Abstract
Introduction
Multiple intravenous doses of ponezumab, an anti-amyloid antibody, were evaluated in subjects with mild-to-moderate Alzheimer's disease (AD).
Methods
In part A, 77 subjects were randomized to ponezumab 0.1, 0.5, or 1 mg/kg (75 treated) and 26 to placebo (24 treated). In part B, 63 subjects were randomized and treated with ponezumab 3 or 8.5 mg/kg and 32 with placebo. Subjects received 10 infusions over 18 months and were followed for 6 months thereafter.
Results
Ponezumab was generally safe and well tolerated. Most common adverse events were fall (16.7% ponezumab, 21.4% placebo), headache (13.8%, 21.4%), and cerebral microhemorrhage (13.8%, 19.6%). Plasma ponezumab increased dose-dependently with limited accumulation. Cerebrospinal fluid penetration was low. Plasma Aβ1–x and Aβ1–40 showed robust increases, but cerebrospinal fluid biomarkers showed no dose response. Ponezumab had no effects on cognitive/functional outcomes or brain volume.
Conclusions
Multiple-dose ponezumab was generally safe, but not efficacious, in mild-to-moderate AD.
Keywords: Alzheimer's disease, Amyloid β, Biomarkers, Cerebrospinal fluid, Immunotherapy, Monoclonal antibody, Pharmacokinetics, Pharmacodynamics, Phase-II study, Ponezumab
1. Introduction
The accumulation of amyloid β (Aβ) is thought to be integral to the pathogenesis of Alzheimer's disease (AD), contributing to the formation of neuritic plaques [1]. The mean level of soluble Aβ in the brain parenchyma is increased 3-fold in patients with AD compared with age-matched controls and correlates highly with measures of tau reactivity in tangles and plaques, as well as neurofibrillary tangle density [2]. Reducing amyloid deposits in brain may be warranted in some subpopulations of mild-to-moderate AD. However, amyloid is thought to begin accumulating long before the clinical symptoms of AD appear; therefore, removal of Aβ from brains of patients who have already progressed to dementia may have limited value.
The brains of patients with AD also typically display cerebral amyloid angiopathy (CAA), a pathological condition caused by the progressive deposition of Aβ1–40 surrounding cerebral blood vessel walls [3]. Although comorbidity of AD and CAA is almost universal, there are clear distinctions between them, such as the Aβ species being deposited (Aβ1–42 in AD vs. Aβ1–40 in CAA), the location of the Aβ deposits (brain parenchyma vs. brain vasculature), and the presence of cerebral microhemorrhages that are the signature of CAA [3].
Current therapeutic options for AD provide limited clinical benefit. Recent advances in the development of therapies targeting Aβ include the anti-Aβ antibodies bapineuzumab, solanezumab, and aducanumab [4], [5], [6], [7]. Although the approach initially appeared promising, bapineuzumab did not improve clinical outcomes [4]. Similarly, solanezumab failed to significantly improve cognitive or functional ability in patients with mild-to-moderate AD [5], although secondary analyses suggested that it may be associated with less worsening of cognition than placebo in individuals with mild AD [6]. Data from the extension arm of the solanezumab studies using a delayed-start design indicated a potential modifying effect on underlying disease progression [7]. A phase-Ib study is currently under way to evaluate aducanumab (BIIB037) in patients with prodromal or mild AD (PRIME, NCT01677572) [8]. The double-blind portion showed a statistically significant reduction of brain amyloid as assessed by the florbetapir PET scan. Clinical progression also appeared to be slowed, although amyloid-related imaging abnormalities (ARIAs; magnetic resonance imaging [MRI] signal changes thought to represent vasogenic edema and cerebral microhemorrhage) were commonly observed adverse events (AEs), raising some safety concerns [9]. Two phase-III studies of aducanumab are ongoing in subjects with early AD (EMERGE, NCT02484547; ENGAGE, NCT02477800) [10], [11].
Ponezumab is a humanized IgG2Δa anti-Aβ monoclonal antibody that targets specific amino acids (30–40 of Aβ40) in the C-terminus of the Aβ sequence. It binds only to soluble Aβ and has a low propensity to induce immune responses [12]. Ponezumab's primary mechanism of action is believed to be sequestration of Aβ in the blood and shifting the brain-blood equilibrium toward the periphery, thereby depleting central Aβ stores (the peripheral sink hypothesis). Studies of ponezumab in preclinical murine models of amyloid overexpression have reported depletion of insoluble brain Aβ deposits and reversal of cognitive defects [13].
Single intravenous doses of ponezumab 0.1–10 mg/kg were shown to be safe and well tolerated in Western and Japanese subjects with mild-to-moderate AD [14], [15], [16]. This phase-II, double-blind, randomized, placebo-controlled study was conducted to characterize the safety, tolerability, pharmacokinetics (PK), pharmacodynamics (PD), efficacy (secondary objective), and immunogenicity of multiple intravenous doses of ponezumab in subjects with mild-to-moderate AD.
2. Methods
2.1. Subjects
Eligible subjects were males and females of nonchildbearing potential, who were aged ≥50 years with a diagnosis of mild-to-moderate AD based on a Mini–Mental State Examination (MMSE) score of 16 to 26 inclusive, and probable AD consistent with criteria from the National Institute of Neurological and Communicative Disorders and Stroke, Alzheimer's Disease and Related Disorders Association, and the Diagnostic and Statistical Manual of Mental Disorders, 4th edition. Subjects were also required to have a Rosen-Modified Hachinski Ischemic Score ≤4 at enrollment.
