Abstract
Objective(s):
Stroke is known as a main cause of mortality and prolonged disability in adults. Both transient receptor potential V1 (TRPV1) channels and toll-like receptors (TLRs) are involved in mediating the inflammatory responses. In the present study, the effects of TRPV1 receptor activation and blockade on stroke outcome and gene expression of TLR2 and TLR4 were assessed following permanent middle cerebral artery occlusion in rats
Materials and Methods:
Eighty male Wistar rats were divided into four groups as follows: sham, vehicle, AMG9810 (TRPV1 antagonist) -treated and capsaicin (TRPV1 agonist) -treated. For Stroke induction, the middle cerebral artery was permanently occluded and then behavioral functions were evaluated 1, 3 and 7 days after stroke.
Results:
TRPV1 antagonism significantly reduced the infarct volume compared to the stroke group. Also, neurological deficits were decreased by AMG9810 seven days after cerebral ischemia. In the ledged beam-walking test, the slip ratio was enhanced following ischemia. AMG9810 decreased this index in stroke animals. However, capsaicin improved the ratio 3 and 7 days after cerebral ischemia. Compared to the sham group, the mRNA expression of TLR2 and TLR4 was significantly increased in the stroke rats. AMG9810 Administration significantly reduced the mRNA expression of TLR2 and TLR4. However, capsaicin did not significantly affect the gene expression of TLR2 and TLR4.
Conclusion:
Our results demonstrated that TRPV1 antagonism by AMG9810 attenuates behavioral function and mRNA expression of TLR2 and TLR4. Thus, it might be useful to shed light on future therapeutic strategies for the treatment of ischemic stroke.
Keywords: Cerebral ischemia, Inflammation, TLR4, TLR2, TRPV1
Introduction
Ischemic stroke is the most common cause of disability in adults and the second leading cause of mortality in the world (1, 2). It is accompanied by vigorous inflammatory responses (3), oxidative damage (4), glutamate-mediated excitotoxicity (5), initiation of apoptosis (6), brain edema (7), and destruction of brain blood barrier (BBB) (8). Previous studies have shown that inflammation and excitotoxicity play pivotal roles in the pathophysiology of stroke (9, 10). Inflammatory response is a major cause of secondary neuronal cell death following stroke (11). It has been documented that innate immunity plays a critical role in the patho-genesis of inflammatory-based disorders including stroke (12).
Transient receptor potential vanilloid-1 (TRPV1) belongs to TRP (transient receptor potential) family of proteins is a non-selective cation channel with preference for calcium ions. These channels have been validated as a pain target by noxious stimuli, low pH, and capsaicin (a pungent component of hot chili peppers). TRPV1 has been shown to play a pivotal role in the induction of the inflammatory responses (13). Compared to the peripheral nervous system, the function of TRPV1 channels in the central nervous system (CNS) is not well understood. These participate in pain perception, regulation of body temperature, control of locomotion, bladder function (14), and anxiety (15). TRPV1 is expressed in various brain regions including striatum, cerebral cortex, hippo-campus, thalamus, cerebellum, hypothalamus, locus coeruleus, periaqueductal gray, amygdala, and the olfactory bulb (16).
Toll-like receptors (TLRs) are well known as a group of inflammatory immune cell recognition transmembrane receptors (17). The innate immune receptors recognize pathogen- and damage-associated molecular patterns (PAMPs and DAMPs, respectively) (18, 19). There is considerable evidence showing that TLR signaling, as an important arm of inflammation, participates in neural injury in the peripheral and central nervous systems (20, 21). Also, TLRs are involved in the pathogenesis of several immune-related diseases including stroke (17).
Among TLRs, TLR2 and TLR4 significantly mediate various physiological brain functions as well as pathological conditions such as inflammation (22, 23). Activation of TLR2 and TLR4 in microglia has been shown to contribute to the stroke-induced neuronal damage via inducing the production of pro-inflammatory cytokines and excitotoxins (23, 24). TLR2 and TLR4 signaling may also contribute to neuronal death following stroke because expression level of these receptors increases in cerebrocortical neurons in response to ischemia/reperfusion injury (25). TLR4 is the first identified mammalian TLR that activates many signaling proteins and results in transcription of genes encoding inflammation-associated molecules and cytokines (26). Furthermore, the stroke-induced brain damage and neurological deficits have been reported to be reduced in TLR2/TLR4 deficient mice (25). There is evidence showing the existence of a functional interaction between TLRs and TRPV1. The most widely studied endocannabinoid, N-arachidonoyl ethanolamine (AEA), which is an endogenous ligand for TRPV1, has been shown to modulate neuroimmune responses, including those induced following TLR activation. Its activity at different targets may account for the variability in the effects of AEA on the neuroinflammatory responses following TLR active-tion. In addition, following TLR4 activation, sensitiza-tion of TRPV1 and potentiation of TRPV1-dependent neuropeptide production (27, 28) was reported. Furthermore, it has been reported that TLR ligands up-regulate the expression of TRPV1 and enhance calcium (Ca2+) flux mediated by TRPV1 (29). On the other hand, it appears that innate immune receptors such as TLRs and TRPV1 play a role in the pathogenesis of stroke.
