Abstract
Introduction
Mechanisms underlying social determinants of stroke and dementia are unclear and brain-derived neurotrophic factor (BDNF) may contribute as a molecular link.
Methods
Using the Framingham Study, we examined social relationship measures as predictors of higher serum BDNF level and cumulative incidence of stroke and dementia.
Results
Among 3294 participants, controlling for age and sex, isolation trended with lower BDNF (odds ratio = 0.69 [0.47–1.00]). Participants with more companionship had reduced risk for stroke (hazard ratio [HR] = 0.59 [0.41–0.83]) and dementia (HR = 0.67 [0.49–0.92]). Greater emotional support was associated with higher BDNF (odds ratio = 1.27 [1.04–1.54]), reduced dementia risk (HR = 0.69 [0.51–0.94], and among smokers, reduced stroke risk (HR = 0.23 [0.10–0.57]). Associations persisted after additional adjustments. BDNF partly mediated the total effect between emotional support and dementia risk.
Conclusions
Availability of social support appears to be associated with increased BDNF levels and, in certain subsets, reduce risk of subsequent dementia and stroke, thus warranting study of these pathways to understand their role in neuroprotection.
Keywords: Brain-derived neurotrophic factor, Social relationships, Social support, Social networks, Dementia, Stroke, Epidemiology, Cohort studies
1. Introduction
Social environments, particularly social relationships, are strongly linked with physical and mental health [1]. However, little is known about the neurobiological mechanisms underlying the association of social relationships and healthy cognitive aging [2], [3]. Because lower circulating BDNF levels and small social networks have separately been associated with higher risk of incident stroke [3], [4], cognitive dysfunction [2], [5], [6], and the accumulation of Alzheimer's disease pathology [7], [8], we postulated that BDNF may be a biological link between social relationships and a reduced likelihood of developing stroke or dementia. Brain-derived neurotrophic factor (BDNF) may partly mediate observed associations given that it is a neuroprotective molecule critical for synaptic plasticity and neuronal repair [9], which is inducible by lifestyle factors [10], [11] and, in animal models, social enrichment [12]. BDNF crosses the blood-brain barrier with high capacity and is highly stable over time, thus serum BDNF is reflective of central nervous system levels [5], [13], [14]. Our study is motivated by the overarching hypothesis that social relationships alter the biology of the brain and are crucial to reducing stroke and dementia risk through a pathway that involves BDNF, as suggested by the observed effect of social enrichment in animal models and BDNF's association with reduced risk of stroke and dementia-related neuropathology. To our knowledge, there has been no clinical study to date that has systematically examined the associations between social relationships and BDNF in humans as well as how these associations might influence the risk for stroke and dementia. We analyzed a sample from the Framingham Heart Study (FHS)—one of the longest running and most closely monitored community-based cohort studies in the United States—to investigate the association between social relationships, serum BDNF, and risk for stroke and dementia.
2. Methods
2.1. Standard protocol approvals, registrations, and patient consents
Written informed consent was obtained from all participants. The Institutional Review Board of Boston University Medical Center approved the consent form and original study design.
2.2. Participants
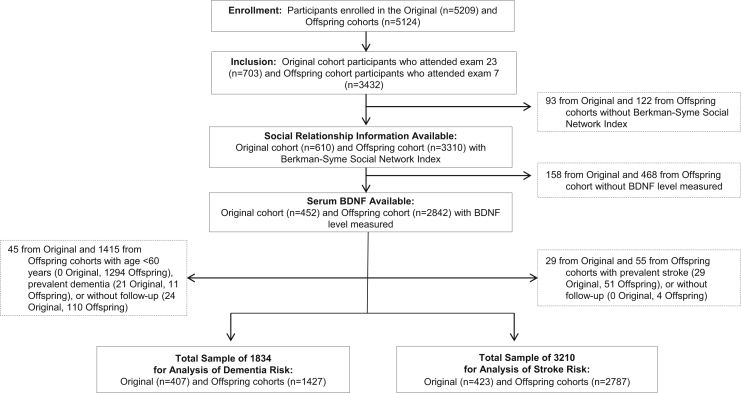
We used data from the community-based, prospective FHS Original (n = 5209, initiated 1948) and Offspring (n = 5124, initiated 1978) cohorts from the general community of Framingham, Massachusetts (Fig. 1). For additional details about the FHS, see previous publications [15]. The analytic group was derived from the 703 Original cohort participants who attended the 23rd biennial examination (1992–1996) and the 3432 who attended the seventh Offspring examination (1998–2001)—when serum samples were drawn for BDNF measurement—and were followed for a median 11 years (range up to 16 years) with minimal loss to follow-up. At examination, 3920 of 4135 (95%) had social relationships assessed and 3294 of 3920 (84%) also had serum drawn for BDNF measurement. For analysis of dementia, persons aged <60 years, those with prevalent dementia, or lack of follow-up for dementia were excluded. For analysis of stroke, those who were aged <45 years, had prevalent stroke, or did not have stroke follow-up were excluded. Thus, a total of 3294 participants were available for cross-sectional analysis of association between social relationships and BDNF, 1834 participants were available for retrospective analyses of association with dementia, and 3210 participants were available for retrospective analyses of association with stroke. On the basis of the effect sizes observed in a previous study that examined association between social relationships and biomarkers of inflammation among 1075 participants, we estimated that it would be possible to detect a small effect size (>8% change in BDNF levels at the alpha level of 0.001) given a sample of more than 3000 participants [16].
