Abstract
Introduction
A novel amyloid β (Aβ) synthetic peptide vaccine (UB-311) has been evaluated in a first-in-human trial with patients of mild-to-moderate Alzheimer's disease. We describe translational research covering vaccine design, preclinical characterization, and phase-I clinical trial with supportive outcome that advances UB-311 into an ongoing phase-II trial.
Methods
UB-311 is constructed with two synthetic Aβ1–14–targeting peptides (B-cell epitope), each linked to different helper T-cell peptide epitopes (UBITh®) and formulated in a Th2-biased delivery system. The hAPP751 transgenic mouse model was used to perform the proof-of-concept study. Baboons and macaques were used for preclinical safety, tolerability, and immunogenicity evaluation. Patients with mild-to-moderate Alzheimer's disease (AD) were immunized by intramuscular route with 3 doses of UB-311 at weeks 0, 4, and 12, and monitored until week 48. Safety and immunogenicity were assessed per protocol, and preliminary efficacy was analyzed by Alzheimer's Disease Assessment Scale–Cognitive Subscale (ADAS-Cog), Mini–Mental State Examination (MMSE), and Alzheimer's Disease Cooperative Study–Clinician's Global Impression of Change (ADCS-CGIC).
Results
UB-311 covers a diverse genetic background and facilitates strong immune response with high responder rate. UB-311 reduced the levels of Aβ1–42 oligomers, protofibrils, and plaque load in hAPP751 transgenic mice. Safe and well-tolerated UB-311 generated considerable site-specific (Aβ1–10) antibodies across all animal species examined. In AD patients, UB-311 induced a 100% responder rate; injection site swelling and agitation were the most common adverse events (4/19 each). A slower rate of increase in ADAS-Cog from baseline to week 48 was observed in the subgroup of mild AD patients (MMSE ≥ 20) compared with the moderate AD subgroup, suggesting that UB-311 may have a potential of cognition improvement in patients with early stage of Alzheimer's dementia.
Discussion
The UBITh® platform can generate a high-precision molecular vaccine with high responder rate, strong on-target immunogenicity, and a potential of cognition improvement, which support UB-311 for active immunotherapy in early-to-mild AD patients currently enrolled in a phase-II trial (NCT02551809).
Keywords: UB-311, UBITh® platform, Amyloid β vaccine, FIH clinical trial, Alzheimer's disease
1. Introduction
The amyloid β (Aβ) peptide, central to the “Amyloid Cascade Hypothesis,” is thought to be the pivot for the onset and progression of Alzheimer's disease (AD), and the toxic forms of oligomers and fibrils are suggested to be responsible for the death of synapses and neurons that leads the pathology of AD and dementia [1], [2]. Although recent findings may suggest a dual protective/damaging role for Aβ peptides in AD pathology [3], [4], a successful disease-modifying therapy for AD will include products that affect the disposition of Aβ in the brain [5], in which the immunotherapeutic strategy has drawn much attention since the first active immunotherapy was investigated in mice in 1999 [6].
AN-1792 vaccine [7], [8], using aggregated full-length Aβ1–42 peptide as immunogen associated with a Th1 adjuvant (QS-21; saponin), was the first immunotherapy tested in AD patients, which generated anti-Aβ antibody responses in <25% of patients with improved memory and decreased level of tau protein in the cerebral spinal fluid of a small subset of patients [7], [8], thus providing encouraging results for development of new Aβ vaccines. AN-1792 was discontinued because of acute meningoencephalitis symptoms in 6% of patients, likely caused by autoreactive T-cell activation and Aβ-reactive T-cell infiltration into the central nervous system [7].
Several second-generation Aβ-targeting vaccines were subsequently designed to minimize Aβ-related T-cell inflammation. These include the following: ACC-001 using Aβ1–7 peptide conjugated to diphtheria toxoid protein [9], CAD106 using Aβ1–6 peptide coupled to Qβ virus-like particle [10], V950 using multivalent Aβ1–15 conjugated to a carrier unknown [11], and affitopes AD01 and AD02 using Aβ mimetics (six amino acids) conjugated to KLH [12]. These vaccines induced variable anti-Aβ antibody titers and responder rates, with most of the immune response directed toward the large carrier molecules. To date, these and related vaccines have not presented convincing clinical efficacy data [13]. Development of AD02, ACC-001, and V950 vaccines have been discontinued for reasons unclear [14]. CAD106, currently in clinical phase-III trial, has completed two phase-II trials reporting acceptable safety and tolerability and evoking a strong serological responses in 80% of patients, and brain PET imaging was suggestive of target engagement [15], [16]. Aβ is the principal target of late-stage development programs with relatively few agents in clinical trials for AD, suggesting a need to amplify the drug discovery ecosystem [17].
We describe in this report a novel design of the UBITh® platform-based, fully synthetic Aβ1–14 peptide vaccine (UB-311), preclinical characterization, and a first-in-human (FIH) phase-I clinical study which enrolled 19 patients with mild-to-moderate AD. UB-311 comprises two Aβ1–14–targeting peptides (B-cell epitopes), each linked to different helper T-cell peptide epitopes (UBITh®) as a chimeric peptide to maximize immunogenicity, which is formulated in a Th2-biased delivery system to minimize T-cell inflammatory reactivity. The UB-311 vaccine was safe and well-tolerated, generating strong site-specific (Aβ1–10) antibodies in all patients. Of note, a subgroup of mild AD patients (Mini–Mental State Examination [MMSE] ≥20) showed a positive trend toward cognition improvement.
2. Methods
2.1. Peptide synthesis and vaccine product
UBITh® Aβ1–14 peptide immunogens for the UB-311 vaccine product and Aβ peptide antigens for immunoassays were synthesized using automated solid-phase synthesis, purified by preparative high performance liquid chromatography (HPLC), and characterized by matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometer, amino acid analysis, and reverse-phase HPLC. UB-311 comprises two Aβ1–14–targeting peptides (B-cell epitope), each synthetically linked to different helper T-cell peptide epitopes (UBITh®), and formulated in an alum-containing Th2-biased delivery system (US patent no. 9,102,752). The two Aβ immunogens are the cationic Aβ1–14-εK-KKK-MvF5 Th [ISITEIKGVIVHRIETILF] and Aβ1–14-εK-HBsAg3 Th [KKKIITITRIITIITID] peptides, in equimolar ratio; they were mixed with polyanionic CpG oligodeoxynucleotide (ODN) to form stable immunostimulatory complexes of micron-size particulates, to which aluminum mineral salt (Adju-Phos, Brenntag Biosector, Denmark), was added to the final formulation, along with sodium chloride for tonicity and 0.25% 2-phenoxyethanol as preservative. The vaccine product was manufactured under “good manufacture practice” (GMP) conditions at United Biomedical as sponsor (New York).