Subjects were required to be in general good health, without known presenilin mutations or a history of familial (early onset) AD and on a stable dose of background cholinesterase inhibitor and/or memantine at least 60 days before dosing. Background therapy was not mandatory for world regions where it was not the standard of care or where intolerant.
The main exclusion criteria are summarized in the Online Supplement. Specific exclusionary brain MRI findings included the following: cortical infarct of any size; >2 microhemorrhages; strategically located subcortical gray-matter infarct (e.g., hippocampus, thalamus, caudate head); and multiple (two or more) white-matter lacunes.
Informed consent was obtained from all subjects, and the study was approved by the institutional review boards and/or independent ethics committees at each investigational center. The study was conducted in compliance with the Declaration of Helsinki and with all the International Conference on Harmonization Good Clinical Practice Guidelines. All the local regulatory requirements were also followed.
2.2. Study design
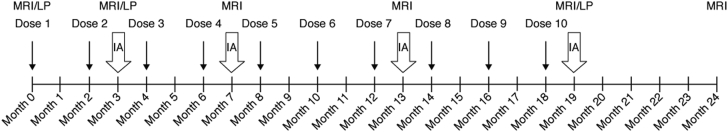
The study was conducted between December 2008 and August 2011 at 30 centers worldwide. The study was composed of two parts, with a total of five ponezumab and two placebo dose arms; in part A, subjects were randomized to receive ponezumab 0.1 mg/kg, 0.5 mg/kg, 1 mg/kg, or placebo, and in part B, three additional cohorts were randomized to receive ponezumab 3 mg/kg, 8.5 mg/kg, or placebo. Treatment was administered as ten 2-hour infusions every 60 days over 18 months. The treatment phase was followed by a 6-month safety follow-up, for a total study duration of 24 months (Fig. 1).
Fig. 1.
Study schematic. Abbreviations: IA, interim analysis; LP, lumbar puncture; MRI, magnetic resonance imaging.
2.3. Study objectives
The primary objectives of the study were to characterize the safety, tolerability, and PK of multiple doses of ponezumab. Secondary objectives included the following: assessment of cognitive efficacy; changes in biomarkers (Aβ species in cerebrospinal fluid [CSF] and plasma, as well as CSF tau and phospho-tau [p-tau] levels); and immunogenicity after repeat dosing.
2.4. Assessments
2.4.1. Safety
Safety evaluations included clinical monitoring, vital signs (heart rate, blood pressure), body weight, 12-lead electrocardiograms (ECGs), AEs, safety laboratory tests, physical examinations, neurological examinations, brain MRIs, continuous cardiac monitoring by telemetry during infusion (for abnormal rhythms), and immunogenicity.
AEs were assessed predose and postdose on all dosing days (months 0, 2, 4, 6, 8, 10, 12, 14, 16, and 18); by telephone at months 1, 9, 11, 15, 17, 21, and 23; and during follow-up visits at months 3, 5, 7, 13, 19, 22, and 24. For all the observed and patient-reported AEs, investigators documented the type and intensity (mild, moderate, or severe), and their opinion of relationship to study treatment. AEs included (but were not limited to) adverse drug reactions, illnesses with onset during the study, exacerbation of previous illnesses, clinically significant changes in physical or neurological examination findings, and clinically significant test findings (ECG, laboratory, etc.).
Safety data were reviewed at regular intervals throughout the study by an external, independent data safety monitoring board. In addition to the scheduled data reviews, four interim analyses were planned for part A and four for part B.
Core safety assessments were supplemented by six mandatory brain MRIs (with no more than 6 months between each MRI) and optional lumbar punctures at baseline, month 3, and month 19. Safety laboratory evaluations and ECGs were conducted at screening; predose on each dosing day; and at months 3, 7, 13, 19, and 24.
Monitoring for intracranial pathology, including cerebral microhemorrhages, superficial siderosis, and vasogenic edema was performed by two external radiologists (central reader) experienced in reading T2* gradient-echo (GRE) images (C.R.J. and K.K.). Microhemorrhages were defined as homogenous hypointense lesions up to 10 mm in diameter in the gray or white matter on T2* GRE images. Superficial siderosis was defined as curvilinear hypointensities overlying the cortical surface, distinct from vascular flow voids [17]. All the scans were read immediately locally for safety but also transmitted to a central reader for neuroimaging analysis and additional safety review.
In addition to the T2* GRE images, sequences in the MRI protocol included 3D MP-RAGE/SPGR sagittal, fluid-attenuated inversion recovery axial, diffusion-weighted imaging axial, and T1 axial pregadolinium (gadolinium contrast optional). Brain volumetrics (hippocampal, ventricular, and whole brain) were measured by MRI at baseline and at months 3, 7, 13, 19, and 24.
2.4.2. Efficacy
Cognitive efficacy was assessed using the 70-point Alzheimer's Disease Assessment Scale–Cognitive Subscale (ADAS-Cog) and Disability Assessment for Dementia (DAD) scale at baseline and at months 3, 7, 13, 19, and 24. Exploratory measures of efficacy included the MMSE, the Cognitive State (CogState) computerized battery, the Neuropsychological Test Battery (NTB), and the Neuropsychiatric Inventory (NPI-12), which were conducted at the same visits as the ADAS-Cog and DAD. In this study, the NTB included the controlled oral word association test, the category fluency test, and the trail-making test. The Clinical Dementia Rating (CDR) Scale global score and CDR Sum of Boxes (CDR-SB) were recorded at baseline and month 19. Quality of life was assessed with the EuroQoL-5D (EQ-5D) instrument at baseline and months 13, 19, and 24.
