Abstract
Objective(s):
The aim of the present study was to investigate the effects of genistein and exercise on the spatial memory and expression of microRNA-132, BDNF, and IGF-1 in the hippocampus of ovariectomized rats.
Materials and Methods:
Sixty animals were divided into six groups of control, sham, ovariectomy (OVX), ovariectomized with 8 weeks of genistein administration (OVX.G), with 8 weeks of swimming training (OVX.E), and with 8 weeks of both of them (OVX.G.E). The effect of genistein and/or exercise was evaluated by measuring microRNA-132, BDNF, and IGF-1 expression levels in the hippocampus tissue. Grafts were analyzed using Real-time polymerase chain reaction for microRNA-132, BDNF, IGF-1, and spatial memory via a Morris water maze (MWM).
Results:
Our findings showed that ovariectomy decreased the expression of microRNA-132, BDNF, and IGF-1 in the hippocampus (P<0.05) in comparison with the sham group as well as performance in the water maze (P<0.05). Also according to results ovariectomized groups that were treated with genistein/exercise or both of them showed significant difference in expression of microRNA-132, BDNF, and IGF-1 in the hippocampus (P<0.05) and decreased latency in MWM (P<0.05) compared with the OVX group but combination treatment was more effective in the OVX.G.E group in comparison with OVX.E and OVX.G groups.
Conclusion:
Overall our results emphasized that combination treatment with genistein and exercise could improve microRNA-132, BDNF, and IGF-1 expression in the hippocampus as well as the spatial memory of ovariectomized rats. These effects may have beneficial impacts on the menopausal period.
Keywords: Exercise, Genistein, MicroRNAs, Ovariectomy, Spatial memory
Introduction
Extensive research has indicated that cognitive disturbances are prevalent among menopausal women suggesting that low estrogen levels may have a close association with these disturbances (1). There is evidence that most of the women who have had bilateral oophorectomy before the natural age of menopause are faced with neurological diseases (2). In this regard, it has been shown that estrogen could have neuroprotective effects in experimental models of neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s (3). Moreover, in rodents, non-human primates, and humans, estrogen can improve cognitive activities (4). According to evidence, brain-derived neurotrophic factor (BDNF) has a significant impact on synaptic plasticity, neural distinction, cell death, learning, and memory (5). One could argue that estrogen has an interfering effect on BDNF and regulates the activity of the neurotrophin system (6). However, studies have revealed that the expression of BDNF in ovariectomized animals decreases; therefore, more estrogen should exist to enhance its expression. High concentration of BDNF is present in the cerebral cortex and hippo-campus (7). It is also well established that an altered expression of BDNF is related to psychiatric disor-ders, and synaptic plasticity (8). Also in the recent years, it is suggested that growth hormone (GH) and its mediator insulin-like growth factor 1 (IGF-1) could have effects on the central nervous system (CNS). In line with this evidence, it has been reported that GH and IGF-1 could improve spatial memory (9). Other studies in this context showed that lack of IGF-1 is a causative factor in the cognitive impair-ment of adults and the elderly as well as rodent models of aging (10). Numerous studies have demons-trated that the hippocampal BDNF is increased by estrogen substitution in ovariectomized rats. So, in order to change the expression of numerous neuro-protective growth factors and growth factor receptor genes, estradiol has been used. Furthermore, IGF-1 binding in rat brain and IGF-1 mRNA levels in a hippocampal cell line and primate frontal cortex can be increased by estradiol (3).
On the other hand, microRNAs are non-coding RNAs which function as post-transcriptional regulators of gene expression. It has been established that microRNA-132, one of the most expressed microRNAs in the brain, influences numerous neuronal functions, including dendritic growth and spinogenesis in cultured neurons in the brain slices, along with the learning behavior of animals. Memory acquisition of trace fear conditioning can be impaired by decreasing the level of hippocampal microRNA-132 expression (11). Studies have revealed that the expression of microRNA-132 can be caused by neurotrophins, including BDNF, which shows the enhancement mechanism of the expression of protein after neurotrophic stimulation (12). It is argued that estradiol replacement is more effective in keeping the expression of microRNAs in the hippocampus and cortex (13). Cognitive disturbances in postmenopausal women are a criticality index for hormone therapy. Moreover, in spite of the helpful influences of estrogen on the brain functions, hormone replacement therapy has augmented adverse oncological effects. Many investigations have been performed to find a natural substitute for estrogen without any side effects. One of the strongest non-pharmacological interventions is exercise, which is argued to improve cognitive functions in menopausal women (8). In fact, studies have revealed that expression of some of the neurotransmitters and neurotrophins have been changed by exercise (14). Also, positive influences of aerobic exercises on learning and memory have been observed in numerous studies (15).
