Abstract
Objective(s):
Studies indicate that major deficiency in the levels of monoaminergic transmitters is a reason for severe depression. On the other hand, it is shown that Carthamus tinctorius L. (CT) may improve neuropsychological injuries by regulation of the monoamine transporter action. Hence, the present study was undertaken to evaluate the involvement of monoaminergic systems in antidepressant-like effect of CT extract in the tail suspension test (TST) in mice.
Materials and Methods:
The mice were intraperitoneally (IP) treated with CT extract (100–400 mg/kg) 1 hr before the TST. To investigate the involvement of monoaminergic systems in antidepressant-like effect, the mice were treated with receptor antagonists 15 min before CT extract treatment (400 mg/kg, IP) and 1 hr before the TST.
Results:
Findings showed that CT extract (100–400 mg/kg, IP), dose-dependently induced antidepressant-like effect (P<0.001), but it was not accompanied by alterations in spontaneous locomotor activity in the open-field test. Pretreatment of mice with SCH23390, sulpiride, haloperidol, WAY100135, cyproheptadine, ketanserin and p-chlorophenylalanine (PCPA) inhibited the antidepressant-like effect of CT extract (400 mg/kg, IP), but not with prazosin and yohimbine. Co-administration of CT extract (100 mg/kg, IP) with sub-effective doses of fluoxetine (5 mg/kg, IP) or imipramine (5 mg/kg, IP) increased their antidepressant-like response.
Conclusion:
Our findings firstly showed that components (especially N-Hexadecanoic acid) of CT extract induce antidepressant-like effects by interaction with dopaminergic (D1 and D2) and serotonergic (5HT1A, 5-HT2A receptors) systems. These findings validate the folk use of CT extract for the management of depression.
Keywords: Antidepressant-like effect Carthamus tinctorius L, Mice, Monoaminergic system, Tail suspension test
Introduction
Depression affects a broad majority of the world population (1). Severe depression is characterized by changes in behavior, energy, appetite, sleep, and weight (2). It believes that a major deficiency in the levels of monoaminergic transmitters is one reason for severe depression (3). Meyers (4) described each of the drugs that can elevate the level of monoaminergic transmi tters in the central nervous system (CNS); it is a suitable choice for antidepressant action. Antidepressant treat-ments increased the synaptic levels of some monoaminer-gic transmitters, serotonin (5HT), noradrenaline (NA), or normalized their levels (5). In addition, the dopaminer-gic system is modulated in depression therapy (6). Drug treatments usually include tricyclic antidepre-ssants (TCAs), selective serotonin reuptake inhibitors (SSRIs), monoamine oxidase inhibitors (MAOIs), and specific serotonin- noradrenaline reuptake inhibitors (SNRIs) (7). These drugs are efficient in the treatment of depression but their usage is restricted due to their side effects (8). It is necessary to find new antidepressants with more efficiency and lower side effects for exploring their efficiency in combination with common antidepressants. Hence, medicinal plants and their extracts may be a suitable choice for treatment of psychiatric disorders, because they have demonstrated psychotherapeutic activity in a broad range of animal models (9).
Carthamus tinctorius L. (CT), safflower, is a member of the family Compositae (or Asteraceae), which is mainly cultivated for its seed and it is suitable for the production of edible oil and birdseed. CT is generally used to promote circulation and for treatment of diabetes in Iranian traditional medicine (10, 11). In addition, our previous studies (12) have demonstrated that CT extract attenuates some aspects of morphine withdrawal signs.
Zhao et al. (13) found a novel 5HT transporter preventer in CT which can improve neuropsychological diseases by regulating the serotoninergic transmit-ssion, thus they advised usage of CT for depression treatment. In another study, Zhao et al. (14) reported that CT extracts interact with activators of dopamine (DA), NA, and/or 5HT transporter preventers. They showed that CT extract may improve neuropsycho-logical injuries by regulating the monoamine-transporter action. The tail suspension test (TST) is used for investigating the likely mechanisms in antidepressant-like effect in animal models. To the best of our knowledge, there is no study that investigates whether CT is modulated in antidepressant-like activity. Thus, our study was performed to evaluate the involvement of the monoaminergic system in antidepressant-like effect of CT hydroethanolic extract in TST in mice.
Materials and Methods
Preparation of carthamus tinctorius L. extract
Fresh CT flowers were procured from a local market and authenticated by a botanist in the School of Pharmacy, Tehran University of Medical Science, Tehran, Iran. The voucher specimen (TEH. 4548) was deposited at the herbarium of the same college. The CT was extracted as described by Zhao et al. for ethanolic extract of CT (14). The percentage yield of the extract was approximately 5.9% w/w dry matter.
The gas chromatography-mass spectrometry analysis (GC-MS)
The GC-MS analysis of chemical constituents of the extract was detected using a Perkin-Elmer GC Clarus 500 system as previously explained by researchers (15). This system was equipped with a VF-5 fused silica capillary column (30×0.25 mm id and film thickness 0.25 μm). For GC-MS detection, an electron ionization system with an ionizing energy of 70 eV was used. Helium gas (99.999% pure) was used as the carrier gas at a constant flow rate of ±1 ml/min and an injection volume of 1 μl was employed (split ratio of 120:1). Mass transfer line temperature was maintained at 220 °C. Injector temperature was 290 °C. The oven temperature was programmed from 50 °C to 150°C at 3 °C/min, then held isothermal for 10 min and finally increased to 300 °C. The relative percentage of the chemical constituents of ethanolic extract (i.e. CT) was expressed as percentage by peak area normalization. The chemical constituents for main components of CT are shown in Table 1.
Table 1.
Main components of the Carthamus tinctorius (CT) extract
| No | Compounds | Amount (%) | No | Compounds | Amount (%) |
|---|---|---|---|---|---|
| 1 | Hexanoic acid | 0.17 | 23 | Humulane-1,6-dien-3-ol | 0.16 |
| 2 | Octanoic acid | 0.11 | 24 | Docosane | 1.15 |
| 3 | Anethole | 0.28 | 25 | Trans-4,4-dimethyl-2-hexene | 0.76 |
| 4 | 1,6,10-dodecatrien-3-ol, 3,7,11-trimethyl | 0.36 | 26 | Hexadecal vinyl ether | 4.17 |
| 5 | Dodecanoic acid | 1.01 | 27 | 9-Octadecenoic acid | 0.24 |
| 6 | Tetradecanal | 0.21 | 28 | Palmitin, 2-mono | 3.67 |
| 7 | Hexadecanal | 0.92 | 29 | Tridecane | 0.43 |
| 8 | Tetradecanoic acid | 2.4 | 30 | Trifluoroacetic acid, n-octadecyl ester | 1.06 |
| 9 | P-allyltoluene | 1.48 | 31 | 9,12-Octadecadienoic acid | 0.89 |
| 10 | 2-Methylindene | 0.52 | 32 | Octadecanoic acid, 9,10-epoxy-, isopropyl | 0.55 |
| 11 | Hexahydrofarnesyl acetone | 1.00 | 33 | Benzene, (2-methyl-1-propenyl) | 0.22 |
| 12 | Pentadecanoic acid | 0.54 | 34 | 14-tricosenyl formate | 0.26 |
| 13 | Neophytadiene | 0.14 | 35 | Vitamin E | 0.62 |
| 14 | Palmitic acid, methyl ester | 0.25 | 36 | 14-beta-h-pregna | 0.31 |
| 15 | 8-Octadecenal | 0.17 | 37 | Campesterol | 0.39 |
| 16 | N-Hexadecanoic acid | 19.88 | 38 | Stigmasterin | 8.15 |
| 17 | Heptadecanoic acid | 0.2 | 39 | Borane, diethylmethyl | 0.19 |
| 18 | Phytol | 0.57 | 40 | Gamma-sitosterol | 9.54 |
| 19 | Linolenic acid, methyl Ester | 10.91 | 41 | Beta-amyrin | 3.35 |
| 20 | Octadecanoic acid | 3.62 | 42 | Alpha-guaiene | 0.39 |
| 21 | 2,3-Dihydro-1h-cylonona | 2.04 | 43 | 2,3-Dihydro-1h-cylonona[defrrbiphenylene | 2.04 |
| 22 | Methyl commate E | 3.08 | 44 | Hop-22(29)-en-3beta-ol | 5.4 |
| 91.76 | |||||
Drugs
The drugs used were as follows: SCH23390 (Sigma-Aldrich, St. Louis, MO, USA), sulpiride (Sigma-Aldrich, St. Louis, MO, USA), haloperidol (Caspian Tamin, Iran), prazosin hydrochloride (Razak Pharmaceutical Co, Iran), yohimbine hydrochloride (Iran Daru Co, Iran), p-chlorophenylalanine (PCPA; Sigma-Aldrich, St. Louis, MO, USA), WAY100635 (Sigma-Aldrich, St. Louis, MO, USA), ketanserin (Sigma -Aldrich, St. Louis, MO, USA), cyproheptadine (Amin Pharmaceutical Co, Isfahan, Iran), fluoxetine hydrochloride (Arya Pharmaceutical Co, Iran), and imipramine hydrochloride (Amin Pharmaceutical Co, Iran). In the present study, all drugs and extract were dissolved in normal saline (0.9%) and administrated intraperitoneally (IP) at a constant volume of 10 ml/kg, but SCH23390 was administrated by subcutaneous (SC) route. TST was seen 30 min after single injection of drugs and extract.
