Abstract
Introduction
The safety, pharmacokinetics, and effect on peripheral and central amyloid β (Aβ) of multiple doses of ponezumab, an anti-Aβ monoclonal antibody, were characterized in subjects with mild-to-moderate Alzheimer's disease treated for 1 year.
Methods
Subjects were aged ≥50 years with Mini–Mental State Examination scores 16 to 26. Cohort Q was randomized to ponezumab 10 mg/kg (n = 12) or placebo (n = 6) quarterly. Cohort M was randomized to a loading dose of ponezumab 10 mg/kg or placebo, followed by monthly ponezumab 7.5 mg/kg (n = 12) or placebo (n = 6), respectively.
Results
Ponezumab was generally well tolerated. Plasma concentrations increased dose dependently, but cerebrospinal fluid (CSF) penetration was low. Plasma Aβ increased dose dependently with ponezumab, but CSF biomarkers, brain amyloid burden, cognition, and function were not affected.
Conclusions
Both ponezumab dosing schedules were generally safe and well tolerated but did not alter CSF biomarkers, brain amyloid burden, or clinical outcomes.
Keywords: Alzheimer's disease, Antibody, Amyloid, Pharmacokinetics, Pharmacodynamics, Ponezumab, PIB, Safety, Tolerability, Anti-drug antibodies
1. Introduction
The amyloid hypothesis of Alzheimer's disease (AD) posits that the accumulation of amyloid β (Aβ) peptide in the brain is the critical pathogenic step [1]. Studies in transgenic murine models of amyloid overexpression have shown reduced cognitive deficits and reversal of some aspects of histopathology with agents that remove Aβ or alter its transport [2], [3]. Monoclonal antibodies that target Aβ include bapineuzumab, solanezumab, and aducanumab [4], [5], [6]. In patients with mild-to-moderate AD, bapineuzumab did not improve clinical outcomes [4] and solanezumab did not significantly improve cognitive or functional ability [5]. However, secondary analyses suggested that in patients with mild AD, solanezumab may have slowed cognitive worsening [7], and a delayed start design in an extension arm indicated a potential modifying effect on underlying disease progression [8]. Aducanumab has been shown to significantly reduce amyloid plaque and slow cognitive decline in patients with prodromal or mild AD [6].
Ponezumab is a humanized IgG2Δa anti-Aβ monoclonal antibody designed for the treatment of AD. It is unique in that (1) it is directed against the C-terminus of the Aβ1–40 peptide derived from the amyloid precursor protein and (2) it has two amino acid substitutions in the Fc region that minimize its ability to activate complement or to support antibody-dependent cell-mediated cytotoxicity. Ponezumab is believed to sequester Aβ in the blood, thereby depleting central Aβ stores (the peripheral sink hypothesis) [2]. Murine analogs of ponezumab reduce brain amyloid burden, as soluble Aβ or in plaques, and reverse cognitive deficits in transgenic murine models [9], [10]. Ponezumab, like many first-generation anti-amyloid monoclonal antibodies, targets soluble Aβ monomers, which is now emerging as a part of the solution, but additional therapies are needed.
Pittsburgh compound B (PIB) is an amyloid imaging ligand with demonstrated specificity and sensitivity to detect central brain amyloid burden in AD [11], [12], [13], as well as utility in following intervention with anti-amyloid therapeutics [14].
To date, ponezumab has been administered as both single and multiple intravenous doses over a 100-fold dose range (0.1–10.0 mg/kg) for durations as long as 18 months [15], [16], [17], [18]. Throughout these clinical studies, ponezumab was generally safe and well tolerated, although the pharmacodynamic (PD) response associated with various dosing intervals has not been established. The current clinical study used both biochemical and neuroimaging methods to evaluate the effect of two dosage schedules (monthly and quarterly) of ponezumab on peripheral and central amyloid burden in subjects with mild-to-moderate AD. The purpose of this study was to inform dose interval for subsequent trials given the adverse events (AEs) of particularly of amyloid-related imaging abnormalities (ARIA) observed with other anti-amyloid approaches. This study was conducted in parallel with a larger multiple-dose study designed and powered to inform efficacy end points.
2. Methods
2.1. Subjects
Subjects were men and women aged ≥50 years with a Mini–Mental State Examination (MMSE) [19] score of 16 to 26 and otherwise healthy. The diagnosis of probable AD was consistent with criteria from the Nation Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association and the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition - Text Revision. Subjects had a Rosen-Modified Hachinski Ischemia Score ≤4 to minimize the potential confounding effect of vascular dementia.
A subset of subjects who received monthly dosing (cohort M) underwent amyloid positron emission tomography (PET) and had to have a brain amyloid burden at screening with radiotracer retention in the range expected for AD subjects. This was defined as three of the five target regions (anterior cingulate, posterior cingulate, frontal cortex, temporal cortex, and parietal cortex) having a ratio of target region radioactivity (kBq/mL) over reference region radioactivity (cerebellar gray matter) > 1.5.
