Abstract
The beneficial effects of fluoride on human oral health are well studied. There are numerous studies demonstrating that a small amount of fluoride delivered to the oral cavity decreases the prevalence of dental decay and results in stronger teeth and bones. However, ingestion of fluoride more than the recommended limit leads to toxicity and adverse effects. In order to update our understanding of fluoride and its potential toxicity, we have described the mechanisms of fluoride metabolism, toxic effects, and management of fluoride toxicity. The main aim of this review is to highlight the potential adverse effects of fluoride overdose and poorly understood toxicity. In addition, the related clinical significance of fluoride overdose and toxicity has been discussed.
Keywords: Fluoridation, Fluoride, Oral health, Toxicity, Water fluoridation
Introduction
Fluoride is the 13th most abundant element present in the earth’s crust. It belongs to the halogen group of elements and is found naturally in water, soil, animals, and plants (1). Fluoride is one of the most reactive and ubiquitously present in nature. It is present in trace amounts in all mineralized tissues of the body such as enamel, dentin, and bone. Fluoride is involved in a number of enzymatic reactions (2). In mineralized tissues and biomaterials, fluoride ions increase the stability of mineralized tissues and materials by decreasing the solubility of hydroxy-apatite mineral phase present in biomaterials and mineralized tissues (3). The protective effects of fluoride on dental health were first observed in 1930 as there was less tooth decay in communities consuming naturally fluoridated water compared to non-fluoridated areas (4). Due to these beneficial effects of fluoride, it was introduced into dentistry in 1940 and since then, it is being added to various consumer products. Water fluoridation is the most successfully adopted method (5-7). Fluoride delivery methods and related sources of dietary fluoride are:
Fluoridated water, beverages, and tea. Water is an important media for fluoride delivery. Fluoride exists either naturally or added during water fluoridation (8-11). Recommended optimal level of fluoride in drinking water is 0.7 mg/l; however, fluoride concentration in water varies based on geographical areas. For instance, fluoride content in drinking waters of Pakistan shows a large variation that ranges from < 0.1 ppm to >3 ppm (12). Another study demonstrated that natural water from certain geographical areas (e.g. Punjab, Pakistan) contains fluoride concentration of up to 21 ppm (13). Therefore, the data suggests a clear need for the careful selection of fluoride products to avoid toxic effects of fluoride (12).
Fluoride containing dentifrices such as tooth-paste, professionally used varnishes/gels, and mouth rinses. Fluoride tooth pastes are available as low fluoride (500 ppm), standard fluoride (1100-1500 ppm) and high fluoride toothpaste (>1500 ppm). Fluoride is added in different forms to toothpastes and mouth rinses such as sodium fluoride (NaF), mono-fluorophosphate (MFP), or stannous fluoride (SnF) (14, 15). The mouth rinses have an advantage over toothpastes because of their low viscosity that results in better delivery to least accessible areas of the teeth such as pits and fissures and interproximal areas (7, 16, 17).
Fluoridated milk including formula milk for infants and table salt fluoridation (7, 17). Fluoride delivery through milk fluoridation is not efficient as compared to other fluoride delivery methods. This is due to fluoride’s tendency to form insoluble complexes with calcium, which makes fluoride absorption difficult.
a) Fluoride releasing dental materials. Some of these biomaterials not only release fluoride but also have a property to recharge them with fluoride once fluoride from other sources is available into the oral cavity. Commonly available fluoride releasing mate-rials are glass ionomer cements (GICs), dental resin composites, compomers (modified dental composites), silicate cement, giomers, elastomeric rings and fluoride delivering mucoadhesive devices (18-21).
The suggestive mechanisms for the beneficial effects of fluoride include following mechanisms (22).
a) Fluorapatite (FA) formation on tooth surface by substitution of hydroxyl with fluoride ion in the hydroxyapatite (HA). FA decreases the solubility of HA and makes the dental enamel more resistant to dissolution from the acid that is produced by the pathogenic bacteria.
b) Inhibition of enzyme enolase, which results in a reduction in lactic acid formation.
Even though the beneficial effects of fluoride on dental health are well-established, it is very crucial to regulate the amount of fluoride intake. In addition to naturally or artificially fluoridated water, fluoride is available in a number of dental products and materials as mentioned earlier. Therefore, fluoride consumption at elevated levels may lead to a range of detrimental effects. The aim of this review is to highlight the potential adverse effects of fluoride overdose and poorly understood toxicity. In addition, the related clinical significance of fluoride overdose and toxicity has been discussed.