Animals
Six-week-old male Naval Medical Research Insti-tute (NMRI) mice, weighing 20–30 g, were procured from the Pasteur Institute (Tehran, Iran). The mice were kept in standard cages (n=6) and 12 hr: 12 hr light/dark cycle. The mice were fed commercial food (supplied by Razi Vaccine and Serum Research Institute, Karaj, Iran), and water ad libitum. All the ethical principles were in accordance with guidelines of the School of Medicine, Tehran University of Medical Sciences (TUMS.REC.3019. Jun 2016) and National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978).
Acute toxicity test
In the present study, the Lorke method (16) was used for acute toxicity test. After 24 hr fasting, thirty-six mice were divided into 6 groups (n=6 mice per group) and the mice received (IP) normal saline (10 ml/kg) and CT extract (500, 1000, 2000, 2500, and 3000 mg/kg of body weight). Thirty minutes post-treatment, the animals were individually considered for possible CNS associated behavioral parameters and signs of toxicity, which include but are not limited to tremors, paw licking, locomotor activity, convulsions, piloerection, bizarre behavior, sleepiness, salivation, injury, changes in physical appearance, any signs of illness or pain, diarrhea, stretching of the entire body, weakness, respiratory distress, coma, and mortality during 24 hr, with special attention given during the first 4 hr. This procedure was carried out three times weekly for 2 weeks, during which animal weights and deaths were also recorded (17).
Tail suspension test
The total duration of immobility induced by tail suspension was calculated on the basis of known procedure (18). Animals, both acoustically and visually isolated, were suspended 50 cm above the floor through adhesive tape positioned approximately 1 cm from the tip of the tail. The mice were separated into six groups and treated as follows (n=6 mice per group); Group I=normal saline (10 ml/kg, IP), Group II–IV= CT extract (100, 200, and 400 mg/kg; IP), respectively, Group V–VI= fluoxetine (20 mg/kg, IP) or imipramine (30 mg/kg, IP), respectively. In this test, the total duration of immobility time was recorded by a chronometer for 4 min after 2 min for accumulation. Reduction in immobility time was taken as a measure of antidepressant activity (19, 20).
Examination of CT extract’s possible mechanism of antidepressant-like effect
Investigation of the role of dopaminergic system in the antidepressant-like effect of CT extract. To examine the possible involvement of dopaminergic system in the antidepressant-like effect of CT extract, mice were pretreated with SCH23390 (a dopamine D1 receptor antagonist; 0.05 mg/kg, SC), sulpiride (a dopamine D2 receptor antagonist; 50 mg/kg, IP), haloperidol (non-selective DA receptor antagonist; 0.2 mg/kg, IP), 15 min before IP treatment of CT (400 mg/kg, IP) or vehicle (10 ml/kg, IP), 1 hr post-treatment, the mice were exposed to TST (21-25).
Investigation of the role of serotonergic system in the antidepressant-like effect of CT extract
To investigate the possible involvement of 5HT in the antidepressant effect of CT extract in the TST, the mice were pretreated with PCPA (an inhibitor of 5HT synthesis; 150 mg/kg, IP) or saline, once a day for 3 consecutive days. Thereafter, the mice were treated with CT (400 mg/kg, IP), 15 min after the last pCPA, one-hour post-treatment mice were exposed to the TST (26). In the other series of experiments, the involvement of the 5HT1 receptor subtype in the antidepressant effect of CT extract in the TST was evaluated. The mice were pretreated with WAY100135 (a selective 5HT1A receptor antagonist; 10 mg/kg, IP), 15 min later, animals were treated with CT (400 mg/kg, IP), 1 hr after treatment the TST was done (23). Also, to investigate the contribution of 5HT2 receptor on the antidepressant effect of CT in the TST, mice were pretreated with ketanserin (a selective 5HT2A/C receptor antagonist; 5 mg/kg, IP) and cyproheptadine (5HT2A receptor antagonist; 3 mg/kg, IP) or saline and 15 min later, CT extract (400 mg/kg, IP) was injected and after 1 hr, TST was performed (24, 27).
Investigation of the role of noradrenergic system in the antidepressant-like effect of CT extract
To examine the possible involvement of noradrener-gic system in the antidepressant-like effect of CT extract, mice were pretreated with prazosin (a α1-adrenorecep- tor antagonist; 1 mg/kg, IP) and yohimbine (a α2-adrenoreceptor antagonist; 1 mg/kg, IP), 15 min prior to IP treatment of CT extract (400 mg/kg, IP) or vehicle (10 ml/kg), 1 hr post-treatments, the mice were exposed to TST (22, 25).
Interaction of CT extract with common antidepressants in the TST
Sub-effective doses of fluoxetine (5 mg/kg, IP) and imipramine (5 mg/kg, IP) were injected 15 min prior to IP treatment of the sub-effective dose of CT extract (100 mg/kg, IP) and after 1 hr of co-administration mice were exposed to TST(28).
Open field test (OFT)
All the mice in the method described by Brown et al. were evaluated for psychomotor stimulant activity. To determine the possible effect of the CT extract on exploratory behavior, the mice were exposed to the OFT (29). Briefly, animals were individually positioned in a Plexiglas box (40×60×50 cm) the floor of which had been separated into 12 squares. We recorded the number of crossings (squares crossed with all paws), and the number of rearings (rising with the front paws) using a counter for 5 min after 1 min for accumulation. The count of squares crossed by the mice with four paws was recorded as sign of locomotor activity, and the count of rearings was sign of exploratory behavior.
