Abstract
Introduction
The exceedingly high rate of failed trials in Alzheimer's disease (AD) calls for immediate attention to improve efficiencies and learning from past, ongoing, and future trials. Accurate, highly rigorous standardized data are at the core of meaningful scientific research. Data standards allow for proper integration of clinical data sets and represent the essential foundation for regulatory endorsement of drug development tools. Such tools increase the potential for success and accuracy of trial results.
Methods
The development of the Clinical Data Interchange Standards Consortium (CDISC) AD therapeutic area data standard was a comprehensive collaborative effort by CDISC and Coalition Against Major Diseases, a consortium of the Critical Path Institute. Clinical concepts for AD and mild cognitive impairment were defined and a data standards user guide was created from various sources of input, including data dictionaries used in AD clinical trials and observational studies.
Results
A comprehensive collection of AD-specific clinical data standards consisting of clinical outcome measures, leading candidate genes, and cerebrospinal fluid and imaging biomarkers was developed. The AD version 2.0 (V2.0) Therapeutic Area User Guide was developed by diverse experts working with data scientists across multiple consortia through a comprehensive review and revision process. The AD CDISC standard is a publicly available resource to facilitate widespread use and implementation.
Discussion
The AD CDISC V2.0 data standard serves as a platform to catalyze reproducible research, data integration, and efficiencies in clinical trials. It allows for the mapping and integration of available data and provides a foundation for future studies, data sharing, and long-term registries in AD. The availability of consensus data standards for AD has the potential to facilitate clinical trial initiation and increase sharing and aggregation of data across observational studies and among clinical trials, thereby improving our understanding of disease progression and treatment.
Keywords: Regulatory science, Translational science, Data science, Drug development and Alzheimer's disease, Data standards, Clinical trials, CDISC, Mild cognitive impairment, Biomarkers, Clinical outcome assessments, Therapeutic Area User Guide
1. Introduction
1.1. Data standards and the current landscape of Alzheimer's disease drug development
Drug development in Alzheimer's disease (AD) is increasingly being aimed at early intervention, with the recognition that such strategies hold the most promise to slow or halt disease progression [1]. New drug development tools such as disease progression models, biomarkers, and outcome measures that can easily and rapidly incorporate new and existing sources of information are urgently needed to accelerate drug development at all stages of the AD disease spectrum. The development and regulatory endorsement of these tools has been hampered by the lack of consensus data standards that cover both clinical and biomarker assessments allowing for rapid integrated analyses derived from multiple data sources.
The inability to compare data across different clinical trials arises in part because of differences between them, including data collection and format.
Data standards enable the integration and analysis of data from multiple sources. This, in turn, allows for development of common open-source tools [2], [3]. Data standards provide the framework for consistent structure and understanding of data. Use of data standards results in an increase in efficiency of studies by maximizing data utility, minimizing reprocessing of data, and expediting regulatory review of new drug applications (NDAs). Standards also enable integrated analyses across different studies by allowing integration of data and reusability of programming statements within analysis software.
Research organizations have responded to the need for data standards by creating many different sets of standards [4]. Pharmaceutical companies have also created their own internal data standards, whereas government agencies have recommended and even required use of specific standards to funders [5].
Given the rapid increase in global data availability [6] and an increasing number of experimental treatment modalities, an efficient way to compare effects on clinically meaningful outcomes is critical for selecting the most promising therapeutics to advance to the clinic. To maximize the knowledge from the growing number of costly and high risk AD intervention studies, it is imperative that the field attend to the importance of data standardization, beginning at study start-up.
1.2. Clinical Data Interchange Standards Consortium data standards
The development and widespread dissemination of universally accepted global clinical data standards is the mission of the Clinical Data Interchange Standards Consortium (CDISC), which has been developing global, platform-independent standards to streamline medical research since 1997 [7]. CDISC is a global nonprofit organization that catalyzes productive collaboration to develop freely available, industry-wide clinical research data standards. The primary CDISC standard governing the structure of data collected in clinical studies is the Study Data Tabulation Model (SDTM), which defines the variables and rules associated with specific observation classes including events, interventions, and findings. SDTM is one of the required standards that sponsors must use for NDAs submitted for the U.S. Food and Drug Administration (FDA) review [8].
The implementation of consensus-based CDISC clinical data standards serves to improve medical research and health care [9]. Such standards support the acquisition, exchange, archiving, and reporting of electronic clinical research data. Notably, CDISC standards are recognized by the FDA and Japan's Pharmaceuticals and Medical Devices Agency as the preferred standards for submission of clinical trial data and enable regulatory reviewers to use sophisticated review tools and conduct more efficient reviews.
Public-private partnerships and precompetitive consortia have emerged as a common strategy to share the cost and risk of development of consensus data standards. The Alzheimer's Disease Neuroimaging Initiative (ADNI), formed in 2004, catalyzed awareness and external recognition of the importance of data standardization in the AD research community [10]. In a parallel effort, two nonprofit organizations, CDISC and the Critical Path Institute (C-Path), created the Coalition for the Acceleration of Standards and Therapies in 2012 to develop Therapeutic Area User Guides (TAUGs) for specific disease areas. The focus of the first CDISC therapeutic specific standard was AD, which used elements from ADNI. AD version 1.0 (V1.0) was completed in 2011. As of January 2017, a total of 27 TAUGs spanning a variety of different disease conditions have been developed by CDISC, most of them under the umbrella of Coalition for the Acceleration of Standards and Therapies.
There are a growing number of public-private partnerships focused on AD [11]. The Coalition Against Major Diseases (CAMD), whose mission is to accelerate the path of drug development, is one of many consortia of C-Path [12]. CAMD is a coalition of stakeholders including industry, government agencies, nonprofit organizations, advocacy organizations, academic experts, and regulatory agencies collaborating to improve the efficiency of drug development for memory disorders [13], [14]. CAMD, in close partnership with CDISC and ADNI, represented the key groups that formed the collaborative framework for stakeholders working across consortia to successfully develop CDISC standards specific for AD.
This study discusses the development of the first therapeutic area-specific CDISC standard, how the CDISC standards are used, the need for additional standards, and, most importantly, the need to implement these standards across clinical studies to maximize knowledge gained from past, current, and future clinical trials.
2. Methods
The primary foundational CDISC data standards are the SDTM, the Analysis Data Model (ADaM), and the Clinical Data Acquisition Standards Harmonization (CDASH) model. SDTM is a standard specification for structuring and organizing data, whereas ADaM is used for the analysis of data sets. CDASH provides traceability from SDTM data sets back to data collection instruments. A complete guide for CDISC SDTM implementation is available [15]. Table 1 identifies these foundational CDISC standards and their descriptions.
