Abstract
People with multiple sclerosis (MS) exhibit pronounced changes in brain structure, activity, and connectivity. While considerable work has begun to elucidate how these neural changes contribute to behavior, the heterogeneity of symptoms and diagnoses makes interpretation of findings and application to clinical practice challenging. In particular, whether MS related changes in brain activity or brain connectivity protect against or contribute to worsening motor symptoms is unclear. With the recent emergence of neuromodulatory techniques that can alter neural activity in specific brain regions, it is critical to establish whether localized brain activation patterns are contributing to (i.e. maladaptive) or protecting against (i.e. adaptive) progression of motor symptoms. In this manuscript, we consolidate recent findings regarding changes in supraspinal structure and activity in people with MS and how these changes may contribute to motor performance. Furthermore, we discuss a hypothesis suggesting that increased neural activity during movement may be either adaptive or maladaptive depending on where in the brain this increase is observed. Specifically, we outline preliminary evidence suggesting sensorimotor cortex activity in the ipsilateral cortices may be maladaptive in people with MS. We also discuss future work that could supply data to support or refute this hypothesis, thus improving our understanding of this important topic.
Keywords: Multiple sclerosis, fMRI, Motor performance, Compensation, Pathology, Transcallosal inhibition
Highlights
-
•
The link between brain activity, connectivity, and motor performance in MS is poorly understood.
-
•
Current evidence for adaptive and maladaptive brain changes is discussed.
-
•
We discuss a possible mechanism for maladaptive increases in ipsilateral brain activity.
1. Introduction
Multiple sclerosis (MS) is an autoimmune pathology that leads to numerous neurological (Nylander and Hafler, 2012) and behavioral (Johansson et al., 2007) changes. In particular, people with MS (PwMS) exhibit multifocal demyelinating lesions or ‘plaques’, that can occur in both grey and white matter (Matthews et al., 2016). These changes are accompanied by grey (Jacobsen and Farbu, 2014) and white (Sbardella et al., 2013) matter atrophy and, perhaps unsurprisingly, numerous changes in brain activity (Tomassini et al., 2012b) and connectivity (Sbardella et al., 2015b). Neuroimaging techniques have furthered our understanding of 1) how structure, activity, and connectivity of the brain change with multiple sclerosis, and 2) how these changes may relate to motor performance. However, Our understanding of which neural changes protect against worsening of symptoms, and which are the result of or contribute to worsening of symptoms remains limited. For the purposes of this review, we operationally define these changes in brain activity and connectivity as: 1) adaptive – a change in brain activity or connectivity that results in fewer symptoms and/or better motor performance or 2) maladaptive – a change in brain activity or connectivity that results in worse symptoms and/or poorer motor performance. There has been considerable interest in this topic, including several reviews and commentaries debating whether neural changes in PwMS are adaptive or maladaptive. However, these reports focus primarily on changes to resting state functional connectivity and its relationship to cognition (Penner and Aktas, 2017, Rocca and Filippi, 2017, Schoonheim, 2017). Whether MS-related changes in brain activity are adaptive vs. maladaptive for motor performance has received less attention.
During motor tasks, the amplitude of brain activity in PwMS is often increased, and this increase is typically interpreted as adaptive, counteracting changes in functional or structural connectivity (Lipp and Tomassini, 2015). However, others (Lenzi et al., 2007, Manson et al., 2006, Tomassini et al., 2012b) suggest an alternative interpretation- that increased activity in some brain regions may be maladaptive; related to reduced transcallosal inhibition (TCI) and callosal degradation. However, a current and comprehensive assessment of the data surrounding this hypothesis is lacking. Neuromodulatory techniques such as repetitive transcranial magnetic stimulation (Hulst et al., 2017), transcranial direct current stimulation (Kasschau et al., 2015, Palm et al., 2014), and deep brain stimulation (Roy and Aziz, 2014) provide the means to facilitate or depress activity in specific brain regions. Understanding whether MS-related changes in brain activity are adaptive or maladaptive is crucial to inform the location and direction of neural manipulation via these methods.
To provide clarity on this issue, we first review the effects of MS on brain structure, activity, and connectivity, including how these neural changes relate to motor performance. Then, we discuss our current understanding of whether changes in neural activity are adaptive or maladaptive for motor performance. In particular, we focus on recent evidence suggesting that increased activity of specific motor regions (e.g. ipsilateral motor areas), may be maladaptive, rather than adaptive, thus contributing to worsening motor symptoms. Finally, we discuss future directions regarding how the described brain changes may contribute to motor performance, including the need for longitudinal studies to clarify these relationships.
2. Structural changes in PwMS
2.1. Grey matter atrophy
PwMS exhibit grey matter atrophy in virtually all brain regions, including the basal ganglia, cortex (frontal, parietal, temporal lobes), cerebellum, and brainstem (for reviews, see: Horakova et al., 2012, Jacobsen and Farbu, 2014, Messina and Patti, 2014, Pirko et al., 2007). This atrophy typically progresses slowly (approximately 0.5–1% per year globally (Filippi, 2015)), and varies by diagnosis, such that progressive forms of MS exhibit faster degradation of brain volume (particularly grey matter) than relapsing forms (Roosendaal et al., 2011). However, atrophy is evident in numerous cortical and subcortical regions even early in the disease (Battaglini et al., 2009). The location of atrophy can also differ across disease types, with RMSS exhibiting predominantly ventricular enlargement, and secondary forms of MS exhibiting more cortical atrophy (Pagani et al., 2005).
Regional grey matter atrophy is related to worse motor performance (see Table 1). For example, brainstem volume was specifically correlated to clinical gait outcomes including the 25 foot walk (Edwards et al., 1999, Jasperse et al., 2007, Shiee et al., 2012), and the ambulation index (Liptak et al., 2008, Liu et al., 1999). Perhaps unsurprisingly, general clinical assessments, such as the EDSS, are related to atrophy of many cortical and subcortical regions (see Table 1). Cerebellar grey matter is also affected in PwMS (Kutzelnigg et al., 2007), with cerebellar atrophy and pathology relating to poorer scores on the EDSS (Damasceno et al., 2014, Tjoa et al., 2005), upper extremity assessments (e.g. nine-hole peg test (Anderson et al., 2009, Henry et al., 2008), and gait (Damasceno et al., 2014, Tjoa et al., 2005). Together, these findings suggest that grey matter volume plays an important role in disability in PwMS, and brainstem and cerebellar atrophy may be specifically related to declines in lower limb performance and locomotion.
Table 1.
Studies describing regional grey matter changes in PwMS, and how these changes correlate to disability.
| Brain region | Study | Cohort |
EDSS | Disability scale | Disability related to | ||||
|---|---|---|---|---|---|---|---|---|---|
| HC | CIS | RR | SP | PP | |||||
| Basal ganglia | (Jacobsen et al., 2014) | 0 | 0 | 62 | 11 | 8 | 3.5 (0–6.0) | EDSS | Cortical & putamen volume |
| (Audoin et al., 2006) | 10 | 0 | 21 | 0 | 0 | 1 (0–3) | EDSS | Bilateral thalamic volume | |
| (Hagemeier et al., 2013) | 0 | 0 | 149 | 61 | 0 | 3.5 (2.1) | EDSS | Caudate, red nucleus, amygdala volume | |
| (Harrison et al., 2015a) | 15 | 0 | 28 | 6 | 3.0 (1.0–6.5) | EDSS | Thalamic lesion burden | ||
| Cortex | (Gorgoraptis et al., 2010) | 12 | 0 | 10 | 1 | 0 | 4.5 (2–6) | 25 ft. walk test | Primary motor cortex volume |
| (Harrison et al., 2015b) | 15 | 0 | 30 | 6 | 3 (1–6.5) | EDSS | Cortical lesion volume | ||
| (Hofstetter et al., 2014) | 0 | 0 | 68 | 0 | 0 | 1.75–3.25 | EDSS | Precuneus, postcentral gyrus volume | |
| (Khaleeli et al., 2007) | 23 | 0 | 0 | 0 | 46 | 4.5 (1.5–7.0) | EDSS | Right primary motor cortex volume | |
| (Charil et al., 2007) | 0 | 0 | 425 | 0 | 0 | 2 (0–8.0) | EDSS | Anterior cingulate; insula; associative cortices volume | |
| (Chen et al., 2004) | 0 | 0 | 24 | 6 | 0 | (1.5–5) | EDSS | Parietal, precentral volume | |
| (Ramasamy et al., 2009) | 38 | 17 | 40 | 14 | 17 | 3.0 (0–7.5) | EDSS | Right parahippocampal, left lateral occipital and postcentral thicknesses; left accumbens and caudate | |
| Brainstem | (Jasperse et al., 2007) | 0 | 0 | 79 | 0 | 0 | 2.0 (2.0–2.5) | EDSS, 25 ft. walk test, 9HPT | Brainstem, cerebellum, peripheral & central cortical atrophy |
| (Edwards et al., 1999) | 0 | 0 | 21 | 20 | 0 | 3.6 (0–6.5) | Spinal EDSS; SNRS; disease duration | Spinal cord volume - EDSS, disease duration; brainstem & cerebellum volume - SNRS | |
| (Liu et al., 1999) | 10 | 0 | 20 | 20 | 0 | 3.5 (1–6) | EDSS; ambulation index | Brainstem volume | |
| (Liptak et al., 2008) | 29 | 0 | 32 | 8 | 5 | Not reported | EDSS; ambulation index | Medulla, upper cervical cord volume | |
| (Shiee et al., 2012) | 37 | 0 | 43 | 9 | 8 | 2.7 (0–6.5) | EDSS; 25 ft. walk test | Thalamus volume; brainstem volume | |
| Cerebellum | (Anderson et al., 2009) | 25 | 29 | 33 | 0 | 11 | 3.25 (1–8) | 9HPT | Cerebellar white matter volume |
| (Henry et al., 2008) | 49 | 41 | 0 | 0 | 0 | 1.1 (0.8) | 9HPT | Cerebellar volume | |
| (Damasceno et al., 2014) | 30 | 42 | 0 | 0 | 0 | 2.5 (0–4) | 25 ft. walk test; 9HPT; EDSS | Cerebellar intracortical lesions | |
| (Tjoa et al., 2005) | 15 | 0 | 41 | 6 | 0 | 3.3 (1–7) | 25 ft. walk test; EDSS | Dentate T2 hypointensity | |
EDSS: Expanded Disability Status Scale; SNRS: Scripts Neurological Rating Scale; 9HPT: 9 hole peg test.