Statistical analysis
The data were presented as mean± standard error of the mean (SEM) (n=6) and analyzed using one-way or two-way analysis of variance (ANOVA), which was continued by Tukey’s comparison test. We considered P<0.05 as statistically significant. All statistical analyses were also performed using Prism, version 7 (GraphPad Software, Inc., San Diego, CA, USA).
Results
GC-MS analysis data
The GC-MS analysis revealed 44 compounds (representing 91.76%) in the CT extract. The main component was N-hexadecanoic acid (19.88%), followed by linolenic acid (10.91%), gama sitosterol (9.54%), stigmasterin or stigmasterol (8.15%), hop-22(29)-En-3. Beta.-Ol (5.4%), hexadecal vinyl ether (4.17%), palmitin,2-mono (3.67), octadecanoic acid or stearic acid (3.62%), beta-amyrin (3.35%), methyl commate E (3.08%), methyl commate E (2.04), 2,3-dihydro-1H-cylonona[Def] biphenylene E (2.04), decosane (1.15%), trifluoroacetic acid, N-octadecyl ester (1.06%), dodecanoic acid (1.01%), and hexahydrofarnesyl acetone(1%) (shown in Table 1).
These components (16) accounted for 79.07% of the yield, while other detected components represented <1%.
Acute toxicity study
Normal behavior without any signs of toxicity was shown in the vehicle treated group. No weight changes were detected in animals. Moreover, we did not observe tremors, paw licking, convulsions, piloerection, bizarre behavior, changes in physical appearance, any signs of illness or pain, diarrhea, weakness, respiratory distress, coma, or any deaths even in acute doses of the CT extract, but behavioral signs of toxicity observed in high (2500–3000 mg/kg) doses of extract included: salivation, urination, stretching of the entire body, decrease locomotor activity, and sleepiness.
Tail suspension test
As shown in Figure 1, acute IP administration of CT extract dose-dependently and significantly decreased [F (5, 30)=52.47; (P<0.001)] the duration of immobility time in the TST in mice. The maximal antidepressant action by the extract was obtained at a dose of 400 mg/kg, thus, this dose was used in later experiments. ANOVA showed that the CT extract and common antidepressants, fluoxetine and imipramine, did not have significant differences for immobility time. On the other hand, the reduction in time spent immobile with CT extract was similar to that of fluoxetine and imipramine-treated mice.
Figure 1.
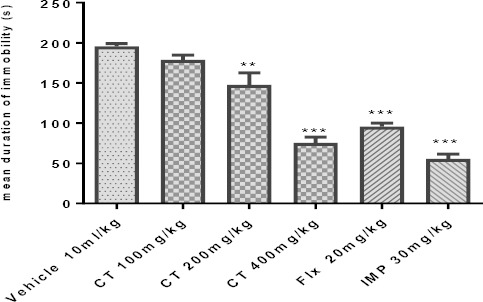
Effect of IP administration of hydroethanolic extract of Carthamus tinctorius (CT) in TST. Values are presented as mean± SEM. ** and *** show significant differences between vehicle (control) and other groups at P<0. 01 and P<0.001, respectively. Significance level was analyzed by one-way ANOVA followed by Tukey’s test
Investigation of mechanism of antidepressant-like effect in TST
Investigation of the dopaminergic system
The effect of Pretreatment of animals with SCH23390 (0.05 mg/kg), sulpiride (50 mg/kg), and haloperidol (0.2 mg/kg) is presented in Figures 2A-C, respectively. Our findings showed that these pre-treatments significantly prevented the antidepressant-like effect induced by the CT extract (400 mg/kg, IP) in the TST. Two-way ANOVA revealed significant differences of SCH23390 Pretreat-ment [F (1, 20) =76.10, P<0.001], extract treatment [F (1, 20) =99.09, P<0.001], and SCH23390 Pretreat-ment×extract interaction [F (1, 20) =77.24, P<0.001]. Similarly, two-way ANOVA revealed significant differences of sulpiride Pretreatment [F (1, 20) =129.3, P<0.001], extract treatment [F (1, 20) =132.9, P<0.001], and sulpiride Pretreatment×extract interaction [F (1, 20) =136.40, P<0.001]. Moreover, two-way ANOVA revealed significant differences of haloperidol Pretreatment [F (1, 20) =92.08, P<0.001], extract treatment [F (1, 20) =172.60, P<0.001], and haloperidol Pretreatment× extract interaction [F (1, 20) = 92.08, P<0.001].
Figure 2.
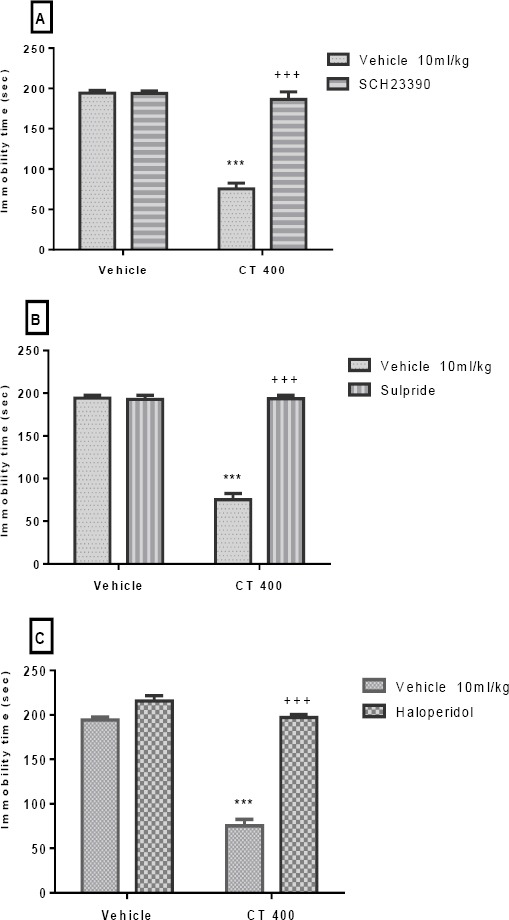
Effect of Pretreatment of animals with SCH23390 (0.05 mg/kg, SC, a dopamine D1 receptor antagonist, panel A), sulpiride (50 mg/kg, IP, a dopamine D2 receptor antagonist, panel B), and haloperidol (0.2 mg/kg, IP, a dopamine D1 receptor antagonist, panel C)on antidepressant-like effect induced by Carthamus tinctorius (CT) extract in the TST. Values are presented as mean± SEM. *** shows significant differences between vehicle (control) and other groups at P<0.001. +++ compared with the extract group. Significance level was analyzed by two-way ANOVA followed by Tukey’s test
Investigation of the serotonergic system
The effect of Pretreatment of animals with an inhibitor of 5HT synthesis (pCPA; 150 mg/kg), WAY100135 (10 mg/kg, IP), 5HT2A receptor antagonist (ketanserin; 5 mg/kg, IP), and cyprohep-tadine (3 mg/kg, IP) are shown in Figures 3A-D, respectively. These Pretreatments significantly inhi- bited the antidepressant-like effect induced by the CT extract (400 mg/kg, IP) in the TST. Two-way ANOVA revealed significant differences of pCPA Pretreatment [F (1, 20) =47.89, P<0.001], extract treatment [F (1, 20) =86.88, P<0.001] and pCPA Pretreatment×extract interaction [F (1, 20) = 85.10, P<0.001].
Figure 3.