Table 1.
Description of relevant CDISC standards
| CDISC terms | Description |
|---|---|
| Study Data Tabulation Model (SDTM) | Specification for creation of data sets for data storage |
| Study Data Tabulation Model Implementation Guide (SDTMIG) | Describes how study sponsors should implement SDTM using “domains” |
| Clinical Data Acquisition Standards Harmonization (CDASH) | Describes the recommended fields to be captured in data acquisition |
| Analysis Data Model (ADaM) | Standard for analysis subsets. Describes the efficient generation, replication, and review of clinical trial statistical analyses, with traceability to SDTM |
| Therapeutic Area User Guide (TAUG) | Document intended to guide users on how to implement the standards in therapeutic area-specific studies |
Abbreviation: CDISC, Clinical Data Interchange Standards Consortium.
The development of the AD CDISC data standard occurred in a series of stages that gathered input from a diverse set of experts including CAMD members, subject-matter experts within ADNI, and CDISC experts. Clinical scientists contributed to an understanding of the clinical concepts and interrelationships that affect the usability and analysis of the data that results from the application of these concepts in a well-controlled study. CDISC experts, whose expertise includes the ability to fit these concepts within the confines of the standard data model, in turn worked with the broader standards community to ensure the resulting specifications were accurate, consistent, and appropriate within the context of the full body of existing CDISC standards and their associated rules.
A TAUG is a compilation of concepts, including concept maps (defined subsequently), brief narratives explaining the concept in the context of a disease area, and implementation examples illustrating the implementation of these concepts across the various CDISC standards (Table 1).
The primary sources of input to the AD TAUG were (1) inventories of clinical concepts identified by consensus with CAMD scientists and (2) ADNI data dictionaries. Subject-matter experts from CAMD and ADNI provided clinical expert input into the development of these standards, whereas working groups of CDISC experts mapped concepts relevant to AD to CDISC SDTM and developed controlled terminology to support the use of these standards in clinical trials. CDASH and ADaM were out of scope for both versions 1.0 and 2.0 of the AD TAUGs.
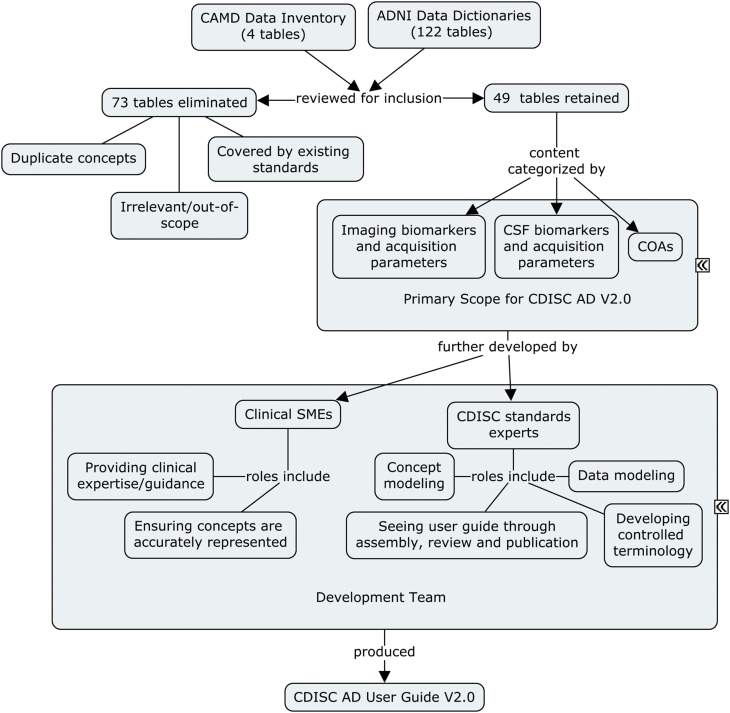
Fig. 1 illustrates the process used by developers of standards for vetting the inputs and for compiling and publishing the content in the CDISC AD TAUG V2.0. The development and final public release of AD V2.0 outlined in Fig. 1 was carried out for more than a period of 12 months.
Fig. 1.
Development process for CDISC AD V2.0 TAUG. CAMD scientists assembled an inventory of the types of data relevant to clinical studies of AD and MCI. These were compared with data dictionaries from ADNI and the pooled items were reduced to 49 tables of data elements based on removing duplicates, concepts already covered by published CDISC standards, and items that were irrelevant or out of scope. The remaining elements were categorized as imaging biomarkers, CSF biomarkers, or COAs. A development team consisting of clinical SMEs and standards experts worked together to capture the relevant details of the scoped concepts and assemble them into a user guide showing how to represent them and relate records in CDISC SDTM. Abbreviations: AD, Alzheimer's disease; ADNI, Alzheimer's Disease Neuroimaging Initiative; CAMD, Coalition Against Major Diseases; CDISC, Clinical Data Interchange Standards Consortium; COA, clinical outcome assessment; CSF, cerebrospinal fluid; MCI, mild cognitive impairment; SDTM, Study Data Tabulation Model; SME, subject-matter expert; TAUG, Therapeutic Area User Guide.