Fluoride absorption, metabolism, and excretion
Fluoride is consumed commonly through the oral cavity and absorbed through the gastrointestinal tract. Other less common routes of fluoride absorp-tion are inhalation and dermal absorption (23, 24). The principal sources of fluoride are fluoridated water and fluoride containing dental products.
The absorption of fluoride starts through the stomach and upper part of the small intestine (1, 25). In the stomach, the absorption of fluoride depends on the pH of the stomach while in the small intestine fluoride absorption is pH independent and absorp-tion is through facilitated diffusion (26). Fluoride absorption depends on numerous factors such as stomach pH, the chemical formula of consumed fluoride, presence of food in the stomach, interaction with other food ingredients present in gastrointestinal tract, aluminum, calcium, and magnesium compounds (23). The unabsorbed fluoride is defecated through feces while the absorbed fluoride is distributed rapidly through the circulation into the intracellular and extracellular fluids and is retained only in the mineralized tissues of the body.
The fluoride uptake by mineralized tissues is more efficient in growing children and progressively declines with age. Retention of fluoride in the mineralized tissues of the body is reversible; fluoride is released back slowly when the fluoride level in plasma falls (25, 27). Fluoride in the plasma is capable of crossing the placenta and is found in placental and fetal tissues. The placenta plays a regulatory role by the accumulation of excess fluoride, which protects the fetal tissues from excess fluoride intake (25). Absorbed fluoride is deposited from serum into mineralized tissues while the remaining is excreted primarily into the urine and to a lesser extent into feces, sweat, saliva, and breast milk. The excretion of fluoride through the urinary system depends upon several factors like plasma levels of fluoride, glomerular filtration rate (GFR), pH of the urine, and its flow (25, 28). The summary of the fluoride absorption, metabolism, and excretion is summarized in Figure 1.
Figure 1.
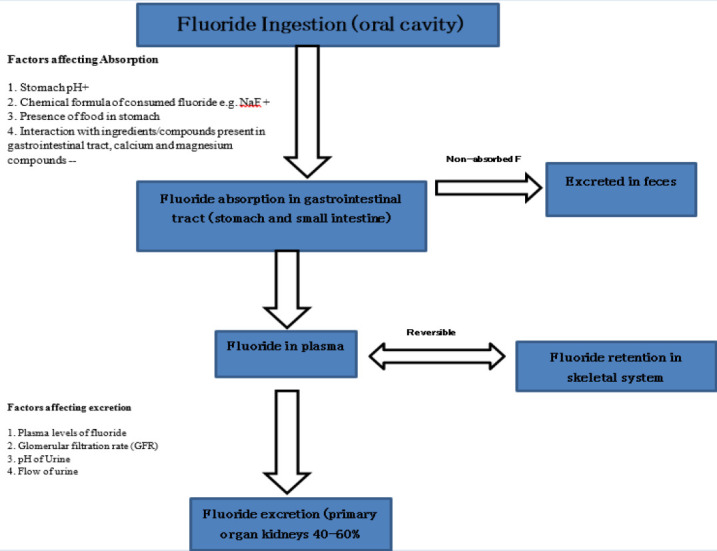
Summary of absorption, metabolism, and excretion of fluoride following oral intake
Toxic effects of fluoride
Excessive ingestion of fluoride may cause toxic and harmful effects. It is important to note that the major source of fluoride toxicity remains oral hygiene products. According to fluoride poisoning data collected by the American Association of Poison Control (AAPC), tooth paste ingestion remains the main source of toxicity followed by fluoride containing mouth washes and supplements (Table 1). The highest proportion (more than 80%) of the cases of fluoride toxicity was reported in children below the age of 6 (29).
Table 1.
Percentage of reported cases of fluoride toxicity (29)
| Cause of fluoride toxicity | Percentage of cases |
|---|---|
| Toothpaste | 68% |
| Mouth rinses | 17% |
| Fluoride supplements | 15% |
These reported toxicities are due to the fact that the swallowing reflex in children is not completely developed and fluoride toothpastes are flavored, which results in voluntary toothpaste swallowing (30). Alternatively, a variety of flavors added to toothpastes may inspire young children to ingest it. Chewing stick (miswak) is another option that is natural and there are no reports of fluoride toxicity from miswak (31). The optimum beneficial dose of fluoride and the fluoride minimal risk levels are summarized in Table 2. These given doses are based on limited data reported in the literature and even at lower than the mentioned doses there are reports of toxic and lethal effects.
