Abstract
Objective(s):
This study was aimed at investigating immune activations of the 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis model in colonic mucosa by immunohistochemical and Western blot methods.
Materials and Methods:
For this purpose, 16 female Wistar albino rats were divided into two random groups of control (n=8) and colitis (n=8). The experimental colitis model was induced by intracolonic administration of TNBS (25 mg/rat). Control animals received only rectal saline for the same time. The animals were sacrificed on the 15th day after TNBS administration, and colon tissue was removed and examined morphologically. Colon samples were stained immunohistochemically with anti-CD3, anti-CD4, anti-CD5, anti-CD8, anti-CD11b, anti-CD45, anti-TNF-α, anti-IL-17, anti-IL-22 and anti-IL-23 antibodies. Additionally, the colonic tissue IL-17 and IL-22 expressions were examined by the Western blot method.
Results:
In the experimental results, it was determined that there was a significant decrease in body weight and an increase in colon weight in the colitis group when comparing initial experiments. The colon tissue ulcerations, inflammation, crypt loss and Goblet cell loss were observed in the colitis group in microscopic examinations. The immunohistochemical positive cell numbers significantly increased in the colitis group. The immunoreactive lymphocytes in the propria, intracryptal and submucosal layers were found to be increased in the colitis group of rats. In addition, IL-17 and IL-23 expressions were increased in colitis colon mucosa found by Western blot analysis.
Conclusion:
The Th17/IL-23 pathway and IL-22 serve important roles in the pathogenesis of ulcerative colitis, and will be further examined by study.
Keywords: Immunohistochemistry; Rat; 2,4,6-trinitrobenzene sulfonic acid; Ulcerative colitis; Western blot
Introduction
Inflammatory bowel diseases (IBD), which are a group of irritable disorder in the gastrointestinal system, include ulcerative colitis (UC), Crohn’s disease (CD) and indeterminate colitis (IC), and are characterized by inflammation of the gut (1). Several etiological factors, such as immunologic, genetic and environmental factors, and their interactions, have been linked with the pathophysiology of IBD (2). Immune dysfunctions and excessive immune activations in immune system cells play critical roles in the pathogenesis of UC (3). Intestinal mucosa has developed complex immune populations that comprise adaptive and innate immune system cells. The innate system consists of cells such as macro-phages, neutrophils, mast cells and natural killer (NK) cells. The adaptive immune system consists of T cells and B lymphocytes (4). On the cell surfaces of
lymphocytes and other immune systems, cells that have critical roles in inflammation have molecules known as “Cluster of Differentiation (CD)” that play an important role in cell signaling, adhesion and various immunological functions (5, 6). Significant changes of the expression of CD molecules may occur in chronic inflammation caused by UC (7). Chronic inflammation also leads to changes in expression of inflammatory cytokines, such as tumor necrosis factor alpha (TNF-α), interferon gamma (IFN-γ) and interleukine 1- beta (IL-1β) (8).
Naive CD4+ T cells, which express CD4 membrane glycoprotein, differentiate into four distinct subsets under the antigenic stimulation, characterized by different cytokine production profiles, including T-helper (Th)1, Th2, Th17 and T-regulator (Treg) (9, 10). Th1 cells produce IFN-γ and they are important for immune response to intracellular pathogens, while Th2 cells produce IL-4, IL-5 and IL-13 for targeting parasites and worms (4, 10). The Th17 cells are different from Th1 and Th2; they secrete mainly IL-17 and other cytokines, including IL- 17-related cytokines, IL-21 and IL-22 (11, 12). IL-17 stimulates fibroblast, macrophages and epithelial cells to produce proinflammatory mediators, such as TNF-α, IL-1, and IL-6 in the inflammatory process (9, 13). Elevated IL-17 and IL-17-related cytokines were shown in intestinal mucosa in IBD patients (11, 14).
IL-22 is a member of the IL-10 family of anti-inflammatory cytokines; it plays a proinflammatory role by inducing the expression of proinflammatory cytokines and matrix metalloproteinase (15, 16). Also, IL-22 promotes Goblet cell hyperplasia, the release of antimicrobial RegIII and mucus production in intestinal mucosa (8, 17). An elevated IL-22 cytokine level is found in intestinal mucosa and serum in active IBD patients (8, 18).
IL-23 is a member of the IL-12 family of cytokines and is a heterodimeric protein composed of IL-12p40/IL-23p19 complex; it has a major proinflammatory role in IBD pathogenesis (19, 20). IL-23 promotes Th17 cell differentiation and controls Th17 cell response (8, 9). Experimental models have shown that IL-23 is required for the development of IBD pathogenesis (20, 21).
Trinitrobenzene sulfonic acid (TNBS)-induced colitis has been frequently used in the experimental colitis model because of its clinical and pathological features similar to human UC. The distinctive feature of our study is the analysis of the relation between Th17/IL-23-related cytokine expression in TNBS-induced colitis model with the activation of both innate and adaptive immune system cells.
Materials and Methods
Animals
Sixteen female Wistar albino rats, weighing 210-250 g, were obtained from the Experimental Animal Research Facility at Trakya University (Edirne, Turkey). The animals were kept in pathogen-free cages and optimum laboratory conditions (temperature: 20±2 °C; humidity: 50%; light/dark: 12/12 hour). The animals were fed a standard laboratory diet and fresh tap water, ad libitum. All experimental procedures were approved by the Local Ethical Committee for Animal Studies of Trakya University (Edirne, Turkey). (Protocol no: TÜHDYEK-2013/65, Date: 27.12.2013).
Experimental design and induction of colitis
Sixteen female rats were divided into two groups, as control (n=8) and colitis (n=8). The control group received saline solution, and the colitis group was administered TNBS dissolved in 50% ethanol solution, under sedation. All rats were weighed and housed in four rats per cage during the experiment. The rats were starved overnight before the induction of colitis.
