Abstract
Objective(s):
The aim of this study was to investigate the effects of mild hypothermia on expression of NF-E2-related factor 2 (Nrf2) and heme-oxygenase-1 (HO-1) of rat cerebral cortex and hippocampus after cardiopulmonary resuscitation and further investigate the possible mechanism of action.
Material and Methods:
To copy an asphyxia heart arrest model, Sprague Dawley rats were randomly divided into normothermia group, mild hypothermia group before restoration of spontaneous circulation (ROSC), and mild hypothermia group after ROSC. Body temperature in normothermia group was maintained at 37.5-39 °C, while in mild hypothermia group maintained at 32-34 °C by surface cooling with the ice pack. Each group then divided into three subgroups: 15 min, 30 min, and 60 min. Reverse transcription-polymerase chain reaction (RT-PCR) was performed to detect the expression of Nrf2 and HO-1 mRNA in cerebral cortex and hippocampus. Hematoxylin-eosin (H&E) staining was performed to observe histological changes. Immunohistochemistry was performed to detect the expression of Nrf2 and HO-1 protein expression.
Results:
The expression of Nrf2 and HO-1 in cerebral cortex and hippocampus after cardiopulmonary resuscitation (CPR) was significantly increased and mild hypothermia up regulated this level. HE staining showed that mild hypothermia significantly improved neuronal injury.
Conclusion:
Mild hypothermia has neuroprotective effects on cerebral ischemia/reperfusion injury after cardiac arrest. The possible mechanism is that Nrf2-ARE pathway in cerebral cortex and hippocampus after CPR is activated.
Keywords: Heart arrest, Heme-oxygenase-1, Mild hypothermia, Neuroprotection, NF-E2-related factor 2, Nrf2
Introduction
At present, heart arrest has become the primary cause of death in industrialized countries (1). In China, around 500,000 people died of heart arrest every year (2).
Cardiopulmonary resuscitation (CPR) is an effect- ive rescue measure to early save patients after heart arrest, which was put forward by Kouwenhoven and his colleagues in 1960. In recent years, CPR theory and techniques have been popularized, promoted and improved. Along with the popularization of CPR theory and the techniques, initial mortality rate has reduced in cardiac arrest (CA) patients. The pathophysiological alter- ations of restoration of spontaneous circulation (ROSC) described as post-cardiac arrest syndrome (PCAS), include post-cardiac arrest brain injury, post-cardiac arrest myocardial dysfunction, and systemic ischemia/reperfusion response (3).
Post-cardiac arrest brain injury accounts for 68% of deaths in patients with out-of-hospital heart arrest and 23% of deaths in patients with in-hospital heart arrest (4). The mechanisms of post-cardiac arrest brain injury include immediate depletion of cellular energy sub- strate and switch to anaerobic metabolism, intra- cellular acidosis, hypercalcemia, mitochondrial dysf- unction, release of glutamate, and neuronal hyperex- citability (5).
Oxidative stress damage plays a crucial role in cerebral ischemia/reperfusion (I/R) pathogenesis. Reactive oxygen species (ROS) generated enormously during cerebral I/R, lead to oxidative stress. The nuclear factor Nrf2/antioxidant response element (ARE) signaling pathway is activated to protect cells from oxidative stress-induced cell death, and it is regarded as the most important pathway to protect against oxidative stress.
The Nrf2/ARE pathway regulates the expression of ARE-mediated phase II and antioxidant enzyme genes, including heme-oxygenase-1 (HO-1) (6, 7).
Mild therapeutic hypothermia has been accepted as a standard therapeutic treatment for post resuscitation care. Hypothermia reduces cerebral metabolic rate, 6-10% for each 1°C drop in body temperature and then alleviates some destructive processes such as free radicals production, mitoch- ondrial injury, the lack of cell membrane, and ion pump dysfunction (8). Animal and clinical studies have shown that hypothermia leads to better functional outcome compared to normothermia (9). Mild hypothermia improved blood pressure, survival, and neurological outcome during hemorrhagic shock in rats (10). Early induction of hypothermia has favorable neurological outcome in patients with out-of-hospital heart arrest (11).
