Abstract
Objective(s):
Hybridization of bioactive natural and synthetic compounds is one of the most promising novel approaches for the design of hit and lead compounds with new molecular structures. In this investigation, a series of novel hybrid structures bearing quinazolinone, benzofuran and imidazolium moieties were designed and synthesized.
Materials and Methods:
Novel hybrid compounds were prepared and their structures were characterized by spectral and analytical data. In order to evaluate the biological activities, the synthesized hybrid compounds were studied for in vitro antibacterial activity against three Gram positive bacteria (Staphylococcus aureu, Bacillus subtilis, Listeria monocitogenes) and three Gram-negative bacteria (Escherichia coli, Pseudomonas aeruginosa, Salmonella entritidis) and also, Candida albicans as one yeast-like fungi strain. Cytotoxic activities of the synthesized compounds were also evaluated by the MTT assay in the human breast cancer cell line (MCF-7) and finally docking studies of cytotoxic derivatives were performed on aromatase enzyme.
Results:
The results of antimicrobial activity showed that compound 14e, with two halogen atoms on quinazolinone and benzofuran was the most active against all the tested strains of microorganisms with the MIC value 16-128 µg/ml. Some of the tested compounds showed good cytotoxicity on MCF-7, and compound 14c with IC50=0.59 micromolar (μM) was found to be the most cytotoxic compound among the studied hybrid derivatives. The docking analysis showed acceptable binding interactions for these compounds.
Conclusion:
Based on the obtained results, the hybrid derivatives of quinazolinone, benzofuran and imidazolium could be regarded as efficient candidates for further molecular developments of anticancer and antimicrobial agents.
Keywords: Antibacterial, Benzofuran, Cytotoxic, Imidazolium salt, QM/MM Docking, Quinazolinone
Introduction
Design of new drug-like small molecules based on the pharmacologically active scaffolds is a rational and a promising direction in modern medicinal chemistry. A number of compounds have been synthesized by the combination of biologically active pharmacophores and utilized by medicinal chemists to develop novel therapeutics agents with a broad range of pharmacological activities (1-3). For example, a series of 1,2,3-triazole bearing chalcones showed notable antimalarial activity against the D10, Dd2 and W2 strains of Plasmodium falciparum (4). 1,2,3-Triazole analogues of flavone displayed antimicrobial activity (5). Some naphthalimide-benzimidazole conjugates showed remarkable anticancer activity with 50% growth inhibition (GI50) values of 0.02 μM and 0.49 μM against central nervous system (SNB-75) and leukemia (K-562) cell lines, respectively (6). Sashidhara et al. (7) synthesized a series of hybrids possessing chalcone and thiazole moieties and evaluated their antibacterial activities. In general, this class of hybrids, exhibit potency against Staphylococcus aureus (7). Additionally, a small library of directly-linked hybrids that contain 2-phenylbenzofuran and imidazole moieties were synthesized by Yang et al. (8) and studied against a panel of human tumor cell lines. The hybrids exhibited outstanding cytotoxic activities, especially against human breast cancer cell line (MCF-7) and myeloid liver carcinoma (SMMC-7721).
The organic compounds containing the quinazoli-none scaffold as pharmacophore exhibit diverse biological and pharmaceutical activities (9). Quinazoli-none, as a heterocyclic aromatic molecule, with electron rich oxygen and nitrogen atoms, is important as a structural motif among biologically active molecules and also, for the combinatorial assembly of heterocyclic scaffolds (10). Compounds bearing a quinazolinone ring residue are reported to show anticancer, anti-inflammatory, analgesic, muscle relaxant, sedative, anti-tubercular, diuretic, antimicrobial, anticonvulsant, anti-allergic, anti-malarial, antiviral, antioxidant and CNS depressant activities (11, 12). On the other hand, benzofurans have received special attention not only in organic chemistry, but also in medicinal chemistry due to their easy synthesis as well as numerous biological activities. Numerous derivatives of benzofuran show good antimicrobial as well as anticancer activities (13, 14).
Imidazole, as an important constituent of natural products including purine, histamine, histidine and nucleic acids, has been accredited with a great amount of attention over the years by medicinal chemists (15). Improving pharmacokinetic characteristics of lead molecules and also, optimizing the solubility and bioavailability of the proposed poorly soluble lead molecules have further attracted the researchers; therefore, new imidazole-based compounds have been envisaged to discover new chemical entities with a potential to afford some promising drugs of the future. On the basis of various literature surveys, imidazole derivatives show various pharmacological activities such as anti-fungal and antibacterial activity, as well as anti-inflammatory, analgesic, anti-tubercular, antiparasitic, antihistaminic, anti-neuropathic, antihypertensive and anti-cancer activities (16). Transformation of imidazole ring into imidazolium would result in more potent antibacte-rial compounds with broadened spectrum, making the study of imidazolium-based antibacterial agents interesting. Positive imidazolium salts are not only helpful in reinforcing affinity, water solubility and membrane permeability, but also they have the ability to prevent migration, leading to improving the antimicrobial efficacy (16).
According to the previous studies, benzofuran-imidazole analog derivatives show good cytotoxic effect on the MCF-7 cell line (8). On the other hand, all three heterocyclic (quinazolinone, benzofuran and imidazole) derivatives show different biological activities, especially cytotoxic and antibacterial activity (11, 16-19). In the continuance of our ongoing research on these hybrid systems, the aim of the present work was the synthesis and evaluation of antibacterial and cytotoxic activities of a series of novel quinazolinone, benzofuran and imidazolium hybrid derivatives (14a-i) (Scheme 1). Our strategy hypothesized that the potency of each of these pharmacophores might be enhanced by putting all three together. For this purpose, it was tried to put these pharmacophores together in such a way to arrange them in a symmetrical shape and subsequently, a routine synthetic procedure was used for the preparation of these novel hybrid compounds. All newly synthesized hybrids derivatives (14a-i) were evaluated for their in vitro antibacterial and antifungal activity, using the microplate Alamar blue assay (MABA) method. Cytotoxic activities of the synthesized compounds were also evaluated by the MTT assay on MCF-7 cell line which is the most studied human breast cancer cell line. Since estrogen plays a major role in breast cancer development and progression, aromatse enzyme could be as a targets for breast cancer treatment (20, 21). So in this study the binding of these novel hybrid compounds to aromatase was theoretically investigated in a docking procedure by applying a combined quantum mechanical/molecular mechanical (QM/MM) method.
Scheme 1.
The general procedure for the synthesis of quinazolinone, benzofurane and imdazoliuom hybrids
Materials and Methods
Chemicals were purchased from Merck and Sigma–Aldrich as ‘synthesis grade’ and used without further purification. Tetrahydrofuran (THF) was distilled from sodium/benzophenoneketyl prior to use. Melting points were recorded in an open capillary using the electrothermal 9200 melting point apparatus. NMR experiments were performed on a Bruker (Germany) Avance 400 instrument. Elemental analysis (CHNS) was performed on a Leco, CHNS-932. Infrared (IR) spectra were recorded on a Jasco-680 (Japan) spectrophotometer as KBr (disc). GC-Mass analysis was carried out using a Fisons Instrument (Rodano, Italy) model 8060. Thin layer chromatography (TLC) was performed on pre-coated silica gel aluminum plates (Kieselgel 60, 254, E. Merck, Germany). Chromatograms were visualized by UV at 254 and 365 nm.
General procedure for the synthesis of derivatives General procedure for the synthesis of 2-methyl-4H-benzo[1,3]oxazin-4-one derivatives (3a-c)
A mixture of anthranilic acid derivatives (1a-c, 20 mmol) and acetic anhydride (2, 40 mmol) was refluxed for 3-8 hours. After confirmation of completion of reaction by TLC, excess acetic anhydride was evaporated under vacuum. The collected solid was washed with petroleum ether. The solid product was kept for next step.
General procedure for the synthesis of quinazolin-4(3H)-one derivatives (5a-c)
The synthesized 2-methyl-4H-benzo[1,3]oxazin-4-one derivatives (3a-c, 8 mmol) was refluxed with hydrazine hydrate (4, 16 mmol) for 3-6 hours. After completion of reactions, excess hydrazine hydrate was evaporated under vacuum and then the collected solid was washed with dilute HCl. The collected solid was filtered and recrystallized from ethanol.
General procedure for the synthesis of (benzofuran-2-yl)ethanone derivatives (8a-c)
A mixture of corresponding salicylaldehyde (6a-c, 3.0 mmol), chloroacetone (7, 3.5 mmol), and anhydrous potassium carbonate (7.0 mmol) in acetone (12 ml) was stirred and heated under reflux condition for an appropriate time. The progress of reaction was monitored by TLC. After completion of the reaction, the mixture was cooled to room temperature and filtered by funnel to get clear solution. Then 10-15 ml acetone was used to wash potassium carbonate on filtrate and then the acetone was removed by vacuum pump to afford the product. Crystallization was achieved by chloroform.
General procedure for the synthesis of (1-Benzofuran-2-yl)-2-bromoethanone derivatives (9a-c)
Substituted 2-acetyl benzofurans (8a-c, 2.0 mmol) was dissolved in acetic acid (100 ml) and then a solution of bromine (2.1 mmole) in acetic acid (10 ml) was added dropwise for about 15-20 min with stirring. After addition of bromine, the mixture was stirred for 45-75 min. Completion of reaction was monitored by TLC. Then, the mixture was decanted in crushed ice, the solid separated was collected and crystallized from ethanol.
General procedure for the synthesis of 3-(2-(benzofuran-2-yl)-2-oxoethylamino)-2-methylquinazolin-4(3H)-one derivatives (10a-i)
A solution of bromoacetylbenzofurans (9a-c, 5mmol) in THF (5 ml) was added dropwise to a mixture of quinazolinone (5a-c, 5 mmol), TEA (5 mmol) and THF (5 ml) under stirring. After dripping, the reaction is lasted for 6-10 hr at reflux temperature. Over time, product was formed as an insoluble substance which had distinctly different Rf on the TLC plate compare with initial compounds. The resulting precipitate was filtered and recrystallized with chloroform and n-heptane to give desired compound 10a-i.
General procedure for the synthesis of 3-(2-(benzofuran-2-yl)-2-hydroxyethylamino)-2-methylquinazolin-4(3H)-one derivatives (11a-i)
To a stirred solution of 3-(2-(benzofuran-2-yl)-2-oxoethylamino)-2-methylquinazolin-4(3H)-one (10a-i, 5 mmol) in MeOH (25 ml) at 0 °C, was added NaBH4 (5 mmol) in small portions and the resulting solution was stirred at ambient temperature for 12 hr. After loss of staring compound spot on TLC plate, a small amount of water was added to neutralize the excess of sodium borohydride and the mixture was stirred for 15 min before rotary evaporation. Then 20 ml of water was added and the product was extracted with chloroform (3×20 ml). The solvent was evaporated under reduced pressure and the residue was recrystallized in ethanol to afford the 3-(2-(benzofuran-2-yl)-2-hydroxyethylamino)-2-methylquinazolin-4(3H)-one derivatives (11a-i).
