Abstract
Multiple sclerosis (MS) is an inflammatory demyelinating disease of the central nervous system (CNS). Riboflavin plays an important role in myelin formation, and its deficiency is implicated as a risk factor for multiple sclerosis. Here, we systematically reviewed the literature concerning the health benefits of riboflavin on MS. The literature recorded within four main databases, including relevant clinical trials, experimental, and case-control studies from 1976 to 2017 were considered. Both human and animal studies were included for review, with no restrictions on age, gender, or ethnicity. Experimental studies demonstrated that riboflavin deficiency triggers neurologic abnormalities related to peripheral neuropathies such as demyelinating neuropathy. Moreover, randomized controlled trials (RCT) and case-control studies in which MS patients received riboflavin supplementation or had higher dietary riboflavin intake showed improvements in neurological motor disability. Riboflavin is a cofactor of xanthine oxidase and its deficiency exacerbates low uric acid caused by high copper levels, leading to myelin degeneration. The vitamin additionally plays a significant role in the normal functioning of glutathione reductase (GR) as an antioxidant enzyme, and conditions of riboflavin deficiency lead to oxidative damage. Riboflavin promotes the gene and protein levels of brain-derived neurotrophic factor (BDNF) in the CNS of an animal model of MS, suggesting that BDNF mediates the beneficial effect of riboflavin on neurological motor disability. Research to date generally supports the role of riboflavin in MS outcomes. However, further observational and interventional studies on human populations are warranted to validate the effects of riboflavin.
Keywords: Brain-derived- neurotrophic factor, Demyelinating disease, Multiple sclerosis, Riboflavin, Riboflavin deficiency
Introduction
Multiple sclerosis (MS) is a demyelinating inflammatory disease of the central nervous system (CNS) (1). The Atlas of MS 2013 has documented an increase in the incidence of MS worldwide from 2.1 million in 2008 to 2.3 million in 2013 (2). On the National MS Society website, more than 900 clinical trials on MS sponsored by the National Institutes of Health have been registered since 2000, including over 300 ongoing or planned studies investigating the effects of different treatments. At present, only eight FDA-approved agents for treatment of relapsing forms of MS are available (3), highlighting the urgent need for new and effective therapeutic approaches for MS (4).
Riboflavin (7, 8-dimethyl-10-ribityl-isoalloxa zine), also known as vitamin B2, is a water soluble vitamin present in a variety of foods, a micronutrient with a key function in maintaining human health (5). The most important biologically active forms of riboflavin, flavin adenine dinucleotide (FAD) and flavin mononucleotide (FMN), participate in a range of redox reactions, some of which are absolutely essential for aerobic cell function (6). Riboflavin is also critical for proper functioning of the nervous, endocrine, cardiovascular, and immune systems. For human subjects and animals that are unable to synthesize riboflavin, the main sources are food and activities of intestinal microflora (5).
Riboflavin deficiency is endemic in many regions worldwide, (7) particularly developing countries, (8) and certain population groups have low dietary intakes (9, 10). Riboflavin deficiency has been implicated as a risk factor in a number of diseases, (10, 11) including MS (12). In addition, the vitamin clearly plays an important role in myelin formation (13-18). However, few reports to date have focused on the effects of inadequate riboflavin intake and /or supplementation on demyelinating diseases. A PubMed search with the keywords “demyelination” and “riboflavin” yields limited citations of studies over the last decade.
Considering the growing interest in the putative role of riboflavin in protecting against demyelinating disease (12, 14, 15, 18-20), evaluation of its metabolic roles, and the public health relevance of low intake of this vitamin on MS disease is warranted. Here, we have provided an updated overview of literature focusing on the correlation between MS and riboflavin, considering recent publications.
Materials and Methods
Search Strategy
The cited publications were generally identified by searching the National Library of Medicine PubMed database (pubmed.gov), MEDLINE, Science Direct, Scopus, and Google Scholar using the terms “demyelination”, “multiple sclerosis”, “encephalo-myelitis, autoimmune, expe- rimental”, “riboflavin”, and “riboflavin deficiency“, in accordance with the Medical Subject Heading (MeSH). Studies on both animals and humans were included, with no restrictions on age, gender, or ethnicity. A manual search of the references cited in the identified studies was additionally conducted.
Firstly, a search strategy was developed using the MeSH terms in MEDLINE (OvidSP) as well as the relevant keywords. All the individual relevant words, phrases, and abbreviations (e.g., “riboflavin”, “multiple sclerosis”, “MS”, and “demyelination”) or a combina -tion were used. All similar phrases (e.g., amyolateral sclerosis, amyotrophic lateral sclerosis, and systemic sclerosis) were excluded. The following broader MeSH terms were added to the search: “riboflavin deficiency”, “Dietary Supplements“, “encephalomye -litis, autoimmune, experimental”, and “Peripheral Nervous System Diseases“ (Figure 1).
Figure 1.
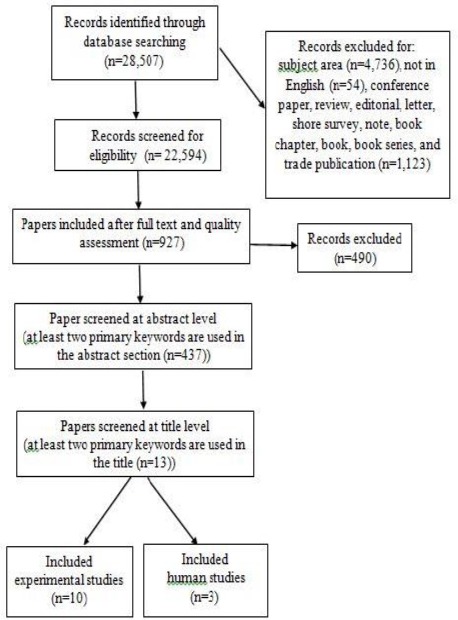
Screening process for papers related to riboflavin, demyelination, and multiple sclerosis
The search for the relevant published papers was accomplished in two phases.