2.2. White matter integrity
In addition to alterations in grey matter, white matter dysfunction remains a hallmark of MS. Diffusion tensor and diffusion weighted imaging (DTI and DWI, respectively) measure the diffusion or movement of water molecules within the brain to characterize the structural integrity of white matter and degree of myelination. Investigations measuring DTI and DWI have demonstrated widespread changes in myelination and white matter microstructural integrity in PwMS compared to people without MS.
White matter lesions (Werring et al., 1999) and changes to “normally appearing white matter” (for review, see Sbardella et al., 2013) in PwMS can be found throughout the brain of PwMS, and damage becomes more pronounced as pathology progresses (Braley et al., 2012, Castriota Scanderbeg et al., 2000, Horsfield et al., 1996). Although there is considerable variability across patients, damage is commonly observed in corticospinal, superior longitudinal fasciculus, periventricular, and corpus callosum tracts (Sbardella et al., 2013). Of these structures, damage is often most pronounced in the corpus callosum, the largest white matter fiber tract in the brain (Bonzano et al., 2008, Warlop et al., 2008).
Although results are somewhat mixed (Fink et al., 2010, Griffin et al., 2001), clinical disability in PwMS (e.g. EDSS) is generally related to lower structural connectivity (i.e. DTI), with corticospinal and proprioceptive tracts being particularly well correlated to EDSS (for review, see: Sbardella et al., 2013). Balance has specifically been related to changes in the corticospinal (Hubbard et al., 2016, Tovar-Moll et al., 2015), brainstem (Peterson et al., 2016) and cerebellar (Anderson et al., 2011, Prosperini et al., 2014) white matter tracts. Finally, integrity of white matter within the corpus callosum has been associated with motor performance and motor learning of the upper extremities (Bonzano et al., 2008, Bonzano et al., 2011, Kern et al., 2011, Peterson et al., 2017, Rimkus et al., 2013). Taken together, these results underscore the widespread and heterogeneous structural dysfunction in the brain directly relating to poorer motor performance in PwMS.
3. Brain activity and connectivity in PwMS
3.1. Brain activity during motor tasks
Task-based functional magnetic resonance imaging (fMRI) is a technique that characterizes brain activity during behavioral tasks. Numerous task-based fMRI analyses have been carried out on PwMS, demonstrating considerable alterations in supraspinal activity during simple and complex motor tasks (for reviews, see: Filippi, 2015, Filippi and Agosta, 2009, Sacco et al., 2013, Sbardella et al., 2013, Tomassini et al., 2012b).
Most imaging studies have investigated brain activity during unilateral upper extremity motor tasks. These studies show that, compared to healthy controls, PwMS exhibit increased activity in typically recruited regions (e.g. contralateral motor areas) as well as brain regions not typically active during unilateral motor tasks in healthy adults (e.g. non-motor cortical and subcortical structures, ipsilateral motor areas, etc.) (Pantano et al., 2005). For example, patients with mild MS (EDSS ≤ 1.5; Colorado et al., 2012), and benign MS (EDSS ≤ 3 at least 15 years after diagnosis) (Giorgio et al., 2010, Rocca et al., 2010b) exhibited increased brain activity in motor and non-motor regions compared to healthy controls. Increases in sensorimotor brain activity during movement were also observed in people with RMSS compared to healthy controls (Rocca et al., 2002). Later in the course of disease (e.g. in people with SPMS) the elevated neural activity spreads to other regions not typically active during simple motor tasks, including secondary sensorimotor regions, and subcortical structures (Rocca et al., 2005). Importantly, the spread of activation is directly related to lesion load in the brain and spinal cord (De Stefano et al., 2006, Lee et al., 2000, Lenzi et al., 2007, Reddy et al., 2000a, Rocca et al., 2006).
Taken together, this work suggests that PwMS exhibit elevated brain activity during motor tasks with respect to people without MS. These results prompted the hypothesis that increased activity in asymptomatic patients may be adaptive or protective, warding off symptoms (Rocca et al., 2005). However, as will be described in detail in Section 4, recent work provides preliminary evidence that region specific increases in brain activity may be, in part, maladaptive. In particular, increased activity in motor regions ipsilateral to the side of the body completing the motor task may be maladaptive, mediated by poorer corpus callosum integrity and reduced TCI.
3.2. Functional connectivity
Functional connectivity MRI (fcMRI) analyses assess the degree to which activity of different parts of the brain oscillate together. This low-frequency activity coherence (typically at frequencies < 1 Hz) can be assessed both at rest and during motor or cognitive tasks, and is thought to represent functional connectivity between structures. As such, it can be, and often is, distinct from changes in the magnitude of brain activation. For example, activity of two brain regions can be elevated, but if their fluctuations are not in synchrony over time, they will not be considered “functionally connected”. Furthermore, when collected at rest, these analyses can reduce the confound of across group differences regarding relative task difficulty and removing attentional demands. Common networks include the default mode, executive function, salience, visual, auditory, sensorimotor, and others (Buckner et al., 2013). For the purpose of this review, we will focus on the effect of MS on the sensorimotor network.
Numerous investigations of resting state-fcMRI (rs-fcMRI) have been conducted in PwMS, and have been reviewed previously (Filippi et al., 2013, Leocani et al., 2016, Sacco et al., 2013, Sbardella et al., 2015a). Although findings are somewhat mixed, studies generally show PwMS exhibit reduced strength of connectivity in motor regions compared to individuals without MS (Table 2). For example, Sbardella and colleagues showed PwMS to exhibit less connectivity in 5 of 11 resting-state networks, including cerebellar, basal ganglia, and sensorimotor networks compared to healthy controls (Sbardella et al., 2015c). Notably, a minority of investigations show PwMS to exhibit no difference in rs-fcMRI (Dogonowski et al., 2013a, Liu et al., 2011, Roosendaal et al., 2010), or even increased functional connectivity strength in motor networks compared to controls (Dogonowski et al., 2013b, Faivre et al., 2012, Roosendaal et al., 2010). While the rationale for this discrepancy remains unclear, of the three studies showing increased connectivity in PwMS, two included patients with mild or transient symptoms. Specifically the cohort in Faivre et al. included individuals with mild MS (EDSS < 1; Faivre et al., 2012) and Roosendaal and colleagues studied people with clinically isolated syndrome (an inflammation or demyelination event in the CNS which leads to transient MS symptoms; Roosendaal et al., 2010).
Table 2.
Studies investigating resting state connectivity strength in motor networks in MS compared to age and gender matched controls. Also shown are correlations between resting state outcomes and symptom severity assessments (e.g. EDSS, balance outcomes, etc.).
| Study | Cohort |
EDSS | Increases in connectivity | Decreases in connectivity | Correlations with behavior | ||||
|---|---|---|---|---|---|---|---|---|---|
| HC | CIS | RR | SP | PP | |||||
| (Roosendaal et al., 2010) | 41 | 0 | 31 | 0 | 0 | 2.5 (2–3.5) | No group differences observed in motor regions (RMSS compared to HC) | Not analyzed | |
| 41 | 14 | 0 | 0 | 0 | 2 (1–2.6) | More connectivity in motor networks in CIS compared to controls and people with RMSS | Not analyzed | ||
| (Fling et al., 2015) | 14 | 0 | 24 | 0 | 0 | 4 (2–4) | Less motor cortex connectivity with cerebellum and striatum in PwMS | More cortico-cerebellar connectivity predicted better balance | |
| (Janssen et al., 2013) | 28 | 0 | 28 | 0 | 0 | 4.0 (1.2) | Less sensorimotor network connectivity in PwMS | More connectivity within the sensorimotor network predicted better EDSS | |
| (Liu et al., 2012) | 35 | 0 | 35 | 0 | 0 | 2.5 (1–6) | No group differences observed in motor regions | More connectivity in the right insula & superior temporal gyrus predicted better EDSS | |
| (Richiardi et al., 2012) | 14 | 0 | 22 | 0 | 0 | (1.5–2) | Less sensorimotor network connectivity in PwMS | Not analyzed | |
| (Dogonowski et al., 2013b) | 30 | 0 | 27 | 15 | 0 | 4.3 (0–7) | More connectivity in the right basal ganglia in PwMS | Not analyzed | |
| (Dogonowski et al., 2013a) | 30 | 0 | 27 | 15 | 0 | 4.3 (0–7) | No group differences observed in motor regions | More connectivity in the left premotor cortex predicted worse motor function | |
| (Dogonowski et al., 2014) | 30 | 0 | 27 | 15 | 0 | 4.3 (0–7) | Less local cerebellar connectivity in PwMS | More local cerebellar connectivity predicted better EDSS | |
| (Faivre et al., 2012) | 14 | 0 | 13 | 0 | 0 | 1.0 (0–3) | More sensorimotor network connectivity in PwMS | More fronto-parietal network connectivity predicted better MSFI | |
| (Sbardella et al., 2015c) | 24 | 0 | 30 | 0 | 0 | 2.5 (0–4) | Less cerebellar, basal ganglia, sensorimotor network connectivity in PwMS | More connectivity was related to information processing, but not motor performance. | |
| (Rocca et al., 2012) | 40 | 0 | 85 | 0 | 0 | 2.0 (0–6) | Less connectivity within sensorimotor I and between sensorimotor I and working memory network in PwMS | More connectivity within non-motor networks predicted better EDSS score (EDSS not correlated to sensorimotor network connectivity) | |
| (Rocca et al., 2017)a | 98 | 13 | 119 | 41 | 13 | 2.0 (0–8.5) | Less connectivity in motor networks including sensorimotor and cerebellar | More connectivity in sensorimotor network predicted better cerebellar functional score of the EDSS | |
RR – relapsing remitting, SP – secondary progressive, PP – primary progressive; CIS – clinically isolated syndrome, HC – healthy controls, EDSS – expanded disability status scale, PASAT – paced auditory serial addition test; MSFI—multiple sclerosis functional index; SDMT – symbol digit modality test.
Also included 29 people with benign MS.