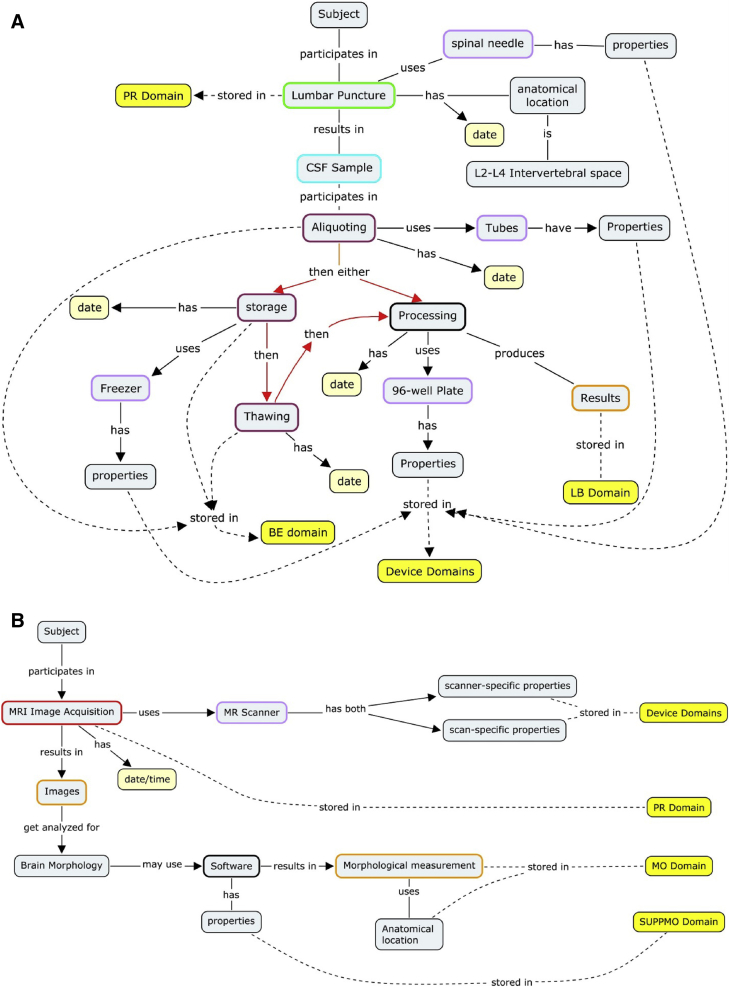
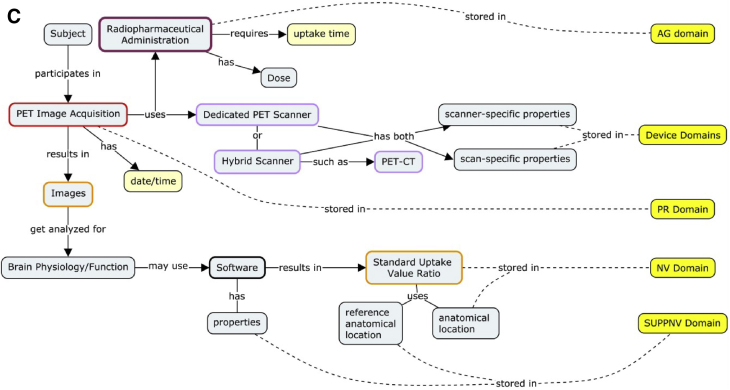
The foremost tool available to developers of standards for building understanding of clinical concepts within these multidisciplinary teams is the “concept map” [16]. Concept maps are the result of concept modeling, whereby knowledge imparted to data modelers from clinical scientists is represented in visual models that describe the process of how a concept is applied and how it results in data elements in a database. These maps enable the experts to ensure that they have reached a common understanding of the concept illustrated. The maps also serve as the first step in fitting the concept into the data model. The interdisciplinary approach ensures that these resulting data models accurately capture the concept and are usable by implementers and analysts. Examples of concept maps developed in the TAUG AD V2.0 are illustrated in Fig. 2.
Fig. 2.
Concept modeling involves iterative discussions between clinical subject-matter experts and data modeling experts to parse the various concepts and relevant qualifiers that describe the pertinent information and data generated within a given research topic. The resulting “concept maps” (examples shown in panels A, B, and C) are the first stage in the development of data models that describe how individual data elements relate to each other so that the resulting data model accounts for and preserves these relationships. (A) A concept map depicting CSF sample processing and the parameters that can impact the results. The color-coding on the perimeter of gray boxes defines observation classes within the CDISC BRIDG model (not discussed). Yellow boxes correspond to the CDISC SDTM domain (data set) where the concepts described reside in SDTM. (B) Concept map depicting MRI for the acquisition of volumetric biomarkers. To fully represent an MRI scan data should be collected regarding subject characteristics, whether contrast enhancement was used, scanner-specific properties, and software properties that determine the anatomic location scanned, pulse-sequence data, and the analysis algorithm to name a few. The color-coding on the perimeter of gray boxes defines observation classes within the CDISC BRIDG model (not discussed). Yellow boxes correspond to the CDISC SDTM domain (data set) where the concepts described reside. (C) Concept map depicting PET scans. Molecular imaging biomarkers in AD V2.0 standards include radiotracers for FDG-PET and amyloid β. PET imaging parameters that are critical to capture include metabolic status/fasting of patient, reference region, and scanner type among others. Abbreviations: AG, procedure agents domain; BE, biospecimen events domain; BRIDG, biomedical research information domain grid; CDISC, Clinical Data Interchange Standards Consortium; CSF, cerebrospinal fluid; CT, computed tomography; FDG-PET, fluorodeoxyglucose–positron emission tomography; LB, laboratory test results domain; MO, morphology domain; MRI, magnetic resonance imaging; NV, nervous system findings domain; PR, procedures domain; SDTM, Study Data Tabulation Model; SUPPMO, supplemental morphology domain; SUPPNV, supplemental nervous systems findings domain.
The AD CDISC V2.0 standard represents broad consensus from the external scientific community. Once the draft therapeutic guide for AD was compiled, it was sent out for a focused review to ensure that the concepts were represented accurately and conformed to rules of the standard. The CDISC standards development team responded to all reviewer comments received and made any necessary changes in an iterative way. This comment resolution process culminated in the first of two reviews by the CDISC Standards Review Council, which is tasked with ensuring the quality of all CDISC TAUGs.
In the later stages of the CDISC standards development process, the draft TAUG was released for a broader public review, during which the global user community was invited to make comments and request changes. The Standards Review Council reviewed all comments and addressed each one to achieve the best standard. Once approved, the new domain was made available for public use. The consensus process that was followed and public availability of the CDISC standard is aimed at encouraging widespread agreement and future implementation.
3. Results
The creation of AD CDISC standards in SDTM format served as the basis for the AD-specific data standards user guide. A number of the included concepts are applicable across different diseases, such as the approach to handling imaging data. In addition to defining a standard format for representing data from common assessments (i.e., medical history), the standards describe how to record a number of factors that are specific for AD trials and may influence the outcome of analyses.
3.1. Data standards are critical to interpreting integrated study data
On completion of V1.0 of the AD TAUG, the AD CDISC standards were prioritized to effectively integrate the AD placebo data from multiple distinct AD trials in the unified AD C-Path Online Data Repository database [17]. The CAMD AD database, which serves as a single integrated database, consists of item-level, patient-level anonymized data from the placebo arm of 24 clinical trials contributed by nine industry sponsors and the Alzheimer's Disease Cooperative Study Group. Development of the integrated database required the remapping of legacy clinical trial data to the CDISC AD standard. The common outcome measure across the different AD trials was Alzheimer's Disease Assessment Scale–Cognitive subscale (ADAS-Cog). Importantly, it was discovered when integrating the data to the AD CDISC standard that the ADAS-Cog instrument was not represented the same in each study. Table 2 illustrates the unique aspects of each sponsor's trial according to the ADAS-Cog measures. The AD CDISC standard served to highlight the differences in ADAS-Cog item level measures across different studies and was used to align the outcome measures across distinct trials in the development of the AD CAMD database.
