Abstract
Introduction
We evaluated whether transcranial direct current stimulation (tDCS) can improve picture-naming abilities in subjects with anomic Alzheimer or frontotemporal dementias.
Methods
Using a double-blind crossover design, 10 participants were trained on picture naming over a series of 10 sessions with either 30 minutes of anodal (2 mA) tDCS stimulation to the left inferior parieto-temporal region (P3) or sham stimulation. We evaluated performance on a trained picture-naming list, an equivalent untrained list, and additional neuropsychological tasks.
Results
Participants improved significantly more receiving real stimulation rather than sham stimulation (40% vs. 19%, P < .01), lasting at least 2 weeks after stimulation. Furthermore, these participants showed a small increase for untrained picture-naming items and digit span when they received real stimulation but a decrease when sham stimulation was received.
Discussion
tDCS stimulation has promise as a treatment for anomia in demented individuals and the effect can generalize to unstudied items as well as other cognitive abilities.
Keywords: tDCS, Anomia, Object naming, PPA, Training
1. Introduction
Neurodegenerative diseases such as Alzheimer's disease (AD) and frontotemporal dementia (FTD) are accompanied by a variety of cognitive impairments involving language, executive function, and memory. Current therapies are limited in their ability to significantly improve these cognitive abilities. Transcranial direct current stimulation (tDCS) has been show to have effects that last beyond the time of stimulation [1]. In a series of experiments in the past decade, tDCS has been shown to impact cognitive performance and emotional states [2]. tDCS has had a benefit sufficient to be used clinically in depression [3] and Parkinson's disease [4]. However, few tDCS studies have targeted any cognitive symptoms in primary progressive aphasia (PPA) living with AD or FTD, and of the few studies done, most have examined memory improvement after a single session of tDCS stimulation without concurrent training. These initial studies were positive [5], [6], [7], but negative results have also been reported [8], [9], [10], [11]. Crucially, these negative results have been attributed to the montage used, the participants included, or the method of evaluation, rather than representing a failure of tDCS itself. Taspkini et al. [12] found that combining training with anodal tDCS stimulation could improve spelling scores in six PPA participants (two nonfluent, four logopenic). These results suggest that tDCS may be especially effective when combined with training, and linguistic abilities such as anomia might be amendable to improvement. For this reason, we conducted a proof-of-concept study with a mixed group of dementia patients having neurodegenerative aphasia syndromes where anomia was prominent. We examined whether language training combined with anodal tDCS stimulation to the inferior parietal lobe would improve naming ability.
2. Method
2.1. Participants
Ten participants were selected for this pilot study, which was designed as a randomized cross-over of tDCS versus sham therapy. Clinically, they had evidence of dementia (AD or FTD), according to either McKhann et al. [13] criteria for AD, or the Rescovsky et al. [14] criteria for FTD. Inclusions criteria were as follows: a diagnosis of dementia (AD or FTD), scoring below a cutoff point for normal performance on the spontaneous naming task of the Cambridge Semantic Battery [15] and a demonstrated ability to do the naming task that was the focus of our investigation. For this reason, we included participants with low Montreal Cognitive Assessment (MoCA) scores and Mini–Mental State Examination (MMSE) scores if they were able to understand and cooperate for the Naming task and could make an effort to name images when prompted to do so. Some patients were taking medication (e.g., cholinesterase inhibitors), but there were no medication differences between the sham and real stimulation rounds. Finally, the study was approved by the research ethics committee of the Jewish General Hospital, Montréal, Canada. At the time of enrollment, all participants also underwent flurodeoxyglucose–positron emission topography scans to examine the level of left temporo-parietal hypometabolism—our target area of stimulation. Clinical reports from the neuroradiologist of hypometabolism in left perisylvian (temporo-parietal) regions were considered an additional inclusion criterion for the study, and the degree of hypometabolism was documented. As can be observed in Table 1, alongside demographic and diagnostic details, all participants had mild-to-severe hypometabolism in the left perisylvian region, but variable hypometabolism on the right.
Table 1.