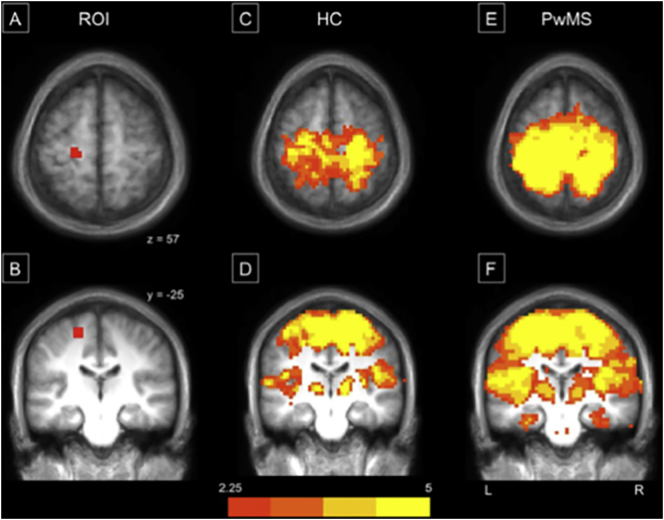
While most of the above-mentioned investigations have investigated changes in the strength of network connectivity in PwMS, a smaller number of studies investigated the size, or breadth, of the functionally connected network (Fling et al., 2015, Roosendaal et al., 2010). This work suggests that in PwMS, the size of the area that is functionally connected is larger (or more “diffuse”; Fig. 1). Notably, older adults also exhibit a similar dedifferentiation of activity, likely related to corpus callosum degradation and reduced TCI (for reviews, see Cabeza, 2001, Seidler et al., 2010). Specifically, older adults exhibit less lateralized brain activity during movements and connectivity during rest, and this reduced lateralization is related to worse upper extremity motor performance (Langan et al., 2010). Although more research is necessary, it is plausible that reduced TCI observed in PwMS (mediated by poorer corpus callosum integrity) may result in more diffuse brain activity as has been suggested in healthy adults.
Fig. 1.
Reprinted from Fling et al. (2015) (NeuroImage: Clinical) - Motor connectivity maps identified from seed regions within the leg region of the left primary motor cortex (M1). Representation of the seed region placed in the left hemisphere's leg region ofM1 (A & B). Panels C–F reflect group connectivity maps from healthy controls (C & D) and PwMS (E & F), respectively.
A number of studies have correlated resting state connectivity and motor behavior. Generally, results show that increased functional connectivity predicts better motor performance. For example, Janssen and colleagues showed that people with stronger connectivity within the right hemisphere motor network exhibited better scores on the EDSS (Janssen et al., 2013). Similarly, Fling and colleagues recently showed that stronger connectivity within cortico-cerebellar circuitry predicted better postural control (Fling et al., 2015). Finally, Roosendaal et al. (2010) showed that people with clinically isolated syndrome had increased functional connectivity in numerous networks (including the sensorimotor network) compared to those with relapsing remitting MS. Although authors did not correlate motor performance with functional connectivity within groups, the increased connectivity in patients with less or no motor dysfunction supports the hypothesis that increased functional connectivity strength may result in better motor outcomes.
Finally, several studies have investigated task-based functional connectivity, defined as the degree to which areas of the brain oscillate together (i.e. are functionally connected) during motor tasks, rather than at rest. These studies have also provided mixed results, showing PwMS to exhibit either similar (Lowe et al., 2008, Lowe et al., 2002), or stronger (Rocca et al., 2009a, Valsasina et al., 2011) functional connectivity during motor tasks compared to healthy controls. For example, two studies utilizing different functional connectivity analyses on the same dataset of 61 PwMS demonstrated an increase in functional connectivity in MS compared to controls across a number of motor and non-motor regions during hand flexion (Rocca et al., 2009a, Valsasina et al., 2011). Valsasina and colleagues further showed that there was an increase in bilateral motor cortex connectivity compared to controls. This is in partial contrast to resting state functional connectivity findings, which often show reduced connectivity in PwMS compared to people without MS. Although the rational for this discrepancy is not fully understood, opposing findings between resting state and task-based functional connectivity analyses in PwMS have been observed before. For example, PwMS have been shown to exhibit increased functional connectivity during cognitive behaviors (Mainero et al., 2006, Rocca et al., 2009b) and decreased activity during rest (Rocca et al., 2010a) in the same frontal and parietal brain regions. Unlike resting state connectivity analyses, only one study has correlated task-based functional connectivity outcomes to motor symptoms (Valsasina et al., 2011), showing no significant relationship between task-based connectivity and EDSS. Therefore, it is currently unclear whether changes in task-based functional connectivity are adaptive or maladaptive. Clearly, additional work is necessary to understand 1) the relationship between task-based and resting state functional connectivity in PwMS, and 2) how task-based connectivity is related to motor performance.
Taken together, these data underscore the complex and heterogeneous effects of MS on functional connectivity. Although exceptions are noted (Dogonowski et al., 2013a, Faivre et al., 2012, Roosendaal et al., 2010), data generally indicate that, at rest, PwMS exhibit a weaker and broader sensorimotor network connectivity map compared to healthy adults. Furthermore, the relationship between increased strength of resting state connectivity and improved motor performance suggests that greater resting state connectivity strength may be, in part, adaptive. However, these investigations are predominantly cross-sectional, making conclusions regarding the adaptive or maladaptive nature of these changes difficult. As noted below, to fully understand the maladaptive or adaptive nature of neural changes, longitudinal studies are necessary (Schoonheim, 2017).
4. Adaptive and maladaptive brain activity in PwMS
4.1. Relating brain activity during motor tasks to motor performance in PwMS
As noted above, PwMS exhibit robust increases in brain activity during motor tasks. However, determining whether these neurophysiological changes are “adaptive” (i.e. protecting against or limiting behavioral dysfunction) or “maladaptive” (i.e. contributing to behavioral dysfunction) remains a challenge. This section will outline current and emerging literature regarding the nature of brain activity during movement in PwMS, and how it relates to motor performance. In particular, we highlight evidence for and against the notion that increased brain activity of some regions, including ipsilateral motor areas, may be maladaptive.
Several approaches have been used to characterize changes in activity as adaptive or maladaptive. First, a number of investigations have related changes in the magnitude of brain activity to structural brain changes (e.g. white matter lesions, grey matter volume, structural integrity, etc.), noting that a correlation between lesion load and brain activity may imply the increased activity compensates for structural changes. Indeed, numerous investigations have shown that lesion load is directly correlated to the increase in activity measured via task-based MRI (e.g. Lee et al., 2000, Pantano et al., 2006, Rocca et al., 2004). Authors hypothesized that increased brain activity may compensate for increases in lesion load, thereby “limiting the functional consequences of tissue injury” (Rocca et al., 2004). However, it is also possible that increased activity could be a downstream maladaptive outcome of lesions rather than compensation for these lesions. In particular, the often-observed increase in ipsilateral motor activity (Lenzi et al., 2007, Reddy et al., 2002) may be a maladaptive outcome of reduced integrity of the corpus callosum (see text below for further details). Therefore, the correlation between lesion load and brain activity, in isolation, provides only partial insight into whether increases in brain activity are adaptive or maladaptive for motor performance.
Another method of determining whether increased brain activity is adaptive or maladaptive is by contrasting the brain activity of PwMS who exhibit more or less pronounced motor dysfunction (Giorgio et al., 2010, Reddy et al., 2002, Rocca et al., 2010a, Rocca et al., 2005). In other words, if patients with fewer symptoms have higher brain activity, this would provide support of an adaptive mechanism of increased brain activity. Alternatively, if people with more pronounced motor symptoms have higher brain activity, activity of this region is maladaptive. Several studies have taken this approach. For example, two investigations by Rocca and colleagues showed that PwMS and less pronounced motor symptoms exhibited more activity in contralateral motor cortices than PwMS and more motor symptoms (Rocca et al., 2010a, Rocca et al., 2005). Specifically, Rocca et al. showed that people with RMSS, compared to SPMS, exhibited more activity in contralateral SMA and putamen, and ipsilateral cerebellum (Rocca et al., 2010a). Rocca et al. (2005) observed that people with RRMS and few motor symptoms (EDSS = 0) exhibited increased activity in motor cortices compared to those with more pronounced symptoms (EDSS ≥ 1). Although increases were seen bilaterally, the most prominent increases were seen in the contralateral sensorimotor cortices (Rocca et al., 2005). Giorgio and colleagues also showed increased brain activity in people with fewer motor symptoms (benign MS compared to RMSS); however in this study, increased activity was observed in non-motor regions (inferior temporal gyrus, central opercular cortex, and lingual gyrus; Giorgio et al., 2010). Interestingly, Reddy and colleagues observed PwMS and fewer motor symptoms to exhibit reduced activity in the ipsilateral motor cortex. Specifically, increasing global disability (assessed via finger tapping) was related to increased ipsilateral pre and primary motor cortex activity. In addition, authors analyzed patients with similar levels of brain lesions but worse motor symptoms. Results again showed higher motor cortex activity in people with worse motor symptoms, although in this case increased activity was more bilateral in nature (Reddy et al., 2002).
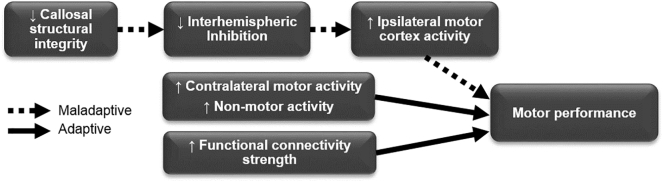
Drawing conclusions from this relatively limited dataset is challenging, and additional longitudinal studies are necessary to support or contradict these findings. However, these results provide preliminary evidence that increased activity in contralateral sensorimotor (Rocca et al., 2010a, Rocca et al., 2005) and non-motor regions (Giorgio et al., 2010) may be adaptive, while activity in ipsilateral motor regions may be maladaptive (Reddy et al., 2002). A number of reports support the hypothesis that contralateral motor and non-motor regions may be adaptive (Rocca et al., 2010a, Rocca et al., 2005). The notion that ipsilateral motor activity may be maladaptive has also gained footing in recent years. As discussed below, this idea comes from converging evidence linking the corpus callosum, TCI, increased ipsilateral activity, and poorer motor performance (Fig. 2; Pantano et al., 2015, Tomassini et al., 2012b).