To create an experimental rat model of colitis, 25 mg/rat TNBS (dissolved in 50% ethanol, total 1 ml) was used. All experimental procedures performed on the rats were completed using ketamine (90 mg/kg) for analgesia and xylazine (10 mg/kg) for sedation. TNBS was administered into the colon with a rubber catheter in a Trendelenburg position (for 45 second). To protect excessive exposure of TNBS, the colons were washed with saline solution for 5 min. The control group of rats received 1 ml saline solution in the same way (22).
Macroscopic and microscopic evaluations
The Macroscopic damage score was evaluated by two independent histologists, as described by McCafferty et al (23) (Table 1). To assess the microscopic damage, distal colon tissues were fixed in a neutral buffered formalin solution for 48 hr and then embedded in paraffin by routine histological methods. Five µm sections were serially cut (Leica, RM-2245) and stained by hematoxylin-eosin (H&E). An Olympus BX51 (Tokyo, Japan) light microscope was used by two independent histologists to assess histopathological changes in the colon. Histological damage scoring was performed using the criteria described by Obermeier et al (24) (Table 2).
Table 1.
Scale for macroscopic damage score (23)
| Characteristics | Score |
|---|---|
| Ulceration | |
| Normal appearance | 0 |
| Focal hyperaemia, no ulcers | 1 |
| Ulceration without hyperaemia or bowel wall thickening | 2 |
| Ulceration with inflammation at one site | 3 |
| Two or more sites with ulceration and inflammation | 4 |
| Adhesions | |
| No adhesions | 0 |
| Minor adhesions (colon can be easily separated from other tissue) | 1 |
| Major adhesions | 2 |
| Diarrhea | |
| No | 0 |
| Yes | 1 |
| Thickness | |
| Maximal bowel wall thickness (x), in millimeters | X |
| Total score | |
Table 2.
Scale for microscopic damage score (24)
| Characteristics | Score |
|---|---|
| Epithelium (E) | |
| Normal morphology | 0 |
| Loss of goblet cells | 1 |
| Loss of goblet cells in large area | 2 |
| Loss of crypts | 3 |
| Loss of crypts in large areas | 4 |
| Infiltration (I) | |
| No infiltrate | 0 |
| Infiltrate around crypt basis | 1 |
| Infiltrate reaching to L. muscularis mucosa | 2 |
| Extensive infiltration reaching to L. muscularis mucosa and thickening of the mucosa with abundant | 3 |
| Edema | |
| Infiltration of the L. submucosa | 4 |
| Total microscopic score represents the sum of the E + I score | Total score |
Immunohistochemical analysis
Anti-CD3 (Bioss bs-0765R), anti-CD4 (Abcam, ab11815), anti-CD5 (Bioss, bs1113R), anti-CD8 (Abbiotec, P0731), anti-CD11b (Abcam, ab75476), anti-CD45 (Abbiotec, ab08575), anti-TNF-α (Abcam, ab199013), anti-IL-17 (Abcam, ab79056), anti-IL-22 (Abcam, ab106773) and anti-IL-23 (Abcam, ab115759) were used as primary antibodies. Incubation time and dilutions of primary antibodies were optimized according to the manufacturers’ instructions. Positive (+) stained cells were calculated for each animal of the groups and expressed as mm2 with the help of Kameram image analysis software (Kameram 2.1; Argenit, Istanbul, Turkey).
Western blot analysis
Colonic tissue samples were added per well and separated by NuPAGE 4-12% Bis-tris Gel (Novex) for 2 hr at 80 V and then blotted on polyvinlydene fluoride (PVDF) membrane (Novex, 2 Transfer Stack, PVDF, Mini) by the iBlot semi-dry transfer system (Invitrogen). Membranes were blocked in 5% non-fat milk in phosphate buffered saline containing 0.01% Tween 20 (TBST) for one hour and then incubated overnight at +4 °C with anti-beta actin (Bioss, bs-0061R, 1:100), anti-IL-17 (Abcam, ab79056, 1:100) and anti- IL-22 (Bioss, bs-2623R, 1:100). Protein bands were detected using an enhanced chemilu-minescence kit (WesternBreeze kit, Life Techno-logies), quantified by ChemiDoc MP System with the Image Lab software (Bio-Rad) and expressed as the relative intensity of target protein to that B-actin control.
Statistical analysis
Data were analyzed using the SPSS 12.0 statistical software package for Windows (SPSS Inc., Chicago, IL, USA). The results were reported as the mean ± standard deviation. Differences among groups were analyzed using the nonparametric Mann-Whitney U-test. P<0.05 was considered statistically significant.
Table 3.
Distribution of positive-stained cell number in groups
| Antibodies | Positive cell number/mm2 | |
|---|---|---|
| Control | Colitis | |
| Anti-TNF-α | 10.1±3.5 | 35.8±8.6* |
| Anti-CD3 | 4.5±1.50 | 8.3±1.8* |
| Anti-CD4 | 3.5±1.8 | 6.6±1.6* |
| Anti-CD5 | 3.2±1.3 | 5.6±1.5* |
| Anti-CD45 | 18.3±3.9 | 40.6±5.3* |
| Anti-CD8 | 2.4±0.9 | 4.6±1.3* |
| Anti-CD11b | 4.1±1.2 | 9.9±1.9* |
| Anti-IL-17 | 4.2±1.5 | 6.0±1.8* |
| Anti-IL-22 | 4.7±1.5 | 9.1±2.0* |
| Anti-IL-23 | 3.3±1.2 | 6.6±1.5* |
The results are expressed as the mean ± standard deviation
P<0.05 compared to control groups
Results
The colitis group of animals showed a significant body weight loss compared to the control group (P<0.05). Additionally, when the colon weight (8 cm) ratio was examined, the colitis group exhibited increased colonic weight compared to the control group (P<0.05, Figure 1f).
