med. 2015;43-44:16–24. doi: 10.1016/j.mam.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 37.Scott T.J., O'Connor A.C., Link A.N., Beaulieu T.J. Economic analysis of opportunities to accelerate Alzheimer's disease research and development. Ann N Y Acad Sci. 2014;1313:17–34. doi: 10.1111/nyas.12417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cummings J.L., Morstorf T., Zhong K. Alzheimer's disease drug-development pipeline: few candidates, frequent failures. Alzheimers Res Ther. 2014;6:37. doi: 10.1186/alzrt269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cook D., Brown D., Alexander R., March R., Morgan P., Satterthwaite G. Lessons learned from the fate of AstraZeneca's drug pipeline: a five-dimensional framework. Nat Rev Drug Discov. 2014;13:419–431. doi: 10.1038/nrd4309. [DOI] [PubMed] [Google Scholar]
- 40.Refolo L.M., Fillit H.M. Partnerships between philanthropy, government and industry are needed to advance drug discovery for neurodegenerative diseases. Curr Alzheimer Res. 2006;3:175–176. doi: 10.2174/156720506777632853. [DOI] [PubMed] [Google Scholar]
- 41.Fernandez J.M., Stein R.M., Lo A.W. Commercializing biomedical research through securitization techniques. Nat Biotechnol. 2012;30:964–975. doi: 10.1038/nbt.2374. [DOI] [PubMed] [Google Scholar]
- 42.Lo A.W., Ho C., Cummings J., Kosik K.S. Parallel discovery of Alzheimer's therapeutics. Sci Transl Med. 2014;6:241cm5. doi: 10.1126/scitranslmed.3008228. [DOI] [PubMed] [Google Scholar]