Subjects were ineligible if they had a personal or family history of presenilin mutations or other contributors to dementia; diagnosis or history of cerebrovascular disease, severe carotid stenosis, cerebral hemorrhage, intracranial tumor, subarachnoid hemorrhage, or subdural hematoma. Subjects were excluded if brain magnetic resonance imaging (MRI) showed cortical infarct, >2 microhemorrhages (ARIA-H), strategically located subcortical gray matter infarct (e.g., hippocampus, thalamus, and caudate head), or multiple (≥2) white matter lacunes. Subjects were on a stable dose of background acetylcholinesterase inhibitor and/or memantine.
2.2. Study design
This was a Phase II, double-blind, randomized, placebo-controlled study conducted at three centers in Sweden. Subjects were enrolled in cohort Q (quarterly) or cohort M (monthly). Cohort Q was fully enrolled before enrollment in Cohort M. A total of 18 subjects/cohorts were randomized in a 2:1 ratio to receive ponezumab or placebo. Subjects in cohort Q received ponezumab 10 mg/kg or placebo quarterly (every 3 months). The sample size was based on feasibility. Cohort M received an initial single loading dose of ponezumab 10 mg/kg or placebo, followed by monthly doses of ponezumab 7.5 mg/kg or placebo, respectively. Study treatment was administered by 10-minute intravenous infusion. Subjects received study treatment for 1 year and were followed for 6 months. The study was conducted in International Council for Harmonization and good clinical practice compliance and reviewed by an external Data Safety Monitoring Board.
2.3. Outcome measures
The primary study objectives were safety, tolerability, pharmacokinetic (PK), and PD (effect on brain amyloid burden and cerebrospinal fluid [CSF] Aβ concentrations) of ponezumab. The secondary objectives were to assess the effect of ponezumab on cognitive and functional status and on plasma Aβ concentrations.
2.3.1. Brain amyloid burden
The percent change from baseline to month 13 in brain amyloid burden was assessed in cohort M by PET. PET data and matched MRI scans were transferred to GE Healthcare Limited for centralized data processing. Brain amyloid burden was assessed according to the PET imaging standard uptake value ratio (SUVR) at the screening visit (baseline) and month 13.
2.3.2. Cognitive and functional status
Cognitive function was assessed using the 70-point Alzheimer's Disease Assessment Scale–Cognitive Subscale (ADAS-Cog) [20] at baseline and months 3, 6, 9, 13, and 18. MMSE was performed at screening, baseline, and at month 13. Functional status was measured using the 100-point Disability Assessment for Dementia (DAD) scale [21] at baseline and months 6, 13, and 18.
2.3.3. Pharmacokinetic and PD evaluations
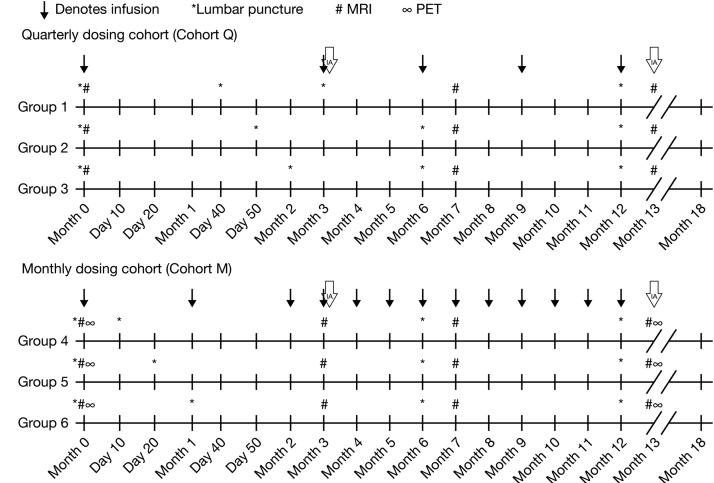
Blood, CSF, and urine samples were collected at prespecified times for analysis of ponezumab PK, PD, and anti-drug antibodies (Fig. 1) up to day 360. Blood samples were collected prior infusion and 5 minutes after completion of infusion.
Fig. 1.
Study schematic. Blood samples were collected for all subjects in cohort Q at months 0, 3, 6, 9, 12, 13, and 18. Additional blood samples were collected on days 40, 50, or 60 in subjects randomized to CSF sampling on those days. In cohort M, samples were collected at month 0 and monthly thereafter for the first year. Blood samples were also collected at months 13 and 18 (follow-up visits). Additional blood samples were collected on days 10 or 20 in subjects randomized to CSF sampling on those days. Blood samples for evaluating immunogenicity (ADAs) were collected before ponezumab infusion on dosing days and at month 18 for all subjects. For CSF sample collection, each cohort was divided into three groups of six subjects (four ponezumab, two placebo) to allow PK/PD analysis at 10 time points over the 12-month treatment period, but with only four LPs for each individual subject. Cohort Q subjects were designated as groups 1, 2, and 3, and cohort M groups were designated as groups 4, 5, and 6. All subjects in these six groups underwent predose LPs at days 0 and 360. In addition, group 1 underwent LPs at days 40 and 90, group 2 days 50 and 180, group 3 on days 60 and 180, group 4 on days 10 and 180, group 5 on days 20 and 180, and group 6 on days 30 and 180. Urine samples were collected before and after the ponezumab infusion at months 0, 3, 6, and 12. Abbreviations: CSF, cerebrospinal fluid; IA, interim analysis; LP, lumbar puncture; MRI, magnetic resonance imaging; PD, pharmacodynamic; PET, positron emission tomography.