Figure 1.
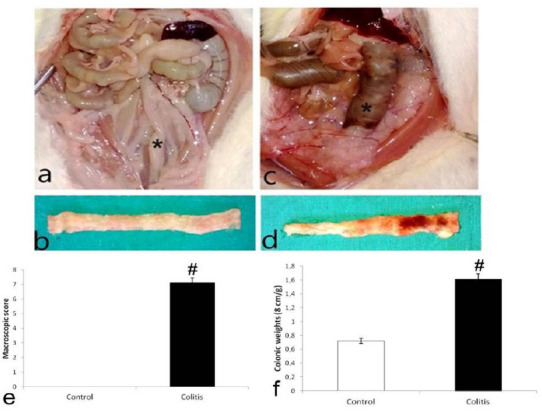
Macroscopic appearance of intraabdominal cavity and colon tissues. Control (a and b); Colitis (c and d) showing hyperemic and ulcerative colon; macroscopic damage score (e); colonic weights of groups (f). (* = colon, #P<0.05 compared with control tissue)
Macroscopic and microscopic evaluations
Macroscopic examination of the colon in the colitis group was observed as normal (Figure 1a-b). Some pathological findings such as mucosal ulcerations, edema and hemorrhage were observed in the colon tissue of the colitis group (Figure 1c-d). Also, the colitis group had a significantly higher rate of macroscopic damage score compared to the control group (P<0.05, Figure 1e).
In microscopic examination, H&E-stained colon tissue preparations were used. Colon tissue showed a normal histological structure in the control group (Figure 2a). Mucosal erosions and ulcerations, disruption of intestinal glands, Goblet cell loss, intense infiltration of mucosa, and histopathological findings of submucosa were observed in colitis tissue (Figure 2b). The colitis group had a significantly higher microscopic damage score when compared to the control group (P<0.05, Figure 2c).
Figure 2.
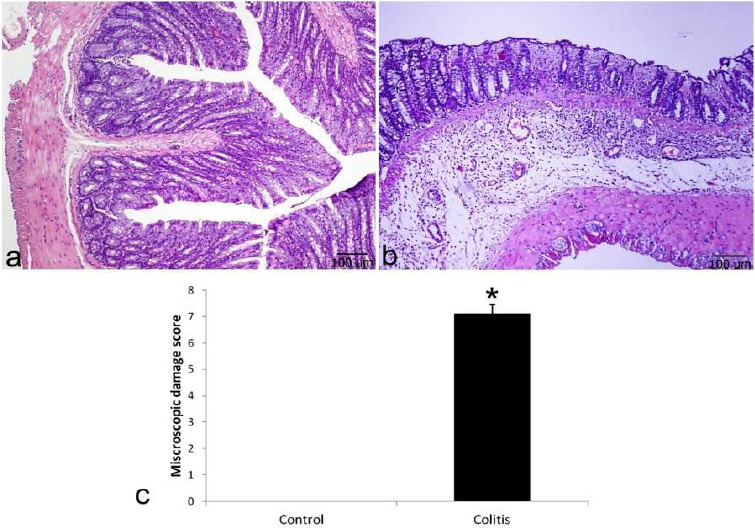
Histological evaluation of colon tissue stained with Hematoxylin and Eosin. Control (a) showing normal histological architecture; Colitis (b) showing mucosal ulcerations, eodema, mucosal and submocosal infiltrations, Goblet cell loss and disrupted cyrpts; Microscopic damage score (c). *P<0.05 compared with control tissue
Immunohistochemical evaluations
Immunohistochemical evaluation of TNF-α expre-ssion in the control group was limited in the subepithelial region of mucosa (Figure 3a). However, in the colitis group, TNF-α- positive cells were filled in the lamina propria of the mucosa (Figure 3b). The colitis group had a significantly higher rate of TNF-α- positive cells compared to the control group (P<0.05, Figure 3c).
Figure 3.

Anti-TNF-α immunohistochemical staining of colon tissue. Control (a) limited expression of TNF-α in lamina epithelialis; Colitis (b) enhanced expression in mucosa and submucosa (Immunoperoxidase, Mayer’s Hematoxylin counter stain, arrows; positive-stained cells)
CD3, CD4, CD8 and CD45-positive stained T lymphocytes were mainly located in the lamina propria of the mucosa, between the crypts of Lieberkuhn; rarely, they were intraepithelial in the control group. However, a significantly higher T lymphocyte accumulation was observed in the colitis group as compared to the control group (P<0.05, Figure 4).
Figure 4.
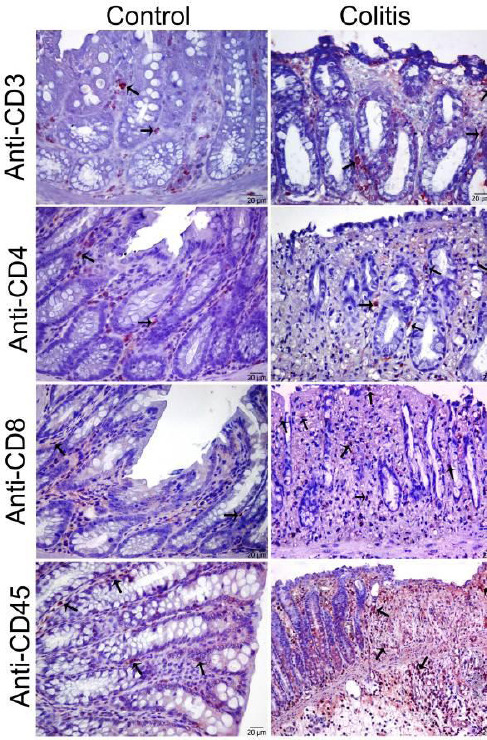
Expression of anti-CD3, CD4, CD8, and CD45 in colon mucosa. Elevated positive-stained cell number in colitis group (Immunoperoxidase, Mayer’s Hematoxylin counter stain, arrows; positive-stained cells)
CD5-positive cells, T-lymphocytes or B-lymphocytes were mainly observed near the crypt of Lieberkuhn in intestinal mucosa in both groups. The colitis group had a significantly higher CD5-positive cell number compared to the control group (P<0.05, Figure 5).