Genistein as a phytoestrogen has a similar structure to 17β –estradiol and is usually applied as the main phytoestrogen agent because of its strong antioxidant features and high affinity to the receptors of estrogen (16). Nevertheless, the effect of exercise and genistein alone or their combination on the expression of microRNA-132, BDNF, and IGF in OVX animals has not been studied yet. Thus, in this study, we examined the effects of genistein and swimming exercise on spatial memory and the expression of microRNA-132, BDNF, and IGF-1 genes and histological changes in the hippocampus of OVX rats.
Materials and Methods
Animal care
In the present study, 60 female Wistar rats (weighing 180–220 g and approximately 10 weeks old) from Experimental Animal Research Center, Tabriz University of Medical Sciences, Tabriz, Iran, were used. All rats were kept under controlled conditions (temperature 22–24 °C with 12:12 hr light-dark cycle) and received standard chow diet and water ad libitum during the experiments. The study was in line with the policies of the university’s ethics committee. After 1 week of adaptation, the rats were randomly divided into six groups (n=10): 1. Control, 2. Sham (which underwent only surgery without ovariectomy), 3. OVX (bilateral ovariectomy), 4. OVX.E (OVX+exercise), 5. OVX.G (OVX+genistein administra-tion), and 6. OVX.G.E (OVX + genistein administration+ exercise). Animals in OVX, OVX.G, OVX.E, and OVX.G.E groups underwent bilateral ovariectomy. Genistein and exercise interventions were applied 10 days after OVX operation.
Ovariectomy
The animals were anesthetized (50 mg/kg ketamine and 10 mg/kg xylazine) and a minor abdominal operation was done. Then, the ovaries were situated and a silk thread was tightly tied around the oviduct, including the ovarian blood vessels. The oviduct was sectioned and the ovary was detached. The skin and muscle walls were then sutured by a silk thread (17).
Exercise training protocol
The animals were familiarized with the swimming pool (5–20 min/day) for 5 successive days; subse-quently, the exercised animals swam for 6 successive days (60 min/day) for 8 weeks. Swimming was not performed by the control group rats. All experiments were carried out 24 hr after the last exercise session. This procedure had been followed in the past and was effective in promoting cardiovascular adaptation (18).
Genistein administration protocol
Genistein (1 mg/kg/day; SC.) [(Sigma Chemical Corporation (St. Louis, MO, USA)] was injected into the animals.
Morris Water Maze
One day after the last genistein and exercise treatment, MWM test was performed for assessment of spatial memory. A black circular water pool with a diameter of 136 cm and a depth of 60 cm was filled with 22±1 °C water and placed in a room with many visual signs on the walls. The pool was conceptually divided into four equal quadrants and had four points designed as starting positions (N, S, W, or E). The behavior of rats was tracked with a camera that was located above the pool and all data was transferred to the computer. Previous to assessing animals in the MWM, rats were allowed to swim in the water for 1 min, in order to familiarize them with the task and its environment. One day after the familiarization session, rats were tested in the MWM task.
Visible platform task
Rats were submitted to a visible platform task to test vision and ability of swimming. Animals were trained to escape to a visible platform, which was transferred to a different maze quadrant on trials. Each rat was provided four trials in which a period of 60 sec was allowed to search the platform.
Hidden platform task
Rats were trained on the hidden platform task to evaluate spatial acquisition. A black escape platform with 10 cm diameter was submerged about 2 cm below the surface of the water in one quadrant area in the pool and remained in the same location for all trials. All rats received three training blocks, each consisting of four trials. Each block considered as a separate experiment session and the blocks of trials were separated by 30 min. All training was finished in one day and took place during the light cycle. In each trial, the rats were allowed to swim up to 60 sec to find the escape platform. Once on the platform, the rats remained there for 15 sec before initiation of the next trial. Rats were removed from the pool and located under a heat lamp after completion of the fourth trial of the block. Path length (the distance traveled to arrive at the platform), escape latency (the time to discover the platform), and speed of swimming were recorded and analyzed.