Plasma, CSF, and urine samples were analyzed for ponezumab concentrations at Prevalere Life Sciences, LLC (Whitesboro, New York, USA) using a validated, sensitive, and specific enzyme-linked immunosorbent assay method. The lower limit of quantification (LLOQ) for ponezumab was 156.0 ng/mL in plasma, 12.0 ng/mL in CSF, and 120 ng/mL in urine.
Plasma and CSF samples were analyzed for Aβ biomarkers and total tau and p-tau (CSF only) at ICON Development Solutions (Manchester, UK). The assay for CSF Aβ1–42 was carried out on a Gyros (Gyros AB, Uppsala, Sweden) platform. The LLOQ was 210 pg/mL. The method for quantifying CSF Aβ1–40 was identical to that for Aβ1–42, with the following exceptions: it included a solid-phase extraction (SPE) step to pretreat the CSF, which resulted in the dissociation of the bound Aβ from ponezumab before analysis. The SPE also inactivated ponezumab to prevent assay interference. The LLOQ was 144 pg/mL. The CSF samples were analyzed for total tau and p-tau using the multiplex xMAP Luminex platform (Luminex Corp, Austin, TX, USA) with INNO-BIA AlzBio3 immunoassay test kits supplied by Innogenetics (Ghent, Belgium). The assay for plasma Aβ1–42 was carried out by an electrochemiluminescence (Meso Scale Discovery [MSD]) platform with LLOQ 15 pg/mL, whereas the assay for plasma Aβ1–40 was carried out on a Gyros platform with SPE. The LLOQ was 5 pg/mL.
Urine samples were analyzed for concentrations of Aβ1–x at Prevalere Life Sciences, LLC (Whitesboro, New York, USA) using a validated electrochemiluminescent assay method with an LLOQ of 300 pg/mL. Lower limits of quantitation for the biomarker assays are shown in Supplementary Table 1.
2.3.4. MRI evaluations
Brain MRI was performed at screening (baseline), and at months (cohort M only) 3, 7, and 13 to check for ARIA-H (with microhemorrhage) or ARIA-E (with vasogenic edema). The MRI consisted of clinically available sequences such as fluid-attenuated inversion recovery, diffusion-weighted imaging, T1-weighted (pregadolinium), and at the investigator's discretion, T1-weighted postgadolinium scans. A central read was used for data reporting.
2.3.5. Safety evaluations
All AEs observed or listed by patients were reported. Clinically significant changes in physical examination findings or vital signs and abnormal laboratory test results were also recorded.
2.3.6. Other evaluations
A blood sample was collected at baseline for apolipoprotein E (APOE) genotyping to determine carrier status for the ε4 allele (APOE ε4).
2.4. Statistical analysis
The target sample size of 18 subjects each for cohort Q and cohort M (12 on active treatment and 6 on placebo) was based on feasibility rather than on hypothesis testing. At this sample size, hypothesis tests for assessment of amyloid plaque burden response to monthly dosing had moderate power (approximately 80%) to detect a change of approximately 20% in PIB-imaged estimates of insoluble amyloid plaque in the brain.
2.4.1. Change in amyloid burden
Analysis of change from baseline to month 13 in amyloid burden (as assessed by the SUVR from PET imaging) was conducted using analysis of covariance after transforming the SUVRs to a log scale. The model included treatment as the main effect and log-transformed baseline SUVR as a covariate. The differences in the least square (LS) means between the ponezumab treatment group and placebo group, P values, standard errors of the differences, and corresponding 90% confidence intervals (CIs) were presented as percentage change from baseline values by back-transforming the change from baseline values on the log scale, subtracting 1, and multiplying by 100. No imputation of missing data was performed.
2.4.2. Cognitive and functional assessments
A mixed model–repeated measures approach was used to analyze data from the ADAS-Cog, MMSE, and DAD scales for the Full Analysis Set (all subjects who received at least one infusion) to compare mean change from baseline in each treatment arm. LS estimates were generated for ADAS-Cog at months 3, 6, 9, and 13, MMSE at month 13, and DAD at months 6 and 13. The structure for the variance-covariance matrix was assumed to be compound symmetry. Missing values were accounted for within the mixed model and were not explicitly imputed. The primary analysis model included terms for treatment and baseline value. Model-based LS mean estimates of the change from baseline and treatment differences of the change from baseline were calculated and presented with 90% CI. No imputation of missing data was performed.
2.4.3. Pharmacokinetic and PD assessments
All PK and PD assessments were summarized at each time point, by treatment group, using descriptive statistics and/or data plots.
3. Results
3.1. Subject disposition
Thirty-six subjects were screened and randomized at three investigative centers in a 3-month period (August to October, 2009). The final assessment was completed on June 1, 2011. Thirty-four subjects completed the study treatment per protocol. One subject in cohort M discontinued treatment due to cerebral ARIA-H, deemed to be a drug-related AE, but remained in the study and was determined after unblinding to be assigned to placebo. He had been enrolled with more baseline ARIA-H (>2) than allowed per protocol, which were noted retrospectively upon the development of post-baseline ARIA-H. The subject withdrew from the study during the post-therapy follow-up phase. One subject randomized to ponezumab in cohort M discontinued treatment due to a non–drug-related serious AE (myocardial infarction) but remained in the study.