The first phase focused on original studies with clinical trials and experimental and observational designs. The original search was conducted in September 2016 and updated at the end of February 2017. This lag time allowed us to incorporate the most recent publications, and thereby, the maximum number of studies. The second phase focused on screening of original studies for eligibility. The relevant full-text manuscripts with high quality were screened at the abstract/title levels. At this level, papers with at least two main keywords (e.g., “riboflavin”, “multiple sclerosis”, “MS”, and “demyelination” or a combination) were included and allocated to experimental and human studies for review. Detailed search strategies are presented in Figure 1.
Inclusion and exclusion criteria
Clinical trials and experimental and case-control studies examining dietary riboflavin, riboflavin supplementations, and deficiencies associated with the clinical, histological, and biochemical nature of the disease involving human subjects and an animal model of MS and demyelination polyneuropathies were included. Articles were excluded based on the following grounds: no specific assessment of demyelinating conditions and other aspects of MS, such as inflammation and axonal loss, e.g., those exam- ining autoimmune and inflammatory diseases, such as human and animal models of brain injuries and amyotrophic lateral sclerosis. No search restrictions were imposed in terms of animal species, age, gender, race, geographical residence, or source of patient participants (e.g., hospital, outpatients, community, registry, or health administrative data).
The outcomes of interest were changes in laboratory and histological biomarkers as well as clinical evaluations, including expanded disability status scale (EDSS) test for assessment of neurol- ogical motor disabilities (Figure 1). EDSS is a 20-point scale (ranging from 0 = normal to 10 = death due to MS, marked by a 0.5 score increase) and currently, the most widely used method to evaluate motor disability in MS (21). This measurement is an expansion of the disability status scale (DSS) that applies the same range without 0.5 increments (22).
Finally, the comprehensive topic of the association between riboflavin status and onset or progression of MS was reviewed and a brief overview provided.
Results
Riboflavin and MS risk
Table 1 summarizes RCTs and case-control studies in which MS patients received riboflavin supplementation or were exposed to high dietary riboflavin intake (12, 23, 24). One RCT demonstrated a significant reduction in EDSS in response to riboflavin supplementation, along with improvement of cognitive function (23). In a double-blind RCT in which MS patients in treatment and control groups received either riboflavin supplementation (10 mg/day) or placebo daily for six months, application of riboflavin did not improve disability status. This effect was not related to serum homocysteine levels (12). A negative linear trend between higher intake of riboflavin and risk of MS was observed in a case-control study (24). In several experiments (14-19, 25, 26) (Table 2), animals maintained on riboflavin-deficient diets showed signs of neuropathy and pathologic changes in the sciatic nerve in a time-dependent manner (26). Similar alterations appeared in cervical and lumbar spinal nerves, characterized by hypertrophic Schwann cells, tomacula (redundant myelin swellings), demyelination/remyelination (14), fibroblastic onion bulb-like structures in sciatic and brachial nerves (19), and acquired primary demyelinating tomaculous neuropathy in sciatic and brachial nerves (16). Pathological evaluation of peripheral nerves revealed demyelinating neuropathy with the formation of paranodal tomacula occurred early in the course of demyelination along with increased frequency of myelinated fibers, indicative of demyelination in avian riboflavin deficiency model of demyelination (25). Peripheral nerve lesions comprising swelling and discoloration of all the peripheral nerve trunks were observed. Moreover, microscopic lesions, including moderate to severe swelling, fragmentation and demyelination of myelin sheaths, atrophy, and loss of axons (17), Schwann cell swelling, perivascular leukocytic infiltration, segmental demyelination, (15, 18) accompanied by accumulation of osmiophilic debris in Schwann cell cytoplasm, and axon degeneration (15, 18) were common.
Table 1.
Summary of human studies on the effects of riboflavin on multiple sclerosis
| Study date (Ref No) | Study type | Riboflavin dose, sample size, and duration of study | Clinical outcomes | Laboratory outcomes |
|---|---|---|---|---|
| Naghashpour et al, 2013 (12) | RCT | A total of 29 MS patients were enrolled. The treatment group received 10 mg/day riboflavin orally for 6 months | No significant differences in EDSS between the treatment and placebo groups | No significant differences between the treatment and placebo groups in terms of EGRAC, riboflavin deficiency levels by EGRAC category and serum homocysteine levels |
| Bisaga et al, 2011 (23) | RCT | A total of 94 patients with RRMS and SPMS in the acute phase. Group 1 (n=53) received cytoflavin (including 5 mg riboflavin as riboflavin mononucleotide) and basic treatment (trental and group B vitamins) and Group 2 (n=41) received basic treatment (trental and group B vitamins). | About 41.5% patients treated with cytoflavin and 34% patients receiving basic treatment did not need corticosteroids. Significant reduction of EDSS and improvement of cognitive function were observed in patients treated with cytoflavin, compared to the group that did not receive this drug. | Decrease in lipid peroxidation levels and content of antibodies to basic myelin protein was evident in patients treated with cytoflavin, compared with the group that did not receive this drug |
| Ghadirian et al, 1998 (24) | Case-Control | 197 incident cases and 202 frequency- matched controls | A negative linear trend was evident between higher intake of riboflavin and risk of MS (<0.001) |
MS, multiple sclerosis; EDSS, expanded disability status scale; RCT, randomized controlled trials; EGRAC, erythrocyte glutathione reductase activity coefficient; RRMS, relapsing-remitting MS; SPMS, secondary-progressive MS
Table 2.