Fig. 2.
Schematic depicting a proposed cascade of events underlying changes in motor performance in PwMS. Converging evidence suggests that the primary physiological insult of reduced callosal structural integrity (left) may contribute to poorer motor performance reduced interhemispheric inhibition and maladaptive increased ipsilateral activity (dashed arrows, top). Increases in contralateral motor and non-motor areas (middle) and increased functional connectivity strength (bottom) may act as adaptive processes, attenuating decreases in performance (solid arrows).
4.2. Longitudinal assessments and the brain activity–motor performance relationship
Another important approach to assess whether changes in motor or non-motor brain activity are maladaptive or adaptive is to track changes in behavior and brain activity over time (e.g. over the course of disease progression or relapse/remit phases). Specifically, detection of covariance between motor performance and imaging outcomes (structural or functional) over time may provide insight into the nature of their relationship.
Several investigations have utilized this approach, with results generally supporting the hypothesis that ipsilateral motor activity is maladaptive. Pantano et al. (2002) investigated brain activity during unilateral finger tapping tasks before and during a relapse. Results showed a large increase in ipsilateral motor and premotor activity during a relapse, when motor symptoms were worst. Furthermore, time since patients' first relapse was directly correlated to both contra- and ipsilateral motor activity, likely reflective of increasing callosal degeneration over the course of disease progression. Reddy et al. (2000b) showed similar results while reporting brain activity of a single patient during the course of recovery from a relapse. Serial fMRI scans showed that over the course of 6 weeks, whole brain axonal damage (measured by MR spectroscopic imaging) was reduced, and hand function improved. In concert with reduced axonal damage and improved motor performance, ipsilateral motor activity (measured via lateralization of activity) was also reduced. Mezzapesa and colleagues reported a similar finding by following 6 individuals with MS after a relapse, showing that persistent activity of the ipsilateral motor cortex was associated with poorer functional recovery (Mezzapesa et al., 2008).
Although additional longer term studies investigating how brain activity and motor performance change over time will provide additional insight on this topic, these results demonstrate a within patient co-variance between motor performance and brain activity specifically in the ipsilateral motor cortex. Though not causal, the finding that across time improvements in motor performance co-varied with reduced ipsilateral activity provides additional evidence supporting the notion that ipsilateral motor cortex activity may be maladaptive.
4.3. Corpus callosum degeneration - a potential link between increased ipsilateral neural activity and poorer motor performance
PwMS exhibit profound degeneration of the corpus callosum, a structure that connects bilateral cortical areas (Bonzano et al., 2008, Warlop et al., 2008). While each primary motor cortex has dense projections to the contralateral muscles controlling movement, the two cortices are also highly interconnected via the corpus callosum. Thus, this structure allows for interhemispheric transfer of information, and plays a key role in the production of coordinated motor responses. For motor behaviors that require precise temporal and spatial coordination between the two sides of the body (e.g. walking, postural control of balance, typing), movement of one limb has an overall inhibitory effect on the ipsilateral motor cortex (Liepert et al., 1998, Sohn et al., 2003, Stinear and Byblow, 2003). This inhibition, termed TCI, is beneficial for motor tasks and is facilitated via the corpus callosum.
PwMS exhibit reduced TCI between both primary motor (Boroojerdi et al., 1998, Hoppner et al., 1999, Schmierer et al., 2002) and pre-motor (Codeca et al., 2010) cortical regions. This reduction in TCI (and subsequent increase in ipsilateral activity) is directly related to corpus callosum degeneration in both MS and non-MS populations (Lenzi et al., 2007, Reddy et al., 2000b, Wahl et al., 2011, Zito et al., 2014). For example, Lenzi et al. (2007) noted that increased ipsilateral motor cortex activity was correlated to both poorer callosal integrity and reduced TCI. This relationship persisted even after correcting for lesion load (Lenzi et al., 2007). Further, a multicenter imaging effort by Manson & colleagues showed that PwMS exhibit more ipsilateral motor cortex activity during finger tapping compared to people without MS, drawing a link between ipsilateral motor activity and reduced TCI in those with MS (Manson et al., 2008).
If corpus callosum degradation does play a role in poorer motor performance via TCI, one would expect TCI to be directly correlated to performance outcomes. Indeed, several investigations show a relationship between reduced TCI and poorer motor outcomes. In a large sample (N = 118) of PwMS, Schmierer et al. (2002) showed that poorer TCI was directly related to worse symptoms as measured by EDSS. Neva et al. (2016) also showed that in 26 PwMS, TCI was directly correlated to symptom severity (EDSS).
Together, these results provide evidence linking poor callosal integrity and TCI to ipsilateral motor cortex activity and poorer motor outcomes. Further, these data provide a framework through which ipsilateral motor cortex activity may be maladaptive, rather than adaptive (Fig. 2). However, as noted above, relatively few studies have been conducted specifically investigating the relationship between activity in ipsilateral motor regions and motor performance. Therefore, additional work will be necessary to support or refute this hypothesis.
5. Future directions
Additional research is necessary to understand the impact of MS related neural changes on motor performance, and to identify neural changes as adaptive or maladaptive. This endeavor is particularly important to characterize disease progression and intervene most effectively. Several approaches, some of which have begun to be applied, will provide insight into this topic.
First, correlating brain structure and function to multiple behaviors (e.g. upper extremity motor performance, postural control, cognitive ability, etc.) will improve our understanding of the relationship between brain activity, connectivity, and motor performance. Although several investigations, noted in Table 2, have examined the relationship between functional connectivity strength and motor outcomes, relatively few studies have correlated task-based activity to motor behavior in PwMS. To our knowledge, no investigations have investigated whether dedifferentiation of connectivity is related to better or worse motor outcomes.
Second, longitudinal assessments of TCI, brain activation and connectivity, and motor performance will be critical to understand the link between neural dysfunction and motor performance. In particular, capturing covariance of changes in these variables in the same participant over time will shed light on this relationship. Similarly, longitudinal assessments of before and after interventions aimed at altering motor function and/or brain function allow investigation of co-variance of these measures. Such investigations have begun to be carried out, demonstrating that motor rehabilitation which improves motor performance also leads to reduced motor cortex activity (Mancini et al., 2009, Tomassini et al., 2012a), and improved corpus callosum structural connectivity (Bonzano et al., 2014, Ibrahim et al., 2011), for review, see Prosperini et al. (2015). However, additional investigations will be necessary to better understand this relationship.
Finally, neuromodulatory tools, such as repetitive TMS, can be used to directly alter neuronal activity through long term potentiation and depression. Thus, manipulation of motor regions along with measurement of activity and motor function may provide insight into the relationship between cortical reorganization and motor function. Several recent investigations have begun to explore these effects, showing that the degree of plasticity of contralateral M1 is directly related to the degree of improvement after relapse (Mori et al., 2014). However, plasticity or heightened activity of ipsilateral M1 and its effect on performance has not been evaluated.
In addition to the experiments noted above, further discussion and consensus is necessary to solidify what types of evidence could constitute adaptive vs. maladaptive neural changes. Similarly, consistency of language regarding adaptive (also described as “compensatory”, “functional reorganization”, etc.) and maladaptive (also described as “pathological”) could reduce confusion in this literature. A series of commentaries have begun to broach this topic (Penner and Aktas, 2017, Rocca and Filippi, 2017, Schoonheim, 2017), however additional discussion will be necessary to create a foundation for continued investigation.
6. Conclusions
Considerable work has demonstrated that PwMS exhibit dysfunction in brain structure (grey matter and white matter), brain activity, and brain connectivity (at rest and during motor tasks), and these changes have direct effects on motor performance. Given the emerging technology that can facilitate local changes in brain activity, it is important to understand whether changes, particularly those in brain activity, contribute to or protect against deficits in motor performance. Evidence to date suggests that increased activity in contralateral motor regions and non-motor regions may be adaptive in nature, thus improving motor performance. Interestingly, recent work provides preliminary data suggesting increased ipsilateral motor activity may be maladaptive (i.e. related to worse motor performance). This relationship may be mediated by poorer structural connectivity of the corpus callosum and reduced TCI. With respect to functional connectivity, increases in the strength of connectivity seem to be adaptive in nature. Although less understood, increases in the breadth or size of functional networks may be maladaptive in those with MS. Additional work is necessary to understand whether changes in brain activity are adaptive or maladaptive. In particular, 1) longitudinal analyses, 2) multimodal neuroimaging assessments, and 3) use of neuromodulatory tools will provide data to support or refute the abovementioned hypotheses. Further, continued discussion regarding the type of evidence that supports adaptive vs. maladaptive activity changes will provide additional clarity on this topic.
Acknowledgments
Acknowledgements
This work was supported by grants from the United States Department of Veteran's Affairs Rehabilitation Research and Development Service (Career Development Award-1: #I01BX007080 (DSP); Small Projects in Rehabilitation Award: #5I21RX001918-02 (BWF)), and the National MS Society (PP-1512-0710 (DSP); RG 5273A1/T (BWF)). The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Conflict of interest
The authors have no conflicts of interest to declare.