Probe trial
Spatial memory retention was assessed in a probe trial that was accomplished 24 hr after the last acquisition trial. After removing the platform, rats were left to swim in the pool about 60 sec. The distance traveled and time spent percentage in each quadrant was recorded (20).
Molecular analysis
RNA isolation and the cDNA synthesis
Rats were sacrificed and their hippocampus was separated at the end of 8 weeks. Total RNA including messenger RNA (mRNA) and microRNA were extracted from the hippocampus using the RNX-Plus solution kit (Fermentase, Cinagen Co. Iran) and mir-amp kit (Parsgenome Co. Iran), respectively in accordance with the manufacturer’s instructions (using chloroform layer separation followed by treatment with isopropanol and ethanol). RNA quantity and A260/280 ratio were measured using the NanoDrop 1000 (Thermo Scientific, Waltham, and Mass) and gel electrophoresis with GelRed (Biotium, Hayward, California) was used to evaluate the integrity of the samples. The IGF-1, BDNF, and miRNA-132 genes expression were quantitatively assessed by real-time polymerase chain reaction. Primers’ sequences for each gene were mentioned in Table 1. The amounts of PCR products were normalized to that for the housekeeping gene -3-phosphate dehydrogenase (GAPDH) mRNA samples and microRNA-191 for microRNA samples (internal control).
Table 1.
The primers sequences for each gene
| Genes | Accession number | Primers Sequence a |
|---|---|---|
| IGF-1 | NM- 001082477 | F: AAG CCT ACA AAG TCA GCT CG |
| R: GGT CTT GTT TCC TGC ACT TC | ||
| BDNF | NM_012513 | F: GCGGCAGATAAA AAGACT GC |
| R: GCAGCCTTCCTTCGTGTA AC | ||
| GAPDH | NM_017008.4 | F: TGCCGCCTGGAGAAACCTGC |
| R: TGAGAGCAATGCCAGCCCCA | ||
| Target sequence b | ||
| microRNA-132 | MIMAT0017123 | ACCGUGGCUUUCGAUUGUUACU |
| microRNA-191a | MIMAT0000866 | CAACGGAAUCCCAAAAGCAGCUG |
Sequences were derived from NCBI (www.ncbi.nlm.nih.gov)
b Sequences were derived from miRBase (http://www.mirbase.org)
For synthesis of cDNA in the mRNA sample, 1 μl of total RNA was reverse transcribed by means of Revert Aid M-MuLV reverse transcriptase (1 μl), DNase I (1 μl) and random hexamer primers (1 μl), dNTPS (2 μl), and RiboLock RNase-inhibitor (0.25 μl), for 10 min at 25 °C, followed by 60 min at 42 °C in a final volume of 20 μl. The reaction was terminated by heating at 70 °C for 5 min. In addition, cDNA in microRNA-RNA sample performance was synthesized according to the microRNA-amp kit (Parsgenome Co. Iran).
Real-time quantitative PCR
A master mix of 25 μl containing 12.5 μl SYBR Green PCR Master Mix (Jena Bioscience, Germany), 1 μl forward primer, 1 μl reverse primer, and 8.5 μl water was prepared to carry out real-time PCR. Two microliters of reverse transcribed cDNA were then added to the PCR master mix to achieve a final volume of 25 μl. Furthermore, to check the accuracy of amplifications, we included a negative control in each run by eliminating the cDNA sample in the tube.
The PCR protocol was used on the real-time PCR machine (Rotor-Gene 3000) in three steps including: 1- initial denaturation (10 min at 95 °C); 2- a three-step amplification program (15 sec at 95 °C followed by 30 sec at 60 °C for BDNF and microRNA-132 and IGF-1 genes and 30 sec at 58°C for Bcl-2 gene, and 30 sec at 72 °C) repeated 40 times; and step 3-melting curve analysis (1 cycle: 72 to 95 °C with temperature transition rate 1 °C/sec for 5 sec). All runs were performed in duplicates. Real-time quantification was monitored by measuring the increase in fluorescence caused by binding of the SYBR Green dye to double-stranded DNA at the end of each amplification cycle. The relative amount of mRNA for each target gene was calculated based on its threshold cycle (Ct) compared to the Ct of the housekeeping (reference) gene (GAPDH). The relative quantification was performed by the 2-ΔΔCt method.