Demographic characteristics were broadly similar among all treatment groups (Table 1). In cohort Q, 83% of ponezumab subjects and 50% of placebo subjects were APOE ε4-positive; the respective proportions in cohort M were 92% and 83%.
Table 1.
Baseline and demographic characteristics
| Demographic | Cohort Q |
Placebo (n = 6) |
Cohort M |
Placebo (n = 6) |
|---|---|---|---|---|
| 10 mg/kg (n = 12) | 10 mg/kg/7.5 mg/kg (n = 12) | |||
| Gender, n | ||||
| Male/female | 8/4 | 3/3 | 9/3 | 1/5 |
| Mean (SD) age, years | 65.1 (7.4) | 71.3 (8.5) | 69.8 (7.5) | 65.8 (8.3) |
| Race, n (%) | ||||
| White | 12 (100) | 6 (100) | 12 (100) | 6 (100) |
| Severity of AD (according to MMSE), n (%) | ||||
| Mild | 10 (83.33) | 3 (50.00) | 8 (66.67) | 4 (66.67) |
| Moderate | 2 (16.67) | 3 (50.00) | 4 (33.33) | 2 (33.33) |
| Mean (SD) screening MMSE | 22.5 (2.75) | 20.8 (2.99) | 21.2 (3.04) | 22.5 (4.04) |
| Mean (SD) baseline ADAS-Cog | 18.6 (8.71) | 23.5 (12.56) | 18.2 (6.57) | 20.1 (9.79) |
| Mean (SD) baseline DAD | 90.2 (12.58) | 85.6 (15.35) | 75.4 (17.85) | 87.3 (19.09) |
Abbreviations: AD, Alzheimer's disease; ADAS-Cog, Alzheimer's Disease Assessment Scale–Cognitive Subscale; DAD, Disability Assessment for Dementia; MMSE, Mini–Mental State Examination; SD, standard deviation.
Seventeen men and seven women received ponezumab, whereas 8 men and 12 women received placebo. Subject ages ranged from 53 to 84 years. Mean MMSE scores in the four groups ranged from 20.8 to 22.5 at screening, consistent with a diagnosis of mild-to-moderate AD. The majority of subjects had mild AD (Table 1). Mean ADAS-Cog scores at baseline ranged from 18.2 to 23.5. Mean DAD scores at baseline ranged from 75.4 to 90.2.
Median time in the study was similar among treatment groups. The median number of infusions was five for both treatment groups in cohort Q and 13 for both treatment groups in cohort M.
3.2. Efficacy
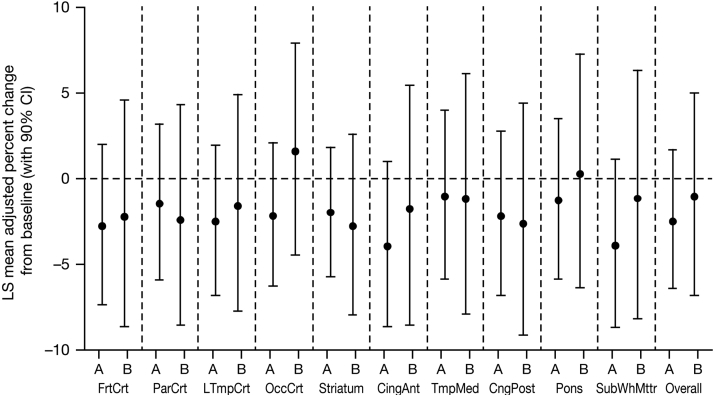
Minimal changes from baseline to month 13 were observed in brain amyloid burden in cohort M, and there were no discernible differences between treatment arms in any region or for the overall brain (Fig. 2). For the overall brain, the LS means and 90% CIs for percent change from baseline in SUVR were −2.48 (−6.47, 1.68) for ponezumab and −1.07 (−6.76, 4.97) for placebo. The between-group difference (ponezumab vs. placebo) in LS means was −1.43 and the 90% CI overlapped zero (−8.35, 6.02; P = .734).
Fig. 2.
PET-adjusted SUVR percent change from baseline to month 13 following monthly doses of ponezumab (n = 12) or placebo (n = 6). Abbreviations: A, ponezumab; B, placebo; CI, confidence interval; LS, least square; PET, positron emission tomography; SUVR, standard uptake volume ratio. Brain regions: CingAnt, cingulum anterior; CingPost, posterior cingulate; FrtCrt, frontal cortex; LTmpCrt, lateral temporal cortex; OccCrt, occiptal cortex; ParCrt, parietal cortex; SubWhMttr, subcortical white matter; TmpMed, medial temporal cortex.