References
- Anderson V.M., Fisniku L.K., Altmann D.R., Thompson A.J., Miller D.H. MRI measures show significant cerebellar gray matter volume loss in multiple sclerosis and are associated with cerebellar dysfunction. Mult. Scler. 2009;15:811–817. doi: 10.1177/1352458508101934. [DOI] [PubMed] [Google Scholar]
- Anderson V.M., Wheeler-Kingshott C.A., Abdel-Aziz K., Miller D.H., Toosy A., Thompson A.J., Ciccarelli O. A comprehensive assessment of cerebellar damage in multiple sclerosis using diffusion tractography and volumetric analysis. Mult. Scler. 2011;17:1079–1087. doi: 10.1177/1352458511403528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audoin B., Davies G.R., Finisku L., Chard D.T., Thompson A.J., Miller D.H. Localization of grey matter atrophy in early RRMS: a longitudinal study. J. Neurol. 2006;253:1495–1501. doi: 10.1007/s00415-006-0264-2. [DOI] [PubMed] [Google Scholar]
- Battaglini M., Giorgio A., Stromillo M.L., Bartolozzi M.L., Guidi L., Federico A., De Stefano N. Voxel-wise assessment of progression of regional brain atrophy in relapsing-remitting multiple sclerosis. J. Neurol. Sci. 2009;282(1-2):55–60. doi: 10.1016/j.jns.2009.02.322. (Jul 15, Epub 2009 Mar 16, https://www.ncbi.nlm.nih.gov/pubmed/19286193) [DOI] [PubMed] [Google Scholar]
- Bonzano L., Tacchino A., Roccatagliata L., Abbruzzese G., Mancardi G.L., Bove M. Callosal contributions to simultaneous bimanual finger movements. J. Neurosci. 2008;28:3227–3233. doi: 10.1523/JNEUROSCI.4076-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonzano L., Tacchino A., Roccatagliata L., Sormani M.P., Mancardi G.L., Bove M. Impairment in explicit visuomotor sequence learning is related to loss of microstructural integrity of the corpus callosum in multiple sclerosis patients with minimal disability. NeuroImage. 2011;57:495–501. doi: 10.1016/j.neuroimage.2011.04.037. [DOI] [PubMed] [Google Scholar]
- Bonzano L., Tacchino A., Brichetto G., Roccatagliata L., Dessypris A., Feraco P., Lopes De Carvalho M.L., Battaglia M.A., Mancardi G.L., Bove M. Upper limb motor rehabilitation impacts white matter microstructure in multiple sclerosis. NeuroImage. 2014;90:107–116. doi: 10.1016/j.neuroimage.2013.12.025. [DOI] [PubMed] [Google Scholar]
- Boroojerdi B., Hungs M., Mull M., Topper R., Noth J. Interhemispheric inhibition in patients with multiple sclerosis. Electroencephalogr. Clin. Neurophysiol. 1998;109:230–237. doi: 10.1016/s0924-980x(98)00013-7. [DOI] [PubMed] [Google Scholar]
- Braley T.J., Lee Y.H., Mohan S., Segal B.M., Berini S., Srinivasan A. Differences in diffusion tensor imaging-derived metrics in the corpus callosum of patients with multiple sclerosis without and with gadolinium-enhancing cerebral lesions. J. Comput. Assist. Tomogr. 2012;36:410–415. doi: 10.1097/RCT.0b013e31825c6cee. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Krienen F.M., Yeo B.T. Opportunities and limitations of intrinsic functional connectivity MRI. Nat. Neurosci. 2013;16:832–837. doi: 10.1038/nn.3423. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Cognitive neuroscience of aging: contributions of functional neuroimaging. Scand. J. Psychol. 2001;42:277–286. doi: 10.1111/1467-9450.00237. [DOI] [PubMed] [Google Scholar]
- Castriota Scanderbeg A., Tomaiuolo F., Sabatini U., Nocentini U., Grasso M.G., Caltagirone C. Demyelinating plaques in relapsing-remitting and secondary-progressive multiple sclerosis: assessment with diffusion MR imaging. AJNR Am. J. Neuroradiol. 2000;21:862–868. [PMC free article] [PubMed] [Google Scholar]
- Charil A., Dagher A., Lerch J.P., Zijdenbos A.P., Worsley K.J., Evans A.C. Focal cortical atrophy in multiple sclerosis: relation to lesion load and disability. NeuroImage. 2007;34:509–517. doi: 10.1016/j.neuroimage.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Chen J.T., Narayanan S., Collins D.L., Smith S.M., Matthews P.M., Arnold D.L. Relating neocortical pathology to disability progression in multiple sclerosis using MRI. NeuroImage. 2004;23:1168–1175. doi: 10.1016/j.neuroimage.2004.07.046. [DOI] [PubMed] [Google Scholar]
- Codeca C., Mori F., Kusayanagi H., Monteleone F., Boffa L., Paolillo A., Bernardi G., Koch G., Centonze D. Differential patterns of interhemispheric functional disconnection in mild and advanced multiple sclerosis. Mult. Scler. 2010;16:1308–1316. doi: 10.1177/1352458510376957. [DOI] [PubMed] [Google Scholar]
- Colorado R.A., Shukla K., Zhou Y., Wolinsky J.S., Narayana P.A. Multi-task functional MRI in multiple sclerosis patients without clinical disability. NeuroImage. 2012;59:573–581. doi: 10.1016/j.neuroimage.2011.07.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasceno A., Damasceno B.P., Cendes F. The clinical impact of cerebellar grey matter pathology in multiple sclerosis. PLoS One. 2014;9 doi: 10.1371/journal.pone.0096193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Stefano N., Battaglini M., Stromillo M.L., Zipoli V., Bartolozzi M.L., Guidi L., Siracusa G., Portaccio E., Giorgio A., Sorbi S., Federico A., Amato M.P. Brain damage as detected by magnetization transfer imaging is less pronounced in benign than in early relapsing multiple sclerosis. Brain. 2006;129:2008–2016. doi: 10.1093/brain/awl152. [DOI] [PubMed] [Google Scholar]
- Dogonowski A.M., Siebner H.R., Soelberg Sorensen P., Paulson O.B., Dyrby T.B., Blinkenberg M., Madsen K.H. Resting-state connectivity of pre-motor cortex reflects disability in multiple sclerosis. Acta Neurol. Scand. 2013;128:328–335. doi: 10.1111/ane.12121. [DOI] [PubMed] [Google Scholar]
- Dogonowski A.M., Siebner H.R., Sorensen P.S., Wu X., Biswal B., Paulson O.B., Dyrby T.B., Skimminge A., Blinkenberg M., Madsen K.H. Expanded functional coupling of subcortical nuclei with the motor resting-state network in multiple sclerosis. Mult. Scler. 2013;19:559–566. doi: 10.1177/1352458512460416. [DOI] [PubMed] [Google Scholar]
- Dogonowski A.M., Andersen K.W., Madsen K.H., Sorensen P.S., Paulson O.B., Blinkenberg M., Siebner H.R. Multiple sclerosis impairs regional functional connectivity in the cerebellum. Neuroimage Clin. 2014;4:130–138. doi: 10.1016/j.nicl.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S.G., Gong Q.Y., Liu C., Zvartau M.E., Jaspan T., Roberts N., Blumhardt L.D. Infratentorial atrophy on magnetic resonance imaging and disability in multiple sclerosis. Brain. 1999;122(Pt 2):291–301. doi: 10.1093/brain/122.2.291. [DOI] [PubMed] [Google Scholar]
- Faivre A., Rico A., Zaaraoui W., Crespy L., Reuter F., Wybrecht D., Soulier E., Malikova I., Confort-Gouny S., Cozzone P.J., Pelletier J., Ranjeva J.P., Audoin B. Assessing brain connectivity at rest is clinically relevant in early multiple sclerosis. Mult. Scler. 2012;18:1251–1258. doi: 10.1177/1352458511435930. [DOI] [PubMed] [Google Scholar]
- Filippi M. MRI measures of neurodegeneration in multiple sclerosis: implications for disability, disease monitoring, and treatment. J. Neurol. 2015;262:1–6. doi: 10.1007/s00415-014-7340-9. [DOI] [PubMed] [Google Scholar]
- Filippi M., Agosta F. Magnetic resonance techniques to quantify tissue damage, tissue repair, and functional cortical reorganization in multiple sclerosis. Prog. Brain Res. 2009;175:465–482. doi: 10.1016/S0079-6123(09)17531-3. [DOI] [PubMed] [Google Scholar]
- Filippi M., Agosta F., Spinelli E.G., Rocca M.A. Imaging resting state brain function in multiple sclerosis. J. Neurol. 2013;260:1709–1713. doi: 10.1007/s00415-012-6695-z. [DOI] [PubMed] [Google Scholar]
- Fink F., Klein J., Lanz M., Mitrovics T., Lentschig M., Hahn H.K., Hildebrandt H. Comparison of diffusion tensor-based tractography and quantified brain atrophy for analyzing demyelination and axonal loss in MS. J. Neuroimaging. 2010;20:334–344. doi: 10.1111/j.1552-6569.2009.00377.x. [DOI] [PubMed] [Google Scholar]
- Fling B.W., Gera Dutta G., Horak F.B. Functional connectivity underlying postural motor adaptation in people with multiple sclerosis. Neuroimage Clin. 2015;8:281–289. doi: 10.1016/j.nicl.2015.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio A., Portaccio E., Stromillo M.L., Marino S., Zipoli V., Battaglini M., Blandino A., Bartolozzi M.L., Siracusa G., Amato M.P., De Stefano N. Cortical functional reorganization and its relationship with brain structural damage in patients with benign multiple sclerosis. Mult. Scler. 2010;16:1326–1334. doi: 10.1177/1352458510377333. [DOI] [PubMed] [Google Scholar]
- Gorgoraptis N., Wheeler-Kingshott C.A., Jenkins T.M., Altmann D.R., Miller D.H., Thompson A.J., Ciccarelli O. Combining tractography and cortical measures to test system-specific hypotheses in multiple sclerosis. Mult. Scler. 2010;16:555–565. doi: 10.1177/1352458510362440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin C.M., Chard D.T., Ciccarelli O., Kapoor B., Barker G.J., Thompson A.I., Miller D.H. Diffusion tensor imaging in early relapsing-remitting multiple sclerosis. Mult. Scler. 2001;7:290–297. doi: 10.1177/135245850100700504. [DOI] [PubMed] [Google Scholar]
- Hagemeier J., Weinstock-Guttman B., Heininen-Brown M., Poloni G.U., Bergsland N., Schirda C., Magnano C.R., Kennedy C., Carl E., Dwyer M.G., Minagar A., Zivadinov R. Gray matter SWI-filtered phase and atrophy are linked to disability in MS. Front. Biosci. (Elite Ed.) 2013;5:525–532. doi: 10.2741/e634. [DOI] [PubMed] [Google Scholar]