The specificity of the PCR reactions was verified by the generation of a melting curve analysis followed by gel electrophoresis, stained with GelRed (Biotium, Hayward, California) (19).
Statistical analysis
Comparison of groups was accomplished by ANOVA followed by Tukey. All analyses were carried out using IBM SPSS version 20. In all comparisons, P≤0.05 was considered significant. Results are expressed as means±SEM.
Results
Because of no statistically significant difference among control and sham animal groups, we only discussed the sham group.
MicroRNA-132 expression
The level of expression of microRNA-132 in the hippocampus is presented in Figure 1. Our results showed that microRNA-132 expression level was significantly decreased in the hippocampus of the OVX group compared with the sham group (P<0.01). Also, in the hippocampus of both OVX.G and OVX.E groups, the microRNA-132 expression levels were significantly increased in comparison with the OVX group (P<0.05). Additionally, exercise and genistein treatment in the OVX.E.G group increased hippocampal expression of microRNA-132 approximately to the level of the sham group.
Figure 1.
Level of microRNA-132 expression in the hippocampus of the different experimental groups
OVX: ovariectomized group, OVX.E: ovariectomized with 8-weeks exercise group. OVX.G: ovariectomized group with 8-weeks genistein administration. OVX.G.E: ovariectomized with 8-weeks genistein administration and swimming training. Data are expressed as mean±SEM
* Significant difference compared with Sham (P<0.01). # Significant difference compared with sham and OVX (P<0.05). ¥ Significant difference compared with OVX (P<0.01) and with OVX.E and OVX.G (P<0.05)
BDNF expression
The level of hippocampus BDNF expression is presented in Figure 2. BDNF expression level was significantly decreased in the hippocampus of OVX in comparison with the sham group (P<0.01). At the end of the experiment, expression of BDNF up-regulated in the hippocampus of OVX.G and OVX.E animal groups significantly compared with the OVX group (P<0.05). BDNF expression level in the hippocampus of the OVX.G.E group was increased approximately to the level of the sham group.
Figure 2.
Level of BDNF expression in hippocampus of the different experimental groups
OVX: ovariectomized group, OVX.E: ovariectomized with 8-weeks exercise group. OVX.G: ovariectomized group with 8-weeks genistein administration. OVX.G.E: ovariectomized with 8-weeks genistein administration and swimming training. Data are expressed as mean±SEM
* Significant difference compared with Sham (P<0.01). # Significant difference compared with sham and OVX (P<0.05). ¥ Significant difference compared with OVX (P<0.01) and with OVX.E and OVX.G (P<0.05)
IGF-1 expression
The level of expression of hippocampus IGF-1 is presented in Figure 3. Data analysis showed that IGF-1 expression level was significantly lesser in the hippocampus of OVX in comparison with the sham group (P<0.01). Also, expression of IGF-1 in the hippocampus of the OVX.E and OVX.G groups were significantly higher in comparison with the OVX group (P<0.05). Interestingly, the IGF-1 expression level in the hippocampus of OVX.G.E group was approximately equal to that of the sham group.
Figure 3.
Level of IGF-1 expression in hippocampus of the different experimental groups
OVX: ovariectomized group, OVX.E: ovariectomized with 8-weeks exercise group. OVX.G: ovariectomized group with 8-weeks genistein administration. OVX.G.E: ovariectomized with 8-weeks genistein administration and swimming training. Data are expressed as mean±SEM
* Significant difference compared with Sham (P<0.05). # Significant difference compared with sham and OVX (P<0.05). ¥ Significant difference compared with OVX (P<0.05) and with OVX.E and OVX.G (P<0.05)
The effect of genistein and swimming on spatial memory in experimental groups
Results of our study indicated that physical exercise and injection of genistein produced and improved spatial learning in the OVX group as decreased path length (the distances (cm) to reach the hidden platform for all groups) (Figure 4A) and decreased latencies of searching for the hidden platform on acquisition trial (Figure 4B) and enhanced percent of time in the target quarter (Figure 4C) and this difference was statistically significant (P<0.05). These results were not confounded by swimming speed because there was no significant difference between the groups (Figure 4D).
Figure 4.