2.4.3. Pharmacokinetics and pharmacodynamics
Plasma, urine, and optional CSF samples were collected to define ponezumab PK profile and its PD effects on Aβ species, total tau, and p-tau. Plasma and urine samples were collected predose and postdose on each dosing day (months 0, 2, 4, 6, 8, 10, 12, 14, 16, and 18) and at months 3, 5, 7, 13, 19, 22, and 24. Optional CSF samples for PK and PD analyses were collected at baseline and at months 3 and 19.
Plasma, urine, and CSF samples were analyzed for ponezumab concentrations using validated, sensitive, and specific enzyme-linked immunosorbent assay methods. The lower limits of quantification were 78.1 ng/mL for plasma, 60 ng/mL for urine, and 12 ng/mL for CSF.
Plasma samples were analyzed for Aβ1–x, Aβ1–40, and Aβ1–42. Urine samples were analyzed for Aβ1–x only. The CSF samples were analyzed for all Aβ species, total tau, and p-tau using validated assays (Supplementary Table 1).
2.4.4. Immunogenicity assessments
Blood samples were collected for assessment of immunogenicity (antidrug antibodies [ADAs]) before ponezumab infusion on each dosing day, as well as at months 3, 5, 7, 13, 19, 22, and 24. Serum was analyzed following a tiered approach using screening, confirmation, and titer/quantitation assays, as applicable. A validated semiquantitative enzyme-linked immunosorbent assay method was used. Assay precision was <8% coefficient of variation (CV) for the positive control and <10.2% CV for the negative control (used to calculate cut point).
2.4.5. Pharmacogenomics
A blood sample was obtained at baseline for apolipoprotein E (APOE) genotyping. Subjects who were classified as APOE ɛ4 positive had a genotype that included at least one copy of the APOE ɛ4 allele.
2.5. Statistical analysis
All analyses were performed for each study part separately. A mixed model repeat measures approach was applied to compare the mean change from baseline for ADAS-Cog total score, DAD total score, MMSE total score, CogState individual tasks and composite score, NTB individual tests and composite score, NPI total score, EQ-5D visual analogue scale, and brain volumes between each active dose and placebo for each visit for the full analysis set (FAS; all subjects who were randomized and received at least one infusion of study medication). The primary assessment was the change from baseline at the month 19 visit (approximately 30 days following the last study drug infusion). The fixed effects in the model were time (as categorical), treatment, treatment-by-time interaction, baseline value, and country. An unstructured variance-covariance matrix was assumed for the within-subject errors. Analysis of covariance was used to compare the mean change from baseline for CDR-SB between each active dose and placebo at month 19 using the FAS. The effects in the model were treatment, baseline CDR-SB, and country.
Two-sided hypothesis tests comparing each active treatment with placebo were conducted for each end point at the nominal α = 0.10 level without adjustment for multiple treatment contrasts or multiple end points. Least squares means, with standard errors, for the change from baseline and treatment differences from placebo were estimated along with 90% confidence intervals.
3. Results
3.1. Subject disposition
A total of 198 subjects were randomized and 194 received at least one infusion of blinded study medication; these subjects comprised the FAS (99 in study part A and 95 in study part B). They received a median of 10 infusions (range: 1–10 infusions), with a similar number in each treatment group. Of the 194 treated subjects, 146 completed the study and 48 discontinued the study.
Demographic and baseline characteristics were comparable across treatment groups (Table 1). There were 105 females and 89 males, and the proportions were generally similar across treatment groups. Most subjects were white (n = 138), and ages ranged from 51 to 90 years. At screening, 121 subjects (62.4%) had mild dementia (MMSE 21–26) and 73 subjects (37.6%) had moderate dementia (MMSE 16–20). APOE ɛ4 carrier status was positive for 129 subjects (66.5%), negative (non-APOE ɛ4 carrier status) for 63 subjects (32.5%), and unknown for two subjects (1%). These proportions were also similar across treatment groups.
Table 1.
Subjects' baseline and demographic characteristics
| Demographic characteristic | Ponezumab |
Placebo A |
Ponezumab |
Placebo B |
|||
|---|---|---|---|---|---|---|---|
| 0.1 mg/kg |
0.5 mg/kg |
1.0 mg/kg |
3.0 mg/kg |
8.5 mg/kg |
|||
| n = 25 | n = 25 | n = 25 | n = 24 | n = 32 | n = 31 | n = 32 | |
| Gender, n | |||||||
| Male | 13 | 10 | 14 | 11 | 12 | 14 | 15 |
| Female | 12 | 15 | 11 | 13 | 20 | 17 | 17 |
| Mean (SD) age, years | 70.8 (8.2) | 71.9 (9.4) | 72.2 (8.4) | 70.0 (7.8) | 70.5 (8.9) | 71.8 (7.3) | 70.4 (10.3) |
| Race, n | |||||||
| White | 16 | 17 | 19 | 17 | 23 | 21 | 25 |
| Black | 0 | 0 | 0 | 1 | 2 | 0 | 0 |
| Asian | 9 | 8 | 6 | 6 | 7 | 9 | 7 |
| Other | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Mean (SD) years of education | 12.4 (3.5) | 12.2 (4.7) | 11.7 (4.2) | 12.1 (3.3) | 12.4 (3.6) | 12.2 (4.2) | 13.7 (4.0) |
| Mean (SD) screening MMSE | 21.5 (2.9) | 21.4 (3.6) | 20.8 (3.0) | 21.0 (3.4) | 22.5 (2.5) | 20.9 (3.1) | 21.9 (3.4) |
| Mean (SD) baseline ADAS-Cog | 20.0 (7.9) | 20.4 (8.2) | 20.8 (6.1) | 20.0 (7.0) | 19.4 (7.0) | 24.5 (10.1) | 18.4 (7.5) |
Abbreviations: SD, standard deviation; MMSE, Mini–Mental State Examination; ADAS-Cog, Alzheimer's Disease Assessment Scale–Cognitive Subscale.
