Abstract
Introduction
Alzheimer's disease (AD) patients are at risk of nutritional insufficiencies because of physiological and psychological factors. Recently, we showed the results of the meta-analyses indicating lower plasma levels of vitamins A, B12, C, E, and folate in AD patients compared with cognitively intact elderly controls (controls). Now, additional and more extensive literature searches were performed selecting studies which compare blood and brain/cerebrospinal fluid (CSF) levels of vitamins, minerals, trace elements, micronutrients, and fatty acids in AD patients versus controls.
Methods
The literature published after 1980 in Cochrane Central Register of Controlled Trials, Medline, and Embase electronic databases was systematically analyzed using Preferred Reporting Items for Systematic reviews and Meta-Analyses guidelines to detect studies meeting the selection criteria. Search terms used are as follows: AD patients, Controls, vitamins, minerals, trace elements, micronutrients, and fatty acids. Random-effects meta-analyses using a linear mixed model with correction for age differences between AD patients and controls were performed when four or more publications were retrieved for a specific nutrient.
Results
Random-effects meta-analyses of 116 selected publications showed significant lower CSF/brain levels of docosahexaenoic acid (DHA), choline-containing lipids, folate, vitamin B12, vitamin C, and vitamin E. In addition, AD patients showed lower circulatory levels of DHA, eicosapentaenoic acid, choline as phosphatidylcholine, and selenium.
Conclusion
The current data show that patients with AD have lower CSF/brain availability of DHA, choline, vitamin B12, folate, vitamin C, and vitamin E. Directionally, brain nutrient status appears to parallel the lower circulatory nutrient status; however, more studies are required measuring simultaneously circulatory and central nutrient status to obtain better insight in this observation. The brain is dependent on nutrient supply from the circulation, which in combination with nutrient involvement in AD-pathophysiological mechanisms suggests that patients with AD may have specific nutritional requirements. This hypothesis could be tested using a multicomponent nutritional intervention.
Keywords: Alzheimer's disease, Brain, Cerebrospinal fluid, Plasma, Nutrient, Nutritional requirement, Metabolism, Omega-3 polyunsaturated fatty acids, DHA, Choline, Vitamins, Phosphatidylcholine, Phospholipid synthesis, Synapse, Neuronal membrane
Highlights
-
•
First systematic comparison of brain nutrient levels in Alzheimer's disease (AD) patients versus controls.
-
•
Specific nutrients show lower levels in AD, whereas other nutrients show no difference.
-
•
Directionally, brain nutrient status seems to parallel blood nutrient status in AD.
1. Introduction
The link between nutrition and the risk of developing Alzheimer's disease (AD) has been recognized for several decades. Specific dietary patterns have been associated with increased risk of developing AD, whereas others are linked to protection. Dietary patterns such as the Mediterranean diet that is characterized by high intakes of legumes, fruits, fish, unsaturated fats, and high in antioxidants offer protection [1]. Conversely, diets high in saturated fats, high trans-fat, and low antioxidants levels have been linked to an increased risk for developing AD [2]. In addition, diet-related disorders such as obesity, hypertension, hypercholesterolemia, and diabetes have consistently been shown to be associated with AD [3], [4], [5].
Since understanding the pivotal importance of B-vitamins for neuronal functioning and cognition, at the beginning of the 20th century [6], [7], [8], several nutrients, including antioxidants, choline, and omega-3 fatty acids, have been suggested to influence cerebral functioning (reviewed in Bourre [9] and in Smith and Blumenthal [10]). It is no surprise, therefore, that these nutrients have been postulated to play roles in the pathophysiological processes in AD. For example, antioxidants reduce reactive oxygen species–induced damage and stabilize membranes; the fatty acid docosahexaenoic acid (DHA) affects abnormal membrane–located protein processing (amyloid-b, tau); and DHA, choline, and uridine modulate neuronal membrane formation (reviewed in van Wijk et al. [11]). Neuronal membrane function has been shown to be dependent on its phospholipid composition, and alterations could lead to membrane instability and synaptic loss and, in that way, contribute to AD pathology [12]. Recent evidence suggests that a multinutrient intervention which enhances phospholipid formation comprising DHA, eicosapentaenoic acid (EPA), uridine monophosphate, choline, folate, vitamin B6, vitamin B12, vitamin C, vitamin E, selenium, and phospholipids modulated functional connectivity measures (assessed by electroencephalography) in AD, indicative of preserved synaptic function [13], [14]. These data suggest that adequate supply of specific nutrients may preserve synaptic function, prevent neurodegeneration, and eventually neuronal loss, while previous work showed that people with AD have lower systemic availability of several nutrients that may limit optimal brain function [15]. Publications on brain nutrients in AD compared with non-AD suggest differences in brain nutrient levels as well, but the available evidence is not fully consistent and systematic reviews are lacking.
The main objective of this review was to evaluate the presence of differences in brain nutrient levels between AD patients and cognitively intact elderly controls. In addition, systemic availability of nonvitamin nutrients will be evaluated to extend our earlier work on plasma vitamin availability in AD [15]. All relevant literature studies published after 1980 in Medline, Embase, and the Cochrane Central Register of Controlled Trials were reviewed using the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines [16].
2. Methods
2.1. Search strategy and selection criteria
Relevant literature published from 1980 to June 26, 2015 was systematically screened in the Cochrane Central Register of Controlled Trials, Medline, and Embase electronic databases according to PRISMA guidelines [16] using the following search terms in the title, abstract, or descriptors: (Alzheimer* and [humans or patients or inpatients or outpatients or persons or volunteers or participants or subjects] and [nutrition or nutritional or nutrient or nutrients or micronutrient or micronutrients or diet or diets or dietary or vitamin or vitamins or mineral or minerals or trace-element or trace-elements or fatty-acid or fatty-acids or PUFA or PUFAs or 1H-MRS (proton magnetic resonance spectroscopy) or MRS or MR spectroscopy]).
The search in the Cochrane Central Register of Controlled Trials, Embase, and Medline resulted in 11,389 published studies that were imported into Endnote (see Fig. 1 for the breakdown of publication selection). Duplicate references were automatically removed, followed by manual examination, which retrieved another 173 duplicate references. Conference abstracts (1474), non-English publications (640), publications with nonhuman data (1719), and Reviews, Editorials, Letters, Notes, Short Surveys, Comments, Case Reports (2180) were all removed from the database. The title and abstract of the remaining 5203 publications were evaluated according to predefined exclusion and inclusion criteria.
Fig. 1.
Breakdown of the retrieved publications leading to the selection of the 116 publications suitable for meta-analysis. Abbreviation: AD, Alzheimer's disease.
Included were those papers that might contain plasma or brain nutrient levels of AD patients, even if not explicitly mentioned in the abstract. Five hundred twenty-eight of the retrieved publications were identified as being of potential relevance. The full text of these publications was analyzed according to the following inclusion criteria: contained AD patient population according to the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer's Disease and Related Disorders Association criteria [17] and/or the Diagnostic and Statistical Manual for Mental Disorders [18] criteria for AD and mentioned the use of a cognitively intact elderly control group, mean and standard deviation, plasma or serum or brain or cerebrospinal fluid (CSF) levels of nutrients, number of AD patients and controls, and mean age or age range of AD and control groups.
Analysis of the retrieved documents revealed that some of these publications concerned data that were derived from the same population of patients. In this case, the publication with the most complete set of data was included. The following studies were excluded: those concerning other cognitive disorders (e.g., mild cognitive impairment, vascular dementia, frontotemporal dementia, or psychogeriatric disease); those that included subjects using vitamin supplements; and those not written in English. Botanicals, resveratrol, curcumin, beta-carotene, and heavy metals (aluminum and mercury) were also excluded because these are not considered micronutrients.
For circulatory nutrient status plasma, serum and erythrocyte levels (in order of preference) were eligible for inclusion. Exception was made for circulatory choline status where, in the context of AD, plasma phosphatidylcholine (PC) level is considered a better choline status indicator than free or total choline levels [19]. For brain nutrient status, both the tissue and CSF levels (in order of preference) were eligible for inclusion. Brain choline status was evaluated by including publications reporting choline levels based on 1H-MRS. Publications reporting relative or absolute choline levels were included, whereas publications reporting choline as a ratio over creatine were excluded for the meta-analysis. For publications reporting tissue/1H-MRS nutrient levels in different brain regions, only one brain region per nutrient per study was selected to perform statistical analysis. Selection criteria for brain regions follow the order: hippocampus, dentate gyrus of the hippocampus, parahippocampal cortex, temporal lobe/cortex and subdivisions, piriform cortex, frontal lobe/cortex and subdivisions, prefrontal cortex, parietal lobe/cortex and subdivisions, whole brain, and pituitary gland. Furthermore, levels of brain DHA were reported in different phospholipid fractions for which the following order of preference was used: PC, phosphatidylethanolamine, choline glycerophospholipid, phosphatidylserine, phosphatidylinositol, diacylphosphatidylcholine, choline phosphoglyceride.
2.2. Statistical analysis
All reported comparisons of plasma/serum and brain/CSF nutrient levels in AD patients and controls were integrated and summarized into a final result per nutrient, using meta-analysis (regression) methods [20], according to the PRISMA statement [16]. For each nutrient, we extracted from the articles the mean and standard deviation of plasma/serum or brain/CSF levels in AD patients and controls as well as the number of subjects and the average age per group. Comparison across publications did not require conversion to the same unit because our analysis was based on the difference between groups, that is, not on the absolute value. These data were analyzed using the random-effects meta-analysis model [20] fitted by restricted maximum likelihood using the program metareg of Stata (StataCorp. 2001. Statistical Software: Release 12.1; College Station, TX, USA). For this analysis, a minimum of four studies is generally required [21], [22]. No meta-analysis was performed when less than four publications were identified; however, a mention is made in Section 3 indicating the number of publications found. No mention is made for those nutrients for which no publications were identified for both circulatory and central levels.
Because it is known that the plasma/serum and brain/CSF levels of several nutrients can vary with age [23], meta-analyses were conducted with and without a correction by meta-regression for differences in mean age between AD patients and controls. The final result for a given nutrient was based on the results of the meta-analysis with this centralized age adjustment.
3. Results
The objectives of this study were to assess whether circulatory (see Section 3.1) and brain (see Section 3.2) nutrient levels differ between AD patients and cognitively intact elderly controls. The current investigation resulted in the identification of 116 publications (Fig. 1) that met all inclusion criteria, some of which described plasma and/or brain levels of multiple nutrients. Meta-analyses were performed with a correction by meta-regression for centralized age differences between AD patients and controls. Table 1 provides a summary of the included publications, listing for each nutrient the number of publications reporting significant lower or higher nutrient levels and the number of publications showing no difference in nutrient levels between AD patients and controls.
Table 1.
Summary of the included publications for the meta-analysis
| Compartment | Nutrient | Total number of publications | Total number of AD patients/average age (years) | Total number of control subjects/average age (years) | Studies reporting significantly lower levels in AD patients than in controls | Studies reporting no significant differences between AD patients and controls | Studies reporting significantly higher levels in AD patients than in controls |
|---|---|---|---|---|---|---|---|
| Circulation | DHA | 13 | 488/78 | 1245/72 | Six studies [24], [25], [26], [27], [28], [29] | Seven studies [30], [31], [32], [33], [34], [35], [36] | |
| EPA | 13 | 488/78 | 1245/72 | Six studies [24], [25], [26], [28], [32], [35] | Seven studies [27], [29], [30], [31], [33], [34], [36] | ||
| Choline (as PC) | 4 | 87/76 | 76/73 | Two studies [37], [38] | Two studies [24], [39] | ||
| Vitamin B6 | 6 | 192/76 | 199/75 | Two studies [40], [41] | Four studies [34], [42], [43], [44] | ||
| Selenium | 17 | 660/77 | 536/72 | Seven studies [34], [45], [46], [47], [48], [49], [50] | Eight studies [51], [52], [53], [54], [55], [56], [57], [58] | Two studies [59], [60] | |
| Brain | DHA | 12 | 237/77 | 220/75 | Four studies [61], [62], [63], [64] | Eight studies [65], [66], [67], [68], [69], [70], [71], [72] | |
| Choline (1H-MRS) | 31 | 828/73 | 791/70 | Four studies [73], [74], [75], [76] | Twenty-seven studies [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93], [94], [95], [96], [97], [98], [99], [100], [101], [102], [103] | ||
| Folate | 9 | 307/73 | 538/65 | Four studies [104], [105], [106], [107] | Five studies [108], [109], [110], [111], [112] | ||
| Vitamin B12 | 4 | 92/70 | 208/69 | Two studies [110], [113] | Two studies [114], [115] | ||
| Vitamin C | 5 | 102/74 | 79/70 | Four studies [41], [116], [117], [118] | One study [119] | ||
| Vitamin E | 5 | 127/73 | 100/70 | Three studies [116], [120], [121] | Two studies [117], [118] | ||
| Selenium | 13 | 487/76 | 353/76 | Three studies [122], [123], [124] | Ten studies [46], [58], [125], [126], [127], [128], [129], [127], [128], [129], [130], [131], [132] | ||
| Zinc | 16 | 496/73 | 306/71 | Five studies [122], [123], [133], [134], [135] | Ten studies [46], [125], [127], [128], [130], [132], [136], [137], [138], [139] | One study [126] | |
Abbreviations: AD, Alzheimer's disease; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid.
