Abstract
Objective(s):
In the present study the effect of stress on monkeys that had learned to retrieve food from a five-chamber receptacle, as well as the relationship between their behavior and the serum cortisol and epinephrine levels and relative size of the amygdala was evaluated.
Materials and Methods:
Six male rhesus monkeys were individually given access to the food reward orderly. They could easily retrieve the rewards from all chambers except for the chamber 4, which a brief, mild electric shock (3 V) was delivered to them upon touching the chamber’s interior. The coping behaviors were video-recorded and analyzed offline. Baseline serum cortisol and epinephrine levels were measured before the experiments using monkey enzyme-linked immunosorbent assay kit. One week after the behavioral experiment, the monkeys’ brains were scanned using magnetic resonance imaging under general anesthesia. The cross-sectional area of the left amygdala in sagittal plane relative to the area of the whole brain in the same slice was evaluated by the planimetric method using ImageJ software.
Results:
Exposure to the distressing condition caused different behavioral responses. Monkeys with higher baseline levels of serum cortisol and epinephrine and larger amygdala behaved more violently in the face of stress, indicating adopting emotion-focused stress-coping strategies. Conversely, those with low plasma epinephrine, moderate cortisol, and smaller amygdala showed perseverative behavior, indicating a problem-focused coping style.
Conclusion:
In dealing with the same stress, different responses might be observed from nonhuman primates according to their cortisol and epinephrine levels as well as their amygdala dimensions.
Keywords: Amygdala, Coping strategies, Cortisol, Epinephrine, Rhesus macaque, Stress
Introduction
Stress is considered as any change in the internal or external environment to which a living organism must be adopted. Stress and its effects on human health have been the focus of intense research in various disciplines, including biology, physiology, psychology, and sociology (1). Adaptation to the growing number of stressors brought about by the modern lifestyle requires efficient coping strategies (2). Coping is defined as a set of cognitive, autonomic, and behavioral responses to perceived stress that leads to diminished adverse consequences of stress (3). From a psycho-logical perspective, resilience to stressful situations varies among individuals, as they may show an emotion-focused or problem-focused coping strategy. Emotion-focused coping involves efforts to maintain emotional balance and minimize distress generated by stressors. It includes a wide range of responses from self-soothing to expression of negative emotions (4, 5). Problem-focused coping strategies which are applied directly against the stressor involve constructive measures of the individual in relation to the stressful situations in order to remove, evade, or change the source of stress or to diminish its impact (6-10).
Biologically, when a distressing factor challenges the organism, some mechanisms are activated in the brain that eventually lead to increased readiness to resist the threatening state (11, 12). Among these mechanisms is the activation of two stress systems: the Hypothalamic-Pituitary-Adrenal (HPA) axis and the Sympathetic-Adrenal Medullary (SAM) axis. The HPA axis consists of the hypothalamic paraventricular nucleus which contains neurons that release vaso-pressin and corticotropin-releasing hormone (CRH). These hormones stimulate the secretion of adreno-corticotropic hormone (ACTH) from the anterior lobe of the pituitary gland. ACTH acts on the adrenal cortex which produces glucocorticoid hormones including cortisol. Glucocorticoids, in turn, suppress CRH and ACTH production by acting back on the hypothalamus and pituitary in a negative feedback process (13). The SAM axis includes preganglionic sympathetic fibers that, in response to environmental stimuli, activate the adrenal medulla to release epinephrine and norepinephrine directly into the blood (14). Stress hormones released into the bloodstream from the adrenal cortex (glucocorticoids including cortisol) and medulla (epinephrine) lead to several physiological and mental consequences, which cause the individual to fight with or flight from the stressor. Increases in cardiac output and blood pressure which enhance blood flow to the skeletal muscles and other vital organs, increased blood glucose, and improved awareness are among these consequences (15-17).