3.2. Safety
All subjects who received at least one infusion of study medication were included in the safety analysis set (n = 194). Ponezumab was generally safe and well tolerated. A total of 183 subjects had at least one treatment-emergent AE, including 86 who had an AE that was considered by the investigator to be treatment related. The most common all-causality AEs were fall (16.7% ponezumab, 21.4% placebo), headache (13.8% ponezumab, 21.4% placebo), and cerebral microhemorrhage (13.8% ponezumab, 19.6% placebo) (Table 2). The most frequently reported treatment-related AEs were incident cerebral microhemorrhage (ARIA with microhemorrhage) (8.7% ponezumab, 16.1% placebo), headache (5.1% and 7.1%, respectively), and fatigue (5.1% and 3.6%, respectively) (Table 2). No treatment-related brain macrohemorrhage or meningoencephalitis was noted. In the MRI analysis, the incidence of microhemorrhages was 16.4% in the pooled ponezumab group and 21.4% in the pooled placebo group over the 24-month observation period. Note that not all microhemorrhages identified by MRI were reported by the investigator as AEs, and the incidences are based only on subjects with a postbaseline MRI. Thus, the incidence of microhemorrhages may differ between the AE reports and the MRI analyses. Incident brain abnormalities noted on MRI included cerebral edema (one subject receiving ponezumab 0.5 mg/kg), cerebral/meningeal enhancement (one subject receiving ponezumab 0.5 mg/kg), subdural hematoma (one subject receiving ponezumab 0.5 mg/kg), cortical infarcts (one subject receiving ponezumab 0.1 mg/kg, two receiving 1 mg/kg, and one receiving 8.5 mg/kg), subcortical gray-matter infarcts (one subject receiving ponezumab 3 mg/kg and one receiving 8.5 mg/kg), white-matter infarcts (one subject receiving ponezumab 0.5 mg/kg and two receiving placebo), and white-matter hyperintensities (three subjects receiving ponezumab 0.5 mg/kg, two receiving 1 mg/kg, four receiving 3 mg/kg, and four receiving placebo).
Table 2.
Incidence of treatment-emergent, all-causality adverse events occurring in ≥10% of any treatment group
| MedDRA (v14.0) preferred term |
Ponezumab |
Placebo A |
Ponezumab |
Placebo B |
|||
|---|---|---|---|---|---|---|---|
| 0.1 mg/kg |
0.5 mg/kg |
1.0 mg/kg |
3.0 mg/kg |
8.5 mg/kg |
|||
| n (%) | n = 25 | n = 25 | n = 25 | n = 24 | n = 32 | n = 31 | n = 32 |
| Cerebral microhemorrhage∗ | 4 (16.0) | 6 (24.0) | 1 (4.0) | 6 (25.0) | 2 (6.3) | 6 (19.4) | 5 (15.6) |
| Confusional state | 0 | 0 | 2 (8.0) | 1 (4.2) | 3 (9.4) | 6 (19.4) | 2 (6.3) |
| Fall | 2 (8.0) | 3 (12.0) | 4 (16.0) | 3 (12.5) | 9 (28.1) | 5 (16.1) | 9 (28.1) |
| Headache | 4 (16.0) | 3 (12.0) | 1 (4.0) | 4 (16.7) | 6 (18.8) | 5 (16.1) | 8 (25.0) |
| Fatigue | 4 (16.0) | 3 (12.0) | 5 (20.0) | 1 (4.2) | 4 (12.5) | 4 (12.9) | 3 (9.4) |
| Agitation | 1 (4.0) | 4 (16.0) | 3 (12.0) | 3 (12.5) | 2 (6.3) | 4 (12.9) | 2 (6.3) |
| Decreased appetite | 0 | 1 (4.0) | 2 (8.0) | 0 | 1 (3.1) | 4 (12.9) | 1 (3.1) |
| Nasopharyngitis | 4 (16.0) | 0 | 2 (8.0) | 1 (4.2) | 5 (15.6) | 3 (9.7) | 4 (12.5) |
| Nausea | 3 (12.0) | 4 (16.0) | 1 (4.0) | 1 (4.2) | 4 (12.5) | 3 (9.7) | 1 (3.1) |
| Constipation | 3 (12.0) | 2 (8.0) | 2 (8.0) | 0 | 3 (9.4) | 3 (9.7) | 1 (3.1) |
| Anxiety | 3 (12.0) | 5 (20.0) | 1 (4.0) | 3 (12.5) | 3 (9.4) | 3 (9.7) | 2 (6.3) |
| Weight decreased | 2 (8.0) | 3 (12.0) | 4 (16.0) | 1 (4.2) | 2 (6.3) | 3 (9.7) | 1 (3.1) |
| Contusion | 1 (4.0) | 4 (16.0) | 1 (4.0) | 2 (8.3) | 6 (18.8) | 2 (6.5) | 5 (15.6) |
| Urinary tract infection | 4 (16.0) | 3 (12.0) | 2 (8.0) | 3 (12.5) | 4 (12.5) | 2 (6.5) | 2 (6.3) |
| Insomnia | 0 | 1 (4.0) | 1 (4.0) | 3 (12.5) | 4 (12.5) | 2 (6.5) | 0 |
| Back pain | 2 (8.0) | 2 (8.0) | 4 (16.0) | 1 (4.2) | 3 (9.4) | 2 (6.5) | 4 (12.5) |
| Upper respiratory tract infection | 5 (20.0) | 4 (16.0) | 5 (20.0) | 7 (29.2) | 1 (3.1) | 2 (6.5) | 1 (3.1) |
| Irritability | 1 (4.0) | 2 (8.0) | 0 | 3 (12.5) | 0 | 2 (6.5) | 0 |
| Depression | 2 (8.0) | 0 | 4 (16.0) | 0 | 0 | 2 (6.5) | 3 (9.4) |
| Hypertension | 4 (16.0) | 3 (12.0) | 0 | 1 (4.2) | 4 (12.5) | 1 (3.2) | 2 (6.3) |
| Dizziness | 2 (8.0) | 2 (8.0) | 1 (4.0) | 3 (12.5) | 3 (9.4) | 1 (3.2) | 3 (9.4) |
| Cough | 2 (8.0) | 2 (8.0) | 8 (32.0) | 4 (16.7) | 3 (9.4) | 1 (3.2) | 1 (3.1) |
| Diarrhea | 3 (12.0) | 3 (12.0) | 5 (20.0) | 1 (4.2) | 2 (6.3) | 1 (3.2) | 5 (15.6) |
| Pneumonia | 0 | 1 (4.0) | 3 (12.0) | 1 (4.2) | 1 (3.1) | 1 (3.2) | 2 (6.3) |
| Aggression | 3 (12.0) | 2 (8.0) | 1 (4.0) | 1 (4.2) | 1 (3.1) | 1 (3.2) | 0 |
Abbreviation: MedDRA, Medical Dictionary for Regulatory Activities.