3.1. Circulatory nutrient meta-analysis
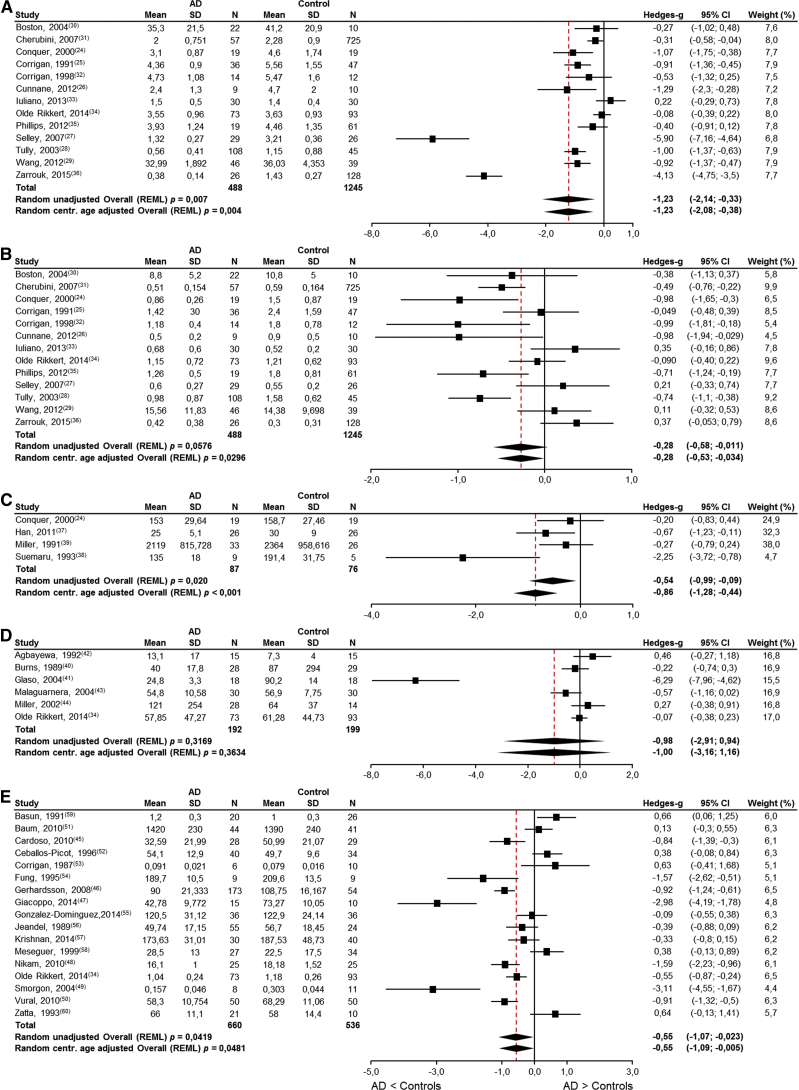
Thirty-seven publications reporting on circulatory nutrient levels in patients with AD and cognitively intact elderly controls were identified. Meta-analyses were possible for circulatory DHA (13 studies), EPA (13 studies), PC (4 studies), vitamin B6 (6 studies), and selenium (17 studies) (Fig. 2A–E and Table 1) because the literature search had yielded four or more publications for each of these nutrients. In addition, the systematic literature search yielded recent meta-analyses on circulatory iron [140], magnesium [141], and zinc [142]. Unfortunately, insufficient number of publications were retrieved reporting on circulatory manganese (two studies [51], [55]). Significantly lower levels of circulatory DHA (Fig. 2A, P < .004), EPA (Fig. 2B, P < .03), PC levels (Fig. 2C, P < .001), and selenium (Fig. 2E, P < .05) were found in AD patients compared with cognitively intact elderly controls. No significant differences were observed in circulatory vitamin B6 levels (Fig. 2D, P = .3634). For completeness, the results of meta-analyses without centralized age correction are also provided in the figures.
Fig. 2.
Results of meta-analyses for circulatory nutrient levels in AD patients and cognitively intact elderly controls. (A) DHA levels are significantly lower in AD patients (P < .05) and approximately 75% of the absolute amount in the control subjects; (B) EPA levels are significantly lower in AD patients (P < .05) and approximately 89% of the absolute amount in the control subjects; (C) choline (as PC) levels are significantly lower in AD patients (P < .05) and approximately 88% of the absolute amount in the control subjects; (D) vitamin B6 levels are not significantly different in AD patients (P = .3634); (E) Selenium levels are significantly lower in AD patients (P < .05) and approximately 93% of the absolute amount in the control subjects. Abbreviations: AD, Alzheimer's disease; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; LCL, lower confidence limit; PC, plasma phosphatidylcholine; REML, restricted maximum likelihood; SD, standard deviation; UCL, upper confidence limit.
3.2. Brain nutrient meta-analysis
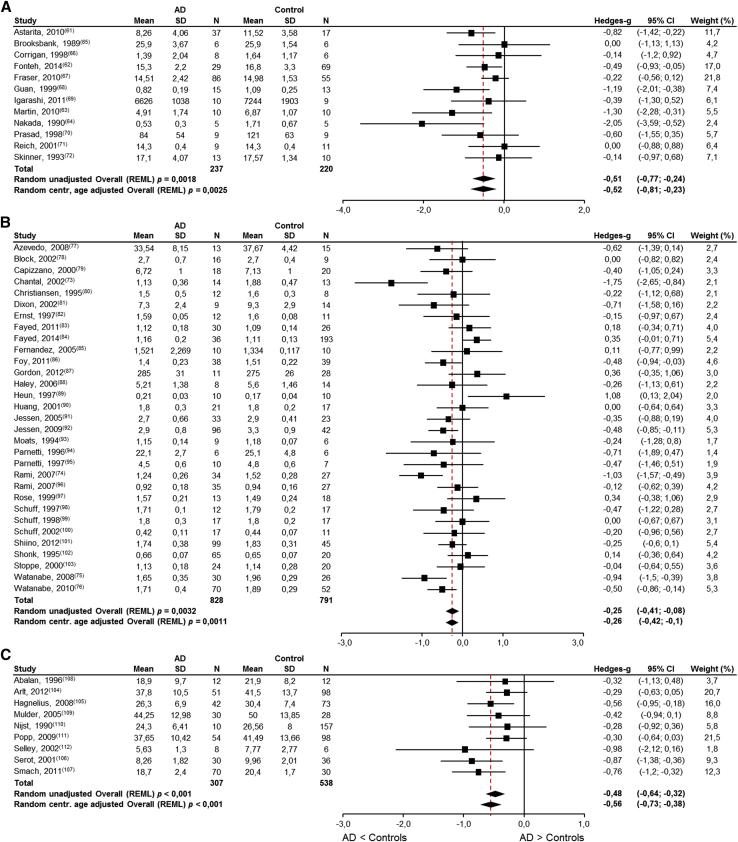
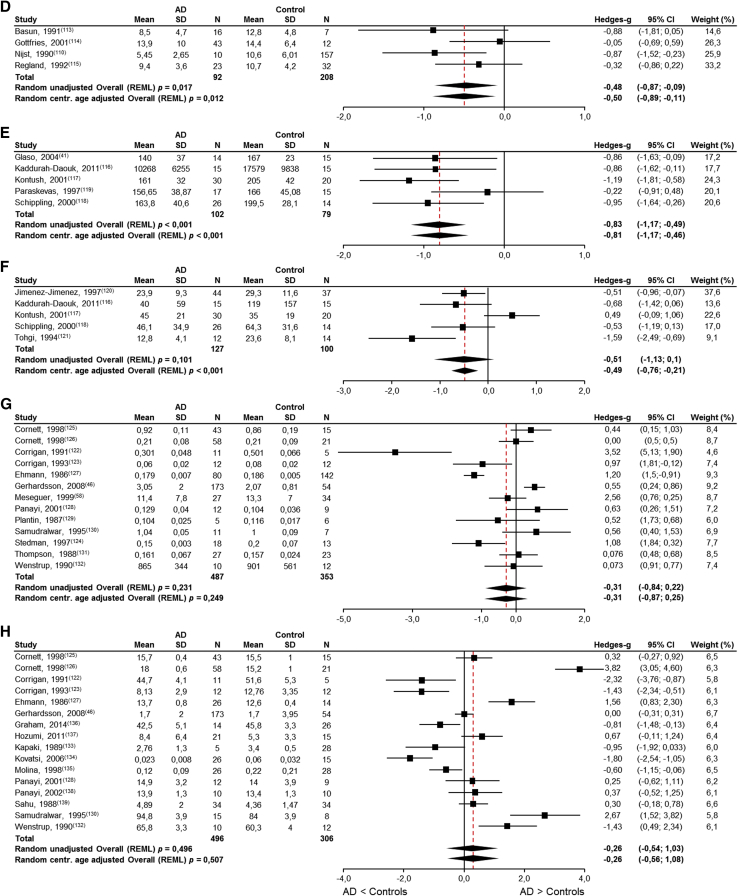
Eighty-two publications reporting on brain nutrient levels in patients with AD and cognitively intact elderly controls were identified. Meta-analyses were possible for brain DHA (12 studies), brain choline-containing lipids (1H-MRS, 31 studies), CSF folate (9 studies), CSF vitamin B12 (4 studies), CSF vitamin C (5 studies), CSF vitamin E (5 studies), brain selenium (13 studies), and brain zinc (16 studies) (Fig. 3A–H and Table 1). Unfortunately, the systematic literature search did not yield sufficient number of publications reporting on brain EPA (three studies [61], [66], [69]), vitamin A (zero studies), vitamin B1 (zero studies), vitamin B6 (zero studies), vitamin D (one study [144]), calcium (two studies [59], [134]), magnesium (two studies, see Veronese et al. [141]), and manganese (three studies [123], [135], [137]).
Fig. 3.
Meta-analyses results for brain nutrient levels in AD patients and cognitively intact elderly controls. (A) DHA levels are significantly lower in AD patients (P < .003) and approximately 85% of the absolute amount in the control subjects; (B) choline-containing lipids (1H-MRS) levels are significantly lower in AD patients (P < .05) and approximately 95% of the absolute amount in the control subjects; (C) folate levels are significantly lower in AD patients (P < .001) and approximately 89% of the absolute amount in the control subjects; (D) vitamin B12 levels are significantly lower in AD patients (P < .012) and approximately 78% of the absolute amount in the control subjects; (E) vitamin C levels between AD patients and cognitively intact elderly controls were significant (P < .01) and approximately 80% of the absolute amount in the control subjects; (F) vitamin E levels are significantly lower in AD patients (P < .001) and approximately 81% of the absolute amount in the control subjects; (G) selenium levels are not significant different in AD patients (P = .249); (H) zinc levels are not significantly different in AD patients (P = .507). Abbreviations: AD, Alzheimer's disease; DHA, docosahexaenoic acid; LCL, lower confidence limit; REML, restricted maximum likelihood; SD, standard deviation; UCL, upper confidence limit.
Significantly lower levels of brain DHA (Fig. 3A, P < .003), brain choline-containing lipids (Fig. 3B, P < .0011), CSF folate (Fig. 3C, P < .001), CSF vitamin B12 (Fig. 3D, P = .012), CSF vitamin C (Fig. 3E, P < .001), and CSF vitamin E (Fig. 3F, P < .001) were found in AD patients compared with cognitively intact elderly controls. No significant differences were observed for brain selenium (Fig. 3G, P = .249) and brain zinc (Fig. 3H, P = .507) levels. For completeness, the results of meta-analyses without centralized age correction are also provided in the figures.
3.3. Summary of the overall nutrient status in patients with AD
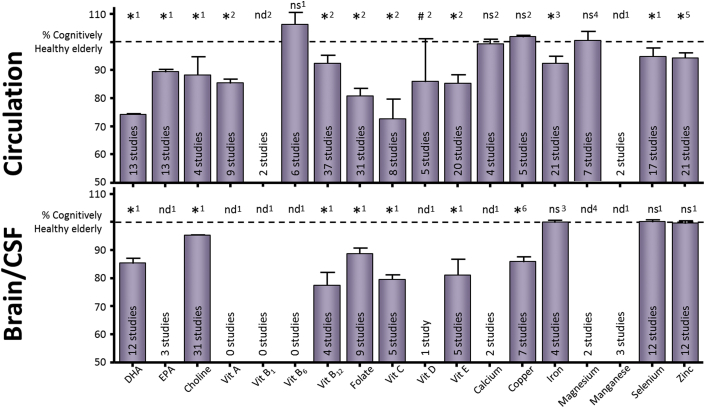
Figure 4 shows the collective results of this meta-analysis combined with previous work from our group, as well as three recent meta-analyses on mineral status in AD. The levels of the respective nutrients are expressed as a percentage of cognitively healthy elderly. Significantly lower blood levels of DHA, EPA, choline, and selenium (75%, 89%, 88%, and 93% of control levels, respectively) and significantly lower brain levels of DHA, choline, folate, vitamin B12, vitamin C, vitamin E, and copper (85%, 95%, 89%, 78%, 80%, 81%, and 86% of control levels, respectively) were found in patients with AD.