Cortisol has been implicated in learning, memory, and emotion (18), which are in turn involved in strategy adoption. Circulating epinephrine, which is a part of the so-called “fight-or-flight” response to stress (19), also affects learning and memory processes, and consequently contributes to strategy selection. Behavior is driven by brain’s cognitive and emotional processes. The brain areas involved in the emotional control of behavior include parts of the limbic system such as the amygdala and the ventromedial prefrontal cortex. The amygdala has long been implicated both in positive and negative emotional states, including fear and anxiety reac-tions. Bilateral lesions of the amygdala in macaque monkeys result in a lack of fear responses to inanimate objects and a socially uninhibited pattern of behavior. The amygdala-lesioned monkeys also experience much less social stress and immediately engage in social interactions (20). The ability to regulate and cope with emotions in complex social and emotional situations is an essential skill for normal social interaction (21). Selective lesions of the central nucleus of the amygdala in rats prevent freezing in response to a conditioned stimulus (CS) associated with shock (passive coping). However, the ability to learn responses that terminate or prevent the CS (active coping) is not affected. On the other hand, selective lesions of the basal amygdala have no effect on freezing but impair learning of the active coping response. Damage to the lateral nucleus of the amygdala prevents both forms of learning (21). Therefore, the amygdala may contribute to strategy selection in stressful conditions. In this context, we attempted to evaluate different coping strategies in male rhesus monkeys in response to a reward-hazard environmental challenge. In addition, we examined whether certain coping styles are associa-ted with basal plasma cortisol and epinephrine levels or the relative amygdala size.
Materials and Methods
Animals
Six male rhesus macaques (named G, I, Jo, Ji, L, and M; aged 2−6 years) were housed in separate cages (80 × 80 × 100 cm) under standard conditions (temperature 23±2 °C; 12 hr light/dark cycle). All monkeys were vaccinated against polio, rabies, and tetanus. The animals had free access to water, and were fed three times a day a recipe prescribed by our veterinarian. All procedures were approved by the Animal Care and Use Committee at Baqiyatallah University of Medical Sciences.
Stress application
A five-chamber food receptacle mounted on a wheeled trolley tray was used for applying hazard-reward challenge to the animals. The size of each chamber was 10 × 10 × 5 cm, so that the animals touched the walls when retrieving the rewards (Figure 1). The animals could easily pick up the rewards from the chambers, unless from the fourth one where an instantaneous, mild DC electric shock (3 V) was delivered to them as they touched the interior surface of the wall. The shock was not damaging to the animals as some of them made several attempts to retrieve the reward from the chamber.
Figure 1.

The animal is being tested in a hazard-and-reward condition
Experimental design
One day before the experiments, blood samples (5 ml) were collected from the femoral vein of the monkeys under general anesthesia using ketamine (10 mg/kg, IM). Blood collection was performed between 8−9 AM. The samples were immediately injected into vacationer tubes containing a procoagulant, and allowed to clot for 2 hr at room temperature. The samples were then centrifuged at 3000 rpm for 8 min, and the serum was isolated and stored at −70 °C for hormone assays. In the next four days, four behavioral trials (episode or session of the experiment) were performed (one trial a day). Each monkey was individually presented from outside of the cage in a separate room by the receptacle baited with one peanut in the chambers individually in succession. The monkeys were required to retrieve the peanuts placed one by one in chambers 1 through 5. Retrieving the peanut from chamber 4 was linked with a mild shock. The animals’ behaviors and their strategies to deal with the stressful situation (retrieving the favorite reward from a fearful well) were recorded by CCTV cameras. When the monkey made no more effort to retrieve the reward from chamber 4, chamber 5 was immediately baited by putting another peanut in it. The use of animal equivalents of the emotion-focused and problem-focused coping strategies was evaluated and compared among the monkeys. Magnetic resonance imaging (MRI) of the monkeys’ brains was performed one week after the behavioral tests for evaluation of the amygdala dimensions.
Hormone assays
Serum cortisol and epinephrine concentrations were measured using monkey enzyme-linked immunosorbent assay (ELISA) kit (Mybiosource, USA), according to the manufacturer’s instruction. A standard curve was plotted and the cortisol and epinephrine levels (ng/ml) of the samples were determined.
MRI scanning and planimetric study of the amygdala
Brain MRI scanning was carried out one week after the behavioral experiments. The monkeys were anesthetized by intramuscular injection of ketamine hydrochloride (10–20 mg/kg) and xylazine (0.2–0.4 mg/kg) and scanned with a 3-Tesla Magnetom (Siemens, Erlangen) using T2 weighted protocol with 3 mm slice thickness. The cross-sectional area of the left amygdala was measured on sagittal slices. The amygdala was visible on one slice on each side of the brain. The DICOM image of the MRI slice was opened in the ImageJ program (NIH, USA). The scaling of the image was corrected automatically. The left amygdala was outlined and the cross-sectional area was calculated (22). The cross-sectional area of the whole brain was also calculated similarly on the same slice, and used for normalizing the area of the amygdala.