In this study, we tested the hypothesis that the mechanism of neuroprotective effect of hypothermia is the activation of Nrf2/ARE signaling pathway, which alleviates oxidative stress damage.
Materials and Methods
The experimental protocol approved by the Animal Committee of Anhui Medical University and conducted in accordance with the requirements of Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health (NIH Publication.No.80-23.1996). Adult male Sprague-Dawley rats weighing 250-400 g were used in these experiments.
Experimental design
36 adult male Sprague-Dawley rats were assigned at random to three groups: normothermia group, mild hypothermia before ROSC group, and mild hypothermia after ROSC group (n=12). Each group then divided into three subgroups: 15 min, 30 min, and 60 min (n=4).
Four sham rats also included. Body temperature in normothermia group was maintained at a normal body temperature of 37.5~39°C (9, 12), while mild hypothermia group maintained at 32~34°C by surface cooling with the ice pack.
Experimental asphyxia-CA model
The rats were fasted overnight and anesthetized with an intraperitoneal injection of 10% chloral hydrate (0.35 ml/100g). Tracheotomy and tracheal intubation was performed with a 14-gauge plastic catheter, the cannula was advanced for a distance of 10 mm into the trachea. Controlled intermittent positive pressure ventilation (HX-300S, Chengdu Technology and Market Co Ltd) with tidal volumes of 1.0 ml/100g at a rate of 50 breaths/min was used. Electrocardiography (ECG) limb leads were attached for cardiac monitoring. A 22G close-vein indwelling needle was placed into the left femoral artery for continuous monitoring of mean arterial blood pressure (MAP). A 24G close-vein indwelling needle was placed into the right femoral vein for intravenous drug administration. The T-branch pipe was connected to the remaining arterial needle. One end was connected to the physiological monitoring recorder (BL-420S, Chengdu Technology and Market Co Ltd), and the other end was connected to an injector with heparin.
Asphyxia was induced by clamping the tubing at the end of expiratory. As demonstrated in Figure 1, the standard criteria for CA were: ① the systolic arterial pressure decreased below 25 mmHg; ② the arterial pulse wave disappeared from blood pressure monitoring; ③ the electrocardiographic wave indicated ventricular fibrillation (VF), pulseless electrical (PEA), or asystole (13).
Figure 1.
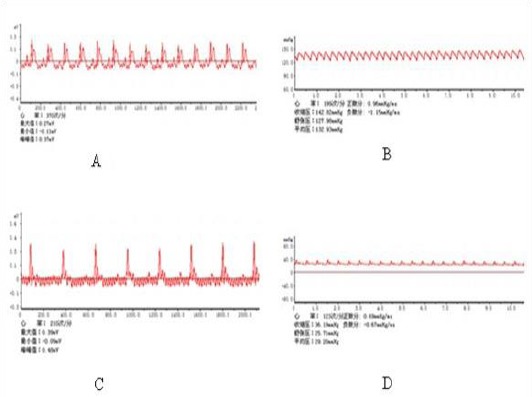
A Normal electrocardiograph of the rat, B Normal atrial pressure of the rat, C Electrocardiograph of the rat when cardiac arrest occurs, D Atrial pressure of the rat when cardiac arrest occurs
Cardiopulmonary resuscitation model
CPR was initiated at 3 min after heart arrest and lasted for a maximum of 10 minutes. Chest compressions were carried out at a rate of 200 compressions/min with equal compression-relaxation, and depth of compression to 1/3 of the anteroposterior chest diameter. Intermittent positive pressure ventilation were carried out with tidal volumes of 0.65 ml/100 g at a rate of 70 breaths/min. Adrenalin (2 ug/100 g) was immediately administrated at the beginning of CPR and repeated at 3 min if necessary. The criterion of ROSC was: ① supraventricular cardiac rhythm was restored; ② the average arterial was more than 60 mmHg for at least 10 min (13).
Extraction of brain tissue
Animals were anesthetized and euthanized at 15 min, 30 min, and 60 min. Brain was extracted rapidly and rinsed thoroughly with 0.9% sodium chloride at 4 °C. Then brain tissue was separated into left and right hemisphere.