Fig. 1.
Derivation of analytic sample used in each analysis. Abbreviation: BDNF, brain-derived neurotrophic factor.
2.3. Study design
Social relationship measures were examined as cross-sectional predictors of higher serum BDNF levels as well as prospective predictors of cumulative stroke and dementia incidence with assessment for mediation effects by BDNF in identified associations. Results are reported in accordance with STROBE guidelines [17]. The primary purpose of this observational study was to investigate whether there is an association between social relationships, higher BDNF levels, and reduced likelihood of developing stroke or dementia.
2.4. Measurement of BDNF
Existing mature serum BDNF data were measured from serum samples drawn in the fasting state, aliquoted, and immediately frozen to −80°C. Samples were thawed only once at assay. Assays used enzyme-linked immunosorbent assay kits from R&D Systems. The intra-assay and interassay coefficients of variation were 4.8% and 7.6%, respectively.
2.5. Social relationships
Although social relationships are defined and operationalized variably between studies, these relationships broadly consist of structural and functional components [18]. Simultaneously studying social relationship structural measures (e.g., social network size) and functional measures (e.g., emotional, instrumental, or informational support provided by members of the network) allows for a more robust assessment of social relationships [1]. This approach is meaningful because of the wide degree of variation that can exist in the intensity, frequency, extent, and type of support provided throughout a social network, particularly when some ties may not be supportive at all [18]. The self-reported Berkman-Syme Social Network Index (SNI) measures both social network size and the degree of social support provided to the individual and predicts mortality independent of age, self-reported physical health and socioeconomic status, lifestyle (i.e., smoking, alcohol, obesity, and physical activity), and use of preventive health services [1], [19]. With regard to social networks, the SNI battery measures four types of social connections that reflect differences in the type and extent of social contact: marriage (no = 0; yes = 1); sociability with close friends and relatives (for close friends and relatives participants separately select “None,” “1 or 2,” “3–5,” “6–9,” or “10 or more”; scored as ≤2 friends and ≤2 relatives = 0 and all other scores = 1); participation in religious meetings or services (less than or equal to every few months = 0; greater than or equal to once or twice a month = 1); or group participation in other community organizations (no = 0; yes = 1). The SNI measure of social connections can be used to assess for social isolation and level of social connectedness. Consistent with extant studies of social relationships using the SNI, showing both positive and null relationships between exposure groups of interest [19], [20], [21], the level of social connectedness was based on summed score cutoffs (0 or 1 = least socially connected; 2, 3, or 4 corresponds to increasing levels of connectedness) and social isolation was a dichotomous variable (no = 0; yes = 1) defined as individuals with very few intimate contacts (where marriage = 0, <6 friends or relatives total based on specific responses to sociability with close friends and relatives, and membership in church or community groups = 0).
With regard to function of social relationships, the following additional items in the SNI battery measure how often dimensions of social support are provided (modeled dichotomously as “most or all of the time” versus “none, a little, or some of the time”): listening (Can you count on anyone to listen to you when you need to talk?); advice (Is there someone available to give you good advice about a problem?); affection (Is there someone available to you who shows you love and affection?); and emotional support (Can you count on anyone to provide you with emotional support?). Given the study's primary aim to examine associations between social support constructs, BDNF, and risk of stroke and dementia, we investigated individual dimensions of social support over a composite social support score [18]. Psychometrics on the SNI and additional evidence for the scale's predictive validity are available in previous publications [19], [20].
2.6. Ascertainment of stroke and dementia
Full details regarding the FHS stroke surveillance protocol, including diagnosis, classification, and assessment of severity have been published previously [22]. As previously outlined in the screening and surveillance methods for the development of incident all-cause dementia, all FHS participants are under continuous surveillance for impairment in cognitive function [23]. Dementia was diagnosed according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) [24]. Median duration of follow-up was 11 years for both stroke and dementia cases.
2.7. Covariates
Information regarding covariates was collected during the visit at which BDNF was measured. Because factors such as age, sex, education, physical activity, and smoking status may determine social relationships and also influence BDNF levels, dementia risk, and stroke risk, we parsimoniously adjusted for a priori variables [4], [25], [26]. Covariates included components of the Framingham Stroke Risk Profile [27] that have been previously described, including age in years, systolic blood pressure, current smoking status, and antihypertensive treatment (both categorized as yes/no), and the presence or absence of diabetes, atrial fibrillation, and cardiovascular disease [28]. Depressive symptoms were assessed using the Center for Epidemiologic Studies Depression Scale with established cutoff score ≥16 used to indicate high depressive symptomatology [29], [30]. Educational achievement was assessed using a three-level variable (no high-school diploma, high-school diploma only, or at least a college degree). The Physical Activity Index was based on information collected from a structured questionnaire at examination 7 (Offspring cohort only) and calculated as a composite score [31]. Apolipoprotein E (APOE) genotype was determined by isoelectric focusing of the plasma and confirmed by DNA genotype [32].
