Abstract
Objective(s):
Inflammatory bowel disease (IBD) results from dysregulation of intestinal mucosal immunity. It is an incurable disease that affects millions of people worldwide. Developing new strategies for the treatment of colitis has been a major challenge. Here, we report the effect of calycosin, a plant-derived flavonoid, in successfully managing colitis in murine model.
Material and Methods:
In vivo model of colitis was induced using 2.5% (w/v) dextran sodium sulfate (DSS, 36,000 to 50,000 Mw). Body weight and disease activity index (DAI) were evaluated every day. Hematoxylin-Eosin (H&E) staining was used to estimate the effect of calycosin on DSS-induced colon damage. The levels of proinflammatory genes and mRNA expression were determined using real-time PCR, whereas the proinflammatory cytokines were assessed with ELISA. The content of other parameters including myeloperoxidase (MPO), glutathione (GSH), superoxide dismutase (SOD) and malondialdehyde (MDA) were also evaluated. Western blot assay was further used to determine the effect of calycosin on both NF-κB and mitogen activated protein kinases (MAPK) pathways.
Results:
The results showed that calycosin prevented weight loss and shortening of the colon length, maintained an intact mucosa, increased GSH and SOD activities, and decreased MDA levels. The drug also significantly inhibited proinflammatory cytokine mRNA expression and decreased MPO activity. Additionally, it remarkably inhibited NF-κB pathway and c-Jun N-terminal kinase (JNK) phosphorylation with no effect on p38 and extracellular signal-regulated kinase (ERK1/2) phosphorylation levels in colon tissue.
Conclusion:
These findings revealed that calycosin successfully ameliorated the effect of DSS-induced colitis in mice, which could be associated with NF-κB and JNK pathway modulations.
Keywords: Colitis, Calycosin, Free radical, Inflammatory cell, NF-κB, Signaling pathway
Introduction
Inflammatory bowel disease (IBD), which includes ulcerative colitis (UC) and Crohn’s disease (CD), is linked to chronic intestinal inflammatory disease of unknown etiology mediated by genetic, immune and environmental factors in the world (1). The symptoms of IBD include diarrhea, abdominal pain, rectal bleeding and weight loss. The histomorphology of IBD is characterized by diffused mucosal infiltration of inflammatory cells, hyperplasia of muscularis mucosae and irregular villiform surface (2).
Many animal models of experimental colitis have been used to investigate the pathogenesis and novel therapeutic approaches of IBD. Presently, dextran sulfate sodium (DSS)-induced colitis is one of the most widely employed animal models for the study of IBD, which is similar to the symptom of human UC. Current treatment in colitis mostly depends on glucocortico- steroids, 5-aminosalicylic acid, azathio- prine and cyclosporine (3).
However, additional therapeutic options are required due to the drug-induced toxicity and side-effect. There is an urgent need to develop new drugs, which are more effective and non-toxic in treating colitis.
Flavonoids, a group of secondary metabolites with variable phenolic structures, widely exist in vegetables, fruits, grass and medicinal plants during the process of long-term natural selection (4). Flavonoids have broad spectrum pharmacological activities including superoxide scavengers and antioxidants (5), anti-tumor (6), and anti-inflamma- tory properties (7) as well as low toxicity. Calycosin is an isoflavone derived from Radix astragali, a traditional herb that has a long history of medicinal use in China and Southeast Asia. Recent reports have shown that calycosin inhibited osteoclast via blocking mitogen activated protein kinases (MAPK) and NF-κB signal pathways (8).
The calycosin modulates estrogen receptor, microRNA-95, insulin-like growth factor receptor 1 and phosphoinositide 3-kinase (PI3K)/Protein kinase B (PKB) to promote apoptosis in SW480 (9). It also exhibits neuroprotective effects through potent antioxidant and radical scavenging activities in ischemia reperfusion (10).
Additionally, the compound inhibits both U87 and U251 cell migrations, as well as invasion mediated by TGF-beta (11). The present study investigated the effects of calycosin on the development of DSS-induced colitis in mice. Due to the low cytotoxity of calycosin, this study could provide a potential natural-based medicine for new therapeutic appro- ach in treating colitis.