Results
We evaluated different coping strategies in male rhesus monkeys in response to a challenging condition. In addition, we examined whether certain coping styles are associated with basal plasma cortisol and epinephrine levels or with amygdala size.
Behavioral responses to stress
Avoidance response
Monkeys Jo and G retrieved the peanuts from the apparatus in succession until they received the first electric shock where they stopped trying on, kept away from the apparatus, and made no more effort to retrieve the rewards even from the remaining innocuous ones. Monkey Jo fled from the test device and sat backward at a corner of the cage, showing a terror reaction. Monkey G was the dominant animal, and similar to what he had always done when distressed, kept patting on his sex organ probably to prove his dominance and fully avoided looking at the experimenter. The animals behaved similarly on trial 2 (the next day) but refused to do the task in trials 3 and 4.
Aggressive response
Monkeys I and L showed aggressive behavior and attempted to attack the experimenter after receiving the shock as if they recognized the experimenter as the source of the insult. They also took their anger out on the test device by shaking it vigorously. After the first shock and the associated aggressive outburst, they did not carry on the task and made no more efforts to reach the risky reward. Monkey L was also not willing to receive food from the experimenter for a while after the test. The same behavior was shown in trial 2, and monkeys did not perform the task in trials 3 and 4.
Perseverative response
Monkeys Ji and M struggled to deal with the hazard-reward conflict. They tried to take the peanut in such a quick way as to avoid the shock, or they jumped on the wall of the cage and tried out different directions to approach the reward, sometimes from the top of the cage. Another strategy was to shape the hand against the chamber walls in a way to prevent receiving the shock. Monkey Ji exhibited more perseverance and made numerous efforts. He completed the task and accomplished to retrieve the reward on all of the four trials. Monkey M was very calm and spent more time evaluating the under surface of the apparatus diligently to find a solution. He succeeded to earn the reward on trial 1, failed to do so in trial 2, and did not carry on subsequent trials.
Serum levels of cortisol and epinephrine
The baseline concentrations of cortisol and epinephrine were assayed using monkey ELISA kits. The highest serum cortisol and epinephrine levels were detected in monkeys I and L which showed aggressive outbursts in response to the shock. The monkeys Ji and M, which behaved more efficiently in the hazard-reward condition, had low epinephrine but moderate cortisol levels. Monkeys G had a similar hormone profile. Monkeys Jo, which showed a fear reaction to the shock, had the lowest cortisol and a high epinephrine level (Table 1).
Table 1.
Baseline serum cortisol and epinephrine, amygdala area, and coping response in monkeys
| Monkeys | Weight (Kg) | Cortisol (ng/ml) | Epinephrine (ng/ml) | Amygdala cross-sectional area (mm2) | Normalized amygdala area (%)* | Coping behavior |
|---|---|---|---|---|---|---|
| G | 6 | 44.43 | 20.13 | 18.35 | 0.84 | Avoidance |
| Jo | 3.2 | 29.72 | 29.25 | 14.84 | 0.63 | Avoidance |
| I | 4.9 | 89.45 | 30.43 | 15.06 | 0.64 | Aggression |
| L | 4.2 | 98.3 | 28.98 | 16.50 | 0.72 | Aggression |
| Ji | 2.9 | 39.58 | 20.29 | 9.01 | 0.46 | Perseverance |
| M | 2.7 | 50.76 | 17.79 | 9.37 | 0.47 | Perseverance |
Percent of amygdala cross-sectional area relative to the area of the whole brain
Amygdala cross-sectional area
The cross-sectional area of the left amygdala was estimated and normalized relative to that of the whole brain on the same MRI slice in the sagittal plane (Figure 2). The normalized amygdala area was lowest in monkeys Ji and M, which exhibited perseverative responses. Monkeys that showed avoidance or aggressive responses had higher amygdala areas, with the highest values found in Monkeys G and L (Table 1).
Figure 2.