Not all microhemorrhages identified by magnetic resonance imaging were reported by the investigator as adverse events.
Abnormalities in laboratory parameters, vital signs, and ECG findings showed no clinically meaningful differences among treatment groups, and immunogenicity testing revealed no ADAs in any serum sample.
A total of 58/1296 (4.5%) AEs were severe, of which three were considered treatment-related by the investigator. These were meningeal thickening and bilateral subdural hygromas (resolved 6 months after onset) in one subject randomized to ponezumab 0.1 mg/kg and headache in one subject randomized to placebo.
Serious AEs (SAEs) occurred in 45 subjects and were more common in the ponezumab groups than in the placebo groups. In part A, all-causality SAEs occurred in seven (28%) subjects receiving ponezumab 0.1 mg/kg, eight (32%) subjects receiving ponezumab 0.5 mg/kg, seven (28%) subjects receiving ponezumab 1 mg/kg, and three (12.5%) subjects receiving placebo. In part B, all-causality SAEs occurred in seven (21.9%) subjects receiving ponezumab 3 mg/kg, 10 (32.3%) subjects receiving ponezumab 8.5 mg/kg, and three (9.4%) subjects receiving placebo. Three SAEs were considered treatment related by the investigator: meningeal thickening/subdural hygromas in one subject (as mentioned previously); asymptomatic vasogenic cerebral edema (ARIA with edema) and superficial siderosis in one subject randomized to ponezumab 0.5 mg/kg, which were identified 175 days after the last dose of ponezumab and resolved approximately 2 months after onset; and prostate cancer in one subject randomized to placebo, which was still present at the last follow-up.
A total of 20 subjects discontinued treatment or withdrew from the study because of AEs; of these, four discontinued because of treatment-related AEs: exertional dyspnea (one subject receiving 3 mg/kg), cerebral microhemorrhage (one subject receiving 0.1 mg/kg), and rash (one subject receiving 0.1 mg/kg and one receiving 8.5 mg/kg). Treatment was temporarily discontinued in 13 subjects due to an AE (one receiving 0.1 mg/kg, two receiving 0.5 mg/kg, one receiving 1 mg/kg, three receiving 3 mg/kg, one receiving 8.5 mg/kg, and five receiving placebo). In two of these patients, the AE was considered treatment related (thalamic infarction in one subject receiving 3 mg/kg and cerebral microhemorrhage in one subject receiving placebo). There were no dose reductions due to AEs.
There were three deaths: two during active treatment and one during post-treatment follow-up. They were attributable to a traffic accident (ponezumab 0.5 mg/kg), intracranial hemorrhage (placebo), and acute coronary syndrome (placebo). None were considered treatment related.