Fig. 4.
Brain and circulatory nutrient levels in AD versus cognitively intact elderly controls. Summary of the circulatory (top panel) and brain (lower panel) nutrient status of AD patients (including [1] current meta-analyses; [2] previous meta-analyses results from Lopes da Silva et al. [15]; [3] recent meta-analyses for circulatory and brain iron Tao et al. [140]; [4] recent meta-analysis for circulatory magnesium Veronese et al. [141]; [5] recent meta-analysis for circulatory zinc Ventriglia et al. [142]; [6] recent meta-analysis for brain copper Schrag et al. [143] status; respectively presented as numbers above the bars). Data are represented as the weighted average percentage of controls ± standard error of the mean. *Indicates statistical significance; # indicates trend for significance; ns indicates nonsignificance; nd indicates not determined with meta-analysis. Abbreviation: AD, Alzheimer's disease.
4. Discussion
To our knowledge, this is the first systematic comparison of brain nutrient levels in AD patients to those in cognitively intact elderly controls. Several studies suggest lower brain nutrient levels in AD, but conclusive data that their levels are different in AD patients compared with controls have thus far been lacking. Integrating the results of all published studies in the current meta-analysis provides an unequivocal answer to the research objective for brain DHA, choline, folate, vitamin B12, vitamin C, and vitamin E. In addition, meta-analyses showing lower levels of circulatory DHA, EPA, PC, and selenium complement prior work on lower circulatory vitamin status in AD patients [15]. The results as a whole disclose directionally aligned results between brain and circulatory nutrient status, that is, nutrients found to be lower in the circulation also show lower status in the brain of AD patients. The magnitude, 5%–25% lower concentrations in AD, of the differences was found to be comparable between circulation and brain nutrient status. The factors that could contribute to the lower circulatory (Section 4.1) and brain (Section 4.2) nutrient levels are discussed in the following section, along with the potential implications (Section 4.4) for people with AD.
4.1. Factors contributing to lower circulatory nutrient levels
Collectively, the current and previous meta-analyses show lower circulatory status for specific nutrients (respectively, DHA + EPA + PC + selenium and vitamins A, B12, C, E, folate). A logical origin for these lower levels may be lower intake, which could be explained from an epidemiological point of view. Epidemiological studies suggest that better adherence to healthy diets, for example, a Mediterranean diet, is associated with reduced risk of developing AD (see, for review, van de Rest et al. [145]) and high adherence to a Western dietary pattern is associated with elevated AD risk [146]. High adherence to the Mediterranean diet, compared with the Western dietary pattern, is associated with higher intakes of omega-3 PUFAs, B-vitamins and antioxidants, and specific nutrient biomarker patterns [147], [148]. In addition, lower circulatory nutrient levels might also be explained by lower food intake associated with malnutrition and weight loss (indicative for protein/energy malnutrition) which are common problems in AD, occurring especially at the moderate-to-severe stages of the disease [149], [150], [151]. Although eating difficulties are also associated with mild-to-moderate and early stages of AD [152], signs of protein/energy malnourishment are less prevalent, with 2.5%–3% of the mild-to-moderate patients being malnourished and ∼18% at risk of malnutrition [153] while, in newly diagnosed AD, prevalence of protein/energy malnutrition was 0% and 14.1% was at risk of malnutrition [154]. The lower nutrient levels in AD do not necessarily originate from protein/energy malnourishment as demonstrated in previous meta-analyses showing lower plasma vitamin levels in AD patients without signs of malnutrition [15]. Other factors associated with AD may also influence circulatory nutrient levels, such as nutrient liberation from food, nutrient uptake from the gut, efficiency with which nutrients are transported over physiological barriers, and the major controlling influence of the liver. A growing number of publications underscore the impact of AD on nutrient-related pathways.
First, apolipoprotein E (APOE) ε4 allele is one of the strongest genetic risk factor for AD. apoE plays a crucial role in the transport and homeostasis of lipids in the bloodstream, where it participates in the delivery and clearance of serum triglycerides, phospholipids, and cholesterol [155], [156] and as such it is involved in the transport of DHA in the circulation [157]. In addition, apoE has been indicated in transport and regulation of lipid-soluble vitamins, for example, vitamins A, E, and K [158], [159], [160]. A recent meta-analysis shows lower circulatory apoE levels in AD patients compared with healthy controls [161]. Lower apoE levels could compromise the availability of lipids and lipid-soluble vitamins and thereby contributing to the lower DHA and EPA levels observed in the current meta-analysis and the lower vitamin A and E in previous work from our group [15]. Unfortunately, most of the publications included in this meta-analysis lacked APOE-status data thereby preventing the possibility to perform meta-analyses to study the impact of APOE status on nutrient-level differences.
Second, several gene polymorphisms influencing the risk of AD are found for genes-encoding enzymes involved in the metabolism and transport of nutrients. Polymorphisms in the genes encoding for MTHFR (methylenetetrahydrofolate reductase) [162] and MTHFD1L (methylenetetrahydrofolate dehydrogenase [NADP+ dependent] 1–like protein) [163] negatively influence key enzymes involved in the metabolism of homocysteine to form methionine. Similarly, polymorphisms in the gene encoding for an important carrier of vitamin B12 in the circulation (transcobalamin II) are suggested to have a protective role against AD and are found to be occurring less often in patients with AD [164]. These polymorphisms provide a genetic basis for the long observed elevated homocysteine levels in AD indicative for compromised one-carbon metabolism and suggestive for higher requirements for vitamin B12 and folate.
Third, studies using metabolomics approaches indicate that abnormalities in metabolic pathways accumulate with disease progression. The affected pathways range from fatty acid metabolism, one-carbon metabolism, energy metabolism, Krebs cycle, mitochondrial function, nucleic acid metabolism, but also neurotransmitter and amino acid metabolism, and lipid biosynthesis [165], [166]. In line with these findings, patients with AD also show altered phospholipid metabolism [33], [167], [168]. As most of these metabolic pathways use nutrients, the aforementioned findings indirectly confirm the lower nutrient status observed in the current and former meta-analyses of circulatory nutrient levels.
Finally, reduced functionality of specific liver-residing enzymes involved in nutrient conversion, for example, compromised formation of DHA from α-linolenic acid [61] or the reduced capacity to form PC from phosphatidylethanolamine [27]. As phosphatidylethanolamine is rich in DHA, a compromised conversion to PC reduces the total DHA content in blood phospholipids. Together with the reduced capacity to synthesize DHA from α-linolenic acid, these data provide a mechanistic basis that could contribute to the lower DHA levels in patients with AD as observed in this meta-analysis.
The aforementioned findings suggest that differences in circulatory nutrient levels in AD are not only dependent on dietary intake but may be affected by several AD-associated differences in genetic, metabolic, and transport mechanisms related to nutrient handling/metabolism. As the brain has very limited capacity for de novo nutrient synthesis, it depends on the circulation to maintain an adequate nutrient availability [9], [169]. Taken together, these AD-associated differences in the periphery may impose the brains of patients with AD with an inadequate nutrient supply.
4.2. Factors contributing to lower brain nutrient levels
In the brain, multiple, separate carrier systems at the blood-brain barrier and at the blood-CSF barrier mediate the transport of fatty acids, vitamins, minerals, and nucleosides from the circulation into the CSF and extracellular space of the brain [170], [171], [172], [173], [174], [175]. Indeed, the current meta-analysis results suggest that brain nutrient status is reflecting the status in the periphery. Yet, comparable to the periphery where dietary intake is not the only factor determining nutrient levels, in the brain, other factors than uptake from the circulation also can influence nutrient levels. In fact, several of the peripheral AD-associated differences in genetic, metabolic, and transport mechanisms related to nutrient handling/metabolism are also observed in the brain. First, apoE levels are found to be reduced in CSF of patients with AD [176], thereby probably impacting lipid and lipid-soluble vitamin availability to neurons [155]. Second, a polymorphism within the gene-encoding transcobalamin II is associated with lower CSF transcobalamin concentrations in patients with AD, which could explain the lower vitamin B12 status resulting from this meta-analysis. Third, metabolomics studies in CSF of AD patients show alterations in metabolic pathways [165], [177] partly overlapping with the altered pathways observed in the periphery, including altered phospholipid metabolism which has been observed from the mid 1980s [178]. Finally, although the brain cannot synthesize DHA, a recent publication observed that lower CSF Aβ42 levels were associated with lower transport of DHA to CSF, suggesting that the presence of amyloid pathology may limit the delivery of DHA to the brain in AD [179].
In addition, brains of people with AD are vulnerable to loss of membrane PC as a result of free choline generation for the synthesis of acetylcholine [180]. This process of choline liberation exclusively operates at the synapse [181] and when excessive destruction of membrane PC might lead to changes in membrane composition and loss in total membrane surface [180], [182] and are in line with the aforementioned alterations in brain phospholipid metabolism. Prolonged duration of these alterations is likely to impact neuronal function, first at the synaptic level and later spreading to the neuronal cell body [180]. Indeed, AD is associated with loss of synaptic function and lower synapse numbers [183], [184], and these changes are the major correlates of cognitive impairment observed in AD [185].
4.3. Strengths and limitations
The strength and uniqueness of the current meta-analysis approach is that it allows demonstration of lower levels of a variety of nutrients both in the brain and in the circulation of patients with AD compared with cognitively intact elderly. Further strengths are the inclusion of case-control studies only and the correction for age differences as a possible confounding factor. Several limitations that could have influenced the overall result of the present meta-analysis warrant mention. First, habitual dietary intake information of the AD and control groups is not provided in all publications. However, our previous meta-analysis showed that differences in plasma nutrient levels were consistent albeit analyzing only studies with no difference in measures of protein/energy malnourishment between AD patients and controls [15]. In all cases, we excluded publications that involved subjects using vitamin supplements. Between-study heterogeneity was also observed which can be attributed to variation in sample size, gender, and the ethnic backgrounds of the participants. Another source of heterogeneity would be the stage of disease. However, most of the publications included for the current meta-analyses studied AD populations in the early and mild-to-moderate phase of AD limiting the impact of malnutrition induced by eating difficulties [152], [153], [154]. Third, there is limited number of eligible studies for circulatory vitamin B6 and choline and for brain vitamins B12, C, and E levels which warrant caution to interpretation of the meta-analyses results. Fourth, although the current meta-analyses offer insight in the status for a number of nutrients, there is still insufficient information available for a range of vitamins such as vitamin D, B-vitamins, and for minerals. This is more apparent for nutrient status in the brain compared with the circulation. Fifth, very few studies simultaneously measured circulatory and central nutrient status thereby limiting the possibility to detect parallels or differences between the two compartments. Such studies would advance the understanding of nutritional needs of the brain in AD. Finally, most of the included studies do not offer the possibility to study the temporal relationship between AD-pathophysiology and nutrient status, to determine when in the disease spectrum the lower levels begin to manifest.
4.4. Interpretation
Collectively, this body of evidence suggests that AD is associated with specific compromised nutrient availability. Although dietary intake is an important factor influencing nutrient levels, AD is also associated with several differences in genetic, metabolic, and transport mechanisms related to nutrient handling/metabolism that could contribute to the lower nutrient status. The consequences of these differences are unknown but possibly have a negative influence on disease progression. The importance of adequate nutrient levels is illustrated briefly by highlighting a number of mechanisms associated with the development of AD neuropathology and involving nutrients.
First, adequate nutrient levels as a necessity for the synthesis of phospholipids that make up synaptic membranes (reviewed in van Wijk et al. [11]) are emphasized by the notion that disrupted phospholipid homeostasis in AD is recognized and confirmed in many studies since the 80s both in the brain and in the circulation [33], [64], [69], [70], [168], [178], [180], [186], [187], [188], [189], [190], [191], [192]. Disruption of phospholipid homeostasis and the loss of synaptic function and synapse numbers [184], [185], [193] appear to go hand-in-hand [194], [195] as co-occurring hallmarks of AD and disease progression. The declining synapse numbers in AD suggest that patients may have a higher need for forming new synapses which may increase the need for adequate phospholipid synthesis.
Second, nutrients also serve crucial roles in epigenetic mechanisms via DNA methylation, histone, and microRNAs modifications (reviewed in Athanasopoulos et al. [196]). Especially, the B-vitamins (B12, folate, and B6) are used as cofactors in the methionine cycle (or one-carbon cycle metabolism) and are critical for the regeneration of S-adenosylmethionine from homocysteine. Lower generation of S-adenosylmethionine, which is a methyl donor, could lead to lower DNA methylation capacity, resulting in overexpression of genes involved in AD pathology [197]. Other nutrients, such as vitamins A, C, and E, through their antioxidant properties can affect the proteins involved in the transmethylation reactions necessary for the methylation/demethylation of DNA [196]. In addition, lower antioxidant capacity may also lead to modifications of histone deacetylases thereby limiting deacetylase activity [198].