Table 2.
| Important doses of fluoride | |
|---|---|
| Optimal dose of fluoride (for children & adults) | 0.05 - 0.07 mg F/kg body weight |
| Toxic dose of fluoride (for children & adults) | 5 mg F/kg body weight |
| Lethal dose of fluoride (children) | 16 mg F/kg body weight |
| Lethal dose of fluoride (adults) | 32 mg F/kg body weight |
The possible mechanisms of fluoride toxicity are (29):
(a) As the fluoride comes in contact with mois ture this results in the formation of hydrofluoric acid and this acid formation results in burning of tissues due to low pH.
(b) Inhibition of nerve impulse or nerve function is due to the fact that calcium forms chemical complexes with fluoride leading to hypocalcemia and ultimately results in inhibition of physiological nerve functioning.
(c) Cellular poisoning results due to inhibition of enzymes required for the physiological functioning of cells.
(d) Hypocalcemia and hyperkalemia result in elec-trolyte imbalance and eventually result in distur-bances in cardiac rhythm.
(e) Fluoride is one of the most reactive elements. In the case of a toxic amount of fluoride in the body, fluoride attacks oxygen and disrupt the metabolism resulting in the production of hydrogen peroxide as a product. In addition, fluoride results in excessive production of free radicles that disrupt the antioxidant formation (23).
The toxicity of fluoride due to excessive ingestion is classified into acute toxic effects and chronic effects (Figure 2).
Figure 2.
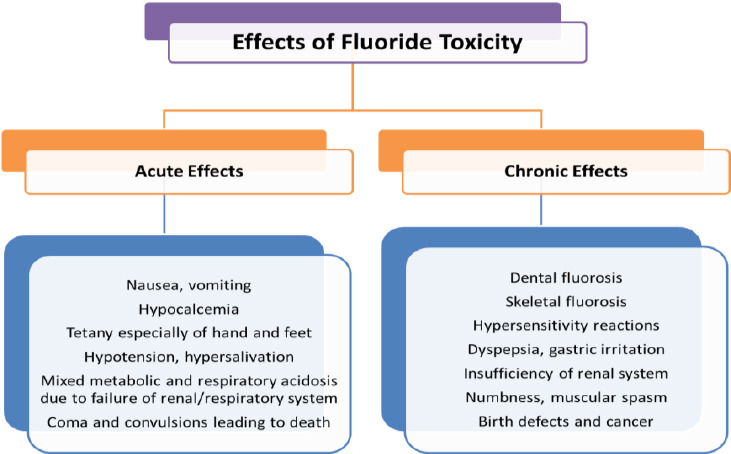
Classification of toxic effects due to excessive ingestion of fluoride
Acute toxic effects
Acute fluoride poisoning although occasionally reported, however, may be fatal. Acute fluoride toxicity usually occurs due to the accidental consumption of fluoride solution or fluoride salts wrongly perceived as sugar solution or powdered eggs (33). The symptoms of acute fluoride toxicity depend upon the type and chemical nature of the ingested compound, the age, and the elapsed time between exposure and the beginning of management (34). For instance, NaF is more toxic as it is more soluble and releases more amounts of fluoride compared to calcium fluoride (CaF) that is a less soluble compound (28). The acute toxic dose range is 5-8 mg/kg body weight. In the case of acute fluoride toxicity, one or a combination of the following symptoms such as gastric disturbances (nausea, vomiting occasionally with blood, abdominal pain, diarrhea, weakness, and hypocalcemia) are observed. These symptoms result in generalized or localized muscle tetany especially of hand and feet. In addition, hypotension, bronchospasm, fixed and dilated pupils, and hyperkalemia are also linked with fluoride toxicity, which may result in ventricular arrhythmias and cardiac arrest. Loss of body fluid contributes to an electrolyte imbalance, a state of hypovolemic shock, and decreased blood pressure. Acute fluoride poisoning may induce in some individuals a polyuria resembling diabetes insipidus, which may persist for days to months. In a few instances, the acute polyuric renal failure has terminated fatally (33). A progressive, mixed metabolic and respiratory acidosis may develop because of the failure of renal and respiratory systems, coma and convulsions terminating in death (28, 29).