Patient diagnostic and general cognition data
| Patient code | Diagnosis, PPA type | Left TP | Right TP | Age | Sex (M/F) | Education (years) | MoCA | MMSE | C. naming score |
|---|---|---|---|---|---|---|---|---|---|
| BaM | FTD, nf PPA | ++ | + | 74 | M | 18 | 3 | 9 | 59 |
| BeJ | FTD, nf PPA | + | 72 | M | 15 | 24 | 28 | 57 | |
| CaM | FTD, nf PPA | + | + | 62 | M | 11 | 13 | 17 | 59 |
| DiC | FTD, nf PPA | ++ | ++ | 75 | M | 11 | 21 | 26 | 58 |
| LaD | FTD, nf PPA | ++ | + | 62 | M | 18 | 9 | 14 | 34 |
| MaA | AD, nf PPA | ++ | 63 | F | 18 | 24 | 27 | 56 | |
| McD | AD, logo PPA | ++ | ++ | 69 | M | 11 | 10 | 17 | 30 |
| OuL | AD, logo PPA | +++ | ++ | 70 | M | 11 | 6 | 10 | 30 |
| LaA | FTD, sv PPA | ++ | ++ | 56 | F | 18 | 3 | 8 | 11 |
| TrL | FTD, sv PPA | ++ | ++ | 71 | F | 18 | 22 | 26 | 9 |
Abbreviations: PPA, primary progressive aphasia; TP, temporal-parietal area; MoCA, Montreal Cognitive Assessment; MMSE, Mini–Mental State Examination; FTD, frontotemporal dementia; PPA subtypes: nf PPA, nonfluent PPA; logo PPA, logopenic PPA; sv PPA, semantic variant PPA; AD, Alzheimer's disease; hypometabolism levels: +, mild; ++, moderate; +++, severe, based on FDG-PET scans collected on participants at the time of study enrollment.
NOTE. C. naming is the spontaneous naming score obtained on the naming task from the Cambridge Semantic Battery (max = 64, normal elderly control mean = 62, standard deviation = 2).
2.2. Experimental design
Five participants were given anodal stimulation (10 daily sessions) during the first round of testing and had 10 sessions of sham stimulation delivered at least 2 months later. For the other five participants, this order was reversed. They were first given 10 sessions of sham stimulation, with anodal stimulation administered during the second round of testing at least 2 months later. All participants and raters were blind to the stimulation condition (active vs. sham). Furthermore, regardless of condition, all participants experienced an initial ramp-up of the machine to 2 mA, which remained at 2 mA for 1 minute in the sham condition. Furthermore, a simulated ramp-up also occurred in the final 30 seconds of the sham condition. In this manner, regardless of condition, all participants felt an initial prickling sensation during the first minute which indicated that stimulation had started. In a real stimulation condition, after this initial ramp-up, participants begin to notice the sensations less as impendence becomes sufficiently low, to the extent that many participants report no longer feeling the stimulation. Thus, in a sham stimulation condition, when stimulation effectively ceases, participants in turn perceive this change as similar to the one felt in the real stimulation condition. In other words, participants have great difficulty distinguishing real and sham stimulation. Consequently, despite being informed after the experiment that some rounds had been sham, participants by and large indicated that they had considered all rounds to contain real stimulation, albeit also reporting that certain rounds were more effective than others.
2.3. Primary outcome measure: Spontaneous naming
Stimuli for the picture-naming task were taken from the Snodgrass and Vanderwart image set [16], with familiarity and agreement norms obtained from a group of 20 normal elderly adults (mean age 72 years). They were asked to name the image and provide a familiarity rating from one (not at all familiar) to 7 (very familiar). We then eliminated any images that were incorrectly named, as well as any image where the agreement for a particular label was less than 95%. The remaining images were then organized into their semantic categories, ranked for familiarity, and divided into three lists hereafter called naming 1, 2, and 3. In this manner, each list was equally familiar and had a similar number of exemplars from each semantic category. There were 60 pictures in each list, and there was no difference between lists in terms of word frequency or picture familiarity (P > .90 for both).