2.2. FIH phase-I clinical trial with patients of mild-to-moderate Alzheimer's disease
2.2.1. Patients and study design
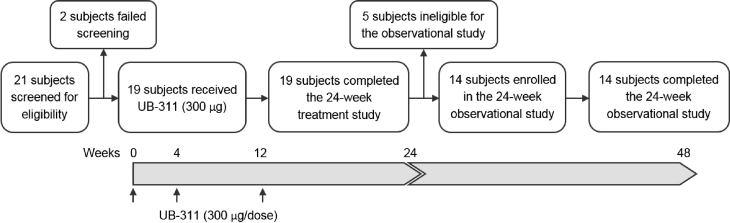
An FIH phase-I clinical trial (NCT00965588) with a 24-week intervention phase (Fig. 1) was conducted at National Taiwan University Hospital and Taipei Veterans General Hospital in Taipei, Taiwan. A total of 19 adults with mild-to-moderate AD were enrolled, including 9 males and 10 females with 50–85 years of age (Table 1). Each patient was immunized three times with UB-311 (300 μg/dose) at weeks 0 (baseline), 4, and 12 by intramuscular injection. An observational 24-week extension study (NCT01189084), from weeks 24 to 48, was included to monitor long-term immunogenicity and efficacy with 14 eligible subjects (Fig. 1).
Fig. 1.
Patient disposition and study design. The first-in-human (FIH) clinical trial enrolled 19 patients (50–80 years old) with mild-to-moderate Alzheimer's disease in a 24-week interventional study, and all 19 subjects received three UB-311 vaccine doses by intramuscular injection (300-μg immunogen peptide) at 0, 4, and 12 weeks and completed the treatment study (identifier no.: NCT00965588). After the first five subjects passed the safety evaluation at week 48, the remaining 14 subjects enrolled in and completed a 24-week observational extension study added to the end of the interventional study to monitor long-term safety, tolerability, immunogenicity, and efficacy (identifier no.: NCT01189084).
Table 1.
Baseline demographics and characteristics of patients with Alzheimer's disease (first-in-human trial with UB-311 vaccine)
| Baseline demographics and disease assessment∗ | Value |
|---|---|
| Age, years | |
| Mean (SD) | 64.0 (8.3) |
| Median (range) | 64.0 (51.0–78.0) |
| Gender | |
| Male (%) | 9 (47.4) |
| Female (%) | 10 (52.6) |
| Race | |
| Asian (%) | 19 (100.0) |
| Other (%) | 0 (0.0) |
| BMI, kg/m2 | |
| Mean (SD) | 23.1 (2.1) |
| Median (range) | 22.6 (20.0–27.2) |
| Time since Alzheimer's disease diagnosis, years | |
| Mean (SD) | 2.4 (2.3) |
| Median (range) | 2.0 (0.3–10.7) |
| ADCS-CGIC | |
| Borderline (%) | 5 (26.3) |
| Mild (%) | 11 (57.9) |
| Moderate (%) | 3 (15.8) |
| MMSE | |
| Mean (SD) | 19.2 (3.2) |
| Median (range) | 19.0 (15.0–25.0) |
| HIS | |
| Mean (SD) | 1.4 (1.2) |
| Median (range) | 1.0 (0.0–4.0) |
| CDR | |
| Mean (SD) | 1.1 (0.3) |
| Median (range) | 1.0 (1.0–2.0) |
Abbreviations: SD, standard deviation; BMI, body mass index; ADCS-CGIC, Alzheimer's Disease Cooperative Study–Clinical Global Impression of Change; MMSE, Mini–Mental State Examination; HIS, Hachinski Ischemic Score; CDR, Clinical Dementia Rating.
The enrolled patients were free of psychiatric, medical or substance abuse problems, history of severe systemic, autoimmune disease or anaphylaxis, hepatic insufficiency, poor control of blood sugar, positive human immunodeficiency virus, hepatitis C virus antibodies, or hepatitis B surface antigen enzyme immunoassay test results. Patients receiving stable treatment with cholinesterase inhibitors were not excluded from the trial.
2.2.2. Objectives of the FIH trial
The primary objectives were to evaluate safety and tolerability; the secondary objectives were to assess immunogenicity and the preliminary vaccine efficacy after a three-dose regimen. Safety assessments included vital signs, physical and neurological examinations, laboratory parameters, electrocardiogram, and brain magnetic resonance imaging (MRI) scans were performed at the initial screening and at the end of the interventional study (weeks 24–26). No abnormal MRI brain imaging scans were reported. Blood samples were collected periodically for clinical laboratory analysis, screening, and characterization of anti-Aβ antibodies, and for in vitro lymphocyte proliferation and cytokine production.
2.2.3. Preliminary evaluation of efficacy
The effect of the vaccine on cognition responses was evaluated using three scales: two cognitive scales, Alzheimer's Disease Assessment Scale–Cognitive Subscale (ADAS-Cog) and MMSE; and a global rating scale, Alzheimer's Disease Cooperative Study–Clinician's Global Impression of Change (ADCS-CGIC). ADAS-Cog and ADCS-CGIC were performed at weeks 0, 16, 24, and 48; MMSE was performed at screening visit, and at weeks 16, 24, 36, and 48.
2.2.4. Statistical analyses
For the UB-311 FIH trial, 19 treated patients were recruited to demonstrate initial safety; the probability of observing at least one case of meningoencephalitis with a true incidence of 6% was calculated to be 69.1%. Pearson's correlation coefficient was calculated for the relationship assessment between anti-Aβ antibody titer and ADAS-Cog and MMSE change. For potential heterogeneity in treatment effects, subgroup analyses were conducted on the efficacy end points by baseline age and MMSE. Repeated-measures mixed-effects model was used to estimate the rates of change (slope) in ADAS-Cog and MMSE scores and to determine the difference in slope between different subgroups. The model terms included baseline efficacy variable, subgroup variable, visit time, and the interaction between subgroup variable and visit time. First-order autocorrelation was used as the covariance matrix based on the Akaike's information criterion. All statistical analyses were based on two-sided tests at a significance level of 0.05 and conducted with R version 2.14.1 (R Foundation for Statistical Computing, Vienna, Austria).
2.3. Supplementary methods online
Supplementary methods and study results are provided online to support specific preclinical studies in animals and additional clinical data: (1) experimental animals for immunogenicity, pharmacology, and toxicity studies; (2) hAPP751 transgenic mouse model study; (3) enzyme-linked immunosorbent assay (ELISA) test for detection of antibodies to synthetic peptides; (4) specificity analyses of anti-Aβ antibodies by epitope mapping; (5) solid-phase ELISA for detection of Aβ antigens; (6) immunohistochemical analysis of human tissue cross-reactivity; (7) Biochemical extraction of Aβ from hAPP751 mouse brain tissues; (8) lymphocyte proliferation and cytokine analyses; and (9) dot-blot analysis of Aβ forms reactive with human antisera.
3. Results
3.1. High levels of anti-Aβ antibody response and responder rate
UB-311 comprises two peptide immunogens, each with an N-terminal Aβ1–14 peptide, synthetically linked through an amino acid spacer to different T helper (Th) cell peptides (UBITh® epitopes) derived from two pathogen proteins, hepatitis B surface antigen (Supplementary Fig. 1A), and measles virus fusion protein (Supplementary Fig. 1B).