Left hemisphere was separated into cerebral cortex and hippocampus, and frozen in liquid nitrogen immediately for RT-PCR. Right hemisphere was fixed in 4% paraformaldehyde for 24-48 hr, then moved into 70% alcohol for hematoxylin-eosin (H&E) staining and immunohistochemistry.
H&E staining
Approximately 1 µm thick sections of the paraffin-embedded brain tissues were stained with H&E and examined under a light microscope (from Olympus, Japan, original magnification×2000). The brain samples underwent washing, dehydration, clearing, wax dipping, embedding, slicing, coating, grilling, and staining with H&E.
RT-PCR
Total RNA from cerebral cortex and hippocampus was isolated from TRIzol homogenate following the Invitrogen protocol. Total RNA (2 μg) was used for reverse transcription (RT) using the Promega RT kit to synthesize double-stranded complementary DNA (cDNA). The following primer pairs were used to evaluate the resultant cDNA: β-actin, 5′-CACCCGC-GAGTACAACCTTC-3′ and 5′-CCCATACCCACCATCA-CACC-3′; Nrf2, 5′-CCAGCACATCCAGACAGAC-3′ and 5′-TATCCAGGGCAAGCGACTC-3′; HO-1, 5′-GCTCTATCGTGC TCGCATGA-3′ and 5′-AATTCCCACTGCCACGGTC-3′. PCR was carried out in a 50 μl reaction volume using PCR Master Mix (Fermentas). Reactions conditions were as follows: initial denaturation for 4 min at 94 °C, 35 cycles of denaturation (30 sec, 94 °C), annealing (30 sec, 57 °C), and extension (30 sec, 72 °C). Amplification products were run in 2% agarose gel and the average band intensities were determined using a Gel Doc 1,000 image analysis system. The mean density per band was estimated by the standard method with housekeeping gene β-actin as the reference gene.
Immunohistochemistry
Immunohistochemical characterization of Nrf2 and HO-1 was performed using polyclonal goat antibody (from Beijing Biosynthesis Biotechnology Co. LTD., Beijing, China). In brief, sections were washed three times by Tris-buffer saline (TBS) and incubated in blocking solution of 10% goat serum for 1 hr, and then incubated overnight at 4 °C with primary antibody (from Beijing Biosynthesis Biotechnology Co. LTD, Beijing, China, dilution 1:100), followed by three times washing with TBS. Sections were then incubated in appropriate secondary antibodies (from Beijing Biosynthesis Biotechnology Co. LTD, Beijing, China, dilution 1:200) for 1 hr at room temperature. 3, 3’-diaminobenzidine (DAB) (Beijing Zhongshan Golden Bridge Biotechnology Co, Ltd., Beijing, China) was used as a chromogen to make the color reaction.
Statistical analysis
SPSS 16.0 for Windows was used for statistical analysis. Results were expressed as Mean ± SD, and P<0.05 was defined as significant. Statistical analysis was performed by one-way analysis of variance followed by post hoc test for multiple comparisons.
Results
Expression of Nrf2 and HO-1 in cerebral cortex and hippocampus increased after CPR in rats in early stage
We analyzed the expression of Nrf2 and HO-1 mRNA and protein in cerebral cortex and hippocampus in early stage of 15 min, 30 min, and 60 min. There was a statistically significant difference between sham group and each model group (P<0.05, respectively); expression of Nrf2 and HO-1 mRNA and protein had significant increase in model group (Table 1 and Table 2).
Table 1.