In each round of stimulation, one list of items would be used for daily training sessions carried out simultaneously with an initial 30 minutes of tDCS stimulation, whereas another naming list was left untrained (as described in Section 2.2). More specifically, in the first round of the experiment, naming 1 was trained, and naming 2 was left untrained, whereas for round 2, naming 2 was trained and naming 3 was left untrained. This allowed us to assess changes in naming pre- and post-tDCS, for both “trained” and “untrained” picture items.
2.4. Secondary outcome measures: Language and cognition
An additional set of general cognition tasks was also included to assess participant's abilities in these domains and to check if parietal tDCS stimulation would lead to higher scores compared to sham. No training for these tasks was ever carried out, and they were only administered during an evaluation session (i.e., before tDCS stimulation, the final session of tDCS stimulation, and 2 weeks after stimulation). The tasks included forward and backward digit span from the WAIS-IV [17], verbal fluency (F, A, S, Animals), and two assessments of general cognition: the MoCA [18] and the MMSE [19]. In addition, informal “exit interviews” were carried out with family members after study, to glean anecdotal information on any observed change in mood, cognition, or day-to-day function observed by the family.
2.5. tDCS montage
A DC STIMULATOR PLUS (NeurConn, Germany) was used to administer anodal stimulation for 30 minutes at 2 mA. The time (30 minutes) and magnitude (2 mA) have been used often with dementia participants (e.g., [20]). The inferior parietal lobe was chosen for stimulation because this area has shown to be highly involved in semantic control processes, in particular when naming objects [21]. However, considering the size of the tDCS sponges (5 × 7 cm), the area was always roughly P3 (in the 10-20 system for EEG electrode placement) and included both the angular gyrus and supramarginal gyrus, as well as the superior areas of Brodmann area 37. We view these additional areas of stimulation as positive as these brain areas have also been noted in semantic control processes, and being able to simultaneously stimulate multiple areas could be a strength when treating people living with dementia. The reference electrode was placed on the right fronto-orbital area; the bottom edge of the sponge was brought down to the person's right eyebrow. Finally, sponges were soaked in a saline (0.9%) solution before application, and straps were placed over the sponges along the person's scalp to ensure sponges remained in place throughout the session. Both the anodal stimulation and sham condition had an initial ramp-up to 2 mA lasting 10 seconds. It is during this initial phase, where impedance is high, that tingling and burning sensations are most felt. To bring impedance levels below 5.0 for all participants, where side effects are perceived substantially less, syringes were used to apply further solution to the area between the sponges and the person's scalp regardless of the condition. In the sham condition, stimulation ceased after 60 seconds, whereas it continued for 30 minutes in the anodal stimulation condition. However, a ramp-down was experienced in both conditions during the final 30 seconds. For this reason, participants reported being unable to tell apart sham and real conditions as all sessions have started and finished with a ramp-up which seemed to indicate that stimulation had occurred. Fig. 1 below displays the montage used.
Fig. 1.
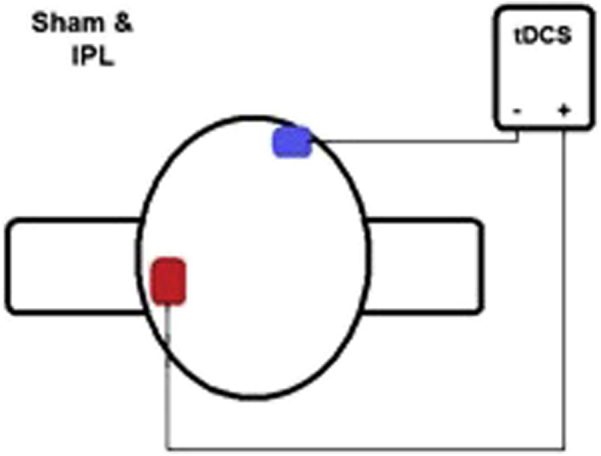
Montage applied. Abbreviation: tDCS, transcranial direct current stimulation.