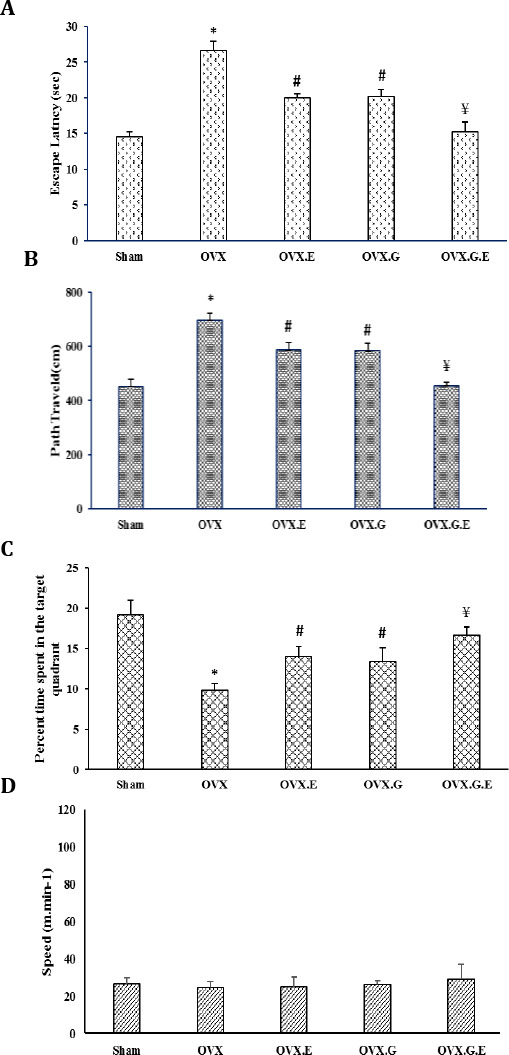
The effects of exercise and genistein on learning acquisition as measured by escape latencies (A), memory retention during the probe trial (B), the percentage of time spent in the target quadrant (C), swimming speed, and (D) observed in the MWM. Ovariectomy significantly impaired spatial learning performance in the MWM (P<0.05). Spatial learning in OVX rats was enhanced also by exercise and genistein administration in relation to the OVX animal group (P<0.05). In the OVX.G.E group spatial learning was approximately restored level of sham group. There were no significant differences between the groups in swimming speed
OVX: ovariectomized group, OVX.E: ovariectomized with 8-weeks exercise group. OVX.G: ovariectomized group with 8-weeks genistein administration. OVX.G.E: ovariectomized with 8-weeks genistein administration and swimming training. Data are expressed as mean±SEM
* Significant difference compared with Sham group (P<0.05). # Significant difference compared with all other groups (P<0.05). ¥ Significant difference compared with OVX, OVX.E, and OVX.G (P<0.05)
Discussion
The relationship between menopause and cognitive disturbance has been recognized (1). It is observed that oophorectomy presents risks of rapid cognitive decline and dementia when it is done before the natural age of menopause (2). It has been also argued that estrogen treatment would reverse this risk. However, estrogen treatment often has serious side effects and may increase the risk of breast or ovarian cancer (3, 8). A search for an alternative has been undertaken and exercising or phytoestrogens have been considered as the possible candidates (8, 16). Thus far, the specific neural mechanisms by which these alternatives may affect cognitive function in women have not been completely recognized.
In this study, we were interested in the expression patterns of microRNA-132, BDNF, and IGF-1 hippocampal genes at the early stages of ovarian hormone loss in a postmenopausal animal model. We examined the swimming training and genistein administration as a probable modality for reducing ovariectomy-induced changes in these hippocampus expressions.
Our major findings were as follows: (1) Compa-red with the sham surgery rats, ovariectomized rats downregulated the hippocampal expressions of micro-RNA-132 gene associated with anti-neurodegenera-tive effects, which may be related to the increased expressions of BDNF and IGF-1; (2). exercise or genistein administration increased the expression of microRNA-132, BDNF, and IGF-1; and (3) exercise and genistein administration further increased the expression of microRNA-132, BDNF, and IGF-1 expressions. In addition, to evaluate the effect of genistein and exercise on spatial memory, Morris water maze (MWM) test was performed. Our results suggested that genistein, as well as exercise, improved memory acquisition (escape latency time and swimming distance to reach the platform) and retention (a probe trial was performed, during which the platform was removed from the pool, and the percentage of time spent in each quadrant was calculated) in the ovariectomized rats, while in the OVX.G.E group, this improvement was even more.