Third, multiple lines of evidence provide strong support for the involvement of oxidative stress in the development of AD [199]. Mitochondrial dysfunction and increased production of reactive oxygen species are believed to be a contributing factor in the progression of AD. Especially, vitamins C and E are known for their antioxidant properties, both found to be lower in the circulation and the brain in the current meta-analyses. However, also metal ions such as selenium, copper, and zinc have their links to antioxidant capacity through their involvement as cofactor in the endogenous antioxidant enzymes glutathione peroxidase, superoxide dismutase, and catalase [50].
Finally, the anti-inflammatory properties of omega-3 long-chain PUFAs have been recognized for long, as evidenced in many studies. Lipid mediator derivates from omega-3 long-chain PUFAs, such as resolvins, protectins, and maresins, have revealed their potential of being a good alternative in the therapy of several pathophysiological models associated with chronic inflammation (see, for review, Lorente-Cebrian et al. [200]). For example, the neuroprotective and regenerative properties of DHA-derived neuroprotectin D1 bioactivity potently downregulate inflammatory signaling, amyloidogenic APP cleavage and apoptosis [201], and inflammation in peripheral blood mononuclear cells from AD patients [202].
The combined data of this meta-analysis and prior work [15] show that many of the nutrients that play functional roles in phospholipid synthesis, oxidative stress, inflammation, and epigenetic mechanisms are lower in the circulation and the brain of AD patients. This suggests that patients with AD might have a functional deficiency for these nutrients limiting them to counteract the effects of phospholipid loss, elevated oxidative stress, and inflammation and epigenetic modifications.
4.5. Implications
The previously outlined differences in nutrient status and the metabolic alterations observed in various other reports suggest potential for nutritional intervention in slowing disease progression. Indeed, over the years, several randomized double-blind clinical studies have been performed in patients with AD supplementing omega-3 fatty acids, B-vitamins, or antioxidants. However, recent systematic reviews/meta-analyses show no clear benefits of omega-3 fatty acids (seven eligible studies) [203], vitamin E [204], folic acid alone, or multivitamin B supplements (three eligible studies) [205] in the treatment of AD. In light of the findings of these meta-analyses and the metabolic alterations observed in other studies, it may not be so surprising that interventions with single nutrients are resulting in little beneficial effects. Moreover, it suggests that nutritional intervention in AD should comprise intervention with multiple nutritional components at once to be effective [206].
4.6. Conclusion
The current investigation suggests that patients with AD have impaired availability of DHA, choline, vitamin B12, folate, vitamin C, and vitamin E in the brain. These lower levels are in line with the lower circulatory levels observed in this and previous studies [15]. The brain is dependent on nutrient supply from the circulation, which in combination with nutrient involvement in AD-pathophysiological mechanisms suggests that patients with AD may have specific nutritional requirements. This hypothesis could be tested using a multicomponent nutritional intervention as suggested previously. In addition, to further improve our understanding when in the disease spectrum the lower nutrient levels begin to manifest, the field would benefit from studies investigating the temporal relationship between AD-pathophysiology and nutrient status.
Research in Context.
-
1.
Systematic review: Literature was reviewed to evaluate differences in brain and circulatory micronutrient and fatty acid availability between Alzheimer's disease (AD) patients and cognitively intact elderly controls. Except for previous work on circulatory vitamin status, to date, no meta-analysis is available that is inclusive for many publications reporting results for brain and/or circulatory nutrient status in AD.
-
2.
Interpretation: Significant effects across a range of nutrients provide evidence that brain and circulatory nutrient levels are lower in AD. Provided the postulated roles for these nutrients in AD-pathological processes (e.g., synapse loss and disrupted phospholipid homeostasis), the concept is emerging that these nutrients become conditionally essential for AD patients.
-
3.
Future directions: Additional research is needed investigating AD pathophysiology in relation to lower nutrient levels, including furthering understanding of AD-specific changes in eating behavior and nutrient metabolism, to determine when in the disease spectrum the lower levels begin to manifest.
Footnotes
M.C.d.W., E.G., A.C.Y., and J.W.S. are employees of Nutricia Advanced Medical Nutrition, Nutricia Research.
References
- 1.Castro-Quezada I., Roman-Vinas B., Serra-Majem L. The Mediterranean diet and nutritional adequacy: a review. Nutrients. 2014;6:231–248. doi: 10.3390/nu6010231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu N., Yu J.T., Tan L., Wang Y.L., Sun L., Tan L. Nutrition and the risk of Alzheimer's disease. Biomed Res Int. 2013;2013:524820. doi: 10.1155/2013/524820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosendorff C., Beeri M.S., Silverman J.M. Cardiovascular risk factors for Alzheimer's disease. Am J Geriatr Cardiol. 2007;16:143–149. doi: 10.1111/j.1076-7460.2007.06696.x. [DOI] [PubMed] [Google Scholar]
- 4.Martins I.J., Hone E., Foster J.K., Sunram-Lea S.I., Gnjec A., Fuller S.J. Apolipoprotein E, cholesterol metabolism, diabetes, and the convergence of risk factors for Alzheimer's disease and cardiovascular disease. Mol Psychiatry. 2006;11:721–736. doi: 10.1038/sj.mp.4001854. [DOI] [PubMed] [Google Scholar]
- 5.Schrijvers E.M., Witteman J.C., Sijbrands E.J., Hofman A., Koudstaal P.J., Breteler M.M. Insulin metabolism and the risk of Alzheimer disease: the Rotterdam Study. Neurology. 2010;75:1982–1987. doi: 10.1212/WNL.0b013e3181ffe4f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lanska D.J. Chapter 30: historical aspects of the major neurological vitamin deficiency disorders: the water-soluble B vitamins. Handb Clin Neurol. 2010;95:445–476. doi: 10.1016/S0072-9752(08)02130-1. [DOI] [PubMed] [Google Scholar]
- 7.Reynolds E. Vitamin B12, folic acid, and the nervous system. Lancet Neurol. 2006;5:949–960. doi: 10.1016/S1474-4422(06)70598-1. [DOI] [PubMed] [Google Scholar]
- 8.Hopkins F.G. Feeding experiments illustrating the importance of accessory factors in normal dietaries. J Physiol. 1912;44:425–460. doi: 10.1113/jphysiol.1912.sp001524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bourre J.M. Effects of nutrients (in food) on the structure and function of the nervous system: update on dietary requirements for brain. Part 1: micronutrients. J Nutr Health Aging. 2006;10:377–385. [PubMed] [Google Scholar]
- 10.Smith P.J., Blumenthal J.A. Diet and neurocognition: review of evidence and methodological considerations. Curr Aging Sci. 2010;3:57–66. doi: 10.2174/1874609811003010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Wijk N., Broersen L.M., de Wilde M.C., Hageman R.J., Groenendijk M., Sijben J.W. Targeting synaptic dysfunction in Alzheimer's disease by administering a specific nutrient combination. J Alzheimers Dis. 2014;38:459–479. doi: 10.3233/JAD-130998. [DOI] [PubMed] [Google Scholar]
- 12.Kosicek M., Hecimovic S. Phospholipids and Alzheimer's disease: alterations, mechanisms and potential biomarkers. Int J Mol Sci. 2013;14:1310–1322. doi: 10.3390/ijms14011310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Waal H., Stam C.J., Lansbergen M.M., Wieggers R.L., Kamphuis P.J., Scheltens P. The effect of Souvenaid on functional brain network organisation in patients with mild Alzheimer's disease: a randomised controlled study. PLoS One. 2014;9:e86558. doi: 10.1371/journal.pone.0086558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scheltens P., Twisk J.W., Blesa R., Scarpini E., von Arnim C.A., Bongers A. Efficacy of Souvenaid in mild Alzheimer's disease: results from a randomized, controlled trial. J Alzheimers Dis. 2012;31:225–236. doi: 10.3233/JAD-2012-121189. [DOI] [PubMed] [Google Scholar]
- 15.Lopes da Silva S., Vellas B., Elemans S., Luchsinger J., Kamphuis P., Yaffe K. Plasma nutrient status of patients with Alzheimer's disease: systematic review and meta-analysis. Alzheimers Dement. 2014;10:485–502. doi: 10.1016/j.jalz.2013.05.1771. [DOI] [PubMed] [Google Scholar]
- 16.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E.M. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 18.American Psychiatric Association . 3rd ed, rev. American Psychiatric Press; Washington, DC: 1987. Diagnostic and statistical manual of mental disorders. [Google Scholar]
- 19.Whiley L., Sen A., Heaton J., Proitsi P., Garcia-Gomez D., Leung R. Evidence of altered phosphatidylcholine metabolism in Alzheimer's disease. Neurobiol Aging. 2014;35:271–278. doi: 10.1016/j.neurobiolaging.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Houwelingen H.C., Arends L.R., Stijnen T. Advanced methods in meta-analysis: multivariate approach and meta-regression. Stat Med. 2002;21:589–624. doi: 10.1002/sim.1040. [DOI] [PubMed] [Google Scholar]
- 21.Borenstein M., Hedges L.V., Higgins J.P.T., Rothstein H.R. Wiley; Hoboken, NJ: 2009. Introduction to Meta-analysis. [Google Scholar]
- 22.The-Cochrane-Collaboration . Wiley-Blackwell; Hoboken, NJ: 2008. Cochrane Handbook for Systematic Reviews of Interventions. [Google Scholar]
- 23.US Centers For Disease Control And Prevention . National Center for Environmental Health; Atlanta, GA: 2008. National Report on Biochemical Indicators of Diet and Nutrition in the U.S. Population 1999-2002. [Google Scholar]
- 24.Conquer J.A., Tierney M.C., Zecevic J., Bettger W.J., Fisher R.H. Fatty acid analysis of blood plasma of patients with Alzheimer's disease, other types of dementia, and cognitive impairment. Lipids. 2000;35:1305–1312. doi: 10.1007/s11745-000-0646-3. [DOI] [PubMed] [Google Scholar]
- 25.Corrigan F.M., Van Rhijn A.G., Ijomah G., McIntyre F., Skinner E.R., Horrobin D.F. Tin and fatty acids in dementia. Prostaglandins Leukot Essent Fatty Acids. 1991;43:229–238. doi: 10.1016/0952-3278(91)90035-4. [DOI] [PubMed] [Google Scholar]
- 26.Cunnane S.C., Schneider J.A., Tangney C., Tremblay-Mercier J., Fortier M., Bennett D.A. Plasma and brain fatty acid profiles in mild cognitive impairment and Alzheimer's disease. J Alzheimers Dis. 2012;29:691–697. doi: 10.3233/JAD-2012-110629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Selley M.L. A metabolic link between S-adenosylhomocysteine and polyunsaturated fatty acid metabolism in Alzheimer's disease. Neurobiol Aging. 2007;28:1834–1839. doi: 10.1016/j.neurobiolaging.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Tully A.M., Roche H.M., Doyle R., Fallon C., Bruce I., Lawlor B. Low serum cholesteryl ester-docosahexaenoic acid levels in Alzheimer's disease: a case-control study. Br J Nutr. 2003;89:483–489. doi: 10.1079/BJN2002804. [DOI] [PubMed] [Google Scholar]
