Abstract
Over the past few years interest has greatly increased in how the lipid mediator sphingosine-1-phosphate (S1P) influences bone homeostasis. Recent work has postulated multiple effects of S1P on osteoblasts and osteoclasts. Based on these findings, S1P has been proposed as a potential osteoporosis treatment. However, to date, there has been only a single study investigating S1P signalling in the cells that co-ordinate bone metabolism: osteocytes. This study aimed to elucidate the role of S1P signalling in osteocyte mechanotransduction.
Utilising 3D cell culture we established the expression profile of all genes related to the S1P signalling system in the Ocy454 osteocyte cell line. Exposure to mechanical loading resulted in a downregulation in Sost, Spns2, the S1P transporter, Sgpl1 and Sgppl1 the enzymes responsible for degradation and dephosphorylation of S1P. These findings, in conjunction with fluid-flow induced upregulation of Sphk1, the kinase responsible for phosphorylation of sphingosine, suggest that mechanical stimulation of osteocytes leads to an increase in intracellular S1P. This was confirmed with mechanical loading of Ocy454 cells rapidly increasing S1P production in conditioned media and protein lysates. These findings strongly suggest an important role for S1P in the response to mechanical loading of bone.
Keywords: Osteocyte, Ocy454, S1P, Mechanical loading
Highlights
-
•
Osteocytes form a cellular network throughout bone ideally suited for sensing the needs of the skeleton and responding to them.
-
•
Over the past few years interest has greatly increased in how S1P influences bone homeostasis.
-
•
Exposure to mechanical loading significantly modifies osteocyte S1P signalling.
-
•
This suggests an important role for S1P production in the response to mechanical loading of bone.
1. Introduction
Osteocytes form a cellular communication network throughout the bone matrix ideally suited for sensing the needs of the skeleton and responding to them. Indeed, targeted ablation of these cells demonstrated their central role in mechanosensing as this resulted in a diminished response of bone to mechanical unloading (Tatsumi et al., 2007). If osteocytes orchestrate the adaptation of bone to mechanical loading the question arises: how is this biological action performed?
Numerous studies have demonstrated that interstitial fluid flow (Piekarski and Munro, 1977, Weinbaum et al., 1994) stimulates osteocytic cell processes, leading to a cascade of intracellular events and production of local factors that regulate activities of osteoclasts and osteoblasts (Nakashima et al., 2011) to maintain adequate bone mass and architecture (Huiskes et al., 2000). However, stimuli of these signalling pathways remain to be determined. One significant candidate is sphingosine-1-phosphate (S1P) which has been shown to be potentially involved in bone mechanotransduction (Ishii et al., 2009, Karagiosis and Karin, 2007) with S1P receptor stimulation eliciting many of the same cell signalling responses that influence osteocyte mechanotransduction (Ishii et al., 2009, You et al., 2001, Alford et al., 2003).
Over the past few years interest has greatly increased in how S1P influences bone homeostasis. Early work postulated a role for S1P in bone remodelling as a coupling factor (a class of osteoclast-derived factors that stimulate osteoblast activity) (Ryu et al., 2006, Lotinun et al., 2013, Pederson et al., 2008). Subsequently multiple effects of S1P on osteoblasts and osteoclasts have been suggested (Ishii et al., 2009, Ryu et al., 2006, Lotinun et al., 2013). Based on these findings, S1P has been proposed as a potential osteoporosis treatment due to its anabolic effect on bone. Keller et al. proposed that increased bone formation seen in Calcr−/− mice was due to locally increased S1P concentrations resulting from increased Spns2-mediated transport (Keller et al., 2014). They also reported that S1PR3 null mice displayed an osteopenic (low bone mass) phenotype due to impaired bone formation, due to lack of osteoblast S1P responsiveness, further demonstrating a role for S1P in bone. Finally, a recent study (Zhang et al., 2015) utilising the MLO-Y4 osteocyte cell line suggested that treatment with S1P increased intracellular calcium and PGE2 release. Given the accepted central role for osteocyte control of bone formation, our focus on osteocyte-derived S1P is therefore all the more important.
This study aimed to elucidate the role of S1P signalling in osteocyte mechanotransduction. Here we report that exposure to mechanical loading significantly modifies osteocyte S1P signalling. These findings strongly suggest an important role for S1P production in the response to mechanical loading of bone.