By the refinement of gene expressions at the post-transcriptional level, microRNAs could regulate a variety of cell functions such as neuronal functions, developmental patterning, apoptosis, cell prolifera-tion, and metabolism (21). Investigations of expre-ssion profiles demonstrated that over 70% of experi-mentally detectable microRNAs existed in the brain. Half of them were expressed in a brain-specific or brain-enriched way, such as microRNA-9, microRNA-124, microRNA-128, microRNA-125, microRNA-7, microRNA-34, and microRNA-132 (22). Numerous actions, such as neuronal cell growth, synaptic smooth ness, swelling, and angiogenesis are associated with microRNA-132. It has been shown that the microRNA-132 expression is caused by neurotrophins, including the neurotrophic factor which is derived from the brain (BDNF) (23). BDNF is a growth factor that augments dendritic spines, improves memory function, and has a significant role in promoting proliferation, survival, and differentiation for a wide range of neuronal cell types (24). IGF-1, another growth factor structurally associated with proinsulin, is a potent survival factor for neurons and oligodendrocytes and is also involved in the growth of neurons and separation in the brain (25). Upregulation of microRNA-132 is involved in BDNF-augmented synaptic proteins (26); however, their probable expression changes are not well-known. Our results showed that ovariectomy causes the dysregulation of microRNA-132 expressions asso-ciated with anti-neurodegeneration, which is perhaps related to the decreased expressions of BDNF and IGF-1 in the hippocampus of rats. Similar findings on the expression of BDNF in the postmenopausal phase have been indicated in previous studies (14). In addition, MWM has shown that, in OVX rats, compared with sham animals, memory acquisition and retention are disturbed.
Numerous investigations have suggested that estrogen replacement treatment in postmenopausal women improves cognition, prevents the develop-ment of dementia, and reduces the severity of dementia, whereas other investigations have not found an advantage in estrogen application (27). On the other hand, the results of large-scale experimental trials have revealed that hormone substitution treatment including estrogen therapy increases concerns, including the increased risk of breast cancer in postmenopausal women (3, 8). Many attempts have been made in order to stop cognitive decline including learning and memory disturbances in postmenopausal women, among which genistein and exercise training have been suggested as natural substitutes for estrogen replacement in preventing and/or enhancing cogni-tive failure (8, 16).
According to our results exercise improved the expression of microRNA-132, and enhanced BDNF and IGF-1 expressions in the ovariectomized rats. In addition, exercise enhanced spatial memory in the aforementioned group. These findings are in line with the previous studies, showing that exercise training can affect the risk factors of cognitive failure. Moreover, this investigation indicates that exercise can raise the expressions of BDNF and IGF by increasing the expression of microRNA-132. Also, training can reduce cell death in the hippocampal cells (28). Learning can be enhanced by exercise through the mechanisms that induce BDNF gene expression. Furthermore, IGF-1 levels in both the periphery and brain can be increased by exercise. Since the peripheral administration of IGF-1 persuades BDNF mRNA in the brain, BDNF is perhaps a downstream agent that facilitates some of the protective effects of IGF-1. These results propose that peripheral IGF-1 initiates growth-factor cascades in the brain that can change the ongoing plasticity mechanisms (29). In addition, the expressions of microRNA-132, BDNF, and IGF-1 were enhanced by genistein administration in the ovariec-tomized rats and it also enhanced spatial memory. It has been shown that genistein, which has antioxidant features like estradiol, could be used as an alternative to estradiol to prevent CNS dysfunction in postmenopausal women. The addition of genistein to ovariectomized female rats’ diet augmented the rate of protein synthesis in the brain (30). In fact, in the past decade, studies have revealed the effects of genistein on microRNAs. Soy germ phytoestrogens enhance the function of spatial memory, which might be related to the rise in BDNF and the expression of synaptic formation proteins in the hippocampus of the brain in ovariectomized rats. Also, the effects of genistein and daidzein on hippocampus neuronal cell proliferation and BDNF expression have been observed. Furthermore, our observation for the first time revealed that the combination of genistein and exercise improved spatial memory and expression of microRNA-132, BDNF, and IGF-1 in a synergistic pattern in the OVX.G.E group in comparison with groups that were treated only with genistein or exercise. These findings are in agreement with the results obtained by other studies, which showed that a combination of soy isoflavones and exercise advanced defensive impacts on cognitive failure in ovariectomized rats (31). More investigation is needed to clarify the underlining cellular and molecular mechanisms of these effects.