Reverse transcription-polymerase chain reaction results of NF-E2-related factor 2 and heme-oxygenase-1 (HO-1) in cerebral cortex and hippocampus. There was significant difference between sham group and each model group (P<0.05); expression of NF-E2-related factor 2 and heme-oxygenase-1 mRNA had significant increase in model group
| Group | Nrf2 in cortex | Nrf2 in hippocampus | HO-1 in cortex | HO-1 in hippocampus |
|---|---|---|---|---|
| Sham | 0.22±0.05 | 0.25±0.03 | 0.40±0.07 | 0.30±0.05 |
| Normothermia group 15 min | 0.43±0.03 | 0.43±0.09 | 0.58±0.09 | 0.49±0.06 |
| Mild hypothermia before ROSC 15 min | 0.49±0.03 | 0.47±0.06 | 0.76±0.08 | 0.61±0.06 |
| Mild hypothermia after ROSC 15 min | 0.49±0.07 | 0.48±0.02 | 0.70±0.04 | 0.65±0.03 |
| Normothermia group 30 min | 0.36±0.04 | 0.42±0.07 | 0.58±0.10 | 0.46±0.07 |
| Mild hypothermia before ROSC 30 min | 0.48±0.05 | 0.54±0.05 | 0.75±0.11 | 0.63±0.09 |
| Mild hypothermia after ROSC 30 min | 0.47±0.07 | 0.56±0.09 | 0.78±0.07 | 0.63±0.10 |
| Normothermia group 60 min | 0.34±0.05 | 0.39±0.05 | 0.64±0.06 | 0.48±0.09 |
| Mild hypothermia before ROSC 60 min | 0.52±0.06 | 0.57±0.03 | 0.82±0.09 | 0.74±0.08 |
| Mild hypothermia after ROSC 60 min | 0.48±0.04 | 0.45±0.06 | 0.75±0.07 | 0.62±0.08 |
| F | 14.535 | 8.953 | 9.720 | 12.139 |
| P | 0.000 | 0.000 | 0.000 | 0.000 |
ROSC: Restoration of spontaneous circulation. F and P are value of statistical analysis
Table 2.
Immunohistochemistry results of NF-E2-related factor 2 and heme-oxygenase-1 (HO-1) in cerebral cortex and hippocampus. There was significant difference between sham group and each model group (P<0.05); expression of NF-E2-related factor 2 and heme-oxygenase-1 protein had significant increase in model group
| Group | Nrf2 in cortex | Nrf2 in hippocampus | HO-1 in cortex | HO-1 in hippocampus |
|---|---|---|---|---|
| Sham | 0.28±0.07 | 0.35±0.06 | 5.23±0.77 | 4.08±0.96 |
| Normothermia group 15 min | 0.62±0.05 | 0.65±0.07 | 10.47±1.93 | 9.23±2.51 |
| Mild hypothermia before ROSC 15 min | 0.74±0.19 | 0.70±0.11 | 14.88±1.90 | 13.90±2.60 |
| Mild hypothermia after ROSC 15 min | 0.81±0.15 | 0.63±0.05 | 13.19±1.86 | 13.30±1.29 |
| Normothermia group 30 min | 0.67±0.16 | 0.58±0.16 | 9.66±1.60 | 10.33±1.73 |
| Mild hypothermia before ROSC 30 min | 0.95±0.11 | 0.84±0.07 | 12.37±2.13 | 16.98±3.63 |
| Mild hypothermia after ROSC 30 min | 0.80±0.10 | 0.81±0.13 | 14.53±2.66 | 13.38±3.38 |
| Normothermia group 60 min | 0.56±0.12 | 0.57±0.14 | 10.01±0.90 | 10.45±2.14 |
| Mild hypothermia before ROSC 60 min | 1.06±0.17 | 0.90±0.13 | 15.03±3.79 | 15.33±2.78 |
| Mild hypothermia after ROSC 60 min | 0.76±0.22 | 0.71±0.08 | 14.32±2.65 | 15.83±2.37 |
| F | 9.546 | 8.520 | 6.722 | 9.636 |
| P | 0.000 | 0.000 | 0.000 | 0.000 |
ROSC: Restoration of spontaneous circulation.
Mild hypothermia improved histopathological change of brain tissues
Pathological structure of brain tissue have significantly improved in mild hypothermia group compared with normothermia group; hippocampus neurons arranged closely with clear structure, neuron structure was more complete, the widened extent of neurons and glial cells around the gap was reduced. The nuclei of nerve cells pyknosis were not evident and no significant degeneration and atrophy was observed. But no significant difference was found between mild hypothermia before ROSC group and mild hypothermia after ROSC (Figure 2).
Figure 2.