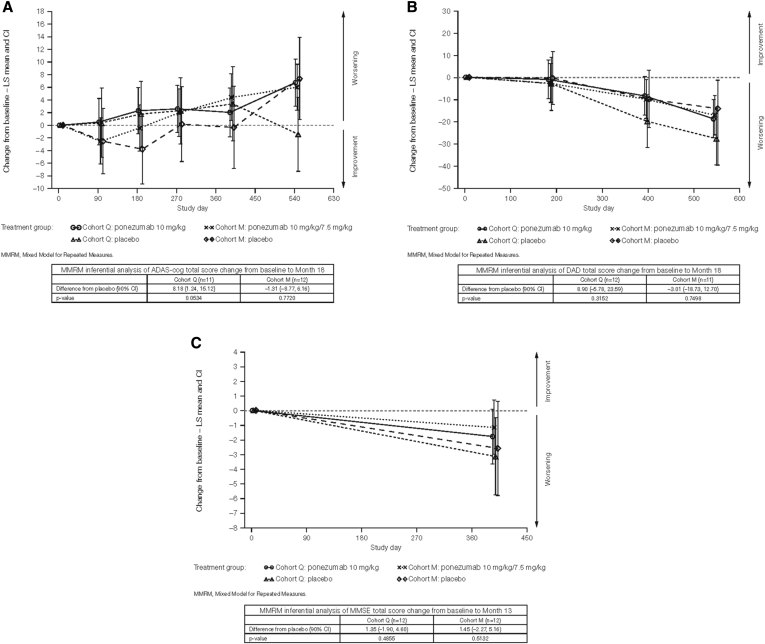
In both cohorts, cognitive and functional abilities worsened from baseline to month 13 (Fig. 3), and there were no significant differences between treatment arms in either cohort (all 90% CIs for the between-group differences overlapped zero). In cohort Q at month 13, the LS mean difference between ponezumab and placebo in change from baseline in ADAS-Cog total score was −1.36 points (90% CI: −8.38, 5.66; P = .7486), whereas in cohort M at month 13, the LS mean difference was 4.74 points (90% CI: −2.77, 12.25; P = .2968). Increases on this scale represent worsening.
Fig. 3.
Cognitive and functional changes from baseline to month 13: (A) ADAS-Cog scores; (B) DAD scores; and (C) MMSE scores.
Similarly, in cohort Q, the difference in LS means between groups in change from baseline to month 13 in DAD total score was 11.37 points (90% CI: −3.31, 26.06; P = .2007). In cohort M, the difference between groups in LS means was −0.10 points (90% CI: −15.72, 15.52; P = .9918). Increases on this scale represent improvement. The differences were not significant in either cohort.
In cohort Q, the difference in LS means between groups in change from baseline to month 13 in MMSE total score was 1.35 points (90% CI: −1.90, 4.60; P = .4855). In cohort M, the difference in LS means was 1.45 points (90% CI: −2.27, 5.16; P = .5132). Increases on this scale represent improvement. The differences were not significant in either cohort.
Owing to the small number of APOE ε4–negative subjects, their outcomes were not compared with those of APOE ε4–positive subjects.
3.3. Safety
Ponezumab was safe and well tolerated. Overall, 29/36 (81%) subjects had an all-causality AE, including 13/36 (36%) whose AEs were considered treatment related. Most AEs were mild or moderate in severity. The most common treatment-related AEs were hypertension/increased blood pressure, cerebral ARIA-H, irritability, and depression (Table 2). The incidence of treatment-related AEs was similar in the ponezumab and placebo groups, occurring in 2/12 (17%) of the cohort Q ponezumab subjects and 1/6 (17%) of the cohort Q placebo subjects; the respective proportions in cohort M were 7/12 (58%) and 3/6 (50%).
Table 2.
Most common treatment-related adverse events (reported in ≥2 subjects)
| Adverse event | Cohort Q |
Placebo (n = 6) |
Cohort M |
Placebo (n = 6) |
|---|---|---|---|---|
| 10 mg/kg (n = 12) | 10 mg/kg/7.5 mg/kg (n = 12) | |||
| Hypertension | 0 | 0 | 4 | 1 |
| Cerebral ARIA-H at any location | 1 | 0 | 1 | 1 |
| Depression | 0 | 0 | 1 | 1 |
| Increased BP | 0 | 0 | 2 | 0 |
| Irritability | 0 | 0 | 1 | 1 |
Abbreviations: ARIA-H, amyloid-related imaging abnormalities with microhemorrhage; BP, blood pressure.
Four subjects had serious AEs, none of which were treatment related: myocardial infarction (n = 1 in the cohort M ponezumab group), hip fracture and urinary tract infection (n = 1 each in the cohort Q ponezumab group), and worsening of dementia Alzheimer's type (n = 1 in the cohort Q placebo group).
Only two subjects had severe AEs, neither of which was treatment related (the hip fracture and dementia Alzheimer's type described previously). There were no deaths.
Owing to the small number of APOE ε4–negative subjects, ponezumab safety and tolerability profiles were not compared with those of APOE ε4–positive subjects. However, all three cases of treatment-related cerebral ARIA-H were in APOE ε4–positive subjects.
No anti-drug antibodies against ponezumab were detected.