Table 2.
Comparison of different study sponsors' implementation of ADAS-Cog
| Item | Study 1 | Study 2 | Study 3 | Study 4 | Study 5 | Study 6 | Study 7 |
|---|---|---|---|---|---|---|---|
| 1 | Word Recall | Word Recall | Word Recall | Word Recall | Word Recall | Word Recall | Word Recall |
| 2 | Commands | Name Obj./Fingers | Name Obj./Fingers | Commands | Name Obj./Fingers | Name Obj./Fingers | Name Obj./Fingers |
| 3 | Constr. Praxis | Delayed Recall | Commands | Constr. Praxis | Commands | Commands | Commands |
| 4 | Delayed Recall | Commands | Constr. Praxis | Delayed Recall | Delayed Recall | Constr. Praxis | Constr. Praxis |
| 5 | Name Obj./Fingers | Constr. Praxis | Idea. Praxis | Name Obj./Fingers | Constr. Praxis | Idea. Praxis | Idea. Praxis |
| 6 | Idea. Praxis | Idea. Praxis | Orientation | Idea. Praxis | Idea. Praxis | Orientation | Orientation |
| 7 | Orientation | Orientation | Word Recog. | Orientation | Orientation | Word Recog. | Word Recog. |
| 8 | Word Recog. | Word Recog. | Remem. Instr. | Word Recog. | Word Recog. | Remem. Instr. | Spoken Lang. Abil. |
| 9 | Remem. Instr. | Remem. Instr. | Spoken Lang. Abil. | Remem. Instr. | Remem. Instr. | Spoken Lang. Abil. | Comprehension |
| 10 | Comprehension | Spoken Lang. Abil. | Word Finding Dif. | Spoken Lang. Abil. | Spoken Lang. Abil. | Word Finding Dif. | Word Finding Dif. |
| 11 | Word Finding Dif. | Word Finding Dif. | Comprehension | Diff. Spont. Speech | Word Finding Dif. | Comprehension | Remem. Instr. |
| 12 | Spoken Lang. Abil. | Comprehension | Concentration | Comprehension | Comprehension | Concentration | |
| 13 | Number Cancel | Concentration | Concentration | Concentration |
Abbreviations: ADAS-Cog, Alzheimer's Disease Assessment Scale–Cognitive subscale; Constr. Praxis, constructional praxis; Diff. Spont. Speech, difficulty with spontaneous speech; Idea. Praxis, ideational praxis; Name Obj./Fingers, naming objects and fingers; Number Cancel., number cancellation; Remem. Instr., remembering instructions; Spoken Lang. Abil., spoken language ability; Word Finding Dif., word finding difficulty; Word Recog., word recognition.
NOTE. Seven Clinical Report Forms (CRFs) from seven individual sponsors were provided to the Coalition Against Major Diseases team for analysis. Each study originates from distinctive sponsors. All points of contact for the different data sources could not confirm with certainty that the order of the ADAS-Cog items in the CRFs reflected the order in which they were administered in the clinical trials. The order in which ADAS-Cog items were reported across the seven CRFs varied for a total number of items ranging from two to 10. Four CRFs reported the trials as having used the 13-item ADAS-Cog scale, two CRFs reported using 12 items of the 13-item ADAS-Cog, and one CRF reported the use of the 11-item ADAS-Cog scale. All CRFs reported the administration of word recall first, and no CRF reported administering word recognition last (which is recommended in the ADAS-Cog administration instructions); three CRFs reported administering word recognition as the seventh item/cognitive test, and four CRFs reported administering word recognition as the eighth item/cognitive test.
3.2. V1.0 as the cornerstone to V2.0
V1.0 of the AD TAUG was published in 2011 and focused on standards from mild-to-moderate stages of AD, the target population for most therapeutic trials that had been carried out historically [18]. Concepts covered in V1.0 included items from the ADAS-Cog, apolipoprotein E (APOE) genotype, and laboratory tests for cerebrospinal fluid (CSF), amyloid β (Aβ), and tau biofluid protein biomarkers. The process required development of multiple new SDTM domains as part of the standards development project, definition of new controlled terminology such as for the various APOE haplotypes, and the rules associated with organizing and relating the data in SDTM.
With the advent of diagnostic criteria for early AD [19], [20] and the initiation of clinical trials in earlier stages of the disease spectrum, there was a necessity to update the AD CDISC standards. The primary focus of the revised clinical data standards was on AD biomarkers, which have posed challenges for sponsors in terms of standardization [21], [22], [23]. V2.0, published in 2013, expanded on V1.0 to include neuroimaging biomarkers and an extended set of sample handling for CSF. Biomarker acquisition parameters were a key focus in expanding the relevance of the TAUG to earlier stages of AD, namely studies of mild cognitive impairment (MCI). Clinical outcome assessments were also added to V2.0 and include Clinical Dementia Rating scale, Functional Assessment Questionnaire, Disability Assessment for Dementia, Alzheimer's Disease Cooperative Study–Activities of Daily Living–MCI, Neuropsychiatric Inventory, Clinical Global Impression, and Geriatric Depression Scale. Table 3 highlights the concepts and domains included in the AD TAUG V2.0.
Table 3.
Concepts covered by the Alzheimer's CDISC User Guide, V2.0
| Concept | Comments |
|---|---|
| APOE genotype | Shows how to represent genotype as one record per allele |
| Family history of AD | |
| Volumetric MRI | Total brain volume, hippocampus volume (left and right), ventricular space volume, and BBSI |
| PET, PET-CT (FDG, Florbetapir, PiB) | SUVR, scan parameters, radiotracers, and procedure details (fasting status, and so forth) |
| CSF biomarkers and sampling | Procedure details (location of puncture, time of day, needle gauge, and so forth.), storage tube composition, freeze thaw cycles, Aβ, total tau, and phosphor-tau measures |
| Clinical outcome assessment instruments | |
| ADAS-Cog | Alzheimer's Disease Assessment Scale–Cognitive subscale. Includes item- and subitem-level scores |
| MMSE | Mini–Mental State Examination. Will be made available to licensees through the copyright-holder's website |
| CDR | Clinical Dementia Rating. Standard specifications developed. Negotiations ongoing with copyright holder on how to publish these specifications |
| FAQ | Functional Assessment Questionnaire |
| MHIS-NACC | Modified Hachinski Ischemic Scale—NACC Version |
| DAD | Disability Assessment for Dementia |
| ADCS-ADL MCI | Alzheimer's Disease Cooperative Study–Activities of Daily Living–MCI |
| NPI | Neuropsychiatric Inventory |
Abbreviations: AD, Alzheimer's disease; APOE, apolipoprotein E; BBSI, brain boundary shift integral; CSF, cerebrospinal fluid; CT, computed tomography; MCI, mild cognitive impairment; MRI, magnetic resonance imaging; NACC, National Alzheimer's Coordinating Center version; PET, positron emission tomography; PiB, Pittsburgh compound B; SUVR, standard uptake value ratio.