2.8. Statistical analysis
We used multivariable logistic regression models to examine cross-sectional associations of social relationship measures with circulating BDNF levels. On the basis of the previous FHS findings suggesting nonlinear functional form of BDNF effects in reducing dementia risk and acknowledging the possibility of detecting potential dose-response as part of an exploratory study, circulating BDNF was modeled as a dichotomous variable (above versus below median) [5]. To examine the association of social relationship measures with incident dementia and stroke, we used Cox (proportional hazards) models. Given previous association of smoking with increased risk of stroke and increased serum BDNF levels [26], we tested for interaction effects of social relationship measures with smoking status. We used interaction terms in individual Cox regression models and stratified by smoking status if the interaction term was significant (P < .10).
All primary analyses were first adjusted for age and sex. We then additionally adjusted for baseline BDNF levels (model B) to see if this partially attenuated and thus contributed the association observed. In secondary models (model C), we additionally adjusted for educational achievement, Center for Epidemiologic Studies Depression Scale, smoking status, and either for APOE ε4 genotype in the dementia analysis or for the following stroke-predominant risk factors in the stroke analysis: systolic blood pressure, atrial fibrillation, diabetes mellitus, current antihypertensive treatment, and prevalent cardiovascular disease. The covariates adjusted for in model C were available for all participants. Model D for dementia and stroke was additionally adjusted for Physical Activity Index, which was available only for the Offspring cohort.
In mediation analysis, we derived estimators of direct and indirect effects with binary outcome of new-onset stroke and dementia, in separate models for each, using the Cox proportional hazard model adjusted for age and sex, and the continuous mediator (BDNF) was modeled using linear regression [33]. To address the possibility of reverse causality from mild cognitive impairment affecting social relationships before diagnosis of dementia, sensitivity analysis was performed by excluding participants with prevalent mild cognitive impairment [34]. Statistical significance for regressions was set at a two-sided α ≤ 0.05 without adjusting for multiple comparisons given the study's aim for hypothesis generation in which results would need to be validated in additional studies. All analyses were conducted using SAS version 9.4 (SAS Institute, Inc, Cary, NC).
3. Results
In our analytic sample of 3294 participants, 116 (4%) were socially isolated. The mean (standard deviation) serum BDNF of the analytic sample was 23,754 (8279) pg/mL, the mean (standard deviation) age was 65 (11) years, and 1849 (56%) were women. Baseline characteristics are presented in Table 1. Controlling for age and sex, social isolation trended with lower serum BDNF (for BDNF above the median: odds ratio, 0.69; 95% confidence interval [CI], 0.47–1.00; P = .052) (Table 2). Social connectedness (either as a multilevel or continuous variable), however, was unassociated with serum BDNF. With regard to dimensions of social support, having someone provide emotional support most or all of the time was the only dimension associated with higher BDNF (for BDNF above the median: odds ratio, 1.27; 95% CI, 1.04–1.54; P = .021).
Table 1.
Baseline characteristics
| Variable | Total sample | Dementia sample | Stroke sample |
|---|---|---|---|
| Number of participants | 3294 | 1834 | 3210 |
| Age, y, mean (SD) | 65 (11) | 72 (8) | 65 (11) |
| Women, n (%) | 1849 (56) | 809 (44) | 1808 (56) |
| Education, n (%) | |||
| No high-school diploma | 239 (7) | 189 (11) | 223 (7) |
| High-school diploma only | 1008 (32) | 637 (36) | 982 (32) |
| Some college | 902 (28) | 484 (27) | 881 (28) |
| College graduate | 1050 (33) | 482 (27) | 1031 (33) |
| CES-D, median (IQR) | 3 (1–7) | 3 (1–6) | 3 (1–7) |
| CES-D ≥16, n (%) | 263 (8) | 134 (7) | 257 (8) |
| BMI, mean (SD), kg/m2 | 28 (5) | 28 (5) | 28 (5) |
| Current smoker, n (%) | 360 (11) | 134 (7) | 353 (11) |
| Physical Activity Index, mean (SD) | 38 (6) | 38 (6) | 38 (6) |
| Prevalent comorbidity, n (%) | |||
| Cardiovascular disease∗ | 569 (17) | 446 (24) | 489 (15) |
| Stroke | 80 (2) | 61 (3) | 3210 (100) |
| Atrial fibrillation | 193 (6) | 153 (8) | 177 (6) |
| Diabetes mellitus | 408 (13) | 275 (17) | 384 (13) |
| Stage 1 or higher JNC-VII hypertension | 1671 (51) | 1143 (62) | 1602 (50) |
| Systolic blood pressure, mean (SD), mm Hg | 129 (20) | 134 (20) | 129 (20) |
| Total cholesterol, mean (SD), mg/dL | 199 (37) | 197 (37) | 199 (37) |
| BDNF by Social Network Index,† median (IQR), pg/mL | |||
| Low | 24,112 (18,926–29,314) | 22,708 (17,342–28,067) | 24,112 (19,045–29,314) |
| Medium | 23,237 (17,807–28,944) | 22,612 (17,579–28,154) | 23,623 (17,887–28,969) |
| Medium-high | 23,804 (18,267–29,287) | 22,961 (17,996–29,122) | 23,789 (18,266–29,286) |
| High | 22,759 (17,647–28,236) | 22,801 (17,767–28,530) | 22,759 (17,675–28,287) |
Abbreviations: BDNF, brain-derived neurotrophic factor; BMI, body mass index; CES-D, Center for Epidemiologic Studies Depression Scale; JNC, Joint National Committee; IQR, interquartile range; SD, standard deviation.