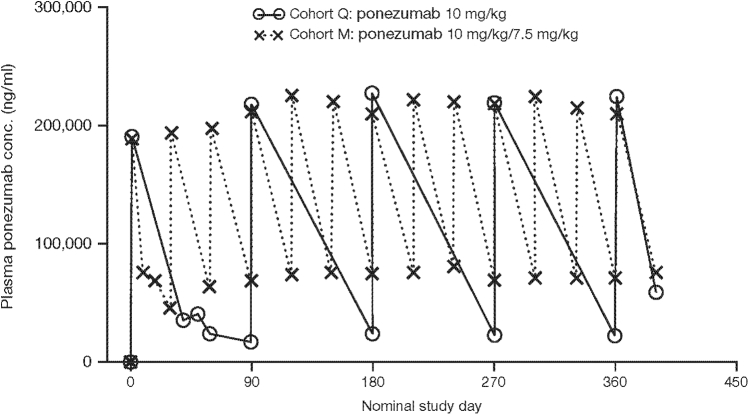
3.4. Pharmacokinetics
Ponezumab exhibited dose-dependent increases in plasma concentrations. Accumulation was limited following multiple dosing, ranging from 1.0- to 1.5-fold across cohorts (Fig. 4). Ponezumab was quantifiable in the CSF of all subjects who received active treatment, but penetration was low (CSF:plasma <1.0%). Ponezumab was not quantifiable in the urine of any subject.
Fig. 4.
Mean plasma ponezumab concentration–time profiles by treatment.
3.5. Biomarkers
Among subjects receiving active treatment, robust increases were observed from baseline to day 360 in mean plasma levels of Aβ1–40 and Aβ1–x. For Aβ1–40, the increases were about 788-fold and 320-fold in cohorts M and Q, respectively; similar increases were observed for plasma Aβ1–x. Minimal increases were noted for either biomarker among subjects receiving placebo. Plasma Aβ1–42 was quantifiable for only 9 of 24 subjects who received ponezumab, and the majority of those values were close to the LLOQ of the bioanalytical assay.
Although CSF Aβ biomarkers, tau, and p-tau were measurable in most subjects, concentrations were highly variable, and there was substantial overlap between ponezumab and placebo treatments both at baseline and following treatment.
Urine Aβ1–x was not detected in any subject.
4. Discussion
In this phase II study, monthly and quarterly ponezumab administration for 1 year was generally safe and well tolerated in subjects with mild-to-moderate AD. Ponezumab exhibited dose-dependent increases in plasma levels, limited plasma accumulation, and low CSF penetration (CSF:plasma <1.0%), consistent with other monoclonal antibodies [22].
Plasma Aβ1–40 and Aβ1–x showed robust increases from baseline in subjects receiving ponezumab, but plasma Aβ1–42 was not quantifiable for the majority (13/24) of subjects. None of the CSF Aβ species, p-tau, or tau were altered when compared with placebo.
No differences between ponezumab and placebo in brain amyloid burden were discernible by PIB for any individual brain region or overall. Cognitive and functional decline was noted over the course of the study, but changes from baseline did not differ between treatment arms.
These results are consistent with those of a separate phase II study in which 198 subjects with mild-to-moderate AD were randomized to receive ponezumab 0.1, 0.5, 1, 3, or 8.5 mg/kg or placebo every 60 days for 18 months [18]. Although active treatment was generally safe and well tolerated, no significant differences from placebo were observed in the change from baseline to month 19 in measures of cognition and function.
The lack of progression of brain amyloid burden in either treated or placebo subjects over the period evaluated in the present study is consistent with data showing that the most rapid deposition of brain amyloid is an early pathologic event, greatest during the periods preceding and during mild cognitive impairment [23], [24]. This may explain the apparent lack of translation from preclinical studies with ponezumab, in which a reduction in central amyloid burden, together with improvements in memory and function, were demonstrated in transgenic mouse models of amyloid overexpression [2], [3]. The small sample size was a limitation of the present study, which was underpowered to detect differences between treatment arms in biochemical, cognitive, or functional end points. With an 80% power to detect a 20% change in the PIB-imaged estimates of insoluble amyloid plaque in the brain, it is important to consider the results of this study alongside the magnitude of changes in brain amyloid burden in studies of other monoclonal antibodies.
In studies of bapineuzumab, PIB showed significant differences in amyloid burden between the treatment and control groups; there were also significant differences in CSF p-tau concentrations between the two groups among patients who were carriers of the APOE ε4 allele [4]. Furthermore, bapineuzumab caused an increase in the rate of change in whole brain and hippocampal ventricular volume as compared with placebo, although no corresponding changes in clinical outcomes were observed [25]. Solanezumab studies revealed large, sustained increases in plasma Aβ and reductions in CSF levels of free (unbound) Aβ40 in conjunction with increased CSF levels of total (bound and unbound) Aβ40 [5]. Total levels of Aβ42 in CSF also increased [5].
Furthermore, clinical evidence with AN1792 suggests that the benefits of treatment on Aβ clearance might be delayed up to 60 months [24], indicating the possible need for longer follow-up periods. In theory, higher doses of ponezumab might have increased CSF drug concentrations or potentially enhanced the peripheral sink effect.
A recent review of the amyloid hypothesis by Selkoe [26] suggests that Aβ dyshomeostasis has emerged as the most extensively validated and compelling therapeutic target. Furthermore, solanezumab that also recognizes soluble, monomeric amyloid has showed a trend in three successive phase III pivotal trials for modest clinical meaningful improvement albeit nonsignificant on the primary end point. The third trial, Expedition3 reported and 11% (nonsignificant) reduction (improvement) in the ADAS-cog 14 in the mild population [27]. Solanezumab is now being advanced for an earlier population. Aducanumab, which has selectivity for fibrillary amyloid has demonstrated convincing phase II results in support of the amyloid hypothesis [28]. Musuek and Holzman [29] point out that it is possible that the appearance of fibular Aβ may represent a point in the cascade where it is too late for effective anti-Aβ therapy. Although the current ponezumab study reports negative results, these data add to the important literature on anti-amyloid therapies to continue to progress the understanding of each antibody's unique mechanism and the potential for therapy alone or in combination.