Therefore, we aimed to investigate the effect of interaction between TRPV1 and TLR2 or TLR4 on the outcome of stroke in a rat model of permanent middle cerebral artery occlusion (pMCAO) by measuring the infarct volume, brain edema, neurological deficits, motor function, and the expression of TLR2 and TLR4 genes.
Materials and Methods
Animals and experiments
Adult male Wistar rats (200–250 g) were housed in Plexiglas cages (10 rats per cage) with the temperature at (23±2 °C) and a 12 hr light/dark cycle. Except during the experiments, food and water were provided ad libitum. Animals were handled considering criteria outlined in the Guide for Care and Use of Laboratory Animals (National Institutes of Health publication, 7th edition) (30). The method of this study was approved by the Animal Ethics Committee of Rafsanjan University of Medical Sciences (Code of Ethics 9/3042).
Two sets of experiments were done in this study. In the first part, rats were randomly assigned to four experimental groups as follows: (1) sham, (2) vehicle (stroke), (3) AMG9810 (selective TRPV1 antagonist, 0.5 mg/kg), and (4) capsaicin (TRPV1 agonist, 1 mg/kg). Each experimental group included ten rats. The drugs were purchased from Tocris Bioscience (Bristol, UK) and dissolved in sterile 0.9% saline, dimethyl sulfoxide (DMSO), and Tween 80 (80/10/10 %, v/v). Vehicle or drug solutions were administrated intraperitoneally (IP) 3 hr after induction. These animals were used for assessment of the infarct volume, brain edema, and behavioral functions. In the second part of the study, forty rats were randomly divided into four equal groups as aforementioned and used for the evaluation of TLR2 and TLR4 mRNA expression through real-time PCR analysis. The doses of capsaicin and AMG9810 were selected based on the previous investigations (31, 32).
MCAO induction
Rats were anesthetized by intraperitoneal injection of ketamine (60 mg/kg) and xylazine (8 mg/kg). Induction of MCAO was performed by occluding the left MCA (middle cerebral artery) as previously reported (33). In brief, a small incision was made between the left ear and the left eye. The temporalis muscle was gently retracted and the skull was exposed. A small hole, about 1 mm in diameter, was drilled in the temporal bone above the MCA at the level of the inferior cerebral vein. The dura was removed and the MCA was carefully cauterized permanently using a thermo coagulator. After the procedure, the muscle was replaced and the skin was sutured. At the end, animals were returned to individual cages. The surgeon was blinded to the treatment assignments.
Measurement of the infarct volume and brain edema
One week after the MCA occlusion, all animals were sacrificed. The brains were removed and the forebrains were sliced into six coronal 2-mm-thick sections using a brain matrix. Slices were then stained with 2% 2,3,5-triphenyltetrazolium chloride (TTC; Sigma chemical Co., St. Louis, MO, USA) at 37 °C for 30 min followed by overnight immersion in 10% formalin. The infarcted tissue remained unstained (white), whereas the normal tissue was stained red. The infarct area of each brain slice was quantified using image analysis software Image J (NIH Image, version 1.61, Bethesda, Maryland, USA). The infarct volumes were calculated by multiplying each area by the slice thickness. In order to compensate the effect of brain edema, the corrected infarct volume was calculated as follows: corrected infarct area= measured infarct area ×{1- [(ipsilateral hemisphere area - contralateral hemisphere area)/contralateral hemisphere]} (34). Brain edema was determined using the following formula: edema= (volume of the left hemisphere - volume of the right hemisphere)/volume of the right hemisphere. The brain edema was reported as a percentage (35) and the examiner was blinded to the treatment assignments.
Evaluation of neurological deficits
Neurological deficits were recorded 1, 3, and 7 days after stroke using a modified 5-point scoring system (36) as follows: 0, no observable deficit; 1, forelimb flexion; 2, forelimb flexion plus decreased resistance to lateral push; 3, unidirectional circling; 4, unidirectional circling plus decreased level of consciousness; and 5, death.
Motor function evaluation
A ledged beam-walking test was performed to evaluate motor function (37). The ledged beam-walking apparatus consists of a beam connected to a platform on one end. The beam was wide at the starting point, taper gradually to a narrow end near the goal, which made the task more sensitive. A black box opening toward the beam was installed on the platform. Rats were pre-trained for three days to traverse the beam and were tested 24 hr before surgery as well as 1, 3, and 7 days after ischemia. The performance was videotaped and later analyzed by calculating the slip ratio of the impaired hind limb (number of slips/number of total steps).