Materials and Methods
Reagents
Calycosin, carboxymethylcellulose sodium (CMC), 5-aminosalicylic acid (5-ASA), dextran sodium sulfate (DSS, 36,000 to 50,000 Mw), sodium dodecyl sulphate (SDS), ammonium persulfate (APS), TritonX-100, Tris-base, Glycine, hematoxylin and eosin (H&E) were purchased from Sigma Chemical Co (St Louis, MO, USA). Reduced glutathione (GSH) assay kit (A006-2), superoxide dismutase (SOD) assay kit (WST-1 method) (A001-3), malondialdehyde (MDA) assay kit (TBA method) (A003-1) and myeloperoxidase (MPO) assay kit (A044) were all obtained from the Institute of Biological Engineering of Nanjing Jiancheng (Nanjing, China). Reactive oxygen species (ROS) detection kit was purchased from Beyotime (Haimen, China). Bicinchoninic acid (BCA) protein assay, TRIzol, Enha- nced chemiluminescence (ECL) detection kit, SYBR Green Master Mix, Tetramethylethylenediamine (TEMED) and Tween 20 were purchased from Thermo Fisher Scientific (Walthan, MA). 30% Acrylamide/Bis-acrylamide solution (37.5:1) and 2× loading buffer were obtained from Bio-Rad Laboratories (Richmond, Calif., USA). Pre-stained protein marker was obtained from Fermentas (Glen Burnie, MD, USA). TNF-α, IL-6, IL-1β and MCP-1 ELISA kits were purchased from R&D systems, Inc. (Minneapolis, MN, USA). PVDF membrane was purchased from Millipore (Bedford, MA, USA). Antibodies to p-IKKα (11930), KKβ (#8943), p-IKKα/β (#2697), IκBα(#4814), p-IκBα(#2859), p65 (#8242), p-p65 (#3033), SAPK/JNK (#9295), p-SAPK/JNK (#4668), ERK1/2 (#4695), p-ERK1/2 (#4370), p38 (#8690), p-p38 (#4511), β-actin (#5174), goat anti-rabbit horseradish peroxidase (HRP) (#7074) and RIPA cell lysis buffer (#9806) were purchased from CST (Boston, MA).
Animal studies
Male Balb/c mice (6-8 weeks old, 18-20 grams, n=35) were obtained from Laboratory Animal Center of Henan Province (Henan, China), and housed under constant conditions (12-hr light/dark cycle, room temperature 21±1 °C) with clean water and food at all times.
DSS-induced experimental colitis was conducted as previously described (12). DSS acts as a colitis inductive agent that damages the mucosa. Briefly, mice were treated with 2.5% DSS aqueous solution for 7 days and kept on water for additional 4 days to exhibit colitis. The normal group was exposed to only water. Body weight changes and disease activity index (DAI) were recorded from the first day to the end of the study. The DAI is the sum of weight loss, rigidity of stool specimens and the extent of hematochezia (13). Scores of weight loss were defined as follows: 0 = no changes, 1 = Weight loss in 1% to 5%, 2 = Weight loss in 5% -10%, 3 = Weight loss in 10% - 20%, 4 = Weight loss over 20%. Scores of rigidity of stool specimens were also defined as follows: 0 = moderate, 2 = less hard but not stick to the anus, 4 = soft and stick to the anus. The scores of hematochezia were defined as follows: 0 = none, 2 = slightness, 3 = moderate, 4 = in quantity.
The mice were randomly divided into five groups, namely normal group (n = 7), vehicle (0.5% CMC-Na) + DSS group (n = 7), 25 mg/kg calycosin + DSS group (n=7), 50 mg/kg calycosin + DSS group (n = 7) and 50 mg/kg 5-aminosalicylic acid (5-ASA) + DSS group (n = 7) according to their body weights. Calycosin and 5-ASA were separately suspended in 0.5% CMC-Na, and orally administered 0.1 ml per 10 gram in mice from the first day to the end of the study.
Myeloperoxidase (MPO) activity
Colon tissues from the same site were weighed and homogenized in ice-cold phosphate-buffered saline (PBS). The homogenate was centrifuged for 10 min at 8000 g and the supernatant collected for the measurement of MPO activity in intestinal mucosa using microplate reader according to the manufac- turer’s instructions on the kit.
Colon oxidative stress
The levels of GSH, SOD and MDA in the supernatant from the homogenate were respectively measured according to the manufacturer’s instructions.
Histology
A histological examination was performed on three samples of the colon from the same position of each mouse. The samples were fixed in 10% formalin and embedded in paraffin. 5-μm-thick section was cut and stained with H&E for analysis.
