Abstract
Pseudomonas aeruginosa an opportunistic pathogen regulates its virulence through Quorum sensing (QS) mechanism comprising of Las and Rhl system. Targeting of QS mechanism could be an ideal strategy to combat infection caused by P. aeruginosa. Silver nanoparticles (AgNPs) have been broadly applied as antimicrobial agents against a number of pathogenic bacterial and fungal strains, but have not been reported as an anti-QS agent. Therefore, the aim of present work was to show the computational analysis for the interaction of AgNPs with the QS system using an In silico approach. In silico studies showed that AgNPs got ‘locked’ deeply into the active site of respective proteins with their surrounding residues. The molecular docking analysis clearly demonstrated that AgNPs got bound to the catalytic cleft of LasI synthase (Asp73-Ag = 3.1 Å), RhlI synthase (His52-Ag = 2.8 Å), transcriptional receptor protein LasR (Leu159-Ag = 2.3 Å) and RhlR (Trp10-Ag = 3.1 Å and Glu34-Ag = 3.2 Å). The inhibition of LasI/RhlI synthase by AgNPs blocked the biosynthesis of AHLs, thus no AHL produced, no QS occurred. Further, interference with transcriptional regulatory proteins led to the inactivation of LasR/RhlR system that finally blocked the expression of QS-controlled virulence genes. Our findings clearly demonstrate the anti-QS property of AgNPs in P. aeruginosa which could be an alternative approach to the use of traditional antibiotics for the treatment of P. aeruginosa infection.
Keywords: In silico, Molecular docking, Quorum sensing, Pseudomonas aeruginosa, Virulence, Silver nanoparticles
Introduction
Quorum sensing (QS) is a phenomenon of a cell to cell communication using diffusible chemical signaling molecules (Reading and Sperandio 2006; Banik et al. 2009). QS molecules control an array of pathogenic behavior such as the production of virulence factors and biofilm formation (Davies 2003) in many gram negative and gram positive bacteria. Pseudomonas aeruginosa is opportunistic pathogens whose most of the virulence characters are QS-controlled and cause acute infection in respiratory systems, particularly in cystic fibrosis and HIV patients (Palmer et al. 2005). However, treatment with antibiotics makes the antibiotic treatment ineffective as it develops resistance within a short period of time (Su et al. 2010; Juan et al. 2010). P. aeruginosa controls its pathogenicity by producing, detecting and responding to extracellular signaling molecules called as autoinducers (Longo et al. 2013). Studies regarding Quorum sensing signalling molecules (QSSMS) or autoinducers in P. aeruginosa have confirmed that these autoinducers in P. aeruginosa are called as acyl homoserine lactones (AHLs) which control the production of virulence factors, antibiotic resistance and biofilm formation (Pearson et al. 1997; Delden and Iglewski 1998; Pesci et al. 1999). The two QS-systems which are involved in the pathogenicity of P. aeruginosa using autoinducers are Las and Rhl systems. The Las QS system through its LasI gene synthesizes N-(3-oxododecanoyl)-L-homoserine lactone (3-oxo-C12-HSL) which is detected by its homolog LasR and Rhl system through its RhlI gene synthesize N-butyryl-L-homoserine lactone (C4-HSL), which is recognized by its receptor RhlR. When a certain threshold concentration of 3-oxo-C12-HSL is reached, it binds to the receptor protein LasR and activates the expression of virulence genes as proteases, elastases and exotoxin A (Passador et al. 1993). In addition, the LasI homolog RhlI is regulated by LasR-3-oxo-C12HSL complex. RhlI after the threshold level of C4-HSL binds to the receptor RhlR and activates target genes, responsible for the production of pyocyanin, elastases, siderophores, and rhamnolipids (Wagner et al. 2008). The combined effect of QS complex leads to the infection although antibiotics are available against the infection but the development of antibiotic resistance makes this bacterium more harmful. Therefore, there is an urgent need to search for the cost-effective and alternative remedy for the treatment of P. aeruginosa infection. Interfering with these bacterial QS systems could have broad application in biological control of disease since these QS molecules are used to regulate virulence (Alvarez et al. 2012). Nanotechnology could provide an answer to this where small particles of nano size called as nanoparticles could interfere with these QS molecules. Nanoparticles have many advantages like reduce toxicity which overcomes resistance and are also cost effective (Pal et al. 2007; Weir et al. 2008). It has been proved that naturally occurring bacteria do not develop resistance to nanoparticles (Mühling et al. 2009). Some of the previous reports have shown the down regulation of QS mediated virulence factors by AgNPs (Singh et al. 2015; Ali et al. 2017). Vyshnava et al. (2016) have also shown the antibiofilm potential of AgNPs using In silico approach. Most of the reports claiming AgNPs to be anti-QS agents are available but very few reports have focused onto to the computational analysis of Ag with QS. Keeping in view about the importance of computational analysis and limited availability of literature about AgNPs and QS controlled virulence genes we have designed few objectives as (1) In silico study of anti-QS activity of AgNPs, (2) molecular docking analysis of interaction of AgNPs with QS-controlled proteins LasI/RhlI AHL synthase, (3) molecular docking analysis of interaction of AgNPs with QS-controlled transcriptional regulatory proteins LasR/RhlR and virulence genes.