3.3. Addition of specific biomarker standards
Protein biomarkers measured in CSF have been a key focus for AD researchers and are used frequently in AD randomized controlled clinical trials [24]. Aβ, total tau, and phospho-tau show the most promise as prognostic biomarkers [25], [26] and have been qualified by the European Medicines Agency for use in clinical trials at the predementia stage [27], [28]. A large number of factors contribute to the measure of each protein and there has been increasing recognition of the importance of a multitude of parameters involved in collection and sample handling [29]. Such parameters are relevant across multiple central nervous system disease states, which has led to the development of consensus guidelines [30].
CSF biomarker values can vary according to a multitude of acquisition and processing parameters, including site of the lumbar puncture, the tube type used for sample storage, and the analytical measurement technique [31]. Such biomarker variables are oftentimes not reported in peer-reviewed publications or clinical protocols. The parameters included in the CSF concept map are known to impact the predictive accuracy of AD CSF analytes in identifying early AD subjects who are more likely to progress to AD dementia [32], [33]. Fig. 2A shows which parameters are relevant to contributing to the final biomarker analyte value and should therefore be controlled for and documented at each step along the way.
Standardization of neuroimaging biomarkers is also a focus of the AD CDISC standards. Neuroimaging concepts were defined to cover multiple modalities including fluorodeoxyglucose–positron emission tomography (PET), structural magnetic resonance imaging, and PET neuroimaging. Fig. 2A and B shows the concept maps used to drive the CDISC standards development for neuroimaging in AD. Table 3 outlines the concepts and parameters that are included in the AD V2.0 TAUG. Developers identified ways to represent a variety of imaging parameters including PET scan tracer administration, scanner type, radiolabeled tracers, software type, scanner-specific features, and reference region in addition to fundamental parameters at collection time (time of day, fasting level, and so forth). Many of the factors captured in the concepts are defined in protocols such as ADNI, yet not predefined or even identified as important to capture in other studies.
4. Discussion
Clinical trials in AD have been conducted with a diversity of approaches in the way in which data are acquired and reported. This confounds cross-comparison between studies and makes it difficult to pool and share data for integrated analyses of multiple trials. Efficiency can be gained through the use of consensus data standards. The current V2.0 AD CDISC standards encompass outcome measures and biomarkers that are relevant to AD clinical trials of drugs targeting AD dementia and predementia stages including mild AD and MCI.
ADNI set out early ambitious goals to meet one of its primary objectives of improving the detection of AD at the earliest stages through the use of biomarkers. The success of ADNI can be attributed to the early agreement that defined data collection standards would be implemented at all sites and all data would be shared with researchers around the world [10], [34], [35]. ADNI protocols served as the foundation for the development of the AD CDISC standards.
The CDISC TAUG for AD V1.0 enabled CAMD to develop the first standardized database of AD clinical trials [14]. This database, available to qualified researchers, is being used to provide novel insights into AD and served as the foundation for the development of the first-ever regulatory-endorsed clinical trial simulation tool (for mild and moderate AD) [36]. This tool, now being used by academics and industry, could not have been developed based on meta-analyses of disparate data.
Although it was assumed that ADAS-Cog represents a standardized clinical outcome, particularly given its widespread use, it was clear to the CAMD developers that the item-level data varied significantly across the different trials. This included variations in total number of items (11-, 12-, and 13-item versions), item order, and word lists. Such differences posed significant challenges for analysis. By remapping the data to CDISC standards, all 11 common ADAS-Cog items are aligned across studies, whereas still retaining an indication of their original implementation.
CDISC standards are evolving entities. Revisions of the standards are a mechanism to integrate new scientific advances. The evolution of V1.0 to V2.0 of the AD CDISC standard provides a prime example of this. It is anticipated that future versions of the AD CDISC standard (V3.0) will focus on prevention trials, particularly given that treatment at presymptomatic disease states represent a focus of ongoing and prospective clinical trials [1]. Future revisions may include elements related to novel outcome measures [37], [38] and biomarkers such as functional magnetic resonance imaging used to assess connectivity and cognitive reserve [39].
4.1. Precompetitive initiatives
Collaborative networks have become a mainstay in AD based on the success of flagship public-private partnerships such as ADNI and CAMD. Presently there are >30 consortia with a focus on AD [11] and many new initiatives being launched in other disease areas. Precompetitive forums like the Collaboration for Alzheimer's Prevention provide an effective platform to champion the implementation of AD CDISC standards [40], [41]. The success of these consortia depends on the ability to easily analyze data available from AD trials. International initiatives such as the European Medical Information Framework, the European Prevention of Alzheimer's Dementia, the Alzheimer's Prevention Initiative, and the Global Alzheimer Platform have all recognized the critical value of data standards and have committed to their implementation so that data from these efforts can be integrated. In addition, the Global Alzheimer's Association Interactive Network is “advancing research into the causes, prevention and treatment of Alzheimer's and other neurodegenerative diseases through a global cooperative of sharing, investigation and discovery” (http://www.gaain.org) [6], [42].
4.2. Focus on biomarkers
AD biomarkers that have been a focus of standardization include CSF analytes [22], [31], [43], plasma proteins [44], [45], and neuroimaging parameters [46], [47]. Given that >70% of the variability in biofluid measurements in blood is attributable to preanalytical factors [44], it is critical to standardize sample collection procedures. CDISC data standards should not be confused with protocol standards for biomarker acquisition. However, having a set of consensus-based data standards that capture the concepts relevant to these protocols serves to highlight the importance of acquisition parameters and provides a standardized way of representing data collected according to a given protocol. This allows analysts to quickly filter subsets of aggregated data that were collected in a similar fashion.