Hematoxylin-eosin staining results of cerebral cortex and hippocampus after cardiopulmonary resuscitation in rats (original magnification×2000). A Hematoxylin-eosin staining of cerebral cortex, B Hematoxylin-eosin staining of hippocampus. a sham group, neurons in hippocampus is well arranged, the body of the neuron has integrity, and the cytoplasm is rich, dyeing uniformity is demonstrated. The nucleus is big and round, light dye, nuclear membrane integrity and nucleoli are clear. b normothermia group, pathological structure have significantly changed, the normal neurons in hippocampus were significantly reduced, neuron shrinkage, cell body smaller, nucleus condensation, the nucleolus disappears, cells with various shapes, coloring dark, neurons and glial cells around the gap widened. c mild hypothermia before restoration of spontaneous circulation, d mild hypothermia after restoration of spontaneous circulation. Compare to b, pathological structure have significantly improved, hippocampus neurons arranged closely, clear structure, neuron structure is more complete, neurons and glial cells around the gap widened extent reduced, the nuclei of nerve cells pyknosis were not evident, no significant degeneration and atrophy. There are no significant changes in pathological patterns between c and d
Mild hypothermia increased expression of Nrf2 and HO-1 in cerebral cortex and hippocampus after CPR
RT-PCR analysis showed that expression pattern of Nrf2 and HO-1 mRNA in cerebral cortex and hippo-campus of normothermia and mild hypother- mia groups was significantly different after CPR (P<0.05); expression in mild hypothermia was higher than that in normothermia group (Figure 3). In accordance with the results of RT-PCR, immune- histochemistry analysis showed that the expression of Nrf2 and HO-1 protein in cerebral cortex and hippocampus after CPR had the same trend in normothermia and mild hypothermia groups (Figure 4).
Figure 3.
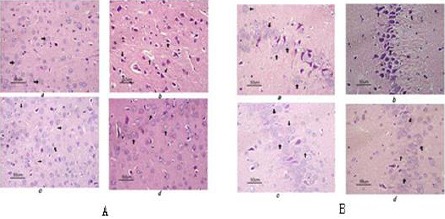
Electrophoresis of the expression of NF-E2-related factor 2 and heme-oxygenase-1 mRNA in cerebral cortex and hippocampus in rat. A expression of NF-E2-related factor 2 and heme-oxygenase-1 mRNA in cerebral cortex, B expression of NF-E2-related factor 2 and heme-oxygenase-1 mRNA in hippocampus. a sham group, b normothermia group, c mild hypothermia before restoration of spontaneous circulation, d mild hypothermia after restoration of spontaneous circulation, e 15 min after restoration of spontaneous circulation with mild hypothermia, f 30 min after restoration of spontaneous circulation with mild hypothermia, g 60 min after restoration of spontaneous circulation
Figure 4.
Immunohistochemisty results of NF-E2-related factor 2 and heme-oxygenase-1 in cerebral cortex and hippocampus after cardiopulmonary resuscitation in rat. A NF-E2-related factor 2 in cerebral cortex, B heme-oxygenase-1 in cerebral cortex, C NF-E2-related factor 2 in hippocampus, D heme-oxygenase-1 in hippocampus. a sham group, b normothermia group, c mild hypothermia before restoration of spontaneous circulation, d mild hypothermia after restoration of spontaneous circulation. There is a significant increase in b compared to a, and c, d compare to b; expression of NF-E2-related factor 2 and heme-oxygenase-1 increased notably in c, and d
But the expression of Nrf2 and HO-1 mRNA and protein had no significant difference (P>0.05) between mild hypothermia before ROSC group and mild hypothermia after ROSC group; the expression of Nrf2 and HO-1 mRNA and protein had no significant difference (P>0.05) at three time points of 15 min, 30 min, and 60 min (Table 3 and Table 4).
Table 3.
Post hoc test for multiple comparisons in expression of NF-E2-related factor 2 and heme-oxygenase-1 (HO-1) mRNA in cerebral cortex and hippocampus after cardiopulmonary resuscitation
| Factor | Nrf2 in cortex | Nrf2 in hippocampus | HO-1 in cortex | HO-1 in hippocampus |
|---|---|---|---|---|
| F P | F P | F P | F P | |
| Time | 1.537 0.233 | 1.630 0.215 | 1.280 0.294 | 1.088 0.351 |
| Temperature | 20.697 0.000 | 10.083 0.001 | 15.752 0.000 | 21.369 0.000 |
| Time*temperature | 1.715 0.176 | 2.119 0.106 | 0.472 0.756 | 1.362 0.273 |
Table 4.