Materials and methods
In silico analysis
Preparation of Las synthase and Las receptor molecules
The 3D structure of LasI Synthase (PDB ID: 1RO5) and transcriptional activator protein LasR (PDB ID: 2UV0) was retrieved from Protein Data Bank (www.rcsb.org). Unfortunately, the crystal 3D structures of RhlI Synthase and regulatory protein RhlR were not available in the Protein Databank, so, we have modelled the 3D structure of RhlI Synthase and RhlR using SWISS-MODEL homology method. PROMOD-II tool integrated into SWISS-MODEL has been used in the model building on the basis of target-template alignment (Sali and Blundell 1993; Guex et al. 2009). QMEAN scoring function has been used by SWISS MODEL to estimate the overall quality of modelled 3D structure (Benkert et al. 2011). All the water molecules and hetero atoms were removed. The Model validation analysis was completed by RAMGAGE Ramachandran Plot analysis server (http://mordred.bioc.cam.ac.uk/~rapper/rampage.php) (Lovell et al. 2002).
Preparation of 3D structure of Ag
The .mol file of Silver (Ag) was taken from Protein Data Bank and converted into .pdb files using Open Babel software. Discovery Studio makes it easier to examine the properties of large and small molecules. Gasteiger charge was added to the ligand. Further, the ligand was submitted for minimization using Chimera version 1.10 using Genetic Algorithm Steps 2000 and 0.5 grid units Optimized (Pettersen et al. 2011).
Prediction of docking/active site of Las and Rhl systems
Prediction of the Ag binding sites for generated LasI/R and RhlI/R models was done by the 3D Ligand site developed by the structural bioinformatics groups, Imperial College London. The pdb files were uploaded in the space provided in the tool, and these pdb files were processed for the identification of active binding sites for Ag ligands based on the critical assessment of techniques for protein structure prediction (Wass et al. 2010).
Molecular docking
Docking was performed over rigid protein structures of LasI Synthase, LasR receptor, RhlI Synthase and RhlR receptor. The interaction of AgNPs with AHL Synthase LasI, LasR receptor, AHL Synthase RhlI and RhlR receptor was explored through PatchDock tool (Schneidman-Duhovny et al. 2005). PatchDock is a geometry-based molecular docking algorithm which is Critical Assessment of Prediction of Interactions (CAPRI). The default RMSD value for this parameter was 4 Å. Based on the ranking, the scores of the docked file were selected and subjected to the post-docking 3D simulation using discovery studio visualize 4.5 and PyMol 3D visualization software.
Results
In silico studies
The 3D crystal structure of LasI Synthase (PDB ID: 1RO5; Fig. 1a) and transcriptional activator protein LasR (PDB ID: 2UV0; Fig. 2a) was retrieved from Protein Data Bank. Due to the unavailability of a 3D crystal structure of RhlI Synthase and regulatory protein in the Protein Databank, we modelled the 3D structure of RhlI Synthase (Fig. 3a) and RhlR (Fig. 4a) using SWISS-MODEL homology method. The Model validation of conformation of RhlI Synthase and RhlR was done by Ramachandran Plot (Hollingsworth and Karplus 2010) based on the Chi (Φ) and Psi (Ψ) analysis; Ramachandran Plot of RhlI Synthase showed 94.5% of the residues in favored region, 4.9% residues in the allowed regions and 0.5% residues in outlier region (Fig. 3b). Similarly, Ramachandran Plot of RhlR showed 97.2% of the residues in the favored region, 2.1% residues in the allowed regions and 0.6% residues in outlier region (Fig. 4b).