RNA extraction and quantitative real-time PCR
Seven days after the induction of stroke, mice were sacrificed by decapitation and the penumbra area was micro dissected under aseptic conditions and immediately stored at −80 °C until use. Total RNA was extracted using the TRIzol reagent (Cinnaclon Co., Iran) according to the manufacturer’s guidelines. RNA was quantitated by measuring absorption at 260/280 nm via a UV spectrophoto-meter. 260/280 ratio of >1.8 was considered an acceptable measure of RNA purity, and checked by electrophoresis on an ethidium bromide pretreated agarose gel. Five micrograms of RNA were converted to cDNA using a cDNA synthesis kit (Parstous Co., Tehran, Iran) with both oligo (dT) and random hexamer primers.
Real-time PCR (polymerase chain reaction) was performed using 10 µl of SYBR green master mix (Bionner, Korea) and 200 ng of template cDNA with the appropriate primers in a final volume of 20 µl by a Bio-Rad CFX96 system (Bio-Rad Company, Foster City, USA). Primers were synthesized by the Bioneer Company (South Korea). The sequences of the used primers are shown in Table 1. On the other hand, β-actin housekeeping gene was used for normalization of amplification signals of target genes. By the 2^-ΔCt formula, the relative amounts of PCR products were determined. The melting curves, dissociation stages and quantitative analysis of the data were performed using the CFX manager software version 1.1.308.111 (Bio-Rad, Foster City, USA).
Table 1.
Gene-specific primers for amplification of rat TLR2, TLR4, and β-actin mRNAs by real-time PCR
| Gene | Sequence |
|---|---|
| TLR2 | Forward: 5′-CTGATGGAGGTGGAGTTTGA -3′ |
| Reverse: 5′-TCCGTATTGTTACCGTTTCTA -3′ | |
| TLR4 | Forward: 5′- GAATTGTATCGCCTTCTTAG -3′ |
| Reverse: 5′-TGTGAGGTCGTTGAGGTTAG -3′ | |
| β-actin | Forward: 5′- ATGGTGGGTATGGGTCAGAAGG-3′ |
| Reverse: 5′- TGGCTGGGGTGTTGAAGGTC -3′ |
Statistical analysis
Data were analyzed using one-way ANOVA and expressed as mean±SEM Individual differences were determined using Tukey’s test. Neurological deficits are reported as medians and interquartile ranges (25th and 75th percentiles) and were analyzed using the Kruskal–Wallis test. A value of P<0.05 was considered to be significant.
Results
Brain infarction and edema
The infarct volume and brain edema percentage are shown in Figures 1 and 2. Administration of the TRPV1 receptor agonist, capsaicin, (1 mg/kg IP) at 3 hr after stroke, significantly increased the infarct volume compared to the stroke group (P<0.05; Figure 1). Administration of the TRPV1 receptor antagonist, AMG9810, (0.5 mg/kg IP) at 3 hr after stroke, significantly reduced the infarct volume compared to the stroke group (P<0.01; Figure 1). No significant difference was observed in brain edema among the stroke-, capsaicin-, and AMG9810-treated groups (Figure 2).
Figure 1.
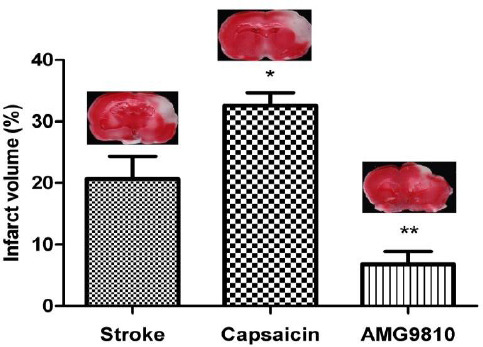
The effects of capsaicin and AMG9810 on infarct volume. Infarct volumes were measured at one week after the MCA occlusion in TTC-stained brain sections and expressed as a percentage of affected ipsilateral hemisphere volumes. Data expressed as mean±SEM * P<0.05 and ** P<0.01 vs. the stroke group. n = 10 in each group
Figure 2.
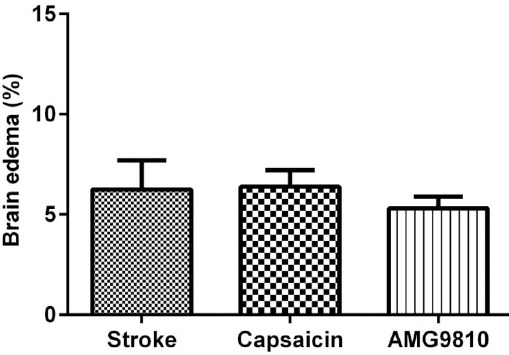
The effects of capsaicin and AMG9810 on brain edema. Brain edema volumes were assessed by comparison of the groups as a percentage of affected ipsilateral hemisphere volumes. Data expressed as mean±SEM, n = 10 in each group
Neurological deficits
A five-point ranking test was performed by a blinded observer to evaluate neurological deficits 1, 3, and 7 days after MCAO induction (Table 2). Compared to the stroke group, AMG9810 decreased neurological deficits 7 days after cerebral ischemia (P<0.01).
Table 2.