- 29.Wang D.C., Sun C.H., Liu L.Y., Sun X.H., Jin X.W., Song W.L. Serum fatty acid profiles using GC-MS and multivariate statistical analysis: potential biomarkers of Alzheimer's disease. Neurobiol Aging. 2012;33:1057–1066. doi: 10.1016/j.neurobiolaging.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 30.Boston P.F., Bennett A., Horrobin D.F., Bennett C.N. Ethyl-EPA in Alzheimer's disease–a pilot study. Prostaglandins Leukot Essent Fatty Acids. 2004;71:341–346. doi: 10.1016/j.plefa.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Cherubini A., Andres-Lacueva C., Martin A., Lauretani F., Iorio A.D., Bartali B. Low plasma N-3 fatty acids and dementia in older persons: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2007;62:1120–1126. doi: 10.1093/gerona/62.10.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corrigan F.M., Mowat B., Skinner E.R., Van Rhijn A.G., Cousland G. High density lipoprotein fatty acids in dementia. Prostaglandins Leukot Essent Fatty Acids. 1998;58:125–127. doi: 10.1016/s0952-3278(98)90151-x. [DOI] [PubMed] [Google Scholar]
- 33.Iuliano L., Pacelli A., Ciacciarelli M., Zerbinati C., Fagioli S., Piras F. Plasma fatty acid lipidomics in amnestic mild cognitive impairment and Alzheimer's disease. J Alzheimers Dis. 2013;36:545–553. doi: 10.3233/JAD-122224. [DOI] [PubMed] [Google Scholar]
- 34.Olde Rikkert M.G., Verhey F.R., Sijben J.W., Bouwman F.H., Dautzenberg P.L., Lansink M. Differences in nutritional status between very mild Alzheimer's disease patients and healthy controls. J Alzheimers Dis. 2014;41:261–271. doi: 10.3233/JAD-131892. [DOI] [PubMed] [Google Scholar]
- 35.Phillips M.A., Childs C.E., Calder P.C., Rogers P.J. Lower omega-3 fatty acid intake and status are associated with poorer cognitive function in older age: a comparison of individuals with and without cognitive impairment and Alzheimer's disease. Nutr Neurosci. 2012;15:271–277. doi: 10.1179/1476830512Y.0000000026. [DOI] [PubMed] [Google Scholar]
- 36.Zarrouk A., Riedinger J.M., Ahmed S.H., Hammami S., Chaabane W., Debbabi M. Fatty acid profiles in demented patients: identification of hexacosanoic acid (C26:0) as a blood lipid biomarker of dementia. J Alzheimers Dis. 2015;44:1349–1359. doi: 10.3233/JAD-142046. [DOI] [PubMed] [Google Scholar]
- 37.Han X., Rozen S., Boyle S.H., Hellegers C., Cheng H., Burke J.R. Metabolomics in early Alzheimer's disease: identification of altered plasma sphingolipidome using shotgun lipidomics. PLoS One. 2011;6:e21643. doi: 10.1371/journal.pone.0021643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suemaru S., Suemaru K., Hashimoto K., Ogasa T., Hirasawa R., Makino S. Cerebrospinal fluid corticotropin-releasing hormone and ACTH, and peripherally circulating choline-containing phospholipid in senile dementia. Life Sci. 1993;53:697–706. doi: 10.1016/0024-3205(93)90246-y. [DOI] [PubMed] [Google Scholar]
- 39.Miller B.L., Read S., Tang C., Jenden D. Differences in red blood cell choline and lipid-bound choline between patients with Alzheimer disease and control subjects. Neurobiol Aging. 1991;12:61–64. doi: 10.1016/0197-4580(91)90040-q. [DOI] [PubMed] [Google Scholar]
- 40.Burns A., Marsh A., Bender D.A. Dietary intake and clinical, anthropometric and biochemical indices of malnutrition in elderly demented patients and non-demented subjects. Psychol Med. 1989;19:383–391. doi: 10.1017/s0033291700012423. [DOI] [PubMed] [Google Scholar]
- 41.Glaso M., Nordbo G., Diep L., Bohmer T. Reduced concentrations of several vitamins in normal weight patients with late-onset dementia of the Alzheimer type without vascular disease. J Nutr Health Aging. 2004;8:407–413. [PubMed] [Google Scholar]
- 42.Agbayewa M.O., Bruce V.M., Siemens V. Pyridoxine, ascorbic acid and thiamine in Alzheimer and comparison subjects. Can J Psychiatry. 1992;37:661–662. doi: 10.1177/070674379203700912. [DOI] [PubMed] [Google Scholar]
- 43.Malaguarnera M., Bella R., Alagona G., Ferri R., Carnemolla A., Pennisi G. Helicobacter pylori and Alzheimer's disease: a possible link. Eur J Intern Med. 2004;15:381–386. doi: 10.1016/j.ejim.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 44.Miller J.W., Green R., Mungas D.M., Reed B.R., Jagust W.J. Homocysteine, vitamin B6, and vascular disease in AD patients. Neurology. 2002;58:1471–1475. doi: 10.1212/wnl.58.10.1471. [DOI] [PubMed] [Google Scholar]
- 45.Cardoso B.R., Ong T.P., Jacob-Filho W., Jaluul O., Freitas M.I., Cozzolino S.M. Nutritional status of selenium in Alzheimer's disease patients. Br J Nutr. 2010;103:803–806. doi: 10.1017/S0007114509992832. [DOI] [PubMed] [Google Scholar]
- 46.Gerhardsson L., Lundh T., Minthon L., Londos E. Metal concentrations in plasma and cerebrospinal fluid in patients with Alzheimer's disease. Dement Geriatr Cogn Disord. 2008;25:508–515. doi: 10.1159/000129365. [DOI] [PubMed] [Google Scholar]
- 47.Giacoppo S., Galuppo M., Calabro R.S., D'Aleo G., Marra A., Sessa E. Heavy metals and neurodegenerative diseases: an observational study. Biol Trace Elem Res. 2014;161:151–160. doi: 10.1007/s12011-014-0094-5. [DOI] [PubMed] [Google Scholar]
- 48.Nikam S.V., Nikam P.S., Ahaley S.K. Lipid peroxidation and antioxidants in Alzheimer's disease. Int J Pharma Bio Sci. 2010;1:1–4. [Google Scholar]
- 49.Smorgon C., Mari E., Atti A.R., Dalla Nora E., Zamboni P.F., Calzoni F. Trace elements and cognitive impairment: an elderly cohort study. Arch Gerontol Geriatr Suppl. 2004:393–402. doi: 10.1016/j.archger.2004.04.050. [DOI] [PubMed] [Google Scholar]
- 50.Vural H., Demirin H., Kara Y., Eren I., Delibas N. Alterations of plasma magnesium, copper, zinc, iron and selenium concentrations and some related erythrocyte antioxidant enzyme activities in patients with Alzheimer's disease. J Trace Elem Med Biol. 2010;24:169–173. doi: 10.1016/j.jtemb.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 51.Baum L., Chan I.H., Cheung S.K., Goggins W.B., Mok V., Lam L. Serum zinc is decreased in Alzheimer's disease and serum arsenic correlates positively with cognitive ability. Biometals. 2010;23:173–179. doi: 10.1007/s10534-009-9277-5. [DOI] [PubMed] [Google Scholar]
- 52.Ceballos-Picot I., Merad-Boudia M., Nicole A., Thevenin M., Hellier G., Legrain S. Peripheral antioxidant enzyme activities and selenium in elderly subjects and in dementia of Alzheimer's type–place of the extracellular glutathione peroxidase. Free Radic Biol Med. 1996;20:579–587. doi: 10.1016/0891-5849(95)02058-6. [DOI] [PubMed] [Google Scholar]
- 53.Corrigan F.M., Finlayson J.D., Stevenson G. Aluminum, zinc and other elements in serum in senile dementia of Alzheimer's type. Trace Elem Med. 1987;4:117–119. [Google Scholar]
- 54.Fung Y.K., Meade A.G., Rack E.P., Blotcky A.J., Claassen J.P., Beatty M.W. Determination of blood mercury concentrations in Alzheimer's patients. J Toxicol Clin Toxicol. 1995;33:243–247. doi: 10.3109/15563659509017991. [DOI] [PubMed] [Google Scholar]
- 55.Gonzalez-Dominguez R., Garcia-Barrera T., Gomez-Ariza J.L. Homeostasis of metals in the progression of Alzheimer's disease. Biometals. 2014;27:539–549. doi: 10.1007/s10534-014-9728-5. [DOI] [PubMed] [Google Scholar]
- 56.Jeandel C., Nicolas M.B., Dubois F., Nabet-Belleville F., Penin F., Cuny G. Lipid peroxidation and free radical scavengers in Alzheimer's disease. Gerontology. 1989;35:275–282. doi: 10.1159/000213037. [DOI] [PubMed] [Google Scholar]
- 57.Krishnan S., Rani P. Evaluation of selenium, redox status and their association with plasma amyloid/tau in Alzheimer's disease. Biol Trace Elem Res. 2014;158:158–165. doi: 10.1007/s12011-014-9930-x. [DOI] [PubMed] [Google Scholar]
- 58.Meseguer I., Molina J.A., Jimenez-Jimenez F.J., Aguilar M.V., Mateos-Vega C.J., Gonzalez-Munoz M.J. Cerebrospinal fluid levels of selenium in patients with Alzheimer's disease. J Neural Transm. 1999;106:309–315. doi: 10.1007/s007020050160. [DOI] [PubMed] [Google Scholar]
- 59.Basun H., Forssell L.G., Wetterberg L., Winblad B. Metals and trace elements in plasma and cerebrospinal fluid in normal aging and Alzheimer's disease. J Neural Transm Park Dis Dement Sect. 1991;3:231–258. [PubMed] [Google Scholar]
- 60.Zatta P., Cervellin D., Mattiello G., Gerotto M., Lazzari F., Gasparoni G. Plasma multielemental analysis in Alzheimer's disease and multi-infarctual dementia. Trace Elem Med. 1993;10:85–89. [Google Scholar]
- 61.Astarita G., Jung K.M., Berchtold N.C., Nguyen V.Q., Gillen D.L., Head E. Deficient liver biosynthesis of docosahexaenoic acid correlates with cognitive impairment in Alzheimer's disease. PLoS One. 2010;5:e12538. doi: 10.1371/journal.pone.0012538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fonteh A.N., Cipolla M., Chiang J., Arakaki X., Harrington M.G. Human cerebrospinal fluid fatty acid levels differ between supernatant fluid and brain-derived nanoparticle fractions, and are altered in Alzheimer's disease. PLoS One. 2014;9:e100519. doi: 10.1371/journal.pone.0100519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martin V., Fabelo N., Santpere G., Puig B., Marin R., Ferrer I. Lipid alterations in lipid rafts from Alzheimer's disease human brain cortex. J Alzheimers Dis. 2010;19:489–502. doi: 10.3233/JAD-2010-1242. [DOI] [PubMed] [Google Scholar]
- 64.Nakada T., Kwee I.L., Ellis W.G. Membrane fatty acid composition shows delta-6-desaturase abnormalities in Alzheimer's disease. Neuroreport. 1990;1:153–155. doi: 10.1097/00001756-199010000-00018. [DOI] [PubMed] [Google Scholar]
- 65.Brooksbank B.W., Martinez M. Lipid abnormalities in the brain in adult Down's syndrome and Alzheimer's disease. Mol Chem Neuropathol. 1989;11:157–185. doi: 10.1007/BF03160049. [DOI] [PubMed] [Google Scholar]
- 66.Corrigan F.M., Horrobin D.F., Skinner E.R., Besson J.A., Cooper M.B. Abnormal content of n-6 and n-3 long-chain unsaturated fatty acids in the phosphoglycerides and cholesterol esters of parahippocampal cortex from Alzheimer's disease patients and its relationship to acetyl CoA content. Int J Biochem Cell Biol. 1998;30:197–207. doi: 10.1016/s1357-2725(97)00125-8. [DOI] [PubMed] [Google Scholar]
- 67.Fraser T., Tayler H., Love S. Fatty acid composition of frontal, temporal and parietal neocortex in the normal human brain and in Alzheimer's disease. Neurochem Res. 2010;35:503–513. doi: 10.1007/s11064-009-0087-5. [DOI] [PubMed] [Google Scholar]
- 68.Guan Z., Wang Y., Cairns N.J., Lantos P.L., Dallner G., Sindelar P.J. Decrease and structural modifications of phosphatidylethanolamine plasmalogen in the brain with Alzheimer disease. J Neuropathol Exp Neurol. 1999;58:740–747. doi: 10.1097/00005072-199907000-00008. [DOI] [PubMed] [Google Scholar]
- 69.Igarashi M., Ma K., Gao F., Kim H.W., Rapoport S.I., Rao J.S. Disturbed choline plasmalogen and phospholipid fatty acid concentrations in Alzheimer's disease prefrontal cortex. J Alzheimers Dis. 2011;24:507–517. doi: 10.3233/JAD-2011-101608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Prasad M.R., Lovell M.A., Yatin M., Dhillon H., Markesbery W.R. Regional membrane phospholipid alterations in Alzheimer's disease. Neurochem Res. 1998;23:81–88. doi: 10.1023/a:1022457605436. [DOI] [PubMed] [Google Scholar]
- 71.Reich E.E., Markesbery W.R., Roberts L.J., 2nd, Swift L.L., Morrow J.D., Montine T.J. Brain regional quantification of F-ring and D-/E-ring isoprostanes and neuroprostanes in Alzheimer's disease. Am J Pathol. 2001;158:293–297. doi: 10.1016/S0002-9440(10)63968-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Skinner E.R., Watt C., Besson J.A., Best P.V. Differences in the fatty acid composition of the grey and white matter of different regions of the brains of patients with Alzheimer's disease and control subjects. Brain. 1993;116:717–725. doi: 10.1093/brain/116.3.717. [DOI] [PubMed] [Google Scholar]
- 73.Chantal S., Labelle M., Bouchard R.W., Braun C.M., Boulanger Y. Correlation of regional proton magnetic resonance spectroscopic metabolic changes with cognitive deficits in mild Alzheimer disease. Arch Neurol. 2002;59:955–962. doi: 10.1001/archneur.59.6.955. [DOI] [PubMed] [Google Scholar]