Figure 5.

Anti-CD5+ cells in control (a) and colitis (b) group in lamina propria and submucosal layer
Moreover, CD11b (also as known Macrophage-1 antigen [Mac-1] or Integrin alpha M) immunoreactivi-ty findings showed that positive cells were located under the lamina epithelialis and base of the crypt of Lieberkuhn in the control group. The number of CD11b positive cells in the colitis group was significantly higher than in the control group (P<0.05, Figure 6).
Figure 6.
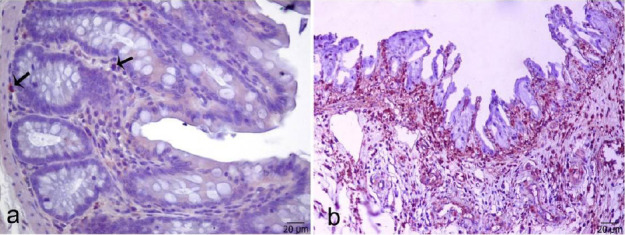
Expression of CD11b in the colonic mucosa, control (a), colitis (b) (Immunoperoxidase, Mayer’s Hematoxylin counter stain, arrows; positive-stained cells)
IL-17 and IL-22 positive stained cells were observed in the lamina propria in the control group, but in the colitis group, they were located in inflammation areas. Both IL-17 and IL-22 cytokines expression were significantly higher in the colitis group when compared to the control group (P<0.05, Figure 7). IL-23-positive stained cells were observed as intraepithelial in colonic mucosa in both groups. Increased IL-23-positive cells were detected in the colitis group as compared to the control group (P<0.05, Figure 7).
Figure 7.

Enhanced IL-17, IL-22 and IL-23-positive stained cells in colitis group in colon tissue (Immunoperoxidase, Mayer’s Hematoxylin counter stain, arrows; positive-stained cells)
Western blot evaluations
Colonic IL-17 and IL-22 protein expressions were quantified by Western blot. In the colitis group, significantly increased IL-17 and IL-22 expressions were observed in colonic tissue compared to the control group (P<0.05, Figure 8). Also, IL-17 and IL-22 immunohistochemical findings were supported by the Western blot results.
Figure 8.
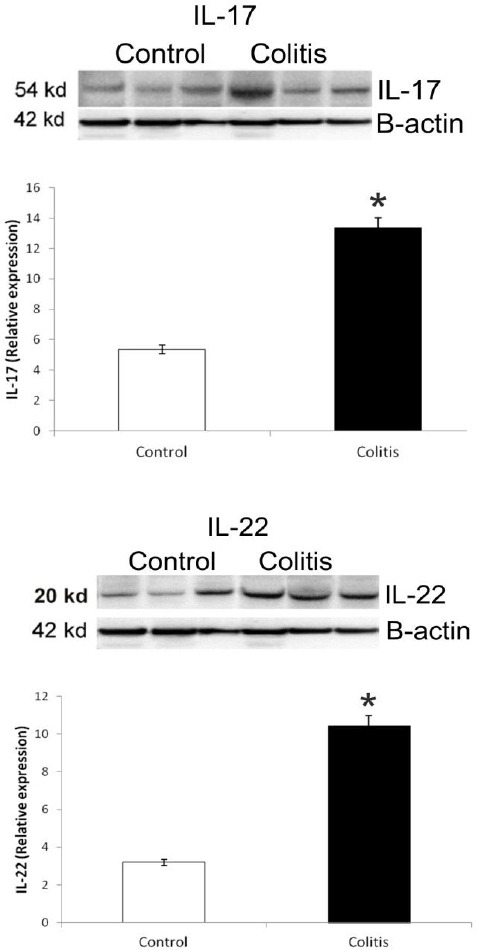
Panels show IL-17 and IL-22 expressions in colonic tissue. *P<0.05 compared with control tissue
Discussion
TNBS-induced colitis represents an experimental model that is able to attain characteristics resembling both acute and chronic human UC. Similar to the case in human UC, TNBS-induced colitis is associated with alterations in body weight and weight loss. Consistent with previous results, rats in the colitis group experienced a significant reduction in average weight as compared to controls (22, 25).
Similar to previous reports, the macroscopic injury scores in the colitis group were higher (26). In contrast, with the absence of macroscopic injury in the control group, the macroscopic injury scale score was 6.8 ± 1.5 in the colitis group, corresponding to a statistically significant difference.
While colon tissue samples in control rats had normal histological structure, many pathological findings, such as epithelial cell erosion, Goblet cell loss, ulceration and inflammation, were observed in the colitis group. Our microscopic findings are consistent with those reported by different researchers (27, 28). The microscopic scores in the colitis group were 7.1 ± 0.8, and the difference in the controls was statistically significant.
It has been reported that oral anti-CD3 administration resulted in the cytokine response in T cell-induced colitis, and this approach may pave the way for novel UC therapies (29). The intestinal mucosa in the colitis group exhibited a significant increase in mucosal CD3+ cells within the lamina propria and submucosal layer. In a study on TNBS-induced colitis model, CD3+ cells were labeled using the immunofluorescence method, and positive cell infiltration in all layers of the distal colonic tissue was more intense in the colitis group than in controls (30). Similarly, findings have shown an increased colonic CD3+ cells in the TNBS-induced colitis model as compared to controls (22). The same finding was also observed in our study with a statistically significant increase in the mucosal CD3+ cell count in the colitis group.