Post hoc test for multiple comparisons in expression of NF-E2-related factor 2 and heme-oxygenase-1 (HO-1) protein in cerebral cortex and hippocampus after cardiopulmonary resuscitation
| Factor | Nrf2 in cortex | Nrf2 in hippocampus | HO-1 in cortex | HO-1 in hippocampus |
|---|---|---|---|---|
| F P | F P | F P | F P | |
| Time | 1.266 0.298 | 1.859 0.175 | 0.526 0.597 | 1.522 0.236 |
| Temperature | 12.118 0.000 | 10.558 0.000 | 12.239 0.000 | 14.353 0.000 |
| Time*temperature | 2.209 0.095 | 2.468 0.069 | 0.850 0.506 | 0.700 0.599 |
Discussion
The pathophysiology of heart arrest is myocardial pump function that is suddenly interrupted leading to viscera ischemia. Immediate CPR makes the body to regain part of blood flow. However, it is not enough to satisfy organs blood supply and can even cause reperfusion injury. Thus, some scholars put forward ‘two-stage’ theory of ischemia and reperfusion after heart arrest: low blood flow stage and reperfusion stage. The pathological processes of brain injury after heart arrest include the primary injury in low blood flow stage and the secondary injury in reperfusion stage. Brain tissues were ischemic and anoxic after heart arrest, and then decreased ATP production, Na+-K+ pump dysfunction, the cellular structure damage, release of the glutamic acid to promote Ca+ flowing into the cell that leads to excitability damage and mitochondrial dysfunction, which subsequently generate a large number of free radicals to cause a serious of harmful proteins reaction, result in the nerve cell death (14, 15).
Nrf2-ARE signaling pathway has been regarded as the most important pathway in the cell to protect against oxidative stress. Study in vivo and in vitro found that Nrf2-ARE pathway has neuroprotective function, which can block neurotoxicity resulting from the lack of glutathione, lipid peroxidation, intracellular calcium overload, excitotoxins, and mitochondrial electron transport chain disruption (16-18). In recent years, the protective action on cerebral ischemia and hypoxia disease of Nrf2-ARE has come out. One study found that compared to wild type, GPX1-/- mice with middle cerebral artery occlusion (MCAO) showed higher ROS production, increased microvascular perfusion, and aggravated cerebral ischemia/reperfusion injury. However, Nrf2-ARE pathway is activated and enhanced gene expression of numerous cytoprotective, antioxidant and detoxifying proteins and enzymes, to attenuate cerebral ischemia (19).
CPR guidelines recommended mild hypothermia in the treatment of heart arrest syndrome. On the one hand, hypothermia can reduce cerebral metabolic rate to reduce the oxygen consumption. On the other hand, it can inhibit response to reperfusion injury, including generation of free radicals, release of the excitability neurotransmitter and lipid peroxidation. However, it is shown that mild hypothermia did not reduce cerebral metabolic rate significantly (20). Therefore, restraining ischemia/reperfusion injury may be more reasonable as a protection mechanism for mild hypothermia.
In view of above, we hypothesized that: ① endogenous antioxidant system has different reactive between low blood flow stage of CPR and reperfusion stage after ROSC, which result in mild hypothermia intervention time may induce different result; ② mild hypothermia induces numerous physiological pathology process, and it has influences on Nrf2-ARE pathway, which is regarded as the most important pathway to protect against oxidative stress.
In our study, compared with normothermia group, hippocampus neurons arranged closely with clear structure, neuron structure was more complete, the widened extent of neurons and glial cells around the gap was reduced, the nuclei of nerve cells pyknosis were not evident, and no significant degeneration and atrophy was observed in mild hypothermia group. These rsults confirmed that mild hypothermia has protective effects on cerebral.