2.6. Experiment protocol
Before the experimental sessions, all participants underwent a structural magnetic resonance imaging (MRI). In a subsequent session, with the aid of a TMS neural-navigation device (Magstim Rapid stimulator, double 70-mm coil, UK), the approximate location of the participants' inferior parietal lobe vis- à-vis their scalp was located via the Brainsight software package, marked, and used in all subsequent sessions as the location for the anodal stimulation sponge; regardless of experimental condition (anodal or sham). Location was guided by an anatomic target on the participant's MRI: the area below the intraparietal sulcus and roughly where the angular and supramarginal gyri meet. Each person subsequently underwent two rounds of 11 sessions at the Jewish General Hospital in Montreal. In the first session, the person was administered the tasks used to evaluate the primary and secondary outcome measures as described earlier. Administering all of these tasks took approximately 2 hours. Participants were unaware if they were in the parietal or sham condition, and for all evaluations, the person administering and scoring the tasks was also blind to the participant's condition. In other words, this was a double-blind crossover design.
For the second to tenth session of the first round, each session followed the same procedure. First, the tDCS machine was started to provide either anodal or sham stimulation. Second, each participant was presented the 60 images from either naming 1 or naming 2, depending on whether this was their first or second round of participation; each image was presented for 6 seconds and the participant was asked to name the picture. The items the participant failed to name during this initial naming task were divided into groups of five images. For example, if a participant failed to name 30 images, there would be six groups created. We then presented a group of five images one-by-one, noting if it was now correctly named or missed, and told the participant the image's name when he or she failed to do so. The images were then cycled until all images in a group were correctly named three cycles in a row. The next group of unnamed images was then presented, and so on, until all untrained images were trained. Note, however, that the unnamed images trained were always specific to that particular session because they were based on the participant's initial performance when asked to name the initial set of 60 images. In other words, the items to be trained in any particular session were always the images that the participant failed to name during that session's initial naming task. For this reason, the length of the session varied greatly as it depended on the number of unnamed items made by the participant at a particular session, and milder participants made fewer errors.
Despite these differences, training always lasted at least 30 minutes (the length of the stimulation given), but no longer than 2 hours to avoid exhaustion, even if some unnamed items were left untrained.
In the final 11th session, tDCS stimulation was administered as in previous sessions, but evaluation rather than training was administered. Therefore, the results for this session could be interpreted as checks for possible online effects because this evaluation was identical to that given in the first session except for the initial tDCS stimulation. Note, however, that participants were again presented both naming lists, despite being trained on only one of these. In this manner, we could compare improvement for a list on which participants were trained versus one where no training was given. This evaluation was repeated 2 weeks later without tDCS stimulation in the interval, to examine the duration of stimulation effects. All evaluations took roughly 2 hours.
In summary, evaluation took place before the first stimulation session, at the final stimulation session accompanied with an initial 30 minutes of real or sham tDCS stimulation, and 2 weeks later without stimulation. Round 2 for a participant began at least 2 months later to ensure washout of any residual effects of the first round. Participants returned to receive the alternate type of stimulation but otherwise underwent the same procedure as done in round 1. Fig. 2 displays the experimental protocol.
Fig. 2.
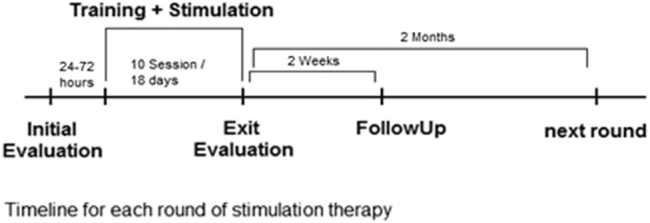
tDCS paradigm used in present study. Abbreviation: tDCS, transcranial direct current stimulation.
2.7. Data analysis
For the primary outcome measures, a one-way ANOVA with repeated measure was used to compare results across the conditions for the same set of participants. Stimulation type (anodal vs. sham) and time (session 1, session 11, after 2 weeks) were included as within-subject factors. To maximize power due to the small sample size, we also ran this ANOVA separately for trained and untrained items. Similarly, for secondary outcome measures, separate one-way ANOVAs with repeated measures were used for each task. Finally, baselines scores across conditions for trained and untrained items were comparable.