Fig. 1.
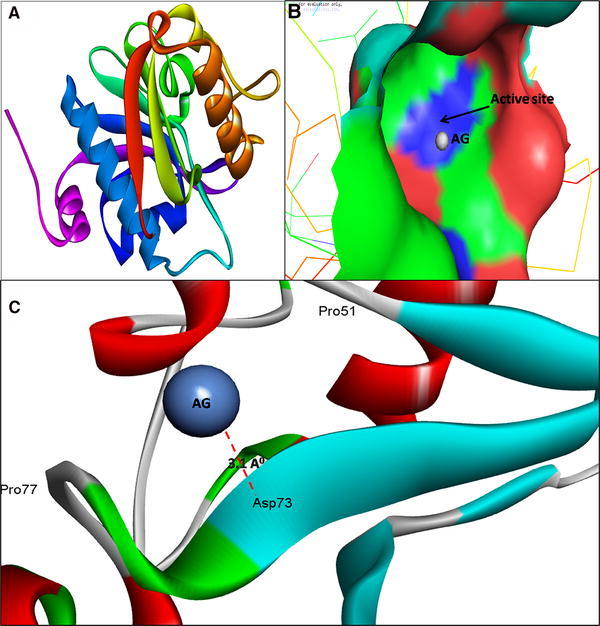
3D structure of Acyl-homoserine lactone (AHL) Synthase LasI (a); binding of AgNPs with the active site of LasI (b); close view of catalytic site of LasI, with bound Ag represented as blue sphere. Amino acids residues (Asp73) of LasI involved in the interaction with AgNPs (c). LasI active site has been depicted by PyMol viewer. Interaction analysis of AgNPs with LasI has been explored through PatchDock
Fig. 2.
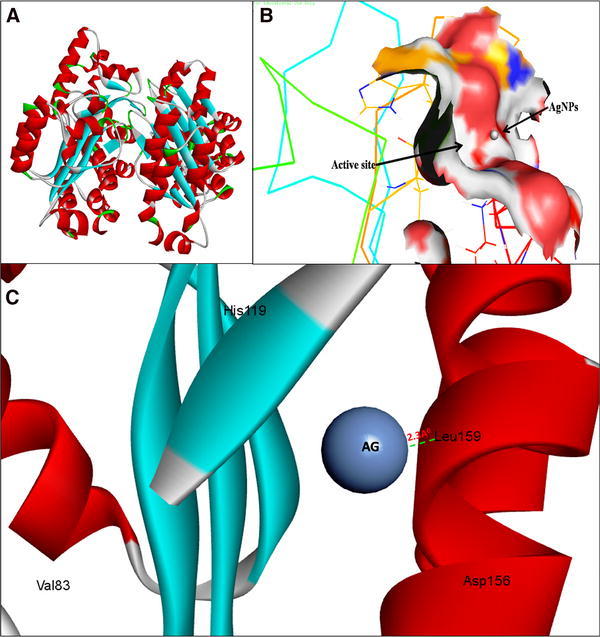
3D structure of transcriptional activator protein LasR (a); binding of AgNPs with the active site of LasR (b); close view of catalytic site of LasR, with bound Ag represented as blue sphere. Amino acids residues (Leu159) of LasR involved in the interaction with AgNPs (c). LasR active site has been depicted by PyMol viewer. Interaction analysis of AgNPs with LasR has been explored through PatchDock
Fig. 3.
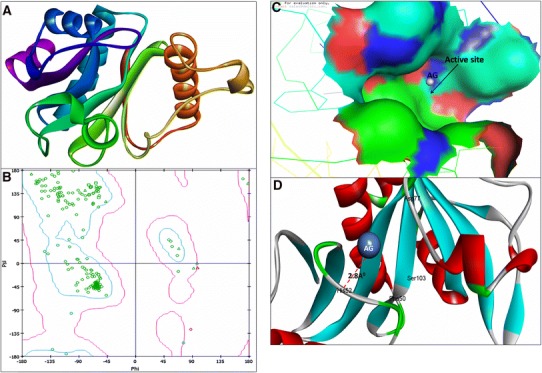
3D structure of Acyl-homoserine lactone (AHL) Synthase RhlI (a); RAMPAGE validation of conformation of RhlI (b); binding of AgNPs with the active site of RhlI (c); close view of catalytic site of RhlI, with bound Ag represented as blue sphere. Amino acids residues (His52) of RhlI involved in the interaction with AgNPs (d). RhlI active site has been depicted by PyMol viewer. Interaction analysis of AgNPs with RhlI has been explored through PatchDock
Fig. 4.