Chronic toxic effects
Chronic toxicity of fluoride is more common than acute toxicity. The effects of chronic ingestion of fluoride depend not only on the duration and dose but also on several other factors such as nutritional status, renal function, and interactions with other trace elements (24, 28).
Dental fluorosis
The association between excessive ingestion of fluoride and dental mottling (fluorosis) was initially discovered over a century ago by Frederick Sumner McKay a practicing dentist in Colorado Springs area and G. V. Black (22). Dental fluorosis is the most sensitive and the earliest indicator of chronic fluoride toxicity (1). Although fluoride is an impor-tant element for caries prevention, the chronic intake of fluoride greater than 1 mg/l or 0.1 mg/kg daily during the period of tooth development interferes with the process of enamel and dentin formation and leads to dental fluorosis (1, 35, 36).
The mechanism of dental fluorosis is very complex and not fully understood. The excess amount of fluoride impedes normal enamel maturation and the dental enamel formed is hypomineralized with more surface and subsurface porosity in comparison with normal enamel. In dentine due to excessive fluoride during dentin formation, the dentinal tubules have an irregular distribution and the lumina of the tubules become narrow and disrupted (36, 37). Clinically, the appea-rance ranges from mild opaque white to brown mottling of enamel associated with pits and enamel fracture in both deciduous and permanent dentitions, and the lesions are generally symmetrical bilaterally (28, 38, 39). The severity of dental fluorosis not only depends on excessive consumption of fluoride but also on the timing and duration of excessive fluoride consumption, the plasma concentration of fluoride, type of fluoride consumed, renal function, and genetic factors (36).
Therefore, in order to prevent fluorosis the following measures should be instituted:
The fluoride level in the drinking water should be regulated between 0.5 to 1 ppm as suggested by the World Health Organization (5).
Low fluoride dentifrices (500 ppm) are indicated for children living in fluoridated areas (35).
Supervised brushing and a smear layer of low fluoride toothpaste should be applied on the brush (40).
Following these precautionary measurements, the chances of fluorosis and related lesions will be reduced.
Skeletal fluorosis
Chronic fluoride exposure at more than the recommended levels either by ingestion, inhalation, or a combination of both results in skeletal fluorosis. This condition is characterized by an increase in bone mass and density because of deposition of excess fluoride within the bone matrix (24). The primary phase of skeletal fluorosis is associated with symptoms such as sporadic pain, joints stiffness due to fluoride deposition with resultant difficulty in mobility, kyphosis of back bone, tingling sensation, muscle weakness, and fatigue. The advanced stage of skeletal fluorosis is linked with signs of arthritis and osteoporosis in long bones, spinal cord compression and calcification of ligaments with resulting neuro-logical defects and muscle wasting (41).
Radiographically, skeletal fluorosis may appear as osteosclerosis and calcification of ligaments (24, 27, 42, 43). The neurological symptoms that occur because of fluoride toxicity are due to abnormal bone outgrowths (1). Primary symptoms of skeletal fluorosis usually occur in fluoride doses greater than 4 mg/l. While the crippling skeletal fluorosis is rare and is associated with intake of water with fluoride level greater than 10 mg/l, it results in a remarkable limitation of joint movements, and deformities of major joints and spine leading to neurological problems (10, 27, 28). The severity of skeletal fluorosis depends on the amount of water intake, quality of water, renal disease, and dietary factors for instance calcium rich diet, which has a protective effect and prevents toxic effects of fluoride on bones (1, 27).
Renal effects
The kidney is the major organ that has a major role in fluoride excretion (50-60% excretion). It is the most commonly affected organ due to the uptake of fluoride within the kidney tubules (24). A prolonged exposure to concentrated fluoridated drinking water (8 ppm or higher) has been reported to increase renal diseases due to structural and functional changes in the kidney (23, 41). Structural changes due to fluoride toxicity include swelling, degeneration of tubular epithelium, fibrosis, atrophy of glomeruli, and tubular necrosis. All these structural changes result in increased serum creatinine and urea nitrogen (41).