2. Materials and methods
2.1. Materials
Phosphate buffered saline (PBS, Cat. No. 17-512F), Dulbecco's Modified Eagle Medium High Glucose (DMEM, Cat. No. 12-604F), Alpha modification of Eagle's medium (αMEM, Cat. No. 12-169F), and penicillin-streptomycin mixture (Cat. No. 17-602E) were obtained from Lonza (Mount Waverly, Australia). Fetal Bovine Serum was obtained from Bovogen (Keilor East, Australia). Trizol Reagent and TrypLE Express were purchased from Life Technologies (Thermo Fisher Scientific, MA, USA). IScript™ Reverse Transcription Super mix for RT-qPCR and iTaq™ Universal SYBR® Green Super mix were purchased from Bio-Rad (Gladesville, Australia). qRT-PCR primers purchased from Integrated DNA Technologies (Baulkham Hills, Australia).
2.2. Cell cultures
Ocy454 murine osteocytic cells were cultured as previously described (Wood et al., 2017). Briefly, for three dimensional cell cultures, 1.6 × 106 Ocy454 cells were plated on a 200 μm polystyrene Alvetex (Reinnervate) well insert scaffolds. Cells were allowed to grow at the permissive temperature (33 °C) for 2 days prior to transferring to (37 °C) for differentiation and fluid flow experiments.
2.2.1. 24 h fluid flow experiment
Ocy454 cells were differentiated for 14 days prior to transferring to the Reinnervate Perfusion Plate. The perfusion plates were attached to a Masterflex Peristaltic Pump (#7520-57) with a Masterflex Standard Pump Head (#7014-20) and were exposed to 2 dynes/cm2 for 24 h prior to extraction for mRNA analysis. Conditioned media was collected for S1P ELISA.
2.2.2. Short-term fluid flow experiment
Ocy454 cells were differentiated for 14 days prior to transferring to the Reinnervate Perfusion Plate. The perfusion plates were attached to a Masterflex Peristaltic Pump (#7520-57) with a Masterflex Standard Pump Head (#7014-20) and were exposed to 5 dynes/cm2 for 1 h. Trizol extraction was performed immediately or 3 h after fluid flow exposure for mRNA analysis.
2.3. S1P ELISA
S1P levels in conditioned media from either fluid flow stimulated Ocy454 cells or unstimulated control cells were analysed using a S1P competitive ELISA kit (Echelon Biosciences) according to the manufacturer's instructions. Cells were lysed in RIPA lysis buffer containing protease and phosphatase inhibitors and frozen at − 20 °C prior to analysis. Sensitivity of the assay was 30 nM. Either 20 μl of conditioned media or 50 μg protein of was added to the ELISA to assess S1P levels.
2.4. Real-time PCR (qPCR)
RNA was extracted from Ocy454 cells lysed in Trizol (Invitrogen, Australia), and cDNA was prepared using random hexamers (Promega, Australia) and Superscript III (Invitrogen, Australia) according to the manufacturer's protocol. Real-time RT-PCR was performed using the StratageneMX3000P (Agilent Technologies) as previously described (Wood et al., 2017, Gooi et al., 2014). Primers designed using Primer-BLAST are listed in Table 1. Primer sets were obtained from Integrated DNA Technologies (Coraville, IA). Post-run samples were analysed using Stratagene MxPro software and are expressed as linear ΔCT values normalized to Hypoxanthine Phosphoribosyltransferase 1 (HPRT1). The level of housekeeping gene did not vary significantly between treatment groups.
Table 1.
Primer sequences for PCR analysis.
| Primer | Forward sequence (5′ to 3′) | Reverse sequence (5′ to 3′) |
|---|---|---|
| SOST | GAGAACAACCAGACCATGAAC | GCTCGCGGCAGCTGTACT |
| SPHK1 | TCCTGGAGGAGGCAGAGATA | GCTACACAGGGGTTTCTGGA |
| SPHK2 | AAATCACCCCTGAATTGCTG | ATGCCTTCCCACTCACTCAG |
| SGPPL1 | CCGCTGGCAGTATCCTCTTA | TTGTCAATCAGGTCCACCAA |
| SGPPL2 | TGGCTGTGGTGTTCTCTACG | TGACACACACAGGGAAGAGG |
| SGPL1 | TGAGCTTATCTTCCAGCCAGA | TACCCTGAGCAGGCAGAGTT |
| SPNS2 | GGCATCTTCTTCTGGTCTGC | AGCATCAATGTGCGTGTGTT |
| S1PR1 | ACCTAGCCCTCTCGGACCTATT | CCCAGACAACAGCAGGTTAGC |
| S1PR2 | GCCATCGCCATCGAGAGA | TGTCACTGCCGTAGAGCTTGA |
| S1PR3 | GTGTGTTCATTGCCTGTTGG | TTGACTAGACAGCCGCACAC |
| S1PR4 | CAAGACCAGCCGTGTGTATG | AAGAGCACATAGCCCTTGGA |
| S1PR5 | GCTGCTGAATCCCATCATCT | TAGAGCTGCGATCCAAGGTT |
| HPRT1 | TGATTAGCGATGATGAACCAG | AGAGGGCCACAATGTGATG |
2.5. Statistics
All experiments were performed a minimum of 3 times, using independent preparations of conditioned medium (n = 3 biological replicates). Data were analysed for statistical significance by Student's t-test or ANOVA as indicated in figure legends followed by Dunnett's multiple comparisons test, using Prism 6.0 (GraphPad). For all graphs, bars represent the mean/group and error bars indicate standard error of the mean (SEM).