Conclusion
Our results were novel as they provided the first direct evidence that exercise and/or genistein administration may predominately employ the actions of microRNA-132, BDNF, and IGF-1 to enhance cognitive function. Furthermore, given the extensive involvement of these genes in some disorders of cognitive function such as learning and memory, the findings suggest exercise and genistein as the highly accessible form of intervention that could be used by menopausal women.
Acknowledgment
This study was financially supported by Drug Applied Research Center of Tabriz University of Medical Sciences, Tabriz, Iran.
References
- 1.Henderson VW. Cognitive changes after menopause:influence of estrogen. Clin Obstet Gynecol. 2008;51:618–626. doi: 10.1097/GRF.0b013e318180ba10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shuster LT, Rhodes DJ, Gostout BS, Grossardt BR, Rocca WA. Premature menopause or early menopause:long-term health consequences. Maturitas. 2010;65:161–166. doi: 10.1016/j.maturitas.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lovekamp-Swan T, Glendenning ML, Schreihofer DA. A high soy diet enhances neurotropin receptor and Bcl-X L gene expression in the brains of ovariectomized female rats. Brain Res. 2007;1159:54–66. doi: 10.1016/j.brainres.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gasbarri A, Tomaz C. Estrogen influences on cognition:INTECH Open Access Publisher. 2012 [Google Scholar]
- 5.Park H, Poo MM. Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci. 2013;14:7–23. doi: 10.1038/nrn3379. [DOI] [PubMed] [Google Scholar]
- 6.Sohrabji F, Lewis DK. Estrogen–BDNF interactions:implications for neurodegenerative diseases. Front Neuroendocrinol. 2006;27:404–414. doi: 10.1016/j.yfrne.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scharfman HE, Mercurio TC, Goodman JH, Wilson MA, MacLusky NJ. Hippocampal excitability increases during the estrous cycle in the rat:a potential role for brain-derived neurotrophic factor. J Neurosci. 2003;23:11641–11652. doi: 10.1523/JNEUROSCI.23-37-11641.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saadati H, Sheibani V, Esmaeili-Mahani S, Darvishzadeh-Mahani F, Mazhari S. Prior regular exercise reverses the decreased effects of sleep deprivation on brain-derived neurotrophic factor levels in the hippocampus of ovariectomized female rats. Regul Pept. 2014;194:11–15. doi: 10.1016/j.regpep.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Grönbladh A, Johansson J, Nöstl A, Nyberg F, Hallberg M. GH improves spatial memory and reverses certain anabolic androgenic steroid-induced effects in intact rats. J Endocrinol. 2013;216:31–41. doi: 10.1530/JOE-12-0315. [DOI] [PubMed] [Google Scholar]
- 10.Deak F, Sonntag WE. Aging, synaptic dysfunction, and insulin-like growth factor (IGF)-1. J Gerontol A Biol Sci Med Sci. 2012;67:611–625. doi: 10.1093/gerona/gls118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang RY, Phang RZ, Hsu PH, Wang WH, Huang HT, Liu IY. In vivo knockdown of hippocampal miR-132 expression impairs memory acquisition of trace fear conditioning. Hippocampus. 2013;23:625–633. doi: 10.1002/hipo.22123. [DOI] [PubMed] [Google Scholar]
- 12.Yi LT, Li J, Liu B-B, Luo L, Liu Q, Geng D. BDNF–ERK–CREB signalling mediates the role of miR-132 in the regulation of the effects of oleanolic acid in male mice. J Psychiatry Neurosci. 2014;39:348. doi: 10.1503/jpn.130169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klinge CM. Estrogen regulation of microRNA expression. Curr Genomics. 2009;10:169–183. doi: 10.2174/138920209788185289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu J, Xu Y, Hu W, Gao Y, Ni X, Sheng H, et al. Exercise ameliorates depression-like behavior and increases hippocampal BDNF level in ovariectomized rats. Neurosci Lett. 2014;573:13–18. doi: 10.1016/j.neulet.2014.04.053. [DOI] [PubMed] [Google Scholar]