The present study demonstrated that ponezumab was generally safe and well tolerated at doses of 7.5 mg/kg administered monthly or 10 mg/kg quarterly for 1 year in subjects with mild-to-moderate AD. Plasma ponezumab accumulation was limited following multiple dosing and showed low CSF penetration. Although plasma Aβ biomarkers showed robust increases, there was no apparent effect on CSF biomarkers, cognition, function, or brain amyloid burden. For these reasons, development of ponezumab for mild-to-moderate AD has been discontinued.
Research in Context.
-
1.
Systematic review: The authors reviewed the scientific literature on amyloid-targeted therapies in patients with mild-to-moderate Alzheimer's disease, using traditional sources (e.g., PubMed) and congress presentations. Preclinical and early clinical evidence suggested that anti-amyloid beta (Aβ) therapies could offer cognitive and functional benefits with a manageable safety profile.
-
2.
Interpretation: Multiple-dose regimens of the anti-Aβ antibody ponezumab were generally safe and well tolerated on both monthly and quarterly dosing schedules. After a year of treatment, plasma Aβ was increased, but cerebrospinal fluid biomarker concentrations were highly variable, and there was substantial overlap between ponezumab and placebo. There were no cognitive or functional effects. These findings are generally consistent with those of other investigational anti-Aβ antibodies.
-
3.
Future directions: More remains to be learned about the optimal dose, frequency, and duration of amyloid-reducing treatment and when it should be initiated during the course of the disease.
Acknowledgments
The authors express their gratitude to all study investigators and the subjects and caregivers who participated in this study. The authors also thank James W. Kupiec for his review of the manuscript.
This study was sponsored by Pfizer Inc. Writing assistance was provided by Susanne Gilbert at ACUMED (New York, NY, USA) and was funded by Pfizer Inc.
Footnotes
J.W.L., C.L.C., C.A.S., J.W. Kupiec, B.B., and M.M.B. are Pfizer employees with equity ownership. P.F.S. is a Pfizer employee. N.A., A.B.-H., and H.Ö. have no conflicts of interest to disclose.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.trci.2017.05.003.
Supplementary data
References
- 1.Hardy J., Selkoe D.J. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 2.DeMattos R.B., Bales K.R., Cummins D.J., Dodart J.C., Paul S.M., Holtzman D.M. Peripheral anti-A beta antibody alters CNS and plasma A beta clearance and decreases brain A beta burden in a mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2001;98:8850–8855. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gelinas D.S., DaSilva K., Fenili D., St George-Hyslop P., McLaurin J. Immunotherapy for Alzheimer's disease. Proc Natl Acad Sci U S A. 2004;101:14657–14662. doi: 10.1073/pnas.0404866101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salloway S., Sperling R., Fox N.C., Blennow K., Klunk W., Raskind M. Two Phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer's disease. N Engl J Med. 2014;370:322–333. doi: 10.1056/NEJMoa1304839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doody R.S., Thomas R.G., Farlow M., Iwatsubo T., Vellas B., Joffee S. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer's disease. N Engl J Med. 2014;370:311–321. doi: 10.1056/NEJMoa1312889. [DOI] [PubMed] [Google Scholar]
- 6.Sevigny J, Chiao P, Williams L, Chen T, Ling Y, O'Gorman J, et al. Aducanumab (BIIB037), an anti-amyloid beta monoclonal antibody, in patients with prodromal or mild Alzheimer's disease: Interim results of a randomized, double-blind, placebo-controlled, Phase 1B study. Presented at the Alzheimer's Association International Conference 2015; Abstract 4484.
- 7.Siemers E.R., Sundell K.L., Carlson C., Case M., Sethuraman G., Liu-Seifert H. Phase 3 solanezumab trials: Secondary outcomes in mild Alzheimer's disease patients. Alzheimers Dement. 2016;12:110–120. doi: 10.1016/j.jalz.2015.06.1893. [DOI] [PubMed] [Google Scholar]
- 8.Liu-Seifert H, Aisen PS, Andersen SW, Holdridge KC, Siemers ER. Delayed start analyses of up to 3.5 years in the Phase 3 solanezumab Expedition program in mild Alzheimer's disease. Presented at the Alzheimer's Association International Conference 2015; Abstract 4769.