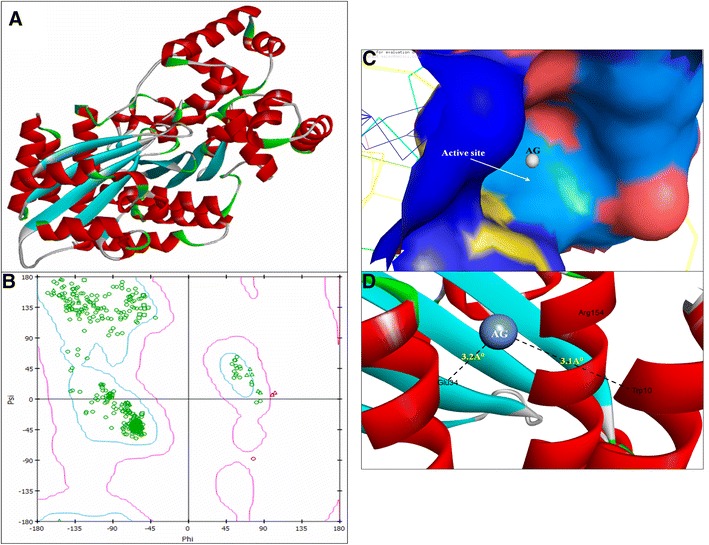
3D structure of regulatory protein RhlR (a); RAMPAGE validation of conformation of RhlR (b); binding of AgNPs with the active site of RhlR (c); close view of catalytic site of RhlR, with bound Ag represented as blue sphere. Specific residues (i.e., Trp10 & Glu34) of RhlR involved in the interaction with AgNPs (d). RhlR active site has been depicted by PyMol and the interaction analysis of AgNPs with RhlR has been explored by PatchDock
The molecular docking results showed that AgNPs were docked into AHLs synthase LasI (Fig. 1b) and RhlI (Fig. 3c) as well as regulatory activator proteins LasR (Fig. 2b) and RhlR (Fig. 4c) successfully. Based on this docking pose, we speculated that the AgNPs got ‘‘locked’’ deeply into the active site of respective proteins with their surrounding residues (Figs. 1c, 2c, 3d, 4d). The molecular docking analysis showed that the Ag(0) got bound to the catalytic Aspartate residue of synthase LasI (Asp73-Ag = 3.1 Å; Fig. 1c), Leucine residue of LasR receptor (Leu159-Ag = 2.3 Å; Fig. 2c), Histidine residue of RhlI synthase (His52-Ag = 2.8 Å; Fig. 3d) and Tryptophan (Trp10-Ag = 3.1 Å) and Glutamate (Glu34-Ag = 3.2 Å) residues of RhlR receptor (Fig. 4d).
Discussion
The molecular docking is a powerful computational based approach that is used to model the interaction between a small molecule and a protein at the atomic level to characterize and elucidate the behavior of small molecules in the binding site of target proteins. We were therefore interested in carrying out exploratory studies into the QS-modulatory activity of AgNPs by In silico approach. It is well known that P. aeruginosa utilizes QS by producing AHLs molecules to control numbers of virulence factors. For example, the Las and Rhl system regulates the production of the virulence factors protease, elastases, exotoxin A, pyocyanin, rhamnolipid, hydrogen cyanide and biofilms formation (Hodgkinson et al. 2012). Thus, interfering with AHL-based QS by AgNPs is expected to become a master plan to attenuate the virulence in P. aeruginosa and can be utilized as a novel chemotherapeutic agent to overcome the QS-controlled infections. The LasI and RhlI synthase direct the synthesis of 3-oxo-C12-HSL and C4-HSL, respectively in P. aeruginosa which triggers the LasR and RhlR-encoded transcriptional activator (LasR and RhlR) to induce the virulence genes. Therefore, Las and Rhl systems were considered as a molecular target for AgNPs. To understand the mechanism of AgNPs mediated inhibition of AHL synthase (LasI/RhlI) and suppression of transcriptional regulatory receptors proteins (LasR/RhlR); molecular docking analysis was performed to predict the probable AgNPs binding site in AHL synthase and LasR/RhlR receptors.