Gastrointestinal tract (GIT)
High concentration fluoride reacts chemically with gastric acid (hydrochloric acid) in the stomach to form hydrogen fluoride. Gastric mucosa is irritated by this excessive formation of hydrofluoric acid (6). Non-ulcer dyspeptic symptoms have been observed in populations consuming high fluoride concentration (3.2 ppm) water. Animal studies reveal that fluoride has a potential to stimulate the secretion of gastric acids, diminish blood supply away from the stomach lining, and may result in the death of epithelial cells of GIT. The fluoride ingestion required to elicit such responses in humans could not be documented. For instance, adverse GIT symptoms are common in areas of endemic fluorosis where nutrition is generally poor or the individuals have gastrointestinal hypersensitivities (27).
Central nervous system
Fluoride can cross the blood brain barrier prior to birth and has been reported to affect mental development, learning disorders, and decrease intelligence and hyperactivity in children. In fetal brain, the levels of the neurotransmitters and the number of receptors are also reported to decrease in endemic fluoride areas (41). In addition, fluoride results in degenerative changes in neural tissues. These changes might account for neurological alterations (such as numbness, pain, and muscle spasm) and decreased memory and learning ability of the experimental animals (44). These neurological changes due to fluoride toxicity may be exacerbated by the deficiency of some other essential elements, for example, iodine or toxicity of other neurotoxic pollutants (41). A few studies have suggested that ingestion of dietary fluoride influences the intellectual capabilities of children. Children inges-ting high levels of fluoride (>2 mg/l) scored more poorly on intelligence tests compared with children ingesting lower amounts of fluoride (<1 mg/l). In addition, fluoride influences the reaction times and visuospatial capabilities, hence lowering the IQ scores during the time sensitive tests (27).
Fetal defects
Fluoride crosses the placental barrier and incorporates into the fetal tissues. This may lead to teratogenic effects. In addition, elevated fluoride exposures may result in disturbances in bone ossification (6). The genotoxic effects of fluoride are due to an aberration in chromosomes (41). These findings are suggestive of toxic effects of fluoride to fetal tissues hence extreme care required while prescribing to pregnant women. Any accidental ingestion of high amounts of dentifrices can lead to harmful effects on the fetus (45). The fetal brain is also susceptible to fluoride poisoning. Fluoride effects the fetal brain tissues and results in remark-able neurological damage, neuronal degeneration, and reduced secretion of neurotransmitters such as norepinephrine. In addition, fluoride disrupts the secretion of certain neurotransmitters and nerve cell receptors and results in neural dysplasia (46).
Table 3.
Summary of treatment protocol for fluoride overdose (48)
| Fluoride/kilogram body weight* | Treatment |
|---|---|
| < 5.0 mg/kg | |
| 1.Oral administration of soluble calcium (milk) to relieve GIT symptoms | |
| 2.Observe for a few hours | |
| 3.Induced vomiting not required | |
| > 5 mg/kg | |
| 1.Require hospital admission | |
| 2.Use emetic to empty the stomach. However, if the patient has depressed gag reflex for instance in the case of babies (<6 months old), Down’s syndrome, or mental retardation, endotracheal intubation should be performed before gastric lavage. | |
| 3.Oral administration of soluble calcium (e.g. milk, calcium lactate, or gluconate solution). | |
| 4. Keep under observation for a few hours. | |
| >15 mg/kg | |
| 1.Immediate hospital admission | |
| 2.Immediate stomach emptying and gastric lavage | |
| 3.Begin cardiac monitoring and be prepared for cardiac arrhythmias | |
| 4.Intravenous administration of 10% calcium gluconate solution | |
| 5.Electrolytes (calcium and potassium) should be monitored and corrected as required | |
| 6.Maintenance of adequate urine output by diuretics if required | |
| 7.General supportive measures for shock | |
Average weight/age: 1–2 years= 10 kg; 2–4 years= 15 kg; 4–6 years= 20 kg; 6–8 years= 23 kg
Miscellaneous effects
Besides the adverse effects mentioned earlier, excessive fluoride ingestion affects multiple body systems with disturbances in respiratory functions, the gastrointestinal system, liver, and excretory system, causes hematological manifestations including red blood cell deformation, neurological manifestations such as depression, abnormal sensations in toes and fingers, excessive thirst, headache, and reduction in immune response (24). Key harmful effects are summarized below:
I. Fluoride level (greater than 3 ppm) affects the reproductive system resulting in a decrease in mean birth rates (6). In animal models, the male reproductive system is more susceptible to chronic fluoride toxicity because of the production of free radicals that result in histological and structural changes in the reproductive system that disturb sperm production and sexual functions (41, 47).