Neurological deficits of the stroke-, capsaicin-, and AMG9810-treated groups at 1, 3, and 7 days following the onset of stroke
| Groups | Stroke | Capsaicin | AMG9810 |
|---|---|---|---|
| Day 1 | 2(2-3) | 2(1-2) | 1(1-2.5) |
| Day 3 | 2(2-3) | 2(2-3) | 2(1-2) |
| Day 7 | 3(2-3) | 3(2-3) | 1(1-2)** |
Neurological deficits were measured by a five-point scale at 1, 3, and 7 days following the onset of stroke. Data are presented as median±25th and 75th percentiles (percentiles in the parentheses). Non-parametric Kruskal-Wallis test showed a significant difference between stroke and AMG9810-treated groups (**P<0.01)
P<0.01 compared to the stroke group at the same time
Motor function evaluation
The motor function was tested using the ledged beam-walking test. Stroke rats showed a significant increase in the slip ratio compared to the sham group at 1, 3, and 7 days after the stroke (P<0.05). Stroke rats treated with AMG9810 showed a significant decrease in slip ratio with the impaired hind limb compared to the stroke group. However, capsaicin increased this index 3 and 7 days after cerebral ischemia (P<0.05). The results are presented in Figure 3.
Figure 3.
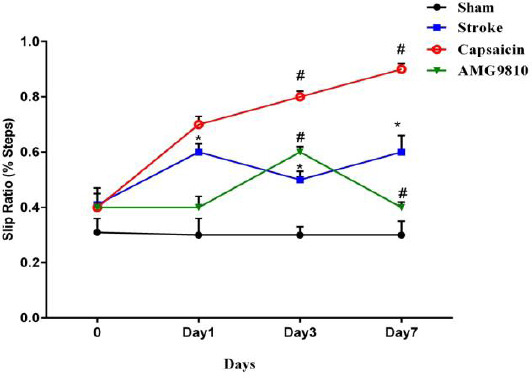
Slip ratio for hind limbs of the stroke-, capsaicin-, and AMG9810-treated groups before (0) and at 1, 3, and 7 days following the onset of stroke. Significant differences in the slip ratio percentage between the stroke and sham groups 1, 3, and 7 days after stroke (*P< 0.05 vs. sham group and # P< 0.05 vs. stroke group). Data are expressed as mean±SEM. n = 10 in each group
The mRNA levels of TLR2 and TLR4 following induction of stroke
The gene expressions of TLR2 and TLR4 in different groups are presented in Figure 4. Seven days after the induction of stroke, the mRNA levels of TLR2 increased in the stroke group compared to the sham group (13.29-fold, P<0.05). Administration of AMG9810 decreased the gene expression of TLR2 compared to the stroke group (0.41-fold, P<0.05). Capsaicin had no significant effect on gene expression of TLR2 (Figure 4A). The mRNA levels of TLR4 increased in the stroke group compared to the sham group (87.45-fold, P<0.001). Administration of AMG9810 decreased the gene expression of TLR4 compared to the stroke group (3.06-fold, P<0.001) while capsaicin had no significant effect on gene expression of TLR4 (Figure 4B).
Figure 4.
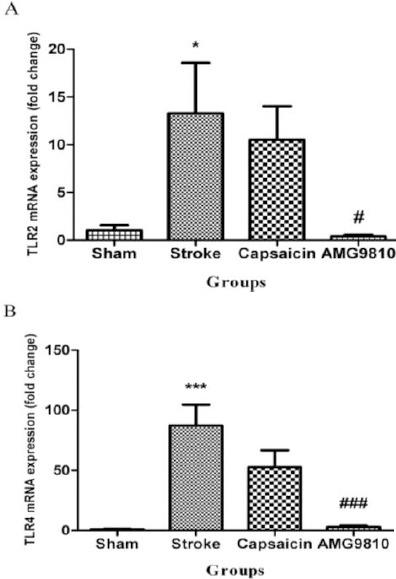
The TLR2 and TLR4 mRNA expression in different experimental groups. (A) The effect of capsaicin and AMG9810 on TLR2 expression. *P< 0.05 vs. sham group; # P< 0.05 vs. stroke group. (B) The effect of capsaicin and AMG9810 on TLR4 expression. ***P< 0.001 vs. sham group; ### P< 0.001 vs. stroke group. Data expressed as mean±SEM. n = 10 in each group
Discussion
In this study, the main aim was to find possible relationships among TRPV1, TLR2, and TLR4 in an animal model of stroke induced by permanent middle cerebral artery occlusion. For this purpose, we investigated the effects of both TRPV1 agonist and antagonist on infarct volume, brain edema, neurological deficits, motor function, and the expression of TLR2 and TLR4 genes in the penumbra area following stroke in rats. The obtained results demonstrated that administration of AMG9810, as a TRPV1 antagonist, significantly reduces the infarct volume. In addition, capsaicin, as a TRPV1 agonist, increased the infarct volume. No significant difference was observed in brain edema among the stroke-, capsaicin-, and AMG9810-treated groups. Furthermore, TLR2 and TLR4 mRNA expression in the area around the infarction was increased in ischemic rats. Also, our results showed that AMG9810 decreases neurological deficits after the induction of permanent MCAO. In the present study, we employed the ledged beam-walking test to evaluate motor integration and found that stroke leads to motor impairment as the slip ratio increased following MCAO. AMG9810 improved this impair-ment in rats that underwent a stroke. Previous studies have also reported similar results for the effect of ischemia on the ledged beam walking test (37).