The vaccine product, formulated in CpG ODN and alum-containing Th2-biased delivery system, could maintain long-term chemical stability as demonstrated after storage for 3 years at 2°C–8°C (Supplementary Fig. 1C). Each peptide was purified to a well-defined molecular entity with an exact mass close to its respective theoretical value (Supplementary Figs. 1D and 1E).
The unique design with UBITh® peptide epitopes (MvF5 and HBsAg3) allows for maximal coverage of the major histocompatibility complex class II binding motifs that can facilitate a high responder rate among the patient population with diverse genetic backgrounds (Supplementary Fig. 2). Of interest, in B-cell antigenicity, UB-311 elicited a strong antibody response that was exclusively directed to the vaccine target Aβ1–14 but not to the UBITh® epitopes, as shown representatively in guinea pigs (Supplementary Table 1). UB-311 can elicit rapid recall immune responses as shown in vaccinated baboons after a long rest period of 72 weeks [18].
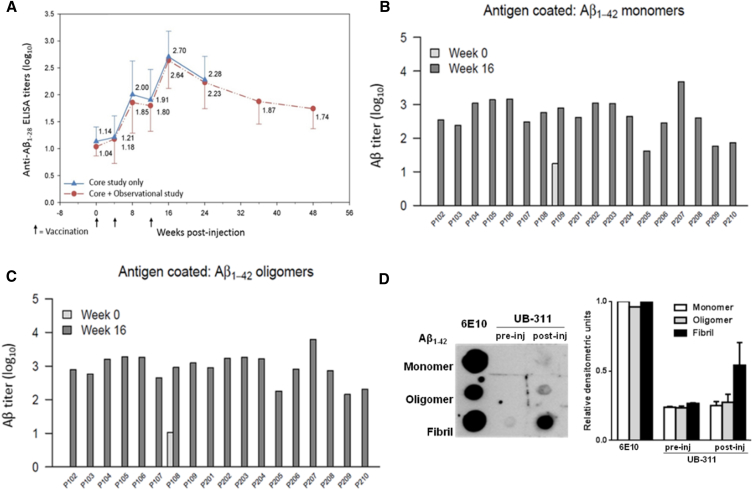
UB-311 generated high immune responses with 100% responder rate in diverse animal species (Supplementary Table 2) and durable, high anti-Aβ antibody response (Fig. 2A) in all of 19 AD patients in the FIH trial (Figs. 2B and 2C; Table 2). UB-311 generated, over-the-threshold, a mean anti-Aβ antibody titer of 1.7 log10 at week 8 after two immunizations (weeks 0 and 4); the immune response peaked with 2.7 log10 at week 16 (after booster injection at week 12) and remained elevated at week 48 (Fig. 2A).
Fig. 2.
Serum anti-Aβ antibody titers assayed by ELISA and preferable targeted Aβ species visualized by dot plot after UB-311 immunization in AD patients. (A) Mean antibody response during the 24-week interventional study (solid line) in 19 AD patients treated with UB-311; 14 of 19 patients (dashed line) were followed up in an additional 24-week observational study, whose mean ADAS-Cog score was 4.9 (improvement). The mean baseline titer (pretreatment) was 1.0 log10; and 4 weeks after last vaccine boost at week 16, the titer peaked at 2.7 log10 (range: 1.8–3.7 log10). At week 48, all patients had decreasing but still positive antibody titers, measured by Aβ1–28 ELISA test. At week 16, serum samples recognized Aβ1–42 monomers (B) and oligomers (C); preimmune serum samples collected at week 0 had anti-Aβ1–42 antibody levels below quantification limit (not shown on log scale), except subjects P109 (monomer) and P108 (oligomer). (D) At week 16, analysis of serum dot plot (left panel), flanked by the positive control 6E10 mAb, reveals that the vaccine-induced anti-Aβ antibodies bind preferentially to Aβ fibrils, followed by oligomers, and the least to monomers; the densitometric measures (right panel) for the three Aβ species are the mean scales from three representative AD subjects, P105, P108, and P206. Abbreviations: AD, Alzheimer's disease; ADAS-Cog, Alzheimer's Disease Assessment Scale–Cognitive Subscale; ELISA, enzyme-linked immunosorbent assay.
Table 2.
Site specificity of anti-Aβ antibody in patients by peptide epitope mapping
| Peptide sequence | Aβ position | Subjects |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P101 | P102 | P103 | P104 | P105 | P106 | P107 | P108 | P109 | P201 | P202 | P203 | P204 | P205 | P206 | P207 | P208 | P209 | P210 | ||
| TEEISEVKMD | −9 to 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| EEISEVKMDA | −8 to 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| EISEVKMDAE | −7 to 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ISEVKMDAEF | −6 to 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| SEVKMDAEFR | −5 to 5 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| EVKMDAEFRH | −4 to 6 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 58 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| VKMDAEFRHD | −3 to 7 | 31 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 13 |
| KMDAEFRHDS | −2 to 8 | 94 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 7 | 0 | 12 | 0 | 0 | 0 | 0 | 0 | 52 |
| MDAEFRHDSG | −1 to 9 | 420 | 12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 19 | 116 | 0 | 17 | 0 | 0 | 2 | 6 | 0 | 121 |
| DAEFRHDSGY | 1–10 | 1983 | 1669 | 36 | 1721 | 3840 | 5193 | 2838 | 948 | 1675 | 1034 | 42,313 | 1284 | 274 | 81 | 1797 | 6024 | 4093 | 27,596 | 20,968 |
| AEFRHDSGYE | 2–11 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| EFRHDSGYEV | 3–12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| FRHDSGYEVH | 4–13 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| RHDSGYEVHH | 5–14 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| HDSGYEVHHQ | 6–15 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| DSGYEVHHQK | 7–16 | 214 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| SGYEVHHQKL | 8–17 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| GYEVHHQKLV | 9–18 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 16 | 0 | 0 | 0 |
| YEVHHQKLVF | 10–19 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 0 | 0 | 0 |
| EVHHQKLVFF | 11–20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| VHHQKLVFFA | 12–21 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 27 | 0 | 0 | 0 |
| HHQKLVFFAE | 13–22 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| HQKLVFFAED | 14–23 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| QKLVFFAEDV | 15–24 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| DAEFRHDSGYEVHHQKLVFFAEDVGSNK | 1–28 | 685 | 161 | 220 | 295 | 450 | 715 | 1163 | 478 | 430 | 339 | 509 | 279 | 284 | 39 | 226 | 906 | 1157 | 893 | 1321 |
NOTE. The in vitro anti-Aβ antibody epitope mapping is a competitive binding inhibition enzyme-linked immunoassay for determination of binding residues of anti-Aβ antibody within the target region; the method is described in Section 2. The N-terminal Aβ1–10 (DAEFRHDSGY) peptide (given in bold) was identified to react most strongly with the serum samples collected on week 16 from each of the 19 patients immunized with UB-311; the results show 100% responder rate after immunization at weeks 0, 4, and 12. The numbers represent the dilution factor of each serum sample that corresponds to the 50% binding inhibition (IC50) by each of the 24 Aβ 10-mer peptides.