The Model validation of conformation of RhlI Synthase and RhlR was done by Ramachandran Plot based on the inspection of Chi (Φ) and Psi (Ψ). Our data of Ramachandran Plot of RhlR are almost similar to the previous report of Gnanendra et al. (2010). Modelled 3D structures of LasI/R and RhlI/R was submitted to minimization process. Chimera 1.10 was used for energy minimization, removal of steric collision with the steepest descent steps 1000, steepest descent size 0.02 Å, conjugated gradient steps 1000 and the conjugate gradient step size 0.02 Å for the conjugate gradient minimization (Pettersen et al. 2011; Wang et al. 2004, 2006). Our data on molecular docking analysis (Figs. 1, 2, 3, 4) clearly shows that silver binds in the catalytic cleft of AHLs synthase (LasI/RhlI) receptor proteins (LasR/RhlR) and finally, inhibits the QS-controlled virulence genes and biofilm formation in P. aeruginosa. Our molecular docking results on the interaction and bindings of Ag with LasR and RhlR are consistent with the findings of Vyshnava et al. (2016). Although the interaction and binding of AgNPs with LasI and RhlI has not been previously reported in the literature, herein for the first time, we have shown the binding of AgNPs with the LasI (Fig. 1) and RhlI signal synthase (Fig. 3).
Based on In silico findings, we have suggested following possible mechanisms of AgNPs interruption with QS circuits, including (1) blocking of biosynthesis of S-adenosyl methionine (SAM), SAM acts as an amino donor for generation of the homoserine lactone ring moiety. It is supposed that AgNPs may bind to SAM, which leads to depletion of SAM and finally no AHL produced (Fig. 5-1). (2) Inhibition of signal biosynthesis i.e., inhibition LasI/RhlI synthase. The AgNPs may bind to AHL synthase, thereby inhibiting the enzymatic activity. Thus, if no AHL produced, no quorum sensing occurs (Fig. 5-2). (3) Interference with signal receptor proteins LasR and RhlR. When the bacterial cell density reaches a particular threshold, the LasI/R QS system gets initiated. Ag binds with the active site of LasR. The Ag-LasR complex so formed inhibits the expression of RhlI as well as LasR-controlled genes i.e., LasI signal synthase, which then leads to inactivation of the RhlI/R system that finally blocks and inhibit the expression of QS-controlled virulence genes (Fig. 5-3).
Fig. 5.
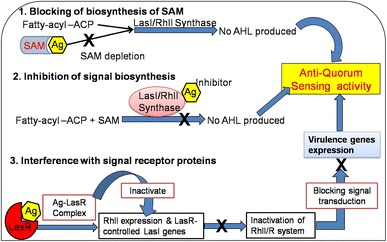
Schematic representation of anti-QS activity of green synthesized AgNPs
Conclusion
It has been concluded that jamming bacterial cell-to-cell communication could be a master plan in developing a novel chemotherapeutic agents. To the best of our knowledge, this is the first In-silico report showing that AgNPs can inhibit AHLs production by interfering with AHL Synthase LasI/RhlI and regulatory receptor proteins LasR/RhlR. Interestingly, AgNP`s have no chemical structure similarity to AHLs analogues, which again suggests a novel mechanism of inhibition of QS-controlled genes and can be an alternative to treat P. aeruginosa infections. The molecular docking analysis result showed that AgNPs got bound to the active site of LasI (Asp73)/RhlI (His52) and LasR (Leu159)/RhlR (Trp10 and Glu34) of the proteins. Thus, the inhibition of quorum sensing in P. aeruginosa using nanoparticles can be recognized as an alternative approach to the use of traditional antibiotics for the treatment of infections caused by this organism. Overall, In silico findings, reveals that the AgNPs can combat with bacterial infections and can act as a novel target for inhibition of QS mediated virulence in P. aeruginosa.
Acknowledgments
Mr. Syed Ghazanfar Ali is grateful to UGC, New Delhi, India for research assistance. Authors thank to Aligarh Muslim University, Aligarh, India and IRMC, University of Dammam, Saudi Arabia for providing instruments facilities and other items used in this study.
