Abstract
Objective(s):
In previous studies, researchers observed that doxepin could improve cognitive processes and has protective effects on the central nervous system. Thus, this study was designed to analyze the effects of doxepin on β-amyloid (Aβ)-induced memory impairment and neuronal toxicity in rat and to explore the underlying mechanism.
Materials and Methods:
Rats were treated with Aβ1-42 and doxepin was injected to validate its effects on cognitive function. The Morris water maze test was performed to detect memory function. Aβ1-42-treated SH-SY5Y human neuroblastoma cell line was also used to detect the effects of doxepin and to explore the underlying mechanism. Western blotting analysis was used to detect the protein expression levels of PSD-95, synapsin 1, p-AKT and p-mTOR in rats.
Results:
After treated with 1 mg/kg of doxepin, Aβ1-42-treated rats showed markedly lower escape latency and higher platform-finding strategy score. Low doses of doxepin significantly reversed the effects of Aβ1-42 on the protein expression levels of PSD-95, synapsin 1, p-AKT and p-mTOR in rats. In vitro experiment showed the consistent results. Besides, PI3K inhibitor (LY294002) treatment could markedly reversed the effects of doxepin on Aβ1-42-treated SH-SY5Y cells.
Conclusion:
Our results demonstrated that doxepin could protect against the Aβ1-42-induced memory impairment in rats. The protective effect of doxepin was associated with the enhancement of PSD-95 and synapsin 1 expression via PI3K/AKT/mTOR signaling pathway.
Keywords: Alzheimer’s disease, Doxepin, Memory injury, PI3-K/AKT/mTOR- signaling, β-amyloid1-42
Introduction
Alzheimer’s disease (AD) influences 13% of the population older than 65 years and 43% older than 85 years (1). AD is a genetically complex multifactorial neurodegenerative disorder and can be regarded as the most common type of dementia, defined by the formation of extracellular amyloid-β (Aβ) plaques, the accumulation of intracellular neurofibrillary tangles (NFTs) in the brain, as well as extensive neuronal and synapses loss (2). The progressive neurodegeneration leads to severe memory loss and other cognitive deficits such as judgment, reasoning and orientation (3). Neuropsychiatric symptoms such as depression, apathy, and aggression/agitation occur in the majori-ty of patients with dementia (4). Different studies have suggested that antidepressant drugs (ATD) are frequently prescribed to patients with AD by reducing cognitive decline and improving cognitive function (5, 6).
Doxepin is a tricyclic antidepressant that inhibits reuptake of serotonin and norepinephrine (7). Local application of doxepin has therapeutic effects in atopic dermatitis (8) and it is used as second-line therapy for chronic urticarial (9). Doxepin has also been proposed as a protective factor against oxidative stress. Doxepin has the ability to reduce calcium signaling, which is a key factor in the oxidative stress-induced damages, exerting protective effects against oxidative stress (10). Moreover, doxepin reduced lipid peroxidation and increased antioxidants such as superoxide dismutase (11). Particularly, in previous studies, researchers observed that doxepin could improve cognitive processes and has protective effects on the central nervous system (12, 13). Thus, this study was designed to explore the underlying mechanism.
Investigation of the status of the PI3-K/AKT system in brains of individuals who have had AD shows aberrant and sustained activation of neuronal PI3-K/AKT/mTOR signaling to be an early feature of the disease (14). Thus, we hypothesize that the effects of doxepin on cognitive function may be associated with the PI3-K /AKT/mTOR signaling pathway. It is well- accepted that synapse loss and synaptic impairment were appeared in the early stages of AD (15). Synaptic proteins, like synapsin and post-synaptic density-95 (PSD-95), play crucial roles in synapse maturation and plasticity (16). Soluble Aβ
treatment was reported to significantly reduce the level of these proteins in rat hippocampal neurons (17). Therefore, the effects of doxepin on synapse loss and synaptic impairment were analyzed by the detection of the protein levels of PSD-95 and synapsin 1 in our study.
Here, rats were treated with Aβ1-42 and doxepin was injected to validate its effects on cognitive function. The Morris water maze test was performed to detect memory function, and the Western blotting analysis was used to detect the protein expression of PSD-95, synapsin 1, p-AKT and p-mTOR in rats. Besides, the mechanism underlying the effects of doxepin was explored in the in vitro experiment. To sum up, our results showed that doxepin could protect against the Aβ1-42-induced memory impairment and neuronal toxicity in rats and the underlying mechanism may be associated with the mTOR/AKT signaling pathway.