- Harrison D.M., Oh J., Roy S., Wood E.T., Whetstone A., Seigo M.A., Jones C.K., Pham D., van Zijl P., Reich D.S., Calabresi P.A. Thalamic lesions in multiple sclerosis by 7T MRI: clinical implications and relationship to cortical pathology. Mult. Scler. 2015;21:1139–1150. doi: 10.1177/1352458514558134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison D.M., Roy S., Oh J., Izbudak I., Pham D., Courtney S., Caffo B., Jones C.K., van Zijl P., Calabresi P.A. Association of cortical lesion burden on 7-T magnetic resonance imaging with cognition and disability in multiple sclerosis. JAMA Neurol. 2015;72:1004–1012. doi: 10.1001/jamaneurol.2015.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry R.G., Shieh M., Okuda D.T., Evangelista A., Gorno-Tempini M.L., Pelletier D. Regional grey matter atrophy in clinically isolated syndromes at presentation. J. Neurol. Neurosurg. Psychiatry. 2008;79:1236–1244. doi: 10.1136/jnnp.2007.134825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstetter L., Naegelin Y., Filli L., Kuster P., Traud S., Smieskova R., Mueller-Lenke N., Kappos L., Gass A., Sprenger T., Penner I.K., Nichols T.E., Vrenken H., Barkhof F., Polman C., Radue E.W., Borgwardt S.J., Bendfeldt K. Progression in disability and regional grey matter atrophy in relapsing-remitting multiple sclerosis. Mult. Scler. 2014;20:202–213. doi: 10.1177/1352458513493034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppner J., Kunesch E., Buchmann J., Hess A., Grossmann A., Benecke R. Demyelination and axonal degeneration in corpus callosum assessed by analysis of transcallosally mediated inhibition in multiple sclerosis. Clin. Neurophysiol. 1999;110:748–756. doi: 10.1016/s1388-2457(98)00075-3. [DOI] [PubMed] [Google Scholar]
- Horakova D., Kalincik T., Dusankova J.B., Dolezal O. Clinical correlates of grey matter pathology in multiple sclerosis. BMC Neurol. 2012;12:10. doi: 10.1186/1471-2377-12-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsfield M.A., Lai M., Webb S.L., Barker G.J., Tofts P.S., Turner R., Rudge P., Miller D.H. Apparent diffusion coefficients in benign and secondary progressive multiple sclerosis by nuclear magnetic resonance. Magn. Reson. Med. 1996;36:393–400. doi: 10.1002/mrm.1910360310. [DOI] [PubMed] [Google Scholar]
- Hubbard E.A., Wetter N.C., Sutton B.P., Pilutti L.A., Motl R.W. Diffusion tensor imaging of the corticospinal tract and walking performance in multiple sclerosis. J. Neurol. Sci. 2016;363:225–231. doi: 10.1016/j.jns.2016.02.044. [DOI] [PubMed] [Google Scholar]
- Hulst H.E., Goldschmidt T., Nitsche M.A., de Wit S.J., van den Heuvel O.A., Barkhof F., Paulus W., van der Werf Y.D., Geurts J.J.G. rTMS affects working memory performance, brain activation and functional connectivity in patients with multiple sclerosis. J. Neurol. Neurosurg. Psychiatry. 2017;88:386–394. doi: 10.1136/jnnp-2016-314224. [DOI] [PubMed] [Google Scholar]
- Ibrahim I., Tintera J., Skoch A., Jiru F., Hlustik P., Martinkova P., Zvara K., Rasova K. Fractional anisotropy and mean diffusivity in the corpus callosum of patients with multiple sclerosis: the effect of physiotherapy. Neuroradiology. 2011;53:917–926. doi: 10.1007/s00234-011-0879-6. [DOI] [PubMed] [Google Scholar]
- Jacobsen C.O., Farbu E. MRI evaluation of grey matter atrophy and disease course in multiple sclerosis: an overview of current knowledge. Acta Neurol. Scand. Suppl. 2014:32–36. doi: 10.1111/ane.12234. [DOI] [PubMed] [Google Scholar]
- Jacobsen C., Hagemeier J., Myhr K.M., Nyland H., Lode K., Bergsland N., Ramasamy D.P., Dalaker T.O., Larsen J.P., Farbu E., Zivadinov R. Brain atrophy and disability progression in multiple sclerosis patients: a 10-year follow-up study. J. Neurol. Neurosurg. Psychiatry. 2014;85:1109–1115. doi: 10.1136/jnnp-2013-306906. [DOI] [PubMed] [Google Scholar]
- Janssen A.L., Boster A., Patterson B.A., Abduljalil A., Prakash R.S. Resting-state functional connectivity in multiple sclerosis: an examination of group differences and individual differences. Neuropsychologia. 2013;51:2918–2929. doi: 10.1016/j.neuropsychologia.2013.08.010. [DOI] [PubMed] [Google Scholar]
- Jasperse B., Vrenken H., Sanz-Arigita E., de Groot V., Smith S.M., Polman C.H., Barkhof F. Regional brain atrophy development is related to specific aspects of clinical dysfunction in multiple sclerosis. NeuroImage. 2007;38:529–537. doi: 10.1016/j.neuroimage.2007.07.056. [DOI] [PubMed] [Google Scholar]
- Johansson S., Ytterberg C., Claesson I.M., Lindberg J., Hillert J., Andersson M., Widen Holmqvist L., von Koch L. High concurrent presence of disability in multiple sclerosis. Associations with perceived health. J. Neurol. 2007;254:767–773. doi: 10.1007/s00415-006-0431-5. [DOI] [PubMed] [Google Scholar]
- Kasschau M., Sherman K., Haider L., Frontario A., Shaw M., Datta A., Bikson M., Charvet L. A protocol for the use of remotely-supervised transcranial direct current stimulation (tDCS) in multiple sclerosis (MS) J. Vis. Exp. 2015:e53542. doi: 10.3791/53542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern K.C., Sarcona J., Montag M., Giesser B.S., Sicotte N.L. Corpus callosal diffusivity predicts motor impairment in relapsing-remitting multiple sclerosis: a TBSS and tractography study. NeuroImage. 2011;55:1169–1177. doi: 10.1016/j.neuroimage.2010.10.077. [DOI] [PubMed] [Google Scholar]
- Khaleeli Z., Cercignani M., Audoin B., Ciccarelli O., Miller D.H., Thompson A.J. Localized grey matter damage in early primary progressive multiple sclerosis contributes to disability. NeuroImage. 2007;37:253–261. doi: 10.1016/j.neuroimage.2007.04.056. [DOI] [PubMed] [Google Scholar]
- Kutzelnigg A., Faber-Rod J.C., Bauer J., Lucchinetti C.F., Sorensen P.S., Laursen H., Stadelmann C., Bruck W., Rauschka H., Schmidbauer M., Lassmann H. Widespread demyelination in the cerebellar cortex in multiple sclerosis. Brain Pathol. 2007;17:38–44. doi: 10.1111/j.1750-3639.2006.00041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langan J., Peltier S.J., Bo J., Fling B.W., Welsh R.C., Seidler R.D. Functional implications of age differences in motor system connectivity. Front. Syst. Neurosci. 2010;4:17. doi: 10.3389/fnsys.2010.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M., Reddy H., Johansen-Berg H., Pendlebury S., Jenkinson M., Smith S., Palace J., Matthews P.M. The motor cortex shows adaptive functional changes to brain injury from multiple sclerosis. Ann. Neurol. 2000;47:606–613. [PubMed] [Google Scholar]
- Lenzi D., Conte A., Mainero C., Frasca V., Fubelli F., Totaro P., Caramia F., Inghilleri M., Pozzilli C., Pantano P. Effect of corpus callosum damage on ipsilateral motor activation in patients with multiple sclerosis: a functional and anatomical study. Hum. Brain Mapp. 2007;28:636–644. doi: 10.1002/hbm.20305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leocani L., Rocca M.A., Comi G. MRI and neurophysiological measures to predict course, disability and treatment response in multiple sclerosis. Curr. Opin. Neurol. 2016;29:243–253. doi: 10.1097/WCO.0000000000000333. [DOI] [PubMed] [Google Scholar]
- Liepert J., Classen J., Cohen L.G., Hallett M. Task-dependent changes of intracortical inhibition. Exp. Brain Res. 1998;118:421–426. doi: 10.1007/s002210050296. [DOI] [PubMed] [Google Scholar]
- Lipp I., Tomassini V. Neuroplasticity and motor rehabilitation in multiple sclerosis. Front. Neurol. 2015;6:59. doi: 10.3389/fneur.2015.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liptak Z., Berger A.M., Sampat M.P., Charil A., Felsovalyi O., Healy B.C., Hildenbrand P., Khoury S.J., Weiner H.L., Bakshi R., Guttmann C.R. Medulla oblongata volume: a biomarker of spinal cord damage and disability in multiple sclerosis. AJNR Am. J. Neuroradiol. 2008;29:1465–1470. doi: 10.3174/ajnr.A1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Edwards S., Gong Q., Roberts N., Blumhardt L.D. Three dimensional MRI estimates of brain and spinal cord atrophy in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry. 1999;66:323–330. doi: 10.1136/jnnp.66.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Liang P., Duan Y., Jia X., Yu C., Zhang M., Wang F., Zhang M., Dong H., Ye J., Butzkueven H., Li K. Brain plasticity in relapsing-remitting multiple sclerosis: evidence from resting-state fMRI. J. Neurol. Sci. 2011;304:127–131. doi: 10.1016/j.jns.2011.01.023. [DOI] [PubMed] [Google Scholar]
- Liu Y., Duan Y., He Y., Yu C., Wang J., Huang J., Ye J., Parizel P.M., Li K., Shu N. Whole brain white matter changes revealed by multiple diffusion metrics in multiple sclerosis: a TBSS study. Eur. J. Radiol. 2012;81:2826–2832. doi: 10.1016/j.ejrad.2011.11.022. [DOI] [PubMed] [Google Scholar]
- Lowe M.J., Phillips M.D., Lurito J.T., Mattson D., Dzemidzic M., Mathews V.P. Multiple sclerosis: low-frequency temporal blood oxygen level-dependent fluctuations indicate reduced functional connectivity initial results. Radiology. 2002;224:184–192. doi: 10.1148/radiol.2241011005. [DOI] [PubMed] [Google Scholar]