General procedure for the synthesis of 1-[[1-(benzofuran-2-yl)-2-(2-methylquinazolin-4(3H)-one-3-yl)] ethylamino -1-yl]-3- methyl imidazol-1-ium chloride derivatives (14a-i)
Thionyl chloride (5.1 mmol) was added in a dropwise manner to a solution of 3-(2-(benzofuran- 2-yl)-2-hydroxyethylamino)-2-methylquinazolin 4(3H)-one derivatives (11a-i, 5 mmol) in chloroform (10 ml) at 0 °C. The resulting mixture was stirred at room temperature for 30 min and then at reflux for 4-8 hr. After completion of the reaction as indicated by TLC, the solvent was evaporated under reduced pressure and the residue was washed twice with n-hexane. The resulting residue was dissolved in acetonitrile and then a solution of methyl imidazole (13, 5.2 mmol) was added. The mixture was stirred at reflux temperature. After 24-48 hr an insoluble substance was formed, which was filtered and washed with cold acetonitrile and then dried to afford imidazolium salts 14a-i.
Biology Antimicrobial activity
The antimicrobial activity of the synthesized compounds (14a-i) was determined against three Gram positive bacteria, three Gram-negative bacteria and also, Candida albicans as yeast-like fungi strains by using MABA test method (22, 23). Serial solutions of the compounds (14a-i) were prepared in H2O and dimethyl sulfoxide (below 1% DMSO) to give a final concentration ranging from 16 to 512 μM for determining the MIC value.
The antibiotic ciprofloxacin and ketoconazole were used as reference antibacterial and antifungal agents, respectively, for comparison. Inoculated plates in triplicate were then incubated at 37 °C to check antibacterial activity for 24 hr; the same was done for 48 hr at 25 °C to probe antifungal activity. After incubation, the minimum inhibitory concentra-tions (MICs) were noted. The MIC was the lowest concentration of the tested compound which prevented a color change from blue to pink. Following a broth microdilution MIC test, from each well that showed no growth, contents were removed and spread onto Muller Hinton agar plates for bacteria and sabouraud dextrose agar for fungi to determine MBC and MFC results. The plates were incubated for 24 hr at 37 °C for bacteria, and 25 °C for fungi (23).
Cytotoxic assay (MTT)
The in vitro cytotoxicity of the synthesized hybrid compounds was measured using the MTT assay. The assay was carried out according to the known protocol (24, 25). Exponentially growing cells were harvested and plated in 96-well plates at a concentration of 1×104 cells/well. After 24 hr incubation at 37 °C under a humidified 5% CO2 to allow cell attachment, the cells in the wells were treated with target compounds at various concentrations for 48 hr, respectively. The concentration of DMSO was always kept below 1.00%, which was found to be non-toxic to the cells. A solution of 3-(4,5-dimethylthiazo1-2-y1)-2,5-diphenyltetrazolium bromide (MTT) was pre-pared at 5 mg/ml in the phosphate buffered saline (PBS) and 20 μl of this solution was added to each well. After incubation for 4 hr at 37 °C in a humidified incubator with 5% CO2, the medium/MTT mixtures were removed, and the formazan crystals formed by the mitochondrial dehydrogenase activity of vital cells were dissolved in 150 μl of DMSO per well. The absorbance of the wells was read with a microplate reader (Elisa-reader) at 570 nm. Cell survival was calculated using the formula (25).
Survival (%)= [(absorbance of treated cells − absorbance of culture medium)/(absorbance of untreated cells − absorbance of culture medium)] × 100.
The experiments were done in triplicate and the inhibitory concentration (IC50), which was the concentration required for 50% inhibition of cell growth, as compared to that of control values, was calculated from a dose response curve. Evaluation was based on mean values from three independent experiments, each comprising at least four micro-cultures per concentration level.
Molecular docking and QM/MM methodology
The binding of novel synthesized hybrid com-pounds to aromatase was investigated in a docking procedure by applying a combined quantum mechanical/molecular mechanical (QM/MM) method. Due to the positive charge on the imidazole ring of the designed ligands and also, the presence of Heme iron in the active site of the enzyme, QM/MM calculations in which partial charges of the ligand were re-fitted according to the polarized active site environment of the enzyme were performed to increase the accuracy of the docking results (26). The X-ray crystal structure of aromatase (PDB code 3EQM) was retrieved for docking studies. The structure was checked for missing atoms and bonds. The structures of ligands were optimized using the PM6 semi-empirical method in Gaussian 09 quantum chemistry package. AutoDock Tools version 1.5.1 was used to generate both grid and docking parameter files. A grid box size of 60× 60 × 60 Å points with a grid spacing of 0.375 Å was considered and its center was defined as the center of the co-crystallized inhibitor. At the end of docking the synthesized ligand with AutoDock with 100 runs, a cluster analysis was performed. Several clusters and binding energies were obtained for docked hybrid compounds in which the best conformers were selected according to the lower docked free energy and the top-ranked cluster to perform docking analysis with AutoDock Tools and PyMOL.
“Our own N-layered integrated molecular orbital and molecular mechanic”, abbreviated as ONIOM method, was implemented in Gaussian 09 and used for QM/MM calculations (27). This method enables different ab initio or semi-empirical methods to be applied to different parts of a system. QM/MM methods can help to offer a superior estimation of the electronic interactions. Previous studies have shown that docking program gives better results if the ligand partial charges are refitted with QM/MM (26). To obtain the partial atomic charge of ligand atoms in the active site of aromatase, PM6 semi-empirical method, one of the best semi-empirical methods in quantum mechanics, was used to represent the QM region (ligand) and the universal force field (UFF) was used for the MM region (aromatase). Therefore, two layer ONIOM calculation (PM6:UFF) was used. The partial atomic charges of the MM region were assigned using the QEq formalism (28). Then, the ligands with improved charges were employed for re-docking into the aromatase enzyme.
Results
Characterization of synthesized compounds
2-Methyl-4H-benzo [1,3]oxazin-4-one (3a)
Yellow powder, Yield: 80%, m.p.: 80-81°C (lit. (29), mp 78-80 °C), FT-IR (KBr) ν (cm-1): 1665.64 (C=O), 1605.91 (C=N), 1169.84 (C-O), 1H NMR (DMSO-d6) δ (ppm): 2.39 (3H, s, CH3), 6.91-8.11 (4H aromatic).
6-Chloro-2-methyl-4H-benzo [1,3]oxazin-4-one (3b)
Pale yellow solid, Yield: 85%, m.p.: 144-145°C (lit. (30), mp 145-147°C), FT-IR (KBr) ν (cm-1): 1695.33 (C=O), 1625.14 (C=N), 1209.28 (C-O), 1H NMR (DMSO-d6) δ (ppm): 2.43 (3H, s, CH3), 7.49 (1H, d, aromatic, J=5.60 Hz), 7.92 (1H, s, aromatic), 8.23 (1H, d, aromatic, J=6.70 Hz).
6-Methoxy-2-methyl-4H-benzo[d][1,3]oxazin-4-one (3c)
Pale yellow solid, Yield: 75%, m.p.: 154–155°C (lit. (31), m.p.: 157–158 °C), FT-IR (KBr) ν (cm-1): 1667.23 (C=O), 1615.79 (C=N), 1183.24 (C-O), 1H NMR (DMSO-d6) δ (ppm): 2.35 (3H, s, CH3), 3.72 (3H, s, OCH3), 7.08 (1H, d, aromatic, J=6.40 Hz), 7.35 (1H, s, aromatic), 7.53 (1H, d, aromatic, J=6.80 Hz).
3-Amino-2-methyl-3H-quinazolin-4-one (5a)
White powder, Yield: 93%, m.p.: 139-141 °C (lit. (29), mp 140-142 °C), FT-IR (KBr) ν (cm-1): 3310.14-3262.25 (NH2), 1677.18 (C=O), 1617.80 (C=N), 1595.74 (C=C), 1H NMR (DMSO-d6) δ (ppm): 2.38 (3H, s, CH3), 4.63 (2H, broad, NH2), 6.71-8.18 (4H, m, aromatic).
6-Chloro-3-amino-2-methyl-3H-quinazolin-4-one (5b)
White powder, Yield: 89%, m.p.:157-158°C, FT-IR (KBr) ν (cm-1): 3360.54-3382.11 (NH2), 1681.28 (C=O), 1630.37 (C=N), 1605.51 (C=C), 1H NMR (DMSO-d6) δ (ppm): 2.46 (3H, s, CH3), 4.92 (2H, broad, NH2), 7.49 (1H, d, aromatic, J=6.90 Hz), 7.96 (1H, s, aromatic), 8.40 (1H, d, aromatic, J=6.90 Hz)
6-Methoxy-3-amino-2-methyl-3H-quinazolin-4-one (5c)
White powder, Yield: 86%, m.p.:148-149 °C, FT-IR (KBr) ν (cm-1): 3281.09-3386.71 (NH2), 1675.34 (C=O), 1619.92 (C=N), 1592.58 (C=C), 1H NMR (DMSO-d6) δ (ppm): 2.39 (3H, s, CH3), 3.70 (3H, s, OCH3), 4.86 (2H, broad, NH2), 7.11 (1H, d, aromatic, J=7.00 Hz), 7.31 (1H, s, aromatic), 7.55 (1H, d, aromatic, J=6.80 Hz).
1-(Benzofuran-2-yl)ethanone (8a)
Transparent crystals, Yield: 75%, m.p.: 79.0-79.5 °C (lit. (32), 78°C), FT-IR (KBr) ν (cm-1): 3021.34 (C-H, aromatic), 2812.92 (C-H, aliphatic), 1698.44 (C=O, ketone), 1544.77, 1501.28 (C=C, aromatic), 1324.75 (C-O), 1H NMR (DMSO-d6) δ (ppm): 2.58 (3H, s, CH3), 6.68 (1H, s, aromatic), 7.28–7.47 (2H, m, aromatic), 7.54 (1H, d, aromatic, J=7.20 Hz), 7.68 (1H, d, aromatic, J=7.10 Hz).
1-(5-bromobenzofuran-2-yl)ethanone (8b)
White solid, Yield: 85%, m.p.: 110°C, FT-IR (KBr) ν (cm-1): 3106.34 (C-H, aromatic), 2932.92 (C-H, aliphatic), 1710.21 (C=O, ketone), 1590.63, 1571.70 (C=C, aromatic), 1368.55(C-O), 864.25 (C-Br), 1H NMR (DMSO-d6) δ (ppm): 2.65(3H, s, CH3), 7.41 (1H, s, aromatic), 7.47 (1H, d, aromatic, J=6.90 Hz), 7.54 (1H, d, aromatic, J=6.90 Hz), 7.77 (1H, s, aromatic).