Materials and Methods
Animals
A total of 40 specific pathogen free (SPF) SD male rats (8 weeks old), weighing 180 ~ 220 g, were obtained from Shanghai West Poole-Bikey Experimental Animal Co., Ltd. (Shanghai, China). The animals were kept on a 12-hr light/dark cycle, housed in plastic cages under the temperature (22 ± 3 °C) and humidity (55 ± 5%), and were allowed to have free access to food and water. The experimental procedures were approved by the Animal Ethics Committee of Jinshan Hospital, Fudan University (Shanghai, China).
Treatment and groups
Rats were randomly divided into 4 groups (n=10 in each group): Group A, rats were sham operated and injected with the same volume of normal saline and with an injection rate of 2 μl / min; Group B, rats were operated and injected with Aβ1-42; Group C, rats were operated and injected with Aβ1-42 and injected intraperitoneally with 1 mg/kg doxepin; and Group D, rats were operated and injected intraventri-cularly with Aβ1-42 and injected intraperitoneally with 5 mg/kg doxepin. Aβ1-42 and doxepin were purchased from Aladdin Chemicals (Shanghai, China). Aβ1-42 preparation was performed as previously described (18).
Rats were anesthetized with 10% chloral hydrate with an intraperitoneal injection of 300-350 mg/kg. Rats were fixed on a stereotaxic apparatus (Second Military Medical University, China), and the top of the head was cut to expose the anterior fontanel. Group B, C and D were injected with 5 μl of peptidoglycan Aβ1-42 into the lateral ventricle by microinjector. The stereotaxic coordinates for the injection are as follows: 0.8 mm posterior to the bregma, 1.3 mm left/right to the midline, and with a depth of 3.5 mm. The rats of group A were sham operated and injected with the same volume of normal saline and with an injection rate of 2 μl/min. For doxepin treatment, rats in group A and B were injected intraperitoneally with 2 ml normal saline, and rats in group C and D were given an intraperitoneal injection of 1 mg / kg and 5 mg / kg doxepin once a day for 21 days, respectively.
Morris water maze test
On next day of final drug treatment, the water maze apparatus (150 cm in diameter, 12 cm in platform diameter, 45 cm high) was filled to a depth of 35 cm with 22 ± 1 °C water. The pool was divided into four equal quadrants with an invisible platform (10 cm in diameter, 2 cm below the water surface) in the northern quadrant. All rats were received the Morris water maze test in the next day after drug treatment and were kept in the laboratory for 7 days to adapt to the laboratory environment. Training consisted of 20 consecutive trials (4 trials each day for 5 consecutive days). The rat was placed in the water facing the pool wall at one of four randomly determined starting locations. The rat was then allowed 120 sec to search for the platform. Once the rat located the platform, it was permitted to remain on it for 30 sec. If the rat did not locate the platform within this time, it was placed on the platform for 30 sec. Between trials rats were removed from the water and placed on a dry surface for 60 sec before the start of the next trial. At the end of the experiment, the time required to locate the escape platform (escape latency, in seconds) and the strategy of rats to find the hidden platform were recorded in each trial for a given animal. The strategy of rats to find the hidden platform was scored: (marked 1 score, aimless way and around the pool border; marked 2 scores, random way; marked 3 scores, hesitating first and then straight way; marked 4 scores, prompt and straight way) (19).
Cell culture
SH-SY5Y human neuroblastoma cell line was pur-chased from American Type Tissue Culture (Manassas, VA, USA), and cultured in Dulbecco minimum essential medium (DMEM) supplemented with 12% heat-inactivated fetal bovine serum (FBS), 100 U/ml penicillin and 100 ug/ml streptomycin in a humidified incubator with 5% CO2 at 37 °C. Cells were trypsinized and seeded onto 12-well plates with 2×105 cells/well. Cells were divided into 4 groups: normal cells (Control group), cells cultured with Aβ1-42 (Aβ1-42 group), cells cultured with Aβ1-42 and doxepin (Aβ1-42+ doxepin group) and cells cultured with Aβ1-42 and LY294002 (Aβ1-42+LY294002 group). Aβ1-42 preparation was performed as previously described (18) and cells were with 5 μM Aβ1-42 for 48 hr. To evaluate the protection of doxepin on SH-SY5Y cells, the cells were detached, re-seeded on plates and then were preincubated with 10 ng/ml of doxepin for 2 hr. In experiments with 10 μM PI3K inhibitor LY294002 (Cell Signaling Technology, Beverly, MA, USA), cells were pretreated with the inhibitor for 1 hr prior to the addition of doxepin. LY294002 was dissolved in dimethyl sulfoxide (DMSO) and the final concen-tration of DMSO in culture medium was 0.1%.