3. Results
3.1. Expression of S1P signalling components in Ocy454 cells
RT-PCR gene expression analysis confirmed the presence of all S1P signalling components (S1P receptors, kinases, lyase and phosphatases) in the Ocy454 cell line (Fig. 1). Sphingosine-1-phosphate receptor 1 (S1Pr1) expression rose significantly at Day 3 and maintained a steady level of expression throughout differentiation and remained the most highly expressed receptor subtype. Sphingosine-1-phosphate receptor 2 (S1Pr2) showed steady, continual expression, 1.3 fold less than S1Pr1. Sphingosine-1-phosphate receptors 3–5 expression significantly increased throughout differentiation until Day 10 where they remained at a high level of expression. The expression of S1Pr3, S1Pr4 and S1Pr5 were 5, 4.2 and 17.8 fold lower than S1Pr1 at peak levels respectively (Fig. 1A).
Fig. 1.
Expression of S1P signalling components in Ocy454 cells.
Differentiation of Ocy454 cell line over 14 days was shown to modify the expression profile of mRNA for S1P signalling components. A) Sphingosine-1-phosphate receptor 1–5, S1Pr1–5. B) Sphingosine kinase 1 (Sphk1) sphingosine kinase 2 (Sphk2), Spinster homolog 2 (Spns2). C) Sphingosine-1-phosphate lyase 1 (Sgpl1), S1P-specific phosphatase 1 (Sgppl1), S1P-specific phosphatase 2 (Sgppl2). n = 3. Data is mean ± SEM assessed by one-way ANOVA where *p < 0.05, **p < 0.01, ***p < 0.001vs Day 0.
Sphingosine kinase 1 (Sphk1) and Sphingosine kinase 2 (Sphk2) expression was steady and high throughout differentiation. Expression of Sphk2 was 1.1 fold higher than Sphk1 at peak levels. Spinster homolog 2 (Spns2), expressed at significantly lower levels than both Sphk1 and Sphk2, maintained a uniform level of expression throughout differentiation (Fig. 1B).
Sphingosine-1-phosphate lyase 1 (Sgpl1) expression was maintained at similar levels throughout differentiation. In contrast, S1P-specific phosphatase 1 (Sgppl1) showed significantly increased expression from Day 10 onwards. S1P-specific phosphatase 2 (Sgppl2) expression was an order of magnitude lower than Sgpl1 and Sgppl1, 529 fold less than Sgppl1. Expression was significantly increased on Days 5,7 and 12 of differentiation compared to Day 0 (Fig. 1C).
3.2. Long-term fluid flow exposure modulates Sost, Sphk1, Sphk2 and Spns2 gene expression
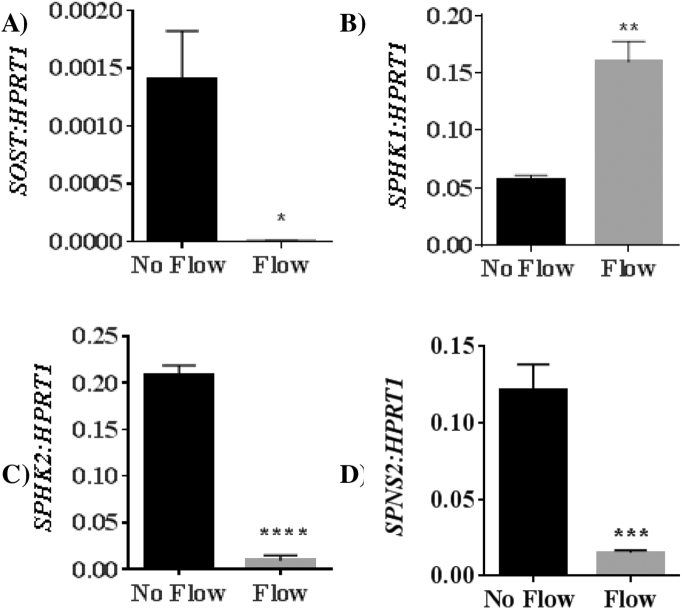
Exposure of differentiated Ocy454 cells to 2 dynes/cm2 of fluid flow for 24 h resulted in a significant downregulation of Sost mRNA expression (449 fold, p = 0.0151, Fig. 2A). Sphk1 expression was significantly upregulated following exposure to fluid flow (2.8 fold, p = 0.0013, Fig. 2B). In contrast, Sphk2 was downregulated relative to no flow control (22 fold, p < 0.0001, Fig. 2C). Spns2 was also downregulated relative to no flow controls (8.6 fold, p = 0.0280, Fig. 2D).