- 74.Rami L., Gomez-Anson B., Sanchez-Valle R., Bosch B., Monte G.C., Llado A. Longitudinal study of amnesic patients at high risk for Alzheimer's disease: clinical, neuropsychological and magnetic resonance spectroscopy features. Dement Geriatr Cogn Disord. 2007;24:402–410. doi: 10.1159/000109750. [DOI] [PubMed] [Google Scholar]
- 75.Watanabe T., Shiino A., Akiguchi I. Absolute quantification in proton magnetic resonance spectroscopy is superior to relative ratio to discriminate Alzheimer's disease from Binswanger's disease. Dement Geriatr Cogn Disord. 2008;26:89–100. doi: 10.1159/000144044. [DOI] [PubMed] [Google Scholar]
- 76.Watanabe T., Shiino A., Akiguchi I. Absolute quantification in proton magnetic resonance spectroscopy is useful to differentiate amnesic mild cognitive impairment from Alzheimer's disease and healthy aging. Dement Geriatr Cogn Disord. 2010;30:71–77. doi: 10.1159/000318750. [DOI] [PubMed] [Google Scholar]
- 77.Azevedo D., Tatsch M., Hototian S.R., Bazzarella M.C., Castro C.C., Bottino C.M. Proton spectroscopy in Alzheimer's disease and cognitive impairment no dementia: a community-based study. Dement Geriatr Cogn Disord. 2008;25:491–500. doi: 10.1159/000128275. [DOI] [PubMed] [Google Scholar]
- 78.Block W., Traber F., Flacke S., Jessen F., Pohl C., Schild H. In-vivo proton MR-spectroscopy of the human brain: assessment of N-acetylaspartate (NAA) reduction as a marker for neurodegeneration. Amino Acids. 2002;23:317–323. doi: 10.1007/s00726-001-0144-0. [DOI] [PubMed] [Google Scholar]
- 79.Capizzano A.A., Schuff N., Amend D.L., Tanabe J.L., Norman D., Maudsley A.A. Subcortical ischemic vascular dementia: assessment with quantitative MR imaging and 1H MR spectroscopy. AJNR Am J Neuroradiol. 2000;21:621–630. [PMC free article] [PubMed] [Google Scholar]
- 80.Christiansen P., Schlosser A., Henriksen O. Reduced N-acetylaspartate content in the frontal part of the brain in patients with probable Alzheimer's disease. Magn Reson Imaging. 1995;13:457–462. doi: 10.1016/0730-725x(94)00113-h. [DOI] [PubMed] [Google Scholar]
- 81.Dixon R.M., Bradley K.M., Budge M.M., Styles P., Smith A.D. Longitudinal quantitative proton magnetic resonance spectroscopy of the hippocampus in Alzheimer's disease. Brain. 2002;125:2332–2341. doi: 10.1093/brain/awf226. [DOI] [PubMed] [Google Scholar]
- 82.Ernst T., Chang L., Melchor R., Mehringer C.M. Frontotemporal dementia and early Alzheimer disease: differentiation with frontal lobe H-1 MR spectroscopy. Radiology. 1997;203:829–836. doi: 10.1148/radiology.203.3.9169712. [DOI] [PubMed] [Google Scholar]
- 83.Fayed N., Modrego P.J., Rojas-Salinas G., Aguilar K. Brain glutamate levels are decreased in Alzheimer's disease: a magnetic resonance spectroscopy study. Am J Alzheimers Dis Other Demen. 2011;26:450–456. doi: 10.1177/1533317511421780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fayed N., Andres E., Viguera L., Modrego P.J., Garcia-Campayo J. Higher glutamate+glutamine and reduction of N-acetylaspartate in posterior cingulate according to age range in patients with cognitive impairment and/or pain. Acad Radiol. 2014;21:1211–1217. doi: 10.1016/j.acra.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 85.Fernandez A., Garcia-Segura J.M., Ortiz T., Montoya J., Maestu F., Gil-Gregorio P. Proton magnetic resonance spectroscopy and magnetoencephalographic estimation of delta dipole density: a combination of techniques that may contribute to the diagnosis of Alzheimer's disease. Dement Geriatr Cogn Disord. 2005;20:169–177. doi: 10.1159/000087094. [DOI] [PubMed] [Google Scholar]
- 86.Foy C.M., Daly E.M., Glover A., O'Gorman R., Simmons A., Murphy D.G. Hippocampal proton MR spectroscopy in early Alzheimer's disease and mild cognitive impairment. Brain Topogr. 2011;24:316–322. doi: 10.1007/s10548-011-0170-5. [DOI] [PubMed] [Google Scholar]
- 87.Gordon M.L., Kingsley P.B., Goldberg T.E., Koppel J., Christen E., Keehlisen L. An open-label exploratory study with memantine: correlation between proton magnetic resonance spectroscopy and cognition in patients with mild to moderate Alzheimer's disease. Dement Geriatr Cogn Dis Extra. 2012;2:312–320. doi: 10.1159/000341604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Haley A.P., Knight-Scott J., Simnad V.I., Manning C.A. Increased glucose concentration in the hippocampus in early Alzheimer's disease following oral glucose ingestion. Magn Reson Imaging. 2006;24:715–720. doi: 10.1016/j.mri.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 89.Heun R., Schlegel S., Graf-Morgenstern M., Tintera J., Gawehn J., Stoeter P. Proton magnetic resonance spectroscopy in dementia of Alzheimer type. Int J Geriatr Psychiatry. 1997;12:349–358. doi: 10.1002/(sici)1099-1166(199703)12:3<349::aid-gps508>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 90.Huang W., Alexander G.E., Chang L., Shetty H.U., Krasuski J.S., Rapoport S.I. Brain metabolite concentration and dementia severity in Alzheimer's disease: a (1)H MRS study. Neurology. 2001;57:626–632. doi: 10.1212/wnl.57.4.626. [DOI] [PubMed] [Google Scholar]
- 91.Jessen F., Traeber F., Freymann N., Maier W., Schild H.H., Heun R. A comparative study of the different N-acetylaspartate measures of the medial temporal lobe in Alzheimer's disease. Dement Geriatr Cogn Disord. 2005;20:178–183. doi: 10.1159/000087095. [DOI] [PubMed] [Google Scholar]
- 92.Jessen F., Gur O., Block W., Ende G., Frolich L., Hammen T. A multicenter (1)H-MRS study of the medial temporal lobe in AD and MCI. Neurology. 2009;72:1735–1740. doi: 10.1212/WNL.0b013e3181a60a20. [DOI] [PubMed] [Google Scholar]
- 93.Moats R.A., Ernst T., Shonk T.K., Ross B.D. Abnormal cerebral metabolite concentrations in patients with probable Alzheimer disease. Magn Reson Med. 1994;32:110–115. doi: 10.1002/mrm.1910320115. [DOI] [PubMed] [Google Scholar]
- 94.Parnetti L., Lowenthal D.T., Presciutti O., Pelliccioli G.P., Palumbo R., Gobbi G. 1H-MRS, MRI-based hippocampal volumetry, and 99mTc-HMPAO-SPECT in normal aging, age-associated memory impairment, and probable Alzheimer's disease. J Am Geriatr Soc. 1996;44:133–138. doi: 10.1111/j.1532-5415.1996.tb02428.x. [DOI] [PubMed] [Google Scholar]
- 95.Parnetti L., Tarducci R., Presciutti O., Lowenthal D.T., Pippi M., Palumbo B. Proton magnetic resonance spectroscopy can differentiate Alzheimer's disease from normal aging. Mech Ageing Dev. 1997;97:9–14. doi: 10.1016/s0047-6374(97)01877-0. [DOI] [PubMed] [Google Scholar]
- 96.Rami L., Gomez-Anson B., Bosch B., Sanchez-Valle R., Monte G.C., Villar A. Cortical brain metabolism as measured by proton spectroscopy is related to memory performance in patients with amnestic mild cognitive impairment and Alzheimer's disease. Dement Geriatr Cogn Disord. 2007;24:274–279. doi: 10.1159/000107487. [DOI] [PubMed] [Google Scholar]
- 97.Rose S.E., de Zubicaray G.I., Wang D., Galloway G.J., Chalk J.B., Eagle S.C. A 1H MRS study of probable Alzheimer's disease and normal aging: implications for longitudinal monitoring of dementia progression. Magn Reson Imaging. 1999;17:291–299. doi: 10.1016/s0730-725x(98)00168-4. [DOI] [PubMed] [Google Scholar]
- 98.Schuff N., Amend D., Ezekiel F., Steinman S.K., Tanabe J., Norman D. Changes of hippocampal N-acetyl aspartate and volume in Alzheimer's disease. A proton MR spectroscopic imaging and MRI study. Neurology. 1997;49:1513–1521. doi: 10.1212/wnl.49.6.1513. [DOI] [PubMed] [Google Scholar]
- 99.Schuff N., Amend D.L., Meyerhoff D.J., Tanabe J.L., Norman D., Fein G. Alzheimer disease: quantitative H-1 MR spectroscopic imaging of frontoparietal brain. Radiology. 1998;207:91–102. doi: 10.1148/radiology.207.1.9530304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schuff N., Capizzano A.A., Du A.T., Amend D.L., O'Neill J., Norman D. Selective reduction of N-acetylaspartate in medial temporal and parietal lobes in AD. Neurology. 2002;58:928–935. doi: 10.1212/wnl.58.6.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shiino A., Watanabe T., Shirakashi Y., Kotani E., Yoshimura M., Morikawa S. The profile of hippocampal metabolites differs between Alzheimer's disease and subcortical ischemic vascular dementia, as measured by proton magnetic resonance spectroscopy. J Cereb Blood Flow Metab. 2012;32:805–815. doi: 10.1038/jcbfm.2012.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shonk T.K., Moats R.A., Gifford P., Michaelis T., Mandigo J.C., Izumi J. Probable Alzheimer disease: diagnosis with proton MR spectroscopy. Radiology. 1995;195:65–72. doi: 10.1148/radiology.195.1.7892497. [DOI] [PubMed] [Google Scholar]
- 103.Stoppe G., Bruhn H., Pouwels P.J., Hanicke W., Frahm J. Alzheimer disease: absolute quantification of cerebral metabolites in vivo using localized proton magnetic resonance spectroscopy. Alzheimer Dis Assoc Disord. 2000;14:112–119. doi: 10.1097/00002093-200004000-00009. [DOI] [PubMed] [Google Scholar]
- 104.Arlt S., Schwedhelm E., Kolsch H., Jahn H., Linnebank M., Smulders Y. Dimethylarginines, homocysteine metabolism, and cerebrospinal fluid markers for Alzheimer's disease. J Alzheimers Dis. 2012;31:751–758. doi: 10.3233/JAD-2012-112138. [DOI] [PubMed] [Google Scholar]
- 105.Hagnelius N.O., Wahlund L.O., Nilsson T.K. CSF/serum folate gradient: physiology and determinants with special reference to dementia. Dement Geriatr Cogn Disord. 2008;25:516–523. doi: 10.1159/000129696. [DOI] [PubMed] [Google Scholar]
- 106.Serot J.M., Christmann D., Dubost T., Bene M.C., Faure G.C. CSF-folate levels are decreased in late-onset AD patients. J Neural Transm. 2001;108:93–99. doi: 10.1007/s007020170100. [DOI] [PubMed] [Google Scholar]
- 107.Smach M.A., Jacob N., Golmard J.L., Charfeddine B., Lammouchi T., Ben Othman L. Folate and homocysteine in the cerebrospinal fluid of patients with Alzheimer's disease or dementia: a case control study. Eur Neurol. 2011;65:270–278. doi: 10.1159/000326301. [DOI] [PubMed] [Google Scholar]
- 108.Abalan F., Zittoun J., Boutami C., Dugrais G., Manciet G., Decamps A. Plasma, red cell, and cerebrospinal fluid folate in Alzheimer's diseaseEncephale. 1996;22:430–434. [PubMed] [Google Scholar]
- 109.Mulder C., Schoonenboom N.S., Jansen E.E., Verhoeven N.M., van Kamp G.J., Jakobs C. The transmethylation cycle in the brain of Alzheimer patients. Neurosci Lett. 2005;386:69–71. doi: 10.1016/j.neulet.2005.03.073. [DOI] [PubMed] [Google Scholar]
- 110.Nijst T.Q., Wevers R.A., Schoonderwaldt H.C., Hommes O.R., de Haan A.F. Vitamin B12 and folate concentrations in serum and cerebrospinal fluid of neurological patients with special reference to multiple sclerosis and dementia. J Neurol Neurosurg Psychiatry. 1990;53:951–954. doi: 10.1136/jnnp.53.11.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Popp J., Lewczuk P., Linnebank M., Cvetanovska G., Smulders Y., Kölsch H. Homocysteine metabolism and cerebrospinal fluid markers for Alzheimer's disease. J Alzheimers Dis. 2009;18:819–828. doi: 10.3233/JAD-2009-1187. [DOI] [PubMed] [Google Scholar]
- 112.Selley M.L., Close D.R., Stern S.E. The effect of increased concentrations of homocysteine on the concentration of (E)-4-hydroxy-2-nonenal in the plasma and cerebrospinal fluid of patients with Alzheimer's disease. Neurobiol Aging. 2002;23:383–388. doi: 10.1016/s0197-4580(01)00327-x. [DOI] [PubMed] [Google Scholar]
- 113.Basun H., Forsell L.G., Bendz R., Wahlund L.O., Wetterberg L., Winblad B. Cobalamin in blood and cerebrospinal fluid in Alzheimer's disease and related disorders. Dement Geriatr Cogn Disord. 1991;2:324–332. [Google Scholar]