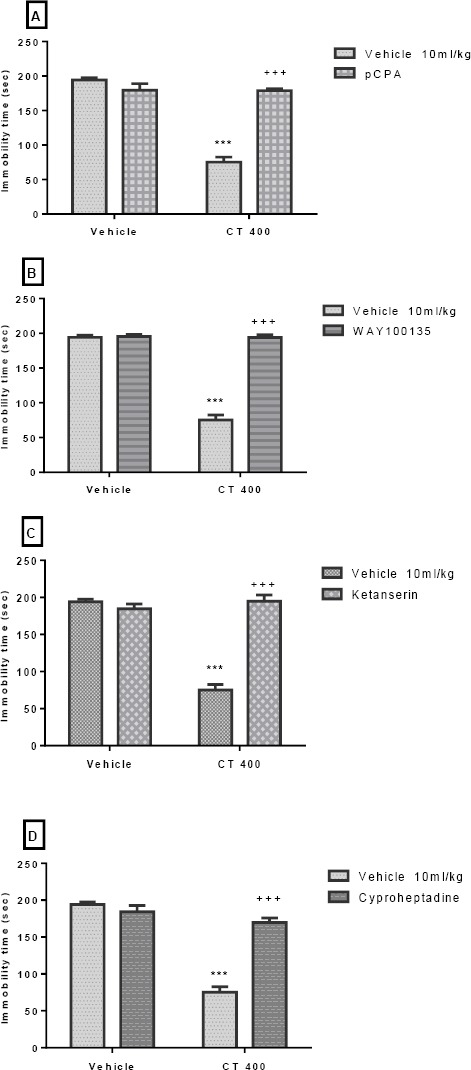
Effect of Pretreatment of animals with pCPA (150 mg/kg, IP, an inhibitor of serotonin synthesis, panel A), WAY100135 (10 mg/kg, IP, 5HT1A receptor antagonist, panel B), ketanserin (5mg/kg, IP, preferential 5HT2A receptor antagonist, panel C), and cyproheptadine (3mg/kg, IP, a 5HT2 receptor antagonist, panel D) on antidepressant-like effect induced by Carthamus tinctorius (CT) extract in the TST. Values are presented as mean± SEM. *** shows significant differences between vehicle (control) and other groups at P<0.001. +++ compared with the extract group. Significance level was analyzed by two-way ANOVA followed by Tukey’s test
Similarly, two-way ANOVA revealed significant differences of WAY100135 Pretreatment [F (1, 20) = 158.40, P<0.001], extract treatment [F (1, 20) =160.0, P<0.001] and WAY100135 Pretreatment×extract interact-tion [F1, 20) =151.70, P<0.001]. Also, two-way ANOVA revealed significant differences of ketanserin pretreat-ment [F(1,20)=65.99, P<0.001], extract treatment [F(1,20)=65.20, P<0.001] and ketanserin pretreat-ment×extract interaction [F(1,20)=91.17, P<0.001]. Moreover, two-way ANOVA revealed significant diffe-rences of cyproheptadine Pretreatment [F(1,20)=41.82, P<0.001], extract treatment [F (1,20)=104.1, P<0.001] and cyproheptadine Pretreatment×extract interaction [F(1,20)= 63.64, P<0.001].
Investigation of the noradrenergic system
The effect of Pretreatment of animals with α1- and α2-adrenoceptor antagonists is presented in Figures 4A and 4B, respectively. Pretreatment of animals with an α1-adrenoceptor antagonist (prazosin; 1 mg/kg, IP), and an α2-adrenoceptor antagonist (yohimbine; 1 mg/kg, IP) could not inhibit the antidepressant-like effect induced by the CT extract (400 mg/kg, IP) in the TST. Two-way ANOVA revealed no significant differences of prazosin Pretreatment [F (1, 20) =0.45, P>0.05], extract treatment [F (1, 20) =219.50, P<0. 001], and prazosin pretreatment×extract interaction [F (1, 20) =4.15, P>0.05]. Similarly, two-way ANOVA revealed no significant differences of yohimbine Pretreatment [F(1,20)=23.11, P<0.001], extract treatment [F(1,20)=45.61, P<0.001], and yohimbine pretreatment×extract interaction [F(1,20)=1.31, P>0.05].
Figure 4.
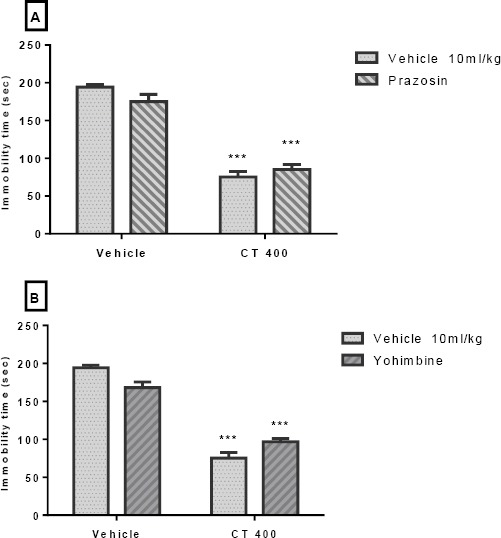
Effect of Pretreatment of animals prazosin (1mg/kg, IP, a α1-adrenoceptor antagonist, panel A) and yohimbine (1 mg/kg, IP, a α2-adrenoceptor antagonist, panel B) on antidepressant-like effect induced by Carthamus tinctorius (CT) extract in the TST. Values are presented as mean± SEM. *** shows significant differences between vehicle (control) and other groups at P<0.001. +++ compared with the extract group. Significance level was analyzed by two-way ANOVA followed by Tukey’s test
Interaction of CT with sub-effective doses of common antidepressants in the TST
The results depicted in Figures 5A and 5B indicate that pretreatment with a sub-effective dose of CT (100 mg/kg, IP) was capable of increasing the effect of sub-effective doses of fluoxetine (5 mg/kg, IP) and imipramine (5 mg/kg, IP). Two-way ANOVA revealed significant differences of fluoxetine [F (1, 20) =118.0, P<0.001], extract treatment [F (1, 20) =156.50, P<0.001], and fluoxetine pretreatment×extract interaction [F (1, 20) =96.24, P<0.001]. Similarly, two-way ANOVA revealed significant differences of imipramine [F (1, 20) =80.22, P<0.001], extract treatment [F (1, 20) =12.66, P=0.002], and imipramine pretreatment×extract interaction [F (1, 20) =18.90, P<0.001].
Figure 5.
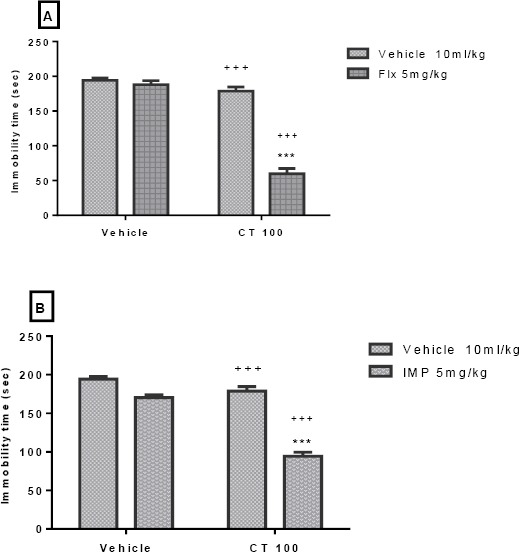
Effect of sub-effective doses of fluoxetine (5 mg/kg, IP, panel A) and sub-effective doses of imipramine (5 mg/kg, IP, panel B) on sub-effective dose of Carthamus tinctorius (CT) extract (100 mg/kg, IP) in the FST in mice. ***P<0.001 compared with vehicle treated control. +++ compared with the extract group. Statistical level of significance analyzed by two-way analysis
Open field test
Our findings did not show significant changes (P>0.05) in the counts of crossings (Figure 6A) and rearings (Figure 6B) in animals treated with CT (100-400 mg/kg; IP) as compared to the control group.