Includes coronary heart disease, congestive heart failure, peripheral vascular disease, ischemic cardiomyopathy, stroke, and transient ischemic attack.
Social Network Index score summed to categorize individuals into four levels of social connection based on summed score cutoffs: 0 or 1 being the low socially connected category and 2 (medium), 3 (medium-high), or 4 (high) corresponded to categories of increasing social connectedness.
Table 2.
Association between social relationships and serum BDNF levels (N = 3294)
| Independent variable∗ | Above versus below median BDNF |
||
|---|---|---|---|
| No. (%) | Odds ratio (95% CI) | P value | |
| Social isolation† | 116 (4) | 0.69 (0.47–1.00) | .05 |
| Support items‡ | |||
| Listening | 2817 (86) | 1.09 (0.90–1.33) | .38 |
| Advice | 2607 (80) | 1.05 (0.89–1.25) | .56 |
| Affection | 2854 (87) | 1.18 (0.96–1.46) | .12 |
| Emotional | 2804 (86) | 1.27 (1.04–1.54) | .02 |
Abbreviations: BDNF, brain-derived neurotrophic factor; CI, confidence interval.
Adjusted for age and sex.
Not married, fewer than six friends or relatives, and no membership in either church or community groups.
Separate models for each dimension of social support based on frequency provided (i.e., most or all of the time versus some, little, or none of the time): Listening, “Can you count on anyone to listen to you when you need to talk?”; Advice, “Is there someone available to give you good advice about a problem?”; Affection, “Is there someone available to you who shows you love and affection?”; and Emotional, “Can you count on anyone to provide you with emotional support?”
During a median 11 years of follow-up, 243 of 1834 (13%) participants developed incident dementia and 183 of 3210 (6%) had incident stroke. Social isolation and social connectedness were unassociated with incident stroke or dementia (Table 3). Adjusting for age and sex, participants who had someone available to listen to them most or all of the time had a reduced risk for stroke (hazard ratio [HR], 0.59; 95% CI, 0.41–0.83; P = .003) and dementia (HR, 0.67; 95% CI, 0.49–0.92; P = .012). This reduced risk persisted after additional adjustment for potential confounders.
Table 3.
Association between social relationships and risk for incident dementia and stroke
| Independent variable∗ | Dementia† 243/1834 |
Stroke‡ 183/3210 |
||
|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | |
| Model A§ | ||||
| Social isolation¶ | 1.12 (0.59–2.13) | .73 | 1.24 (0.58–2.64) | .58 |
| Listening | 0.67 (0.49–0.92) | .01 | 0.59 (0.41–0.83) | .003 |
| Advice | 0.87 (0.65–1.15) | .32 | 0.80 (0.57–1.12) | .19 |
| Affection | 0.89 (0.62–1.26) | .50 | 0.79 (0.53–1.19) | .26 |
| Emotional | 0.69 (0.51–0.94) | .02 | 0.73 (0.50–1.06) | .10 |
| Model B# | ||||
| Social isolation | 1.13 (0.60–2.15) | .71 | 1.24 (0.58–2.64) | .58 |
| Listening | 0.68 (0.50–0.92) | .01 | 0.59 (0.41–0.83) | .003 |
| Advice | 0.87 (0.65–1.15) | .33 | 0.80 (0.58–1.12) | .20 |
| Affection | 0.88 (0.62–1.26) | .49 | 0.79 (0.53–1.20) | .27 |
| Emotional | 0.69 (0.51–0.94) | .02 | 0.73 (0.51–1.07) | .11 |
| Model C∗∗ | ||||
| Social isolation | 0.87 (0.45–1.68) | .68 | 1.15 (0.42–3.19) | .79 |
| Listening | 0.72 (0.52–0.99) | .05 | 0.55 (0.35–0.87) | .01 |
| Advice | 0.98 (0.73–1.32) | .88 | 0.80 (0.52–1.24) | .32 |
| Affection | 0.93 (0.65–1.34) | .70 | 0.73 (0.43–1.23) | .23 |
| Emotional | 0.78 (0.56–1.09) | .14 | 0.79 (0.48–1.29) | .35 |
| Model D†† | ||||
| Social isolation | 1.54 (0.37–6.40) | .56 | 1.17 (0.42–3.23) | .77 |
| Listening | 0.59 (0.35–0.99) | .05 | 0.55 (0.35–0.87) | .01 |
| Advice | 1.00 (0.62–1.62) | .99 | 0.80 (0.51–1.24) | .31 |
| Affection | 1.18 (0.62–2.25) | .61 | 0.73 (0.43–1.23) | .24 |
| Emotional | 0.85 (0.48–1.50) | .58 | 0.79 (0.48–1.30) | .35 |
Abbreviation: CI, confidence interval.
Participants aged 45 years and older.