Finally, there is a need to consider developing CDISC standards for nascent promising biomarkers including novel imaging methodologies, metabolomics, proteomics, electroencephalogram, and even digital health platform technologies. This will reduce the time for validation and encourage data integration across studies. It is anticipated that prospective use of CDISC standards will expedite regulatory endorsement of biomarkers in the future for related central nervous system conditions and provide a path for the development of future standards [43].
4.3. Regulatory implications of data standards
Regulatory agencies have encouraged consistent data collection and aggregation across multiple disease areas [48], [49]. Since December 2016, FDA and Japan's Pharmaceuticals and Medical Devices Agency require the use of CDISC data standards for all NDA and certain investigational new drug submissions [50].
In recognition of the challenges posed by the sharing and aggregation of large data sets, regulatory agencies have launched the Letter of Support Initiative to highlight promising biomarkers, encourage data sharing, and stimulate additional studies [51], [52]. Two of the first three letters of support issued for clinical biomarkers were issued to CAMD and focused on AD for exploratory prognostic CSF biomarkers (Aβ, tau, and phospho-tau) and the use of low baseline volume of the hippocampus as a biomarker for enrichment in trials at early stages of AD [53]. Notably, these letters, signed by FDA leadership, clearly recommended the use of AD CDISC standards in future AD trials.
5. Conclusions
The AD CDISC data standard holds the promise of implementing a reproducible research framework that spans from first studies in man through the approval of new medicines. The use of CDISC standards aids in the understanding of the course of disease progression, improves the ability to detect statistically significant signals, and maximizes our ability to learn from both successes and failures. CDISC standards can be leveraged to enable the extraction of knowledge from ongoing and future AD trials. Table 4 highlights the key recommendations for the efficient execution and the development of drug development tools that accelerate the delivery of novel AD treatments. The use of the AD data standard will permit complex data modeling of disease progression from asymptomatic to dementia stages of this devastating condition in urgent need of effective intervention.
Research in Context.
-
1.
Systematic review: The first therapeutic area user guide for AD CDISC standards had not previously integrated biomarkers. Given the growing importance of biomarker assays to understand disease progression, and the requirement of the FDA to have trials submitted using CDISC standards, we initiated an effort towards developing global consensus CDISC data standards for key AD biomarkers.
-
2.
Interpretation: AD data standards promote the acceleration of our understanding of AD. They provide a reproducible research framework that spans from first studies in man though the launch of new medicines. CDISC standards increase our ability to improve our ability to detect signals in new compounds, and maximize our ability to share learnings from both successes and failures.
-
3.
Future directions: Future use of the AD data standards (v2.0) will permit complex data modeling of disease progression from asymptomatic to dementia stages of this devastating disease, and improve the efficiency of future regulatory reviews.
Table 4.
Future recommendations to enable efficient execution of novel AD treatments
|
Abbreviations: AD, Alzheimer's disease; CDE, common data element; CDISC, Clinical Data Interchange Standards Consortium; RFA, request for application; TBI, traumatic brian injury.
Acknowledgments
The authors acknowledge Dr Kewei Chen, Dr Patricia Cole, Dr Mark Forrest Gordon, Dr Susan DeSanti, Dr Adam Fleisher, Dr Andreas Jeromin, Dr Gerald Novak, Roberta Rosenberg, Dr Erin Muhlbradt, and Dr Jessica Langbaum who were critical in providing input to the development of the AD Therapeutic Area User Guide. The aforementioned colleagues served as subject-matter experts providing input on clinical science concepts and controlled terminology to support the use of standards in clinical trials. We acknowledge the leadership of Dr Rebecca Kush at Clinical Data Interchange Standards Consortium (CDISC) for her support of this initiative and Dr Amy Porter, Lisa Bain, and Dr Volker Kern for their role as medical writers and editors. This work was partially funded by the U.S. Food and Drug Administration's Critical Path Public Private-Partnerships Grant Program (grant number 1U18FD005320).
References
- 1.Sperling R., Mormino E., Johnson K. The evolution of preclinical Alzheimer's disease: implications for prevention trials. Neuron. 2014;84:608–622. doi: 10.1016/j.neuron.2014.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kush R., Goldman M. Fostering responsible data sharing through standards. N Engl J Med. 2014;370:2163–2165. doi: 10.1056/NEJMp1401444. [DOI] [PubMed] [Google Scholar]
- 3.FitzGerald G.A. Measure for measure: biomarker standards and transparency. Sci Transl Med. 2016;8:343fs10. doi: 10.1126/scitranslmed.aaf8590. [DOI] [PubMed] [Google Scholar]
- 4.Kahn M.G., Bailey L.C., Forrest C.B., Padula M.A., Hirschfeld S. Building a common pediatric research terminology for accelerating child health research. Pediatrics. 2014;133:516–525. doi: 10.1542/peds.2013-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grinnon S.T., Miller K., Marler J.R., Lu Y., Stout A., Odenkirchen J. National Institute of Neurological Disorders and Stroke Common Data Element Project—approach and methods. Clin Trials. 2012;9:322–329. doi: 10.1177/1740774512438980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toga A.W., Neu S.C., Bhatt P., Crawford K.L., Ashish N. The Global Alzheimer's Association Interactive Network. Alzheimers Dement. 2016;12:49–54. doi: 10.1016/j.jalz.2015.06.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huser V., Sastry C., Breymaier M., Idriss A., Cimino J.J. Standardizing data exchange for clinical research protocols and case report forms: an assessment of the suitability of the Clinical Data Interchange Standards Consortium (CDISC) Operational Data Model (ODM) J Biomed Inform. 2015;57:88–99. doi: 10.1016/j.jbi.2015.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.FDA . Food and Drug Adminstration; Silver Spring, MD: 2016. FDA-Study Data Technical Conformance Guide v3.2.1.pdf. U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER) [Google Scholar]
- 9.The Clinical Data Interchange Standards Consortium. CDISC vision and mission [Internet]. [cited 2016 Aug 15]. Available at: http://www.cdisc.org/system/files/all/CDISC-4-Pager_pages_web.pdf.