Western blot
Hippocampal and temporal lobe tissues were collected and used for protein extract. Western blott-ing analysis was carried out as described previously (20). Proteins were lysed in radioimmunoprecipitation buffer (RIPA) containing protease inhibitors at 4 °C for 30 min. Lysates were prepared with a RIPA lysis buffer kit (Santa Cruz Biotechnology, Inc.), and the protein concentrations were quantified using a Bio-Rad protein assay (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Proteins (30 μg) were separated on 8% SDS-PAGE and transferred to polyvinylidene difluoride membranes (Amersham; GE Healthcare, Chicago, IL, USA). The membranes were blocked in 5% non-fat milk (Merck KGaA) overnight at 4 °C, followed by overnight incubation with the primary antibodies, anti-synapsin 1 (1:1500, (Chemicon International Inc., Temecula, CA, USA), anti-PSD-95 (1: 500; Cedarlane, Hornby, Ontario, Canada), anti- p-mTOR and p-AKT (all obtained from Cell Signaling Technology, Beverly, MA, USA). The membranes were washed and incubated with a horseradish peroxidase-conjugated secondary antibody (1: 1,000; Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China) at room temperature for 1 hr. Immuno-labeled protein bands were detected using an enhanced chemiluminiscence system (Amersham Biosciences, Freiburg, Germany) following an autoradio-graphic exposure to HyperfilmECL (Amersham Bio-sciences). The quantification of the signals was performed by densitometry using the program ImageMaster I-D (Pharmacia, Uppsala, Sweden).
Statistical analysis
Statistical analyses were conducted with the SPSS 16.0 software packages (SPSS Inc., Chicago, IL, USA). Data were represented as mean±SD. Comparisons between experimental and control groups were performed by one-way ANOVA with Tukey’s post-hoc analysis. Differences were considered to be statistically significant when P-value was less than 0.05.
Results
The results of Morris water maze test of Aβ1-42-treated rats after doxepin treatment
The memory function of rats was examined by observing the time to find the hidden platform (escape latency) and the platform-finding strategy score via the Morris water maze test. As shown in Table 1, the escape latency in Group B was significantly higher, and platform-finding strategy score was significantly lower than that in Group A (P<0.05), which may indicate the successful induction of AD model by Aβ1-42 treatment. After treated with 1 mg/kg of doxepin, Aβ1-42-treated rats showed markedly lower escape latency and higher platform-finding strategy score as compared to Group B (P<0.05), which suggests the effective effects of doxepin on memory function of Aβ1-42-treated rats. However, after treatment with 5 mg/kg of doxepin, we found that these alterations were not obvious.
Table 1.
The results of Morris water maze test after doxepin treatment
| Control (Group A) | Aβ1-42 (Group B) | Doxepin (1 mg/kg) +Aβ1-42 (Group C) | Doxepin (5 mg/kg) +Aβ1-42 (Group D) | |
|---|---|---|---|---|
| Eescape latency (seconds) | 21.45±4.41 | 34.68±5.02* | 31.50±5.85*# | 33.68±4.32* |
| The platform-finding strategy scores | 2.93±0.66 | 1.80±0.69* | 2.15±0.74*# | 1.93±0.66* |
P<0.05 compared to Group A, and
P<0.05 compared to Group B
The protein expression levels of PSD-95, synapsin 1, p-AKT and p-mTOR in hippocampus and temporal lobe of Aβ1-42-treated rats after doxepin treatment
As shown in Figure 1, the protein expression levels of PSD-95 and synapsin 1 in hippocampus and tem-poral lobe of Aβ1-42-treated rats were significantly decreased as compared to the control group (P<0.05). The protein expression level of p-AKT in hippocampus and temporal lobe of Aβ1-42-treated rats was signi-ficantly decreased (P<0.05), but the protein expre-ssion level of p-mTOR in hippocampus and tempo-ral lobe of Aβ1-42-treated rats was significantly increased (P<0.05). Indeed, after treatment of 1 mg/kg of doxepin, the expression alterations of these proteins were significantly reversed. However, after treatment of 5 mg/kg of doxepin, the expression alterations of these proteins were not significantly reversed.