Separate models for social isolation and for each dimension of social support based on frequency provided (i.e., most or all of the time versus some, little, or none of the time): Listening, Can you count on anyone to listen to you when you need to talk?; Advice, Is there someone available to give you good advice about a problem?; Affection, Is there someone available to you who shows you love and affection?; and Emotional, Can you count on anyone to provide you with emotional support?
Participants aged 45 years and older.
Model A was adjusted for age and sex.
Not married, fewer than six friends or relatives, and no membership in either church or community groups.
Model B was additionally adjusted for serum brain-derived neurotrophic factor level.
Model C for dementia was additionally adjusted for apoliproprotein ε4 genotype and educational achievement, Center for Epidemiologic Studies Depression Scale, and current smoking status. Model C for stroke was additionally adjusted for systolic blood pressure, atrial fibrillation, diabetes mellitus, current antihypertensive treatment, and prevalent cardiovascular disease as well as educational achievement, Center for Epidemiologic Studies Depression Scale, and current smoking status.
Model D, for both dementia (106 of 1324 cases) and stroke (114 of 2604 cases), was additionally adjusted for Physical Activity Index, which was available only for the Offspring cohort.
Having someone provide emotional support most or all of the time was associated with reduced risk of dementia (HR, 0.69; 95% CI, 0.51–0.94; P = .018) (Table 3). The association with dementia persisted unchanged after adjusting for the serum BDNF level, thus suggesting serum BDNF is likely not a significant mediator of this relationship. Although, in causal mediation analysis, the size of the indirect effect suggests that about 1.6% of the total effect (standardized β = 0.69; 95% CI, 0.51–0.94; P = .017) between emotional support and dementia risk is mediated through an increase in serum BDNF.
For interactions between social relationships and smoking status on stroke risk, we found significant associations between decreased risk of stroke and having someone available to give advice (HR, 0.39; 95% CI, 0.16–0.95; P = .037), listen (HR, 0.21; 95% CI, 0.09–0.53; P < .001), and provide emotional support (HR, 0.23; 95% CI, 0.10–0.57; P < .001) (Supplementary Table 1). These stratum-specific associations remained unchanged after additional adjustment. Sensitivity analysis performed by excluding participants with mild cognitive impairment revealed minimal change in β estimates for Cox proportional hazards models.
4. Discussion
In our community-based cohort, we explored the associations between social relationships and risk of stroke and dementia. A key finding was that social isolation trended with lower serum BDNF after controlling for age and sex; meanwhile, having someone available to provide emotional support most or all of the time was associated with higher BDNF. The association with BDNF was not observed with other social support items. A second key finding was that, among individuals free of dementia at baseline, social isolation was not associated with incident dementia in follow-up yet having someone available to listen and to provide emotional support most or all of the time each independently reduced dementia risk. In addition, stroke-free participants who reported having someone available to listen to them had a lower risk of subsequent stroke when compared with others. The associations between certain elements of social support and risk of stroke were greater in smokers.
Strengths of this exploratory study include its community-based design, large sample of stroke-free and dementia-free participants, and continuous, rigorous surveillance for clinical end points with minimal loss to follow-up. Although the possibility of reverse causality cannot be eliminated in observational studies, a community-based prospective cohort is ideal to identify novel pathways that might be involved in brain health because studies of prospectively collected data are better able to distinguish the temporal direction of any observed association to help separate probable cause and effect. For example, given that cognitive impairment can affect social relationships, social determinants would need to be ascertained years before the onset of age-related neurologic disease. Results, however, must be interpreted in the context of observational study limitations. Although adjustment was possible for multiple potential confounders, other unknown factors may have biased results. Moreover, the effect size of associations with BDNF in the brain may have been more pronounced if plasma BDNF, pro-BDNF, or postmortem BDNF levels had been used. Hence, our findings should be considered hypothesis generating and replicated in other cohorts. With regard to generalizability, our sample in some of the stratified categories was small and largely of European ancestry so our results also need replication in other race and ethnic groups.
The principal findings contribute to existing literature in three major areas. First, to our knowledge, this is the first study to investigate the relationship between social isolation and serum BDNF levels in humans; our findings are consistent with similar results in animal models [12]. This is also the first study to associate higher serum BDNF with a specific type of social support, emotional support. Second, results for the association between social support measures and the risk for stroke and dementia have not been previously reported in the Framingham Study. Our findings build on previous studies demonstrating an association between social relationships and reduced risk of dementia by identifying two specific forms of social support associated with lower dementia risk: having someone available to listen and having someone available to provide emotional support. One previous study of 1189 participants aged 70 to 79 years found an association between emotional support (although not social ties) and better cognitive function assessed by a neuropsychological battery [35]. Our findings not only support this study but also suggest a potential relationship with BDNF given that emotional support, in particular, was associated with higher serum BDNF levels. The association of serum BDNF with social isolation as a measure of extreme lack of social connectivity and the small mediation effect of BDNF on the association between emotional support and dementia risk may be a result of using serum BDNF rather than BDNF levels. Although serum BDNF parallels relative difference in BDNF levels, BDNF levels may be substantially higher, have attenuated effects over time, and may be subject to variability of effect because of differences in the expression levels of BDNF receptors [8]. Moreover, our findings suggest an additional association specifically between having someone available to listen and reduced risk of dementia. Although we did not find an association between social isolation and risk of incident dementia, this may be because of the social isolation measure consisting of a smaller segment of our total sample and its construct using deficiency across several types of intimate contacts (i.e., marriages, friends, relatives, and community groups) given that previous studies have found that specific types of social isolation were associated with increased dementia risk [36], [37]. Although we did not find an association between social isolation and increased stroke risk, specific aspects of social support reduced stroke risk. The stronger impact of social support in smokers may indicate an underlying mechanism in determining stroke risk that involves BDNF, such as greater sensitivity to BDNF effects on neuronal repair or synaptogenesis potentially induced by both smoking and the absence of social isolation in the setting of chronic neurovascular endothelial injury [3], [26].