Fig. 2.
Long-term fluid flow exposure modulates Sost, Sphk1, Sphk2 and Spns2 gene expression. Expression of A) Sost, B) SphK1, C) SphK2, and D) Spns2 mRNA in Ocy454 cells differentiated for 14 days and exposed to fluid shear stress (FSS) (2 dynes/cm2) for 24 h. Data is mean ± SEM, n = 4, *p < 0.05, **p < 0.01, ****p < 0.0001 vs no flow.
3.3. Long-term fluid flow exposure downregulates S1P receptor expression
Expression of S1Pr1 and S1Pr2 mRNA were not altered following exposure to 2 dynes/cm2 flow for 24 h (Fig. 3A & B). In contrast, fluid flow stimulated Ocy454 cells displayed a significant downregulation in S1Pr3 (5.2 fold, p = 0.0101, Fig. 3C), S1Pr4 (9 fold, p = 0.0091, Fig. 3D) and S1Pr5 (3.2 fold, p = 0.0001, Fig. 3E) mRNA compared to no flow control.
Fig. 3.
Mechanical loading of Ocy454 cells (2 dynes/cm2, 24 h) modulated S1P receptor expression. S1P receptor subtypes A) 1, B) 2, C) 3, D) 4, and E) 5. Data is mean ± SEM, n = 4, *p < 0.05, **p < 0.01, ***p < 0.001 vs no flow.
3.4. Long-term fluid flow exposure downregulates S1P lysase and phosphatase expression
24 h of fluid flow stimulation resulted in a significant downregulation of Sgpl1 (2.1 fold, p = 0.003, Fig. 4A), Sgppl1 (2.5 fold, p = 0.0225, Fig. 4B) and Sgppl2 (6.4 fold, p = 0.005, Fig. 4C) gene expression compared to no flow control group.
Fig. 4.
Mechanical loading of Ocy454 cells (2 dynes/cm2, 24 h) modulated S1P lyase and phosphatase mRNA expression. A) S1P lyase (Sgpl1), B) S1P-specific phosphatase 1 (Sgppl1), and C) S1P-specific phosphatase 2 (Sgppl2). Data is mean ± SEM, n = 4, **p < 0.01 vs no flow.
3.5. Short-term fluid flow modulates S1P signalling gene expression
Gene expression changes following short-term exposure to fluid flow (5 dynes/cm2 for 1 h) identified that many of the effects observed in the long-term fluid flow studies occur within a rapid time frame. Extractions were performed immediately or 3 h after fluid flow exposure to examine the changes to gene expression over time.
Sost expression (Fig. 5A) was downregulated immediately upon cessation of fluid flow and 3 h post fluid flow treatment. Spns2 mRNA expression (Fig. 5B) was similarly downregulated, both immediately and 3 h after fluid flow exposure.
Fig. 5.
Short-term fluid flow exposure modulates genes related to S1P signalling.
Expression of A) Sost and genes related to S1P signalling B) Spns2, C) S1Pr2, D) S1Pr3, E) S1Pr5, F) Sgpl1, G) Sgppl1, H) Sgppl2, I) SphK1 were modulated in Ocy454 cells were exposed to fluid shear stress (FSS) (5 dynes/cm2) 5 dynes of fluid flow for 1 h. Extractions were performed immediately or 3 h after fluid flow exposure. Data is mean ± SEM, n = 4, *p < 0.05, **p < 0.01, ****p < 0.0001 vs no flow. #p < 0.05, ##p < 0.01 vs immediate extract.