Real-time PCR analysis
RNA was prepared using 1 ml of TRIzol and the cDNA generated with reverse transcriptase. The qPCR was carried out using the DyNAmo SYBR Green 2-step qRT-PCR kit (Finnzymes, F430L). The primer sequences of the genes were as follows: TNF-α (Fwd, CTTCTGTCTACTGAACTTCGGG; Rev, CAGGCTTGTCACT CGAATTTTG), IL-1β (Fwd, ACGGACCCCAAAAGAT GAAG; Rev, TTCTCCACAGCCACAATGAG), IL-6 (Fwd, CAAAGCCAGAGTCCTTCAGAG; Rev, GTCCTTAGCCAC TCCTTCTG), IFN-γ (Fwd, CCTAGCTCTGAGACAA TGAACG; Rev, TTCCACATCTATGCCACTTGAG), MCP-1 (Fwd, GTCCCTGTCATGCTTCTGG; Rev, GCTCTCCAGCCT ACTCATTG), iNOS (Fwd, GCAAACATCACATTCA GATCCC; Rev, TCAGCCTCATGGTAAACACG). Expression was normalized relative to β-actin (Fwd, ACCTTCTA CAATGAGCTGCG; Rev, CTGGATGGCTACGTACATGG). Data collection was performed using ABI PRISM 7000 Sequence Detection System with the SYBR Green PCR Master Mix (Applied Biosystems).
Enzyme-linked immunosorbent assay (ELISA)
Colon tissues were collected from the same position and 500 µl of homogenization buffer containing protease inhibitors and phosphatase inhibitors added to homogenize for 30 sec. The mixture was incubated on ice for 30 min and centrifuged at 12000 g for 5 min. The supernatant was obtained and used to determine the cytokines according to the manufacturer’s instructions on the ELISA kit.
Preparation of cellular lysates and Western blot analysis
Colon tissues were cut into small pieces and put in Eppendorf tubes. The samples were then homogenized using a lysis buffer containing protease cocktail inhibitors (Roche). The homogenate was centrifuged at 14000 g for 15 min at 4°C and the protein concentration was determined using BCA protein kit. Whole tissue lysate (20 μg) was loaded onto 12.5% SDS-PAGE. Electrophoresis was performed using a stacking gel at 80 V for 20 min and a separating gel at 110 V for 70 min. The proteins were transferred to PVDF membranes (Millipore, MA, USA) using an electro-blotting apparatus (Bio-Rad, CA, USA) at 300 mA for 90 min. The membranes were blocked for 1 hr in TBST containing 0.1% Tween-20 and 5% dry milk and then incubated overnight with primary antibodies. Afterwards, the membranes were washed using TBST containing 0.1% Tween-20 for 5 min for 3 times. Then, incubation for 2 hours with horseradish peroxidase-conjugated secondary antibodies was performed and washed again for 5 min for 3 times. The optical densities of the antibody-specific bands were analyzed using a Luminescent Image Analyzer (Alpha, USA). The relative quantity of protein expression was normalized with actin using an optical density ratio of targeted protein/β-actin.
Statistical analysis
The experimental data were expressed as mean ± SD of at least three independent experiments. Statistical analysis was performed using one-way analysis of variance (ANOVA) with the SPSS18.0 statistical software. P values less than 0.05 were considered statistically significant.
Results
Calycosin attenuated DSS-induced intestinal injury in mice
The body weight of mice in the normal group increased, whereas that of the mice in DSS model group decreased from the 4th day to the 7th day (Figure 1A). The inhibition of weight loss in DSS-treated group by calycosin (50 mg/kg) was comparable to the 5-ASA (positive drug) group (Figure 1A).
Figure 1.
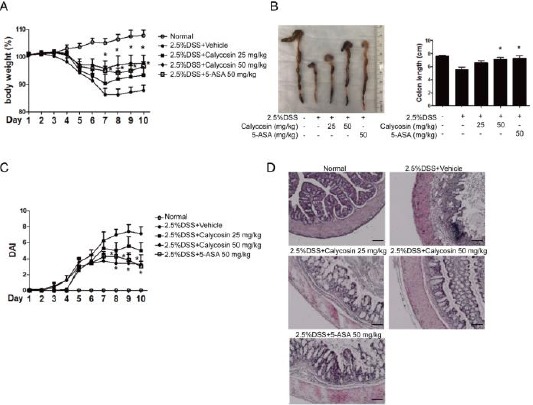
Calycosin attenuates dextran sulfate sodium (DSS)-induced intestinal injury in mice.