3.2. Site-specificity epitope mapping
The UBITh® platform can generate a high-precision molecular vaccine as UB-311 has been shown, with immunized sera from baboons [18], to bind a precise peptide sequence DAEFRHDSGY, which represents the N-terminal 10-mer peptide of the full-length Aβ1–42. In an additional competitive ELISA (Supplementary Table 3), significant reactions were seen with Aβ1–28 and Aβ1–10 peptides only (equivalent high inhibition level), but not with adjoining residue masking “D” (Aβ[−1]–9) or N-terminal “D” removed (Aβ2–11, Aβ3–12) or nonimmunogen peptide Aβ17–43. The exactness of binding-site specificity at DAEFRHDSGY was further confirmed by 10-mer epitope mapping using sera from all 19 patients with AD (Table 2).
3.3. Preferential Aβ forms targeted by the vaccine
At week 16 after vaccination in AD patients where peak anti-Aβ responses were observed (Fig. 2A), antisera on ELISA was found to recognize Aβ1–42 monomers (Fig. 2B) and oligomers (Fig. 2C). Further analysis with dot blot (Fig. 2D) reveals that the vaccine-induced, anti-Aβ IgGs (purified from antisera) bind preferentially to Aβ fibrils, followed by oligomers, and the least to monomers.
3.4. Safety features in animals and human subjects
In UB-311-treated hAPP751 transgenic mice and cynomolgus macaques, no evidence was found for microglial cell activation (anti-CD11b antibody marker) or for T-cell infiltration (anti-CD3 antibody marker) of brain tissue; no changes in lymphocyte proliferation or upregulation of cytokine secretion were noted in macaque peripheral blood mononuclear cells (PBMCs) incubated with Aβ1–14 peptide [18]. In the FDA-approved panel of 30 normal adult human tissues stained with purified guinea pig anti-Aβ1–14 IgG, all tissues were negative other than some positive Aβ plaques and cerebral vessels in some brain samples from elderly individuals (Supplementary Fig. 3), indicative of no nonspecific binding by immunoglobulins generated after UB-311 vaccination.
UB-311 was safe and well tolerated in the 19 AD patients of FIH trial. Injection site swelling (4/19, 21%), agitation (4/19, 21%), and transient increase in alanine aminotransferase levels in blood (3/19, 16%) were the most common adverse effects. Treatment-emergent adverse events in 19 AD patients were reported in Table 3. No significant changes were notable with the electrocardiograms, brain magnetic resonance imaging scans, and neurology examination. Laboratory measures of hematological tests, blood clinical chemistry tests, and coagulation factors showed stable trends throughout the study period. No change of lymphocyte proliferation responses (Supplementary Table 4) or cytokine upregulation (Supplementary Table 5) was observed in the presence of Aβ1–14 or Aβ1–28 peptides. Of special note, no evidence of meningoencephalitis was recorded during or after the study.
Table 3.
Phase-I treatment-emergent adverse events in 19 patients with AD
| Adverse event by preferred term |
No. of subjects = 19 | |
|---|---|---|
| System organ class∗ | Preferred term | |
| Gastrointestinal disorders | Toothache | 1 (5.3%) |
| Vomiting | 1 (5.3%) | |
| General disorders and administration site conditions | Injection site hemorrhage | 1 (5.3%) |
| Injection site pain | 1 (5.3%) | |
| Injection site swelling | 4 (21.1%) | |
| Infections and infestations | Herpes zoster | 1 (5.3%) |
| Upper respiratory tract infection | 2 (10.5%) | |
| Injury, poisoning, and procedural complications | Burns first degree | 1 (5.3%) |
| Open wound | 1 (5.3%) | |
| Investigations† | Alanine aminotransferase increased | 3 (15.8%) |
| Aspartate aminotransferase increased | 2 (10.5%) | |
| Autoantibody positive | 2 (10.5%) | |
| Neutrophil count increased | 1 (5.3%) | |
| Neutrophil percentage increased | 1 (5.3%) | |
| Red blood cell sedimentation rate increased | 2 (10.5%) | |
| White blood cell count increased | 1 (5.3%) | |
| Metabolism and nutrition disorders | Decreased appetite | 1 (5.3%) |
| Hyperglycemia | 1 (5.3%) | |
| Nervous system disorders | Poor quality sleep | 1 (5.3%) |
| Dizziness | 1 (5.3%) | |
| Psychiatric disorders | Agitation | 4 (21.1%) |
| Obsessive-compulsive disorder | 1 (5.3%) | |
| Renal and urinary disorders | Genitourinary tract infection | 1 (5.3%) |
| Nephrolithiasis | 1 (5.3%) | |
| Reproductive system and breast disorders | Epididymitis | 1 (5.3%) |
| Respiratory, thoracic and mediastinal disorders | Cough | 2 (10.5%) |
| Skin and subcutaneous tissue disorders | Herpes zoster | 1 (5.3%) |
| Cellulitis | 1 (5.3%) | |
| Tinea cruris | 1 (5.3%) | |
Abbreviation: AD, Alzheimer's disease.
NOTE. Summary of treatment-emergent adverse events (AEs). The MedDRA system was used to code the AEs.
The AEs were summarized descriptively by system organ class and preferred terms. AEs were classified into pretreatment and treatment-emergent AEs according to the time of occurrence. Treatment-emergent AEs, which were definitely, probably, or possibly related to the test drug, were regarded as treatment-related AEs. There were a total of 16 treatment-related AEs in 9 subjects (47.4%); the treatment-related AEs are indicated in bold type.
Laboratory evaluation.
3.5. Functional immunogenicity in animals and human subjects
UB-311 reduced plaque size in the cortex and hippocampus of hAPP751 aged responder transgenic mice (Supplementary Fig. 4A). The reducing effects correlated with the lowering of Aβ1–42 levels in brain tissue extracts, in which young responder transgenic mice were found to have fewer protofibrils (Triton X-100 fraction) and fewer large oligomers and fibrils (sodium dodecyl sulfate [SDS] fraction) compared with nontreated or placebo control animals (Supplementary Fig. 4B).
In cynomolgus macaques, UB-311 promoted brain-to-blood Aβ efflux as demonstrated with increased plasma Aβ1–40 levels in the presence of elevated anti-Aβ1–14 antibody levels (Supplementary Table 6); Aβ1–40 levels remained steady state in the cerebral spinal fluid [18]. This was also observed in AD patients, although anti-Aβ1–14 antibody titers were much higher in the macaques after six vaccine doses than those in human subjects after three immunizations. Serum Aβ1–40 levels from 9 of 12 patients were measured for net change; anti-Aβ1–14 antibody titers greater than 2.4 log10 in serum showed measurable efflux (Supplementary Table 7). Of note, serum antibodies from all AD patients could bind Aβ1–42 monomers and oligomers (Figs. 2B and 2C), an activity that may lead to reduction of soluble oligomers in the brain.