S1Pr2 (Fig. 5C) was upregulated immediately after fluid flow treatment returning to no flow levels by 3 h post flow. S1Pr3 expression (Fig. 5D) was significantly downregulated immediately and 3 h post fluid flow treatment change compared to no flow control. S1Pr5 expression was not significantly altered immediately following cessation of fluid flow, however was significantly downregulated 3 h post fluid flow (Fig. 5E).
Expression of Sgpl1 (Fig. 5F) was upregulated immediately after fluid flow treatment returning to no flow levels by 3 h post flow. Sgppl1 (Fig. 5G) and Sgppl2 (Fig. 5H) expression were unchanged immediately flowing flow treatment however were significantly downregulated 3 h after fluid flow exposure.
The expression of SphK1 (Fig. 5I) was significantly upregulated immediately upon cessation of 1 h of fluid flow returning to no flow levels by 3 h post flow.
The expression of S1Pr1, S1Pr4, and Sphk2 was not significantly altered from no flow control in any fluid flow treatment groups (data not shown).
3.6. Detection of S1P in conditioned media from fluid flow exposed Ocy454 cells
We compared the levels of S1P in conditioned media (CM) collected from Ocy454 cells exposed to5 dynes of fluid flow for 1 h compared to no flow CM. S1P levels were measured by a competitive ELISA, and up to 3.47 ± 0.25 μM (mean ± SEM) of S1P was detected in CM, significantly higher than those observed in no flow CM which had an undetectable level of S1P (p < 0.05). As the levels of S1P detected in Flow conditioned media could be complicated by the presence of S1P in the fetal calf serum component of the cell culture media we tested fresh cell culture media which had not been exposed to Ocy454 cells. We did not detect any S1P, thus ruling out any potential interference from serum in our ELISA (Fig. 6A).
Fig. 6.
Mechanical loading of Ocy454 cells (5 dynes/cm2, 1 h) rapidly increased S1P (ELISA) in (A) conditioned media and (B) protein lysates. Data is mean ± SEM. N = 3. *p < 0.05, **p < 0.01 vs no flow. Housekeeping gene (HPRT1). ND: not detected.
3.7. Detection of S1P in cell lysates from fluid flow exposed Ocy454 cells
S1P was not detected in no flow lysates however was significantly increased in flow treated Ocy454 lysates (30.27 ± 10.24 pmol/well (p < 0.05) (Fig. 6B)).
4. Discussion
This study investigated the effect of mechanical loading on osteocyte S1P signalling. We identified that exposure of Ocy454 osteocyte cells to pulsatile fluid flow resulted in a significant increase in S1P production. Furthermore we characterised the expression profile of S1P signalling related genes in Ocy454 cells and their response to mechanical loading.
As a mechanosensitive cell, it was important to investigate how S1P related gene expression was modified in osteocytes, given the known role of S1P as a stimulator of migration and survival of osteoblasts (Ryu et al., 2006). S1P has already been implicated as a key mediator of mechanosensitive processes in vascular cell (Bolz et al., 2003). We observed a significant upregulation of the gene required for intracellular S1P production (Sphk1) in Ocy454 cells exposed to pulsatile fluid flow. This increase in SphK1 mRNA was reflected in increased S1P levels in both conditioned media and protein lysates from mechanically-loaded Ocy454 cells. We also confirmed that S1P lyase (Sgpl1) and S1P phosphatases 1 and 2 (Sgppl1, Sgppl2) the signalling components responsible for S1P degradation and Spinster-homolog-2 (Spns2), the transporter protein responsible for S1P transport, are down-regulated by mechanical loading, providing robust confirmation that both production of S1P and its stability is elevated by mechanical load and may therefore play a significant role in the bone formation response.
It has been suggested that SphK1 upregulation has a negative effect on osteoclastogenesis with correlated changes in ERK and p38 signalling (Ryu et al., 2006). Additionally, overexpression of Sphk1 in calvarial osteoblasts stimulated migration and survival in S1P conditioned media (Ryu et al., 2006). However, these studies utilised a co-culture of bone marrow derived macrophages (osteoclast precursor cells) and calvarial osteoblasts, finding that SphK1 had dichotomous role in osteoclastogenesis depending on RANKL concentration and the microenvironment (Ryu et al., 2006). With our focus on osteocyte-like cells, a more accurate reflection how SphK1 is modulated in mechanical load could be investigated, given the accepted place of the osteocyte as the master regulator of bone remodelling. We propose that the upregulation of SphK1 in Ocy454 cells represents an increase in intracellular phosphorylation of Sphingosine which, coupled with the downregulation of Spns2, Sgpl1, Sgppl1, and Sgppl2 is used as a summation of the amount of mechanical force that the osteocyte has endured. By modulating S1P production and secretion, osteocytes have a number of means to translate the force they have experienced into a biological response. Firstly, osteocytes could respond to the amount of stress by releasing stored S1P in response to fluid flow, allowing paracrine stimulation osteoblasts and osteoclasts to an extent directly proportional to the amount of bone remodelling needed.