Body changes in each group (n=7 per group). The mice were given fresh tap water, DSS or DSS combined with different concentrations of calycosin by oral gavage for 10 days. DSS combined 5-aminosalicylic acid (5-ASA) group is as a positive group. The weight of each mouse was then followed daily (A). The morphology of colon and colon length on day 10 in each group (B). The changes of Disease Activity Index during DSS-induced colitis (C). The histologic findings in DSS-induced colitis. Colons were excised after 10 days and stained with hematoxylin and eosin (HE). The data represent means ± SD (n = 7 mice/group). −P < 0.05 compared with DSS+vehicle group, −−P < 0.01 with DSS+vehicle group
Significant increases in body weight were observed in calycosin+DSS group (25 mg/kg and 50 mg/kg) and 5-ASA+DSS group compared with DSS+vehicle group from the 8th day till the end of the experiment. The average colon length was 7.54 ± 0.39 cm in the normal group, but decreased in the DSS group to 5.57 ± 0.38 cm (Figure 1B). Calycosin (50 mg/kg) and 5-ASA significantly blocked the colon shortening to produce 7.11 ± 0.41 cm and 7.2 7± 0.42 cm, respectively (Figure 1B).
The mice in DSS group appeared hematochezia and also observed to have soft faeces on the 5th day. The DAI of these mice reached the highest value on the 8th day. However, the hematochezia was decreased and the faeces was harder in calycosin (50 mg/kg) and 5-ASA (50 mg/kg) group. The DAI was shown in Figure 1C.
There were decreased goblet cells and fossae, severely damaged mucosa and infiltrated inflammatory cells in the colon of DSS-treated mice (Figure 1D). However, the colon morphology was improved with goblet cells and fossae being intact in calycosin groups (25 mg/kg; 50 mg/kg) and 5-ASA group (50 mg/kg). Additionally, the mucosa damage was attenuated with inflammatory cells being fewer in the colon for the drug-treated groups.
These observations collectively demonstrated that calycosin has a protective effect on colitis.
Calycosin inhibited proinflammatory response in DSS-treated mice
As depicted in Figure 2A, the mRNA levels of proinflammatory factors such as IL-1β, IL-6, TNF-α, iNOS, MCP-1 and IFN-γ in DSS-treated group were significantly enhanced compared with the normal group. Calycosin suppressed the mRNA levels of proinflammatory factors under colitis condition. The 5-ASA, positive drug, also significantly inhibited the increasing mRNA of proinflammatory factors. The production of proinflammatory factors including IL-1β, IL-6, TNF-α and MCP-1 in colon tissue were detected in each group using ELISA kits. As in mRNA expression levels for each group, both calycosin (50 mg/kg) and 5-ASA substantially decreased the production of proinflammatory factors in colon tissue.
Figure 2.
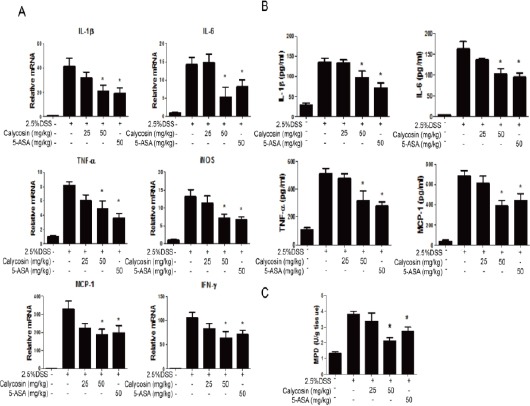
Calycosin alleviates dextran sulfate sodium (DSS)-induced proinflammatory cytokine production in the mouse colon.
RNA were isolated using TRIzol and reversed transcribed to cDNA for real-time PCR analysis of cytokine mRNA levels such as IL-1β, IL-6, TNF-α, iNOS, MCP-1 and IFN-γ in each group (n=3) (A), The cytokine mRNA expression level in normal group was set as 100%, and mRNA expression levels in other groups were compared with normal group. Colon tissues were collected via homogenate lysis buffer to detect cytokine protein levels including IL-1, IL-6, TNF-α, and MCP-1 using ELISA kits (B). MPO activities of colon were measured by MPO assay kit (C). The data represent means ± SD (n = 3 mice/group). −P < 0.05 compared with DSS+vehicle group
The results further showed that the MPO activity in DSS-induced colitis was 3.8 times higher than the normal group. Compared with colitis group, the content of MPO (3.35 U/g tissue) in the calycosin group (25 mg/kg) presented no obvious change, but that in calycosin (50 mg/kg) and 5-ASA group largely reversed the increasing MPO activity.