3.6. Potential efficacy for mild Alzheimer's disease
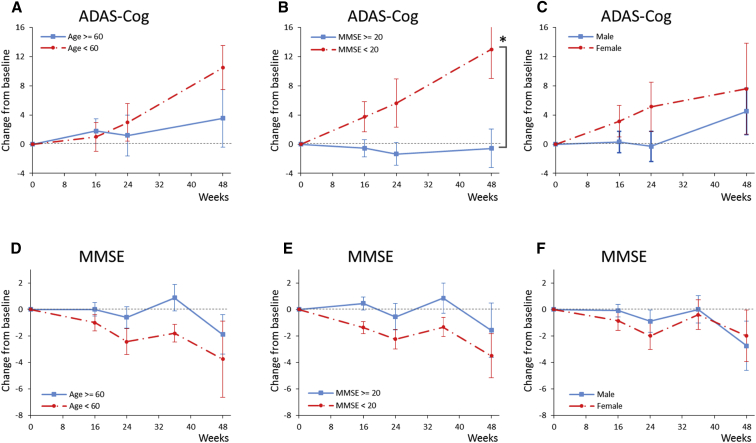
The UB-311 vaccine regimen of three immunizations (300 μg/dose at weeks 0, 4, and 12) by intramuscular route and monitored through week 24 (19 patients in interventional study phase) and week 48 (14 patients in observational extension study phase) (Fig. 1) was safe, well-tolerated, immunogenic and showed encouraging ADAS-Cog scores in a subset of patients. Observed in the 14 patients, ADAS-Cog scores increased by 3.2 points, MMSE scores decreased by 2.2 points, and about 39% of patients showed improvement or no change in ADCS-CGIC scores derived based on the clinician's perception of the patient's change in global clinical status over time. Possible confounder effects in the subgroups based on the age component, baseline MMSE scores, or gender were further analyzed in the post hoc subgroup analyses (Fig. 3). A significantly lower rate of change in ADAS-Cog from baseline to week 48 was observed in the subgroup of mild AD patients with baseline MMSE ≥ 20 (P = .0002, while P < .0083 is considered significant after adjusted for multiple comparisons with Bonferroni correction) (Fig. 3B), whereas there is no statistically significant difference in the rate of change of either ADAS-Cog or MMSE scores when grouped by age or gender of the subjects (Figs. 3A, 3C, 3D, and 3F). This suggests that UB-311 may have a potential of cognition improvement in patients with early stage of Alzheimer's dementia.
Fig. 3.
Mean change over time in ADAS-Cog and MMSE scores by age, MMSE at baseline, or gender. For assessment of ADAS-cog and MMSE scores, UB-311-treated patients were stratified by (A and D) age in years (<60 and ≥60), (B and E) MMSE score (<20 and ≥ 20), or (C and F) gender (male and female). A positive change from baseline in ADAS-Cog score represents cognitive deterioration. For MMSE, a negative change indicates deterioration. Error bars are standard error. (B) MMSE score (<20 and ≥20); *P < .0002 for the difference in the rate of disease progression. Abbreviations: ADAS-Cog, Alzheimer's Disease Assessment Scale–Cognitive Subscale; MMSE, Mini–Mental State Examination.
4. Discussion
By design, UB-311 vaccine has several safety features. First, site-specific immunogenicity is directed to the N-terminus (Aβ1–10) (Table 2) and is devoid of intrinsic Aβ-specific T-cell epitopes located between amino acids 16–33 [19], the Aβ T-cell sites possibly responsible for the adverse T-cell autoimmunity in the case of AN-1792 (Aβ1–42) vaccine [20]. Second, UB-311 is formulated in a Th2-biased delivery system containing CpG ODN, known to favor generalized B-cell mitogenicity with a preference for regulatory Th2 responses rather than Th1 proinflammatory T-cell responses (US patent number 8,088,388); and alum is known to induce Th2-polarized immune responses [21].
The meningoencephalitis associated with AN-1792 vaccine may have also been exacerbated by a Th1-biased adjuvant composition that included QS-21 (saponin) adjuvant and polysorbate-80 surfactant in the formulation [8]. Thus, it is important to design a vaccine product that favors Th2-only while inhibiting without abrogating Th1 immunity [22], [23]. Of note, Alzheimer's disease represents a state of unhealthy aging, termed as “inflamm-aging,” marked by a decline of Th2 responses and chronic upregulation of Th1 proinflammatory mediators such as IL-6, TNF-α, and C-reactive protein in the brain [24], [25] and by an inflammation-driven breakdown of blood brain barrier beginning in the hippocampus that could contribute to cognitive impairment [26].
UB-311 is safe and well tolerated in the chronic toxicity study in cynomolgus macaques and in FIH trial with AD patients, where no meningoencephalitis was reported. Of note, neither microglial cell activation nor T-cell infiltration was observed in brain tissue–staining studies with hAPP751 transgenic mice and cynomolgus macaques [18]. Furthermore, lack of PBMC proliferation responses and cytokine secretion in AD patients (Supplementary Tables 4 and 5) indicate that UB-311 did not generate inflammatory anti-self, cell-mediated immune responses.
The two UBITh® T-helper cell peptide epitopes, MvF5 Th and HBsAg3 Th, could maximize both anti-Aβ antibody titers and responder rates without generating significant anti-UBITh® antibody responses (Supplementary Table 1). The epitopes are expected to provide broader and stronger T-cell help than the incidental intrinsic T-helper epitopes of aggregated Aβ1–42, which may improve immunogenicity in an elderly population. This was observed that UB-311 generated a 100% responder rate for all AD patients in FIH trial (Figs. 2B and 2C; Table 2), and good antibody responses sustained throughout the 48-week study period (Fig. 2A). One additional important feature of the UBITh® platform–based vaccine design is that the responses to chimeric UBITh® anti-self-immunogens are reversible and can be maintained by booster immunizations [27], [28], [29], [30].
Achieving a 100% responder rate is rare in the vaccine development history. AN-1792 vaccine in AD patients (with QS-21 as adjuvant) had a responder rate of <25% [7], [8] but increased to 60% after additional injections with polysorbate-80 added in the formulation [31]. The CAD106 (Aβ1–6) vaccine with a bacteriophage Qβ carrier protein had improved anti-Aβ antibody response in 82% of AD patients but only for a short period with anti-Qβ antibodies still detected [10]. Overall, in contrast with UB-311, the current second-generation Aβ vaccines were reported with a 20%–80% responder rate, including ACC-001, CAD106, V950, affitopes, AD01, and AD02 [9], [10], [11], [12].