To date, there has been limited work examining S1P signalling in osteocytes, however, it is possible to extrapolate meaning from literary findings in other cell lines. Spns2, a member of the facilitator superfamily of non-ATP-dependent transporters, has been identified as a transporter of S1P both in vivo and in vitro (Hisano et al., 2012, Fukuhara et al., 2012, Kawahara et al., 2009). Bradley et al. confirmed, in the human NSCLC cell lines A549 and H1299, that Spns2 knockdown increases intracellular S1P levels (Bradley et al., 2014). The intracellular accumulation of S1P has a number of implications. While the direct targets of intracellular S1P are not known, there is evidence that the Sph:S1P ratio has a critical impact on cell cycle regulation, apoptosis and calcium homeostasis (Spiegel and Milstien, 2003). Increases in intracellular calcium have shown to be directly correlated to the amount of fluid flow shear stress exposed to MLO-Y4 cells (Rath et al., 2010). Activity of MAPK and p38 stress signalling pathways has also been associated with intracellular calcium mobilization (Kamioka et al., 2006). Zhang et al. have suggested that S1P mediated intracellular calcium concentration is linked with PGE2 synthesis and release (Zhang et al., 2015). Each theory represents a potential mechanosensitive mechanism of endogenous S1P.
It is also worth noting that western blot analyses have shown that Spns2 expression is intrinsically linked with the Akt/PI3K and Jak/Stat3 pathways; increased expression of Spns2 decreases their activity while Spns2 knockdown increased their activity (Bradley et al., 2014). Both of these signalling pathways are crucial for cell migration (Teng et al., 2009, Jope et al., 2007). Inhibition of either pathway or S1P synthesis using an SphK1 inhibitor, removed the observed enhanced migration, strengthening the case that intracellular S1P has a direct influence on PI3K/Akt and Jak/Stat3 mediated cell migration, albeit in A549 and H1299 cell lines (Bradley et al., 2014). This adds another potential layer to the multifaceted role of S1P in bone remodelling, as Spns2 downregulation could trigger orchestrated movements which assist the osteocyte in bone remodelling mediation by regulating osteoblast and osteoclast cell migration within the bone matrix.
An important component in the mediation of both intracellular and extracellular S1P is the role of Sppl1 and Sgpl1 expression in dephosphorylation and cleaving S1P bases respectively. Both enzymes reside in the endoplasmic reticulum and have been associated with disease and cellular responses. siRNA induced knockdown of human Sphingosine-1-phosphatase 1 (Sgppl1) caused accumulation of S1P within cells and media, leading to suggestions that it has important role in apoptosis and cell proliferation (Johnson et al., 2003). These results endorse our hypothesis that a mechanotransductive response from osteocytes is incumbent on an increase of intracellular and secreted S1P, which is reinforced by a mechanically-induced downregulation of the enzymes responsible for its degradation and dephosphorylation.
5. Conclusions
S1P signalling components were demonstrated to be expressed in Ocy454 cells and significantly modulated by pulsatile fluid flow. We established the expression profile of all genes related to the S1P signalling system in culture conditions most appropriate to in vivo osteocytes. This study represents the first reporting of a number of observations regarding the modulation of the S1P system following pulsatile fluid flow. Fluid flow mediated downregulation of Spns2, Sgpl1 and Sgppl1, along with upregulation of SphK1, collectively reinforcing a mechanically induced increase in intracellular S1P in osteocytes. The implications of this response include an increase in intracellular calcium, leading to increase PGE2 synthesis and release. The evidence of S1P signalling involvement in the osteocyte's ability to transduce a mechanical signal into a biochemical response is captivating. Further discovery and investigation could help identify new therapeutic targets for treatment of pathological disruption to the bone remodelling process.
Competing financial interests statement
The authors have no competing financial interests.
Author contributions statement
Study design and execution C.D. and J.H.G.; data collection and analysis C.D. and J.H.G.; data interpretation C.D. and J.H.G.; manuscript draft C.D. and J.H.G. All authors reviewed the manuscript.