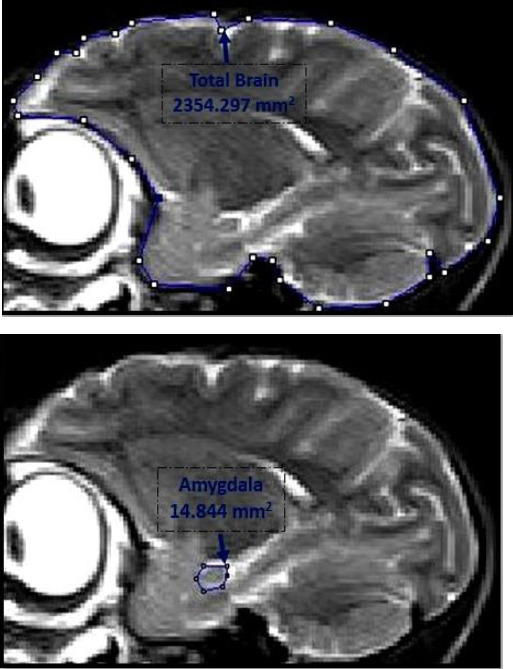
Planimetric study of the amygdala showing the outlines of the left amygdala (right) and the whole brain (left) in monkey Jo
Discussion
The main finding of this study was that exposure to a novel distressing condition caused different behavioral responses and adopting dissimilar coping strategies in nonhuman primates. The behavioral responses were associated with variations in the baseline plasma levels of stress hormones cortisol and epinephrine, as well as the relative amygdala dimensions.
Failure to cope with the stressors, when they are too demanding or uncontrollable, might give rise to negative health consequences, including an increaseed susceptibility to stress-related diseases. Compelling evidence strongly suggests that coping capacity varies widely among individuals presumably because of variations in genotype, development, early experience, and social support (6). Therefore, we hypothesized that nonhuman primates may also have dissimilar coping styles. There have been extensive attempts to classify variant responses to stress as distinct coping styles. Based on their reactions to social stress, two coping styles were distinguished in rodents, including proactive and reactive styles (23). The proactive coping involves the fight-or-flight response and is characteri-zed behaviorally by territorial control and aggression, whereas the reactive style involves the conservation-withdrawal response and is characterized behaviorally by immobility and low levels of aggression (23). On the other hand, humans adopt either problem-focused or emotion-focused coping strategies when confronted with a stressful situation. The problem-focused coping involves efforts to actively master stressful circums-tances, whereas emotion-focused coping involves efforts to regulate the emotional consequences of stressful events (5). The proactive coping in animals seems to be equivalent to the problem-focused coping in humans, as both involve directing the behavior to the stressor. On the other hand, the reactive coping appears to be roughly an animal counterpart of the human emotion-focused coping, as they involve avoiding the stressor and shaping emotions to avert or diminish its negative consequences.
In the present study, monkeys showed both the proactive and reactive coping styles in response to the same unprecedented challenge. More specifically, two monkeys struggled to retrieve the reward in spite of the hazard of being startled by the electric shock (proactive/problem-focused style), two monkeys avoided the reward-hazard condition (reactive/emotion-focused style), and two others exhibited aggressive outbursts (assumed paradoxically as a proactive/emotion-focused style). The exhibition of two different types of proactive responses can be explained by the bi-aspectual nature of the stress applied to the subjects. The use of a free choice condition, seeking the reward at the expense of being shocked or ignoring it, instead of using a pure stressful condition allowed for the differentiation in the expression of coping styles. Indeed, tests that measure aspects of initiative or proactivity seem to be most discriminative (23). On the other hand, the way animals behave in response to a stressor is influenced by the way they appraise the salience of the stress. Humans also select their coping style based on the stress appraisal, which depends as such on the personality type and the type of the stressor. For example, people typically use problem-focused coping to deal with potentially controllable problems such as work- and family-related problems, whereas less controllable stressors such as certain kinds of physical health problems, prompt more emotion-focused coping (24). Therefore, monkeys could give prominence either to the electric shock or to the fear of missing the reward. Then, the desired coping could be avoiding the shock or achieving the reward. As a result, both the monkeys that struggled for the reward and those that showed aggressive outbursts had the proactive coping style since they all directed their behavior to the source of their appraised stress. Classification of the latter nonhuman primate behavior from the human psychological perspective is, however, more of a challenge. Unlike the classification of animal coping strategies, aggressive reactions are mostly related to the emotional state and are categorized as an emotion-focused strategy (25).