The goal for active immunotherapy is to induce antibodies specific for both Aβ monomers and oligomers. Aβ peptides circulate in blood, cerebrospinal fluid, and brain interstitial fluid mainly as soluble Aβ1–40 [32], [33]. Senile plaques contain both Aβ1–40 and Aβ1–42 [34], while vascular amyloid is predominantly the shorter Aβ1–40. Measurement of plasma Aβ1–40 could thus serve as a pharmacodynamic marker. The observation of plasma Aβ1–40 in cynomolgus macaques and human subjects (Supplementary Tables 6 and 7) suggests that UB-311 may operate through the mechanism of promoting efflux of Aβ peptides from brain, supporting the “amyloid sink hypothesis” [35].
In AD patients, UB-311 can generate antibodies able to bind both Aβ1–42 monomers and oligomers (Figs. 2B and 2C); and, in fact, the antibodies preferentially bind to fibrils, followed by oligomers, and weakly to monomers per dot-blot analysis (Fig. 2D). This is of considerable clinical interest as amyloid oligomers, rather than plaques, play a more important role for the disease development [36], [37]; and Aβ1–42 oligomers are most toxic to neurons compared to Aβ1–42 monomers or amyloid plaques [1], [2], [38]. Aβ oligomers can contribute to AD pathogenesis by affecting synaptic plasticity and inhibition of long-term potentiation [38]. Of note, Aβ aggregates can cause certain inflammatory responses that lead to formation of tau aggregates and neurofibrillary tangles [39]; and oligomeric Aβ-induced IL-1β secretion by microglia may also augment neuroinflammation and increase neuronal cell death [40].
The phase-I observations suggest that UB-311 immunization may have a potential of cognition improvement in patients with mild AD. Compared with patients with moderate AD, those with mild AD (MMSE ≥ 20) revealed a stable trend in ADAS-Cog scores with a significant slowdown in deterioration and a slower worsening in MMSE (Fig. 3B). These findings indicate that patients with mild AD have a better cognitive response to UB-311 (P = .0002), and UB-311 may have a positive therapeutic effect in patients with early stage of Alzheimer's disease.
The last two decades have seen robust development of disease-modifying, passive, and active anti-Aβ immunotherapy [5], [13], [14], [41], [42]; however, there has been very limited convincing clinical benefits of cognitive improvement [13], [38]. Bapineuzumab [43] and solanezumab [44] failed to improve cognition or functional ability in patients with mild-to-moderate AD. Bapineuzumab was limited to lower doses, which were believed to be suboptimal for efficacy, due to high incidence of amyloid-related imaging abnormalities–edema [45]. Although potential benefits of both antibodies were observed in patients with mild dementia when stratified by disease severity, solanezumab in the recent EXPEDITION3 phase-III trial disappointingly failed to meet the primary end point in mild AD patients (http://www.medscape.com/viewarticle/873143?src=wnl_edit_tpal).
The chance to overcome mild AD may still exist. Aducanumab in a phase-Ib trial has shown a tantalizing but tenuous evidence that the mAb dose-dependently reduces Aβ plaque that correlates with cognitive improvement [46]. Unlike solanezumab that binds tightly to Aβ monomers but not to the more toxic soluble oligomers or insoluble fibrils [47], aducanumab selectively binds aggregated forms of Aβ, including soluble oligomers and insoluble fibrils, but does not bind monomers [48]. The cognitive efficacy by aducanumab implies that a similar positive effect may be achieved via active immunotherapy. UB-311 immunotherapy in patients with mild AD patients (Fig. 3B) may be mediated through a target-binding mechanism similar to that by aducanumab, that is, preferential binding to oligomers (Fig. 2C) and fibrils (Fig. 2D). UB-311 binds to Aβ monomers as well (Fig. 2B). Overall, the potential of cognition improvement observed with aducanumab [46] also support the amyloid cascade theory [39], [48] including Aβ-induced tau pathology that underlies the Alzheimer's disease [49], [50], [51].
Based on the favorable results of the UB-311 phase I trial (24-week treatment plus 24-week follow-up) in safety and preliminary efficacy, a randomized, double-blind, placebo-controlled, 78-week multicenter phase IIa trial (identifier no.: NCT02551809) was initiated in 2015 to characterize longer term safety, tolerability, immunogenicity, and efficacy. After the initial 0, 4, and 12 week priming doses, additional booster doses are given at 12-week or 24-week intervals in 45 patients with mild AD and a positive Aβ PET imaging scan at baseline. The phase-IIa trial is expected to conclude in 2018. No serious drug-related adverse events have been reported to date.
Research in Context.
-
1.
Systematic review: We searched PubMed, ClinicalTrials.gov, and recent literature reviews that track product development for antibodies and vaccines with anti-Aβ disease–modifying features for Alzheimer's disease (AD). None of these immunotherapeutics, active or passive, in clinical trial has been shown to effectively improve cognition of AD patients; however, that reduced Aβ plaque load correlates with improved cognition scores in mild AD patients has been reported in early aducanumab clinical trial.
-
2.
Interpretation: UB-311 represents a new-generation vaccine design that has potential to prevent or delay the onset of Alzheimer disease. The utility of the vaccine, if successful, would be more cost-effective and easier for dose administration than an antibody drug for treatment of Alzheimer's disease.
-
3.
Future directions: UB-311's preclinical Aβ reduction features and favorable early clinical outcome of potential cognition improvement in mild AD patients have led to the ongoing phase-II trial for early-to-mild Alzheimer's dementia.
Acknowledgments
The authors wish to thank the study patients and their family members and the clinical investigators P.-N.W. and M.-J.C. and their site personnel. The FIH studies reported in this study were supported by United Biomedical, Inc., Hauppauge, New York, United Biomedical, Inc., Asia, Taiwan, and the Ministry of Economic Affairs Grant, Taiwan (grant proposal: UBITh AD Immunotherapeutic Vaccine [UB-311] for phase-1 clinical trial; the fund no. 98-EC-17-A-20-1-0014). The authors also want to thank Dr. Ruby Chen at Academia Sinica whose laboratory performed the dot-blot analytical assay.
Authors' contributions: C.Y.W. conceived the design of the peptide vaccine and directed the UB-311 vaccine program. P.-N.W. and M.-J.C. were principal investigators and contributed equally to the clinical trial. C.L.F., F.L., X.D.F., K.Z., C.H.H., W.-J.P., J.W., S.L., Y.-H.T., Y.T., and C.-C.Y. designed and performed the experiments and also collected and analyzed output data. C.Y.W., C.L.F., B.S.K., and P.A.F. wrote the article. All authors discussed the results and implications and commented on the article at all stages. All authors contributed extensively to the work presented in this study.