References
- Alford A.I., Jacobs C.R., Donahue H.J. Oscillating fluid flow regulates gap junction communication in osteocytic MLO-Y4 cells by an ERK1/2 MAP kinase-dependent mechanism. Bone. 2003;33(1):64–70. doi: 10.1016/s8756-3282(03)00167-4. (Epub 2003/08/16) [DOI] [PubMed] [Google Scholar]
- Bolz S.S., Vogel L., Sollinger D., Derwand R., Boer C., Pitson S.M. Sphingosine kinase modulates microvascular tone and myogenic responses through activation of RhoA/Rho kinase. Circulation. 2003;108(3):342–347. doi: 10.1161/01.CIR.0000080324.12530.0D. (Epub 2003/07/09) [DOI] [PubMed] [Google Scholar]
- Bradley E., Dasgupta S., Jiang X., Zhao X., Zhu G., He Q. Critical role of Spns2, a sphingosine-1-phosphate transporter, in lung cancer cell survival and migration. PLoS One. 2014;9(10) doi: 10.1371/journal.pone.0110119. (Epub 2014/10/21) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara S., Simmons S., Kawamura S., Inoue A., Orba Y., Tokudome T. The sphingosine-1-phosphate transporter Spns2 expressed on endothelial cells regulates lymphocyte trafficking in mice. J. Clin. Invest. 2012;122(4):1416–1426. doi: 10.1172/JCI60746. (Epub 2012/03/13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooi J.H., Chia L.Y., Walsh N.C., Karsdal M.A., Quinn J.M., Martin T.J. Decline in calcitonin receptor expression in osteocytes with age. J. Endocrinol. 2014;221(2):181–191. doi: 10.1530/JOE-13-0524. (Epub 2014/02/12) [DOI] [PubMed] [Google Scholar]
- Hisano Y., Kobayashi N., Yamaguchi A., Nishi T. Mouse SPNS2 functions as a sphingosine-1-phosphate transporter in vascular endothelial cells. PLoS One. 2012;7(6) doi: 10.1371/journal.pone.0038941. (Epub 2012/06/23) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huiskes R., Ruimerman R., van Lenthe G.H., Janssen J.D. Effects of mechanical forces on maintenance and adaptation of form in trabecular bone. Nature. 2000;405(6787):704–706. doi: 10.1038/35015116. (Epub 2000/06/23) [DOI] [PubMed] [Google Scholar]
- Ishii M., Egen J.G., Klauschen F., Meier-Schellersheim M., Saeki Y., Vacher J. Sphingosine-1-phosphate mobilizes osteoclast precursors and regulates bone homeostasis. Nature. 2009;458(7237):524–528. doi: 10.1038/nature07713. (Epub 2009/02/11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K.R., Johnson K.Y., Becker K.P., Bielawski J., Mao C., Obeid L.M. Role of human sphingosine-1-phosphate phosphatase 1 in the regulation of intra- and extracellular sphingosine-1-phosphate levels and cell viability. J. Biol. Chem. 2003;278(36):34541–34547. doi: 10.1074/jbc.M301741200. (Epub 2003/06/20) [DOI] [PubMed] [Google Scholar]
- Jope R.S., Yuskaitis C.J., Beurel E. Glycogen synthase kinase-3 (GSK3): inflammation, diseases, and therapeutics. Neurochem. Res. 2007;32(4–5):577–595. doi: 10.1007/s11064-006-9128-5. (Epub 2006/09/01) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamioka H., Sugawara Y., Murshid S.A., Ishihara Y., Honjo T., Takano-Yamamoto T. Fluid shear stress induces less calcium response in a single primary osteocyte than in a single osteoblast: implication of different focal adhesion formation. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2006;21(7):1012–1021. doi: 10.1359/jbmr.060408. (Epub 2006/07/04) [DOI] [PubMed] [Google Scholar]
- Karagiosis S.A., Karin N.J. Lysophosphatidic acid induces osteocyte dendrite outgrowth. Biochem. Biophys. Res. Commun. 2007;357(1):194–199. doi: 10.1016/j.bbrc.2007.03.121. (Epub 2007/04/10) [DOI] [PubMed] [Google Scholar]
- Kawahara A., Nishi T., Hisano Y., Fukui H., Yamaguchi A., Mochizuki N. The sphingolipid transporter spns2 functions in migration of zebrafish myocardial precursors. Science. 2009;323(5913):524–527. doi: 10.1126/science.1167449. (Epub 2008/12/17) [DOI] [PubMed] [Google Scholar]