Summary of experimental studies on animal models of demyelination
| Study date | Riboflavin dose, sample size, and duration of study | Clinical outcomes | Histological outcomes | Laboratory outcomes |
|---|---|---|---|---|
| Naghashpour et al, 2016 (35) | Ten week-old C57BL/6 female EAE mice were administered riboflavin at 10 mg/kg body weight or INFβ-1a at 150 IU/g body weight for two weeks. | Peak disease score was reduced by riboflavin in both effector and chronic phases of the disease (P<0.05). Riboflavin delayed the onset of disease in EAE mice (P<0.05). | Riboflavin in combination with INFβ-1a increased BDNF mRNA and protein levels in the brain and spinal cord of EAE mice (P<0.01) IL-6 expression was elevated in the brains of the EAE group receiving riboflavin and INFβ-1a (P<0.01) | |
| Naghashpour et al, 2016 (37) | Ten week-old C57BL/6 female EAE mice were administered riboflavin at 10 mg/kg body weight and/or INFβ-1a at 150 IU/g body weight for two weeks. The Morris water maze (MWM) test to evaluate cognitive function and clinical monitoring were performed in mice. | Riboflavin and a combination of riboflavin and INF-β1a reduced the clinical scores. Riboflavin enhanced the swim speed of EAE mice in the MWM test (P<0.05) | Results obtained from brain revealed increased BDNF mRNA expression in EAE mice treated with a combination of riboflavin and INF-β1a (P<0.01). A combination of riboflavin and INF-β1a enhanced the BDNF levels in brains of EAE mice (P<0.05) | |
| Cai et al, 2009 (14) | One day-old broiler chickens were fed a riboflavin-deficient diet (1.8 mg/kg) while control chickens were administered a conventional diet containing 5.0 mg/kg riboflavin. | Pathologic changes were evident in sciatic, cervical, and lumbar spinal nerves of riboflavin-deficient chickens characterized by hypertrophic Schwann cells, tomacula (redundant myelin swellings), and demyelination/remyelination. | ||
| Cai et al, 2007 (19) | One-day old broiler chickens were fed a riboflavin-deficient diet (1.8 mg/kg) and killed on postnatal days 6, 11, 16, 21, and 31, whereas control chickens were fed a conventional diet containing 5.0 mg/kg riboflavin | Fibroblastic onion bulb-like structures were detected in sciatic and brachial nerves of riboflavin-deficient chickens from day 11 onwards, consisting of long cytoplasmic processes of hypertrophied fibroblasts surrounding demyelinated, remyelinated and normally myelinated axons. | ||
| Cai et al, 2006 (16) | Rapidly growing broiler chickens were fed either a riboflavin-deficient diet (containing 1.8 mg/kg riboflavin) or a conventional diet (containing 5.0 mg/kg riboflavin) and killed on postnatal days 6 (n = 6), 11 (n = 14), 16 (n = 6), and 21 (n = 5). | Acquired primary demyelinating tomaculous neuropathy was observed in the sciatic and brachial nerves of riboflavin-deficient chickens from day 11 onwards. | ||
| Cai et al, 2006 (25) | Newborn broiler chickens were maintained either on routine diet containing 5.0 mg/kg riboflavin (control group) or a riboflavin-deficient diet (containing 1.8 mg/kg riboflavin). | Riboflavin-deficient chickens showed signs of neuropathy from day 8 and pathological examination of peripheral nerves revealed demyelinating neuropathy with paranodal tomacula formation, starting on day 11. After day 16, paranodal swellings showed prominent degenerative changes accompanied by increased frequency of demyelination of fibers. | ||
| Johnson and Storts, 1998 (15) | One day-old chickens were fed a riboflavin-deficient diet (containing 1.65 mg/g riboflavin) for 52 days, followed by the control diet for 14 days. | Demyelinating peripheral neuropathy in young, rapidly growing chickens, including leg weakness and paralysis as early as 12 days of age. | Significant microscopic lesions were confined to peripheral nerves and included tissue separation (suggesting interstitial edema), Schwann cell swelling, perivascular leukocytic infiltration, and segmental demyelination accompanied by accumulation of osmiophilic debris in Schwann cell cytoplasm. Axon degeneration was observed. | Acid phosphatase enzyme activity of Schwann cells was increased in the affected nerves |
| Wada et al, 1996 (17) | Nine 14- to 55-day-old racing pigeons were maintained on a riboflavin-deficient diet (riboflavin concentration of 0.9 mg/kg feed). | DiarrhoeaDiarrhea, and leg and wing paralysis | Peripheral nerve lesions, including discoloration and swelling of all the peripheral nerve trunks, were observed. Microscopic lesions comprising swelling, fragmentation, demyelination of myelin sheaths, and proliferation of Schwann cells were evident in the peripheral nerves of all birds examined. These changes were associated with moderate to severe swelling, fragmentation, atrophy, and loss of axons. | |
| Jortner et al, 1987 (18) | A strain of rapidly growing meat-type chickens were fed a diet deficient in riboflavin (1.8 mg/kg riboflavin) from 1–40 days of age. | Diminished growth rate, progressive gait abnormality and reluctance to move | Neurologic abnormalities related to peripheral neuropathy characterized by Schwann cell hypertrophy and degeneration with cytoplasmic lipid droplets and segmental demyelination. Sequestration of myelin debris within Schwann cells was common. Presence of endoneurial edema and axonal degeneration involving small numbers of fibers. | Liver concentrations of riboflavin in deficient birds were significantly reduced on day 13 but not day 26. |
| Norton et al, 1976 (26) | Eighteen adult male rats were distributed into three equal groups. Each group was maintained on a specific diet: Group 1, complete diet (control); Group 2, riboflavin-deficient diet; and Group 3, riboflavin-deficient diet plus galactoflavin, 2 g/kg of diet. | Sciatic nerve fibers demyelinated in animals maintained on riboflavin- deficient diets in a time-dependent manner. Cellular organelles of both myelinated and nonmyelinated nerve fibers remained intact and presumably functional. |
PBS, phosphate buffer saline; EAE, experimental autoimmune encephalomyelitis; INFβ-1a, Interferon beta-1a; BDNF, brain-derived neurotrophic factor; IL-6, Interleukin-6; IL-17A, Interleukin-17A
The collective findings support the utility of riboflavin as a clinically effective treatment option for MS patients and animal models. However, further RCTs are required to provide solid evidence of the benefits of riboflavin supplementation.