1-(5-Methoxybenzofuran-2-yl)ethanone (8c)
Light yellow crystal, Yield: 80%, m.p.: 91-92 °C, FT-IR (KBr) ν (cm-1): 3091.34 (C-H, aromatic), 2928.92 (C-H, aliphatic), 1705.54 (C=O, ketone), 1568.13, 1521.36 (C=C, aromatic), 1335.74 (C-O), 1H NMR (DMSO-d6) δ (ppm): 2.64 (3H, s, CH3), 3.75 (3H, s, OCH3), 6.94 (1H, s, aromatic), 7.18 (1H, d, aromatic, J=7.10 Hz), 7.28 (1H, d, aromatic, J=7.10 Hz), 7.47 (1H, s, aromatic).
1-(1-Benzofuran-2-yl)-2-bromoethanone (9a)
Light green crystals, Yield: 80%, m.p.: 93-94°C (lit (32). mp 94°C), FT-IR (KBr) ν (cm-1): 3101.04 (C-H, aromatic), 2935.12 (C-H, aliphatic), 1708.31 (C=O, ketone), 1584.21, 1573.85 (C=C, aromatic), 1320.75 (C-O), 831.13 (C-Br), 1H NMR (DMSO-d6) δ (ppm): 4.37 (2H, s, CH2), 7.21 (1H, s, aromatic), 7.575-7.617 (2H, m, aromatic), 7.78 (1H, d, aromatic, J=7.10 Hz), 7.83 (1H, d, aromatic, J=7.30 Hz).
Bromo-1-(5-bromo-1-benzofuran-2-yl) ethanone (9b)
White solid, Yield: 76%, m.p.:140–142°C, FT-IR (KBr) ν (cm-1): 3111.04 (C-H, aromatic), 2942.93 (C-H, aliphatic), 1720.65 (C=O, ketone), 1612.14, 1575.24 (C=C, aromatic), 1371.11 (C-O), 857.41 (C-Br), 1H NMR (DMSO-d6) δ (ppm): 4.55 (2H, s, CH2), 7.48 (1H, s, aromatic), 7.58 (1H, d, aromatic, J=7.10), 7.63 (1H, d, aromatic, J=7.10 Hz), 8.13 (1H, s, aromatic).
2-Bromo-1-(5-methoxybenzofuran-2-yl)ethanone (9c)
Yellow crystal, Yield: 70%, m.p.: 115-116°C, FT-IR (KBr) ν (cm-1): 3095.63 (C-H, aromatic), 2938.25 (C-H, aliphatic), 1711.05 (C=O, ketone), 1612.36, 1573.72 (C=C, aromatic), 1357.88 (C-O), 844.21 (C-Br), 1H NMR (DMSO-d6) δ (ppm): 3.83 (3H, s, OCH3), 4.44 (2H, s, CH2), 7.04 (1H, s, aromatic), 7.34 (1H, d, aromatic, J=7.00 Hz), 7.49 (1H, d, aromatic, J=6.90 Hz), 7.63 (1H, s, aromatic).
3-(2-(Benzofuran-2-yl)-2-oxoethylamino)-2- methylquinazolin -4(3H)-one (10a)
White solid, Yield: 65%, m.p.: 102-103.5 °C, FT-IR (KBr) ν (cm-1): 3290 (NH), 3103.83, 3076.61 and 2917.72 (C-H, aromatic and aliphatic), 1709.93 (C=O), 1673.27 (C=O), 1619.25 (C=N), 1278.57 (C-O), 1H NMR (DMSO-d6) δ (ppm): 2.32 (3H, s, CH3), 4.19 (2H, s, CH2), 4.87 (1H, broad, NH), 6.73 (1H, s, aromatic), 7.25-7.31 (2H, m, aromatic), 7.40 (1H, d, aromatic, J=7.20 Hz), 7.51-7.77 (4H, m, aromatic), 8.18 (1H, d, aromatic, J=7.30 Hz).
3-(2-(Benzofuran-2-yl)-2-oxoethylamino)-6-chloro-2-methylquinazolin-4(3H)-one (10b)
Off-white solid, Yield: 67%, m.p.: 135-136°C, FT-IR (KBr) ν (cm-1): 3310 (NH), 3110.83, 3079.21 and 2941.18 (C-H, aromatic and aliphatic), 1711.48 (C=O), 1680.47 (C=O), 1632.33 (C=N), 1286.17 (C-O), 1H NMR (DMSO-d6) δ (ppm): 2.48 (3H, s, CH3), 4.40 (2H, s, CH2), 4.75 (1H, broad, NH),6.94 (1H, s, aromatic), 7.13-7.19 (2H, m, aromatic), 7.42 (1H, d, aromatic, J=7.10 Hz), 7.49 (1H, d, aromatic, J=7.30 Hz), 7.55(1H, d, aromatic, J=7.00 Hz), 7.96 (1H, s, aromatic), 8.4(1H, d, aromatic, J=7.00 Hz).
3-(2-(Benzofuran-2-yl)-2-oxoethylamino)-6-methoxy-2-methylquinazolin-4(3H)-one (10c)
White solid, Yield: 60%, m.p.:107-108°C, FT-IR (KBr) ν (cm-1): 3307 (NH), 3113.02, 3068.46 and 2907.24 (C-H, aromatic and aliphatic), 1709.06 (C=O), 1675.87 (C=O), 1620.24 (C=N), 1269.47 (C-O), 1H NMR (DMSO-d6) δ (ppm): 2.35 (3H, s, CH3), 3.70 (3H, s, OCH3), 4.32 (2H, s, CH2), 5.2 (1H, broad, NH), 6.89 (1H,s, aromatic), 7.11 (1H, d, aromatic, J=6.90 Hz), 7.25-7.31 (3H, m, aromatic), 7.42-7.51 (3H, m, aromatic).
3-(2-(5-Bromobenzofuran-2-yl)-2-oxoethylamino)-2-methylquinazolin-4(3H)-one (10d)
Pale green solid, Yield: 65%, m.p.:136-137°C, FT-IR (KBr) ν (cm-1):3320 (NH), 3109.45, 3089.16 and 2927.24 (C-H, aromatic and aliphatic), 1717.06 (C=O), 1673.28 (C=O), 1620.30 (C=N), 1289.41 (C-O), 1H NMR (DMSO-d6) δ (ppm):2.37 (3H, s, CH3), 4.50 (2H, s, CH2), 5.2 (1H, broad, NH), 7.48-7.8 (6H,m, aromatic), 8.14-8.21 (2H, m, aromatic).
3-(2-(5-Bromobenzofuran-2-yl)-2-oxoethylamino)-6-chloro-2-methylquinazolin-4(3H)-one (10e)
Pale green solid, Yield: 70%, m.p.: 140-141°C, FT-IR (KBr) ν (cm-1): 3322 (NH), 3117.21, 3097.41 and 2939.17 (C-H, aromatic and aliphatic), 1715.31 (C=O), 1682.51 (C=O), 1635.28 (C=N), 1295.47 (C-O), 1H NMR (DMSO-d6) δ (ppm): 2.51 (3H, s, CH3), 4.83 (2H, s, CH2), 5.25 (1H, broad, NH), 7.62 (1H,d, aromatic, J=6.70 Hz), 7.7-7.9 (3H, m, aromatic), 8.03 (1H, s, aromatic), 8.12 (1H, s, aromatic), 8.20 (1H, d, aromatic, J=6.70 Hz).
3-(2-(5-Bromo benzofuran-2-yl) -2-oxo ethyl amino)-6-methoxy-2- methyl quinazolin-4(3H)-one (10f)
Pale green crystals, Yield: 58%, m.p.: 110-111.5°C, FT-IR (KBr) ν (cm-1): 3287 (NH), 3104.74, 3079.49 and 2947.74 (C-H, aromatic and aliphatic), 1712.49 (C=O), 1672.39 (C=O), 1621.99 (C=N), 1292.88 (C-O), 1H NMR (DMSO-d6) δ (ppm): 2.39 (3H, s, CH3), 3.81 (3H, s, OCH3), 4.65 (2H, s, CH2), 5.25 (1H, broad, NH), 7.14 (3H, m, aromatic), 7.51-7.62 (3H, m, aromatic), 8.12 (1H, s, aromatic).
3-(2-(5-Methoxybenzofuran-2-yl)-2-oxoethylamino)-2-methylquinazolin-4(3H)-one (10g)
White crystals, Yield: 65%, m.p.: 116-118°C, FT-IR (KBr) ν (cm-1): 3290 (NH), 3979.18, 3084.40 and 2907.24 (C-H, aromatic and aliphatic), 1705.67 (C=O), 1675.19 (C=O), 1617.30 (C=N), 1267.71 (C-O), 1H NMR (DMSO-d6) δ (ppm): 2.51 (3H, s, CH3), 3.70 (3H, s, OCH3), 4.29 (2H, s, CH2), 5.15 (1H, broad, NH), 6.65 (1H,s, aromatic), 6.70 (1H, s, aromatic), 7.05 (2H, m, aromatic), 7.5-7.8 (3H, m, aromatic), 8.3 (1H, d, aromatic, J=7.10 Hz).
3-(2-(5-Methoxy benzofuran-2-yl)-2-oxo ethyl amino)-6-chloro-2-methyl quinazolin -4(3H)-one (10h)
Off-white crystals, Yield: 68%, m.p.: 125-126°C, FT-IR (KBr) ν (cm-1): 3310 (NH), 3106.55, 3080.74 and 2947.76 (C-H, aromatic and aliphatic), 1709.21 (C=O), 1677.28 (C=O), 1629.31 (C=N), 1273.71 (C-O), 1H NMR (DMSO-d6) δ (ppm): 2.41 (3H, s, CH3), 3.78 (3H, s, OCH3), 4.46 (2H, s, CH2), 5.61 (1H, broad, NH), 6.64 (1H,s, aromatic), 6.73 (1H, s, aromatic), 7.04 (2H, m, aromatic), 7.6 (1H, d, aromatic, J=7.00 Hz), 7.88 (1H, s, aromatic), 8.12 (1H, d, aromatic, J=7.00 Hz).
3-(2-(5-Methoxy benzofuran-2-yl)-2-oxo ethyl amino)-6-methoxy-2-methyl quinazolin -4(3H)-one (10i)
Pale yellow solid, Yield: 63%, m.p.: 121-122 °C, FT-IR (KBr) ν (cm-1): 3301 (NH), 3091.15, 3078.99 and 2907.74 (C-H, aromatic and aliphatic), 1699.21 (C=O), 1663.78 (C=O), 1615.90 (C=N), 1265.09 (C-O), 1H NMR (DMSO-d6) δ (ppm): 2.38 (3H, s, CH3), 3.80 (3H, s, OCH3), 3.89 (3H, s, OCH3), 4.39 (2H, s, CH2), 5.02 (1H, broad, NH), 6.63 (1 H,s, aromatic), 6.89 (1H, s, aromatic), 7.05-7.10 (3H, m, aromatic) 7.43 (1H, d, aromatic, J=7.20 Hz), 7.68 (1H, s, aromatic).