Our findings might extend observed effects of BDNF and lifestyle factors on human studies [38] as well as on animal models associating BDNF with the broader construct of social enrichment [12]. Emotional support and, in particular, having someone available to listen are forms of social support that may independently help reduce the risk of stroke and dementia. Animal and human studies suggest that different aspects of social support may have variable effects because of differences in how different forms of social support influence other mediating pathways, such as cognitive stimulation [12], systemic inflammation [20], or lifestyle habits [39], [40]. Clinically, these findings suggest that the relatively simple intervention of developing and implementing strategies that provide greater social support (i.e., increasing availability of persons who can provide companionship and emotional support to older adults at risk of stroke and dementia) may help reduce risks. Although the availability of emotional support was associated with both increased serum BDNF levels and decreased risk of dementia and stroke, BDNF levels only explained a small portion of the observed association with disease. Although social interaction and relationships may reduce the risk of stroke and dementia through several candidate behavioral, psychological, and physiological mechanisms, our exploratory findings help to begin disentangling the relationship for observed associations between social isolation and increased risk of age-related neurologic disease by supporting a role for specific types of social support and potential mediating effects through neuronal repair or synaptogenesis tied to the expression of BDNF. For example, individuals in small social networks who have limited social support may be more likely to participate in adverse health behaviors, such as cigarette smoking, poor diet, physical inactivity, and substance abuse [41]. There are also associations between social isolation and both psychological and physiological stress (e.g., dysfunction of platelets and endothelium, increased markers of chronic inflammation, and increased activity of the neuroendocrine system) [16], [42]. Hence, further study of the biological and social mechanisms through which support from social networks may protect against age-related neurologic disease is needed.
Admittedly, it is not possible to clearly establish a causal relationship between social relationships and risk of stroke and dementia from observational studies alone [43]. Our results and the findings of previous studies may reflect residual confounding from the inability to accurately measure and adequately adjust for factors such as diet, health service use, longstanding psychiatric symptoms, and additional social and environmental exposures that are challenging to capture, may have differed between comparison groups, and could affect the risk of incident stroke or dementia. Our results may also reflect some residual reverse causality bias from homophily or propinquity that are challenging to account for in observational studies whereby lifestyle choices, health-related behaviors, or geography may contribute to determining social relationships [1]. These complex relationships need to be explored further with techniques such as Mendelian randomization and in interventional trials.
Research in Context.
-
1.
Systematic review: The authors reviewed the literature using traditional sources (e.g., PubMed). Recent studies support associations between social integration and reduced risk of stroke and dementia, and these are appropriately cited. This is the first study building on animal models to investigate the association between social isolation and BDNF levels in humans. This is also the first study using the Framingham Study to investigate links between social relationships and risk for stroke and dementia.
-
2.
Interpretation: Our findings contribute to the literature by proposing that (1) lower BDNF is associated with social isolation; (2) individual social support subtypes are associated with lower dementia risk; and (3) BDNF may partly mediate associations between greater availability of social relationships and reduced risk of stroke and dementia.
-
3.
Future directions: Our results can inform studies aimed at disentangling how social determinants of stroke and dementia may alter neurobiology.
Acknowledgments
This work was supported by the Boston University School of Medicine and Massachusetts General Hospital; contracts from the National Heart, Lung, and Blood Institute [N01-HC 25195, HHSN268201500001I]; and by grants from the National Institute for Neurologic Disease and Stroke [NS017950, T32NS048005] and National Institute on Aging [AG031287-funded BDNF assays, AG008122, AG033193].
We are most grateful to the Framingham Study participants who have committed so much of their time and effort.
Conflicts of Interest: The authors have no conflicts of interest to disclose.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.trci.2017.03.001.