- 15.Cetinkaya C, Sisman AR, Kiray M, Camsari UM, Gencoglu C, Baykara B, et al. Positive effects of aerobic exercise on learning and memory functioning, which correlate with hippocampal IGF-1 increase in adolescent rats. Neurosci Lett. 2013;549:177–181. doi: 10.1016/j.neulet.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 16.Peng Y, Jiang B, Wu H, Dai R, Tan L. Effects of genistein on neuronal apoptosis, and expression of Bcl-2 and Bax proteins in the hippocampus of ovariectomized rats. Neural Regen Res. 2012;7:2874–2881. doi: 10.3969/j.issn.1673-5374.2012.36.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Habibi P, Alihemmatti A, Alipour M, Nourazar A, Yousefi H, Andalib S, et al. Effects of exercise on mir-29 and igf-1 expression and lipid profile in the heart of ovariectomized rat. ACTA ENDOCRINOLOGICA-BUCHAREST. 2016;12:130–136. doi: 10.4183/aeb.2016.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DA Silva ND, Jr, Fernandes T, Soci U, Monteiro A, Phillips MI, DE Oliveira EM. Swimming training in rats increases cardiac MicroRNA-126 expression and angiogenesis. Med Sci Sports Exerc. 2012;44:1453–1462. doi: 10.1249/MSS.0b013e31824e8a36. [DOI] [PubMed] [Google Scholar]
- 19.Habibi P, Alihemmati A, Nasirzadeh M, Yousefi H, Habibi M, Ahmadiasl N. Involvement of microRNA-133 and-29 in cardiac disturbances in diabetic ovariectomized rats. Iran J Basic Med Sci. 2016;19:1177–1185. [PMC free article] [PubMed] [Google Scholar]
- 20.Hashemi Nosrat Abadi T, Vaghef L, Babri S, Mahmood-Alilo M, Beirami M. Effects of different exercise protocols on ethanol-induced spatial memory impairment in adult male rats. Alcohol. 2013;47:309–316. doi: 10.1016/j.alcohol.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 21.M Ardekani A, Moslemi Naeini M. The role of microRNAs in human diseases. Avicenna J Med Biotechnol. 2011;2:161–180. [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, Lin L, Jin P. The microRNA pathway and fragile X mental retardation protein. Biochim Biophys Acta. 2008;1779:702–705. doi: 10.1016/j.bbagrm.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y, Kannan G, Pletnikov MV, Yolken RH, Xiao J. Chronic infection of Toxoplasma gondii downregulates miR-132 expression in multiple brain regions in a sex-dependent manner. Parasitology. 2015;142:623–632. doi: 10.1017/S003118201400167X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murray PS, Holmes PV. An overview of brain-derived neurotrophic factor and implications for excitotoxic vulnerability in the hippocampus. Int J Pept. 2011;2011:12. doi: 10.1155/2011/654085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MOROVIĆ S, Demarin V. Role of physical activity on human brain functions. Period Biol. 2014;116:219–221. [Google Scholar]
- 26.Numakawa T, Yamamoto N, Chiba S, Richards M, Ooshima Y, Kishi S, et al. Growth factors stimulate expression of neuronal and glial miR-132. Neurosci Lett. 2011;505:242–247. doi: 10.1016/j.neulet.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 27.Yaffe K, Sawaya G, Lieberburg I, Grady D. Estrogen therapy in postmenopausal women:effects on cognitive function and dementia. JAMA. 1998;279:688–695. doi: 10.1001/jama.279.9.688. [DOI] [PubMed] [Google Scholar]
- 28.Kim TW, Kim CS, Kim JY, Kim CJ, Seo JH. Combined exercise ameliorates ovariectomy-induced cognitive impairment by enhancing cell proliferation and suppressing apoptosis. Menopause. 2016;23:18–26. doi: 10.1097/GME.0000000000000486. [DOI] [PubMed] [Google Scholar]
- 29.Cotman CW, Berchtold NC. Exercise:a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- 30.Lee J, Cho HS, Kim DY, Cho JY, Chung JS, Lee HK, et al. Combined effects of exercise and soy isoflavone diet on paraoxonase, nitric oxide and aortic apoptosis in ovariectomized rats. Appetite. 2012;58:462–469. doi: 10.1016/j.appet.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 31.Riesco E, Aubertin-Leheudre M, Maltais ML, Audet M, Dionne IJ. Synergic effect of phytoestrogens and exercise training on cardiovascular risk profile in exercise-responder postmenopausal women:a pilot study. Menopause. 2010;17:1035–1039. doi: 10.1097/gme.0b013e3181da7915. [DOI] [PubMed] [Google Scholar]