II. Higher fluoride level affects thyroid function due to rise in calcitonin activity. Excess fluoride also results in decreased glucose tolerance (1, 6).
III. Chronic fluoride toxicity adversely affects both cell mediated and humoral immunity, for instance, it destroys the white cell energy reservoirs that are required for phagocytosis of foreign agents and by inhibition of antibody formation (41).
All the above-reported toxicities may also be a result of the presence of heavy metals and other substances present in fluoridation chemicals added during fluoridation of water or related dental products. Therefore, it is important to purify fluoride from heavy metals and any impurities before adding it to the drinking water or other fluoride containing products (22).
Basis of the treatment
The management of fluoride toxicity consists of: i. The fluoride toxicity case must be evaluated immediately for the type and amount of ingested fluoride. The minimum optimal dose likely to cause toxicity and requiring therapeutic intervention has been set at 5 mg/kg of body weight. Regarding the chemical type, NaF and hydrogen fluoride are more soluble, resulting in faster absorption. On the other hand, CaF and magnesium fluoride are the less soluble fluoride compounds and absorption may be relatively slow (29).
ii. Milk has a proven role in reducing the absorption of fluoride (rich in calcium which has a fluoride binding effect). Further absorption can be minimized using, calcium gluconate, calcium lactate, or milk of magnesia and aluminum, which form insoluble complexes that decrease the absorption of fluoride. Therefore, calcium containing compounds are used in acute fluoride toxicity (25,40). Gastric lavage is recommended instead of an emetic agent because of the danger of aspiration of gastric contents and burning of the esophagus due to hydrofluoric acid present in the stomach (40).
iii. Alkalization of the body fluids results in the faster removal of the ingested fluoride from the body fluids because of the faster flux of fluoride out of the cells and its elimination into the urine (25).
iv. Supporting the vital signs by oxygen therapy, artificial respiration, and hemodialysis are highly recommended. These measures should be continued until the stabilization of vital signs and serum chemistry (25).
Conclusion
The beneficial role of fluoride for the maintenance of good oral health has been known for many decades and strongly evidenced by scientific research. However, it must be emphasized that tooth decay (dental caries) is not caused by fluoride deficiency and fluoride supplementation will never reverse the active or gross carious lesions. Since the level of safety of fluoride is low, products that contain a high level of fluoride should be stored and used according to the recommend-dation and should be monitored by a qualified dental professional especially in children and pregnant women. In children, the swallowing reflex is not very well developed and the fluoride containing dental products are flavored hence increasing the possibility of a child to consume an excessive dose of fluoride. In areas with high fluoride levels in the drinking water, alternative dental products with low fluoride levels should be prescribed and monitored.
Acknowledgment
We are grateful to Prof SM Kefi Iqbal, Dean Sindh Institute of Oral Health Sciences, Jinnah Sindh Medical University, Karachi, Pakistan for his valuable suggestions and guidance for the completion of this manuscript. This work did not receive any financial support.
Conflicts of interest
The authors declared no conflicts of interest for conducting this research.