In summary, calycosin protected against neutron-phils and monocytes infiltration into the colon, thereby decreasing the inflammation.
Calycosin suppressed DSS-induced oxidative stress in mice
As exhibited in Figure 3A and 3B, the DSS caused an imbalance in the redox reactions and damaged the activity of antioxidant enzymes, which resulted in significant decrease in the concentration of GSH and SOD. However, compared with DSS group, the concentration of GSH and SOD were significantly enhanced in calycosin (50 mg/kg) and 5-ASA groups. Additionally, MDA, as a byproduct of oxidative stress, increased in DSS-treated group, whereas both calycosin (50 mg/kg) and 5-ASA groups significantly downregulated the production of MDA. Thus, calycosin protected against DSS-induced oxidative damage in the colon.
Figure 3.
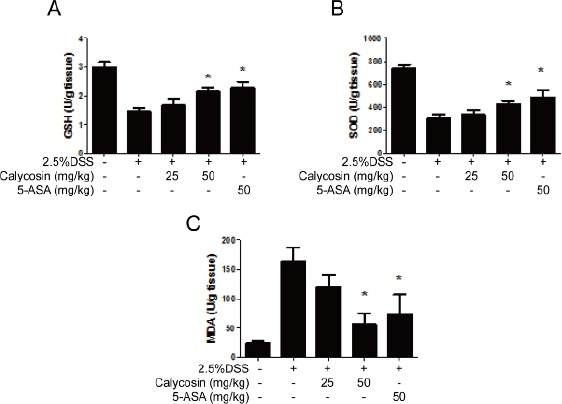
Calycosin suppress oxidative stress in colon tissue in dextran sulfate sodium (DSS)-treated mice.
Histogram shows glutathione (GSH) levels (A), superoxide dismutase (SOD) activities (B) and malondialdehyde (MDA) levels (C) in different treatment groups in colon tissue. The data represent means ± SD (n = 7 mice/group)
Calycosin inhibited NF-kB pathway activation
Western blot analysis revealed that the DSS stimulated the activation of IKKα/β, IκBα and p65 via phosphorylation.
The calycosin (50 mg/kg) and 5-ASA inhibited the phosphorylation of IKKα/β, IκBα and p65 to attenuate the inflammation, which was consistent with the suppression of mRNA expressions (Figure 4A). The protein expression was quantified as the ratio of targeted protein and β-actin (Figure 4B).
Figure 4.
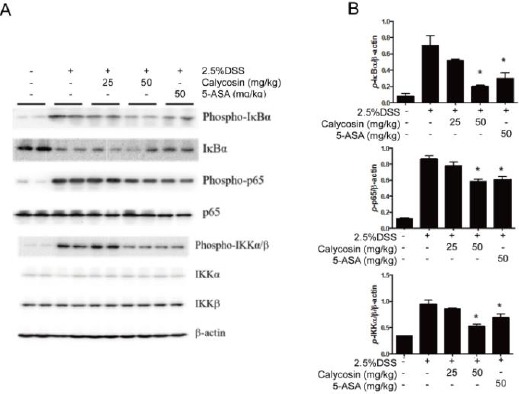
Calycosin suppresses NF-κB signal pathway.
Total colon tissue lysates were prepared for detecting the activation states of IκBα, p65 and IKKα/β by Western blot (A). The action was used as protein loading control. The relative density of the signaling band on Western blot was compared with the actin band in each group (B). The data based on 3 independent experiments represent means ± SD, and −P < 0.05 compared with dextran sulfate sodium (DSS)+vehicle group
In short, calycosin blocked NF-κB pathway to suppress the production of proinflammatory cytokines.
Calycosin inhibited MAPK/JNK pathway activation
The protein kinases such as extracellular signal-regulated kinase (ERK1/2), p38 and c-Jun N-terminal kinase (JNK) were phosphorylated in DSS-stimulated colitis in mice, but both calycosin (50 mg/kg) and 5-ASA (50 mg/kg) significantly inhibited the phosphory- lation of JNK with no effect on p38 and ERK1/2 (Figure 5A). The ratio of targeted protein and β-actin is also as shown in Figure 5B.
Figure 5.