The expression of different coping styles by the monkeys might be attributable to various behavioral traits. It has been suggested that the individual level of aggressive behavior is associated with the way individual males react to environmental challenges. For example, when male wild-type rats were confronted with electrical stimulation in their home cages, aggressive males spent most of the test time burying the shock prod with the bedding material (a proactive strategy), while non-aggressive males showed immobility behavior and hid in a corner of the cage (a reactive strategy) (26, 27). A relatively similar pattern of behavior was observed in our study. The monkeys that showed proactive coping by aggressive outbursts were more aggressive during the adaptation period before the initiation of the study, whereas monkeys that showed reactive avoidance behavior were less aggressive. However, monkeys that showed proactive reward-seeking response were also nonaggressive. This might be explained by the fact that behavioral traits of the subjects may affect their stress appraisal, hence their desired coping.
The expression of different coping styles can be further explained by variations in the neuroendocrine characteristics of the subjects. The prominent active-ty of stress axes including HPA axis and the SAM is mirrored respectively by the plasma levels of cortisol and epinephrine. The results of our study showed that proactive aggressive responses to the shock were consistently associated with high baseline serum cortisol and epinephrine. Proactive reward-directed behaviors were accompanied by low serum epinephrine but moderate cortisol. The reactive avoidance response to the shock was linked to low cortisol and high epinephrine levels (with the exception of monkey G). Prior studies also suggested that differences in HPA axis activity under baseline conditions might contribute to different coping styles in different species. It has been shown that the level of aggressive behavior in Mangrove rivulus fish was correlated with the baseline cortisol concentrations (10). On the other hand, both acute and basal HPA axis activity has been shown to influence aggressive behavior in rats (28). Kruk et al. identified a positive feedback cycle, in which the activation of the HPA axis causes enhanced aggressive behavior, which in turn further activates the HPA axis. However, low basal activity of the HPA axis in rats is causally involved in abnormal forms of aggressive behavior (29). Furthermore, reduced circadian peak plasma corticosterone levels have been observed in aggre-ssive mice as compared to non-aggressive mice (30). The results of our study were consistent with pre-vious findings from human subjects. While positive coping characteristics such as personal mastery and sense of control in adult humans were associated with steeper cortisol slopes over the course of the day, problem engagement and support seeking were associated with lower overall cortisol levels (31). In children affected by parental HIV/AIDS, greater positive coping was associated with higher morning cortisol, whereas greater negative coping was associated with lower morning cortisol and a flatter diurnal cortisol slope (32).
In our study, different stress-coping styles were also associated with relative amygdala size. Animals with avoidance or aggressive responses had a larger amygdala, whereas those with perseverative res-ponses had smaller amygdala. The amygdala has been implicated in coping behavior, as it was shown that oxytocin release in the central amygdala was involved in the generation of passive stress-coping strategies in response to swim stress (33).
Furthermore, bilateral selective depletion of sero-tonin in the medial prefrontal cortex of mice reduced stress-induced GABA release in the basolateral amygdala and immobility (passive coping) in the forced swimming test. Disconnecting the medial prefrontal cortex and the amygdala bilaterally also decreased immobility in the forced swimming test. Thus, prefrontal-amygdala connectivity mediated by sero-tonin and GABA transmission is a critical neural mechanism in stress-induced behavior (34). Moreover, findings in humans have shown larger amygdala volumes, partly related to early stress exposure. Children reared in orphanages and those of mothers with depressive symptomatology were found to present enlarged amygdala volumes, suggesting that the amygdala may be particularly sensitive to severely disturbed care in infancy (35).
Conclusion
It seems that negative or positive coping strategies in male rhesus monkeys in response to stressful conditions are determined by the behavioral traits, the neuroendocrine characteristics, and the relative amygdala size. Proactive, problem-focused strategy (perseverative behavior) was associated with low basal plasma epinephrine, moderate cortisol, and smaller relative amygdala size. Reactive, emotion-focused coping (avoidance behavior) was related to low cortisol and high epinephrine concentrations, and larger amygdala. Finally, proactive, but emotion-focused style (aggressive behavior) was consistently accompanied by high cortisol and epinephrine and larger amygdala.