Figure 1.
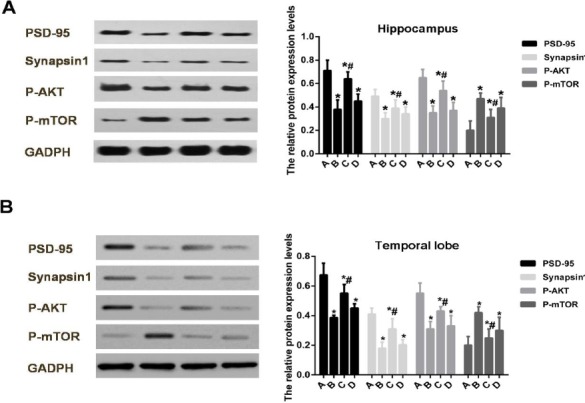
The protein expression levels of PSD-95, synapsin 1, p-AKT and p-mTOR in hippocampus and temporal lobe of Aβ1-42-treated rats after doxepin treatment
(A) The protein expression levels of PSD-95, synapsin 1, p-AKT and p-mTOR in hippocampus of AD rats after doxepin treatment. (B)The protein expression levels of PSD-95, synapsin 1, p-AKT and p-mTOR in temporal lobe of AD rats after doxepin treatment. *P<0.05 compared to Group A, and #P<0.05 compared to Group B. Group A, rats were sham operated and injected with the same volume of normal saline and with an injection rate of 2 μl/min; Group B, rats were operated and injected with Aβ1-42; Group C, rats were operated and injected with Aβ1-42 and injected intraperitoneally with 1 mg/kg doxepin; and Group D, rats were operated and injected intraventricularly with Aβ1-42 and injected intraperitoneally with 5 mg/kg doxepin
The protein expression levels of PSD-95, synapsin 1, p-AKT and p-mTOR in SH-SY5Y cells after PI3K inhibitor LY294002 treatment
To further validate the effects of doxepin, the in vitro experiment was performed, which were shown in Figure 2. Firstly, we found that the protein expre-ssion levels of PSD-95, synapsin 1 and p-AKT in SH-SY5Y cells were significantly decreased as compared to the control group (P<0.05). The protein expression level of p-mTOR in SH-SY5Y cells was significantly increased (P<0.05). Doxepin treatment significantly reversed the expression levels of these proteins (P<0.05). After PI3K inhibitor LY294002 treatment, we found that the protein expression levels of PSD-95, synapsin 1 and p-AKT in SH-SY5Y cells were significantly decreased, and the protein expression level of p-mTOR was significantly increased as com-pared to the SH-SY5Y cells that treated with Aβ1-42 and doxepin (P<0.05).
Figure 2.
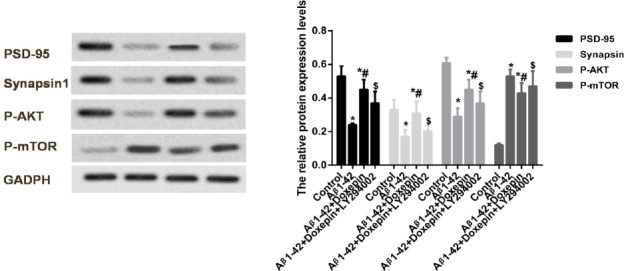
The protein expression levels of PSD-95, synapsin 1, p-AKT and p-mTOR in SH-SY5Y cells after PI3K inhibitor LY294002 treatment. *P<0.05 compared to the Control group, and #P<0.05 compared to Aβ1-42 group, and $P<0.05 compared to Aβ1-42 + doxepin group. Normal cells (Control group), cells cultured with 5 μM Aβ1-42 (Aβ1-42 group), cells cultured with 5 μM Aβ1-42 and 10 ng/ml doxepin (Aβ1-42 + doxepin group) and cells cultured with 5 μM Aβ1-42 and 10 μM LY294002 (Aβ1-42 + LY294002 group)
Discussion
Pathologically, the most recognized mechanism is that by which amyloid precursor protein (APP) can be cleaved by β – and γ -secretase generating A β1-42 oligomers (21, 22). Aβ deposition in neocortex and hippocampus may cause neuronal death, neuronal toxicity and synaptic failure, ultimately resulting in irreversible behavioral alterations and cognitive impairment (23). Thus, in our study, Aβ1-42 was used for the induction of AD rat model. We found that the escape latency in Group B was significantly higher, and platform-finding strategy score was significantly lower than that in Group A. To some extent, these results may indicate the successful induction of AD model.