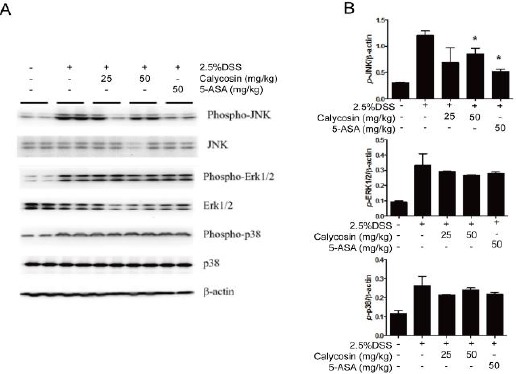
Calycosin suppresses c-Jun N-terminal kinase (JNK) signaling pathways-mediated proinflammatory cytokine production in colon tissue of dextran sulfate sodium (DSS)-treated mice.
Total colon tissue lysates were prepared for detecting the activation states of mitogen activated protein kinases (MAPK) signal pathway including JNK, extracellular signal-regulated kinase (ERK) and p38 by Western blot (A). The action was used as protein loading control. The relative density of the signaling band on Western blot was compared with the actin band in each group (B). Data ratio is based on 3 independent experiments, and −P < 0.05 compared with DSS+vehicle group
The results therefore demonstrated that calycosin suppressed JNK pathway to decrease the production of proinflammatory factors.
Discussion
IBD, a complicated multi-factor disease of unknown etiology, is associated with environmental stimulation, genes and abnormal immune responses. The IBD cannot fully simulate the onset process at the cellular level. At present, animal models induced by immune stimulus have significantly made an outstanding contribution to the research of IBD. Therefore, the present study employed DSS-stimulated colitis to investigate the effect of calycosin on IBD and also to establish the mechanism of the drug. DSS, a polyanion derived from dextran, has been reported to induce acute colitis in hamsters (14). Recently, DSS has been widely used to induce experimental colitis in mice, rats, hamsters and guinea pigs. Moreover, DSS-induced colitis in mice has some common characteristics with IBD. Furthermore, the DSS has several advantages such as cheap price, easy induction and high reproducibility in animal models for experimental colitis. Thus, the DSS colitis could be suitable for colon cancer research. However, more research is needed to further ascertain the exact mechanism of DSS.
A possible mechanism could be that DSS directly decreases intestinal permeability through downregulating zonula occluden (ZO-1) expression and damaging colon mucosa in a concentration dependent manner. The initial damage in colitis is most likely caused by the epithelial cells, and the inflammation is usually expanded from the damaged epithelial cells. Downregulation of ZO-1 on the first day of DSS administration has been reported, and also the intestinal permeability increases with production of inflammation on the third day(15, 16). After the colon mucosa injury, the changes of integrin α4 and M290 subunits in epithelial cells broke up the interaction with Gamma-delta (γδ) T cells. Although the role of Gamma-delta (γδ) T cells remains unknown, it has been reported to protect against DSS-induced mucosa damage (17). Intestinal bacteria also play an important role in DSS-stimulated colitis. Metronidazole and ciprofloxacin can improve DSS-induced acute colitis but not in the case of chronic colitis (18). The findings revealed that both calycosin (50 mg/kg) and 5-ASA significantly inhibited the weight loss, colon length shortening and DAI increases in DSS-treated mice. H&E staining analysis showed that the calycosin protected the intact mucosa and depleted the infiltrated inflammatory cells.
Macrophages play an important role in DSS-induced experimental colitis. Following the intestinal epithelial cell injury, the cells were broken with the release of inflammatory cytokines and chemokines to activate macrophage, a scavenger. Additionally, a large number of inflammatory cytokines including IL-1β, IL-6 and TNF-α were released from macrophage to exert a second attack on intestinal epithelial cells. The IL-10 produced by macrophages as an anti-inflammatory factor has been reported to attenuate DSS-induced colitis via ROS-NO pathway (19). The IL-10 deficient mice are more sensitive to DSS and T cell-induced colitis. These mice develop colitis spontaneously, which can be reversed by the administration of IL-10 (20, 21). Additionally, the IL-10 can regulate the levels of ROS. An appropriate production of ROS can prevent bacteria entry into the mucosa, but excessive production of ROS also leads to damage to vital cells in the body. The ROS as a strong oxidizer can trigger lipid peroxidation directly or indirectly. The normal function of cells is greatly affected by the production of oxide or peroxide when ROS intereacts with nucleic acid, protein and fatty acids (22). The findings showed that the oral administration of calycosin (50 mg/kg) or 5-ASA significantly inhibited the activity of MPO, upregulated the activities of GSH and SOD, and decreased the production of MDA. As flavonoids, calycosin exhibited strong antioxidant activity, which likely prevented epithelial cell injury. The study also revealed that calycosin (50 mg/kg) or 5-ASA suppressed the phosphorylation of IKKα/β, IκBα and p65, and significantly inhibited the phosphorylation of JNK. Some reports supported the fact that ROS promotes the activation of NF-κB and MAPK signal pathways, and as to whether the downregulation of ROS inhibits NF-κB and MAPK signal in calycosin treatment needs further investigations.