C.Y.W., C.L.F., F.L., X.D.F., K.Z., W.-J.P., J.W., S.L., C.H.H., Y.-H.T., Y.T., C.-C.Y., B.-S.K., and P.A.F. are salaried employees and shareholders of the respective companies within the United Biomedical, Inc. group, the developer and owner of the UBITh® AD Immunotherapeutic Vaccine (UB-311) and the sponsor of the clinical trials. In addition, C.Y.W. is a cofounder of the respective companies within the United Biomedical, Inc. group. C.Y.W. is an inventor on two filed patents titled “Immunogenic peptide composition as vaccines for the prevention and treatment of Alzheimer's disease.” US patent nos. 6,713,301/7,951,909/8,232,373, United States: United Biomedical Inc., 2005/2011/2012, and “Peptide vaccine for prevention and immunotherapy of dementia of the Alzheimer's Type.” US patent no. 9,102,752. United States: United Biomedical Inc., 2015. All other authors declare no competing financial interests.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.trci.2017.03.005.
Supplementary data
References
- 1.Hardy J., Selkoe D.J. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 2.Kayed R., Lasagna-Reeves C.A. Molecular mechanisms of amyloid oligomers toxicity. J Alzheimers Dis. 2013;33:S67–S78. doi: 10.3233/JAD-2012-129001. [DOI] [PubMed] [Google Scholar]
- 3.Kumar D.K., Choi S.H., Washicosky K.J., Eimer W.A., Ghofrani J., Leftkowitz A. Amyloid-β peptide protects against microbial infection in mouse and worm models of Alzheimer's disease. Sci Transl Med. 2016;8:340ra72. doi: 10.1126/scitranslmed.aaf1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soscia S.J., Kirby J.E., Washicosky K.J., Tucker S.M., Ingelsson M., Hyman B. The Alzheimer's disease-associated amyloid-beta-protein is an antimicrobial peptide. PLoS One. 2010;5:e9505. doi: 10.1371/journal.pone.0009505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qian X.X., Hamad B., Dias-Lalcaca G. The Alzheimer disease market. Nat Rev Drug Discov. 2015;14:675–676. doi: 10.1038/nrd4749. [DOI] [PubMed] [Google Scholar]
- 6.Schenk D., Barbour R., Dunn W., Gordon G., Grajeda H., Guido T. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 7.Orgogozo J.M., Gilman S., Dartigues J.F., Laurent B., Puel M., Kirby L.C. Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology. 2003;61:46–54. doi: 10.1212/01.wnl.0000073623.84147.a8. [DOI] [PubMed] [Google Scholar]
- 8.Gilman S., Koller M., Black R.S., Jenkins L., Griffith S.G., Fox N.C. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005;64:1553–1562. doi: 10.1212/01.WNL.0000159740.16984.3C. [DOI] [PubMed] [Google Scholar]
- 9.Pasquier F., Sadowsky C., Holstein A., Leterme G.L., Peng Y., Jackson N. Two phase 2 multiple ascending-dose studies of vautide cridificar (ACC-001) and QS-21 adjuvant in mild-to-moderate Alzheimer's disease. J Alzheimers Dis. 2016;51:1131–1143. doi: 10.3233/JAD-150376. [DOI] [PubMed] [Google Scholar]
- 10.Winblad B., Andreasen N., Minthon L., Floesser A., Imbert G., Dumortier T. Safety, tolerability and antibody response of active Aβ immunotherapy with CAD106 in patients with Alzheimer's disease: randomised, double-blind, placebo-controlled, first-in-human study. Lancet Neurol. 2012;11:597–604. doi: 10.1016/S1474-4422(12)70140-0. [DOI] [PubMed] [Google Scholar]
- 11.Savage M.J., Wu G., McCampbell A., Wessner K.R., Citron M., Ling X. A novel multivalent Abeta peptide vaccine with preclinical evidence of a central immune response that generates antisera recognizing a wide range of Abeta peptide species. Alzheimers Dement. 2010;6:S142. Abstract No. O3-07-03. [Google Scholar]
- 12.Hendrix S., Ellison N., Stanworth S., Tierney L., Mattner F., Schmidt W. Methodological aspects of the phase II study AFF006 evaluating amyloid-beta-targeting vaccine AFFITOPE AD02 in early Alzheimer's disease—prospective use of novel composite scales. J Prev Alzheimers Dis. 2015;2:91–102. doi: 10.14283/jpad.2015.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marciani D.J., Ellison N., Stanworth S., Tierney L., Mattner F., Schmidt W. A retrospective analysis of the Alzheimer's disease vaccine progress—the critical need for new development strategies. J Neurochem. 2016;137:687–700. doi: 10.1111/jnc.13608. [DOI] [PubMed] [Google Scholar]
- 14.Agadjanyan M.G., Petrovsky N., Ghochikyan A. A fresh perspective from immunologists and vaccine researchers: active vaccination strategies to prevent and reverse Alzheimer's disease. Alzheimers Dement. 2015;11:1246–1259. doi: 10.1016/j.jalz.2015.06.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farlow M.R., Andreasen N., Riviere M.E., Vostiar I., Vitaliti A., Sovago J. Long-term treatment with Aβ immunotherapy with CAD106 in mild Alzheimer's disease. Alzheimers Res Ther. 2015;7:23. doi: 10.1186/s13195-015-0108-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vandenberghe R., Riviere M.E., Caputo A., Sovago J., Maguire R.P., Farlow M. Active Aβ immunotherapy CAD106 in Alzheimer's disease: A phase 2b study. Alzheimers Dement (N Y) 2017;3:10–22. doi: 10.1016/j.trci.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cummings J., Morstort T., Lee G. Alzheimer's drug development pipeline: 2016. Alzheimers Dement (N Y) 2016;2:222–232. doi: 10.1016/j.trci.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang C.Y., Finstad C.L., Walfield A.M., Sia C., Sokoll K.K., Chang T.Y. Site-specific UBITh® amyloid-β vaccine for immunotherapy of Alzheimer's disease. Vaccine. 2007;25:3041–3052. doi: 10.1016/j.vaccine.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 19.Monsonego A., Zota V., Karni A., Krieger J.I., Bar-Or A., Bitan G. Increased T cell reactivity to amyloid beta protein in older humans and patients with Alzheimer disease. J Clin Invest. 2003;112:415–422. doi: 10.1172/JCI18104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenberg S.M., Bacskai B.J., Hyman B.T. Alzheimer disease's double edged vaccine. Nat Med. 2003;9:389–390. doi: 10.1038/nm847. [DOI] [PubMed] [Google Scholar]
- 21.Ghochikyan A., Mkrtichyan M., Petrushina I., Movsesyan N., Karapetyan A., Cribbs D.H. Prototype Alzheimer's disease epitope vaccine induced strong Th2-type anti-Abeta antibody response with Alum to Quil A adjuvant switch. Vaccine. 2006;24:2275–2282. doi: 10.1016/j.vaccine.2005.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pellicano M., Bulati M., Buffa S., Barbagallo M., Di Prima A., Misiano G. Systemic immune responses in Alzheimer's disease: in vitro mononuclear cell activation and cytokine production. J Alzheimers Dis. 2010;21:181–192. doi: 10.3233/JAD-2010-091714. [DOI] [PubMed] [Google Scholar]