Figure 6.
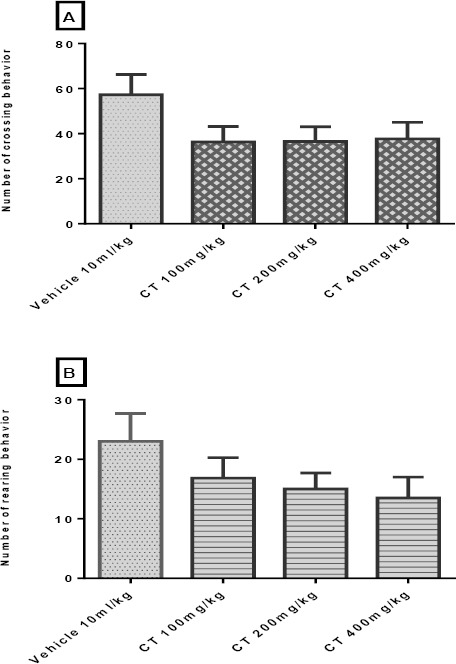
Effect of Carthamus tinctorius (CT) extract in open field test on crossing (panel A) and rearing (panel B) in mice. Values are expressed as mean± SEM. P<0.05, as compared with vehicle treated control. Statistical level of significance analyzed by one-way ANOVA followed by Tukey’s comparison test
Figure 7.
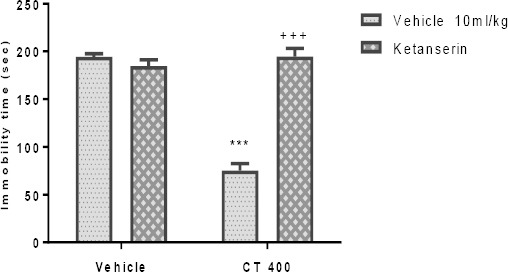
Effect of Pretreatment of animals with ketanserin (5mg/kg, IP, preferential 5HT2A receptor antagonist) on antidepressant-like effect induced by Carthamus tinctorius (CT) extract in the TST. Values are presented as mean± SEM. *** shows significant differences between vehicle (control) and other groups at P<0.001. +++ compared with the extract group. Significance level was analyzed by two-way ANOVA followed by Tukey’s test
Figure 8.
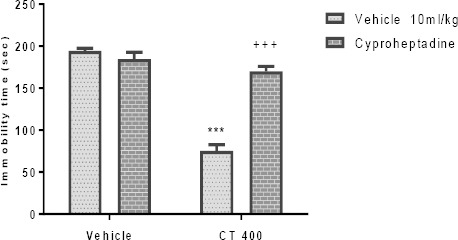
Effect of Pretreatment of animals with cyproheptadine (3mg/kg, IP, a 5HT2 receptor antagonist) on antidepressant-like effect induced by Carthamus tinctorius (CT) extract in the FST. Values are presented as mean± SEM. *** shows significant differences between vehicle (control) and other groups at P<0.001. +++ compared with the extract group. Significance level was analyzed by two-way ANOVA followed by Tukey’s test
Figure 9.
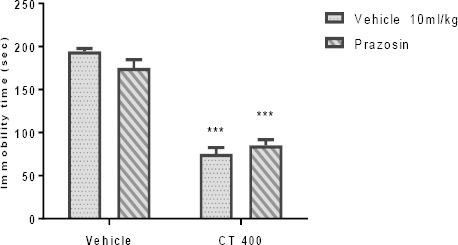
Effect of Pretreatment of animals with prazosin (1mg/kg, IP, a α1-adrenoceptor antagonist) on antidepressant-like effect induced by Carthamus tinctorius (CT) extract in the TST. Values are presented as mean± SEM. *** shows significant differences between vehicle (control) and other groups at P<0.001. +++ compared with the extract group. Significance level was analyzed by two-way ANOVA followed by Tukey’s test
Figure 10.
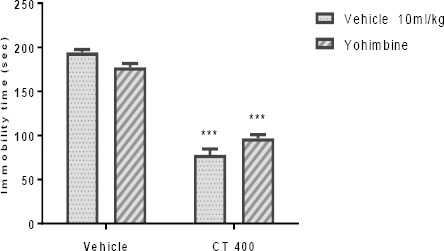
Effect of Pretreatment of animals with yohimbine (1 mg/kg, IP, a α2-adrenoceptor antagonist) on antidepressant-like effect induced by Carthamus tinctorius (CT) extract in the TST. Values are presented as mean± SEM. *** shows significant differences between vehicle (control) and other groups at P<0.001. +++ compared with the extract group. Significance level was analyzed by two-way ANOVA followed by Tukey’s test
Figure 11.
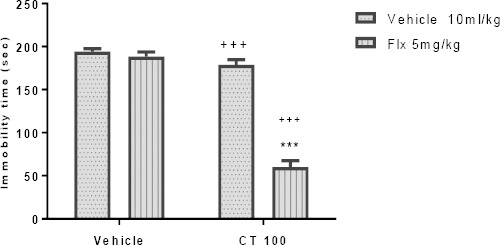
Effect of sub-effective doses of fluoxetine (5 mg/kg, IP) on sub-effective dose of Carthamus tinctorius (CT) extract (100 mg/kg, IP) in the TST in mice. ***P<0.001 compared with vehicle treated control. +++ compared with the extract group. Statistical level of significance analyzed by two-way analysis
Figure 12.
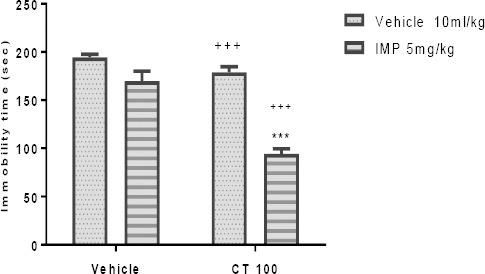
Effect of sub-effective doses of imipramine (5 mg/kg, IP) on sub-effective dose of Carthamus tinctorius (CT) extract (100 mg/kg, IP) in the FST in mice. ***P<0.001 compared with vehicle treated control. +++ compared with the extract group. Statistical level of significance analyzed by two-way analysis.
Figure 13.
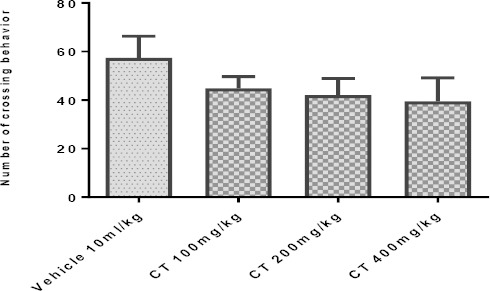
Effect of Carthamus tinctorius (CT) extract in open field test (crossing) in mice. Values are expressed as mean± SEM. P<0.05, as compared with vehicle treated control. Statistical level of significance analyzed by one-way ANOVA followed by Tukey’s comparison test
Figure 14.
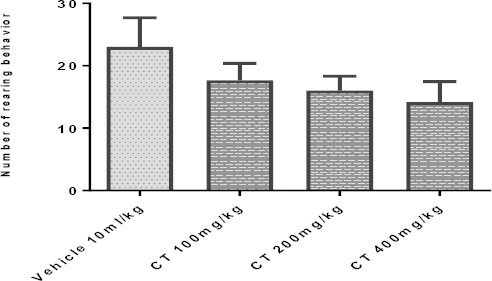
Effect of Carthamus tinctorius (CT) extract in open field test (rearing) in mice. Values are expressed as mean± SEM. P<0.05, as compared with vehicle treated control. Statistical level of significance analyzed by one-way ANOVA followed by Tukey’s comparison test.