As mentioned, a significant difference was observ-ed in TLR2 and TLR4 mRNA expressions in the area around the infarction compared to the sham group. This implies that TLR2 and TLR4 are involved in stroke. Also, stroke triggers the activation of TLRs which in turn leads to the production of cytokines, cellular adhesion molecules, chemokines, and the expression of surface antigens that result in the activation of a nervous system immune cascade. Therefore, TLRs also invoke inflammation in the nervous system (38). It has been demonstrated that the expressions of TLR2 and TLR4 are increased throughout the mouse brain following cerebral ischemia (23, 39). Tang et al. reported that MCAO increases the mRNA levels of TLR4 and also the relevant inflammatory cytokines such as TNF-α (25). All these findings show that TLRs especially types 2 and 4 have crucial roles in the pathophysiology of stroke. Thus, the inhibition of these receptors or related signaling pathways might be a potential strategy to decrease the inflammatory response following stroke and also to overcome some stroke-associated neurological deficits. Consistently, it has been revealed that TLR2 and TLR4 gene deficiency attenuates brain damage induced by cerebral ischemia in mice (23, 39). Similar results have also been reported by Hamanaka and Hara (40). In addition, administration of TAK-242 as a TLR4 antagonist has been shown to reduce brain infarction and neurological abnormalities after transient middle cerebral artery occlusion (41).
In this study, neither capsaicin nor AMG9810 changed brain edema. However, in agreement with the present finding, some studies showed that the neuroprotective effects during brain ischemia may be presented without a significant change in the brain edema (42-44).
However, some contradictory results have been reported. For example, TLR2 knockout mice have been reported to exhibit higher mortality and increased brain infarct size (45). The same authors have used knockout mice to show differential roles for TLR2 and TLR4 in cerebral ischemia. Their study showed that the sizes of the brain infarct region were significantly less in TLR4 knockout mice compared to TLR2 knockout ones. In addition, Stevens et al. (2011) have reported that the preconditioned stimulation of TLR4 by lipopolysaccharide leads to protection against ischemia in MCAO (46).
TRPV1 has an important role in the induction of inflammation (13). For example, TPRV1 is involved in the development of pain and hyperalgesia (47). Miyanohara et al. have shown that neurological and motor deficits, as well as the infarct volume, were reduced in TRPV1 knock-out mice compared to those of wild-type mice after cerebral ischemia. In addition, they showed that capsazepine, as a TRPV1 antagonist, exerts protective effects on infarct volume and neurological deficits (48). In the present study, the mRNA expression of TLR2 and TLR4 in the area around the infarction was significantly elevated in ischemic rats. However, AMG9810, as a TRPV1 antagonist, significantly suppressed this elevation. Qi and colleagues (2011) have revealed that TLR ligands could up-regulate the expression of TRPV1 (29).
The interaction between TLRs and TRPV1 has also been suggested by some studies. Li et al. showed that the chemotherapeutic drug paclitaxel, induced pain that was mediated by sensitizing transient receptor potential vanilloid subtype 1 (TRPV1) through Toll-like receptor 4 (TLR4) signaling. They showed that administration of AMG9810, as a TRPV1 antagonist, reversed paclitaxel-induced pain (49). Concomitant down-regulation of TRPV1 and TLR4 in samples obtained from patients with chronic periodontitis is another finding that implies the existence of an interaction (50). It is a possibility that TRPV1 antagonists not only change TLRs expression but they may interfere with TLRs signaling. In accor-dance, preincubation with the TRPV1 antagonist capsazepine or TRPV1 gene silencing with siRNA abolished or markedly attenuated the TLR agonist induced increase in the phosphorylation of trans-forming growth factor kinase1 and mitogen-activated protein kinases and release rates of interleukin 6 and 8 (51). Moreover, similar to TRPV1 ligands, TLR ligands can change the expression of TRPV1 in cultures of primary mouse DRGNs (29). Similarly, LPS is capable of directly activating trigeminal neurons, and sensitizing TRPV1 via a TLR4-mediated mechanism (27). Of note, the present real-time PCR results do not reflect a change in TLR protein expression. Due to some technical restrictions, we did not do Western blotting to observe TLR protein expression and this is a major limitation of the present study.
Future studies should be directed to human models in order to develop immunotherapy against stroke complications.
Conclusion
Results of this study demonstrated an increase in expression levels of TLR2 and TLR4 genes following stroke induction. Based on the present results and previous studies, TRPV1 has an important role in the pathophysiology of stroke and might be considered as a potential therapeutic target in future investigations.