Our study demonstrated that the expression of Nrf2 and HO-1 in cerebral cortex and hippocampus after CPR increased in early stage of 15 min, 30 min, and 60 min, and mild hypothermia upregulated the expression of these genes. It drew a conclusion that Nrf2-ARE pathway was activated in cerebral cortex and hippocampus after CPR, and one possible mechanism of mild hypothermia for neuroprotection was the activation of Nrf2-ARE pathway. Also, there was no significant difference in different intervention time; it inferred that there was no difference in endogenous antioxidant system at different stage, and low blood flow stage and reperfusion stage. It may be partly due to the short observation time that the difference was not significant. We also found that the expression of Nrf2 and HO-1 has no significant difference at three time points of 15 min, 30 min, and 60 min, illustrating that there was no time dependence in early stage after CPR on expression of Nrf2 and HO-1.
Conclusion
In different period of low blood flow stage and completely reperfusion stage, whether the endogenous antioxidant system has difference, is still need to be further explored. There is no time dependence in early stage after CPR on expression of Nrf2 and HO-1 in cerebral cortex. Also in later stage, along with the time, the expression of Nrf2 and HO-1 would be increased or reduced, needs to be further investigated to explore the effects of activated Nrf2-ARE pathway on neuroprotection with mild hypothermia.
Mild hypothermia has neuroprotective effects on cerebral ischemia/reperfusion injury after heart arrest. The possible mechanism is the activation of Nrf2-ARE pathway in cerebral cortex and hippocampus after CPR.
Acknowledgment
This work was supported by a national key specialty construction of clinical of China. The results described in this paper were part of student thesis.
References
- 1.Ali B, Zafari AM. Narrative review:cardiopulmonary resuscitation and emergency cardiovascular care:review of the current guidelines. Ann Intern Med. 2007;147:171–179. doi: 10.7326/0003-4819-147-3-200708070-00006. [DOI] [PubMed] [Google Scholar]
- 2.Hua W, Zhang LF, Wu YF, Liu XQ, Guo DS, Zhou XL, et al. Incidence of sudden cardic death in China-analysis of 4 regional populations. JACC. 2009;54:1110–1118. doi: 10.1016/j.jacc.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 3.Robert WN, Jerry PN, Christophe A. Post–cardiac arrest syndrome:epidemiology, pathophysiology, treatment, and Prognostication a consensus statement from the international liaison committee on resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa);the American Heart Association Emergency Cardiovascular Care Committee;the Council on Cardiovascular Surgery and Anesthesia;the Council on Cardiopulmonary, Perioperative, and Critical Care;the Council on Clinical Cardiology;and the Stroke Council. Circulation. 2008;118:2452–2483. doi: 10.1161/CIRCULATIONAHA.108.190652. [DOI] [PubMed] [Google Scholar]
- 4.Laver S, Farrow C, Turner D, Nolan J. Mode of death after admission to an intensive care unit following cardiac arrest. Intensive Care Med. 2004;30:2126–2128. doi: 10.1007/s00134-004-2425-z. [DOI] [PubMed] [Google Scholar]
- 5.Navaz K, Romergryko GG. Post-cardiac arrest syndrome update on brain injury management and prognostication. Curr Treat Options Neurol. 2011;13:191–203. doi: 10.1007/s11940-011-0112-2. [DOI] [PubMed] [Google Scholar]
- 6.Kobayash M, Yamamoto M. Nrf2-keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv Enzyme Regul. 2006;46:113–140. doi: 10.1016/j.advenzreg.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Chan KH, Ng MK, Stocker R. Haem oxygenase-1 and cardiovascular disease:mechanisms and therapeutic potential. Clin Sci. 2011;120:493–504. doi: 10.1042/CS20100508. [DOI] [PubMed] [Google Scholar]