3. Results
3.1. Trained naming list
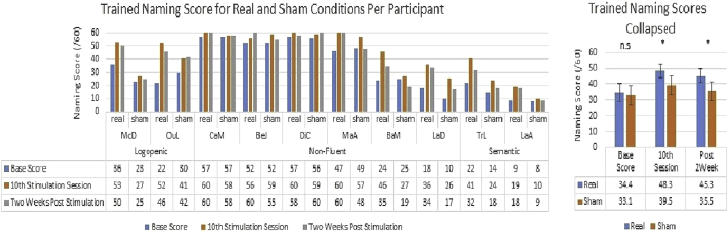
For trained items, both stimulation (F = 13.41, P < .01, partial η = 0.60) and the stimulation by time interaction were significant (F = 9.98, P < .05, partial η = 0.53). Participants in the real anodal tDCS stimulation condition improved from a prestimulation baseline of 34.4/60 (SD = 17.71) to 48.3/60 (SD = 13.22), representing a 40% improvement, with a slight decrease to 45.3/60 (SD = 14.86) 2 weeks later. In contrast, the same participants improved only from 33.1/60 (SD = 19.2) to 39.5/60 (SD = 17.92), in the sham condition, a 19% improvement, followed by a small decrease 2 weeks later to 35.5/60 (SD = 19.29). The results strongly suggest although participants benefited from training in both conditions, the improvement was twice as large when it was combined with tDCS stimulation (40% increase vs. 19% increase). Planned comparisons, paired t-tests, also found base scores for the real and sham conditions were similar [t (9) = 0.84, P = .43] but then significantly different at the 10th stimulation session [t (9) = 3.75, P < .01], as well as 2 weeks later [t (9) = 4.18, P < .01]. These results are displayed in Fig. 3.
Fig. 3.
Naming score improvement for trained items per stimulation round. Abbreviation: n.s., not significant. *P <.05.
3.2. Untrained naming list
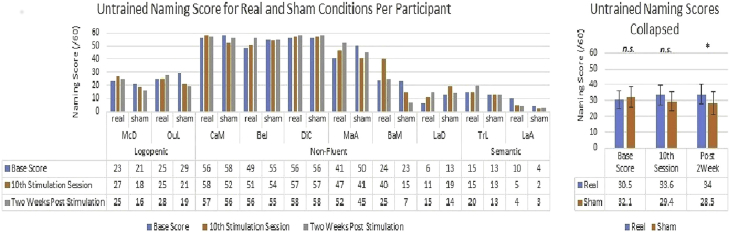
For untrained items, only the stimulation by time interaction was significant (F = 5.83, P < .05, partial η = 0.39). Examining the results per condition, we found that participants in the real stimulation condition improved slightly from a baseline of 30.5/60 (SD = 18.69) to 33.6/60 (SD = 19.60), and then to 34.0/60 (SD = 19.91) 2 weeks after stimulation. In contrast, the same participants receiving sham stimulation actually showed a small decrease from a baseline score of 32.1/60 (SD = 20.76) to a score of 29.4/60 (SD = 19.56) and further decreased to 28.5/60 (SD = 22.27) 2 weeks later. Planned comparisons, paired t-tests, found real versus sham scores were similar at baseline [t (9) = 1.03, P = .33] and at the 10th session [t (9) = 1.51, P = .17] but significantly different 2-weeks after stimulation [t (9) = 3.07, P < .05] because participants in the real condition continued to increase as opposed to the decrease experienced in the sham condition. We present the results in Fig. 4 and provide possible explanations for these results in Section 4.
Fig. 4.
Naming score improvement for untrained items per stimulation round. Abbreviation: n.s., not significant. *P <.05.