CD5 is a membrane glycoprotein expressed in T cells and in B1 lymphocytes, a subgroup of B lymphocytes, which synthesize IgM (31, 32). Previous studies showed increased CD5 expression in experimental UC models (7, 22). Anti-CD5 staining results in our study showed increased CD5 expression, similar to previous reports. In this regard, it may be assumed that therapeutic CD5 antagonists may be useful in reducing the severity of IBD (31).
Recruitment of circulating leukocytes into the site of inflammation is dependent on the expression of various adhesion molecules and a serious of consecutive adhesion processes that occur as a result of an interaction between leukocytes and endothelial cells. Intercellular adhesion molecule (I-CAM) and vascular cell adhesion molecule (V-CAM), expressed on endothelium, are used as ligands by LFA-1 and Mac-1 (CD11b) expressed in leukocytes (33). Our results showed a significant increase in CD11b expression in the colitis group as compared to controls. Other findings also showed that CD11b+ cells in control and UC subjects were closely located around the mucosal intestinal crypts, as shown by their immunohistochemical examinations performed in colonic biopsy samples (34). Conversely, the results of a study on dextran sulphate sodium (DSS)-induced mice model of colitis showed a proportional decrease in CD11b+ cells in the lamina propria (35). In that study, the number of CD11b+ cells was counted separately in mesenteric lymph nodes, lamina propria, intraepithelial lymphocytes and Peyer’s patches. Except for the lamina propria, increased CD11b expression was reported in other tissues and sites (35).
CD8+ T cell subpopulation plays an important role in the immunogenicity of intracellular pathogens and tumors (36, 37). The common characteristic of CD8+ T cells, which may have pathogenic or protective roles, is the production of IFN-γ and TNF-α, in addition to their cytotoxic functions. In IBD, they have been shown to play a pathogenic role (38, 39). According to the results of the present study, colonic mucosa of the colitis group exhibited an increased number of CD8+ T lymphocytes as compared to controls. Ghavidel et al. (2013), in their study examining the distribution of CD8+ T lymphocytes in the colonic biopsy samples of patients with IBD, found a significant reduction in the number of these cells in comparison with healthy individuals (40). The results of a study on the serum inflammatory markers as well as CD4+ and CD8+ T lymphocyte counts in IBD patients and healthy subjects indicated higher peripheral blood CD8+ T lymphocyte counts in the former group of individuals (41). Also, investigation on colonic CD3+, CD4+ and CD8+ cells in a rat model of sulfasalazine-induced colitis showed a higher CD8+ T recruitment in the colonic mucosa of rats with colitis (42). In our study, the observation that a higher count of CD8+ T lymphocytes was found in the colonic mucosa is at odds with certain previous studies (40), while it is consistent with others (22, 40).
CD45 (Protein Tyrosine Phosphatase, Receptor type C; PTCRC) is a transmembrane glycoprotein that is known as common leukocyte antigen. It is involved in many different functions, such as cell adhesion, migration, cytokine production and signal conduction (43). Immunohistochemical CD45 expression was higher in the mucosa of our colitis group than in controls. Published literature also points to increased CD45 expression in colitis models. For instance, a significant increase of CD45+ cell infiltration has been reported in the mucosa (44).
Naive CD4+ T lymphocytes differentiate into at least four different major Th cells, i.e. Th1, Th2, Th17 and Treg, which play a major role in the regulation of immune response against a number of different microorganisms through cytokine-mediated signal-ing pathway and T cell receptor (TCR) activation (10). Of these T cell subpopulations classified accor-ding to the cytokine production profile, Th1 cells are associated with highest IFN-gamma production, while Th2 predominantly produces cytokines such as IL-4, IL-5 and IL-13. The Th17 subpopulation is responsible for high levels of IL-17 expression, in addition to IL-21, IL-22 and TNF-α production (10, 45). As compared to the control group, colonic mucosa of the colitis group had higher CD4+ T lymphocyte counts. Carvalho et al. (2007), in a TNBS (at a dose of 20 mg/rat)-induced rat colitis model, identified CD4+ cells in the colonic tissue through immunohistochemical methods (46). In that study, the CD4+ cell count in the colon mucosa of the colitis group was insignificantly higher than that of the control group, while the CD4+ count was significantly higher among colitis rats than among controls in our study. Other findings have also shown that the presence of elevated numbers of peripheral blood CD4+ T lymphocytes in IBD patients was more than in healthy individuals (41).
Increased IL-21 and IL-22 expression was found in association with IL-17 in colon biopsy samples of UC patients, as compared to healthy subjects (11). Moreover, using immunohistochemistry, it was found an increased IL-17 expression in a TNBS-induced model of colitis (47). In the present study, the increase in the number of IL-17 positive cells was also shown using the same staining method in the colon mucosa of the colitis group. In experimental colitis models with IL-17R (IL-17 receptor) knock-out mice, the absence of inflammation indicates the importance of IL-17R in inflammation occurring in UC. Additionally, studies testing the suppression of increased IL-17 expression in experimental animal models with different immunomodulator agents have been published. In this regard, the therapeutic effects of resveratrol have been shown on UC through the suppression of the production of muco-sal IL-10, IL-6, and IL-17 cytokines in an experimen-tal mice model of colitis (48).
In our study, colonic IL-17 expression was tested not only with immunohistochemistry, but also with Western blot analysis. IL-17 expression was higher in the colitis group than in controls. Similarly, increased colonic IL-17 expression has been shown in an experimental TNBS-induced colitis model (49). In genetic studies involving UC patients, polymer-phisms in IL-17 and related genes are also suppor-tive of the role of Th17 pathways in the development of UC (50).