Acknowledgment
This study has been financially sponsored by Neuroscience Research Center of Baqiyatallah University of Medical Sciences, Tehran, Iran.
Conflicts of interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- 1.Driskell JE, Salas E. Stress and human performance:Psychology Press. 2013 [Google Scholar]
- 2.Elliott GR, Eisdorfer C. Stress and human health:analysis and implications of research:a study:Springer Publishing Company. 1982 [Google Scholar]
- 3.Wingfield JC. Control of behavioural strategies for capricious environments. Anim Behav. 2003;66:807–816. [Google Scholar]
- 4.Pfattheicher S, Keller J. Towards a biopsychological understanding of costly punishment:the role of basal cortisol. PLoS One. 2014;9:e85691. doi: 10.1371/journal.pone.0085691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Boer SF, Koolhaas JM. 5-HT1A and 5-HT1B receptor agonists and aggression:a pharmacological challenge of the serotonin deficiency hypothesis. Eur J Pharmacol. 2005;526:125–139. doi: 10.1016/j.ejphar.2005.09.065. [DOI] [PubMed] [Google Scholar]
- 6.Carver CS, Scheier MF, Weintraub JK. Assessing coping strategies:a theoretically based approach. J Pers Soc Psychol. 1989;56:267. doi: 10.1037//0022-3514.56.2.267. [DOI] [PubMed] [Google Scholar]
- 7.Billings AG, Moos RH. The role of coping responses and social resources in attenuating the stress of life events. J Behav Med. 1981;4:139–157. doi: 10.1007/BF00844267. [DOI] [PubMed] [Google Scholar]
- 8.Dumont M, Provost MA. Resilience in adolescents:Protective role of social support, coping strategies, self-esteem, and social activities on experience of stress and depression. J Youth Adolesc. 1999;28:343–363. [Google Scholar]
- 9.Felton BJ, Revenson TA. Coping with chronic illness:a study of illness controllability and the influence of coping strategies on psychological adjustment. J Consult Clin Psychol. 1984;52:343. doi: 10.1037//0022-006x.52.3.343. [DOI] [PubMed] [Google Scholar]
- 10.Montoya ER, Terburg D, Bos PA, van Honk J. Testosterone, cortisol, and serotonin as key regulators of social aggression:A review and theoretical perspective. Motiv Emot. 2012;36:65–73. doi: 10.1007/s11031-011-9264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh-Taylor A, Korosi A, Molet J, Gunn BG, Baram TZ. Neurobiology of Stress 2014. doi: 10.1016/j.ynstr.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wadhwa PD, Sandman CA, Garite TJ. The neurobiology of stress in human pregnancy:implications for prematurity and development of the fetal central nervous system. Prog Brain Res. 2001;133:131–142. doi: 10.1016/s0079-6123(01)33010-8. [DOI] [PubMed] [Google Scholar]
- 13.Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci. 2006;8:383–395. doi: 10.31887/DCNS.2006.8.4/ssmith. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christensen NJ. The biochemical assessment of sympathoadrenal activity in man. Clin Auton Res. 1991;1:167–172. doi: 10.1007/BF01826215. [DOI] [PubMed] [Google Scholar]
- 15.Kudielka BM, Buske-Kirschbaum A, Hellhammer DH, Kirschbaum C. HPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children:impact of age and gender. Psychoneuroendocrinology. 2004;29:83–98. doi: 10.1016/s0306-4530(02)00146-4. [DOI] [PubMed] [Google Scholar]
- 16.Kudielka BM, Schommer NC, Hellhammer DH, Kirschbaum C. Acute HPA axis responses, heart rate, and mood changes to psychosocial stress (TSST) in humans at different times of day. Psychoneuroendocrinology. 2004;29:983–992. doi: 10.1016/j.psyneuen.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 17.Buske-Kirschbaum A, Fischbach S, Rauh W, Hanker J, Hellhammer D. Increased responsiveness of the hypothalamus–pituitary–adrenal (HPA) axis to stress in newborns with atopic disposition. Psychoneuroendocrinology. 2004;29:705–711. doi: 10.1016/S0306-4530(03)00100-8. [DOI] [PubMed] [Google Scholar]