3.3. Efficacy
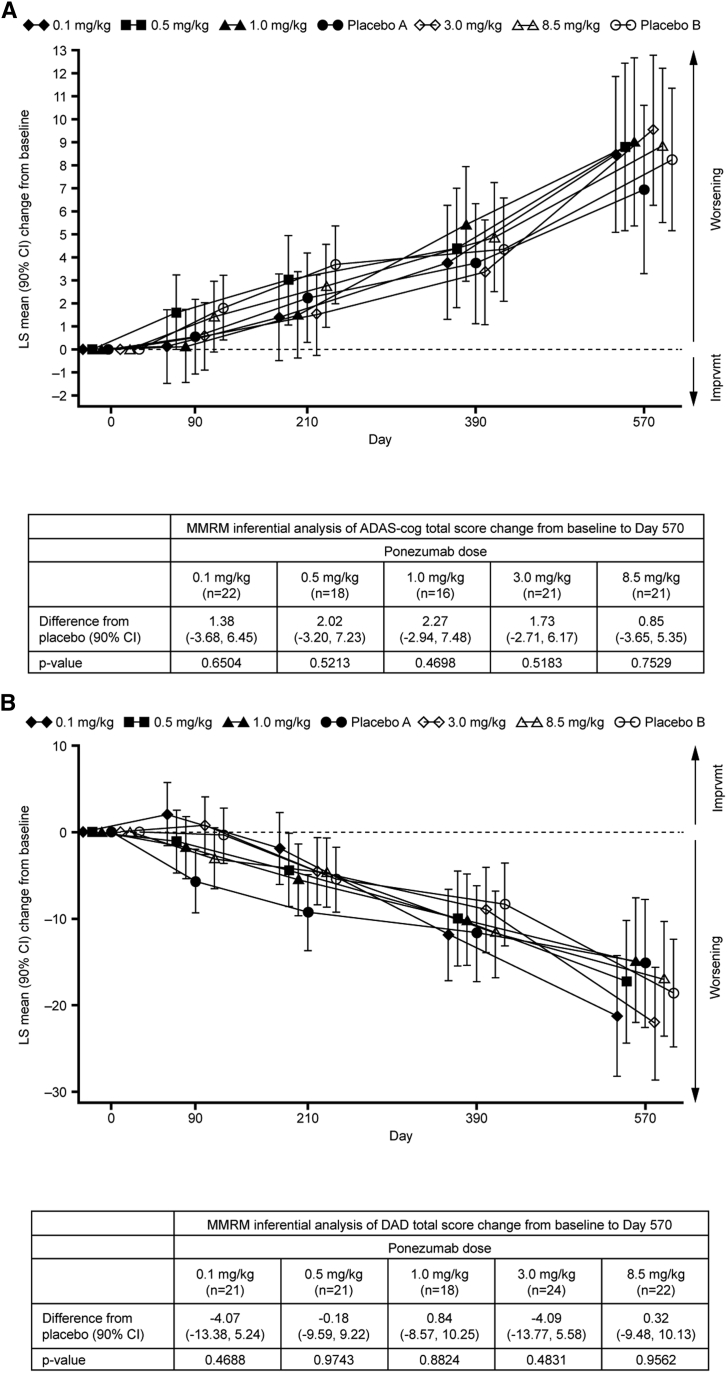
The FAS was the primary analysis set for the efficacy analyses. Cognition and functional ability declined over time in the ponezumab and placebo groups. Overall, the ponezumab groups did not differ from placebo in the change from baseline at month 19 in mean ADAS-Cog total score (Fig. 2A) or mean DAD total score (Fig. 2B). These changes appeared similar regardless of baseline AD severity and APOE ɛ4 status.
Fig. 2.
(A) Alzheimer's Disease Assessment Scale–Cognitive Subscale (ADAS-Cog) total score LS mean (90% CI) change from baseline; (B) Disability Assessment for Dementia (DAD) total score LS mean (90% CI) change from baseline. Abbreviations: CI, confidence interval; LS, least squares.
Generally, there were no differences between ponezumab and placebo in the change from baseline at month 19 in CogState individual items and composite score, global CDR score, CDR-SB score, MMSE total score, NPI total score, NTB score, and EQ-5D score.
In the MRI analyses, ponezumab generally did not differ significantly from placebo in the change from baseline at month 19 in mean whole-brain volume, hippocampal volume, or ventricular volume.
3.4. Pharmacokinetics
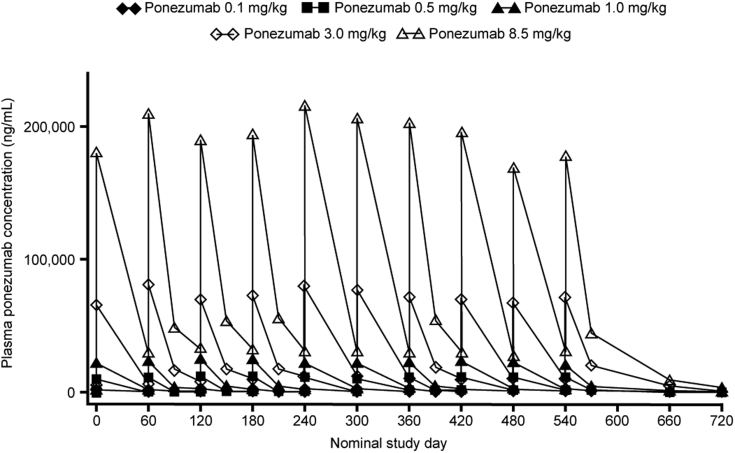
All subjects who received at least one infusion of study medication were included in the PK analyses (n = 194). After multiple dosing, plasma ponezumab concentrations increased in a dose-dependent manner and exhibited limited accumulation (Fig. 3). Mean increases were approximately 1- to 1.3-fold based on the ratio of the concentration at the end of the infusion (Cendinf) at month 18 to that at month 0, and 1.5- to 1.8-fold based on the ratio of trough concentration (Ctrough) at month 18 to that at month 2.
Fig. 3.
Mean plasma ponezumab concentration-time profiles after a 2-hour intravenous infusion every 2 months.
CSF ponezumab concentrations at months 3 and 19 are shown in Supplementary Table 2. The mean CSF concentrations were <1% of mean plasma total concentrations.
Ponezumab was quantifiable in the urine of one subject at month 24 after the 0.1 mg/kg dose (14.9 ng/mL), one subject at month 10 after the 1 mg/kg dose (126 ng/mL), two subjects at month 24 after the 3 mg/kg dose (mean = 26 ng/mL), and one subject at month 19 after the 8.5 mg/kg dose (7.38 ng/mL).