Riboflavin as an immunomodulatory agent
Injection of riboflavin reduces mortality of mice with septic shock (27), enhances resistance to bacterial infection (28), and affects neutrophil migration, but does not alter acquired immune responsiveness (29). On the other hand, riboflavin deficiency results in lower neutrophil phagoc- ytosis (30), increased prostaglandin biosy- nthesis in rat kidney (31), and impaired hemato- poiesis through hindering iron absorption (7). Iron acts as an oxidation agent (via the Fenton reaction) and riboflavin decreases oxidative stress by reducing iron absorption. As discussed in a previous review by our group, riboflavin itself is considered an antioxidant. Other possible mechanisms by which riboflavin protects the body against oxidative stress may be attributed to the glutathione redox cycle and the conversion of reduced riboflavin to the oxidized form (32). Accordingly, we hypothesized that riboflavin could act as an anti-inflammatory agent via decreasing oxidative stress. Removal of riboflavin from the culture medium resulted in lower cell viability and accelerated cell death, resulting in a strikingly high fraction of late apoptotic cells with compromised membranes in a murine monocyte/macrophage cell line; the monocyte/macrophage is sensitive to riboflavin deficiency in culture medium (5). As professional phagocytes, macrophages play a crucial role in pathogen elimination but this ability appears to be significantly limited in macrophages with riboflavin deficiency (5). Macrophages play a dual role in MS pathology. These cells exert neuroprotective and growth promotory effects on the one hand but contribute to tissue damage via production of inflammatory mediators on the other (33). The various effector functions of macrophages in the formation of lesions include signaling events that move these cells into the white matter of CNS and trigger pathways that facilitate the destruction of myelin. For instance, macrophages have been identified as producers of a number of pro-inflammatory cytokines, chemo- kines, and toxic molecules known to promote demyelination, the major responders to CNS chemokines, and the key cells involved in phago- cytosis/degradation of the myelin sheath. Interes-tingly, activated transcription factors, such as nuclear factor kappa B (NF-κB), signal transd- ucer, activator of transcription 1 (STAT1), and STAT6, in both leukocytes and CNS plaques of MS patients, lead to enhanced expression of these inflammatory effectors in macrophages (34).
One RCT demonstrated favorable immunological changes in sera of MS patients administered a pharmaceutical form of riboflavin. In this study, decreased levels of antibodies to the basic myelin protein were detected in MS patients treated with cytoflavin including riboflavin as a component, compared to the group that did not receive the drug. This decrease was associated with significant reduction of EDSS and improvement of cognitive function (23). Moreover, our group showed that riboflavin at 10 mg/kg of body weight reduces motor disability accompanied by concomitant increased expression of IL-6 as a nouropointin immune factor and BDNF as a neurotrophic factor in the whole brain and spinal cord of experimental autoimmune encephalomyelitis (EAE) models of MS (35). IL-6 stimulates proliferation of microglia in culture and potentially promotes survival through inducing BDNF (35, 36). The results collectively demonstrate that riboflavin affects the immune system, and consequently exerts antinociceptive, anti-inflamm- atory (5), antioxidant (32), and neuropro- tective (35, 37) effects.
Riboflavin, genetics, and MS
Riboflavin deficiency causes protein and DNA damage accompanied by cell cycle arrest, increased cell stress, and apoptosis. Furthermore, mutations in the SLC52A2 or SLC52A3 as genes encoding riboflavin transporters that convey riboflavin into neurons and other cells are often associated with chronic, severe, inflammatory demyelinating, and sensory-motor polyneuropathy (20, 38). Nevertheless, treatment with riboflavin can terminate disease progression in most patients with riboflavin transporter disorders (20).
Differential riboflavin status also strongly affects the expression of numerous genes (5).
The relative mRNA levels of antimicrobial peptides (liver-specific antimicrobial peptide 2 and hepcidin), anti-inflammatory cytokines (interleukin 10 and transforming growth factor β1), tight junction proteins (occludin, zonula occludens 1, claudin-c, and claudin-3), signalling molecules (inhibitor of κBα, target of rapamycin, and NF-E2-related factor 2), and antioxidant enzymes and agents (copper, zinc superoxide dismutase, and GR) are significantly decreased in gills of fish fed a riboflavin-deficient diet. Conversely, mRNA levels of proinflammatory cytokines (tumor necrosis factor α, interleukin 8, interferon γ2, and interleukin 1β), signaling molecules (NF-κB p65, IκB kinase β, IκB kinase γ, Kelch-like-ECH-associated protein 1β, and myosin light chain kinase) and tight junction protein, claudin-12, are markedly increased in gills of fish fed a riboflavin-deficient diet. Moreover, relative mRNA expression of regulatory cytokines (IL-6) is significantly increased in EAE mice supplemented with riboflavin (35). In conclusion, riboflavin acts as an antioxidant and potentially regulates cytokines, other antioxidant agents, gill tight junction proteins, and related signaling molecules at the transcriptional level (39).
Discussion
Animal models of riboflavin deficiency display neurologic abnormalities related to peripheral neuropathy, such as demyelinating neuropathy characterized by Schwann cell hypertrophy and segmental demyelination, (14, 16, 19, 25), and riboflavin supplementation is shown to improve neurological motor disabilities, such as central neuropathy, in animal models of MS (37).