- Keller J., Catala-Lehnen P., Huebner A.K., Jeschke A., Heckt T., Lueth A. Calcitonin controls bone formation by inhibiting the release of sphingosine 1-phosphate from osteoclasts. Nat. Commun. 2014;5:5215. doi: 10.1038/ncomms6215. (Epub 2014/10/22) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotinun S., Kiviranta R., Matsubara T., Alzate J.A., Neff L., Luth A. Osteoclast-specific cathepsin K deletion stimulates S1P-dependent bone formation. J. Clin. Invest. 2013;123(2):666–681. doi: 10.1172/JCI64840. (Epub 2013/01/17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima T., Hayashi M., Fukunaga T., Kurata K., Oh-Hora M., Feng J.Q. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat. Med. 2011;17(10):1231–1234. doi: 10.1038/nm.2452. (Epub 2011/09/13) [DOI] [PubMed] [Google Scholar]
- Pederson L., Ruan M., Westendorf J.J., Khosla S., Oursler M.J. Regulation of bone formation by osteoclasts involves Wnt/BMP signaling and the chemokine sphingosine-1-phosphate. Proc. Natl. Acad. Sci. U. S. A. 2008;105(52):20764–20769. doi: 10.1073/pnas.0805133106. (Epub 2008/12/17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piekarski K., Munro M. Transport mechanism operating between blood supply and osteocytes in long bones. Nature. 1977;269(5623):80–82. doi: 10.1038/269080a0. (Epub 1977/09/01) [DOI] [PubMed] [Google Scholar]
- Rath A.L., Bonewald L.F., Ling J., Jiang J.X., Van Dyke M.E., Nicolella D.P. Correlation of cell strain in single osteocytes with intracellular calcium, but not intracellular nitric oxide, in response to fluid flow. J. Biomech. 2010;43(8):1560–1564. doi: 10.1016/j.jbiomech.2010.01.030. (Epub 2010/03/02) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu J., Kim H.J., Chang E.J., Huang H., Banno Y., Kim H.H. Sphingosine 1-phosphate as a regulator of osteoclast differentiation and osteoclast-osteoblast coupling. EMBO J. 2006;25(24):5840–5851. doi: 10.1038/sj.emboj.7601430. (Epub 2006/11/25) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel S., Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat. Rev. Mol. Cell Biol. 2003;4(5):397–407. doi: 10.1038/nrm1103. (Epub 2003/05/03) [DOI] [PubMed] [Google Scholar]
- Tatsumi S., Ishii K., Amizuka N., Li M., Kobayashi T., Kohno K. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab. 2007;5(6):464–475. doi: 10.1016/j.cmet.2007.05.001. (Epub 2007/06/07) [DOI] [PubMed] [Google Scholar]
- Teng T.S., Lin B., Manser E., Ng D.C., Cao X. Stat3 promotes directional cell migration by regulating Rac1 activity via its activator betaPIX. J. Cell Sci. 2009;122(Pt 22):4150–4159. doi: 10.1242/jcs.057109. (Epub 2009/10/29) [DOI] [PubMed] [Google Scholar]
- Weinbaum S., Cowin S.C., Zeng Y. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J. Biomech. 1994;27(3):339–360. doi: 10.1016/0021-9290(94)90010-8. (Epub 1994/03/01) [DOI] [PubMed] [Google Scholar]
- Wood C.L., Pajevic P.D., Gooi J.H. Osteocyte secreted factors inhibit skeletal muscle differentiation. Bone Rep. 2017;6:74–80. doi: 10.1016/j.bonr.2017.02.007. (Epub 2017/04/06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- You J., Reilly G.C., Zhen X., Yellowley C.E., Chen Q., Donahue H.J. Osteopontin gene regulation by oscillatory fluid flow via intracellular calcium mobilization and activation of mitogen-activated protein kinase in MC3T3-E1 osteoblasts. J. Biol. Chem. 2001;276(16):13365–13371. doi: 10.1074/jbc.M009846200. (Epub 2001/03/30) [DOI] [PubMed] [Google Scholar]
- Zhang J.N., Zhao Y., Liu C., Han E.S., Yu X., Lidington D. The role of the sphingosine-1-phosphate signaling pathway in osteocyte mechanotransduction. Bone. 2015;79:71–78. doi: 10.1016/j.bone.2015.05.017. (Epub 2015/05/20) [DOI] [PubMed] [Google Scholar]