Discussion
Our findings, for the first time, revealed that the acute administration of CT extract by IP route induces a significant antidepressant-like activity in TST in mice. It is interesting that CT extract and common antidepressants, fluoxetine and imipramine, did not show significant differences in immobility time. Furthermore, the locomotor activity was not altered with CT extract in OFT. An increase in the locomotor activity is not a suitable response for anti-depressant activity because the increase in the locomotor activity is a response of false positive. Moreover, our results demonstrate that CT extract antidepressant-like effect seems to be mediated by an interaction with the dopaminergic and serotonergic systems, but not with the adrenergic system. The TST is well known as a behavioral model for detection of antidepressant activity. In TST, mice were positioned in a predictable status, knowing that reduction in immobility time is an indicator for antidepressant-like action (18). The TST is the index for discriminating the psycho-stimulant drugs from antidepressants (30). To date, we could not find any study showing that CT extract is modulated in antidepressant-like activity. In agreement with our findings, other researchers demonstrated that some herbal extracts show antidepressant-like effects (31-33).
Based on our GS-MS analysis data, N-Hexadecanoic (palmitic) acid is the major compound of CT extract. Similarly, researchers demonstrated that the N-Hexadecanoic acid was the main component of the CT extract (34). N-Hexadecanoic acid is the most common saturated fatty acid (SFA) found in plants (35). In addition, other SFAs (linolenic acid, octadecanoic acid, 2,3-dihydro-1H-cylonona, and methyl commate E) were separated and identified from CT extract. On the other hand, previous studies reported the antioxidant activity of SFAs (36, 37). Apart from SFAs, phytosterols (i.e. stigmasterin and gamma-sitosterol) were identified from the CT extract. These components may also have contributed to the antioxidant activity of the CT extract (38). However, other compounds were relatively minor.
It is worth mentioning that different studies have demonstrated antidepressant-like activity of anti-oxidants (39, 40). On the other hand, several researchers provide evidence for the cumulative antioxidant-promoting impacts of different classes of antidepressants (e.g. amitriptyline, venlafaxine, and fluoxetine), which may represent a universal mechanism of action that only involves potential target of antidepressant regulation (41). Hence, it seems that antioxidant activity of CT extract components may contribute to its anti-depressant-like activity.
Elhwuegi reported that monoamine neurotrans-mitters, NA, DA, and 5HT, modulate in the pathophysio-logy and treatment of depression (42). It is well documented that most of the drugs used in the treatment of depression elevate levels of these monoamine neurotransmitters (43). Our results showed that Pretreatment of animals with DA receptor antagonists, SCH23390, sulpiride, or haloperidol significantly prevented the reduction in immobility time elicited by the extract; suggesting that the dopaminergic system appears to be implicated in the antidepressant-like effects of the CT extract in TST in mice. This system modulates mood regulation and is efficient in the treatment of depression (6, 44). Based on earlier studies, because the antagonists of the D1 and D2 receptor could inhibit antidepressant-like activity, researchers explained its role in depression (28, 45, 46). Clinical investigations have suggested the use of D2 receptor agonists for the depression treatment (47). Thus, CT extract can partly act by the dopaminergic system in the TST in mice. Monoamine transmitters, i.e. NA and DA, are crucial for the true activity of cognition, emotion, etc. (48). It is important to mention that, Zhao et al. showed CT extracts interact with activators of DA, NA, and/or 5HT transporter preventers (14). Thus, it is reasonable that CT extract, through these activators and inhibitors, may improve neuropsychological injuries (e.g. depression).
Pretreatment of mice with pCPA, WAY100135, ketanserin, and cyproheptadine inhibited the dec-rease in immobility time elicited by the CT extract; suggesting that the serotonergic system appears to be implicated in the antidepressant effects of CT extract in TST in mice. Some studies have introduced 5HT as the main neurotransmitter in depression because it modulates some signs of severe depression (e.g. memory) (49). The 5HT1A receptors are directly involved in the clinical effect of antidepressants (50) because they are placed on the soma and dendrites of 5HT neurons in the dorsal raphe and prevent 5HT release (51). The 5-HT2 receptors that are located in the brain regions such as the hippocampus were associated with etiology of depression (52). Studies have shown that ketanserin inhibits the antidepressant activity of some medicinal plants in the TST in mice (43, 53). Our findings showed that there is a relation between the serotonergic system and CT extract in the TST. It is well accepted that tryptophan is the substrate of the 5HT synthesis and, on the other hand, study showed that CT extract prevents tryptophan breakdown (54). Thus, the involvement of CT extract and serotonergic system may be due to suppression of tryptophan breakdown. Meanwhile, Zhao et al. indicated acoumaroylsper-midine analog isolate of CT, N1, N(5)-(Z)-N(10)-(E)-tri-p-coumaroylspermidine inhibits 5HT uptake (13). It is concluded that CT interacts with the serotonergic system through prevention in tryptophan breakdown or 5HT uptake.
Our results indicated that Pretreatment of mice with an α1-adrenoceptor antagonist (prazosin) and α2-adrenoceptor antagonist (yohimbine) could not reverse the decrease in immobility time elicited by the CT extract in the TST in mice, suggesting noninvolvement of the noradrenergic system in antidepressant-like effect of the CT extract. Several studies have shown that the noradrenergic system is included in the pathophysiology of depression and the mechanism action of antidepressant agents (42, 55, 56, 58), but we cannot, unfortunately, find any study which shows the involvement of CT extract through the noradrenergic system. Considering the obtained results, it seems CT extract does not act by the noradrenergic system.
Our results also indicated that sub-effective dose of the CT extract (100 mg/kg, IP) could increase the antidepressant-like effect of common drugs (e.g. fluoxetine and imipramine) in a sub-effective dose (5 mg/kg, IP). Hence, we can suggest that the combination of CT extract with common antidepre-ssants may be a promising approach for further studies on the treatment of depression. However, the isobologram analysis is needed to clarify any synergistic effects between them. Nevertheless, some researchers consider the adjuvant use of medicinal plants a promising choice for increasing the response and improving the efficacy of synthetic antidepre-ssants and decreasing their side effects by allowing lower doses to be prescribed (57). On the other hand, combining antidepressants from different classes represents an alternative strategy to exploit potential synergies among two or more independent thera-peutic mechanisms for treating resistant patients. The main advantage of the proposed approach is decreased doses of prescribed antidepressants and consequently the adverse side effects (58). Thus, the sub-effective dose of CT extract, without side effects, in combination with common drugs (a sub-effective dose) can be used for depression treatment in commercial form.
Conclusion
The current study suggests that the antidepressant-like effect of the CT extract in the TST may be attributed, at least in part, to N-Hexadecanoic (palmitic) acid as a main component. However, other components could play a role in the antidepressant-like effect of CT extract and further studies should be designed to clarify the bioactive principles responsible for these activities. Moreover, our observations show that the CT extract demonstrates this effect mediated through the dopaminergic and serotoninergic systems. Also, CT extract can increase antidepressant-like effects of common antidepressant drugs, indicating that CT extract can improve the effectiveness of these drugs. Our findings validate the folk use of CT extract for the management of depression.