Previous studies have shown that MAPKs subfamilies, including ERK for regulating cell growth and differentiation, JNK for regulating inflammation, and p38 MAPK for regulating apoptosis, have been implicated in the production of proinflammatory cytokines (23). MAPK cascades, consisting of three protein kinases including MAPK kinase kinase (MAPKKK), MAPK kinase (MAPKK) and MAPK, are highly conserved signaling agents, which modulate receptors or sensors to transduce extracellular stimuli into intracellular responses. MAPKKK phosphorylates and activates MAPKK, then activated MAPKK phosphorylates and activates MAPK (24). As one of MAPKKKs, apoptosis signal-regulated kinase 1 (ASK1) regulates JNK and p38 to trigger apoptosis via upstream MKK4, MKK3 and MKK6 under oxidative stress (25). Additionally, CD4+ and CD8+ T cells may be less important than the macrophages in DSS-induced colitis. Studies have shown that the number of CD8+ T cells was decreased in DSS-induced acute and chronic colitis but the CD4+ T cells did not change (26). Furthermore, the DSS can contribute SCID mice to experimental colitis, which further illustrates that T cells are not truly important(27). In the present study, the protective effect of calycosin may be implicated to JNK inhibition.
Conclusion
In summary, it has been shown that calycosin exerted significant effect on DSS-induced experimental colitis in vivo, and the potential mechanism could be the modulation of inflammation signaling pathway and anti-oxidative stress. As a flavonoid without any significant side-effect, calycosin can be developed into a promising drug for intestinal inflammatory diseases.
Acknowledgment
The authors of the present would like to thank the following: The National Natural Science Foundation of China (grant no. 81370494); the Technological Leading Project of Zhengzhou (grant no. 131PLIRC656).
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Manichanh C, Borruel N, Casellas F, Guarner F. The gut microbiota in IBD. Nat Rev Gastroenterol Hepatol. 2012;9:599–608. doi: 10.1038/nrgastro.2012.152. [DOI] [PubMed] [Google Scholar]
- 2.Erben U, Loddenkennper C, Spieckermann S, Heimesaat MM, Siegmund B, Kuehl AA. Histomorphology of intestinal inflammation in inflammatory bowel diseases (IBD) mouse models and its relevance for IBD in men. Int J Clin Exp Med. 2016;9:408–442. [Google Scholar]
- 3.Podolsky Daniel K. Inflammatory bowel disease. N Engl J Med. 1991;325:928–937. doi: 10.1056/NEJM199109263251306. [DOI] [PubMed] [Google Scholar]
- 4.Kumar S, Pandey AK. Chemistry and biological activities of flavonoids:an overview. ScientificWorldJournal. 2013;2013:162750. doi: 10.1155/2013/162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yuting C, Rongliang Z, Zhongjian J, Yong J. Flavonoids as superoxide scavengers and antioxidants. Free Radic Biol Med. 1990;9:19–21. doi: 10.1016/0891-5849(90)90045-k. [DOI] [PubMed] [Google Scholar]
- 6.Ravishankar D, Rajora AK, Greco F, Osborn HM. Flavonoids as prospective compounds for anti-cancer therapy. Int J Biochem Cell B. 2013;45:2821–2831. doi: 10.1016/j.biocel.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Fu Y, Chen J, Li Y J, Zheng Y F, Li P. Antioxidant and anti-inflammatory activities of six flavonoids separated from licorice. Food Chem. 2013;141:1063–1071. doi: 10.1016/j.foodchem.2013.03.089. [DOI] [PubMed] [Google Scholar]
- 8.Quan G H, Wang H, Cao J, Zhang Y, Wu D, Peng Q, et al. Calycosin suppresses RANKL-mediated osteoclastogenesis through inhibition of MAPKs and NF-κB. Int J Mol Sci. 2015;16:29496–507. doi: 10.3390/ijms161226179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao X, Li X, Ren Q, Tian J, Chen J. Calycosin induces apoptosis in colorectal cancer cells, through modulating the ERβ/MiR-95 and IGF-1R, PI3K/Akt signaling pathways. Gene. 2016;591:123–128. doi: 10.1016/j.gene.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 10.Guo C, Tong L, Xi M, Yang H, Dong H, Wen A. Neuroprotective effect of calycosin on cerebral ischemia and reperfusion injury in rats. J Ethnopharmacol. 2012;144:768–774. doi: 10.1016/j.jep.2012.09.056. [DOI] [PubMed] [Google Scholar]