- 18.Mihai CT, Rotinberg P, Brinza F, Vochita G. Extremely low-frequency electromagnetic fields cause DNA strand breaks in normal cells. J Environ Health Sci Eng. 2014;12:15. doi: 10.1186/2052-336X-12-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong DL. Epinephrine biosynthesis:hormonal and neural control during stress. Cell Mol Neurobiol. 2006;26:891–900. doi: 10.1007/s10571-006-9056-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amaral DG. The primate amygdala and the neurobiology of social behavior:implications for understanding social anxiety. Biol Psychiatry. 2002;51:11–17. doi: 10.1016/s0006-3223(01)01307-5. [DOI] [PubMed] [Google Scholar]
- 21.Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing:from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 22.Shanthi V, Singh D. Estimation of hippocampus volume from MRI using imageJ for alzheimer’s diagnosis. Atlas J Med Biol Sci. 2011;1:15–20. [Google Scholar]
- 23.Scotti MA, Rendon NM, Greives TJ, Romeo RD, Demas GE. Short-day aggression is independent of changes in cortisol or glucocorticoid receptors in male Siberian hamsters (Phodopus sungorus) J Exp Zool A Ecol Genet Physiol. 2015;323:331–341. doi: 10.1002/jez.1922. [DOI] [PubMed] [Google Scholar]
- 24.Thoits PA. Stress, coping, and social support processes:Where are we? What next? J Health Soc Behav. 1995:53–79. [PubMed] [Google Scholar]
- 25.de Quervain DJ, Roozendaal B, Mc Gaugh JL. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature. 1998;394:787–790. doi: 10.1038/29542. [DOI] [PubMed] [Google Scholar]
- 26.Clow A, Hucklebridge F, Stalder T, Evans P, Thorn L. The cortisol awakening response:more than a measure of HPA axis function. Neurosci Biobehav Rev. 2010;35:97–103. doi: 10.1016/j.neubiorev.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 27.Clutton-Brock TH, Parker GA. Punishment in animal societies. Nature. 1995;373:209–216. doi: 10.1038/373209a0. [DOI] [PubMed] [Google Scholar]
- 28.Yahyavi ST, Zarghami M, Naghshvar F, Danesh A. Relationship of cortisol, norepinephrine, and epinephrine levels with war-induced posttraumatic stress disorder in fathers and their offspring. Rev Bras Psiquiatr. 2015;37:93–98. doi: 10.1590/1516-4446-2014-1414. [DOI] [PubMed] [Google Scholar]
- 29.Kruk MR, Halasz J, Meelis W, Haller J. Fast positive feedback between the adrenocortical stress response and a brain mechanism involved in aggressive behavior. Behav Neurosci. 2004;118:1062. doi: 10.1037/0735-7044.118.5.1062. [DOI] [PubMed] [Google Scholar]
- 30.Saltzman W, Abbott DH. Effects of elevated circulating cortisol concentrations on maternal behavior in common marmoset monkeys (Callithrix jacchus) Psychoneuroendo- crinology. 2009;34:1222–1234. doi: 10.1016/j.psyneuen.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bohnke R, Bertsch K, Kruk MR, Naumann E. The relationship between basal and acute HPA axis activity and aggressive behavior in adults. J Neural Transm (Vienna) 2010;117:629–637. doi: 10.1007/s00702-010-0391-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carpenter RE, Korzan WJ, Bockholt C, Watt MJ, Forster GL, Renner KJ, et al. Corticotropin releasing factor influences aggression and monoamines:modulation of attacks and retreats. Neuroscience. 2009;158:412–425. doi: 10.1016/j.neuroscience.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lupien SJ, McEwen BS. The acute effects of corticosteroids on cognition:integration of animal and human model studies. Brain Res Rev. 1997;24:1–27. doi: 10.1016/s0165-0173(97)00004-0. [DOI] [PubMed] [Google Scholar]
- 34.Andolina D, Maran D, Valzania A, Conversi D, Puglisi-Allegra S. Prefrontal/amygdalar system determines stress coping behavior through 5-HT/GABA connection. Neuro-psychopharmacology. 2013;38:2057–2067. doi: 10.1038/npp.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vafaei A, Rashidy-Pour A, Taherian A. Peripheral injection of corticosterone has different effects on consolidation and retrieval spatial memory. Tabriz Pharmaciutical Sci. 2009;14:237–245. [Google Scholar]