Compliance with ethical standards
Funding
None.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human or animals performed by any of the authors.
Footnotes
Syed Ghazanfar Ali, Mohammad Azam Ansari and Qazi Mohd. Sajid Jamal contributed equally.
Contributor Information
Syed Ghazanfar Ali, Email: syedmicro72@gmail.com.
Mohammad Azam Ansari, Email: azammicro@gmail.com.
References
- Ali SG, Ansari MA, Khan HM, Jalal M, Mahdi AA, Cameotra SS. Crataeva nurvala nanoparticles inhibit virulence factors and biofilm formation in clinical isolates of Pseudomonas aeruginosa. J Basic Microbiology. 2017;57:193–203. doi: 10.1002/jobm.201600175. [DOI] [PubMed] [Google Scholar]
- Alvarez MV, Moreira MR, Ponce A. Antiquorum sensing and antimicrobial activity of natural agents with potential use in food. J Food Saf. 2012;32:379–387. doi: 10.1111/j.1745-4565.2012.00390.x. [DOI] [Google Scholar]
- Banik SK, Fenley AT, Kulkarni RV. A model for signal transduction during quorum sensing in Vibrio harveyi. Phys Biol. 2009;6:046008. doi: 10.1088/1478-3975/6/4/046008. [DOI] [PubMed] [Google Scholar]
- Benkert P, Biasini M, Schwede T. Toward the estimation of the absolute quality of individual protein structure models. Bioinformatics. 2011;27:343–350. doi: 10.1093/bioinformatics/btq662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies D. Understanding biofilm resistance to antibacterial agents. Nat Rev Drug Discov. 2003;2:114–122. doi: 10.1038/nrd1008. [DOI] [PubMed] [Google Scholar]
- Delden CV, Iglewski BH. Cell-to-cell signaling and Pseudomonas aeruginosa infections. Emerg Infect Dis. 1998;4:551–560. doi: 10.3201/eid0404.980405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnanendra TS, Geethu G, Arjunan S, Sharmila B, Hussain MIZ, Moorthy K, Jeyakumar N. Theoretical models of quorum sensing dependent regulation of transcriptional activators of Pseudomonas aeruginosa. Inter J Biol Technol. 2010;1:43–49. [Google Scholar]
- Guex N, Peitsch MC, Schwede T. Automated comparative protein structure modeling with SWISS-MODEL and Swiss-PdbViewer: a historical perspective. Electrophoresis. 2009;30:S162–S173. doi: 10.1002/elps.200900140. [DOI] [PubMed] [Google Scholar]
- Hodgkinson JT, Galloway WRJD, Wright M, Mati IK, Nicholson RL, Welch M, Spring DR. Design, synthesis and biological evaluation of non-natural modulators of quorum sensing in Pseudomonas aeruginosa. Org Biomol Chem. 2012;10:6032–6044. doi: 10.1039/c2ob25198a. [DOI] [PubMed] [Google Scholar]
- Hollingsworth SA, Karplus PA. A fresh look at the Ramachandran plot and the occurrence of standard structures in proteins. Biomol Concepts. 2010;1:271–283. doi: 10.1515/bmc.2010.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juan C, Zamorano L, Perez JL, Ge Y, Oliver A. Activity of a new antipseudomonal cephalosporin, CXA-101 (FR264205), against carbapenem resistant and multidrugresistant Pseudomonas aeruginosa clinical strains. Antimicrob Agents Chemother. 2010;54:846–851. doi: 10.1128/AAC.00834-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo F, Rampioni G, Bondì R, Imperi F, Fimia GM, Visca P, Zennaro E, Leoni L. A new transcriptional repressor of the Pseudomonas aeruginosa quorum sensing receptor gene lasR. PLoS ONE. 2013;8:e69554. doi: 10.1371/journal.pone.0069554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell SC, Davis IW, Arendall WB, III, de Bakker PIW, Word JM, Prisant MG, Richardson JS, Richard DC. Structure validation by Calpha geometry: phi, psi and Cbeta deviation. Proteins Struct Funct Genet. 2002;50:437–450. doi: 10.1002/prot.10286. [DOI] [PubMed] [Google Scholar]