IL-22 is expressed in Th17 cells, as well as innate immune cells, and is a cytokine belonging to the family of IL-10 cytokines (51). Investigation on the colonic biopsy samples of patients with UC demons-trated a reduced mucosal Th22 cell counts in com-parison with controls (52). In addition, investigating the mucosal IL-22 and related proteins in patients with UC showed a higher mucosal IL-22 expression in chronic patients than in controls as documented immunohistochemically (53). Our data also showed increased IL-22 in the colonic mucosa of the colitis group, both with immunohistochemistry and Western blot analysis.
The IL-23 is expressed in activated macrophages and dendritic cells (9). IL-23 has been reported to be necessary for the growth and stabilization of Th17 cells (54). Immunohistochemical results of our study showed that IL-23+ cells are mostly located in the intraepithelial segments of the colonic mucosa both in colitis and control groups. Based on the localization of IL-23+ cells and in consistent with previous reports, we may assume that these cells mostly consist of dendritic cells.
Our study is different from other studies in which the activation of innate-adaptive immune system cells involves with Th17-associated cytokines (IL-17, IL-22 and IL-23) in TNBS-induced rat colitis model. In the differentiation of the Th17 subpopulation, Transforming growth factor beta 1(TGF1-B) plays a special role (55). We suggest that leukocytes, which are marked with different markers (CD3, CD5, CD8 and CD45) in the inflamed areas and increased in number, support the development of Th17 subpopulation by increasing TNF-α and TGF1-B expression.
Macrophage and dendritic cell-derived IL-23 is required for the development of UC and the persistence of the Th17 subpopulation. In the colitis group, the increase in the number of CD11b+ stained macrophages in the areas of ulceration accounts for the increase in Th17 subpopulation and related cytokines and the chronic progression of inflammation.
Conclusion
To the best of our knowledge, this study represents the first of its kind, since the activation of the newly identified T cell subpopulations Th17 and Th22 in a TNBS-induced experimental colitis model was compared with the activation of both innate immune cells as well as adaptive immune cells. Further studies utilizing different research methodology are required to shed further light on these associations.
Acknowledgment
The results presented in this paper were part of Doctoral thesis. The project was supported by Research Fund of the Trakya University. Project number: 2014/20 funding agency.
Conflict of interest
The authors have not declared any conflicts of interest.
References
- 1.Azuma K, Osaki T, Kurozumi S, Kiyose M, Tsuka T, Murahata Y, et al. Anti-inflammatory effects of orally administered glucosamine oligomer in an experimental model of inflammatory bowel disease. Carbohydr Polym. 2015;115:448–456. doi: 10.1016/j.carbpol.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 2.Al-Rejaie SS, Abuohashish HM, Al-Enazi MM, Al-Assaf AH, Parmar MY, Ahmed MM. Protective effect of naringenin on acetic acid-induced ulcerative colitis in rats. World J Gastroenterol. 2013;19:5633. doi: 10.3748/wjg.v19.i34.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou YH, Yu JP, Liu YF, Teng XJ, Ming M, Lv P, et al. Effects of Ginkgo biloba extract on inflammatory mediators (SOD, MDA, TNF-α, NF-κBp65, IL-6) in TNBS-induced colitis in rats. Mediators Inflamm. 2006;2006:92642. doi: 10.1155/MI/2006/92642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zenewicz LA, Antov A, Flavell RA. CD4 T-cell differentiation and inflammatory bowel disease. Trends Mol Med. 2009;15:199–207. doi: 10.1016/j.molmed.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Hongbao M, Young M, Yan Y. Cluster of Differentiation (CD) N Y Sci J. 2015;8:49–53. [Google Scholar]
- 6.Zola H, Swart B, Nicholson I, Aasted B, Bensussan A, Boumsell L, et al. CD molecules. 2005:human cell differentiation molecules. Blood. 2005;106:3123–3126. doi: 10.1182/blood-2005-03-1338. [DOI] [PubMed] [Google Scholar]
- 7.Karaca T, Şimşek N, Uslu S, Kalkan Y, Can I, Kara A, et al. The effect of royal jelly on CD3+, CD5+, CD45+T-cell and CD68+cell distribution in the colon of rats with acetic acid-induced colitis. Allergol Immunopathol (Madr) 2012;40:357–361. doi: 10.1016/j.aller.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Műzes G, Molnár B, Tulassay Z, Sipos F. Changes of the cytokine profile in inflammatory bowel diseases. World J Gastroenterol. 2012;18:5848. doi: 10.3748/wjg.v18.i41.5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwakura Y, Ishigame H. The IL-23/IL-17 axis in inflammation. J Clin Invest. 2006;116:1218. doi: 10.1172/JCI28508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu J, Paul WE. Peripheral CD4+T-cell differentiation regulated by networks of cytokines and transcription factors. Immunol Rev. 2010;238:247–262. doi: 10.1111/j.1600-065X.2010.00951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang W, Su J, Zhang X, Cheng X, Zhou J, Shi R, et al. Elevated levels of Th17 cells and Th17-related cytokines are associated with disease activity in patients with inflammatory bowel disease. Inflamm Res. 2014;63:943–950. doi: 10.1007/s00011-014-0768-7. [DOI] [PubMed] [Google Scholar]