Acknowledgment
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. But, we appreciate Dr Ravirajsinh Jadeja, an editorial board member of the American Journal of Pharmacology and Phytotherapy (AJPP), for his help in preparation of this manuscript.
Conflict of interest
The authors declare that no conflict of interest exists.
References
- 1.Nemeroff CB. The burden of severe depression:a review of diagnostic challenges and treatment alternatives. J Psychiatr Res. 2007;41:189–206. doi: 10.1016/j.jpsychires.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Neal MJ. Medical pharmacology at a Glance. 7th ed. John Wiley & Sons; 2012. [Google Scholar]
- 3.Howland RD, Mycek MJ, Harvey RA, Champe PC. Lippincott’s illustrated reviews:Pharmacology. 5th ed. Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 4.Meyers S. Monoaminergic supplements as natural antidepressants. Altern Med Rev. 2000;5:64–71. [PubMed] [Google Scholar]
- 5.Hasler G. Pathophysiology of depression:do we have any solid evidence of interest to clinicians? World Psychiatr. 2010;9:155–161. doi: 10.1002/j.2051-5545.2010.tb00298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papakostas GI. Dopaminergic-based pharmacotherapies for depression. Eur Neuropsychopharmacol. 2006;16:391–402. doi: 10.1016/j.euroneuro.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Trevor AJ, Katzung BG, Masters SB, Kruidering-Hall M. Pharmacology Examination & Board Review. 11th ed. McGraw-Hill Medical; 2010. [Google Scholar]
- 8.Tamminga CA, Nemeroff CB, Blakely RD, Brady L, Carter CS, Davis KL, et al. Developing novel treatments for mood disorders:accelerating discovery. Biol Psychiatry. 2002;52:589–609. doi: 10.1016/s0006-3223(02)01470-1. [DOI] [PubMed] [Google Scholar]
- 9.Zhang ZJ. Therapeutic effects of herbal extracts and constituents in animal models of psychiatric disorders. Life Sci. 2004;75:1659–1699. doi: 10.1016/j.lfs.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 10.Asgary S, Rahimi P, Mahzouni P, Madani H. Antidiabetic effect of hydroalcoholic extract of Carthamus tinctorius L. in alloxan-induced diabetic rats. J Res Med Sci. 2012;17:386. [PMC free article] [PubMed] [Google Scholar]
- 11.Zargari A. Medicinal plants. 8th ed. Tehran University Press; 2014. [Google Scholar]
- 12.Abbasi Maleki S. Effect of ethanolic extract of Safflower on naloxone-induced morphine withdrawal signs in mice. Adv Herb Med. 2015;1:9–15. [Google Scholar]
- 13.Zhao G, Gai Y, Chu WJ, Qin GW, Guo LH. A novel compound N 1, N 5-(Z)-N 10-(E)-tri-p-coumaroylspermidine isolated from Carthamus tinctorius L. and acting by serotonin transporter inhibition. Eur Neuropsychopharmacol. 2009;19:749–758. doi: 10.1016/j.euroneuro.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Zhao G, Zheng XW, Gai Y, Chu WJ, Qin GW, Guo LH. Safflower extracts functionally regulate monoamine transporters. J Ethnopharmacol. 2009;124:116–124. doi: 10.1016/j.jep.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Al Hashmi LS, Hossain MA, Weli AM, Al-Riyami Q, AlSabahi JN. Gas chromatography–mass spectrometry analysis of different organic crude extracts from the local medicinal plant of Thymus vulgaris L. Asian Pac J Trop Biomed. 2013;3:69–73. doi: 10.1016/S2221-1691(13)60026-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lorke D. A new approach to practical acute toxicity. Arch Toxicol. 1983;53:275–289. doi: 10.1007/BF01234480. [DOI] [PubMed] [Google Scholar]
- 17.OECD/OCDE 423.OECD web. Consulted 22 May 2009. http://iccvam.niehs.nih.gov/SuppDocs/FedDocs/OECD/OECD.GL423.pdf .
- 18.Steru L, Chermat R, Thierry B, Simon P. The tail suspension test:a new method for screening antidepressants in mice. Psychopharmacol. 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- 19.Jesse CR, Wilhelm EA, Bortolatto CF, Nogueira CW. Evidence for the involvement of the noradrenergic system, dopaminergic and imidazoline receptors in the antidepressant-like effect of tramadol in mice. Pharmacol Biochem Behav. 2010;95:344–350. doi: 10.1016/j.pbb.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 20.Voiculescu SE, Rosca AE, Zeca V, Zagrean L, Zagrean AM. Impact of maternal melatonin suppression on forced swim and tail suspension behavioral despair tests in adult offspring. J Med Life. 2015;8:202–206. [PMC free article] [PubMed] [Google Scholar]
- 21.Piotrowska A, Siwek A, Wolak M, Pochwat B, Szewczyk B, Opoka W, et al. Involvement of the monoaminergic system in the antidepressant-like activity of chromium chloride in the forced swim test. J Physiol Pharmacol. 2013;64:493–498. [PubMed] [Google Scholar]
- 22.Przegaliński E, Moryl E, Papp M. The effect of 5-HT 1A receptor ligands in a chronic mild stress model of depression. Neuropharmacol. 1995;34:1305–1310. doi: 10.1016/0028-3908(95)00102-c. [DOI] [PubMed] [Google Scholar]
- 23.Tanyeri P, Buyukokuroglu ME, Mutlu O, Ulak G, Akar FY, Celikyurt IK, et al. Involvement of serotonin receptor subtypes in the antidepressant-like effect of beta receptor agonist Amibegron (SR 5↣A):an experimental study. Pharmacol Biochemy Behav. 2013;105:12–16. doi: 10.1016/j.pbb.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 24.Gu L, Liu YJ, Wang YB, Yi LT. Role for monoaminergic systems in the antidepressant-like effect of ethanol extracts from Hemerocallis citrina. J Ethnopharmacol. 2012;139:780–787. doi: 10.1016/j.jep.2011.11.059. [DOI] [PubMed] [Google Scholar]
- 25.BinfaréRW Rosa AO, Lobato KR, Santos AR, Rodrigues AL. Ascorbic acid administration produces an antidepressant-like effect:evidence for the involvement of monoaminergic neurotransmission. Prog Neuropsychopharmacol Biol Psychiatry. 2009;30:530–540. doi: 10.1016/j.pnpbp.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Harkin A, Connor T, Walsh M, St John N, Kelly J. Serotonergic mediation of the antidepressant-like effects of nitric oxide synthase inhibitors. Neuropharmacol. 2003;44:616–623. doi: 10.1016/s0028-3908(03)00030-3. [DOI] [PubMed] [Google Scholar]
- 27.Zheng M, Li Y, Shi D, Liu C, Zhao J. Antidepressant-like effects of flavonoids extracted from Apocynum venetum leaves in mice:the involvement of monoaminergic system in mice. Afr J Pharm Pharmacol. 2014;8:765–774. [Google Scholar]
- 28.Ishola IO, Agbaje EO, Akinleye MO, Ibeh CO, Adeyemi OO. Antidepressant-like effect of the hydroethanolic leaf extract of Alchornea cordifolia (Schumach&Thonn.) Mull Arg(Euphorbiaceae) in mice:Involvement of monoaminergic system. J Ethnopharmacol. 2014;158:364–372. doi: 10.1016/j.jep.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 29.Brown RE, Corey SC, Moore AK. Differences in Measures of Exploration and Fear in MHC-Congenic C57BL/6J and B6-H-2K Mice. Behav Genet. 1999;29:263. [Google Scholar]