3.5. Biomarkers
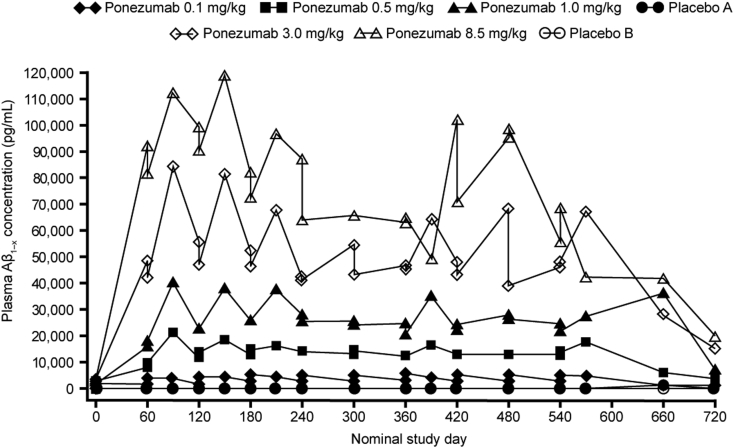
Robust increases in plasma Aβ1–x (Fig. 4) and Aβ1–40 mean concentrations were observed, but plasma Aβ1–42 levels were sporadic and below the lower limits of quantification (20 pg/mL) for most subjects. The appearance of Aβ1–x in the urine was negligible.
Fig. 4.
Mean plasma Aβ1–x concentration-time profiles after a 2-hour intravenous infusion of ponezumab every 2 months. Abbreviation: Aβ, amyloid β.
There was no clear dose response for any CSF biomarker (Aβ1–x, Aβ1–40, Aβ1–42, tau, or p-tau) at months 3 or 19. Similar time courses were observed for these biomarkers in both the placebo and ponezumab groups. Furthermore, the percent change from baseline was highly variable for each biomarker in each dose group, with most CVs greater than 100%.
4. Discussion
Ponezumab was generally safe and well tolerated at multiple doses up to 8.5 mg/kg administered over 18 months. No ADAs were detected. Ponezumab demonstrated dose-dependent increases in plasma concentrations, limited plasma accumulation, low CSF penetration, and negligible appearance in the urine after multiple doses.
Robust increases from baseline were observed for plasma Aβ1–x and Aβ1–40, consistent with ponezumab's likely mechanism of action. However, the time course of CSF biomarkers did not differ substantially for placebo versus ponezumab, nor were any dose responses observed.
There were no differences between ponezumab and placebo in cognitive or functional outcomes. However, because efficacy was assessed as a secondary objective, the results are descriptive in nature rather than inferential, and any conclusions derived from the analysis of these secondary efficacy end points are limited because of the limited sample size.
The results of this study are broadly consistent with those of the phase-III bapineuzumab and solanezumab studies in similar patient populations, which failed to meet their primary end points [4], [5]. However, secondary analyses of some of these studies indicate that defined subpopulations of patients with AD may experience benefit from treatment [6], [7] and further investigation is warranted.
5. Conclusions
Multiple doses of ponezumab over 18 months were generally safe and well tolerated, with no evidence of treatment-related macrohemorrhage or meningoencephalitis and a reduced rate of microhemorrhages compared with placebo. However, treatment did not alter CSF biomarkers, brain volumetrics, or clinical outcomes compared with placebo. For these reasons, development of ponezumab for mild-to-moderate AD has been discontinued.
Research in Context.
-
1.
Systematic review: The authors reviewed the scientific literature on amyloid-targeted therapies in patients with mild-to-moderate Alzheimer's disease, using traditional sources (e.g., PubMed) and congress presentations. Preclinical and early clinical evidence suggested that anti–amyloid beta (Aβ) therapies could offer cognitive and functional benefits with a manageable safety profile.
-
2.
Interpretation: This study of the anti-Aβ antibody, ponezumab, adds to the body of knowledge on the drug class. Multiple-dose regimens were generally safe and well tolerated. Treatment increased plasma Aβ, but CSF biomarkers showed no dose response and there were no cognitive or functional effects. These findings are generally consistent with those of other investigational anti-Aβ antibodies.
-
3.
Future directions: These results should prompt further research into the pathogenic role of Aβ and timing of amyloid-reducing therapeutic interventions.
Acknowledgments
This study was sponsored by Pfizer. Medical writing support was provided by Susanne Gilbert of ACUMED (New York, NY) and was funded by Pfizer.
The authors gratefully acknowledge the contributions of the A9951002 study investigators, study participants, and their caregivers. The authors would also like to thank Dr. Yao Zhang (Pfizer) for his statistical review of the brain infarct and hemorrhage data, and Dr. James W. Kupiec (Pfizer) for his review of the article.
Footnotes
J.W.L., C.B.B., C.C., S.S., A.H.B., C.S., P.F.S., W.T.D., Q.Z., K.S., M.M.B., and B.B. are employees of Pfizer with equity ownership (or were at the time this study was conducted). S.C. has received research grants from Pfizer for conducting ponezumab clinical trials and has received honoraria from Pfizer for talks related to Alzheimer's disease and participating in advisory boards. J.-H.L. has no conflicts to disclose. C.R.J. provides consulting services for Eli Lilly. He also receives research funding from the National Institutes of Health (R01-AG011378, RO1-AG041851, RO1-AG037551, U01-AG032438, and U01-AG024904) and the Alexander Family Alzheimer's Disease Research Professorship of the Mayo Foundation. K.K. serves on the data safety monitoring board for Takeda Global Research & Development Center, Inc., Pfizer., and Janssen Alzheimer Immunotherapy; she is funded by the NIH (R01AG040042, P50 AG16574, and P50 AG44170).