- 8.Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med. 2009;37:S186–202. doi: 10.1097/CCM.0b013e3181aa5241. [DOI] [PubMed] [Google Scholar]
- 9.Zhao H, Chen Y. Effects of mild hypothermia therapy on the levels of glutathione in rabbit blood and cerebrospinal fluid after cardiopulmonary resuscitation. Iran J Basic Med Sci. 2015;18:194–198. [PMC free article] [PubMed] [Google Scholar]
- 10.Deniz T, Agalar C, Ozdogan M, Edremitlioglu M, Eryilmaz M, Devay SD, et al. Mild hypothermia improves survival during hemorrhagic shock without affecting bacterial translocation. J Invest Surg. 2009;22:22–28. doi: 10.1080/08941930802566706. [DOI] [PubMed] [Google Scholar]
- 11.Takeuchi I, Takehana H, Satoh D, Fukaya H, Tamura Y, Nishi M, et al. Effect of hypothermia therapy after outpatient cardiac arrest due to ventricular fibrillation. Circ J. 2009;73:1877–1880. doi: 10.1253/circj.cj-09-0088. [DOI] [PubMed] [Google Scholar]
- 12.Lu J, Shen Y, Qian H, Liu L, Zhou B, Xiao Y, et al. Effects of mild hypothermia on the ROS and expression of caspase-3 mRNA and LC3 of hippocampus nerve cells in rats after cardiopulmonary resuscitation. World J Emerg Med. 2014;4:298–304. doi: 10.5847/wjem.j.issn.1920-8642.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Idris AH, Becker LB, Ornato JP, Hedges JR, Bircher NG, Chandra NC, et al. Utstein-style guidelines for uniform reporting of laboratory CPR research. A statement for healthcare professionals from a task force of the American Heart Association, the American College of Emergency Physicians, the American College of Cardiology, the European Resuscitation Council, the Heart and Stroke Foundation of Canada, the European Resuscitation Care Medicine, the Safar Center for Resuscitation Research, and the Society for Academic Emergency Medicine. Writing Group. Circulation. 1996;94:2324–2336. doi: 10.1161/01.cir.94.9.2324. [DOI] [PubMed] [Google Scholar]
- 14.Bano D, Nicotera P. Ca2+signals and neuronal death in brain ischemia. Stroke. 2007;38:674–676. doi: 10.1161/01.STR.0000256294.46009.29. [DOI] [PubMed] [Google Scholar]
- 15.Geocadin RG, Koenig MA, Stevens RD, Peberdy MA. Intensive care for brain injury after cardiac arrest:therapeutic hypothermia and related neuroprotective strategies. Crit Care Clin. 2006;22:619–636. doi: 10.1016/j.ccc.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 16.Lee JM, Shih AY, Murphy TH, Johnson JA. NF-E2-related factor-2 mediateds neuroproteciton against mitochondrial complex I inhibitors and increased concentrations of intracellular calcium in primary cortical neurons. J Biol Chem. 2003;278:37948–37956. doi: 10.1074/jbc.M305204200. [DOI] [PubMed] [Google Scholar]
- 17.Mizuno K, Kume T, Muto C, Takada-Takatori Y, Izumi Y, Sugimoto H, et al. Glutathione biosynthesis via activation of the nuclear factor E2-related factor 2 (Nrf2)--antioxidant-response element (ARE) pathway is essential for neuroprotective effects of sulforaphane and 6-(methylsulfinyl) hexyl isothiocyanate. J Pharmacol Sci. 2011;115:320–328. doi: 10.1254/jphs.10257fp. [DOI] [PubMed] [Google Scholar]
- 18.Calkins MJ, Jakel RJ, Johnson DA, Chan K, Kan YW, Johnson JA. Protection from mitochondrial complex I inhibition in vitro and in vivo by Nrf2-mediated transcription. Proc Natl Acad Sci USA. 2005;102:244–249. doi: 10.1073/pnas.0408487101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen MJ, Wong CH, Peng ZF, Manikandan J, Melendez AJ, Tan TM, et al. A global transcriptomic view of the multifaceted role of glutathione peroxidase-1 in cerebral ischemic-reperfusion injury. Free Radic Biol Med. 2011;50:736–748. doi: 10.1016/j.freeradbiomed.2010.12.025. [DOI] [PubMed] [Google Scholar]
- 20.Grigore AM, Murray CF, Ramakrishna H, Djaiani G. A Core Review of Temperature Regimens and Neuroprotection During Cardiopulmonary Bypass:Does Rewarming Rate Matter? Anesth. Analg. 2009;109:1741–1751. doi: 10.1213/ANE.0b013e3181c04fea. [DOI] [PubMed] [Google Scholar]