The precise mechanism by which riboflavin deficiency results in disruption of myelin lamellae is yet to be resolved. In addition to its role as an obligatory co-factor in numerous NADH dehydro-genation reactions, riboflavin acts at the first dehydrogenation step during fatty acid oxidation. In livers of weanling rats fed a riboflavin-deficient diet, mitochondrial fatty acid oxidation is suppressed, and conversely, restored after riboflavin supplementa- tion (40). Additionally, within the livers of riboflavin-deficient rats, dehydrogenation of fatty acids is reduced, and consequently, linoleic, linolenic, and arachidonic acid contents in serum and liver decrease. The issue of whether or not the phenom- enon of nerve fiber demyelination is related to altered fatty acid metabolism remains uncertain. Biochemical evaluation of myelinated nerve fibers under experimental conditions of riboflavin deficiency confirmed that demyelination is the result of specific alterations in the synthesis, degradation, or composition of membrane constituents (26).
Riboflavin is a coenzyme for xanthine oxidase, and its deficiency exacerbates low uric acid caused by high copper levels, leading to myelin degeneration (6). Moreover, riboflavin deficiency leads to oxidative damage, which may be attributable to decreased catalase, copper/zinc superoxide dismutase (SOD), glutathione peroxidase (GPx), glutathione-S-transferase, and glutathione reductase (GR) activities and reduced glutathione content (39). Riboflavin plays a critical role in the normal functioning of GR, an antioxidant enzyme required for conversion of oxidized glutathione to reduced glutathione (GSH). The latter is an important intracellular antioxidant that protects against reactive oxygen species (ROS) damage (10). As discussed in an earlier review, it is possible that riboflavin deficiency affects the antioxidant properties of glutathione, leading to impaired antioxidant potential of cells (32). Deactivation of peroxides, such as hydroperoxide, is one of the most important antioxidant activities of glutathione, mediated through the action of GPx (41). GPx transfers a hydrogen ion from reduced glutathione to lipid peroxide and produces oxidized glutathione and alcohol (42). Accordingly, riboflavin deficiency is expected to enhance lipid peroxidation (32). However, an earlier study disclosed no significant differences in serum levels of total antioxidant status (TAS) and dietary intake of antioxidants between MS patients and healthy controls; all subjects had low antioxidant status (43).
Moreover, riboflavin deficiency interferes with electron transport along the respiratory chain (44). Therefore, riboflavin-deficient cells deal with decreased energy production and are unable to maintain the intact cell membrane of early apoptotic cells. Consequently, these cells go through the early apoptosis stage rapidly to yield a higher level of secondary necrosis (5).
Riboflavin additionally enhances the gene and protein levels of BDNF in the whole brain and spinal cord as well as IL-6 gene expression in brains of EAE mice, suggesting that IL-6 and BDNF contribute to the observed beneficial effects of riboflavin on neurological motor disability, therefore presenting targets for strategic therapies (35). BDNF, an important regulator of neuronal maturation and protection, is expressed in and around MS lesions (45). Immune factors regulate the BDNF levels and affect the survival of neuronal cells in vitro (45). In this context, IL-6 may exert a proliferative effect on astrocytes in vitro (46, 47) synergistically with other factors (48). Moreover, in vitro assays have shown that both virus-infected microglia and astrocytes secrete IL-6, which, in turn, triggers secretion of the neurotrophin nerve growth factor (NGF) by astrocytes (49). In addition, microglia proliferate upon stimulation by IL-6 in culture and IL-6 possibly promotes survival through inducing BDNF (36). In vivo, NGF in dorsal root ganglia (DRG) is not upregulated in riboflavin-deficient mice with nerve injury (36). Thus, in many respects, IL-6 behaves in a neurotrophin-like fashion (50). Moreover, riboflavin plays a critical role as a coenzyme for succinate dehydrogenase activity in complex II of the mitochondrial respiratory chain generating intracellular adenosine triphosphate (ATP) (51). Since responses of CNS to circulating neurotrophic factors depend on the availability of intracellular ATP, riboflavin may enhance mitochondrial survival and bio-energy, leading to increased responsiveness to BDNF followed by enhanced myelin production in MS (Figure 2) (52). Furthermore, riboflavin is found to have a crucial role in the production of substrates used for the electron transport chain (ETC). Accordingly, any defect in riboflavin transport would disrupt ETC and consequently lead to neurodegeneration. The schematic mechanism of riboflavin deficiency leading to mitochondrial oxidative stress-mediated neuro-degeneration is summarized in Figure 3 (53).
Figure 2.
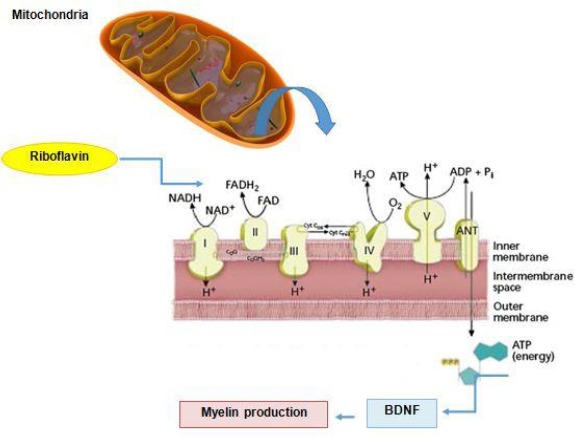
Riboflavin and mitochondrial pathways in MS. Riboflavin as an integral component of two coenzymes: FAD and FMN enhance availability of intracellular ATP, mitochondrial survival, and bio-energy, leading to increased responsiveness to BDNF followed by enhanced myelin production in MS. ADP=Adenosine diphosphate; ATP=Adenosine triphosphate; BDNF= Brain-derived neurotrophic factor; FAD= Flavin adenine dinucleotide; FMN= Flavin mononucleotide; NADH/NADH2= Nicotinamide adenine dinucleotide
Figure 3.