References
- 1.World Health Organization. Trace elements in human nutrition and health. Geneva: World Health Organization; 1996. [Google Scholar]
- 2.Mertz W. The essential trace elements. Science. 1981;213:1332–1338. doi: 10.1126/science.7022654. [DOI] [PubMed] [Google Scholar]
- 3.Tressaud A, Haufe G. Fluorine and health:molecular imaging, biomedical materials and pharmaceuticals. Amsterdam: Elsevier; 2008. pp. 279–331. [Google Scholar]
- 4.Harrison PT. Fluoride in water:a UK perspective. J Fluorine Chem. 2005;126:1448–1456. [Google Scholar]
- 5.Nicholson JW, Czarnecka B Fluoride in dentistry and dental restoratives. Fluoride in dentistry and dental restoratives. Fluorine and health:molecular imaging, biochemical materials and pharmaceuticals. Oxford: Elsevier; 2008. pp. 333–378. [Google Scholar]
- 6.Akyuz S, Yarat A, Alturfan EE, Kaya S. Fluoride in saliva and its impact on health. In: Preedy VR, editor. Fluorine. London: Royal Society of Chemistry; 2015. pp. 173–185. [Google Scholar]
- 7.Ullah R, Zafar MS. Oral and dental delivery of fluoride:a review. Fluoride. 2015;48:195–204. [Google Scholar]
- 8.Harrison PT. Fluoride in water:a UK perspective. J Fluorine Chem. 2005;126:1448–1456. [Google Scholar]
- 9.Spittle B. Fluoride fatigue:fluoride poisoning:is fluoride in your drinking water, and from other sources, making you sick? New Zealand: Paua Press; 2008. [Google Scholar]
- 10.Tahir MA, Rasheed H. Fluoride in the drinking water of Pakistan and the possible risk of crippling fluorosis. Drink Water Eng Sci. 2013;6:17–23. [Google Scholar]
- 11.Ramadan A, Hilmi Y. The influence of climate on the determination of the upper permissible fluoride level in potable water in Sudan. Fluoride. 2014;47:170–180. [Google Scholar]
- 12.Khan AK, Whelton H, O’Mullane D. A map of natural fluoride in drinking water in Pakistan. Int Dent J. 2002;52:291–297. doi: 10.1111/j.1875-595x.2002.tb00633.x. [DOI] [PubMed] [Google Scholar]
- 13.Farooqi A, Masuda H, Firdous N. Toxic fluoride and arsenic contaminated groundwater in the Lahore and Kasur districts, Punjab, Pakistan and possible contaminant sources. Environ Pollut. 2007;145:839–849. doi: 10.1016/j.envpol.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Davies R, Ellwood RP, Davies GW. The rational use of fluoride toothpaste. Int J Dent Hyg. 2003;1:3–8. doi: 10.1034/j.1601-5037.2003.00001.x. [DOI] [PubMed] [Google Scholar]
- 15.Bentley E, Ellwood R, Davies R. Fluoride ingestion from toothpaste by young children. Br Dent J. 1999;186:460–462. doi: 10.1038/sj.bdj.4800140. [DOI] [PubMed] [Google Scholar]
- 16.Künzel W. Systemic use of fluoride--other methods:salt, sugar, milk, etc. Caries Res. 1993;27:16–22. doi: 10.1159/000261597. [DOI] [PubMed] [Google Scholar]
- 17.Marino R. Should we use milk fluoridation? A review. Bull Pan Am Health Organ. 1995;29:287–298. [PubMed] [Google Scholar]
- 18.Zafar MS, Ahmed N. Therapeutic roles of fluoride released from restorative dental materials. Fluoride. 2015;48:184–194. [Google Scholar]
- 19.Zafar MS. Effects of surface pre-reacted glass particles on fluoride release of dental restorative materials. World Appl Sci J. 2013;28:457–462. [Google Scholar]
- 20.Khurshid Z, Zafar M, Qasim S, Shahab S, Naseem M, AbuReqaiba A. Advances in nanotechnology for restorative dentistry. Materials. 2015;8:717–731. doi: 10.3390/ma8020717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shah FA. Fluoride-containing bioactive glasses:glass design, structure, bioactivity, cellular interactions, and recent developments. Mater Sci Eng C Mater Biol Appl. 2016;58:1279–1289. doi: 10.1016/j.msec.2015.08.064. [DOI] [PubMed] [Google Scholar]
- 22.Levine M. Topics in dental biochemistry. Berlin, Germany: Springer Science & Business Media; 2010. [Google Scholar]
- 23.Yang K, Liang X. Fluoride in drinking water:effect on liver and kidney function. New York: Elsevier; 2011. pp. 769–775. [Google Scholar]
- 24.Jha SK, Mishra VK, Sharma DK, Damodaran T. Fluoride in the environment and its metabolism in humans anonymous reviews of environmental contamination and toxicology. New York: Springer; 2011. pp. 121–142. [DOI] [PubMed] [Google Scholar]