Acknowledgment
The results presented in this paper were part of a student thesis. This study was financially supported by Research Vice Chancellor of Rafsanjan University of Medical Sciences, Rafsanjan, Iran (grant no 9/20/794).
Conflicts of interest
The authors declare no conflicts of interest related to this study.
References
- 1.Wang XH, You YP. Epigallocatechin gallate extends therapeutic window of recombinant tissue plasminogen activator treatment for brain ischemic stroke:a randomized double-blind and placebo-controlled trial. Clin Neuropharmacol. 2017;40:24–28. doi: 10.1097/WNF.0000000000000197. [DOI] [PubMed] [Google Scholar]
- 2.Aidar FJ, de Oliveira RJ, de Matos DG, Mazini Filho ML, Moreira OC, de Oliveira CE, et al. A Randomized trial investigating the influence of strength training on quality of life in ischemic stroke. Top Stroke Rehabil. 2016;23:84–89. doi: 10.1080/10749357.2015.1110307. [DOI] [PubMed] [Google Scholar]
- 3.Doyle KP, Simon RP, Stenzel-Poore MP. Mechanisms of ischemic brain damage. Neuropharmacology. 2008;55:310–318. doi: 10.1016/j.neuropharm.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crack PJ, Taylor JM. Reactive oxygen species and the modulation of stroke. Free Radic Biol Med. 2005;38:1433–1444. doi: 10.1016/j.freeradbiomed.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 5.Wang Z, Wei X, Liu K, Zhang X, Yang F, Zhang H, et al. NOX2 deficiency ameliorates cerebral injury through reduction of complexin II-mediated glutamate excitotoxicity in experimental stroke. Free Radic Biol Med. 2013;65:942–951. doi: 10.1016/j.freeradbiomed.2013.08.166. [DOI] [PubMed] [Google Scholar]
- 6.Ramos-Cabrer P, Campos F, Sobrino T, Castillo J. Targeting the ischemic penumbra. Stroke. 2011;42:S7–11. doi: 10.1161/STROKEAHA.110.596684. [DOI] [PubMed] [Google Scholar]
- 7.Darabi S, Mohammadi MT. Fullerenol nanoparticles decrease ischaemia-induced brain injury and oedema through inhibition of oxidative damage and aquaporin-1 expression in ischaemic stroke. Brain Inj. 2017:1–9. doi: 10.1080/02699052.2017.1300835. [DOI] [PubMed] [Google Scholar]
- 8.Zhu M, Xing D, Lu Z, Fan Y, Hou W, Dong H, et al. DDR1 may play a key role in destruction of the blood-brain barrier after cerebral ischemia-reperfusion. Neurosci Res. 2015;96:14–19. doi: 10.1016/j.neures.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Leker RR, Shohami E. Cerebral ischemia and trauma-different etiologies yet similar mechanisms:neuroprotective opportunities. Brain Res Brain Res Rev. 2002;39:55–73. doi: 10.1016/s0165-0173(02)00157-1. [DOI] [PubMed] [Google Scholar]
- 10.del Zoppo G, Ginis I, Hallenbeck JM, Iadecola C, Wang X, Feuerstein GZ. Inflammation and stroke:putative role for cytokines, adhesion molecules and iNOS in brain response to ischemia. Brain Pathol. 2000;10:95–112. doi: 10.1111/j.1750-3639.2000.tb00247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tureyen K, Kapadia R, Bowen KK, Satriotomo I, Liang J, Feinstein DL, et al. Peroxisome proliferator-activated receptor-gamma agonists induce neuroprotection following transient focal ischemia in normotensive, normoglycemic as well as hypertensive and type-2 diabetic rodents. J Neurochem. 2007;101:41–56. doi: 10.1111/j.1471-4159.2006.04376.x. [DOI] [PubMed] [Google Scholar]
- 12.Shichita T, Ago T, Kamouchi M, Kitazono T, Yoshimura A, Ooboshi H. Novel therapeutic strategies targeting innate immune responses and early inflammation after stroke. J Neurochem. 2012;123:29–38. doi: 10.1111/j.1471-4159.2012.07941.x. [DOI] [PubMed] [Google Scholar]
- 13.Zielinska M, Jarmuz A, Wasilewski A, Salaga M, Fichna J. Role of transient receptor potential channels in intestinal inflammation and visceral pain:novel targets in inflammatory bowel diseases. Inflamm Bowel Dis. 2015;21:419–427. doi: 10.1097/MIB.0000000000000234. [DOI] [PubMed] [Google Scholar]
- 14.Moran MM, McAlexander MA, Biro T, Szallasi A. Transient receptor potential channels as therapeutic targets. Nat Rev Drug Discov. 2011;10:601–620. doi: 10.1038/nrd3456. [DOI] [PubMed] [Google Scholar]
- 15.Rubino T, Realini N, Castiglioni C, Guidali C, Vigano D, Marras E, et al. Role in anxiety behavior of the endocannabinoid system in the prefrontal cortex. Cereb Cortex. 2008;18:1292–1301. doi: 10.1093/cercor/bhm161. [DOI] [PubMed] [Google Scholar]