3-(2-(Benzofuran-2-yl)-2-hydroxyethylamino)-2-methylquinazolin-4(3H)-one (11a)
Off-white solid, Yield: 60%, m.p.: 114-115 °C, FT-IR (KBr) ν (cm-1): 3210.74-3350.23 (NH and OH, broad), 3105.32, 3052.41 and 2945.17 (C-H, aromatic and aliphatic), 1673.24 (C=O), 1620.31 (C=N), 1275.31 (C-O), 1H NMR (DMSO-d6) δ (ppm): 2.31 (3H, s, CH3), 4.13 (2H, d, CH2, J=7.60 Hz), 4.51 (1H, t, CH, J=7.60 Hz), 4.78 (1H, broad, OH), 5.47 (1H, broad, NH), 6.82 (1H, s, aromatic), 7.11-7.47 (6H, m, aromatic), 7.51 (1H, d, aromatic), 7.69 (1H, d, aromatic).
3-(2-(Benzofuran-2-yl)-2-hydroxyethylamino)-6-chloro-2-methylquinazolin-4(3H)-one (11b)
Off-white solid, Yield: 64%, m.p.: 148-150°C, FT-IR (KBr) ν (cm-1): 3296.11-3379.64 (NH and OH, broad), 3109.43, 3070.18 and 2963.71 (C-H, aromatic and aliphatic), 1679.78 (C=O), 1635.27 (C=N), 1285.76 (C-O), 1H NMR (DMSO-d6) δ (ppm): 2.40 (3H, s, CH3), 4.37 (2H, d, CH2, J=7.00 Hz),4.69 (1H, t, CH, J=7.00 Hz), 4.82 (1H, broad, OH), 5.50 (1H, broad, NH),6.84 (1H, s, aromatic), 7.11-7.16 (2H, m, aromatic), 7.42-7.50 (3H, m, aromatic), 7.8 (1H, s, aromatic), 8.2(1H, d, aromatic, J=6.90 Hz).
3-(2-(Benzofuran-2-yl)-2-hydroxyethylamino)-6-methoxy-2-methylquinazolin-4(3H)-one (11c)
Off-white solid, Yield: 62%, m.p.: 123-124°C, FT-IR (KBr) ν (cm-1): 3222.59-3384.15 (NH and OH, broad), 3104.28, 3050.19 and 2950.17 (C-H, aromatic and aliphatic), 1674.18 (C=O), 1620.81 (C=N), 1270.42 (C-O), 1H NMR (DMSO-d6) δ (ppm): 2.32 (3H, s, CH3), 3.71 (3H, s, OCH3), 4.28(2H, d, CH2, J=7.10 Hz), 4.39 (1H, t, CH, J=7.10 Hz), 5.02 (1H, broad, OH), 5.35 (1H, broad, NH), 6.90 (1H,s, aromatic), 7.11 (1H, d, aromatic, J=6.90 Hz), 7.21-7.28 (3H, m, aromatic), 7.50 (1H, d, aromatic, J=6.70 Hz), 7.60 (1H, d, aromatic, J=7.10 Hz), 7.62 (1H, s, aromatic).
3-(2-(5-Bromobenzofuran-2-yl)-2-hydroxyethyl amino)-2-methylquinazolin-4(3H)-one (11d)
Pale yellow solid, Yield: 65%, m.p.: 152-153°C, FT-IR (KBr) ν (cm-1): 3252.32-3405.11 (NH and OH, broad), 3110.15, 3079.25 and 2959.84 (C-H, aromatic and aliphatic), 1674.71 (C=O), 1621.38 (C=N), 1288.60 (C-O), 1H NMR (DMSO-d6) δ (ppm): 2.37 (3H, s, CH3), 4.43 (2H, d, CH2, J=7.40 Hz),4.70 (1H, t, CH, J=7.40 Hz), 5.3 (2H, broad, NH & OH), 7.41-7.81 (6H,m, aromatic), 8.11 (2H, m, aromatic).
3-(2-(5-Bromobenzofuran-2-yl)-2- hydroxyethylamino)- 6-chloro-2-methyl quinazolin-4(3H)-one (11e)
Pale yellow solid, Yield: 60%, m.p.: 161-162 °C, FT-IR (KBr) ν (cm-1): 3306.51-3415.21 (NH and OH, broad), 3130.27, 3086.61 and 2967.13 (C-H, aromatic and aliphatic), 1684.61 (C=O), 1638.27 (C=N), 1291.83 (C-O), 1H NMR (DMSO-d6) δ (ppm): 2.45 (3H, s, CH3), 4.75 (2H, d, CH2, J=7.20 Hz), 4.81(1H, t, CH, J=7.20 Hz),5.20 (1H, broad,OH), 5.31 (1H, broad, NH), 7.55 (1H,d, aromatic, J=6.80 Hz), 7.60-7.71 (3H, m, aromatic), 7.98 (1H, s, aromatic), 8.11 (1H, s, aromatic), 8.18 (1H, d aromatic, J=6.80 Hz).
3-(2-(5-Bromobenzofuran-2-yl)-2- hydroxyethylamino)- 6-methoxy-2-methyl quinazolin -4(3H)-one (11f)
Pale yellow solid, Yield: 60%, m.p.: 130-132°C, FT-IR (KBr) ν (cm-1): 3255.40-3709.04 (NH and OH, broad), 3108.46, 3071.21 and 2957.61 (C-H, aromatic and aliphatic), 1670.27 (C=O), 1620.41 (C=N), 1290.30 (C-O), 1H NMR (DMSO-d6) δ (ppm): 2.39 (3H, s, CH3), 3.80 (3H, s, OCH3), 4.60 (2H, d, CH2, J=7.20 Hz),4.73(1H, t, CH, J=7.20 Hz), 5.15 (1H, broad, OH), 5.25 (1H, broad, NH), 7.11-7.20 (2H,m, aromatic), 7.48-7.53 (4H, m, aromatic), 8.09 (1H, s, aromatic).
3-(2-Hydroxy-2-(5-methoxybenzofuran-2- yl)ethylamino)- 2-methylquinazolin-4(3H)-one (11g)
Pale yellow solid, Yield: 59%, m.p.: 129-130°C, FT-IR (KBr) ν (cm-1): 3220.14-3394.36 (NH and OH, broad), 3096.53, 3049.24 and 2942.13 (C-H, aromatic and aliphatic), 1671.78 (C=O), 1619.27 (C=N), 1265.09 (C-O), 1H NMR (DMSO-d6) δ (ppm): 2.46 (3H, s, CH3), 3.73 (3H, s, OCH3), 4.26 (2H, d, CH2, J=7.30 Hz), 4.59 (1H, t, CH, J=7.30 Hz), 5.05 (1H, broad, OH), 5.28 (1H, broad, NH), 6.61 (1H,s, aromatic), 6.76 (1H, s, aromatic), 6.98 (2H, m, aromatic), 7.50-7.68 (3H, m, aromatic), 7.98 (1H, d, aromatic, J=7.20 Hz).
3-(2-Hydroxy-2-(5-methoxybenzofuran-2- yl)ethylamino)- 6-chloro-2-methyl quinazolin -4(3H)-one (11h)
Pale yellow solid, Yield: 67%, m.p.: 137-138°C, FT-IR (KBr) ν (cm-1): 3270.51-3410.86 (NH and OH, broad), 3099.87, 3058.24 and 2960.34 (C-H, aromatic and aliphatic), 1678.46 (C=O), 1630.29 (C=N), 1272.09 (C-O), 1H NMR (DMSO-d6) δ (ppm): 2.40 (3H, s, CH3), 3.73 (3H, s, OCH3), 4.39 (2H, d, CH2, J=7.40 Hz),4.71 (1H, t, CH, J=7.40 Hz), 5.35 (1H, broad, OH), 5.58 (1H, broad, NH), 6.61 (1H, s, aromatic), 6.76 (1H, s, aromatic), 7.04-7.10 (2H, m, aromatic), 7.51 (1H, d, aromatic, J=6.90 Hz), 7.79 (1H, s, aromatic), 8.11 (1H, d, aromatic, J=6.90 Hz).
3-(2-Hydroxy -2-(5-methoxy benzofuran-2-yl) ethyl amino)-6-methoxy -2-methyl quinazolin -4(3H)-one (11i)
Pale yellow solid, Yield: 65%, m.p.: 137-139°C, FT-IR (KBr) ν (cm-1): 3256.34-3396.82 (NH and OH, broad), 3073.91, 3051.18 and 2951.64 (C-H, aromatic and aliphatic), 1663.91 (C=O), 1616.27 (C=N), 1265.98 (C-O), 1H NMR (DMSO-d6) δ (ppm): 2.38 (3H, s, CH3), 3.72 (3H, s, OCH3), 3.84 (3H, s, OCH3), 4.36 (2H, d, CH2, J=7.40 Hz),4.65 (1H, t, CH, J=7.40 Hz), 5.15 (1H, broad, OH), 5.28 (1H, broad, NH), 6.63 (1H,s, aromatic), 6.76 (1H, s, aromatic), 6.98 (2H, m, aromatic), 7.12 (1H, d, aromatic, J=7.20 Hz) 7.28 (1H, d, aromatic, J=7.20 Hz), 7.60 (1H, s, aromatic).