- 23.Marciani D.J. Alzheimer's disease vaccine development: a new strategy focusing on immune modulation. J Neuroimmunol. 2015;287:54–63. doi: 10.1016/j.jneuroim.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 24.Giunta B., Fernandez F., Nikolic W.V., Obregon D., Rrapo E., Town T. Inflammaging as a prodrome to Alzheimer's disease. J Neuroinflammation. 2008;5:51. doi: 10.1186/1742-2094-5-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reale M., Iarlori C., Feliciani C., Gambi D. Peripheral chemokine receptors, their ligands, cytokines and Alzheimer's disease. J Alzheimers Dis. 2008;14:147–159. doi: 10.3233/jad-2008-14203. [DOI] [PubMed] [Google Scholar]
- 26.Montagne A., Barnes S.R., Sweeney M.D., Halliday M.R., Sagare A.P., Zhao Z. Blood-brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85:296–302. doi: 10.1016/j.neuron.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang C.Y., Chang T.Y., Walfield A.M., Ye J., Shen M., Chen S.P. Effective synthetic peptide vaccine for foot-and-mouth disease in swine. Vaccine. 2002;20:2603–2610. doi: 10.1016/s0264-410x(02)00148-2. [DOI] [PubMed] [Google Scholar]
- 28.Wang C.Y., Shen M., Tam G., Fang X.D., Ye J., Shen F. Synthetic AIDS vaccine by targeting HIV receptor. Vaccine. 2002;21:89–97. doi: 10.1016/s0264-410x(02)00432-2. [DOI] [PubMed] [Google Scholar]
- 29.Wang C.Y., Walfield A.M., Fang X.D., Hammerberg B., Ye J., Li M.L. Synthetic IgE peptide vaccine for immunotherapy of allergy. Vaccine. 2003;21:1580–1590. doi: 10.1016/s0264-410x(02)00732-6. [DOI] [PubMed] [Google Scholar]
- 30.Wang C.Y., Walfield A.M. Site-specific peptide vaccines for immunotherapy and immunization against chronic diseases, cancer, infectious diseases, and for veterinary applications. Vaccine. 2005;23:2049–2056. doi: 10.1016/j.vaccine.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 31.Bayer A.J., Bullock R., Jones R.W., Wilkinson D., Paterson K.R., Jenkins L. Evaluation of the safety and immunogenicity of synthetic Abeta42 (AN1792) in patients with AD. Neurology. 2005;64:94–101. doi: 10.1212/01.WNL.0000148604.77591.67. [DOI] [PubMed] [Google Scholar]
- 32.Ghiso J., Frangione B. Amyloidosis and Alzheimer's disease. Adv Drug Deliv Rev. 2002;54:1539–1551. doi: 10.1016/s0169-409x(02)00149-7. [DOI] [PubMed] [Google Scholar]
- 33.Zlokovic B.V. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat Rev Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Masters C.L., Simms G., Weinman N.A., Multhaup G., McDonald B.L., Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985;82:4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeMattos R.B., Bales K.R., Cummins D.J., Dodart J.C., Paul S.M., Holtzman D.M. Peripheral anti-Abeta antibody alters CNS and plasma Abeta clearance and decreases brain A beta burden in a mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2001;98:8850–8855. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao L.N., Long H., Mu Y., Chew L.Y. The toxicity of amyloid oligomers. Int J Mol Sci. 2012;13:7303–7327. doi: 10.3390/ijms13067303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kayed R., Head E., Thompson J.L., McIntire T.M., Milton S.C., Cotman C.W. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- 38.Shankar G.M., Li S., Mehta T.H., Garcia-Munoz A., Shepardson N.E., Smith I. Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stancu I.C., Vasconcelos B., Terwel D., Dewachter I. Models of β-amyloid induced Tau-pathology: the long and “folded” road to understand the mechanism. Mol Neurodegener. 2014;9:51. doi: 10.1186/1750-1326-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parajuli B., Sonobe Y., Horiuchi H., Takeuchi H., Mizuno T., Suzumara A. Oligomeric amyloid beta induces IL-1β processing via production of ROS: implication in Alzheimer's disease. Cell Death Dis. 2013;4:e975. doi: 10.1038/cddis.2013.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wisniewski T., Goni F. Immunotherapeutic approaches for Alzheimer's disease. Neuron. 2015;85:1162–1176. doi: 10.1016/j.neuron.2014.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wisniewski T., Drummond E. Developing therapeutic vaccines against Alzheimer's disease. Expert Rev Vaccines. 2016;15:401–415. doi: 10.1586/14760584.2016.1121815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salloway S., Sperling R., Fox N.C., Blennow K., Klunk W., Raskind M. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer's disease. N Engl J Med. 2014;370:322–333. doi: 10.1056/NEJMoa1304839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Siemers E.R., Sundell K.L., Carlson C., Case M., Sethuraman G., Liu-Seifert H. Phase 3 solanezumab trials: secondary outcomes in mild Alzheimer's disease patients. Alzheimers Dement. 2016;12:110–120. doi: 10.1016/j.jalz.2015.06.1893. [DOI] [PubMed] [Google Scholar]
- 45.Sperling R., Salloway S., Brooks D.J., Tampieri D., Barakos J., Fox N.C. Amyloid-related imaging abnormalities in patients with Alzheimer's disease treated with bapineuzumab: a retrospective analysis. Lancet Neurol. 2012;11:241–249. doi: 10.1016/S1474-4422(12)70015-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sevigny J., Chiao P., Bussiere T., Weinreb P.H., Williams L., Maier M. The antibody aducanumab reduces Aβ plaques in Alzheimer's disease. Nature. 2016;537:50–56. doi: 10.1038/nature19323. [DOI] [PubMed] [Google Scholar]
- 47.Bouter Y., Lopez Noguerola J.S., Tucholla P., Crespi G.A., Parker M.W., Wiltfang J. Abeta targets of the biosimilar antibodies of Bapineuzumab, Crenezumab, Solanezumab in comparison to an antibody against N-truncated Abeta in sporadic Alzheimer disease cases and mouse models. Acta Neuropathol. 2015;130:713–729. doi: 10.1007/s00401-015-1489-x. [DOI] [PubMed] [Google Scholar]
- 48.Reiman E.M. Attack on amyloid-β protein. Nature. 2016;537:36–37. doi: 10.1038/537036a. [DOI] [PubMed] [Google Scholar]
- 49.Zempel H., Luedtke J., Kumar Y., Biermat J., Dawson H., Mandelkow E. Amyloid-β oligomers induce synaptic damage via tau-dependent microtubule severing by TTLL6 and spastin. EMBO J. 2013;32:2920–2937. doi: 10.1038/emboj.2013.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seward M.E., Swanson E., Norambuena A., Reimann A., Cochran J.N., Li R. Amyloid-β signals through tau to drive ectopic neuronal cell cycle re-entry in Alzheimer's disease. J Cell Sci. 2013;126:1278–1286. doi: 10.1242/jcs.1125880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Y., Mandelkow E. Tau in physiology and pathology. Nat Rev Neurosci. 2016;17:5–21. doi: 10.1038/nrn.2015.1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.