After treatment with 1 mg/kg of doxepin, Aβ1-42-treated rats showed markedly lower escape latency and higher platform-finding strategy score as compared to Group B. However, after treatment with 5 mg/kg of doxepin, we found that these alterations were not obvious. Totally, these results suggest that doxepin exerts protective effects on memory impairment of Aβ1-42-treated rats. Consistently, previous study indicates that learning and memory are impaired in stressful conditions and doxepin prevented memory deficit (13). In our study, low concentration (1 mg/kg) of doxepin may have better effects than high concentration (5 mg/kg). Previous study suggested that low doses of doxepin affect the hypothalamus-pituitary-adrenal axis and can prevent the deleterious effects of stress. High doses of doxepin probably reduces the effects of stress on memory (13). Different doses of doxepin may have different effects, for example, doxepin at low doses but not at high doses has hypnagogic effects (24). One underlying mechanism may exist: doxepin at low doses can selectively antagonise H (1) receptors, which is believed to promote the initiation and maintenance of sleep (25).
It has been reported that soluble Aβ treatment significantly reduced the level of PSD-95 and synapsin 1 in rat hippocampal neurons (17). In our study, we found that the protein expression levels of PSD-95 and synapsin 1 in hippocampus and temporal lobe were significantly decreased after Aβ1-42 treatment as compared to the control group. However, low doses of doxepin significantly reversed the expression levels of these proteins. These investigations indicated that low doses of doxepin exert protective role in synapse maturation and plasticity through increasing the protein expression levels of PSD-95 and synapsin 1. However, we found that high doses of doxepin exert no significant effects on the expression levels of PSD-95 and synapsin 1. Previous study indicated that only low doses of doxepin (1 mg/kg) but not high doses (5 mg/kg or 10 mg/kg) decreased TNF-α level in the rat hippocampus (13). This study indicated that high doses of doxepin had no significant effects on the expression levels of PSD-95 and synapsin 1, as well as on the learning or memory ability. Though our study found the fact that low doses of doxepin could increase the protein expression levels of PSD-95 and synapsin 1, the underlying mechanism also needs further exploration.
PI3K, AKT, and mTOR pathways are vital cellular components that determine cell fate during acute and chronic disorders, such as Parkinson’s disease, Huntington’s disease, epilepsy, AD, stroke, and trauma (26). Among them, the kinases of PI3K are vital in the signaling pathways leading to the regulation of protein kinase B (PKB, also termed AKT) and mTOR (26). In our study, the protein expression level of p-AKT was significantly decreased, and the protein expression level of p-mTOR was significantly increased both in AD rats and in Aβ1-42-treated SH-SY5Y cells. And low doses of doxepin significantly reversed the expression levels of these proteins. Importantly, after PI3K inhibitor LY294002 treat-ment, we found that the protein expression levels of PSD-95, synapsin 1and p-AKT in SH-SY5Y cells were significantly decreased, and the protein expression level of p-mTOR was significantly increased as compared to the SH-SY5Y cells that treated with Aβ1-42 and doxepin. In concordance, significant increase in the level of phosphorylation of mTOR was found in AD temporal cortex compared with controls (27). Another study showed that Aβ treatment significantly decreased the protein level of p-AKT in PC12 cells. Pretreatment with isorhynchophylline (IRN) could significantly reverse the effect of Aβ on p-AKT which accounted for the protective mechanism of IRN against Aβ-induced neurotoxicity (28).
Conclusion
In summary, our results demonstrated that doxepin could protect against the Aβ1-42-induced memory impairment in rats. The protective effect of doxepin was associated with the enhancement of PSD-95 and synapsin 1 expression via PI3K/AKT/mTOR signaling pathway. The present study not only advances the knowledge regarding the neuroprotective mechanism of doxepin, but also laid a foundation for future clinical studies to evaluate the potential benefits of doxepin on AD patients.
Acknowledgment
This research was supported by the Health and Family Planning Commission of Jinshan District (Shanghai, China) (JSKJ-KTMS-2014-05).