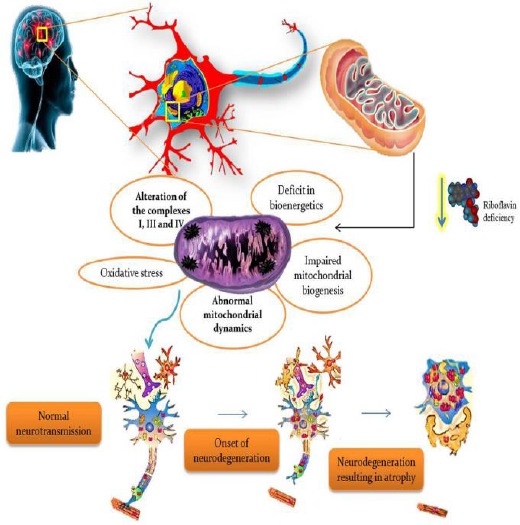
Riboflavin deficiency leads to mitochondrial oxidative stress-mediated neurodegeneration (53) (permission for reuse of this figure was acquired online from the Journal of Clinical Medicine)
These findings strongly suggest that riboflavin acts as a neuroprotective nutrient, particularly against cytokines, ROS, and nitric oxide (NO), which contribute to damaging myelin sheath and neurons that lead to neurological motor disability and development of MS.
Conclusion
Riboflavin may be considered a probable comple-mentary agent to reduce the deleterious effects of neurological disability in MS and animal models. The mechanism by which riboflavin protects the body against demyelination possibly involves neurotro-phins, especially BDNF, a crucial agent for neuronal maturation and protection. Since the majority of research in this area to date is limited to experimental studies, further interventional studies on human populations are required to establish the effects of riboflavin.
Acknowledgment
The results presented in this paper were part of a student thesis. Financial support for this project (Mahshid Naghashpour’s PhD research) was provided by the Vice Chancellor for Research, Ahvaz Jundishapur University of Medical Sciences (grant number: NRC-9208), Ahvaz, Iran, a concurrent research grant from the Vice-Chancellor for Research, Ahvaz Jundishapur University of Medical Sciences and the Academic Center for Education, Culture, and Research-Khuzestan (ACECR- Khuzestan), Iran (grant number: U-89238).
References
- 1.Kutzelnigg A, Lassmann H. Pathology of multiple sclerosis and related inflammatory demyelinating diseases. Handb Clin Neurol. 2014;122:15–58. doi: 10.1016/B978-0-444-52001-2.00002-9. [DOI] [PubMed] [Google Scholar]
- 2.Browne P, Chandraratna D, Angood C, Tremlett H, Baker C, Taylor BV, et al. Atlas of Multiple Sclerosis 2013:A growing global problem with widespread inequity. Neurology. 2014;83:1022–1024. doi: 10.1212/WNL.0000000000000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pappas DJ, Oksenberg JR. Multiple sclerosis pharmacogenomics:maximizing efficacy of therapy. Neurology. 2010;74:S62–69. doi: 10.1212/WNL.0b013e3181c980fb. [DOI] [PubMed] [Google Scholar]
- 4.Harrirchian MH, Mohammadzadeh Honarvar N, Koohdani F, Bitarafan S, Siassi F, Jafarirad S, et al. The effect of vitamin A supplementation on disease progression, cytokine levels and gene expression in multiple sclerotic patients:study protocol for a randomized controlled trial. Acta Med Iran. 2014;52:94–100. [PubMed] [Google Scholar]
- 5.Mazur-Bialy AI, Buchala B, Plytycz B. Riboflavin deprivation inhibits macrophage viability and activity - a study on the RAW 264.7 cell line. Br J Nutr. 2013;110:509–514. doi: 10.1017/S0007114512005351. [DOI] [PubMed] [Google Scholar]
- 6.Johnson S. The possible role of gradual accumulation of copper, cadmium, lead and iron and gradual depletion of zinc, magnesium, selenium, vitamins B2, B6, D, and E and essential fatty acids in multiple sclerosis. Med Hypotheses. 2000;55:239–241. doi: 10.1054/mehy.2000.1051. [DOI] [PubMed] [Google Scholar]
- 7.Powers HJ. Riboflavin (vitamin B-2) and health. Am J Clin Nutr. 2003;77:1352–1360. doi: 10.1093/ajcn/77.6.1352. [DOI] [PubMed] [Google Scholar]
- 8.LeBlanc JG, Rutten G, Bruinenberg P, Sesma F, de Giori GS, Smid EJ. A novel dairy product fermented with Propionibacterium freudenreichii improves the riboflavin status of deficient rats. Nutrition. 2006;22:645–651. doi: 10.1016/j.nut.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Naghashpour M, Nematpour S, Haghighizadeh MH. Dietary, anthropometric, biochemical and psychiatric indices in shift work nurses. Food Nutr Sci J. 2013;4:1239–1246. [Google Scholar]
- 10.Naghashpour M, Amani R, Nutr R, Nematpour S, Haghighizadeh MH. Riboflavin status and its association with serum hs-CRP levels among clinical nurses with depression. J Am Coll Nutr. 2011;30:340–347. doi: 10.1080/07315724.2011.10719977. [DOI] [PubMed] [Google Scholar]
- 11.Vafa MR, Karandish M, Mosavi SM, Alizadeh M, Salehi MH, Maddah M. Evaluation of urinary riboflavin levels of primary School Children in Rafsanjan, Iran. J Biol Sci. 2009;9:389–391. [Google Scholar]
- 12.Naghashpour M, Majdinasab N, Shakerinejad G, Kouchak M, Haghighizadeh MH, Jarvandi F, et al. Riboflavin supplementation to patients with multiple sclerosis does not improve disability status nor is riboflavin supplementation correlated to homocysteine. Int J Vitam Nutr Res. 2013;83:281–290. doi: 10.1024/0300-9831/a000170. [DOI] [PubMed] [Google Scholar]
- 13.Ogunleye AJ, Odutuga AA. The effect of riboflavin deficiency on cerebrum and cerebellum of developing rat brain. J Nutr Sci Vitaminol (Tokyo) 1989;35:193–197. doi: 10.3177/jnsv.35.193. [DOI] [PubMed] [Google Scholar]