- Lowe M.J., Beall E.B., Sakaie K.E., Koenig K.A., Stone L., Marrie R.A., Phillips M.D. Resting state sensorimotor functional connectivity in multiple sclerosis inversely correlates with transcallosal motor pathway transverse diffusivity. Hum. Brain Mapp. 2008;29:818–827. doi: 10.1002/hbm.20576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainero C., Pantano P., Caramia F., Pozzilli C. Brain reorganization during attention and memory tasks in multiple sclerosis: insights from functional MRI studies. J. Neurol. Sci. 2006;245:93–98. doi: 10.1016/j.jns.2005.08.024. [DOI] [PubMed] [Google Scholar]
- Mancini L., Ciccarelli O., Manfredonia F., Thornton J.S., Agosta F., Barkhof F., Beckmann C., De Stefano N., Enzinger C., Fazekas F., Filippi M., Gass A., Hirsch J.G., Johansen-Berg H., Kappos L., Korteweg T., Manson S.C., Marino S., Matthews P.M., Montalban X., Palace J., Polman C., Rocca M., Ropele S., Rovira A., Wegner C., Friston K., Thompson A., Yousry T. Short-term adaptation to a simple motor task: a physiological process preserved in multiple sclerosis. NeuroImage. 2009;45:500–511. doi: 10.1016/j.neuroimage.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Manson S.C., Palace J., Frank J.A., Matthews P.M. Loss of interhemispheric inhibition in patients with multiple sclerosis is related to corpus callosum atrophy. Exp. Brain Res. 2006;174:728–733. doi: 10.1007/s00221-006-0517-4. [DOI] [PubMed] [Google Scholar]
- Manson S.C., Wegner C., Filippi M., Barkhof F., Beckmann C., Ciccarelli O., De Stefano N., Enzinger C., Fazekas F., Agosta F., Gass A., Hirsch J., Johansen-Berg H., Kappos L., Korteweg T., Polman C., Mancini L., Manfredonia F., Marino S., Miller D.H., Montalban X., Palace J., Rocca M., Ropele S., Rovira A., Smith S., Thompson A., Thornton J., Yousry T., Frank J.A., Matthews P.M. Impairment of movement-associated brain deactivation in multiple sclerosis: further evidence for a functional pathology of interhemispheric neuronal inhibition. Exp. Brain Res. 2008;187:25–31. doi: 10.1007/s00221-008-1276-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews P.M., Roncaroli F., Waldman A., Sormani M.P., De Stefano N., Giovannoni G., Reynolds R. A practical review of the neuropathology and neuroimaging of multiple sclerosis. Pract. Neurol. 2016;16:279–287. doi: 10.1136/practneurol-2016-001381. [DOI] [PubMed] [Google Scholar]
- Messina S., Patti F. Gray matters in multiple sclerosis: cognitive impairment and structural MRI. Mult. Scler. Int. 2014;2014:609694. doi: 10.1155/2014/609694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzapesa D.M., Rocca M.A., Rodegher M., Comi G., Filippi M. Functional cortical changes of the sensorimotor network are associated with clinical recovery in multiple sclerosis. Hum. Brain Mapp. 2008;29:562–573. doi: 10.1002/hbm.20418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori F., Kusayanagi H., Nicoletti C.G., Weiss S., Marciani M.G., Centonze D. Cortical plasticity predicts recovery from relapse in multiple sclerosis. Mult. Scler. 2014;20:451–457. doi: 10.1177/1352458513512541. [DOI] [PubMed] [Google Scholar]
- Neva J.L., Lakhani B., Brown K.E., Wadden K.P., Mang C.S., Ledwell N.H., Borich M.R., Vavasour I.M., Laule C., Traboulsee A.L., MacKay A.L., Boyd L.A. Multiple measures of corticospinal excitability are associated with clinical features of multiple sclerosis. Behav. Brain Res. 2016;297:187–195. doi: 10.1016/j.bbr.2015.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylander A., Hafler D.A. Multiple sclerosis. J. Clin. Invest. 2012;122:1180–1188. doi: 10.1172/JCI58649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani E., Rocca M.A., Gallo A., Rovaris M., Martinelli V., Comi G., Filippi M. Regional brain atrophy evolves differently in patients with multiple sclerosis according to clinical phenotype. AJNR Am. J. Neuroradiol. 2005;26:341–346. [PMC free article] [PubMed] [Google Scholar]
- Palm U., Ayache S.S., Padberg F., Lefaucheur J.P. Non-invasive brain stimulation therapy in multiple sclerosis: a review of tDCS, rTMS and ECT results. Brain Stimul. 2014;7:849–854. doi: 10.1016/j.brs.2014.09.014. [DOI] [PubMed] [Google Scholar]
- Pantano P., Iannetti G.D., Caramia F., Mainero C., Di Legge S., Bozzao L., Pozzilli C., Lenzi G.L. Cortical motor reorganization after a single clinical attack of multiple sclerosis. Brain. 2002;125:1607–1615. doi: 10.1093/brain/awf164. [DOI] [PubMed] [Google Scholar]
- Pantano P., Mainero C., Lenzi D., Caramia F., Iannetti G.D., Piattella M.C., Pestalozza I., Di Legge S., Bozzao L., Pozzilli C. A longitudinal fMRI study on motor activity in patients with multiple sclerosis. Brain. 2005;128:2146–2153. doi: 10.1093/brain/awh549. [DOI] [PubMed] [Google Scholar]
- Pantano P., Mainero C., Caramia F. Functional brain reorganization in multiple sclerosis: evidence from fMRI studies. J. Neuroimaging. 2006;16:104–114. doi: 10.1111/j.1552-6569.2006.00029.x. [DOI] [PubMed] [Google Scholar]
- Pantano P., Petsas N., Tona F., Sbardella E. The role of fMRI to assess plasticity of the motor system in MS. Front. Neurol. 2015;6 doi: 10.3389/fneur.2015.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner I.K., Aktas O. Functional reorganization is a maladaptive response to injury - NO. Mult. Scler. 2017;23:193–194. doi: 10.1177/1352458516679895. [DOI] [PubMed] [Google Scholar]
- Peterson D.S., Gera G., Horak F.B., Fling B.W. Corpus callosum structural integrity is associated with postural control improvement in persons with multiple sclerosis who have minimal disability. Neurorehabil. Neural Repair. 2017;31(4):343–353. doi: 10.1177/1545968316680487. (Apr, Epub 2016 Dec 8.PMID: 27932696) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson D.S., Gera G., Horak F.B., Fling B.W. Supraspinal control of automatic postural responses in people with multiple sclerosis. Gait Posture. 2016;47:92–95. doi: 10.1016/j.gaitpost.2016.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirko I., Lucchinetti C.F., Sriram S., Bakshi R. Gray matter involvement in multiple sclerosis. Neurology. 2007;68:634–642. doi: 10.1212/01.wnl.0000250267.85698.7a. [DOI] [PubMed] [Google Scholar]
- Prosperini L., Fanelli F., Petsas N., Sbardella E., Tona F., Raz E., Fortuna D., De Angelis F., Pozzilli C., Pantano P. Multiple sclerosis: changes in microarchitecture of white matter tracts after training with a video game balance board. Radiology. 2014;273:529–538. doi: 10.1148/radiol.14140168. [DOI] [PubMed] [Google Scholar]
- Prosperini L., Piattella M.C., Gianni C., Pantano P. Functional and structural brain plasticity enhanced by motor and cognitive rehabilitation in multiple sclerosis. Neural Plast. 2015;2015:481574. doi: 10.1155/2015/481574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy D.P., Benedict R.H., Cox J.L., Fritz D., Abdelrahman N., Hussein S., Minagar A., Dwyer M.G., Zivadinov R. Extent of cerebellum, subcortical and cortical atrophy in patients with MS: a case-control study. J. Neurol. Sci. 2009;282:47–54. doi: 10.1016/j.jns.2008.12.034. [DOI] [PubMed] [Google Scholar]
- Reddy H., Narayanan S., Arnoutelis R., Jenkinson M., Antel J., Matthews P.M., Arnold D.L. Evidence for adaptive functional changes in the cerebral cortex with axonal injury from multiple sclerosis. Brain. 2000;123(Pt 11):2314–2320. doi: 10.1093/brain/123.11.2314. [DOI] [PubMed] [Google Scholar]
- Reddy H., Narayanan S., Matthews P.M., Hoge R.D., Pike G.B., Duquette P., Antel J., Arnold D.L. Relating axonal injury to functional recovery in MS. Neurology. 2000;54:236–239. doi: 10.1212/wnl.54.1.236. [DOI] [PubMed] [Google Scholar]
- Reddy H., Narayanan S., Woolrich M., Mitsumori T., Lapierre Y., Arnold D.L., Matthews P.M. Functional brain reorganization for hand movement in patients with multiple sclerosis: defining distinct effects of injury and disability. Brain. 2002;125:2646–2657. doi: 10.1093/brain/awf283. [DOI] [PubMed] [Google Scholar]
- Richiardi J., Gschwind M., Simioni S., Annoni J.M., Greco B., Hagmann P., Schluep M., Vuilleumier P., Van De Ville D. Classifying minimally disabled multiple sclerosis patients from resting state functional connectivity. NeuroImage. 2012;62:2021–2033. doi: 10.1016/j.neuroimage.2012.05.078. [DOI] [PubMed] [Google Scholar]
- Rimkus C.D., Junqueira T.D., Callegaro D., Otaduy M.C.G., Leite C.D. Segmented corpus callosum diffusivity correlates with the Expanded Disability Status Scale score in the early stages of relapsing-remitting multiple sclerosis. Clinics. 2013;68:1115–1120. doi: 10.6061/clinics/2013(08)09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca M.A., Filippi M. Functional reorganization is a maladaptive response to injury - YES. Mult. Scler. 2017;23:191–193. doi: 10.1177/1352458516667242. [DOI] [PubMed] [Google Scholar]
- Rocca M.A., Falini A., Colombo B., Scotti G., Comi G., Filippi M. Adaptive functional changes in the cerebral cortex of patients with nondisabling multiple sclerosis correlate with the extent of brain structural damage. Ann. Neurol. 2002;51:330–339. doi: 10.1002/ana.10120. [DOI] [PubMed] [Google Scholar]