1-[[1-(Benzofuran-2-yl)-2-(2-methylquinazolin-4(3H)-one-3-yl)] ethyl amino -1-yl]-3- methyl imidazol-1-ium chloride (14a)
White solid, Yield: 50%, m.p.: 189-190°C, FT-IR (KBr) ν (cm-1): 3315.21 (NH, broad), 3115.47, 3048.41 and 2933.23 (C-H, aromatic and aliphatic), 1675.49 (C=O), 1625.15 (C=N), 1274.19 (C-O), 1H NMR (DMSO-d6) δ (ppm): 2.45 (3H, s, CH3), 2.83 (3H, s, CH3), 4.27 (2H, d, CH2, J=6.50 Hz), 4.92 (1H, t, CH, J=6.50 Hz),5.11 (1H, broad, NH), 6.76 (1H,s, aromatic), 7.20-7.35 (4H, m, aromatic), 7.38-7.48 (4H, m, aromatic), 7.52 (1H, d, aromatic, J=7.00 Hz), 7.82 (1H, d, aromatic, J=7.20 Hz), 8.10 (1H, s, aromatic), m/z Calcd for C23H22N5O2 [M-Cl]+ 400.18, found 400.20, Elemental analysis: Calcd; C: 63.37, H: 5.09, N: 16.07, found; C: 63.49, H: 5.22, N: 16.06
1-[[1-(Benzofuran-2-yl)-2-(6-chloro-2- methylquinazolin- 4(3H)-one-3-yl)] ethyl amino -1-yl]-3- methyl imidazol-1-ium chloride (14b)
White solid, Yield: 60%, m.p.: 201-203°C, FT-IR (KBr) ν (cm-1): 3340.43 (NH, broad), 3117.12, 3065.49 and 2952.80 (C-H, aromatic and aliphatic), 1678.56 (C=O), 1640.47 (C=N), 1288.48 (C-O), 1H NMR (DMSO-d6) δ (ppm): 2.48 (3H, s, CH3), 2.92 (3H, s, CH3), 4.61 (2H, d, CH2, J=6.80 Hz), 5.13 (1H, t, CH, J=6.80 Hz),5.35 (1H, broad, NH), 6.70 (1H,s, aromatic), 7.21-7.30 (4H, m, aromatic), 7.42 (1H, d, aromatic, J=7.10 Hz), 7.55-7.61 (2H, m, aromatic) 7.96 (1H, s, aromatic), 8.14 (1H, d, aromatic, J=6.90 Hz), 8.21 (1H, s, aromatic), m/z Calcd for C23H21ClN5O2 [M-Cl]+ 434.14, found 434.12, Elemental analysis: Calcd; C: 58.73, H: 4.50, N: 14.89, found; C: 58.56, H: 4.55, N: 14.86.
1-[[1-(Benzofuran-2-yl)-2-(6-methoxy-2- methylquinazolin- 4(3H)-one-3-yl)] ethyl amino -1-yl]-3- methyl imidazol-1-ium chloride (14c)
White solid, Yield: 55%, m.p.: 199-201°C, FT-IR (KBr) ν (cm-1): 3327.15 (NH, broad), 3109.41, 3073.49 and 2932.15 (C-H, aromatic and aliphatic), 1673.72 (C=O), 1622.12 (C=N), 1272.94 (C-O), 1H NMR (DMSO-d6) δ (ppm): 2.38 (3H, s, CH3), 2.88 (3H, s, CH3), 3.82 (3H, s, OCH3), 4.57 (2H, d, CH2, J=6.70 Hz), 5.01 (1H, t, CH, J=6.70 Hz),5.27 (1H, broad, NH), 6.98 (1H,s, aromatic), 7.11-7.30 (6H, m, aromatic), 7.52 (1H, m, aromatic), 7.63 (1H, d, aromatic, J=7.00 Hz), 7.68 (1H, s, aromatic), 8.14 (1H, s, aromatic), m/z Calcd for C24H24N5O3 [M-Cl]+ 430.19, found 430.21, Elemental analysis: Calcd; C: 61.87, H: 5.19, N: 15.03, found; C: 61.83, H: 5.35, N: 15.17.
1-[[1-(5-Bromobenzofuran-2-yl)-2-(2- methylquinazolin- 4(3H)-one-3-yl)] ethyl amino -1-yl]-3- methyl imidazol-1-ium chloride (14d)
White solid, Yield: 55%, m.p.: 215-216°C, FT-IR (KBr) ν (cm-1): 3345.15 (NH, broad), 3093.58, 3050.46 and 2936.71 (C-H, aromatic and aliphatic), 1675.82 (C=O), 1624.28 (C=N), 1289.49 (C-O), 1H NMR (DMSO-d6) δ (ppm): 2.39 (3H, s, CH3), 2.93 (3H, s, CH3), 4.71 (2H, s, CH2, J=7.00 Hz), 5.33 (1H, t, CH, J=7.00 Hz), 5.40 (1H, broad, NH), 7.25-7.50 (7H, m, aromatic), 7.73 (1H, s, aromatic, J=6.90 Hz), 7.89 (1H,d, aromatic), 8.08 (1H, d, aromatic, J=7.20 Hz), 8.19 (1H, s, aromatic), m/z Calcd for C23H21BrN5O2 [M-Cl]+ 480.09, found 480.11, Elemental analysis: Calcd; C: 53.66, H: 4.11, N: 13.60, found; C: 53.84, H: 4.13, N: 13.71.
1-[[1-(5-Bromobenzofuran-2-yl)-2-(6-chloro-2-methyl quinazolin-4(3H)-one-3-yl)] ethyl amino -1-yl]-3- methyl imidazol-1-ium chloride (14e)
White solid, Yield: 58%, m.p.: 220-222°C, FT-IR (KBr) ν (cm-1): 3352.45 (NH, broad), 3118.16, 3042.20 and 2956.83 (C-H, aromatic and aliphatic), 1685.44 (C=O), 1641.28 (C=N), 1293.49 (C-O), 1H NMR (DMSO-d6) δ (ppm): 2.51 (3H, s, CH3), 2.94 (3H, s, CH3), 5.04 (2H, d, CH2, J=6.80 Hz), 5.41 (1H, t, CH, J=6.80 Hz), 5.51 (1H, broad, NH), 7.25-7.31 (3H, m, aromatic), 7.42 (2H, m, aromatic), 7.50 (1H, d, aromatic, J=6.70 Hz), 7.92 (1H, s, aromatic),7.96 (1H, s, aromatic), 8.14 (1H, d, aromatic, J=6.70 Hz), 8.24 (1H, s, aromatic), m/z Calcd for C23H20BrClN5O2 [M-Cl]+ 514.05, found 514.09, Elemental analysis: Calcd; C: 50.30, H: 3.67, N: 12.75, found; C: 50.54, H: 3.77, N: 12.77.
1-[[1-(5-Bromobenzofuran-2-yl)-2-(6-methoxy-2-methylquinazolin-4(3H)-one-3-yl)] ethyl amino -1-yl]-3- methyl imidazol-1-ium chloride (14f)
White solid, Yield: 49%, m.p.: 210-212 °C, FT-IR (KBr) ν (cm-1): 3322.47 (NH, broad), 3105.84, 3061.23 and 2940.48 (C-H, aromatic and aliphatic), 1672.60 (C=O), 1625.73 (C=N), 1290.94 (C-O), 1H NMR (DMSO-d6) δ (ppm): 2.41 (3H, s, CH3), 2.98 (3H, s, CH3), 3.82 (3H, s, OCH3), 4.79 (2H, d, CH2, J=7.00 Hz), 5.35 (1H, t, CH, J=7.00 Hz), 5.44 (1H, broad, NH),7.11-7.36 (4H,m, aromatic), 7.51-7.60 (3H, m, aromatic), 7.87 (1H, s, aromatic), 8.11 (1H, s, aromatic), 8.23 (1H, s, aromatic), m/z Calcd for C24H23BrN5O3 [M-Cl]+ 508.10, found 508.13, Elemental analysis: Calcd; C: 52.91, H: 4.26, N: 12.85, found; C: 53.15, H: 4.39, N: 12.94.
1-[[1-(5-Methoxybenzofuran-2-yl)-2-(2-methylquinazolin-4(3H)-one-3-yl)] ethyl amino -1-yl]-3- methyl imidazol-1-ium chloride (14g)
White solid, Yield: 48%, m.p.: 195-197 °C, FT-IR (KBr) ν (cm-1): 3306.65 (NH, broad), 3094.78, 3047.85 and 2931.19 (C-H, aromatic and aliphatic), 1670.60 (C=O), 1623.49 (C=N), 1266.83 (C-O), 1H NMR (DMSO-d6) δ (ppm): 2.48 (3H, s, CH3), 2.95 (3H, s, CH3), 3.76 (3H, s, OCH3),4.40 (2H, d, CH2, J=6.80 Hz), 5.21 (1H, t, CH, J=6.80 Hz),5.30 (1H, broad, NH), 6.69 (1H,s, aromatic), 6.70 (1H, s, aromatic), 7.14-7.32 (4H, m, aromatic), 7.52 (2H, m, aromatic), 7.63 (1H, d, aromatic, J=6.90 Hz), 8.01 (1H, d, aromatic, J=7.10 Hz), 8.21 (1H, s, aromatic), m/z Calcd for C24H24N5O3 [M-Cl]+ 430.19, found 430.20, Elemental analysis: Calcd; C: 61.78, H: 5.19, N: 15.03, found; C: 61.82, H: 5.41, N: 15.17.
1-[[1-(5-Methoxybenzofuran-2-yl)-2-(6-chloro-2-methylquinazolin-4(3H)-one-3-yl)] ethyl amino -1-yl]-3- methyl imidazol-1-ium chloride (14h)
White solid, Yield: 50%, m.p.: 204-206°C, FT-IR (KBr) ν (cm-1): 3339.56 (NH, broad), 3098.29, 3061.67 and 2937.43 (C-H, aromatic and aliphatic), 1675.91 (C=O), 1636.60(C=N), 1274.18 (C-O), 1H NMR (DMSO-d6) δ (ppm): 2.43 (3H, s, CH3), 2.75 (3H, s, CH3), 3.77 (3H, s, OCH3), 4.67 (2H, d, CH2, J=7.10 Hz), 5.42 (1H, t, CH, J=7.10 Hz), 5.36 (1H, broad, NH, f), 6.67 (1H, s, aromatic), 6.73 (1H, s, aromatic), 7.07 (2H, m, aromatic), 7.21-7.26 (2H, dd, aromatic, J=6.70 Hz), 7.49 (1H,d, aromatic, J=6.60 Hz),7.88 (1H, s, aromatic), 8.12 (1H, d, aromatic, J=6.60 Hz), 8.24 (1H, s, aromatic), m/z Calcd for C24H23ClN5O3 [M-Cl]+ 464.15, found 464.17, Elemental analysis: Calcd; C: 57.61, H: 4.63, N: 14.00, found; C: 57.84, H: 4.87, N: 14.05.
1-[[1-(5-Methoxybenzofuran-2-yl)-2-(6-methoxy-2-methyl-quinazolin-4(3H)-one-3-yl)] ethylamino -1-yl]-3- methyl imidazol-1-ium chloride (14i)
White solid, Yield: 40%, m.p.: 190-193°C, FT-IR (KBr) ν (cm-1): 3325.25 (NH, broad), 3085.64, 3036.26 and 2925.59 (C-H, aromatic and aliphatic), 1664.47 (C=O), 1625.14 (C=N), 1268.42 (C-O), 1H NMR (DMSO-d6) δ (ppm): 2.48 (3H, s, CH3), 2.74 (3H, s, CH3), 2.75 (3H, s, OCH3), 3.86 (3H, s, OCH3), 4.51 (2H, d, CH2, J=6.70 Hz), 5.17 (1H, t, CH, J=6.70 Hz), 5.34 (1H, broad, NH), 6.70 (1H, s, aromatic), 6.72 (1H, s, aromatic), 7.18-7.33 (6H, m, aromatic), 7.8 (1H, s, aromatic), 8.16 (1H, s, aromatic), m/z Calcd for C25H26N5O4 [M-Cl]+460.20, found 460.18, Elemental analysis: Calcd; C: 60.54, H: 5.28, N: 14.12, found; C: 60.51, H: 5.51, N: 14.18.