- 114.Gottfries J., Blennow K., Lehmann M.W., Regland B., Gottfries C.G. One-carbon metabolism and other biochemical correlates of cognitive impairment as visualized by principal component analysis. J Geriatr Psychiatry Neurol. 2001;14:109–114. doi: 10.1177/089198870101400302. [DOI] [PubMed] [Google Scholar]
- 115.Regland B., Abrahamsson L., Blennow K., Gottfries C.G., Wallin A. Vitamin B12 in CSF: reduced CSF/serum B12 ratio in demented men. Acta Neurol Scand. 1992;85:276–281. doi: 10.1111/j.1600-0404.1992.tb04044.x. [DOI] [PubMed] [Google Scholar]
- 116.Kaddurah-Daouk R., Rozen S., Matson W., Han X., Hulette C.M., Burke J.R. Metabolomic changes in autopsy-confirmed Alzheimer's disease. Alzheimers Dement. 2011;7:309–317. doi: 10.1016/j.jalz.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kontush A., Donarski N., Beisiegel U. Resistance of human cerebrospinal fluid to in vitro oxidation is directly related to its amyloid-beta content. Free Radic Res. 2001;35:507–517. doi: 10.1080/10715760100301521. [DOI] [PubMed] [Google Scholar]
- 118.Schippling S., Kontush A., Arlt S., Buhmann C., Sturenburg H.J., Mann U. Increased lipoprotein oxidation in Alzheimer's disease. Free Radic Biol Med. 2000;28:351–360. doi: 10.1016/s0891-5849(99)00247-6. [DOI] [PubMed] [Google Scholar]
- 119.Paraskevas G.P., Kapaki E., Libitaki G., Zournas C., Segditsa I., Papageorgiou C. Ascorbate in healthy subjects, amyotrophic lateral sclerosis and Alzheimer's disease. Acta Neurol Scand. 1997;96:88–90. doi: 10.1111/j.1600-0404.1997.tb00245.x. [DOI] [PubMed] [Google Scholar]
- 120.Jimenez-Jimenez F.J., de Bustos F., Molina J.A., Benito-Leon J., Tallon-Barranco A., Gasalla T. Cerebrospinal fluid levels of alpha-tocopherol (vitamin E) in Alzheimer's disease. J Neural Transm. 1997;104:703–710. doi: 10.1007/BF01291887. [DOI] [PubMed] [Google Scholar]
- 121.Tohgi H., Abe T., Nakanishi M., Hamato F., Sasaki K., Takahashi S. Concentrations of alpha-tocopherol and its quinone derivative in cerebrospinal fluid from patients with vascular dementia of the Binswanger type and Alzheimer type dementia. Neurosci Lett. 1994;174:73–76. doi: 10.1016/0304-3940(94)90122-8. [DOI] [PubMed] [Google Scholar]
- 122.Corrigan F.M., Reynolds G.P., Ward N.I. Reductions of zinc and selenium in brain in Alzheimer's disease. Trace Elem Med. 1991;8:1–5. [Google Scholar]
- 123.Corrigan F.M., Reynolds G.P., Ward N.I. Hippocampal tin, aluminum and zinc in Alzheimer's disease. Biometals. 1993;6:149–154. doi: 10.1007/BF00205853. [DOI] [PubMed] [Google Scholar]
- 124.Stedman J.D., Spyrou N.M. Elemental analysis of the frontal lobe of normal brain tissue and that affected by Alzheimer disease. J Radioanal Nucl Chem. 1997;217:163–166. [Google Scholar]
- 125.Cornett C.R., Ehmann W.D., Wekstein D.R., Markesbery W.R. Trace elements in Alzheimer's disease pituitary glands. Biol Trace Elem Res. 1998;62:107–114. doi: 10.1007/BF02820026. [DOI] [PubMed] [Google Scholar]
- 126.Cornett C.R., Markesbery W.R., Ehmann W.D. Imbalances of trace elements related to oxidative damage in Alzheimer's disease brain. Neurotoxicology. 1998;19:339–346. [PubMed] [Google Scholar]
- 127.Ehmann W.D., Markesbery W.R., Alauddin M., Hossain T.I., Brubaker E.H. Brain trace elements in Alzheimer's disease. Neurotoxicology. 1986;7:197–206. [PubMed] [Google Scholar]
- 128.Panayi A.E., Spyrou N.M., Part P. Differences in trace element concentrations between Alzheimer and “normal” human brain tissue using instrumental neutron activation analysis (INAA) J Radioanal Nucl Chem. 2001;249:437–441. [Google Scholar]
- 129.Plantin L.O., Lying-Tunell U., Kristensson K. Trace elements in the human central nervous system studied with neutron activation analysis. Biol Trace Elem Res. 1987;13:69–75. doi: 10.1007/BF02796622. [DOI] [PubMed] [Google Scholar]
- 130.Samudralwar D.L., Diprete C.C., Ni B.F., Ehmann W.D., Markesbery W.R. Elemental imbalances in the olfactory pathway in Alzheimer's disease. J Neurol Sci. 1995;130:139–145. doi: 10.1016/0022-510x(95)00018-w. [DOI] [PubMed] [Google Scholar]
- 131.Thompson C.M., Markesbery W.R., Ehmann W.D., Mao Y.X., Vance D.E. Regional brain trace-element studies in Alzheimer's disease. Neurotoxicology. 1988;9:1–7. [PubMed] [Google Scholar]
- 132.Wenstrup D., Ehmann W.D., Markesbery W.R. Trace element imbalances in isolated subcellular fractions of Alzheimer's disease brains. Brain Res. 1990;533:125–131. doi: 10.1016/0006-8993(90)91804-p. [DOI] [PubMed] [Google Scholar]
- 133.Kapaki E., Segditsa J., Papageorgiou C. Zinc, copper and magnesium concentration in serum and CSF of patients with neurological disorders. Acta Neurol Scand. 1989;79:373–378. doi: 10.1111/j.1600-0404.1989.tb03803.x. [DOI] [PubMed] [Google Scholar]
- 134.Kovatsi L., Touliou K., Tsolaki M., Kazis A. Cerebrospinal fluid levels of calcium, magnesium, copper and zinc in patients with Alzheimer's disease and mild cognitive impairment. Trace Elem Electrolytes. 2006;23:247–250. [Google Scholar]
- 135.Molina J.A., Jimenez-Jimenez F.J., Aguilar M.V., Meseguer I., Mateos-Vega C.J., Gonzalez-Munoz M.J. Cerebrospinal fluid levels of transition metals in patients with Alzheimer's disease. J Neural Transm. 1998;105:479–488. doi: 10.1007/s007020050071. [DOI] [PubMed] [Google Scholar]
- 136.Graham S.F., Nasaruddin M.B., Carey M., Holscher C., McGuinness B., Kehoe P.G. Age-associated changes of brain copper, iron, and zinc in Alzheimer's disease and dementia with Lewy bodies. J Alzheimers Dis. 2014;42:1407–1413. doi: 10.3233/JAD-140684. [DOI] [PubMed] [Google Scholar]
- 137.Hozumi I., Hasegawa T., Honda A., Ozawa K., Hayashi Y., Hashimoto K. Patterns of levels of biological metals in CSF differ among neurodegenerative diseases. J Neurol Sci. 2011;303:95–99. doi: 10.1016/j.jns.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 138.Panayi A.E., Spyrou N.M., Iversen B.S., White M.A., Part P. Determination of cadmium and zinc in Alzheimer's brain tissue using Inductively Coupled Plasma Mass Spectrometry. Neurol Sci. 2002;195:1–10. doi: 10.1016/s0022-510x(01)00672-4. [DOI] [PubMed] [Google Scholar]
- 139.Sahu R.N., Pandey R.S., Subhash M.N., Arya B.Y., Padmashree T.S., Srinivas K.N. CSF zinc in Alzheimer's type dementia. Biol Psychiatry. 1988;24:480–482. doi: 10.1016/0006-3223(88)90190-4. [DOI] [PubMed] [Google Scholar]
- 140.Tao Y., Wang Y., Rogers J.T., Wang F. Perturbed iron distribution in Alzheimer's disease serum, cerebrospinal fluid, and selected brain regions: a systematic review and meta-analysis. J Alzheimers Dis. 2014;42:679–690. doi: 10.3233/JAD-140396. [DOI] [PubMed] [Google Scholar]
- 141.Veronese N., Zurlo A., Solmi M., Luchini C., Trevisan C., Bano G. Magnesium status in Alzheimer's disease: a systematic review. Am J Alzheimers Dis Other Demen. 2016;31:208–213. doi: 10.1177/1533317515602674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ventriglia M., Brewer G.J., Simonelli I., Mariani S., Siotto M., Bucossi S. Zinc in Alzheimer's disease: a meta-analysis of serum, plasma, and cerebrospinal fluid studies. J Alzheimers Dis. 2015;46:75–87. doi: 10.3233/JAD-141296. [DOI] [PubMed] [Google Scholar]
- 143.Schrag M., Mueller C., Oyoyo U., Smith M.A., Kirsch W.M. Iron, zinc and copper in the Alzheimer's disease brain: a quantitative meta-analysis. Some insight on the influence of citation bias on scientific opinion. Prog Neurobiol. 2011;94:296–306. doi: 10.1016/j.pneurobio.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Johansson P., Almqvist E.G., Johansson J.O., Mattsson N., Andreasson U., Hansson O. Cerebrospinal fluid (CSF) 25-hydroxyvitamin D concentration and CSF acetylcholinesterase activity are reduced in patients with Alzheimer's disease. PLoS One. 2013;8:e81989. doi: 10.1371/journal.pone.0081989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.van de Rest O., Berendsen A.A., Haveman-Nies A., de Groot L.C. Dietary patterns, cognitive decline, and dementia: a systematic review. Adv Nutr. 2015;6:154–168. doi: 10.3945/an.114.007617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shakersain B., Santoni G., Larsson S.C., Faxen-Irving G., Fastbom J., Fratiglioni L. Prudent diet may attenuate the adverse effects of Western diet on cognitive decline. Alzheimers Dement. 2015;12:100–109. doi: 10.1016/j.jalz.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 147.Hsiao P.Y., Mitchell D.C., Coffman D.L., Allman R.M., Locher J.L., Sawyer P. Dietary patterns and diet quality among diverse older adults: the University of Alabama at Birmingham Study of Aging. J Nutr Health Aging. 2013;17:19–25. doi: 10.1007/s12603-012-0082-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bowman G.L., Silbert L.C., Howieson D., Dodge H.H., Traber M.G., Frei B. Nutrient biomarker patterns, cognitive function, and MRI measures of brain aging. Neurology. 2012;78:241–249. doi: 10.1212/WNL.0b013e3182436598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gillette-Guyonnet S., Nourhashemi F., Andrieu S., de Glisezinski I., Ousset P.J., Riviere D. Weight loss in Alzheimer disease. Am J Clin Nutr. 2000;71:637S–642S. doi: 10.1093/ajcn/71.2.637s. [DOI] [PubMed] [Google Scholar]
- 150.Gillette Guyonnet S., Abellan Van Kan G., Alix E., Andrieu S., Belmin J., Berrut G. IANA (International Academy on Nutrition and Aging) Expert Group: weight loss and Alzheimer's disease. J Nutr Health Aging. 2007;11:38–48. [PubMed] [Google Scholar]
- 151.White H., Pieper C., Schmader K. The association of weight change in Alzheimer's disease with severity of disease and mortality: a longitudinal analysis. J Am Geriatr Soc. 1998;46:1223–1227. doi: 10.1111/j.1532-5415.1998.tb04537.x. [DOI] [PubMed] [Google Scholar]
- 152.Zhao Q.F., Tan L., Wang H.F., Jiang T., Tan M.S., Tan L. The prevalence of neuropsychiatric symptoms in Alzheimer's disease: systematic review and meta-analysis. J Affect Disord. 2016;190:264–271. doi: 10.1016/j.jad.2015.09.069. [DOI] [PubMed] [Google Scholar]
- 153.Guerin O., Soto M.E., Brocker P., Robert P.H., Benoit M., Vellas B. Nutritional status assessment during Alzheimer's disease: results after one year (the REAL French Study Group) J Nutr Health Aging. 2005;9:81–84. [PubMed] [Google Scholar]
- 154.Droogsma E., van Asselt D.Z., Scholzel-Dorenbos C.J., van Steijn J.H., van Walderveen P.E., van der Hooft C.S. Nutritional status of community-dwelling elderly with newly diagnosed Alzheimer's disease: prevalence of malnutrition and the relation of various factors to nutritional status. J Nutr Health Aging. 2013;17:606–610. doi: 10.1007/s12603-013-0032-9. [DOI] [PubMed] [Google Scholar]
- 155.Lane R.M., Farlow M.R. Lipid homeostasis and apolipoprotein E in the development and progression of Alzheimer's disease. J Lipid Res. 2005;46:949–968. doi: 10.1194/jlr.M400486-JLR200. [DOI] [PubMed] [Google Scholar]
- 156.Getz G.S., Reardon C.A. Apoprotein E as a lipid transport and signaling protein in the blood, liver, and artery wall. J Lipid Res. 2009;50:S156–S161. doi: 10.1194/jlr.R800058-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Samieri C., Lorrain S., Buaud B., Vaysse C., Berr C., Peuchant E. Relationship between diet and plasma long-chain n-3 PUFAs in older people: impact of apolipoprotein E genotype. J Lipid Res. 2013;54:2559–2567. doi: 10.1194/jlr.P036475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.D'Ambrosio D.N., Clugston R.D., Blaner W.S. Vitamin A metabolism: an update. Nutrients. 2011;3:63–103. doi: 10.3390/nu3010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kayden H.J., Traber M.G. Absorption, lipoprotein transport, and regulation of plasma concentrations of vitamin E in humans. J Lipid Res. 1993;34:343–358. [PubMed] [Google Scholar]
- 160.Shearer M.J., Newman P. Metabolism and cell biology of vitamin K. Thromb Haemost. 2008;100:530–547. [PubMed] [Google Scholar]