- Mühling M, Bradford A, Readman JW, Somerfield PJ, Handy RD. An investigation into the effects of silver nanoparticles on antibiotic resistance of naturally occurring bacteria in an estuarine sediment. Mar Environ. 2009;68:278–283. doi: 10.1016/j.marenvres.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Pal S, Tak YK, Song JM. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl Environ Microbiol. 2007;73:1712–1720. doi: 10.1128/AEM.02218-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer KL, Mashburn LM, Singh PK, Whiteley M. Cystic fibrosis sputum supports growth and cues key aspects of Pseudomonas aeruginosa physiology. J Bacteriol. 2005;187:5267–5277. doi: 10.1128/JB.187.15.5267-5277.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passador L, Cook JM, Gambello MJ, Rust L, Iglewski BH. Expression of Pseudomonas aeruginosa virulence genes requires cell-to-cell communication. Science. 1993;260:1127–1130. doi: 10.1126/science.8493556. [DOI] [PubMed] [Google Scholar]
- Pearson JP, Pesci EC, Iglewski BH. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J Bacteriol. 1997;179:5756–5767. doi: 10.1128/jb.179.18.5756-5767.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesci EC, Milbank JB, Pearson JP, McKnight S, Kende AS, Greenberg EP, Iglewski BH. Quinolone signaling in the cell-to-cell communication system of Pseudomonas aeruginosa. Proc Natl Acad Sci. 1999;96:11229–11234. doi: 10.1073/pnas.96.20.11229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem. 2011;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Reading NC, Sperandio V. Quorum sensing: the many languages of bacteria. FEMS Microbiol Lett. 2006;254:1–11. doi: 10.1111/j.1574-6968.2005.00001.x. [DOI] [PubMed] [Google Scholar]
- Sali A, Blundell TL. Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol. 1993;234:779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- Schneidman-Duhovny D, Inbar Y, Nussinov R, Wolfson HJ. PatchDock and SymmDock: servers for rigid and symmetric docking. Nucl Acids Res. 2005;33:W363–W367. doi: 10.1093/nar/gki481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh BR, Singh BN, Singh A, Khan W, Naqvi AH, Singh HB. Mycofabricated biosilver nanoparticles interrupt Pseudomonas aeruginosa quorum sensing systems. Sci Rep. 2015;5:13719. doi: 10.1038/srep13719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su HC, Ramkissoon K, Doolittle J, Clark M, Khatun J, Secrest A, Wolfgang MC, Giddings MC. The development of ciprofloxacin resistance in Pseudomonas aeruginosa involves multiple response stages and multiple proteins. Antimicrob Agents Chemother. 2010;54:4626–4635. doi: 10.1128/AAC.00762-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyshnava SS, Kanderi DK, Panjala SP, Pandian K, Bontha RR, Goukanapalle PKR, Banaganapalli B. Effect of silver nanoparticles against the formation of biofilm by Pseudomonas aeruginosa an in silico approach. Appl Biochem Biotechnol. 2016;180(3):426–437. doi: 10.1007/s12010-016-2107-7. [DOI] [PubMed] [Google Scholar]
- Wagner VE, Filiatrault MJ, Picardo KF, Iglewski BH. Pseudomonas aeruginosa virulence and pathogenesis issues. In: Cornelis P, editor. Pseudomonas genomics and molecular biology. 1. Norfolk: Caister Academic Press; 2008. pp. 129–158. [Google Scholar]
- Wang J, Wolf RM, Caldwell JW, Kollman PA, Case DA. Development and testing of general amber force field. J Comput Chem. 2004;25:1157–1174. doi: 10.1002/jcc.20035. [DOI] [PubMed] [Google Scholar]
- Wang J, Wang W, Kollman PA, Case DA. Automatic atom type and bond type perception in molecular mechanical calculations. J Mol Graph Model. 2006;25:247–260. doi: 10.1016/j.jmgm.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Wass MN, Kelley LA, Sternberg MJ. 3DLigandSite: predicting ligand-binding sites using similar structures. Nucl Acids Res. 2010;38:469–473. doi: 10.1093/nar/gkq406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir E, Lawlor A, Whelan A, Regan F. The use of nanoparticles in antimicrobial materials and their characterization. Analyst. 2008;133:835–845. doi: 10.1039/b715532h. [DOI] [PubMed] [Google Scholar]