- 30.Borsini F, Lecci A, Mancinelli A, D’Aranno V, Meli A. Stimulation of dopamine D-2 but not D-1 receptors reduces immobility time of rats in the forced swimming test:implication for antidepressant activity. Eur J Pharmacol. 1988;148:301–307. doi: 10.1016/0014-2999(88)90107-0. [DOI] [PubMed] [Google Scholar]
- 31.Jafarpoor N, Abbasi-Maleki S, Asadi-Samani M, Khayatnouri MH. Evaluation of antidepressant- like effect of hydroalcoholic extract of Passiflora incarnata in animal models of depression in male mice. J Herb Med Pharmacol. 2014;3:41–45. [Google Scholar]
- 32.Moallem SA, Hosseinzadeh H, Ghoncheh F. Evaluation of antidepressant effects of aerial parts of echium vulgare on mice. Iran J Basic Med Sci. 2007;10:189–196. [Google Scholar]
- 33.Shahamat Z, Abbasi-Maleki S, Mohammadi Motamed S. Evaluation of antidepressant-like effects of aqueous and ethanolic extracts of Pimpinella anisum fruit in mice. Avicenna J Phytomed. 2016;6:322–328. [PMC free article] [PubMed] [Google Scholar]
- 34.Jia LH, Liu Y, Li YZ. Rapid determination of volatile constituents in safflower from Xinjiang and Henan by ultrasonic-assisted solvent extraction and GC–MS. J Pharm Anal. 2011;1:213–218. doi: 10.1016/j.jpha.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bazinet RP, Laye S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat Rev Neurosci. 2014;15:771–785. doi: 10.1038/nrn3820. [DOI] [PubMed] [Google Scholar]
- 36.Henry GE, Momin RA, Nair MG, Dewitt DL. Antioxidant and cyclooxygenase activities of fatty acids found in food. J Agric Food Chem. 2002;50:2231–2234. doi: 10.1021/jf0114381. [DOI] [PubMed] [Google Scholar]
- 37.Elagbar ZA, Naik RR, Shakya AK, Bardaweel SK. Fatty ascids analysis, antioxidant and biological activity of fixed oil of annona muricata L. Seeds. J Chem. 2016;2016:1–6. [Google Scholar]
- 38.Yoshida Y, Niki E. Antioxidant effects of phytosterol and its components. J Nutr Sci Vitaminol (Tokyo) 2003;49:277–280. doi: 10.3177/jnsv.49.277. [DOI] [PubMed] [Google Scholar]
- 39.Scapagnini G, Davinelli S, Drago F, De Lorenzo A, Oriani G. Antioxidants as antidepressants:fact or fiction? CNS Drugs. 2012;26:477–490. doi: 10.2165/11633190-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 40.Slamon ND, Pentreath VW. Antioxidant defense against antidepressants in C6 and 1321N1 cells. Chem Biol Interact. 2000;127:181–199. doi: 10.1016/s0009-2797(00)00172-1. [DOI] [PubMed] [Google Scholar]
- 41.Kolla N, Wei Z, Richardson JS, Li XM. Amitriptyline and fluoxetine protect PC12 cells from cell death induced by hydrogen peroxide. J Psychiatry Neurosci. 2005;30:196–201. [PMC free article] [PubMed] [Google Scholar]
- 42.Elhwuegi AS. Central monoamines and their role in major depression. Prog Neuro-Psychopharmacol Biol Psychiatr. 2004;28:435–451. doi: 10.1016/j.pnpbp.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 43.Risch SC, Nemeroff CB. Neurochemical alterations of serotonergic neuronal systems in depression. J Clin Psychiatry. J Clin Psychiatry. 1992;53:3–7. [PubMed] [Google Scholar]
- 44.Willner P, Hale AS, Argyropoulos S. Dopaminergic mechanism of antidepressant action in depressed patients. J Affect Disord. 2005;86:37–45. doi: 10.1016/j.jad.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 45.Hirano S, Miyata S, Onodera K, Kamei J. Involvement of dopamine D 1 receptors and α1-adrenoceptors in the antidepressant-like effect of chlorpheniramine in the mouse tail suspension test. Eur J Pharmacol. 2007;562:72–76. doi: 10.1016/j.ejphar.2007.01.063. [DOI] [PubMed] [Google Scholar]
- 46.Machado DG, Kaster MP, Binfaré RW, Dias M, Santos AR, Pizzolatti MG, et al. Antidepressant-like effect of the extract from leaves of Schinus molle L. in mice:evidence for the involvement of the monoaminergic system. Prog Neuro-Psychopharmacol Biol Psychiatr. 2007;31:421–428. doi: 10.1016/j.pnpbp.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 47.Wæhrens J, Gerlach J. Bromocriptine and imipramine in endogenous depression:a double-blind controlled trial in out-patients. J Affect Disord. 1981;3:193–202. doi: 10.1016/0165-0327(81)90044-6. [DOI] [PubMed] [Google Scholar]
- 48.Marien MR, Colpaert FC, Rosenquist AC. Noradrenergic mechanisms in neurodegenerative diseases:a theory. Brain Res Rev. 2004;45:38–78. doi: 10.1016/j.brainresrev.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 49.Haider S, Khaliq S, Haleem DJ. Enhanced serotonergic neurotransmission in the hippocampus following tryptophan administration improves learning acquisition and memory consolidation in rats. Pharmacol Rep. 2007;59:53. [PubMed] [Google Scholar]
- 50.Celada P, Puig MV, Amargós-Bosch M, Adell A, Artigas F. The therapeutic role of 5-HT1A and 5-HT2Areceptors in depression. Psychiatry Neurosci. 2004;29:252. [PMC free article] [PubMed] [Google Scholar]
- 51.Shrestha S, Hirvonen J, Hines CS, Henter ID, Svenningsson P, Pike VW, et al. Serotonin-1A receptors in major depression quantified using PET:controversies, confounds, and recommendations. Neuroimage. 2012;59:3243–3251. doi: 10.1016/j.neuroimage.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 53.Machado DG, Bettio LE, Cunha MP, Capra JC, Dalmarco JB, Pizzolatti MG, et al. Antidepressant-like effect of the extract of Rosmarinus officinalis in mice:involvement of the monoaminergic system. Prog Neuro-Psychopharmacol Biol Psychiatr. 2009;33:642–650. doi: 10.1016/j.pnpbp.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 54.Kuehnl S, Schroecksnadel S, Temml V, Gostner JM, Schennach H, Schuster D, et al. Lignans from Carthamus tinctorius suppress tryptophan breakdown via indoleamine 2, 3-dioxygenase. Phytomed. 2013;20:1190–1195. doi: 10.1016/j.phymed.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Frazer A. Norepinephrine involvement in antidepressant action. J Clin Psychiatry. 2000;61:25–30. [PubMed] [Google Scholar]
- 56.Nutt DJ, Baldwin DS, Clayton AH. The role of dopamine and norepinephrine in depression and antidepressant treatment. J Clin Psychiatry. 2006;67:3–8. [PubMed] [Google Scholar]
- 57.Sarris J, Kavanagh DJ, Byrne G. Adjuvant use of nutritional and herbal medicines with antidepressants, mood stabilizers and benzodiazepines. J Psychiatr Res. 2010;44:32–41. doi: 10.1016/j.jpsychires.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 58.Lam RW, Wan DD, Cohen NL, Kennedy SH. Combining antidepressants for treatment resistant depression:a review. J Clin Psychiatry. 2002;63:685–693. doi: 10.4088/jcp.v63n0805. [DOI] [PubMed] [Google Scholar]