- 161.Wang C., Yu J.T., Wang H.F., Jiang T., Tan C.C., Meng X.F. Meta-analysis of peripheral blood apolipoprotein E levels in Alzheimer's disease. PLoS One. 2014;9:e89041. doi: 10.1371/journal.pone.0089041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Rai V. Methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism and Alzheimer disease risk: a meta-analysis. Mol Neurobiol. 2017;54:1173–1186. doi: 10.1007/s12035-016-9722-8. [DOI] [PubMed] [Google Scholar]
- 163.Naj A.C., Beecham G.W., Martin E.R., Gallins P.J., Powell E.H., Konidari I. Dementia revealed: novel chromosome 6 locus for late-onset Alzheimer disease provides genetic evidence for folate-pathway abnormalities. PLoS Genet. 2010;6:e1001130. doi: 10.1371/journal.pgen.1001130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cascalheira J.F., Goncalves M., Barroso M., Castro R., Palmeira M., Serpa A. Association of the transcobalamin II gene 776C-->G polymorphism with Alzheimer's type dementia: dependence on the 5, 10-methylenetetrahydrofolate reductase 1298A-->C polymorphism genotype. Ann Clin Biochem. 2015;52:448–455. doi: 10.1177/0004563214561770. [DOI] [PubMed] [Google Scholar]
- 165.Trushina E., Dutta T., Persson X.M., Mielke M.M., Petersen R.C. Identification of altered metabolic pathways in plasma and CSF in mild cognitive impairment and Alzheimer's disease using metabolomics. PLoS One. 2013;8:e63644. doi: 10.1371/journal.pone.0063644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wang G., Zhou Y., Huang F.J., Tang H.D., Xu X.H., Liu J.J. Plasma Metabolite Profiles of Alzheimer's disease and mild cognitive impairment. J Proteome Res. 2014;13:2649–2658. doi: 10.1021/pr5000895. [DOI] [PubMed] [Google Scholar]
- 167.Gonzalez-Dominguez R., Ruperez F.J., Garcia-Barrera T., Barbas C., Gomez-Ariza J.L. Metabolomic-driven elucidation of serum disturbances associated with Alzheimer's disease and mild cognitive impairment. Curr Alzheimer Res. 2016;13:641–653. doi: 10.2174/1567205013666160129095138. [DOI] [PubMed] [Google Scholar]
- 168.Mapstone M. Plasma phospholipids identify antecedent memory impairment in older adults. Nat Med. 2014;20:415–418. doi: 10.1038/nm.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bourre J.M. Effects of nutrients (in food) on the structure and function of the nervous system: update on dietary requirements for brain. Part 2: macronutrients. J Nutr Health Aging. 2006;10:386–399. [PubMed] [Google Scholar]
- 170.Spector R. Nucleoside and vitamin homeostasis in the mammalian central nervous system. Ann N Y Acad Sci. 1986;481:221–230. doi: 10.1111/j.1749-6632.1986.tb27153.x. [DOI] [PubMed] [Google Scholar]
- 171.Takeda A. Movement of zinc and its functional significance in the brain. Brain Res Brain Res Rev. 2000;34:137–148. doi: 10.1016/s0165-0173(00)00044-8. [DOI] [PubMed] [Google Scholar]
- 172.Spector A.A. Plasma free fatty acid and lipoproteins as sources of polyunsaturated fatty acid for the brain. J Mol Neurosci. 2001;16:159–165. doi: 10.1385/JMN:16:2-3:159. [DOI] [PubMed] [Google Scholar]
- 173.Spector R., Johanson C.E. Vitamin transport and homeostasis in mammalian brain: focus on vitamins B and E. J Neurochem. 2007;103:425–438. doi: 10.1111/j.1471-4159.2007.04773.x. [DOI] [PubMed] [Google Scholar]
- 174.Burk R.F., Hill K.E., Motley A.K., Winfrey V.P., Kurokawa S., Mitchell S.L. Selenoprotein P and apolipoprotein E receptor-2 interact at the blood-brain barrier and also within the brain to maintain an essential selenium pool that protects against neurodegeneration. FASEB J. 2014;28:3579–3588. doi: 10.1096/fj.14-252874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Nguyen L.N., Ma D., Shui G., Wong P., Cazenave-Gassiot A., Zhang X. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature. 2014;509:503–506. doi: 10.1038/nature13241. [DOI] [PubMed] [Google Scholar]
- 176.Talwar P., Sinha J., Grover S., Agarwal R., Kushwaha S., Srivastava M.V. Meta-analysis of apolipoprotein E levels in the cerebrospinal fluid of patients with Alzheimer's disease. J Neurol Sci. 2016;360:179–187. doi: 10.1016/j.jns.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 177.Czech C., Berndt P., Busch K., Schmitz O., Wiemer J., Most V. Metabolite profiling of Alzheimer's disease cerebrospinal fluid. PLoS One. 2012;7:e31501. doi: 10.1371/journal.pone.0031501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Miatto O., Gonzalez R.G., Buonanno F., Growdon J.H. In vitro 31P NMR spectroscopy detects altered phospholipid metabolism in Alzheimer's disease. Can J Neurol Sci. 1986;13:535–539. doi: 10.1017/s0317167100037276. [DOI] [PubMed] [Google Scholar]
- 179.Yassine H.N., Rawat V., Mack W.J., Quinn J.F., Yurko-Mauro K., Bailey-Hall E. The effect of APOE genotype on the delivery of DHA to cerebrospinal fluid in Alzheimer's disease. Alzheimers Res Ther. 2016;8:25. doi: 10.1186/s13195-016-0194-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Blusztajn J.K., Holbrook P.G., Lakher M., Liscovitch M., Maire J.C., Mauron C. “Autocannibalism” of membrane choline-phospholipids: physiology and pathology. Psychopharmacol Bull. 1986;22:781–786. [PubMed] [Google Scholar]
- 181.Löffelholz K. Brain choline has a typical precursor profile. J Physiol Paris. 1998;92:235–239. doi: 10.1016/s0928-4257(98)80025-9. [DOI] [PubMed] [Google Scholar]
- 182.Wurtman R.J. How anticholinergic drugs might promote Alzheimer's disease: more amyloid-β and less phosphatidylcholine. J Alzheimers Dis. 2015;46:983–987. doi: 10.3233/JAD-150290. [DOI] [PubMed] [Google Scholar]
- 183.Overk C.R., Masliah E. Pathogenesis of synaptic degeneration in Alzheimer's disease and Lewy body disease. Biochem Pharmacol. 2014;88:508–516. doi: 10.1016/j.bcp.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.de Wilde M.C., Overk C.R., Sijben J.W., Masliah E. Meta-analysis of synaptic pathology in Alzheimer's disease reveals selective molecular vesicular machinery vulnerability. Alzheimers Dement. 2016;12:633–644. doi: 10.1016/j.jalz.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Terry R.D., Masliah E., Salmon D.P., Butters N., DeTeresa R., Hill R. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 186.Nitsch R.M., Blusztajn J.K., Pittas A.G., Slack B.E., Growdon J.H., Wurtman R.J. Evidence for a membrane defect in Alzheimer disease brain. Proc Natl Acad Sci U S A. 1992;89:1671–1675. doi: 10.1073/pnas.89.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Pettegrew J.W., Panchalingam K., Hamilton R.L., McClure R.J. Brain membrane phospholipid alterations in Alzheimer's disease. Neurochem Res. 2001;26:771–782. doi: 10.1023/a:1011603916962. [DOI] [PubMed] [Google Scholar]
- 188.Grimm M.O., Grösgen S., Riemenschneider M., Tanila H., Grimm H.S., Hartmann T. From brain to food: analysis of phosphatidylcholins, lyso-phosphatidylcholins and phosphatidylcholin-plasmalogens derivates in Alzheimer's disease human post mortem brains and mice model via mass spectrometry. J Chromatogr A. 2011;1218:7713–7722. doi: 10.1016/j.chroma.2011.07.073. [DOI] [PubMed] [Google Scholar]
- 189.Gaudin M., Panchal M., Auzeil N., Duyckaerts C., Brunelle A., Laprevote O. Choline-containing phospholipids in microdissected human Alzheimer's disease brain senile plaque versus neuropil. Bioanalysis. 2012;4:2153–5159. doi: 10.4155/bio.12.189. [DOI] [PubMed] [Google Scholar]
- 190.Wood P.L., Barnette B.L., Kaye J.A., Quinn J.F., Woltjer R.L. Non-targeted lipidomics of CSF and frontal cortex grey and white matter in control, mild cognitive impairment, and Alzheimer's disease subjects. Acta Neuropsychiatr. 2015;27:270–278. doi: 10.1017/neu.2015.18. [DOI] [PubMed] [Google Scholar]
- 191.Wood P.L., Medicherla S., Sheikh N., Terry B., Phillipps A., Kaye J.A. Targeted lipidomics of frontal cortex and plasma diacylglycerols (DAG) in mild cognitive impairment and Alzheimer's disease: validation of DAG accumulation early in the pathophysiology of Alzheimer's disease. J Alzheimers Dis. 2015;48:537–546. doi: 10.3233/JAD-150336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Casanova R., Varma S., Simpson B., Kim M., An Y., Saldana S. Blood metabolite markers of preclinical Alzheimer's disease in two longitudinally followed cohorts of older individuals. Alzheimers Dement. 2016;12:815–822. doi: 10.1016/j.jalz.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Selkoe D.J. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 194.Yuki D., Sugiura Y., Zaima N., Akatsu H., Takei S., Yao I. DHA-PC and PSD-95 decrease after loss of synaptophysin and before neuronal loss in patients with Alzheimer's disease. Sci Rep. 2014;4:7130. doi: 10.1038/srep07130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Bennett S.A., Valenzuela N., Xu H., Franko B., Fai S., Figeys D. Using neurolipidomics to identify phospholipid mediators of synaptic (dys)function in Alzheimer's Disease. Front Physiol. 2013;4:168. doi: 10.3389/fphys.2013.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Athanasopoulos D., Karagiannis G., Tsolaki M. Recent findings in Alzheimer disease and nutrition focusing on epigenetics. Adv Nutr. 2016;7:917–927. doi: 10.3945/an.116.012229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Marques S., Outeiro T.F. Epigenetics in Parkinson's and Alzheimer's diseases. Subcell Biochem. 2013;61:507–525. doi: 10.1007/978-94-007-4525-4_22. [DOI] [PubMed] [Google Scholar]
- 198.Vadnal J., Houston S., Bhatta S., Freeman E., McDonough J. Transcriptional signatures mediated by acetylation overlap with early-stage Alzheimer's disease. Exp Brain Res. 2012;221:287–297. doi: 10.1007/s00221-012-3172-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Tonnies E., Trushina E. Oxidative stress, synaptic dysfunction, and Alzheimer's disease. J Alzheimers Dis. 2017;57:1105–1121. doi: 10.3233/JAD-161088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lorente-Cebrian S., Costa A.G., Navas-Carretero S., Zabala M., Laiglesia L.M., Martinez J.A. An update on the role of omega-3 fatty acids on inflammatory and degenerative diseases. J Physiol Biochem. 2015;71:341–349. doi: 10.1007/s13105-015-0395-y. [DOI] [PubMed] [Google Scholar]
- 201.Zhao Y., Calon F., Julien C., Winkler J.W., Petasis N.A., Lukiw W.J. Docosahexaenoic acid-derived neuroprotectin D1 induces neuronal survival via secretase- and PPARgamma-mediated mechanisms in Alzheimer's disease models. PLoS One. 2011;6:e15816. doi: 10.1371/journal.pone.0015816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Mizwicki M.T., Liu G., Fiala M., Magpantay L., Sayre J., Siani A. 1alpha,25-dihydroxyvitamin D3 and resolvin D1 retune the balance between amyloid-beta phagocytosis and inflammation in Alzheimer's disease patients. J Alzheimers Dis. 2013;34:155–170. doi: 10.3233/JAD-121735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Canhada S., Castro K., Perry I.S., Luft V.C. Omega-3 fatty acids' supplementation in Alzheimer's disease: a systematic review. Nutr Neurosci. 2017:1–10. doi: 10.1080/1028415X.2017.1321813. [DOI] [PubMed] [Google Scholar]
- 204.Farina N., Llewellyn D., Isaac M., Tabet N. Vitamin E for Alzheimer's dementia and mild cognitive impairment. Cochrane Database Syst Rev. 2017;4:CD002854. doi: 10.1002/14651858.CD002854.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Li M.M., Yu J.T., Wang H.F., Jiang T., Wang J., Meng X.F. Efficacy of vitamins B supplementation on mild cognitive impairment and Alzheimer's disease: a systematic review and meta-analysis. Curr Alzheimer Res. 2014;11:844–852. [PubMed] [Google Scholar]
- 206.von Arnim C.A., Gola U., Biesalski H.K. More than the sum of its parts? Nutrition in Alzheimer's disease. Nutrition. 2010;26:694–700. doi: 10.1016/j.nut.2009.11.009. [DOI] [PubMed] [Google Scholar]