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Tan L, Yu JT, Zhang W, Wu ZC, Zhang Q, Liu QY, et al. Association of GWAS-linked loci with late-onset Alzheimer’s disease in a northern Han Chinese population. Alzheimers Dement. 2012;9:546–553. doi: 10.1016/j.jalz.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Tan MS, Yu JT, Jiang T, Zhu XC, Wang HF, Zhang W, et al. NLRP3 polymorphisms are associated with late-onset Alzheimer’s disease in Han Chinese. J Neuroimmunol. 2013;265:91–95. doi: 10.1016/j.jneuroim.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Weintraub D, Rosenberg PB, Drye LT, Martin BK, Frangakis C, Mintzer JE, et al. Sertraline for the treatment of depression in Alzheimer disease:week-24 outcomes. J Geriatr Psychiatry Neurol. 2011;24:222–228. doi: 10.1177/0891988711422527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, Dekosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment:results from the cardiovascular health study. Jama J Am Med Assoc. 2002;288:1475–1483. doi: 10.1001/jama.288.12.1475. [DOI] [PubMed] [Google Scholar]
- 5.Nyth AL, Gottfries CG, Lyby K, Smedegaardandersen L, Gyldingsabroe J, Kristensen M, et al. A controlled multicenter clinical study of citalopram and placebo in elderly depressed patients with and without concomitant dementia. Acta Psychiatr Scand. 1992;86:138–145. doi: 10.1111/j.1600-0447.1992.tb03242.x. [DOI] [PubMed] [Google Scholar]
- 6.Mossello E, Boncinelli M, Caleri V, Cavallini MC, Palermo E, Di BM, et al. Is antidepressant treatment associated with reduced cognitive decline in Alzheimer’s disease? Dement Geriatr Cogn Dis. 2008;25:372–379. doi: 10.1159/000121334. [DOI] [PubMed] [Google Scholar]
- 7.Vermeeren A, Coenen AM. Effects of the use of hypnotics on cognition. Prog Brain Res. 2011;190:189. doi: 10.1016/B978-0-444-53817-8.00005-0. [DOI] [PubMed] [Google Scholar]
- 8.Drake LA, Fallon JD, Sober A. Relief of pruritus in patients with atopic dermatitis after treatment with topical doxepin cream. The Doxepin Study Group. J Am Acad Dermatol. 1994;31:613–616. doi: 10.1016/s0190-9622(94)70225-x. [DOI] [PubMed] [Google Scholar]
- 9.Hajak G, Rodenbeck A, Voderholzer U, Riemann D, Cohrs S, Hohagen F, et al. Doxepin in the treatment of primary insomnia:a placebo-controlled, double-blind, polysomnogra-phic study. J Clin Psychiatr. 2001;62:453–463. doi: 10.4088/jcp.v62n0609. [DOI] [PubMed] [Google Scholar]
- 10.Ray SK, Fidan M, Nowak MW, Wilford GG, Hogan EL, Banik NL. Oxidative stress and Ca2+influx upregulate calpain and induce apoptosis in PC12 cells. Brain Res. 2000;852:326–334. doi: 10.1016/s0006-8993(99)02148-4. [DOI] [PubMed] [Google Scholar]
- 11.Bian-sheng JI, He JI, Guo-qing LIU. Doxepin protects cultured neurons against oxidative stress-induced injury. Acta Pharm Sin. 2004;25:297–300. [PubMed] [Google Scholar]
- 12.Gharzi M, Dolatabadi HR, Reisi P, Javanmard SH. Effects of different doses of doxepin on passive avoidance learning in rats. Adv Biomed Res. 2013;2:66. doi: 10.4103/2277-9175.115823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Azadbakht AA, Radahmadi M, Sh HJ, Reisi P. The effects of doxepin on stress-induced learning, memory impairments, and TNF-αlevel in the rat hippocampus. Res Pharm Sci. 2015;10:460–465. [PMC free article] [PubMed] [Google Scholar]