- 14.Cai Z, Blumbergs PC, Finnie JW, Manavis J, Thompson PD. Selective vulnerability of peripheral nerves in avian riboflavin deficiency demyelinating polyneuropathy. Vet Pathol. 2009;46:88–96. doi: 10.1354/vp.46-1-88. [DOI] [PubMed] [Google Scholar]
- 15.Johnson WD, Storts RW. Peripheral neuropathy associated with dietary riboflavin deficiency in the chicken. I. Light microscopic study. Vet Pathol. 1988;25:9–16. doi: 10.1177/030098588802500102. [DOI] [PubMed] [Google Scholar]
- 16.Cai Z, Finnie JW, Blumbergs PC. Avian riboflavin deficiency:an acquired tomaculous neuropathy. Vet Pathol. 2006;43:780–781. doi: 10.1354/vp.43-5-780. [DOI] [PubMed] [Google Scholar]
- 17.Wada Y, Kondo H, Itakura C. Peripheral neuropathy of dietary riboflavin deficiency in racing pigeons. J Vet Med Sci. 1996;58:161–163. doi: 10.1292/jvms.58.161. [DOI] [PubMed] [Google Scholar]
- 18.Jortner BS, Cherry J, Lidsky TI, Manetto C, Shell L. Peripheral neuropathy of dietary riboflavin deficiency in chickens. J Neuropathol Exp Neurol. 1987;46:544–555. doi: 10.1097/00005072-198709000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Cai Z, Blumbergs PC, Finnie JW, Manavis J, Thompson PD. Novel fibroblastic onion bulbs in a demyelinating avian peripheral neuropathy produced by riboflavin deficiency. Acta Neuropathol. 2007;114:187–194. doi: 10.1007/s00401-007-0215-8. [DOI] [PubMed] [Google Scholar]
- 20.Allison T, Roncero I, Forsyth R, Coffman K, Le Pichon JB. Brown-vialetto-van laere syndrome as a mimic of neuroimmune disorders. J Child Neurol. 2017 doi: 10.1177/0883073816689517. 883073816689517. [DOI] [PubMed] [Google Scholar]
- 21.Kurtzke JF. Rating neurologic impairment in multiple sclerosis:an expanded disability status scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 22.Kurtzke JF. A new scale for evaluating disability in multiple sclerosis. Neurology. 1955;5:580–583. doi: 10.1212/wnl.5.8.580. [DOI] [PubMed] [Google Scholar]
- 23.Bisaga GN, Odinak MM, Boĭko AN, Mel’nik IuB, Popova NF. Possibilities of treatment of multiple sclerosis exacerbations without corticosteroids:a role of metabolic and antioxidant therapy. Zh Nevrol Psikhiatr Im S S Korsakova. 2011;111:44–48. [PubMed] [Google Scholar]
- 24.Ghadirian P, Jain M, Ducic S, Shatenstein B, Morisset R. Nutritional factors in the aetiology of multiple sclerosis:a case-control study in Montreal, Canada. Int J Epidemiol. 1998;27:845–852. doi: 10.1093/ije/27.5.845. [DOI] [PubMed] [Google Scholar]
- 25.Cai Z, Finnie JW, Blumbergs PC, Manavis J, Ghabriel MN, Thompson PD. Early paranodal myelin swellings (tomacula) in an avian riboflavin deficiency model of demyelinating neuropathy. Exp Neurol. 2006;198:65–71. doi: 10.1016/j.expneurol.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 26.Norton WN, Daskal I, Savage HE, Seibert RA, Lane M. Effects of riboflavin deficiency on the ultrastructure of rat sciatic nerve fibers. Am J Pathol. 1976;85:651–660. [PMC free article] [PubMed] [Google Scholar]
- 27.Toyosawa T, Suzuki M, Kodama K, Araki S. Effects of intravenous infusion of highly purified vitamin B2 on lipopolysaccharide-induced shock and bacterial infection in mice. Eur J Pharmacol. 2004;492:273–280. doi: 10.1016/j.ejphar.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Araki S, Suzuki M, Fujimoto M, Kimura M. Enhancement of resistance to bacterial infection in mice by vitamin B2. J Vet Med Sci. 1995;57:599–602. doi: 10.1292/jvms.57.599. [DOI] [PubMed] [Google Scholar]
- 29.Verdrengh M, Tarkowski A. Riboflavin in innate and acquired immune responses. Inflamm Res. 2005;54:390–393. doi: 10.1007/s00011-005-1372-7. [DOI] [PubMed] [Google Scholar]
- 30.Wertman KF, Sypherd PS. The effects of riboflavin deficiency on phagocytosis and susceptibility to infection. J Immunol. 1960;85:511–515. [PubMed] [Google Scholar]
- 31.Pelliccione NJ, Karmali R, Rivlin RS, Pinto J. Effects of riboflavin deficiency upon prostaglandin biosynthesis in rat kidney. Prostaglandins Leukot Med. 1985;17:349–358. doi: 10.1016/0262-1746(85)90126-x. [DOI] [PubMed] [Google Scholar]
- 32.Ashoori M, Saedisomeolia A. Riboflavin (vitamin B2) and oxidative stress:a review. Br J Nutr. 2014;111:1985–1991. doi: 10.1017/S0007114514000178. [DOI] [PubMed] [Google Scholar]
- 33.Vogel DY, Vereyken EJ, Glim JE, Heijnen PD, Moeton M, van der Valk P, et al. Macrophages in inflammatory multiple sclerosis lesions have an intermediate activation status. J Neuroinflamm. 2013;10:1–12. doi: 10.1186/1742-2094-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Christophi GP, Panos M, Hudson CA, Christophi RL, Gruber RC, Mersich AT, et al. Macrophages of multiple sclerosis patients display deficient SHP-1 expression and enhanced inflammatory phenotype. Lab Invest. 2009;89:742–759. doi: 10.1038/labinvest.2009.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naghashpour M, Amani R, Sarkaki A, Ghadiri A, Samarbafzadeh A, Jafarirad S, et al. Brain-derived neurotrophic and immunologic factors:beneficial effects of riboflavin on motor disability in murine model of multiple sclerosis. Iran J Basic Med Sci. 2016;19:439–448. [PMC free article] [PubMed] [Google Scholar]