Biological evaluation : Antimicrobial activity
The antimicrobial activity of the synthesized compounds (14a-i) was determined by using Alamar blue susceptibility test (MABA) method, as recommended by the National Committee for Clinical Laboratory Standards, (NCCLS) (22, 23). The compounds were evaluated for antimicrobial activity against bacteria viz. Staphylococcus aureus PTCC 1337, Bacillus subtilis PTCC 1023, Listeria monocitogenes PTCC 1165 (gram ram positive bacteria), Escherichia coli PTCC 1338, Pseudomonas aeruginosa PTCC 1074, Salmonella entritidis PTCC 1091 (Gram-negative bacteria) and also, Candida albicans PTCC 5027 as yeast-like fungi strains. The results related to the antimicrobial activity of the synthesized compounds (14a-i) are illustrated in Tables 1 and 2.
Table 1.
MIC of the synthesized compounds (14a-i) against Gram-positive, Gram-negative bacteria and Candida albicans
 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Code | R1 | R2 | Gram-negative bacteria µg/ml1 | Gram-positive bacteria µg/ml | Candida albicans µg/ml | ||||
| Escherichia coli | Pseudomonas aeruginosa | Salmonella entritidis | Staphylococcus aureus | Bacillus subtilis | Listeria monocitogenes | ||||
| MIC | MIC | MIC | MIC | MIC | MIC | MIC | |||
| 14a | H | H | G | 512 | 512 | 128 | 256 | 128 | 512 |
| 14b | Cl | H | 512 | 256 | 256 | 64 | 128 | 256 | 256 |
| 14c | OMe | H | G | G | 512 | 128 | 128 | 256 | 256 |
| 14d | H | Br | 512 | 256 | 128 | 128 | 128 | 128 | 256 |
| 14e | Cl | Br | 128 | 128 | 128 | 16 | 32 | 128 | 128 |
| 14f | OMe | Br | G | 256 | 512 | 512 | 256 | 512 | 512 |
| 14g | H | OMe | G | 512 | 256 | 128 | 512 | 256 | 128 |
| 14h | Cl | OMe | 512 | 512 | 512 | 256 | 256 | 256 | 256 |
| 14i | OMe | OMe | G | G | 512 | 512 | 128 | 512 | 512 |
| Ciprofloxacin | 8 | 4 | 4 | 4 | 8 | 8 | - | ||
| Ketoconazol | - | - | - | - | - | - | 8 | ||
G: Growth of bacteria
Table 2.
MBC and MFC of the synthesized compounds (14a-i) against Gram-positive, Gram-negative bacteria and Candida albicans
 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Code | R1 | R2 | Gram-negative bacteria µg/ml1 | Gram-positive bacteria µg/ml | Candida albicans µg/ml | ||||
| Escherichia coli | Pseudomonas aeruginosa | Salmonella entritidis | Staphylococcus aureus | Bacillus subtilis | Listeria monocitogenes | ||||
| MBC | MBC | MBC | MBC | MBC | MBC | MFC | |||
| 14a | H | H | NA | NA | NA | 512 | 512 | 512 | NA |
| 14b | Cl | H | NA | NA | 512 | 256 | 256 | 512 | 512 |
| 14c | OMe | H | NA | NA | NA | 256 | 512 | 512 | NA |
| 14d | H | Br | NA | NA | 512 | 512 | 256 | 256 | 512 |
| 14e | Cl | Br | 512 | 512 | 256 | 128 | 128 | 256 | 256 |
| 14f | OMe | Br | NA | 512 | NA | NA | 512 | NA | NA |
| 14g | H | OMe | NA | NA | 512 | 512 | NA | 512 | 512 |
| 14h | Cl | OMe | NA | NA | NA | 512 | 512 | ND | 512 |
| 14i | OMe | OMe | NA | NA | NA | NA | 512 | ND | NA |
NA: Not applicable
Cytotoxic activity
The in vitro cytotoxicity of the synthesized hybrid compounds was measured on the human breast cancer cell line (MCF-7) using the MTT assay (24, 25). In this assay, cytotoxic activities were expressed as the percentage of cell survival and measured at 4 different concentrations. Doxorubicin was used as a positive control against the MCF7 cell line. Cytotoxicity screening data were showed in Figure 1 and Table 3.
Figure 1.
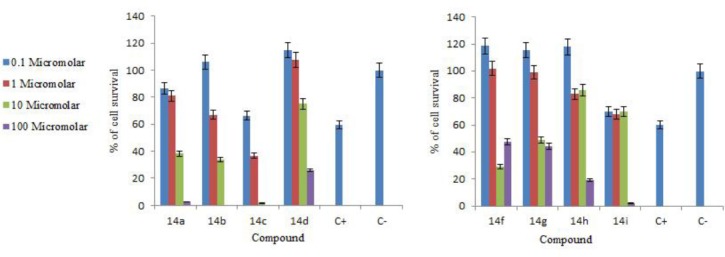
Cytotoxic results of the synthesized compounds on the MCF-7 cell line
Table 3.
Active concentrations and IC50 of the synthesized compounds on the MCF-7 cell line
| Code | R1 | R2 | Active concentrations (μM) | Survival (%) | IC50 (μM) |
|---|---|---|---|---|---|
| 14a | H | H | 0.1, 1, 10, 100 | 86.52, 81.37, 38.25, 2.53 | 7.53 |
| 14b | Cl | H | 1, 10, 100 | 66.93, 33.86, 0.00 | 5.60 |
| 14c | OMe | H | 0.1, 1, 10, 100 | 66.20, 36.73, 1.43, 0.00 | 0.59 |
| 14d | H | Br | 10, 100 | 74.96, 25.87 | 55.76 |
| 14f | OMe | Br | 10, 100 | 70.00, 47.00 | 89.64 |
| 14g | H | OMe | 1, 10, 100 | 98.74, 85.76, 44.46 | 87.97 |
| 14h | Cl | OMe | 1, 10, 100 | 83.00, 48.00, 19.00 | 9.73 |
| 14i | OMe | OMe | 0.1, 1, 10, 100 | 70.16, 68.00, 29.13, 2.07 | 5.16 |
| doxorubicin | 0.5 | 59.70 | 3.62* |
IC50 of doxorubicin was reported based on reference 33
Docking study
According to the cytotoxic studies, quinazolinone -bezofuran-imidazole hybrids, especially derivatives with the methoxy substituent, have a good cytotoxic effect on the MCF-7 cell line. Since aromatase is overexpressed in the MCF-7 cell line, it is thought that these compounds could interact with the aromatase enzyme. The orientation of the target hybrides in the aromatase active site was predicted by docking simulation. According to the previous studies, polarization effects could play a significant role in determining the structures of protein-ligand complexes (26, 34). The free energies of binding (ΔGb) and inhibition constants (Ki) as calculated by AutoDock are summarized in Table 4.
Table 4.
Free binding energy (kcal/mol) and inhibition constants (Ki) of group 2 ligands, after refitting the charges, calculated by AutoDockk
| No | R1 | R2 | cluster | Binding energy(1) | Binding energy(2) | Binding energy(3) | Ki |
|---|---|---|---|---|---|---|---|
| 14a | H | H | 43 | -7.13 | -7.97 | -7.99 | 3.29 µM |
| 14b | Cl | H | 52 | -7.01 | -7.21 | -7.11 | 7.80 µM |
| 14c | OMe | H | 48 | -7.01 | -7.68 | -7.67 | 5.15 µM |
| 14d | H | Br | 48 | -7.32 | -7.65 | -7.62 | 5.35 µM |
| 14f | OMe | Br | 28 | -7.25 | -7.60 | -7.54 | 6.68 µM |
| 14g | H | OMe | 58 | -7.23 | -7.50 | -7.52 | 6.73 µM |
| 14h | Cl | OMe | 50 | -7.23 | -7.85 | -7.81 | 4.28 µM |
| 14i | OMe | OMe | 42 | -8.12 | -8.31 | -8.28 | 730.00nM |
The first binding energy calculated with autodock
The binding energy calculated after refitting charge with the values obtained from QM/MM calculation
The binding energy calculated after fixed change values
Discussion
Chemistry
The general procedure for the synthesis of some 1-[[(1-(benzofuran-2-yl)-2-(2-methylquinazolin-4(3H)-one-3-yl)] ethylamino -1-yl]-3-methyl imidazol-1-ium chloride derivatives (14a-i) is outlined in Scheme 1. At first, acetylation of anthranilic acid derivatives (1a-c) by acetic anhydride yielded 2-methyl-4-benzoxazinone derivatives (3a-c), which were conver-ted to 3-amino-2-methyl-3H-quinazolin-4-one deriva-tives (5a-c) by reaction with hydrazine hydrate. On the other hand, condensation of salicylaldehyde derivatives (6a-c) and chloroacetone (7) was performed in acetone in the presence of potassium carbonate at reflux to give 2-acethylbenzofuran derivatives (8a-c). Subsequently, Compounds 8a-c were brominated using Br2 in acetic acid to give 2-bromo-2-acethylbenzofuran derivatives (9a-c). Quinazolinone-benzofuran hybrids (10a-i) were synthesized by the treatment of 3-amino-2-methyl-3H-quinazolin-4-one derivatives (5a-c) with 2-bromo-2-acethylbenzofuran derivatives (9a-c) in the dry THF in the presence of triethylamine. The ketones 10a-i was selectively reduced by sodium borohydride (NaBH4) to give the corresponding alcohols (11a-i). Reaction of the alcohols 11ai with thionyl chloride in dichloromethane (DCM) converted the hydroxyl group into chloro derivatives (12a-i). Finally, the isolated chloro derivatives 12a-i in acetonitrile were reacted with N-methyl imidazole to furnish the target hybrid compounds (14a-i).
FT-IR characterization
The structures of the synthesized compounds were evaluated by means of the FT-IR technique and the results showed a good agreement with the proposed structures 14a-i (Scheme 1). The characteristic IR bands are presented in the experimental section. The FT-IR of the synthesized quinazolinone derivatives exhibited absorptions at 1667-1681 and 3310-3388 cm-1, indicating the presence of the heterocyclic amide and NH in the structures, respectively. On the other hand, the formation of 2-acethylbenzofuran products (8a-c) was confirmed by the elimination of parent bands at 1668-1676 and 3095-3228 cm−1, which could be originally attributed to the stretching vibrations of carbonyl (CHO) and hydroxyl (OH) moieties of salicilaldehyde derivatives; instead, strong new bands that appeared at 1698-1710 and 1324-1368 cm−1 confirmed the ketone carbonyl (O=C) and O-C linkages, respectively. Bromination of 2-acethyl benzofuran (8a-c) derivatives with bromin in acetic acid resulted in a new band in 831-857 cm-1 which was assigned to the vibration of C-Br group. Two strong bands appeared at 1700-1717 and 1663-1682 cm-1, confirming the presence of both ketone and amid carbonyl groups in the FT-IR spectra of quinazolinoone-benzofuran hybrids (10a-i). After reduction with NaBH4, the bands attributed to the stretching vibrations of ketone were disappeared and the peaks observed at 3280-3404 cm-1 confirmed the reduction of ketone to alcohol. In the FT-IR spectra of the final products, the presence of absorption bands in the range of 3306–3352 cm-1 indicated the presence of NH and the strong absorption bands at 1664–1685 cm−1 confirmed the presence of CON groups in the compounds 14a-i. The absorption bands at 1266–1293 and 1637–1651 cm−1 were ascribed to C-O and C=N groups, respectively. In the spectrum of the final products, as compared to the earlier intermediate, a brief widening was observed. This flattening could be attributed to existence of new bands related to imidazole moiety.