- 10.Weiner M.W., Veitch D.P., Aisen P.S., Beckett L.A., Cairns N.J., Cedarbaum J. Impact of the Alzheimer's Disease Neuroimaging Initiative, 2004 to 2014. Alzheimers Dement. 2015;11:865–884. doi: 10.1016/j.jalz.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Snyder H.M., Bain L.J., Egge R., Carrillo M.C. Alzheimer's disease public-private partnerships: a landscape of the global nonprofit community. Alzheimers Dement. 2013;9:466–471. doi: 10.1016/j.jalz.2013.05.1761. [DOI] [PubMed] [Google Scholar]
- 12.Brumfield M. The Critical Path Institute: transforming competitors into collaborators. Nat Rev Drug Discov. 2014;13:785–786. doi: 10.1038/nrd4436. [DOI] [PubMed] [Google Scholar]
- 13.Romero K., Corrigan B., Neville J., Kopko S., Cantillon M. Striving for an integrated drug-development process for neurodegeneration: the coalition against major diseases. Neurodegener Dis Manag. 2011;1:379–385. [Google Scholar]
- 14.Stephenson D, Brumfield M, Romero K, Woodcock J, Zineh I, Reiman EM, et al. Alzheimer's and Parkinson's diseases face common challenges in therapeutic development: role of the precompetitive consortium, coalition against major diseases [Internet]. J Alzheimers Dis Parkinsonism. Available at: http://www.omicsonline.org/open-access/alzheimers-and-parkinsons-diseases-face-common-challenges-in-therapeutic-development-role-of-the-precompetitive-consortiumcoalition-against-major-diseases-2161-0460-1000183.php?aid=42374. Accessed May 12, 2016.
- 15.CDISC . 2017. Study Data Tabulation Model (SDTM) [Internet]https://www.cdisc.org/standards/foundational/sdtm [cited 2017 Jan 26]. Available at: [Google Scholar]
- 16.CmapTools [Internet]. Florida, USA: Cmap. Available at: http://cmap.ihmc.us/cmaptools/cmaptools-download/. Accessed January 26, 2017.
- 17.Neville J., Kopko S., Broadbent S., Avilés E., Stafford R., Solinsky C.M. Development of a unified clinical trial database for Alzheimer's disease. Alzheimers Dement. 2015;11:1212–1221. doi: 10.1016/j.jalz.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Cummings J.L., Morstorf T., Zhong K. Alzheimer's disease drug-development pipeline: few candidates, frequent failures. Alzheimers Res Ther. 2014;6:37. doi: 10.1186/alzrt269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Albert M.S., DeKosky S.T., Dickson D., Dubois B., Feldman H.H., Fox N.C. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dubois B., Feldman H.H., Jacova C., Hampel H., Molinuevo J.L., Blennow K. Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria. Lancet Neurol. 2014;13:614–629. doi: 10.1016/S1474-4422(14)70090-0. [DOI] [PubMed] [Google Scholar]
- 21.Molinuevo J.L., Blennow K., Dubois B., Engelborghs S., Lewczuk P., Perret-Liaudet A. The clinical use of cerebrospinal fluid biomarker testing for Alzheimer's disease diagnosis: a consensus paper from the Alzheimer's Biomarkers Standardization Initiative. Alzheimers Dement. 2014;10:808–817. doi: 10.1016/j.jalz.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Carrillo M.C., Blennow K., Soares H., Lewczuk P., Mattsson N., Oberoi P. Global standardization measurement of cerebral spinal fluid for Alzheimer's disease: an update from the Alzheimer's Association Global Biomarkers Consortium. Alzheimers Dement. 2013;9:137–140. doi: 10.1016/j.jalz.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Frisoni G.B., Jack C.R., Bocchetta M., Bauer C., Frederiksen K.S., Liu Y. The EADC-ADNI harmonized protocol for manual hippocampal segmentation on magnetic resonance: evidence of validity. Alzheimers Dement. 2015;11:111–125. doi: 10.1016/j.jalz.2014.05.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blennow K. Biomarkers in Alzheimer's disease drug development. Nat Med. 2010;16:1218–1222. doi: 10.1038/nm.2221. [DOI] [PubMed] [Google Scholar]
- 25.Holtzman D.M. CSF biomarkers for Alzheimer's disease: current utility and potential future use. Neurobiol Aging. 2011;32:S4–S9. doi: 10.1016/j.neurobiolaging.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olsson B., Lautner R., Andreasson U., Öhrfelt A., Portelius E., Bjerke M. CSF and blood biomarkers for the diagnosis of Alzheimer's disease: a systematic review and meta-analysis. Lancet Neurol. 2016;15:673–684. doi: 10.1016/S1474-4422(16)00070-3. [DOI] [PubMed] [Google Scholar]
- 27.European Medicines Agency . 2012. Qualification opinion of Alzheimer's Disease Novel Methodologies/Biomarkers for PET Amyloid Imaging (Positive/Negative) as a Biomarker for Enrichment, for use in Regulatory Clinical Trials in Predementia Alzheimer's Disease. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Regulatory_and_procedural_guideline/2012/04/WC500125018.pdf; 2012. Accessed May 9, 2016. [Google Scholar]
- 28.Isaac M., Vamvakas S., Abadie E., Jonsson B., Gispen C., Pani L. Qualification opinion of novel methodologies in the predementia stage of Alzheimer's disease: cerebro-spinal-fluid related biomarkers for drugs affecting amyloid burden—regulatory considerations by European Medicines Agency focusing in improving benefit/risk in regulatory trials. Eur Neuropsychopharmacol. 2011;21:781–788. doi: 10.1016/j.euroneuro.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Kang J.H., Korecka M., Toledo J.B., Trojanowski J.Q., Shaw L.M. Clinical utility and analytical challenges in measurement of cerebrospinal fluid amyloid-β(1-42) and τ proteins as Alzheimer disease biomarkers. Clin Chem. 2013;59:903–916. doi: 10.1373/clinchem.2013.202937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gnanapavan S., Hegen H., Khalil M., Hemmer B., Franciotta D., Hughes S. Guidelines for uniform reporting of body fluid biomarker studies in neurologic disorders. Neurology. 2014;83:1210–1216. doi: 10.1212/WNL.0000000000000809. [DOI] [PubMed] [Google Scholar]