- 16.Kauer JA, Gibson HE. Hot flash:TRPV channels in the brain. Trends Neurosci. 2009;32:215–224. doi: 10.1016/j.tins.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Okun E, Griffioen KJ, Mattson MP. Toll-like receptor signaling in neural plasticity and disease. Trends Neurosci. 2011;34:269–281. doi: 10.1016/j.tins.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Zare-Bidaki M, Tsukiyama-Kohara K, Arababadi MK. Toll-like receptor 4 and hepatitis B infection:molecular mechanisms and pathogenesis. Viral Immunol. 2014;27:321–326. doi: 10.1089/vim.2014.0039. [DOI] [PubMed] [Google Scholar]
- 20.Downes CE, Crack PJ. Neural injury following stroke:are Toll-like receptors the link between the immune system and the CNS? Br J Pharmacol. 2010;160:1872–1888. doi: 10.1111/j.1476-5381.2010.00864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lehnardt S. Innate immunity and neuroinflammation in the CNS:the role of microglia in Toll-like receptor-mediated neuronal injury. Glia. 2010;58:253–263. doi: 10.1002/glia.20928. [DOI] [PubMed] [Google Scholar]
- 22.Abbadie C. Chemokines, chemokine receptors and pain. Trends Immunol. 2005;26:529–534. doi: 10.1016/j.it.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Lehnardt S, Lehmann S, Kaul D, Tschimmel K, Hoffmann O, Cho S, et al. Toll-like receptor 2 mediates CNS injury in focal cerebral ischemia. J Neuroimmunol. 2007;190:28–33. doi: 10.1016/j.jneuroim.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 24.Caso JR, Pradillo JM, Hurtado O, Lorenzo P, Moro MA, Lizasoain I. Toll-like receptor 4 is involved in brain damage and inflammation after experimental stroke. Circulation. 2007;115:1599–1608. doi: 10.1161/CIRCULATIONAHA.106.603431. [DOI] [PubMed] [Google Scholar]
- 25.Tang SC, Arumugam TV, Xu X, Cheng A, Mughal MR, Jo DG, et al. Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proc Natl Acad Sci U S A. 2007;104:13798–13803. doi: 10.1073/pnas.0702553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42:145–151. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 27.Diogenes A, Ferraz CC, Akopian AN, Henry MA, Hargreaves KM. LPS sensitizes TRPV1 via activation of TLR4 in trigeminal sensory neurons. J Dent Res. 2011;90:759–764. doi: 10.1177/0022034511400225. [DOI] [PubMed] [Google Scholar]
- 28.Ferraz CC, Henry MA, Hargreaves KM, Diogenes A. Lipopolysaccharide from Porphyromonas gingivalis sensitizes capsaicin-sensitive nociceptors. J Endod. 2011;37:45–48. doi: 10.1016/j.joen.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qi J, Buzas K, Fan H, Cohen JI, Wang K, Mont E, et al. Painful pathways induced by TLR stimulation of dorsal root ganglion neurons. J Immunol. 2011;186:6417–6426. doi: 10.4049/jimmunol.1001241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gonder JC, Laber K. A renewed look at laboratory rodent housing and management. ILAR J. 2007;48:29–36. doi: 10.1093/ilar.48.1.29. [DOI] [PubMed] [Google Scholar]
- 31.Khatibi NH, Jadhav V, Charles S, Chiu J, Buchholz J, Tang J, et al. Capsaicin pre-treatment provides neurovascular protection against neonatal hypoxic-ischemic brain injury in rats. Acta Neurochir Suppl. 2011;111:225–230. doi: 10.1007/978-3-7091-0693-8_38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fu J, Bottegoni G, Sasso O, Bertorelli R, Rocchia W, Masetti M, et al. A catalytically silent FAAH-1 variant drives anandamide transport in neurons. Nat Neurosci. 2012;15:64–69. doi: 10.1038/nn.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allahtavakoli M, Amin F, Esmaeeli-Nadimi A, Shamsizadeh A, Kazemi-Arababadi M, Kennedy D. Ascorbic acid reduces the adverse effects of delayed administration of tissue plasminogen activator in a rat stroke model. Basic Clin Pharmacol Toxicol. 2015;117:335–339. doi: 10.1111/bcpt.12413. [DOI] [PubMed] [Google Scholar]
- 34.Xing B, Chen H, Zhang M, Zhao D, Jiang R, Liu X, et al. Ischemic postconditioning inhibits apoptosis after focal cerebral ischemia/reperfusion injury in the rat. Stroke. 2008;39:2362–2369. doi: 10.1161/STROKEAHA.107.507939. [DOI] [PubMed] [Google Scholar]