Portions of this material were presented at the International Conference on Alzheimer's Disease, Honolulu, Hawaii, July 15–20, 2010 (Bednar et al. The prevalence of brain microhemorrhages and infarcts in a cohort of patients with mild–moderate Alzheimer's disease. P2-372) and at the Alzheimer's Association International Conference, Vancouver, BC, Canada, July 14–19, 2012 (Landen et al. Safety, efficacy, pharmacokinetics, and pharmacodynamics of multiple doses of ponezumab in subjects with mild-to-moderate Alzheimer's disease. Proposal number 31178).
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.trci.2017.04.003.
Supplementary data
References
- 1.Wippold F.J., 2nd, Cairns N., Vo K., Holtzman D.M., Morris J.C. Neuropathology for the neuroradiologist: plaques and tangles. AJNR Am J Neuroradiol. 2008;29:18–22. doi: 10.3174/ajnr.A0781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McLean C.A., Cherny R.A., Fraser F.W., Fuller S.J., Smith M.J., Beyreuther K. Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer's disease. Ann Neurol. 1999;46:860–866. doi: 10.1002/1531-8249(199912)46:6<860::aid-ana8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 3.Auriel E., Greenberg S.M. The pathophysiology and clinical presentation of cerebral amyloid angiopathy. Curr Atheroscler Rep. 2012;14:343–350. doi: 10.1007/s11883-012-0254-z. [DOI] [PubMed] [Google Scholar]
- 4.Salloway S., Sperling R., Fox N.C., Blennow K., Klunk W., Raskind M. Two Phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer's disease. New Engl J Med. 2014;370:322–333. doi: 10.1056/NEJMoa1304839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doody R.S., Thomas R.G., Farlow M., Iwatsubo T., Vellas B., Joffee S. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer's disease. New Engl J Med. 2014;370:311–321. doi: 10.1056/NEJMoa1312889. [DOI] [PubMed] [Google Scholar]
- 6.Siemers E.R., Sundell K.L., Carlson C., Case M., Sethuraman G., Liu-Seifert H. Phase 3 solanezumab trials: secondary outcomes in mild Alzheimer's disease patients. Alzheimers Dement. 2016;12:110–120. doi: 10.1016/j.jalz.2015.06.1893. [DOI] [PubMed] [Google Scholar]
- 7.Liu-Seifert H, Aisen PS, Andersen SW, Holdridge KC, Siemers ER. Delayed start analyses of up to 3.5 years in the phase 3 solanezumab expedition program in mild Alzheimer's disease. Presented at the Alzheimer’s Association International Conference 2015;Abstract 4769.
- 8.Clinicaltrials.gov. NCT01677572. Available at: https://clinicaltrials.gov/ct2/show/NCT01677572?term=aducanumab&rank=1.
- 9.Sevigny J, Chiao P, Williams L, Chen T, Ling Y, O’Gorman J, et al. Aducanumab (BIIB037), an anti-amyloid beta monoclonal antibody, in patients with prodromal or mild Alzheimer's disease: interim results of a randomized, double-blind, placebo-controlled, Phase 1B study. Presented at the Alzheimer’s Association International Conference 2015;Abstract 4484.
- 10.Clinicaltrials.gov. NCT02484547. Available at: https://www.clinicaltrials.gov/ct2/show?term=aducanumab&rank=3.
- 11.Clinicaltrials.gov. NCT02477800. Available at: https://www.clinicaltrials.gov/ct2/show?term=aducanumab&rank=4.
- 12.Armour K.L., van de Winkel J.G., Williamson L.M., Clark M.R. Differential binding to human FcgammaRIIa and FcgammaRIIb receptors by human IgG wildtype and mutant antibodies. Mol Immunol. 2003;40:585–593. doi: 10.1016/j.molimm.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Wilcock D.M., Rojiani A., Rosenthal A., Subbarao S., Freeman M.J., Gordon M.N. Passive immunotherapy against Abeta in aged APP-transgenic mice reverses cognitive deficits and depletes parenchymal amyloid deposits in spite of increased vascular amyloid and microhemorrhage. J Neuroinflammation. 2004;1:24. doi: 10.1186/1742-2094-1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landen J.W., Zhao Q., Cohen S., Borrie M., Woodward M., Billing C.B., Jr. Safety and pharmacology of a single intravenous dose of ponezumab in subjects with mild-to-moderate Alzheimer disease: a phase I, randomized, placebo-controlled, double-blind, dose-escalation study. Clin Neuropharmacol. 2013;36:14–23. doi: 10.1097/WNF.0b013e31827db49b. [DOI] [PubMed] [Google Scholar]
- 15.Burstein A.H., Zhao Q., Ross J., Styren S., Landen J.W., Ma W.W. Safety and pharmacology of ponezumab (PF-04360365) after a single 10-minute intravenous infusion in subjects with mild to moderate Alzheimer disease. Clin Neuropharmacol. 2013;36:8–13. doi: 10.1097/WNF.0b013e318279bcfa. [DOI] [PubMed] [Google Scholar]
- 16.Miyoshi I., Fujimoto Y., Yamada M., Abe S., Zhao Q., Cronenberger C. Safety and pharmacokinetics of PF-04360365 following a single-dose intravenous infusion in Japanese subjects with mild-to-moderate Alzheimer's disease: a multicenter, randomized, double-blind, placebo-controlled, dose-escalation study. Int J Clin Pharm Ther. 2013;51:911–923. doi: 10.5414/CP201816. [DOI] [PubMed] [Google Scholar]
- 17.Kantarci K., Gunter J.L., Tosakulwong N., Weigand S.D., Senjem M.S., Petersen R.C. Focal hemosiderin deposits and β-amyloid load in the ADNI cohort. Alzheimers Dement. 2013;9:S116–S123. doi: 10.1016/j.jalz.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.