- 9.Wilcock D.M., Rojiani A., Rosenthal A., Subbarao S., Freeman M.J., Gordon M.N. Passive immunotherapy against Aβ in aged APP-transgenic mice reverses cognitive deficits and depletes parenchymal amyloid deposits in spite of increased vascular amyloid and microhemorrhage. J Neuroinflammation. 2004;1:24. doi: 10.1186/1742-2094-1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilcock D.M., Alamed J., Gottschall P.E., Grimm J., Rosenthal A., Pons J. Deglycosylated anti-amyloid-beta antibodies eliminate cognitive deficits and reduce parenchymal amyloid with minimal vascular consequences in aged amyloid precursor protein transgenic mice. J Neurosci. 2006;26:5340–5346. doi: 10.1523/JNEUROSCI.0695-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klunk W.E., Engler H., Nordberg A., Wang Y., Blomqvist G., Holt D.P. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 12.Price J.C., Klunk W.E., Lopresti B.J., Lu X., Hoge J.A., Ziolko S.K. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J Cereb Blood Flow Metab. 2005;25:1528–1547. doi: 10.1038/sj.jcbfm.9600146. [DOI] [PubMed] [Google Scholar]
- 13.Lopresti B.J., Klunk W.E., Mathis C.A., Hoge J.A., Ziolko S.K., Lu X. Simplified quantification of Pittsburgh Compound B amyloid imaging PET studies: a comparative analysis. J Nucl Med. 2005;46:1959–1972. [PubMed] [Google Scholar]
- 14.Koo J., Byun Y. Current status of PET-imaging probes of β-amyloid plaques. Arch Pharm Res. 2013;36:1178–1184. doi: 10.1007/s12272-013-0193-4. [DOI] [PubMed] [Google Scholar]
- 15.Landen J.W., Zhao Q., Cohen S. Safety and pharmacology of a single intravenous dose of ponezumab in subjects with mild-to-moderate Alzheimer's disease: A phase I, randomized, placebo-controlled, double-blind, dose-escalation study. Clin Neuropharmacol. 2013;36:14–23. doi: 10.1097/WNF.0b013e31827db49b. [DOI] [PubMed] [Google Scholar]
- 16.Burstein A.H., Zhao Q., Ross J. Safety and pharmacology of ponezumab (PF-04360365) after a single 10-minute intravenous infusion in subjects with mild-to-moderate Alzheimer's disease. Clin Neuropharmacol. 2013;36:8–13. doi: 10.1097/WNF.0b013e318279bcfa. [DOI] [PubMed] [Google Scholar]
- 17.Miyoshi I., Fujimoto Y., Yamada M. Safety and pharmacokinetics of PF-04360365 following a single-dose intravenous infusion in Japanese subjects with mild-to-moderate Alzheimer's disease: a multicenter, randomized, double-blind, placebo-controlled, dose-escalation study. Int J Clin Pharmacol Ther. 2013;51:911–923. doi: 10.5414/CP201816. [DOI] [PubMed] [Google Scholar]
- 18.Landen J, Cohen S, Billing CB Jr, et al. Safety, efficacy, pharmacokinetics, and pharmacodynamics of multiple doses of ponezumab in subjects with mild-to-moderate Alzheimer's disease. Presented at the Alzheimer's Association International Conference, 2012, Vancouver, BC, Canada (Proposal 31178).
- 19.Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 20.Rosen W.G., Mohs R.C., Davis K.L. A new rating scale for Alzheimer's disease. Am J Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 21.Gelinas I., Gauthier L., McIntyre M., Gauthier S. Development of a functional measure for persons with Alzheimer's disease: the disability assessment for dementia. Am J Occup Ther. 1999;53:471–481. doi: 10.5014/ajot.53.5.471. [DOI] [PubMed] [Google Scholar]
- 22.Lobo E.D., Hansen R.J., Balthasar J.P. Antibody pharmacokinetics and pharmacodynamics. J Pharm Sci. 2004;93:2645–2668. doi: 10.1002/jps.20178. [DOI] [PubMed] [Google Scholar]
- 23.Villemagne V.L., Burnham S., Bourgeat P., Brown B., Ellis K.A., Salvado O. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: a prospective cohort study. Lancet Neurol. 2013;12:357–367. doi: 10.1016/S1474-4422(13)70044-9. [DOI] [PubMed] [Google Scholar]
- 24.Creed M.C., Milgram N.W. Amyloid-modifying therapies for Alzheimer's disease: therapeutic progress and its implications. Age (Dordr) 2010;32:365–384. doi: 10.1007/s11357-010-9142-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Novak G., Fox N., Clegg S., Nielsen C., Einstein S., Lu Y. Changes in brain volume with bapinezumab in mild to moderate Alzheimer's disease. J Alzheimers Dis. 2016;49:1123–1134. doi: 10.3233/JAD-150448. [DOI] [PubMed] [Google Scholar]
- 26.Selkoe D.J., Harty J. The amyloid hypothesis of Alzheimer's disease at 25 years. Embo Mol Med. 2016;8:595–608. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.The Lancet Neurology Solanezumab: too late in mild Alzheimer's disease? Lancet Neurol. 2017;16:97. doi: 10.1016/S1474-4422(16)30395-7. [DOI] [PubMed] [Google Scholar]
- 28.Sevigny J., Chiao P., Bussière T., Weinreb P.H., Williams L., Maier M. The Antibody aducanumab reduces Aβ plaques in Alzheimer's disease. Nature. 2016;537:50–56. doi: 10.1038/nature19323. [DOI] [PubMed] [Google Scholar]
- 29.Musiek E.S., Holtzman D.M. Three dimensions of the amyloid hypothesis: time, space and ‘wingmen’. Nat Neurosci. 2015;18:800–806. doi: 10.1038/nn.4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.