- 11.Nie XH, Ou-yang J, Xing Y, Li DY, Liu RE, Xu RX. Calycosin inhibits migration and invasion through modulation of transforming growth factor beta-mediated mesenchymal properties in U87 and U251 cells. Drug Des Devel Ther. 2016;10:767. doi: 10.2147/DDDT.S90457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541–546. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- 13.Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–249. [PubMed] [Google Scholar]
- 14.Yamada M, Ohkusa T, Okayasu I. Occurrence of dysplasia and adenocarcinoma after experimental chronic ulcerative colitis in hamsters induced by dextran sulphate sodium. Gut. 1992;33:1521–1527. doi: 10.1136/gut.33.11.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kitajima S, Takuma S, Morimoto M. Changes in colonic mucosal permeability in mouse colitis induced with dextran sulfate sodium. Exp Anim Tokyo. 1999;48:137–143. doi: 10.1538/expanim.48.137. [DOI] [PubMed] [Google Scholar]
- 16.Poritz LS, Garver KI, Green C, Fitzpatrick L, Ruggiero F, Koltun WA. Loss of the tight junction protein ZO-1 in dextran sulfate sodium induced colitis. J Surg Res. 2007;140:12–19. doi: 10.1016/j.jss.2006.07.050. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y, Chou K, Fuchs E, Havran WL, Boismenu R. Protection of the intestinal mucosa by intraepithelial γδT cells. Proc Natl Acad Sci U S A. 2002;99:14338–14343. doi: 10.1073/pnas.212290499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hans W, Schölmerich J, Gross V, Falk W. The role of the resident intestinal flora in acute and chronic dextran sulfate sodium-induced colitis in mice. EUR J Gastroen Hepat. 2000;12:267. doi: 10.1097/00042737-200012030-00002. [DOI] [PubMed] [Google Scholar]
- 19.Li B, Alli R, Vogel P, Geiger TL. IL-10 modulates DSS-induced colitis through a macrophage–ROS–NO axis. Mucosal Immunol. 2014;7:869. doi: 10.1038/mi.2013.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol. 2011;29:71–109. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- 21.Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002;16:219–230. doi: 10.1016/s1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- 22.Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial ROS-induced ROS release:an update and review. BBA Biomembranes. 2006;1757:509–517. doi: 10.1016/j.bbabio.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 23.Kyriakis JM, Avruch J. Mammalian MAPK signal transduction pathways activated by stress and inflammation:a 10-year update. Physiol Rev. 2012;92:689–737. doi: 10.1152/physrev.00028.2011. [DOI] [PubMed] [Google Scholar]
- 24.Seger R, Krebs EG. The MAPK signaling cascade. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- 25.Noguchi T, Ishii K, Fukutomi H, Naguro I, Matsuzawa A, Takeda K, et al. Requirement of reactive oxygen species-dependent activation of ASK1-p38 MAPK pathway for extracellular ATP-induced apoptosis in macrophage. J Biol Chem. 2008;283:7657–7665. doi: 10.1074/jbc.M708402200. [DOI] [PubMed] [Google Scholar]
- 26.Stevceva L, Pavli P, Husband AJ, Doe WF. The inflammatory infiltrate in the acute stage of the dextran sulphate sodium induced colitis:B cell response differs depending on the percentage of DSS used to induce it. BMC Clin Pathol. 2001;1:1. doi: 10.1186/1472-6890-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Axelsson LG, Landström E, Goldschmidt TJ, Grönberg A, Bylund-Fellenius AC. Dextran sulfate sodium (DSS) induced experimental colitis in immunodeficient mice:effects in CD4+-cell depleted, athymic and NK-cell depleted SCID mice. J Inflamm Res. 1996;45:181–191. doi: 10.1007/BF02285159. [DOI] [PubMed] [Google Scholar]