- 36.Murphy PG, Borthwick LA, Altares M, Gauldie J, Kaplan D, Richardson PM. Reciprocal actions of interleukin-6 and brain-derived neurotrophic factor on rat and mouse primary sensory neurons. Eur J Neurosci. 2000;12:1891–1899. doi: 10.1046/j.1460-9568.2000.00074.x. [DOI] [PubMed] [Google Scholar]
- 37.Naghashpour M, Amani R, Sarkaki A, Ghadiri A, Samarbaf-Zadeh A, Jafarirad S, et al. Brain-derived neurotrophic factor in murine model of multiple sclerosis:beneficial effects of riboflavin on motor disability. Int J Vitam Nutr Res. 2016;19:439–448. [PMC free article] [PubMed] [Google Scholar]
- 38.Manole A, Houlden H. Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, et al., editors. Riboflavin transporter deficiency neuronopathy. Gene Rev Seattle (WA) 1993 [Google Scholar]
- 39.Chen L, Feng L, Jiang WD, Jiang J, Wu P, Zhao J, et al. Dietary riboflavin deficiency decreases immunity and antioxidant capacity, and changes tight junction proteins and related signaling molecules mRNA expression in the gills of young grass carp (Ctenopharyngodon idella) Fish Shellfish Immunol. 2015;45:307–320. doi: 10.1016/j.fsi.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 40.Sakurai T, Miyazawa S, Furuta S, Hashimoto T. Riboflavin deficiency and beta-oxidation systems in rat liver. Lipids. 1982;17:598–604. doi: 10.1007/BF02535365. [DOI] [PubMed] [Google Scholar]
- 41.Hayes JD, McLellan LI. Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defence against oxidative stress. Free Radic Res. 1999;31:273–300. doi: 10.1080/10715769900300851. [DOI] [PubMed] [Google Scholar]
- 42.Mulherin DM, Thurnham DI, Situnayake RD. Glutathione reductase activity, riboflavin status, and disease activity in rheumatoid arthritis. Ann Rheum Dis. 1996;55:837–840. doi: 10.1136/ard.55.11.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hejazi E, Amani R, SharafodinZadeh N, Cheraghian B. Comparison of antioxidant status and vitamin D levels between multiple sclerosis patients and healthy matched subjects. Mult Scler Int. 2014;2014:539854. doi: 10.1155/2014/539854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Depeint F, Bruce WR, Shangari N, Mehta R, O’Brien PJ. Mitochondrial function and toxicity:role of the B vitamin family on mitochondrial energy metabolism. Chem Biol Interact. 2006;163:94–112. doi: 10.1016/j.cbi.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 45.Azoulay D, Urshansky N, Karni A. Low and dysregulated BDNF secretion from immune cells of MS patients is related to reduced neuroprotection. J Neuroimmunol. 2008;195 doi: 10.1016/j.jneuroim.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 46.Benveniste EN, Whitaker JN, Gibbs DA, Sparacio SM, Butler JL. Human B cell growth factor enhances proliferation and glial fibrillary acidic protein gene expression in rat astrocytes. Int Immunol. 1989;1:219–228. doi: 10.1093/intimm/1.3.219. [DOI] [PubMed] [Google Scholar]
- 47.Selmaj KW, Farooq M, Norton WT, Raine CS, Brosnan CF. Proliferation of astrocytes in vitro in response to cytokines. A primary role for tumor necrosis factor. J Immunol. 1990;144:129–135. [PubMed] [Google Scholar]
- 48.Levison SW, Jiang FJ, Stoltzfus OK, Ducceschi MH. IL-6-type cytokines enhance epidermal growth factor-stimulated astrocyte proliferation. Glia. 2000;32:328–337. doi: 10.1002/1098-1136(200012)32:3<328::aid-glia110>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 49.Frei K, Malipiero UV, Leist TP, Zinkernagel RM, Schwab ME, Fontana A. On the cellular source and function of interleukin 6 produced in the central nervous system in viral diseases. Eur J Immunol. 1989;19:689–694. doi: 10.1002/eji.1830190418. [DOI] [PubMed] [Google Scholar]
- 50.Erta M, Quintana A, Hidalgo J. Interleukin-6, a major cytokine in the central nervous system. Int J Biol Sci. 2012;8:1254–1266. doi: 10.7150/ijbs.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cicek G, Schiltz E, Hess D, Staiger J, Brandsch R. Analysis of mitochondrial antigens reveals inner membrane succinate dehydrogenase flavoprotein subunit as autoantigen to antibodies in anti-M7 sera. Clin Exp Immunol. 2002;128:83–87. doi: 10.1046/j.1365-2249.2002.01816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reese D, Shivapour ET, Wahls TL, Dudley-Javoroski SD, Shields R. Neuromuscular electrical stimulation and dietary interventions to reduce oxidative stress in a secondary progressive multiple sclerosis patient leads to marked gains in function:a case report. Cases J. 2009;2:7601. doi: 10.4076/1757-1626-2-7601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Udhayabanu T, Manole A, Rajeshwari M, Varalakshmi P, Houlden H, Ashokkumar B. Riboflavin responsive mitochondrial dysfunction in neurodegenerative diseases. J Clin Med. 2017;6 doi: 10.3390/jcm6050052. [DOI] [PMC free article] [PubMed] [Google Scholar]