Supplementary data
References
- 1.Berkman L.F., Glass T., Brissette I., Seeman T.E. From social integration to health: Durkheim in the new millennium. Soc Sci Med. 2000;51:843–857. doi: 10.1016/s0277-9536(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 2.Kuiper J.S., Zuidersma M., Oude Voshaar R.C., Zuidema S.U., van den Heuvel E.R., Stolk R.P. Social relationships and risk of dementia: a systematic review and meta-analysis of longitudinal cohort studies. Ageing Res Rev. 2015;22:39–57. doi: 10.1016/j.arr.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Valtorta N.K., Kanaan M., Gilbody S., Ronzi S., Hanratty B. Loneliness and social isolation as risk factors for coronary heart disease and stroke: systematic review and meta-analysis of longitudinal observational studies. Heart. 2016;102:1009–1016. doi: 10.1136/heartjnl-2015-308790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pikula A., Beiser A.S., Chen T.C., Preis S.R., Vorgias D., DeCarli C. Serum brain-derived neurotrophic factor and vascular endothelial growth factor levels are associated with risk of stroke and vascular brain injury: Framingham Study. Stroke. 2013;44:2768–2775. doi: 10.1161/STROKEAHA.113.001447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weinstein G., Beiser A.S., Choi S.H., Preis S.R., Chen T.C., Vorgas D. Serum brain-derived neurotrophic factor and the risk for dementia: the Framingham Heart Study. JAMA Neurol. 2014;71:55–61. doi: 10.1001/jamaneurol.2013.4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buchman A.S., Yu L., Boyle P.A., Schneider J.A., De Jager P.L., Bennett D.A. Higher brain BDNF gene expression is associated with slower cognitive decline in older adults. Neurology. 2016;86:735–741. doi: 10.1212/WNL.0000000000002387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bennett D.A., Schneider J.A., Tang Y., Arnold S.E., Wilson R.S. The effect of social networks on the relation between Alzheimer's disease pathology and level of cognitive function in old people: a longitudinal cohort study. Lancet Neurol. 2006;5:406–412. doi: 10.1016/S1474-4422(06)70417-3. [DOI] [PubMed] [Google Scholar]
- 8.Kao P.F., Banigan M.G., Vanderburg C.R., McKee A.C., Polgar P.R., Seshadri S. Increased expression of TrkB and Capzb2 accompanies preserved cognitive status in early Alzheimer disease pathology. J Neuropathol Exp Neurol. 2012;71:654–664. doi: 10.1097/NEN.0b013e31825d06b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emanueli C., Schratzberger P., Kirchmair R., Madeddu P. Paracrine control of vascularization and neurogenesis by neurotrophins. Br J Pharmacol. 2003;140:614–619. doi: 10.1038/sj.bjp.0705458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee J., Seroogy K.B., Mattson M.P. Dietary restriction enhances neurotrophin expression and neurogenesis in the hippocampus of adult mice. J Neurochem. 2002;80:539–547. doi: 10.1046/j.0022-3042.2001.00747.x. [DOI] [PubMed] [Google Scholar]
- 11.Neeper S.A., Gómez-Pinilla F., Choi J., Cotman C. Exercise and brain neurotrophins. Nature. 1995;373:109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- 12.Branchi I., D'Andrea I., Fiore M., Di Fausto V., Aloe L., Alleva E. Early social enrichment shapes social behavior and nerve growth factor and brain-derived neurotrophic factor levels in the adult mouse brain. Biol Psychiatry. 2006;60:690–696. doi: 10.1016/j.biopsych.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Pan W., Banks W.A., Fasold M.B., Bluth J., Kastin A.J. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology. 1998;37:1553–1561. doi: 10.1016/s0028-3908(98)00141-5. [DOI] [PubMed] [Google Scholar]
- 14.Laske C., Stransky E., Leyhe T., Eschweiler G.W., Maetzler W., Wittorf A. BDNF serum and CSF concentrations in Alzheimer's disease, normal pressure hydrocephalus and healthy controls. J Psychiatr Res. 2007;41:387–394. doi: 10.1016/j.jpsychires.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 15.Dawber T., Meadors G., Moore F. Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health. 1951;41:279–281. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Y.C., Li T., Frenk S.M. Social network ties and inflammation in U.S. adults with cancer. Biodemography Soc Biol. 2014;60:21–37. doi: 10.1080/19485565.2014.899452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147:573–577. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 18.Valtorta N.K., Kanaan M., Gilbody S., Hanratty B. Loneliness, social isolation and social relationships: what are we measuring? A novel framework for classifying and comparing tools. BMJ Open. 2016;6:e010799. doi: 10.1136/bmjopen-2015-010799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berkman L.F., Syme S.L. Social networks, host resistance, and mortality: a nine-year follow-up study of Alameda County residents. Am J Epidemiol. 1979;109:186–204. doi: 10.1093/oxfordjournals.aje.a112674. [DOI] [PubMed] [Google Scholar]
- 20.Loucks E.B., Sullivan L.M., D'Agostino R.B., Sr., Larson M.G., Berkman L.F., Benjamin E.J. Social networks and inflammatory markers in the Framingham Heart Study. J Biosoc Sci. 2006;38:835–842. doi: 10.1017/S0021932005001203. [DOI] [PubMed] [Google Scholar]