- 12.Zhou L, Littman DR. Transcriptional regulatory networks in Th17 cell differentiation. Curr Opin Immunol. 2009;21:146–152. doi: 10.1016/j.coi.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kolls JK, Lindén A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 14.Fantini M, Monteleone G, MacDonald T. IL-21 comes of age as a regulator of effector T cells in the gut. Mucosal Immunol. 2008;1:110–115. doi: 10.1038/mi.2007.17. [DOI] [PubMed] [Google Scholar]
- 15.Aujla S, Kolls J. IL-22:a critical mediator in mucosal host defense. J Mol Med (Berl) 2009;87:451–454. doi: 10.1007/s00109-009-0448-1. [DOI] [PubMed] [Google Scholar]
- 16.Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 17.Seiderer J, Brand S. IL-22:A two-headed cytokine in IBD? Inflamm Bowel Dis. 2009;15:473–474. doi: 10.1002/ibd.20625. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto-Furusho JK, Miranda-Pérez E, Fonseca-Camarillo G, Sánchez-Muñoz F, Dominguez-Lopez A, Barreto-Zuniga R. Colonic epithelial upregulation of interleukin 22 (IL-22) in patients with ulcerative colitis. Inflamm Bowel Dis. 2010;16:1823. doi: 10.1002/ibd.21235. [DOI] [PubMed] [Google Scholar]
- 19.Ahern PP, Schiering C, Buonocore S, McGeachy MJ, Cua DJ, Maloy KJ, et al. Interleukin-23 drives intestinal inflammation through direct activity on T cells. Immunity. 2010;33:279–288. doi: 10.1016/j.immuni.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, Mckenzie B, et al. IL-23 is essential for T cell–mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hue S, Ahern P, Buonocore S, Kullberg MC, Cua DJ, McKenzie BS, et al. Interleukin-23 drives innate and T cell–mediated intestinal inflammation. J Exp Med. 2006;203:2473–2483. doi: 10.1084/jem.20061099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karaca T, Uz YH, Demirtas S, Karaboga I, Can G. Protective effect of royal jelly in 2, 4, 6 trinitrobenzene sulfonic acid-induced colitis in rats. Iran J Basic Med Sci. 2015;18:370. [PMC free article] [PubMed] [Google Scholar]
- 23.McCafferty DM, Sharkey KA, Wallace JL. Beneficial effects of local or systemic lidocaine in experimental colitis. Am J Physiol Gastrointest Liver Physiol. 1994;266:G560–G567. doi: 10.1152/ajpgi.1994.266.4.G560. [DOI] [PubMed] [Google Scholar]
- 24.Obermeier F, Kojouharoff G, Hans W, Scholmerich J, Gross V, Falk W. Interferon-gamma (IFN-)-and tumour necrosis factor (TNF)-induced nitric oxide as toxic effector molecule in chronic dextran sulphate sodium (DSS)-induced colitis in mice. Clin Exp Immunol. 1999;116:238–245. doi: 10.1046/j.1365-2249.1999.00878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang M, Lin HB, Gong S, Chen PY, Geng LL, Zeng YM, et al. Effect of Astragalus polysaccharides on expression of TNF-α, IL-1βand NFATc4 in a rat model of experimental colitis. Cytokine. 2014;70:81–86. doi: 10.1016/j.cyto.2014.07.250. [DOI] [PubMed] [Google Scholar]
- 26.Kolgazi M, Uslu U, Yuksel M, 0Velioglu-Ogunc A, Ercan F, Alican I. The role of cholinergic anti-inflammatory pathway in acetic acid-induced colonic inflammation in the rat. Chem Biol Interact. 2013;205:72–80. doi: 10.1016/j.cbi.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 27.Wakuda T, Azuma K, Saimoto H, Ifuku S, Morimoto M, Arifuku I, et al. Protective effects of galacturonic acid-rich vinegar brewed from Japanese pear in a dextran sodium sulfate-induced acute colitis model. J Funct Foods. 2013;5:516–523. [Google Scholar]
- 28.Watanabe T, Asano N, Murray PJ, Ozato K, Tailor P, Fuss IJ, et al. Muramyl dipeptide activation of nucleotide-binding oligomerization domain 2 protects mice from experimental colitis. J Clin Invest. 2008;118:545–559. doi: 10.1172/JCI33145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forster K, Goethel A, Chan CWT, Zanello G, Streutker C, Croitoru K. An oral CD3-specific antibody suppresses T-cell–induced colitis and alters cytokine responses to T-cell activation in mice. Gastroenterology. 2012;143:1298–1307. doi: 10.1053/j.gastro.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 30.Reinecke K, Eminel S, Dierck F, Roessner W, Kersting S, Chromik AM, et al. The JNK inhibitor XG-102 protects against TNBS-induced colitis. Plos One. 2012;7(3):e30985. doi: 10.1371/journal.pone.0030985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dasu T, Qualls JE, Tuna H, Raman C, Cohen DA, Bondada S. CD5 plays an inhibitory role in the suppressive function of murine CD4+CD25+T reg cells. Immunol Lett. 2008;119:103–113. doi: 10.1016/j.imlet.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perez-Villar JJ, Whitney GS, Bowen MA, Hewgill DH, Aruffo AA, Kanner SB. CD5 negatively regulates the T-cell antigen receptor signal transduction pathway:involvement of SH2-containing phosphotyrosine phosphatase SHP-1. Mol Cell Biol. 1999;19:2903–2912. doi: 10.1128/mcb.19.4.2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gulubova MV, Manolova IM, Vlaykova TI, Prodanova M, Jovchev JP. Adhesion molecules in chronic ulcerative colitis. Int J Colorectal Dis. 2007;22:581–589. doi: 10.1007/s00384-006-0236-0. [DOI] [PubMed] [Google Scholar]