NMR characterization
The structures of the synthesized compounds were also assigned by 1H NMR. All the protons were found to be in their expected region, showing agreement with those obtained from the values of the CHN analyses. Singlet integration for one proton of the NH group of quinazolinones 5a-c was observed between 4.63–4.92 ppm as a broad signal. Aromatic protons of quinazolinone appeared in the range of 6.71-8.40 ppm. In the 1H NMR spectra of 2-acethyl-benzofurane derivatives (8a-c), singlet integration for three protons characteristic of the methyl group was observed in 2.58–2.65 ppm. Aromatic protons of benzofuran rings were found in the region at 6.68-7.77 ppm. The 1H NMR spectrum of quinazolinone-benzofurane hybrids (10a-i) revealed characteristic peaks of methylen (CH2) and NH protons as two singlets at 4.17-4.83 ppm and 4.75-5.61 ppm (broad), respectively, thereby providing evidence for the linkage between quinazolinone and benzofuran systems. After the reduction of carbonyl group with NaBH4, the NMR spectrum of all compounds (11a-i) showed a singlet integrating one proton of the OH group in 4.75-5.35 ppm as a broad signal and also, exhibiting the characteristic peaks of CH and CH2 protons at 4.39-4.81 and 4.13-4.75 ppm as a triplet and doublet peak, respectively. In the final products (14a-i), addition of methylimidazole unit to the structure was confirmed by the appearance of the methyl (CH3) protons of imidazole at 2.83-2.98 ppm as a single peak and also, the increasing number of aromatic protons. It should be mentioned that methoxy substitution of derivatives was found in the region at 3.74-3.85 as a single peak.
Biological evaluation antimicrobial activity
The results related to the antimicrobial activity of the synthesized compounds (14a-i) showed that the compounds 14e were found to be more active than other compounds with MIC 16–128 μM against all tested microorganisms. Other synthesized compounds except 14i showed good to moderate antimicrobial activity with the MIC of 32–512 μM. From the results of antibacterial activity, it was found that the synthesized hybrid compounds were more active against all Gram-positive bacteria, especially S. aureus, but with less potency against gram negative bacteria, especially against E. coli. All compounds showed antibacterial effects at the concentrations of 128-512 μg/ml for B. subtilis, L. monocitogenes and S. entritidis.
From the obtained data, the following conclusions could be drawn with regard to antimicrobial activity: the compounds substituted with halogens on the quinazolinone and benzofurane rings showed the maximum antibacterial activity in comparison to other derivatives, as observed in the case of the compound 14e. It could be suggested that halogen atoms on benzofuran and quinazolinone rings might be responsible for the higher activity of compound 14e, similar to what was observed in benzofuran containing the natural products (35). One possible reason could be the increased hydrophobicity. A decrease in the antimicrobial activity was observed in the case of compounds 14i, probably due to the presence of electron donating substituents (methoxy) on the quinazolinone and benzofurans. One discription to lower activity of compounds 14i might be related to the decreased lipophilicity of the compound due to the presence of methoxy group at C-5 and C-6 position of benzofuran and qunazolinone heterocyclic rings, respectively. Compounds with halogen and methoxy substituents showed moderate activity (Table 1). The results of MBC revealed that the tested compounds had more significant bacteriostatic rather than bactericidal activities (Table 2). The compounds 14e, 14g (MIC=128 µg/ml), 14b, 14c, 14d, 14h (MIC=256 µg/ml) and 14a, 14f, 14i (MIC=512 µg/ml) showed good to moderate in vitro anti-fungal activity against Candida albicans. In general, the anti-fungal activity was lower than the antibacterial activity. Compound 14e and 14g were found to be the most potent members showing lower MIC against C. albicans. The obtained results of MFC revealed that the tested compounds had more significant fungistatic rather than fungicidal activities (Table 2).
Cytotoxic activity
The in vitro cytotoxicity of the synthesized hybrid compounds was measured on the human breast cancer cell line (MCF-7) using the MTT assay (28, 29). The results revealed that the compounds 14c demonstrated the most potent cytotoxicity, with IC50 values of 0.59 μM. It is interesting to note that the compounds 14i, 14b, 14a and 14h also showed appreciable cytotoxicity with IC50 values of 5.16, 5.60, 7.53 and 9.73 μM, respectively. The compounds such as 14d, 14f and 14g showed a moderate level of activity at the concentrations of 55.76, 87.97 and 89.69 μM, respectively (Table 3).
A close look at cytotoxic results of these compounds clearly revealed the inherent cytotoxicity associated with the basic skeleton consisting of quinazolinone, benzofuran and imidazolium moieties, as seen in the case of the unsubstituted compound 14a with the IC50 value of 7.53 μM; in some cases, this was enhanced by the influence of some substituents and decreased by some other substituents. For example, the compounds 14c, which had methoxy substituents on quinazolinone, showed the significantly enhanced activity (IC50=0.59 μM). The compound 14i with two methoxy substituents either at quinazolinone and benzofuran (IC50=5.16 μM) and the compound 14b with the chloro substituent on quinazolinone ring (IC50=5.60 μM) also showed significant activity on the MCF-7 cell line. Substitutions of chloro on quinazolinone and the methoxy group on benzofuran rings resulted in IC50=9.73 μM for the compound 14h.
According to the obtained results, the presence of the methoxy substituent on benzofuuran ring played an important role in the observed growth inhibition. The favorable effect of the methoxy group of benzofuran on activity profiles was authenticated by comparing 14a, 14i and 14h, while compounds with the methoxy group on quinazilinone ring did not show enhanced activity, as compared to the unsubstituted analog (Table 3). In contrast, the halogen substituent on the quinazolinone ring increased the activity (compare 14b to 14a), while the presence of the methoxy substituent on benzofuuran ring resulted in decreasing the cytotoxic activity (compare 14a, 14d and 14f).
Docking study
In this study, owing to the positive charge on the imidazole ring of the designed compounds and also, the presence of Heme iron in the active site, polarization effects were more important in energy calculations. So, to increase the accuracy of docking results, fixed charges of ligands obtained from force field parameterization were replaced by QM/MM calculations in the protein environment, treating only the ligands as the quantum region. According to the docking results, the novel designed compounds could be fitted well within the binding site cavity of aromatase, showing acceptable dG values. Among three heterocyclic rings, quinazolinone nucleus was oriented towards the Heme, providing hydrophobic interaction between quinazolinone moieties and Heme porphyrins. Imidazole and benzofuran rings could foster van der Waals interactions with the gap bounded by Leu372, Phe134, Ile133, Trp224 and Val370 residues of the aromatase active site (Figure 2). The hybrid containing the methoxy substituent on quinazolinone established the hydrogen bond with Met374 residue (Figure 3), while the amino acid Ser 478 was found to play an important role in hydrogen bond interactions with the compounds 14g having the methoxy substituent on the benzofuran ring (Figure 4).
Figure 2.
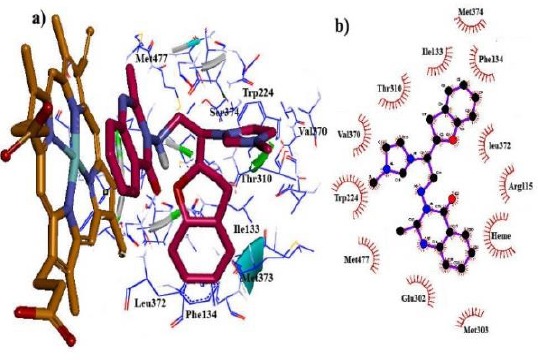
The binding mode of the synthesized hybrid scaffold (compound 14a) in the active site of aromatase obtained from autodock4: (a) 3D structure and (b) 2D structure
Figure 3.
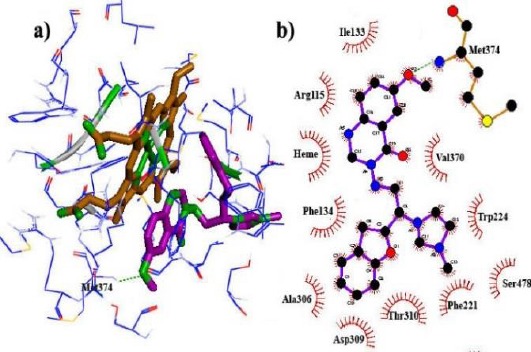
Binding modes and hydrogen bonds interactions of the methoxy group on the quinazolinone ring (compound 14c) with Met374 in aromatase active site: (a) 3D structure and (b) 2D structure
Figure 4.
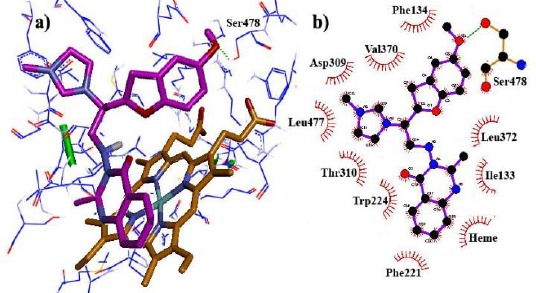
Binding modes and hydrogen bonds interactions of the methoxy group on the benzofuran ring (compound 14g) with Ser 478 in aromatase active site (a) 3D structure and (b) 2D structure
Conclusion
To conclude, a series of novel hybrids containing quinazolinone, benzofuran and imidazole moieties were synthesized and their antimicrobial and cytotoxic activities were evaluated. The results of antibacterial activity showed that the synthesized hybrid compounds were more active against the tested Gram-positive bacteria, especially S. aureus, but with less potency against Gram negative bacteria, especially against E. coli. The compound 14e, with two halogen atoms on quinazolinone and benzofuran, was found to be the most active against all the tested strains of microorganisms.