- 31.Vanderstichele H., Bibl M., Engelborghs S., Le Bastard N., Lewczuk P., Molinuevo J.L. Standardization of preanalytical aspects of cerebrospinal fluid biomarker testing for Alzheimer's disease diagnosis: a consensus paper from the Alzheimer's Biomarkers Standardization Initiative. Alzheimers Dement. 2012;8:65–73. doi: 10.1016/j.jalz.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 32.Fourier A., Portelius E., Zetterberg H., Blennow K., Quadrio I., Perret-Liaudet A. Pre-analytical and analytical factors influencing Alzheimer's disease cerebrospinal fluid biomarker variability. Clin Chim Acta. 2015;449:9–15. doi: 10.1016/j.cca.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 33.Niemantsverdriet E., Goossens J., Struyfs H., Martin J.-J., Goeman J., De Deyn P.P. Diagnostic impact of cerebrospinal fluid biomarker (pre-)analytical variability in Alzheimer's disease. J Alzheimers Dis. 2016;51:97–106. doi: 10.3233/JAD-150953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weiner M.W., Veitch D.P., Aisen P.S., Beckett L.A., Cairns N.J., Green R.C. The Alzheimer's Disease Neuroimaging Initiative: a review of papers published since its inception. Alzheimers Dement. 2013;9:e111–e194. doi: 10.1016/j.jalz.2013.05.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones-Davis D.M., Buckholtz N. The impact of the Alzheimer's Disease Neuroimaging Initiative 2: what role do public-private partnerships have in pushing the boundaries of clinical and basic science research on Alzheimer's disease? Alzheimers Dement. 2015;11:860–864. doi: 10.1016/j.jalz.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Romero K., Ito K., Rogers J.A., Polhamus D., Qiu R., Stephenson D. The future is now: model-based clinical trial design for Alzheimer's disease. Clin Pharmacol Ther. 2015;97:210–214. doi: 10.1002/cpt.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ayutyanont N., Langbaum J.B., Hendrix S.B., Chen K., Fleisher A.S., Friesenhahn M. The Alzheimer's prevention initiative composite cognitive test score: sample size estimates for the evaluation of preclinical Alzheimer's disease treatments in presenilin 1 E280A mutation carriers. J Clin Psychiatry. 2014;75:652–660. doi: 10.4088/JCP.13m08927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Donohue M.C., Sperling R.A., Salmon D.P., Rentz D.M., Raman R., Thomas R.G. The preclinical Alzheimer cognitive composite: measuring amyloid-related decline. JAMA Neurol. 2014;71:961–970. doi: 10.1001/jamaneurol.2014.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farràs-Permanyer L., Guàrdia-Olmos J., Peró-Cebollero M. Mild cognitive impairment and fMRI studies of brain functional connectivity: the state of the art. Front Psychol. 2015;6:1095. doi: 10.3389/fpsyg.2015.01095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reiman E.M., Langbaum J.B., Tariot P.N., Lopera F., Bateman R.J., Morris J.C. CAP—advancing the evaluation of preclinical Alzheimer disease treatments. Nat Rev Neurol. 2016;12:56–61. doi: 10.1038/nrneurol.2015.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weninger S., Carrillo M.C., Dunn B., Aisen P.S., Bateman R.J., Kotz J.D. Collaboration for Alzheimer's Prevention: principles to guide data and sample sharing in preclinical Alzheimer's disease trials. Alzheimers Dement. 2016;12:631–632. doi: 10.1016/j.jalz.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ashish N., Bhatt P., Toga A.W. Global data sharing in Alzheimer disease research. Alzheimer Dis Assoc Disord. 2016;30:160–168. doi: 10.1097/WAD.0000000000000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arnerić S.P., Batrla-Utermann R., Blennow K., Beckett L., Bittner T., Carter L. Cerebrospinal fluid biomarkers for Alzheimer's Disease: a status report from the coalition against major diseases CSF biomarker team. J Alzheimers Dis. 2017;55:19–35. doi: 10.3233/JAD-160573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O'Bryant S.E., Gupta V., Henriksen K., Edwards M., Jeromin A., Lista S. Guidelines for the standardization of preanalytic variables for blood-based biomarker studies in Alzheimer's disease research. Alzheimers Dement. 2015;11:549–560. doi: 10.1016/j.jalz.2014.08.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rowe C.C., Jones G., Doré V., Pejoska S., Margison L., Mulligan R.S. Standardized expression of 18F-NAV4694 and 11C-PiB β-amyloid PET results with the Centiloid Scale. J Nucl Med. 2016;57:1233–1237. doi: 10.2967/jnumed.115.171595. [DOI] [PubMed] [Google Scholar]
- 46.Jack C.R., Barkhof F., Bernstein M.A., Cantillon M., Cole P.E., DeCarli C. Steps to standardization and validation of hippocampal volumetry as a biomarker in clinical trials and diagnostic criteria for Alzheimer's disease. Alzheimers Dement. 2011;7 doi: 10.1016/j.jalz.2011.04.007. 474–485.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buckler A.J., Bresolin L., Dunnick N.R., Sullivan D.C., Aerts H.J.W.L., Bendriem B. Quantitative imaging test approval and biomarker qualification: interrelated but distinct activities. Radiology. 2011;259:875–884. doi: 10.1148/radiol.10100800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Woodcock J., Brumfield M., Gill D., Zerhouni E. The driving role of consortia on the critical path to innovative therapies. Nat Rev Drug Discov. 2014;13:781. doi: 10.1038/nrd4462. [DOI] [PubMed] [Google Scholar]
- 49.Woodcock J., Woosley R. The FDA Critical Path Initiative and its influence on new drug development. Annu Rev Med. 2008;59:1–12. doi: 10.1146/annurev.med.59.090506.155819. [DOI] [PubMed] [Google Scholar]
- 50.US Food and Drug Administration . 2014. Providing Regulatory Submissions In Electronic Format—Standardized Study Data Guidance for Industry. Available at: http://www.fda.gov/downloads/drugs/guidances/ucm292334.pdf; 2014. Accessed May 9, 2016. [Google Scholar]
- 51.Amur S., LaVange L., Zineh I., Buckman-Garner S., Woodcock J. Biomarker qualification: toward a multiple stakeholder framework for biomarker development, regulatory acceptance, and utilization. Clin Pharmacol Ther. 2015;98:34–46. doi: 10.1002/cpt.136. [DOI] [PubMed] [Google Scholar]
- 52.Amur S.G., Sanyal S., Chakravarty A.G., Noone M.H., Kaiser J., McCune S. Building a roadmap to biomarker qualification: challenges and opportunities. Biomark Med. 2015;9:1095–1105. doi: 10.2217/bmm.15.90. [DOI] [PubMed] [Google Scholar]
- 53.US Food and Drug Administration . 2015. Biomarker Letter of Support for use of CSF Analytes Ab1-42, tau and phosphotau, as Exploratory Prognostic Biomarkers for Enrichment in Clinical Trials in Alzheimer's Disease. Available at: http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/UCM439713.pdf; 2015. Accessed May 9, 2016. [Google Scholar]
- 54.Smith D.H., Hicks R.R., Johnson V.E., Bergstrom D.A., Cummings D.M., Noble L.J. Pre-Clinical Traumatic Brain Injury Common Data Elements: Toward a Common Language Across Laboratories. J Neurotrauma. 2015;32:1725–1735. doi: 10.1089/neu.2014.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]