- 34.Vainer B, Nielsen OH, Horn T. Comparative studies of the colonic in situ expression of intercellular adhesion molecules (ICAM-1,-2, and-3), β2 integrins (LFA-1, Mac-1, and p150, 95), and PECAM-1 in ulcerative colitis and Crohn’s disease. Am J Surg Pathol. 2000;24:1115–1124. doi: 10.1097/00000478-200008000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Wang L, Jiang X, Liu X, Qian T, Chu Y. Local immune compartments are related to the severity of dextran sodium sulphate induced colitis. Biosci Trends. 2014;8:242–247. doi: 10.5582/bst.2014.01088. [DOI] [PubMed] [Google Scholar]
- 36.Klenerman P, Hill A. T cells and viral persistence:lessons from diverse infections. Nat Immunol. 2005;6:873–879. doi: 10.1038/ni1241. [DOI] [PubMed] [Google Scholar]
- 37.Lu Y, Hong B, Li H, Zheng Y, Zhang M, Wang S, et al. Tumor-specific IL-9–producing CD8+Tc9 cells are superior effector than type-I cytotoxic Tc1 cells for adoptive immunotherapy of cancers. Proc Natl Acad Sci. 2014;111:2265–2270. doi: 10.1073/pnas.1317431111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen J, Bruce D, Cantorna MT. Vitamin D receptor expression controls proliferation of naive CD8+T cells and development of CD8 mediated gastrointestinal inflammation. BMC Immunol. 2014;15:6. doi: 10.1186/1471-2172-15-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steinhoff U, Brinkmann V, Klemm U, Aichele P, Seiler P, Brandt U, et al. Autoimmune intestinal pathology induced by hsp60-specific CD8 T cells. Immunity. 1999;11:349–358. doi: 10.1016/s1074-7613(00)80110-7. [DOI] [PubMed] [Google Scholar]
- 40.Ghavidel A, Somi MH, Ebrahimzadeh ZA, Halimi M, Tabrizi A. CD4+and CD8+T cell count in colonic mucosa of patients with IBD and IBS. J Am Sci. 2013;9(9s) [Google Scholar]
- 41.Funderburg NT, Stubblefield Park SR, Sung HC, Hardy G, Clagett B, Ignatz-Hoover J, et al. Circulating CD4+and CD8+T cells are activated in inflammatory bowel disease and are associated with plasma markers of inflammation. Immunology. 2013;140:87–97. doi: 10.1111/imm.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu D, Zhao H, Zhao N, Lu C, Lu A. Effect of Bawei Xilei powder on CD3, CD4, CD8 T-lymphocytes of rats with ulcerative colitis. Zhongguo Zhong Yao Za Zhi. 2008;33:1301–1304. [PubMed] [Google Scholar]
- 43.Saunders A, Johnson P. Modulation of immune cell signalling by the leukocyte common tyrosine phosphatase, CD45. Cell Signal. 2010;22:339–348. doi: 10.1016/j.cellsig.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 44.Tobisawa Y, Imai Y, Fukuda M, Kawashima H. Sulfation of colonic mucins by N-acetylglucosamine 6-O-sulfotransferase-2 and its protective function in experimental colitis in mice. J Biol Chem. 2010;285:6750–6760. doi: 10.1074/jbc.M109.067082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 46.Carvalho AT, Souza H, Carneiro AJ, Castelo-Branco M, Madi K, Schanaider A, et al. Therapeutic and prophylactic thalidomide in TNBS-induced colitis:synergistic effects on TNF-alpha, IL-12 and VEGF production. World J Gastroenterol. 2007;13:2166. doi: 10.3748/wjg.v13.i15.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang M, Qiu X, Zhang H, Yang X, Hong N, Yang Y, et al. Faecalibacterium prausnitzii inhibits interleukin-17 to ameliorate colorectal colitis in rats. Plos One. 2014;9:e109146. doi: 10.1371/journal.pone.0109146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yao J, Wei C, Wang JY, Zhang R, Li YX, Wang L-S. Effect of resveratrol on Treg/Th17 signaling and ulcerative colitis treatment in mice. World J Gastroenterol. 2015;21:6572–6581. doi: 10.3748/wjg.v21.i21.6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.He Y, Lin LJ, Zheng CQ, Jin Y, Lin Y. Cytokine expression and the role of Thl7 cells in mice colitis. Hepatogastro-enterology. 2012;59:1809–1813. [PubMed] [Google Scholar]
- 50.Thompson AI, Lees CW. Genetics of ulcerative colitis. Inflamm Bow Dis. 2011;17:831–848. doi: 10.1002/ibd.21375. [DOI] [PubMed] [Google Scholar]
- 51.Rutz S, Eidenschenk C, Ouyang W. IL-22, not simply a Th17 cytokine. Immunol Rev. 2013;252:116–132. doi: 10.1111/imr.12027. [DOI] [PubMed] [Google Scholar]
- 52.Leung J, Davenport M, Wolff M, Wiens K, Abidi W, Poles M, et al. IL-22-producing CD4+cells are depleted in actively inflamed colitis tissue. Mucosal Immunol. 2014;7:124–133. doi: 10.1038/mi.2013.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu LZ, Wang HY, Yang SP, Yuan ZP, Xu FY, Sun C, et al. Expression of interleukin-22/STAT3 signaling pathway in ulcerative colitis and related carcinogenesis. World J Gastroenterol. 2013;19:2638–2649. doi: 10.3748/wjg.v19.i17.2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McKenzie BS, Kastelein RA, Cua DJ. Understanding the IL-23–IL-17 immune pathway. Trends Immunol. 2006;27:17–23. doi: 10.1016/j.it.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 55.Liu Z, Yadav PK, Xu X, Su J, Chen C, Tang M, et al. The increased expression of IL-23 in inflammatory bowel disease promotes intraepithelial and lamina propria lymphocyte inflammatory responses and cytotoxicity. J Leukoc Biol. 2011;89:597–606. doi: 10.1189/jlb.0810456. [DOI] [PubMed] [Google Scholar]