In the cytotoxic assay, compounds 14i, 14c and 14a exhibited a significant activity with the IC50 values 0.1–7.8 μM, while compounds 14b, 14d, 14f, 14g and 14h showed a moderate activity with the IC50 values 1–100 μM. Subsequently, all cytotoxic compounds were subjected to molecular docking to understand their binding mode to aromatase enzyme. The binding mode analysis revealed that the synthesized compounds could easily bind to the aromatase active site with good affinity through hydrophobic and H-bond interactions. Finally, the proposed scaffold of quinazolinone, benzofuran and imidazole hybrid offered the possibility of convenient further modifications, giving rise to lead structures with improved cytotoxic and antimicrobial activities.
Acknowledgment
We gratefully acknowledge the Research Council of Isfahan University of Medical Sciences (193071).
References
- 1.Muregi FW, Ishih A. Next-Generation Antimalarial Drugs:Hybrid Molecules as a New Strategy in Drug Design. Drug Dev Res. 2010;71:20–32. doi: 10.1002/ddr.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rane RA, Telvekar VN. Synthesis and evaluation of novel chloropyrrole molecules designed by molecular hybridization of common pharmacophores as potential antimicrobial agents. Bioorg Med Chem Lett. 2010;20:5681–5685. doi: 10.1016/j.bmcl.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 3.Rizzo S, Riviere C, Piazzi L, Stefano R, Alessandra B, Gobbi S, et al. Benzofuran-based hybrid compounds for the inhibition of cholinesterase activity, amyloid aggregation, and a neurotoxicity. J Med Chem. 2008;51:2883–2886. doi: 10.1021/jm8002747. [DOI] [PubMed] [Google Scholar]
- 4.Guantai EM, Ncokazi K, Egan TJ, Gut J, Rosenthal PJ, Smith PJ, Chibale K. Design, synthesis and in vitro antimalarial evaluation of triazole-linked chalcone and dienone hybrid compounds. Bioorg Med Chem. 2010;18:8243–8256. doi: 10.1016/j.bmc.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 5.Evranos B, Altanlar N, Ertan R. Synthesis and biological activity of some new lavonylazole derivatives. Acta Pharm Sci. 2007;49:231–238. [Google Scholar]
- 6.Ahmed K, Pogula PK, Mohammed NA, Bobburi NS, Olepu S. Hybrid pharmacophore design and synthesis of naphthalimide–benzimidazole conjugates as potential anticancer agents. Lett Drug Des Discov. 2015;12:374–384. [Google Scholar]
- 7.Sashidhara KV, Rao KB, Kushwaha P, Modukuri RK, Singh P, Soni I, et al. Novel chalcone-thiazole hybrids as potentinhibitors of drug resistant staphylococcus aureus. ACS Med Chem Lett. 2015;6:809–813. doi: 10.1021/acsmedchemlett.5b00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang XD, Wan WC, Deng XY, Li Y, Yang LJ, Li L, et al. Design, synthesis and cytotoxic activities of novel hybrid compounds between 2-phenylbenzofuran and imidazole. Bioorg Med Chem Lett. 2012;22:2726–2729. doi: 10.1016/j.bmcl.2012.02.094. [DOI] [PubMed] [Google Scholar]
- 9.Sharma PC, Kaur1 G, Pahwa R, Sharma A, Rajak H. Quinazolinone analogs as potential therapeutic agents. Curr Med Chem. 2011;18:4786–4812. doi: 10.2174/092986711797535326. [DOI] [PubMed] [Google Scholar]
- 10.Mohammadi MA, Askari S, Rohi H, Soorki AA. Design, synthesis and antibacterial evaluation of same novel 3’-(phenylamino)-1’h-spiro[indoline-3,2’- quinazoline] -2,4’(3’h)-dione derivatives. Synth Commun. 2014;44:457–467. [Google Scholar]
- 11.Modh RP, Clercq ED, Pannecouque C, Chikhalia KH. Design, synthesis, antimicrobial activity and anti-HIV activity evaluationof novel hybrid quinazoline–triazine derivatives. J Enzyme Inhib Med Chem. 2014;29:100–108. doi: 10.3109/14756366.2012.755622. [DOI] [PubMed] [Google Scholar]
- 12.Noolvi MN, Patel HM, Bhardwaj V, Chauhan A. Synthesis and in vitro antitumor activity of substituted quinazoline and quinoxaline derivatives:Search for anticancer agent. Eur J Med Chem. 2011;46:2327–2346. doi: 10.1016/j.ejmech.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 13.Nevagi RJ, Dighe SN, Dighe SN. Biological and medicinal significance of benzofuran. Eur J Med Chem. 2015;97:561–581. doi: 10.1016/j.ejmech.2014.10.085. [DOI] [PubMed] [Google Scholar]
- 14.Khodarahmi GA, Asadi P, Hassanzadeh F, Khodarahmi E. Benzofuran as a promising scaffold for the synthesis of antimicrobial and antibreast cancer agents:A review. J Res Med Sci. 2015;20:1094–1104. doi: 10.4103/1735-1995.172835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdel-Wahab BF, Awad GE, Badria FA. Synthesis, antimicrobial, antioxidant, anti-hemolytic and cytotoxic evaluation of new imidazole-based heterocycles. Eur J Med Chem. 2011;46:1505–1511. doi: 10.1016/j.ejmech.2011.01.062. [DOI] [PubMed] [Google Scholar]
- 16.Zhang L, Peng XM, Damu GLV, Geng RX, Zhou CH. Comprehensive review in current developments of imidazole-based medicinal chemistry. Med Res Rev. 2014;34:340–437. doi: 10.1002/med.21290. [DOI] [PubMed] [Google Scholar]
- 17.Chen W, Deng XY, Li Y, Yang LJ, Wan WC, Wang XQ, et al. Synthesis and cytotoxic activities of novel hybrid 2-phenyl-3-alkylbenzofuran and imidazole/triazole compounds. Bioorg Med Chem Lett. 2013;23:4297–4302. doi: 10.1016/j.bmcl.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Zahedifard M, Faraj FL, Paydar M, Looi CY, Hajrezaei M, Hasanpourghadi M, et al. Synthesis, characterization and apoptotic activity of quinazolinone Schiff base derivatives toward MCF-7 cells via intrinsic and extrinsic apoptosis pathways. Sci Rep. 2015;5:11544. doi: 10.1038/srep11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmed MF, Hashim AA. Design, synthesis of novel quinazolin-4-one derivatives and biological evaluation against human MCF-7 breast cancer cell line. Res Chem Intermediates. 2016;42:1777–1789. [Google Scholar]
- 20.Brooks JD, Thompson LU. Mammalian lignans and genistein decrease the activities of aromatase and 17-hydroxysteroid dehydrogenase in MCF-7 cells. J Steroid Biochem Mol Biol. 2005;94:461–467. doi: 10.1016/j.jsbmb.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Bheemanapalli LN, Kaur A, Arora R, Sangeeta Akkinepally RR, Javali NM. Synthesis, evaluation of 6,8-dibromo-2-aryl-2,3-dihydroquinolin-4(1H)-ones in MCF-7 (breast cancer) cell lines and their docking studies. Med Chem Res. 2012;21:1741–1750. [Google Scholar]
- 22.Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity:A review. J Pharma Anal. 2016;6:71–79. doi: 10.1016/j.jpha.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khodarahmi GA, Rahmani Khajouei M, Hakimelahi GH, Abedi D, Jafari E, Hassanzadeh F. Antibacterial, antifungal and cytotoxic evaluation of some new 2,3-disubstituted 4(3H)-quinazolinone derivatives. Res Pharm Sci. 2012;7:151–158. [PMC free article] [PubMed] [Google Scholar]
- 24.Van de Loosdrecht AA, Beelen RHJ, Ossenkoppele GJ, Broekhoven MG, Langenhuijsen MMAC. A tetrazolium-based colorimetric MTT assay to quantitate human monocyte mediated cytotoxicity against leukemic cells from cell lines and patients with acute myeloid leukemia. J Immun Methods. 1994;174:311–320. doi: 10.1016/0022-1759(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 25.Davis JM. Basic cell culture;a practical approach. 57-64. United states: Oxford University;IRL press; 1994. pp. 93–99. [Google Scholar]
- 26.Cho AE, Guallar V, Berne B, Friesner R. Importance of accurate charges in molecular docking:quantum mechanical/molecular mechanical (QM/MM) approach. J Comput Chem. 2005;26:915–931. doi: 10.1002/jcc.20222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, et al. Gaussian development version, revision B.01. Wallingford CT: Gaussian Inc; 2009. [Google Scholar]
- 28.Dapprich S, Komaromi I, Byun KS, Morokuma K, Frisch MJ. The calculation of energies, gradients, vibrational frequencies and electric field derivatives. J Mol Struct. 1999;462:1–21. [Google Scholar]
- 29.Farag DB, Farag NA, Esmat A, Abuelezz SA, Ibrahim EAS, Ella DAAE. Synthesis, 3D pharmacophore, QSAR and docking studies of novel quinazoline derivatives with nitric oxide release moiety as preferential COX-2 inhibitors. Med Chem Commun. 2015;6:283–299. [Google Scholar]
- 30.Ahmed B, Samad A, Hasan M. Molecular modelling studies, synthesis and antimicrobial screening of some novel sulphonamide quinazolin-4(3h)-one fused derivatives. Int J Pharm Pharm Sci. 2014;6:312–317. [Google Scholar]
- 31.Bozdag M, Alafeefy AM, Vullo D, Carta F, Dedeoglu N, Al-Tamimi AM, et al. Benzenesulfonamides incorporating bulky aromatic/heterocyclic tails with potent carbonic anhydrase inhibitory activity. Bioorg Med Chem. 2015;23:7751–7764. doi: 10.1016/j.bmc.2015.11.023. [DOI] [PubMed] [Google Scholar]
- 32.Kamble VS, Habade BM, Patil GK, Agasimundin Y. Synthesis and evaluation of 4-(1- benzofuran-2-yl)-1,3-oxazole-2-amine and its derivatives. Int J Res Pharm Chem. 2012;2:32–36. [Google Scholar]
- 33.Kouznetsov VV, Robles-Castellanos ML, Sojo F, Rojas-Ruiz1 FA, Arvelo F. Diverse C-6 substituted 4-methyl-2-(2-, 3- and 4-pyridinyl) quinolines:synthesis in vitro anticancer evaluation and in silico studies. Med Chem Res. 2017;26:551–561. [Google Scholar]
- 34.Khodarahmi GA, Asadi P, Farrokhpour H, Hassanzadeh F, Dinari M. Design of novel potential aromatase inhibitors via hybrid pharmacophore approach:docking improvement using the QM/MM method. RSC Adv. 2015;5:58055–58064. [Google Scholar]
- 35.Jiang X, Liu W, Zhang W, Jiang F, Gao Z, Zhuang H, et al. Synthesis and antimicrobial evaluation of new benzofuran derivatives. Eur J Med Chem. 2011;46:3526–3530. doi: 10.1016/j.ejmech.2011.04.053. [DOI] [PubMed] [Google Scholar]