- 14.O’ NC. PI3-kinase/Akt/mTOR signaling:impaired on/off switches in aging, cognitive decline and Alzheimer’s disease. Exp Gerontol. 2013;48:647–653. doi: 10.1016/j.exger.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 15.Mairetcoello G, Courchet J, Pieraut S, Courchet V, Maximov A, Polleux F. The CAMKK2-AMPK kinase pathway mediates the synaptotoxic effects of Aβoligomers through Tau phosphorylation. Neuron. 2013;78:94. doi: 10.1016/j.neuron.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stein SC, Woods A, Jones NA, Davison MD, Carling D. The regulation of AMP-activated protein kinase by phosphorylation. Biochem J. 2000;345(Pt 3):437–443. [PMC free article] [PubMed] [Google Scholar]
- 17.Carling D, Thornton C, Woods A, Sanders MJ. AMP-activated protein kinase:new regulation, new roles? Biochem J. 2012;445:11–27. doi: 10.1042/BJ20120546. [DOI] [PubMed] [Google Scholar]
- 18.CM T, SA R, Z X, AC M, TJ C, ML S, et al. beta-Amyloid-induced neuronal apoptosis requires c-Jun N-terminal kinase activation. J Neurochem. 2001;77:157–164. doi: 10.1046/j.1471-4159.2001.t01-1-00218.x. [DOI] [PubMed] [Google Scholar]
- 19.Lin J, Li W, Zhang XL. Effects of Gingko biloba leaf extract on the learning and memory and expression of glial fibrillary acidic protein in hippocampal astrocytes of type 2 diabetic rats. Chinese J Clin Rehabil. 2006;10:176–179. [Google Scholar]
- 20.JämsäA Hasslund K, Cowburn RF, Bäckström A, Vasänge M. The retinoic acid and brain-derived neurotrophic factor differentiated SH-SY5Y cell line as a model for Alzheimer’s disease-like tau phosphorylation. Biochem Biophys Res Commun. 2004;319:993–1000. doi: 10.1016/j.bbrc.2004.05.075. [DOI] [PubMed] [Google Scholar]
- 21.Leblanc AC, Kovacs DM, Chen HY, Villaré F, Tykocinski M, Autilio-Gambetti L, et al. Role of amyloid precursor protein (APP):study with antisense transfection of human neuroblastoma cells. J Neurosci Res. 1992;31:635–645. doi: 10.1002/jnr.490310407. [DOI] [PubMed] [Google Scholar]
- 22.Schonrock N, Matamales M, Ittner LM, Götz J. MicroRNA networks surrounding APP and amyloid-βmetabolism--implications for Alzheimer’s disease. Exp Neurol. 2012;235:447. doi: 10.1016/j.expneurol.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 23.Li S, Yan Y, Jiao Y, Gao Z, Xia Y, Kong L, et al. Neuroprotective effect of osthole on neuron synapses in an alzheimer’s disease cell model via upregulation of microRNA-9. J Mol Neurosci. 2016:1–11. doi: 10.1007/s12031-016-0793-9. [DOI] [PubMed] [Google Scholar]
- 24.Krystal AD, Durrence HH, Scharf M, Jochelson P, Rogowski R, Ludington E, et al. Efficacy and safety of doxepin 1 mg and 3 mg in a 12-week sleep laboratory and outpatient trial of elderly subjects with chronic primary lnsomnia. Sleep. 2010;33:1553–1561. doi: 10.1093/sleep/33.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weber J, Siddiqui MA, Wagstaff AJ, Mccormack PL. Low-dose doxepin:in the treatment of insomnia. CNS Drugs. 2010;24:713–720. doi: 10.2165/11200810-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 26.Chong ZZ, Shang YC, Wang S, Maiese K. A Critical kinase cascade in neurological disorders:PI 3-K, Akt, and mTOR. Future Neurol. 2012;7:733. doi: 10.2217/fnl.12.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Griffin RJ, Moloney A, Kelliher M, Johnston JA, Ravid R, Dockery P, et al. Activation of Akt/PKB, increased phosphorylation of Akt substrates and loss and altered distribution of Akt and PTEN are features of Alzheimer’s disease pathology. J Neurochem. 2005;93:105–117. doi: 10.1111/j.1471-4159.2004.02949.x. [DOI] [PubMed] [Google Scholar]
- 28.Xian YF, Lin ZX, Mao QQ, Chen JN, Su ZR, Lai XP, et al. Isorhynchophylline Protects PC12 Cells Against Beta-Amyloid-Induced Apoptosis via PI3K/Akt Signaling Pathway. Evid Based Complementary Altern Med. 2013;2013:163057. doi: 10.1155/2013/163057. [DOI] [PMC free article] [PubMed] [Google Scholar]