3.3. Secondary outcome measures
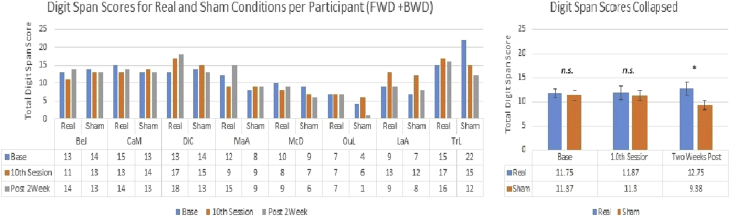
Of the five secondary measures assessed, one significant result emerged: digit span (n = 8). For this task, participant's score was expressed as their total for both forward and backward digit span. When participants received real anodal stimulation to P3, their prestimulation total digit span score changed little from 11.75 (SE = 1.01) to 11.88 (SE = 1.36) but had a small increase 2 weeks later to 12.75 (SE = 1.39). Although these results may seem unimpressive, compare when the same participants received sham stimulation: little change from a first session score of 11.38 (SE = 1.98) to the final session score of 11.38 (SE = 1.27), but then a drop 2 weeks later to 9.38 (SE = 1.52). Consistent with these observations, both the stimulation effect (F = 7.15, P < .05, partial η = 0.51) and the stimulation by time interaction were significant (F = 3.81, P < .05, partial η = 0.35). Post hoc paired t-tests with a Bonferroni correction found total digit span scores were similar at baseline [t (7) = 0.31, P = .77] and at the 10th session [t (7) = 1.0, P = .35] but significantly different 2 weeks later [t (7) = 4.34, P < .01]. These results are displayed in Fig. 5.
Fig. 5.
Score per condition over time for digit span. Abbreviation: n.s., not significant. *P <.05.
3.4. Informal caregiver interviews
We did not formally assess to what extent stimulation results extended to aspects of daily living; however, 7/10 family members in debriefing sessions reported noting positive behavior change that coincided with active parietal stimulation, which were absent when sham stimulation was administered. The only observed side effect was occasional redness where the sponge was placed, which disappeared after a few hours.
4. Discussion
We have found that application of tDCS to the inferior parietal lobe area (P3) during picture-naming training demonstrated significant improvement in picture naming beyond that encountered with training alone. Improvement in performance was greater and more durable when training was undertaken with simultaneous tDCS stimulation. These results suggest tDCS is effective at stimulating the parietal lobe and enhancing cortical networks involved in cognitive function. A similar (albeit less striking) result was found for untrained items. Although the participants showed only small improvement, this was contrasted with a decrease when sham stimulation was received. During sham stimulation, participants would often name an object incorrectly by using a label for which they had been previously trained, and we suggest this greater tendency during the sham condition was the cause for the decrease. For example, a frequently misnamed and therefore frequently practiced item regardless of training session was owl, and when being evaluated on the untrained naming list, participants would produce the verbal label owl in the presence of another bird (e.g., eagle). Therefore, training participants on a certain set of images appeared to cause semantic interference when they were then presented a novel array of images; however, participants receiving real anodal stimulation to the parietal lobe were better able to handle this increased level of semantic interference. Finally, a stimulation effect was found for digit span, which is consistent with previous results suggesting the digit span task was argued to rely on parietal cortices [21], [22].
In summary, for trained items, participants improved more and maintained their scores longer, when stimulation was real rather than sham, whereas an improvement for untrained items and digit span was observed only when stimulation was real. Therefore, we are confident that a tDCS stimulation benefit was produced in our participants. Further studies will be needed to check if the results can be replicated with a larger sample size, but the initial results are also impressive enough to warrant such an investigation.
4.1. tDCS: Is there potential as an ancillary therapy for dementia?