- 25.Buzalaf MA, Whitford GM. Fluoride metabolism. Monogr Oral Sci. 2011;22:20–36. doi: 10.1159/000325107. [DOI] [PubMed] [Google Scholar]
- 26.Barbier O, Arreola-Mendoza L, Del Razo LM. Molecular mechanisms of fluoride toxicity. Chem Biol Interact. 2010;188:319–333. doi: 10.1016/j.cbi.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 27.Ozsvath DL. Fluoride and environmental health:a review. Rev Environ Sci Bio Technol. 2009;8:59–79. [Google Scholar]
- 28.Ponikvar M. Exposure of humans to fluorine and its assessment. Fluorine Health. 2008;7:487–549. [Google Scholar]
- 29.Martínez-Mier EA. Fluoride:its metabolism, toxicity, and role in dental health. J Evid Based Complement Alternat Med. 2012;17:28–32. [Google Scholar]
- 30.World Health Organization. Basic methods for assessment of renal fluoride excretion in community prevention programmes for oral health. Geneva: World Health Organization; 2014. [Google Scholar]
- 31.Niazi F, Naseem M, Khurshid Z, Zafar MS, Almas K. Role of Salvadora persica chewing stick (miswak):a natural toothbrush for holistic oral health. Eur J Dent. 2016;10:301–308. doi: 10.4103/1305-7456.178297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buzalaf MA, Levy SM. Fluoride intake of children:considerations for dental caries and dental fluorosis. Monogr Oral Sci. 2011;22:1–19. doi: 10.1159/000325101. [DOI] [PubMed] [Google Scholar]
- 33.Smith FA. Fluoride toxicity. New York: Handbook of Hazardous Materials; 2012. pp. 277–283. [Google Scholar]
- 34.Whitford GM. Acute toxicity of ingested fluoride. Monogr Oral Sci. 2011;22:66–80. doi: 10.1159/000325146. [DOI] [PubMed] [Google Scholar]
- 35.Limaleite AD, Buzalaf CP, Buzalaf M. Fluoride intake in the context of dental fluorosis. Fluorine: Chemistry, Analysis, Function and Effects; 2015. pp. 22–38. [Google Scholar]
- 36.Denbesten P, Li W. Chronic fluoride toxicity. Dental fluorosis. Monogr Oral Sci. 2011;22:81–96. doi: 10.1159/000327028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kidd EA. Essentials of dental caries. Oxford;New York: Oxford University Press; 2005. [Google Scholar]
- 38.García MG, Borgnino L. Fluoride in the context of the environment. Fluorine: Chemistry, Analysis, Function and Effects; 2015. pp. 3–21. [Google Scholar]
- 39.World Health Organization. Oral health surveys:basic methods. Geneva: World Health Organization; 2013. [Google Scholar]
- 40.Cameron AC, Widmer RP. Handbook of pediatric dentistry. New York: Elsevier Health Sciences; 2013. [Google Scholar]
- 41.Ranjan R, Ranjan A. Fluoride toxicity in animals. New York: Springer; 2015. [Google Scholar]
- 42.Nabavi SF, Daglia M, Sureda A, Nabavi SM. Fluoride-induced oxidative stress in the liver. Fluorine: Chemistry, Analysis, Function and Effects; 2015. p. 271. [Google Scholar]
- 43.Craig L, Lutz A, Berry KA, Yang W. Recommendations for fluoride limits in drinking water based on estimated daily fluoride intake in the upper east region, Ghana. Sci Total Environ. 2015;532:127–137. doi: 10.1016/j.scitotenv.2015.05.126. [DOI] [PubMed] [Google Scholar]
- 44.Valdez-Jiménez L, Fregozo CS, Beltrán MM, Coronado OG, Vega MP. Effects of the fluoride on the central nervous system. Neurología. 2011;26:297–300. doi: 10.1016/j.nrl.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 45.Naseem M, Khurshid Z, Khan HA, Niazi F, Zohaib S, Zafar MS. Oral health challenges in pregnant women:Recommendations for dental care professionals. Saudi J Dental Res. 2016;7:138–146. [Google Scholar]
- 46.Yu Y, Yang W, Dong Z, Wan C, Zhang J, Liu J, et al. Neurotransmitter and receptor changes in the brains of fetuses from areas of endemic fluorosis. Fluoride. 2008;41:134–138. [Google Scholar]
- 47.Feng D, Huang H, Yang Y, Yan T, Jin Y, Cheng X, et al. Ameliorative effects of N-acetylcysteine on fluoride-induced oxidative stress and DNA damage in male rats’testis. Mutat Res Gen Toxicol Environ Mutagen. 2015;792:35–45. doi: 10.1016/j.mrgentox.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 48.Bayless JM, Tinanoff N. Diagnosis and treatment of acute fluoride toxicity. J Am Dent Assoc. 1985;110:209–211. doi: 10.14219/jada.archive.1985.0246. [DOI] [PubMed] [Google Scholar]