- 21.Kawachi I., Colditz G.A., Ascherio A., Rimm E.B., Giovannucci E., Stampfer M.J. A prospective study of social networks in relation to total mortality and cardiovascular disease in men in the USA. J Epidemiol Community Health. 1996;50:245–251. doi: 10.1136/jech.50.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carandang R., Seshadri S., Beiser A., Kelly-Hayes M., Kase C.S., Kannel W.B. Trends in incidence, lifetime risk, severity, and 30-day mortality of stroke over the past 50 years. JAMA. 2006;296:2939–2946. doi: 10.1001/jama.296.24.2939. [DOI] [PubMed] [Google Scholar]
- 23.Satizabal C.L., Beiser A.S., Chouraki V., Chêne G., Dufouil C., Seshadri S. Incidence of dementia over three decades in the Framingham Heart Study. N Engl J Med. 2016;374:523–532. doi: 10.1056/NEJMoa1504327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.American Psychiatric Association, American Psychiatric Association. Task Force on DSM-IV . 4th ed. American Psychiatric Association; Washington, DC: 1994. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. [Google Scholar]
- 25.Andel R., Vigen C., Mack W.J., Clark L.J., Gatz M. The effect of education and occupational complexity on rate of cognitive decline in Alzheimer's patients. J Int Neuropsychol Soc. 2006;12:147–152. doi: 10.1017/S1355617706060206. [DOI] [PubMed] [Google Scholar]
- 26.Bus B.A., Molendijk M.L., Penninx B.J., Buitelaar J.K., Kenis G., Prickaerts J. Determinants of serum brain-derived neurotrophic factor. Psychoneuroendocrinology. 2011;36:228–239. doi: 10.1016/j.psyneuen.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 27.Wolf P.A., D'Agostino R.B., Belanger A.J., Kannel W.B. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22:312–318. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- 28.Cupples L., D'Agostino R. National Heart, Lung, and Blood Institute; Bethesda, MD: 1987. Some Risk Factors Related to the Annual Incidence of Cardiovascular Disease and Death Using Pooled Repeated Biennial Measurements: Framingham Heart Study, 30-year Follow-up. [Google Scholar]
- 29.Radloff L.S. The CES-D scale a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 30.Meader N., Moe-Byrne T., Llewellyn A., Mitchell A.J. Screening for poststroke major depression: a meta-analysis of diagnostic validity studies. J Neurol Neurosurg Psychiatry. 2014;85:198–206. doi: 10.1136/jnnp-2012-304194. [DOI] [PubMed] [Google Scholar]
- 31.Kannel W.B., Sorlie P. Some health benefits of physical activity. The Framingham Study. Arch Intern Med. 1979;139:857–861. [PubMed] [Google Scholar]
- 32.Welty F.K., Lahoz C., Tucker K.L., Ordovas J.M., Wilson P.W., Schaefer E.J. Frequency of APOB and APOE gene mutations as causes of hypobetalipoproteinemia in the Framingham Offspring population. Arterioscler Thromb Vasc Biol. 1998;18:1745–1751. doi: 10.1161/01.atv.18.11.1745. [DOI] [PubMed] [Google Scholar]
- 33.Vansteelandt S., Bekaert M., Lange T. Imputation strategies for the estimation of natural direct and indirect effects. Epidemiol Method. 2012;1:131–158. doi: 10.1093/aje/kwr525. [DOI] [PubMed] [Google Scholar]
- 34.Jefferson A.L., Beiser A.S., Himali J.J., Seshadri S., O'Donnell C.J., Manning W.J. Low cardiac index is associated with incident dementia and Alzheimer disease: the Framingham Heart Study. Circulation. 2015;131:1333–1339. doi: 10.1161/CIRCULATIONAHA.114.012438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seeman T.E., Lusignolo T.M., Albert M., Berkman L. Social relationships, social support, and patterns of cognitive aging in healthy, high-functioning older adults: MacArthur studies of successful aging. Health Psychol. 2001;20:243–255. doi: 10.1037//0278-6133.20.4.243. [DOI] [PubMed] [Google Scholar]
- 36.Bickel H., Cooper B. Incidence and relative risk of dementia in an urban elderly population: findings of a prospective field study. Psychol Med. 1994;24:179–192. doi: 10.1017/s0033291700026945. [DOI] [PubMed] [Google Scholar]
- 37.Fratiglioni L., Wang H.X., Ericsson K., Maytan M., Winblad B. Influence of social network on occurrence of dementia: a community-based longitudinal study. Lancet. 2000;355:1315–1319. doi: 10.1016/S0140-6736(00)02113-9. [DOI] [PubMed] [Google Scholar]
- 38.Weinstein G., Preis S.R., Beiser A.S., Kaess B., Chen T.C., Satizabal C. Clinical and environmental correlates of serum BDNF: a descriptive study with plausible implications for AD research. Curr Alzheimer Res. 2017 doi: 10.2174/1567205014666170203094520. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Christakis N.A., Fowler J.H. The spread of obesity in a large social network over 32 years. N Engl J Med. 2007;357:370–379. doi: 10.1056/NEJMsa066082. [DOI] [PubMed] [Google Scholar]
- 40.Christakis N.A., Fowler J.H. The collective dynamics of smoking in a large social network. N Engl J Med. 2008;358:2249–2258. doi: 10.1056/NEJMsa0706154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watt R.G., Heilmann A., Sabbah W., Newton T., Chandola T., Aida J. Social relationships and health related behaviors among older US adults. BMC Public Health. 2014;14:533. doi: 10.1186/1471-2458-14-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Non A.L., Rimm E.B., Kawachi I., Rewak M.A., Kubzansky L.D. The effects of stress at work and at home on inflammation and endothelial dysfunction. PLoS One. 2014;9:e94474. doi: 10.1371/journal.pone.0094474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hill A.B. The environment and disease: association or causation? Proc R Soc Med. 1965;58:295–300. doi: 10.1177/003591576505800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.