We have demonstrated significant improvement in cognitive functions lasting at least up to 2 weeks following anodal tDCS stimulation to the inferior parietal lobe in individuals with neurodegenerative disease who showed hypometabolism in that region. The improvement was clearly demonstrated on the target picture-naming task as accuracy for naming trained pictures was markedly improved, but improvement against sham was demonstrable for all pictures tested. Furthermore, certain family members in debriefing sessions reported noting positive behavior change that coincided with active parietal stimulation, which were absent when sham stimulation was administered. For this reason, we view these results as sufficient to motivate further work to elucidate the optimal positioning, location, cognitive tasks, montage, and patient characteristics which will produce significant benefit for AD and FTD individuals. For example, Cheng et al. [23] have proposed a tDCS study examining if tDCS could also improve working memory in individuals with Alzheimer's disease. If our results are replicated in formal larger clinical trials, tDCS with training might emerge as a viable therapy for AD and FTD.
Research in Context.
-
1.
Systematic review: Only a few transcranial direct current stimulation (tDCS) studies have included people with dementia and the results have been mixed. We note that combining training with stimulation seems crucial for positive effects. Thus, we ran a proof-of-concept study to investigate if tDCS stimulation combined with training would improve naming ability in people with primary progressive aphasia caused by a neurodegenerative disease (i.e., Alzheimer's disease or frontotemporal dementia).
-
2.
Interpretation: Participants improved when naming training was combined with 30 minutes of anodal stimulation. Furthermore, results were much larger for the items that were trained, which reinforces our hypothesis that effects are greater when stimulation is combined with training.
-
3.
Future directions: Test the paradigm for another domain (e.g., working memory). For example, administering stimulation with training on an N-Back task may lead to working memory improvement. Positive results would suggest our paradigm works across domains, whereas null results would suggest that the paradigm's effectiveness is limited.
Acknowledgments
Argye E. Hillis and Kyrana Tsapkini (Johns Hopkins School of Medicine) provided advice and guidance on the design of the study. Melanie Malus (Jewish General Hospital, Montreal) served as the blind evaluator. Shelley Solomon (Jewish General Hospital, Montreal) coordinated patient visits with the assistance of Fern Collier, Heather Kape, and Reneé Kaminski (Jewish General Hospital, Montreal). Chris Hosein (Jewish General Hospital, Montreal) assisted with ethics approval. Finally, the authors are also grateful to Ziad Nasreddine (Université de Sherbrooke), Pedro Rosanato (McGill University), and Anna Zumbansen (McGill University), and Aphasie Rive-Sud (Sherbrooke, Québec, Canada) for their help recruiting participants.
Study was supported by postdoctoral fellowships from the Alzheimer's Society of Canada to Carlos Roncero and by a grant from the Canadian Institutes of Health Research (CIHR) to Howard Chertkow and Alexander Thiel.
References
- 1.Nitsche M.A., Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527:633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woods A.J., Antal A., Bikson M., Boggio P.S., Brunoni A.R., Celnik P. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin Neurophysiol. 2016;127:1031–1048. doi: 10.1016/j.clinph.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nitsche M.A., Boggio P.S., Fregni F., Pascual-Leone A. Treatment of depression with transcranial direct current stimulation (tDCS): a review. Exp Neurol. 2009;219:14–19. doi: 10.1016/j.expneurol.2009.03.038. [DOI] [PubMed] [Google Scholar]
- 4.Benninger D.H., Lomarev M., Lopez G., Wassermann E.M., Li X., Considine E. Transcranial direct current stimulation for the treatment of Parkinson's disease. J Neurol Neurosurg Psychiatry. 2010;81:1105–1111. doi: 10.1136/jnnp.2009.202556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrucci R., Mameli F., Guidi I., Mrakic-Sposta S., Vergari M., Marceglia S. Transcranial direct current stimulation improves recognition memory in Alzheimer's disease. Neurology. 2008;71:493–498. doi: 10.1212/01.wnl.0000317060.43722.a3. [DOI] [PubMed] [Google Scholar]