- 35.Esmaeeli-Nadimi A, Kennedy D, Allahtavakoli M. Opening the window:Ischemic postconditioning reduces the hyperemic response of delayed tissue plasminogen activator and extends its therapeutic time window in an embolic stroke model. Eur J Pharmacol. 2015;764:55–62. doi: 10.1016/j.ejphar.2015.06.043. [DOI] [PubMed] [Google Scholar]
- 36.Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion:evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- 37.Allahtavakoli M, Jarrott B. Sigma-1 receptor ligand PRE-084 reduced infarct volume, neurological deficits, pro-inflammatory cytokines and enhanced anti-inflammatory cytokines after embolic stroke in rats. Brain Res Bull. 2011;85:219–224. doi: 10.1016/j.brainresbull.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 38.Wang H, Zhou M, Brand J, Huang L. Inflammation and taste disorders:mechanisms in taste buds. Ann N Y Acad Sci. 2009;1170:596–603. doi: 10.1111/j.1749-6632.2009.04480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hua F, Ha T, Ma J, Li Y, Kelley J, Gao X, et al. Protection against myocardial ischemia/reperfusion injury in TLR4-deficient mice is mediated through a phosphoinositide 3-kinase-dependent mechanism. J Immunol. 2007;178:7317–7324. doi: 10.4049/jimmunol.178.11.7317. [DOI] [PubMed] [Google Scholar]
- 40.Hamanaka J, Hara H. Involvement of Toll-like receptors in ischemia-induced neuronal damage. Cent Nerv Syst Agents Med Chem. 2011;11:107–113. doi: 10.2174/187152411796011312. [DOI] [PubMed] [Google Scholar]
- 41.Hua F, Tang H, Wang J, Prunty MC, Hua X, Sayeed I, et al. TAK-242, an antagonist for Toll-like receptor 4, protects against acute cerebral ischemia/reperfusion injury in mice. J Cereb Blood Flow Metab. 2015;35:536–542. doi: 10.1038/jcbfm.2014.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brady JD, Mohr C, Rossi DJ. Vesicular GABA release delays the onset of the Purkinje cell terminal depolarization without affecting tissue swelling in cerebellar slices during simulated ischemia. Neuroscience. 2010;168:108–117. doi: 10.1016/j.neuroscience.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chiba Y, Sasayama T, Miyake S, Koyama J, Kondoh T, Hosoda K, et al. Anti-VEGF receptor antagonist (VGA1155) reduces infarction in rat permanent focal brain ischemia. Kobe J Med Sci. 2008;54:E136–146. [PubMed] [Google Scholar]
- 44.Gu Y, Xi G, Liu W, Keep RF, Hua Y. Estrogen reduces iron-mediated brain edema and neuronal death. Acta Neurochir Suppl. 2010;106:159–162. doi: 10.1007/978-3-211-98811-4_29. [DOI] [PubMed] [Google Scholar]
- 45.Hua F, Ma J, Ha T, Kelley JL, Kao RL, Schweitzer JB, et al. Differential roles of TLR2 and TLR4 in acute focal cerebral ischemia/reperfusion injury in mice. Brain Res. 2009;1262:100–108. doi: 10.1016/j.brainres.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stevens SL, Ciesielski TM, Marsh BJ, Yang T, Homen DS, Boule JL, et al. Toll-like receptor 9:a new target of ischemic preconditioning in the brain. J Cereb Blood Flow Metab. 2008;28:1040–1047. doi: 10.1038/sj.jcbfm.9600606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002;36:57–68. doi: 10.1016/s0896-6273(02)00908-x. [DOI] [PubMed] [Google Scholar]
- 48.Miyanohara J, Shirakawa H, Sanpei K, Nakagawa T, Kaneko S. A pathophysiological role of TRPV1 in ischemic injury after transient focal cerebral ischemia in mice. Biochem Biophys Res Commun. 2015;467:478–483. doi: 10.1016/j.bbrc.2015.10.027. [DOI] [PubMed] [Google Scholar]
- 49.Li Y, Adamek P, Zhang H, Tatsui CE, Rhines LD, Mrozkova P, et al. The Cancer chemotherapeutic paclitaxel increases human and rodent sensory neuron responses to TRPV1 by activation of TLR4. J Neurosci. 2015;35:13487–13500. doi: 10.1523/JNEUROSCI.1956-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ozturk A, Yildiz L. Expression of transient receptor potential vanilloid receptor 1 and toll-like receptor 4 in aggressive periodontitis and in chronic periodontitis. J Periodontal Res. 2011;46:475–482. doi: 10.1111/j.1600-0765.2011.01363.x. [DOI] [PubMed] [Google Scholar]
- 51.Wang Z, Yang H, Wolosin JM, Reinach PS. In human corneal epithelial cells TLR2/4 innate immune responses are fully dependent on TRPV1 activity mediated through both MyD88-dependent and independent signaling pathways. Invest Ophthalmol Visual Sci. 2012;53:1832–1832. [Google Scholar]