- Rocca M.A., Gallo A., Colombo B., Falini A., Scotti G., Comi G., Filippi M. Pyramidal tract lesions and movement-associated cortical recruitment in patients with MS. NeuroImage. 2004;23:141–147. doi: 10.1016/j.neuroimage.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Rocca M.A., Colombo B., Falini A., Ghezzi A., Martinelli V., Scotti G., Comi G., Filippi M. Cortical adaptation in patients with MS: a cross-sectional functional MRI study of disease phenotypes. Lancet Neurol. 2005;4:618–626. doi: 10.1016/S1474-4422(05)70171-X. [DOI] [PubMed] [Google Scholar]
- Rocca M.A., Agosta F., Martinelli V., Falini A., Comi G., Filippi M. The level of spinal cord involvement influences the pattern of movement-associated cortical recruitment in patients with isolated myelitis. NeuroImage. 2006;30:879–884. doi: 10.1016/j.neuroimage.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Rocca M.A., Absinta M., Valsasina P., Ciccarelli O., Marino S., Rovira A., Gass A., Wegner C., Enzinger C., Korteweg T., Sormani M.P., Mancini L., Thompson A.J., De Stefano N., Montalban X., Hirsch J., Kappos L., Ropele S., Palace J., Barkhof F., Matthews P.M., Filippi M. Abnormal connectivity of the sensorimotor network in patients with MS: a multicenter fMRI study. Hum. Brain Mapp. 2009;30:2412–2425. doi: 10.1002/hbm.20679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca M.A., Valsasina P., Ceccarelli A., Absinta M., Ghezzi A., Riccitelli G., Pagani E., Falini A., Comi G., Scotti G., Filippi M. Structural and functional MRI correlates of Stroop control in benign MS. Hum. Brain Mapp. 2009;30:276–290. doi: 10.1002/hbm.20504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca M.A., Ceccarelli A., Rodegher M., Misci P., Riccitelli G., Falini A., Comi G., Filippi M. Preserved brain adaptive properties in patients with benign multiple sclerosis. Neurology. 2010;74:142–149. doi: 10.1212/WNL.0b013e3181c91a00. [DOI] [PubMed] [Google Scholar]
- Rocca M.A., Valsasina P., Absinta M., Riccitelli G., Rodegher M.E., Misci P., Rossi P., Falini A., Comi G., Filippi M. Default-mode network dysfunction and cognitive impairment in progressive MS. Neurology. 2010;74:1252–1259. doi: 10.1212/WNL.0b013e3181d9ed91. [DOI] [PubMed] [Google Scholar]
- Rocca M.A., Valsasina P., Martinelli V., Misci P., Falini A., Comi G., Filippi M. Large-scale neuronal network dysfunction in relapsing-remitting multiple sclerosis. Neurology. 2012;79:1449–1457. doi: 10.1212/WNL.0b013e31826d5f10. [DOI] [PubMed] [Google Scholar]
- Rocca M.A., Valsasina P., Leavitt V.M., Rodegher M., Radaelli M., Riccitelli G.C., Martinelli V., Martinelli-Boneschi F., Falini A., Comi G., Filippi M. Functional network connectivity abnormalities in multiple sclerosis: correlations with disability and cognitive impairment. Mult. Scler. 2017 doi: 10.1177/1352458517699875. [DOI] [PubMed] [Google Scholar]
- Roosendaal S.D., Schoonheim M.M., Hulst H.E., Sanz-Arigita E.J., Smith S.M., Geurts J.J., Barkhof F. Resting state networks change in clinically isolated syndrome. Brain. 2010;133:1612–1621. doi: 10.1093/brain/awq058. [DOI] [PubMed] [Google Scholar]
- Roosendaal S.D., Bendfeldt K., Vrenken H., Polman C.H., Borgwardt S., Radue E.W., Kappos L., Pelletier D., Hauser S.L., Matthews P.M., Barkhof F., Geurts J.J. Grey matter volume in a large cohort of MS patients: relation to MRI parameters and disability. Mult. Scler. 2011;17:1098–1106. doi: 10.1177/1352458511404916. [DOI] [PubMed] [Google Scholar]
- Roy H.A., Aziz T.Z. Deep brain stimulation and multiple sclerosis: therapeutic applications. Mult. Scler. Relat. Disord. 2014;3:431–439. doi: 10.1016/j.msard.2014.02.003. [DOI] [PubMed] [Google Scholar]
- Sacco R., Bonavita S., Esposito F., Tedeschi G., Gallo A. The contribution of resting state networks to the study of cortical reorganization in MS. Mult. Scler. Int. 2013;2013:857807. doi: 10.1155/2013/857807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbardella E., Tona F., Petsas N., Pantano P. DTI measurements in multiple sclerosis: evaluation of brain damage and clinical implications. Mult. Scler. Int. 2013;2013:671730. doi: 10.1155/2013/671730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbardella E., Petsas N., Tona F., Pantano P. Resting-state fMRI in MS: general concepts and brief overview of its application. Biomed. Res. Int. 2015;2015:212693. doi: 10.1155/2015/212693. (Epub 2015 Aug 27, https://www.ncbi.nlm.nih.gov/pubmed/26413509) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbardella E., Petsas N., Tona F., Pantano P. Resting-state fMRI in MS: general concepts and brief overview of its application. Biomed. Res. Int. 2015;2015:212693. doi: 10.1155/2015/212693. Epub 2015 Aug 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbardella E., Tona F., Petsas N., Upadhyay N., Piattella M.C., Filippini N., Prosperini L., Pozzilli C., Pantano P. Functional connectivity changes and their relationship with clinical disability and white matter integrity in patients with relapsing-remitting multiple sclerosis. Mult. Scler. 2015;21:1681–1692. doi: 10.1177/1352458514568826. [DOI] [PubMed] [Google Scholar]
- Schmierer K., Irlbacher K., Grosse P., Roricht S., Meyer B.U. Correlates of disability in multiple sclerosis detected by transcranial magnetic stimulation. Neurology. 2002;59:1218–1224. doi: 10.1212/wnl.59.8.1218. [DOI] [PubMed] [Google Scholar]
- Schoonheim M.M. Functional reorganization is a maladaptive response to injury - commentary. Mult. Scler. 2017;23:194–196. doi: 10.1177/1352458516677593. [DOI] [PubMed] [Google Scholar]
- Seidler R.D., Bernard J.A., Burutolu T.B., Fling B.W., Gordon M.T., Gwin J.T., Kwak Y., Lipps D.B. Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci. Biobehav. Rev. 2010;34:721–733. doi: 10.1016/j.neubiorev.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiee N., Bazin P.L., Zackowski K.M., Farrell S.K., Harrison D.M., Newsome S.D., Ratchford J.N., Caffo B.S., Calabresi P.A., Pham D.L., Reich D.S. Revisiting brain atrophy and its relationship to disability in multiple sclerosis. PLoS One. 2012;7 doi: 10.1371/journal.pone.0037049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn Y.H., Jung H.Y., Kaelin-Lang A., Hallett M. Excitability of the ipsilateral motor cortex during phasic voluntary hand movement. Exp. Brain Res. 2003;148:176–185. doi: 10.1007/s00221-002-1292-5. [DOI] [PubMed] [Google Scholar]
- Stinear C.M., Byblow W.D. Role of intracortical inhibition in selective hand muscle activation. J. Neurophysiol. 2003;89:2014–2020. doi: 10.1152/jn.00925.2002. [DOI] [PubMed] [Google Scholar]
- Tjoa C.W., Benedict R.H., Weinstock-Guttman B., Fabiano A.J., Bakshi R. MRI T2 hypointensity of the dentate nucleus is related to ambulatory impairment in multiple sclerosis. J. Neurol. Sci. 2005;234:17–24. doi: 10.1016/j.jns.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Tomassini V., Johansen-Berg H., Jbabdi S., Wise R.G., Pozzilli C., Palace J., Matthews P.M. Relating brain damage to brain plasticity in patients with multiple sclerosis. Neurorehabil. Neural Repair. 2012;26:581–593. doi: 10.1177/1545968311433208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomassini V., Matthews P.M., Thompson A.J., Fuglo D., Geurts J.J., Johansen-Berg H., Jones D.K., Rocca M.A., Wise R.G., Barkhof F., Palace J. Neuroplasticity and functional recovery in multiple sclerosis. Nat. Rev. Neurol. 2012;8:635–646. doi: 10.1038/nrneurol.2012.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar-Moll F., Evangelou I.E., Chiu A.W., Auh S., Chen C., Ehrmantraut M., Ohayon J.M., Richert N., Bagnato F. Diffuse and focal corticospinal tract disease and its impact on patient disability in multiple sclerosis. J. Neuroimaging. 2015;25:200–206. doi: 10.1111/jon.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valsasina P., Rocca M.A., Absinta M., Sormani M.P., Mancini L., De Stefano N., Rovira A., Gass A., Enzinger C., Barkhof F., Wegner C., Matthews P.M., Filippi M. A multicentre study of motor functional connectivity changes in patients with multiple sclerosis. Eur. J. Neurosci. 2011;33:1256–1263. doi: 10.1111/j.1460-9568.2011.07623.x. [DOI] [PubMed] [Google Scholar]
- Wahl M., Huebers A., Lauterbach-Soon B., Hattingen E., Jung P., Cohen L.G., Ziemann U. Motor callosal disconnection in early relapsing-remitting multiple sclerosis. Hum. Brain Mapp. 2011;32:846–855. doi: 10.1002/hbm.21071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warlop N.P., Fieremans E., Achten E., Debruyne J., Vingerhoets G. Callosal function in MS patients with mild and severe callosal damage as reflected by diffusion tensor imaging. Brain Res. 2008;1226:218–225. doi: 10.1016/j.brainres.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Werring D.J., Clark C.A., Barker G.J., Thompson A.J., Miller D.H. Diffusion tensor imaging of lesions and normal-appearing white matter in multiple sclerosis. Neurology. 1999;52:1626–1632. doi: 10.1212/wnl.52.8.1626. [DOI] [PubMed] [Google Scholar]
- Zito G., Luders E., Tomasevic L., Lupoi D., Toga A.W., Thompson P.M., Rossini P.M., Filippi M.M., Tecchio F. Inter-hemispheric functional connectivity changes with corpus callosum morphology in multiple sclerosis. Neuroscience. 2014;266:47–55. doi: 10.1016/j.neuroscience.2014.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]