- 6.Boggio P.S., Khoury L.P., Martins D.C., Martins O.E., de Macedo E.C., Fregni F. Temporal cortex direct current stimulation enhances performance on a visual recognition memory task in Alzheimer disease. J Neurol Neurosurg Psychiatry. 2009;80:444–447. doi: 10.1136/jnnp.2007.141853. [DOI] [PubMed] [Google Scholar]
- 7.Boggio P.S., Ferruci R., Mameli F., Martins D., Martins O., Vergari M. Prolonged visual memory enhancement after direct current stimulation in Alzheimer's disease. Brain Stimul. 2012;5:223–230. doi: 10.1016/j.brs.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Cotelli M., Manenti R., Brambilla M., Petesi M., Rosini S., Ferrari C. Anodal tDCS during face-name associations memory training in Alzheimer's patients. Front Aging Neurosci. 2014;6:38. doi: 10.3389/fnagi.2014.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huey E.D., Probasco J.C., Moll J., Stocking J., Ko M.H., Grafman J. No effect of DC polarization on verbal fluency in patients with advanced frontotemporal dementia. Clin Neurophysiol. 2007;118:1417–1418. doi: 10.1016/j.clinph.2007.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suemoto C.K., Apolinario D., Nakamura-Palacios E.M., Lopes L., Leite R.E., Sales M.C. Effects of a non-focal plasticity protocol on apathy in moderate Alzheimer's disease: a randomized, double-blind, sham-controlled trial. Brain Stimul. 2014;7:308–313. doi: 10.1016/j.brs.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Bystad M., Gronli O., Rasmussen I.D., Gundersen N., Nordvang L., Wang-Iversen H. Transcranial direct current stimulation as a memory enhancer in patients with Alzheimer's disease: a randomized, placebo-controlled trial. Alzheimers Res Ther. 2016;8:13. doi: 10.1186/s13195-016-0180-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsapkini K., Frangakis C., Gomez Y., Davis C., Hillis A.E. Augmentation of spelling therapy with transcranial direct current stimulation in primary progressive aphasia: preliminary results and challenges. Aphasiology. 2014;28:1112–1130. doi: 10.1080/02687038.2014.930410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKhann G.M., Knopman D.S., Chertkow H.C., Hyman B.T., Jack C.R., Jr., Kawas C.H. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging and the Alzheimer's Association workgroup. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rascovsky K., Hodges J.R., Knopman D., Mendez M.F., Kramer J.H., Neuhaus J. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adlam A.L., Patterson K., Bozeat S., Hodges J.R. The Cambridge Semantic Test Battery: detection of semantic deficits in semantic dementia and Alzheimer's disease. Neurocase. 2010;16:193–207. doi: 10.1080/13554790903405693. [DOI] [PubMed] [Google Scholar]
- 16.Snodgrass J.G., Vanderwart M. A standardized set of 260 pictures: Norms for name agreement, image agreement, familiarity, and visual complexity. J Exp Psychol Hum Learn. 1980;6:174–215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- 17.Wechsler D. 4th ed. Pearson; San Antonio, TX: 2008. Wechsler Adult Intelligence Scale. [Google Scholar]
- 18.Nasreddine Z.S., Phillips N.A., Bedirian V., Charbonneau S., Whitehead V., Collin I. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 19.Folstein M.F., Folstein S.E., McHugh P.R. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 20.Binder J.R., Desai R.H. The neurobiology of semantic memory. Trends Cogn Sci. 2011;15:527–536. doi: 10.1016/j.tics.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koenings M., Barbey A.K., Postle B.R., Grafman J. Superior parietal cortex is critical for the manipulation of information in working memory. J Neurosci. 2009;29:14980–14986. doi: 10.1523/JNEUROSCI.3706-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warrington E.K., Logue V., Pratt R.T. The anatomical localization of selective impairment of auditory verbal short-term memory. Neuropsychologia. 1971;9:377–387. doi: 10.1016/0028-3932(71)90002-9. [DOI] [PubMed] [Google Scholar]
- 23.Cheng C.P., Chan S.S., Mak A.D., Chan W.C., Cheng W.C., Cheng S.T. Would transcranial direct current stimulation (tDCS) enhance the effects of working memory training in older adults with mild neurocognitive disorder due to Alzheimer's disease: study protocol for a randomized controlled trial. Trials. 2015;16:479. doi: 10.1186/s13063-015-0999-0. [DOI] [PMC free article] [PubMed] [Google Scholar]