Abstract
Introduction:
Polycystic ovary syndrome (PCOS) is the most common endocrinopathy in women in reproductive age that is associated with insulin resistance (IR) and metabolic abnormalities which are also a part of metabolic syndrome (Met S). This study was aimed to determine the prevalence of nonalcoholic fatty liver disease (NAFLD) women diagnosed with PCOS based on the Rotterdam criteria from January 2013 to June 2014.
Methods:
In this cross-sectional study, 75 women with PCOS and 75 healthy controls were enrolled. Anthropometric parameters, biochemical and hormonal investigation, were measured in all women. IR was calculated by homeostasis model assessment. Abdominal ultrasonography and biochemical tests were used to determine the NAFLD.
Results:
The level of triglyceride, cholesterol, low-density lipoprotein, aspartate aminotransferase, alkalin phosphatase, fasting insulin, and homeostatic model assessment index in women with PCOS were significantly higher than women without PCOS. High-density lipoprotein and alanine aminotransferase (ALT) in women with PCOS were significantly lower. The frequency of IR women with or without PCOS was 53.3% and 29.3%, respectively (P = 0.003). The frequency of Met S in women with PCOS was 33.3% and in other was 10.7% (P = 0.001). The prevalence of fatty liver in women with or without PCOS was 38.7% and 18.7%, respectively (0.008). In women with PCOS, body mass index (BMI) (odds ratio [OR] = 4.25; P = 0.046), ALT (OR = 1.62; P = 0.005), fasting insulin (OR = 1.32; P = 0.032), and IR (OR = 58.17; P = 0.025) were associated with a higher fatty liver.
Conclusions:
NAFLD is frequent in patients with PCOS with combination with other metabolic derangements. BMI, ALT, fasting insulin, and IR are the risk factors for high prevalence of NAFLD in women with PCOS.
Keywords: Fatty liver, metabolic syndrome, polycystic ovary syndrome
Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrinopathy andpresents in 15.2% Iranian women of reproductive age-based Rotterdam criteria.[1] It is thought that insulin resistance (IR) and compensatory hyperinsulinemia are key pathological factors of PCOS.[2,3] The prevalence of metabolic syndrome (Met S) and IRs was 24.9% and 24.3%, respectively in Iranian women with PCOS, based on Rotterdam criteria.[4] Nonalcoholic fatty liver disease (NAFLD) refers to the presence of hepatic of hepatic steatosis when other secondary causes of hepatic fat accumulation are not present. NAFLD may progress to cirrhosis and is likely an important cause of cryptogenic cirrhosis.[5,6,7]
Identifying women at risk for developing NAFLD using simple diagnostic tools could have a beneficial impact on women health if prevalence measures could be undertaken. The first aim of the present study was to assess the prevalence of NAFLD in a sample of Iranian women with PCOS. The second aim was to define clinical predictive factors to determine PCOS women who should or should not be screened for insulin sensitivity and liver enzyme and liver ultrasonography.
Methods
The present cross-sectional study is conducted from January 2013 to June 2014 in Shahid Beheshti Hospital of Isfahan. Seventy-five women with PCOS and 75 women without PCOS who referred to the clinic of gynecology in Shahid Beheshti Hospital in Isfahan, Iran, were enrolled. Women were included if they were between 18 and 42 years of age. They were diagnosed retrospectively according to the European Society for Human Reproduction and Embryology and American Society for Reproductive Medicine criteria, i.e., the presence of least two of the following conditions: chronic an ovulation, hyperandrogenism, and polycystic ovaries.[8] Inclusion criteria were no symptom of other causes of irregular menstrual cycles, no androgen excess, no pregnancy, and no presence of viral hepatitis, hemochromatosis, liver disease, and other autoimmune hepatic disorder. Furthermore, women who received medications potentially affecting liver function, oral contraceptive pill, or metformin were not eligible. This study is approved by the Ethics Committee at Isfahan University of Medical Sciences and written informed consent was obtained from all the participants.
After a complete history taking, all women underwent a full clinical assessment and had blood pressure and anthropometric measurement (weight, height, waist circumference) by standard protocol and calibrated instrument by the same physician. Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2). After an overnight fast of patient and control group, blood was taken for biochemical profile, including total cholesterol, triglyceride, high-density lipoprotein (HDL), low-density lipoprotein (LDL), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkalin phosphatase (ALP), glucose, and insulin.
Met S was defined according to the National Cholesterol Education Program Adult Treatment Panel III guidelines. Individuals with at least three of the following criteria were diagnosed with Met S: waist circumference >88 cm in women; serum triglyceride level >150 mg/dl; HDL cholesterol concentration <50 mg/dl for women; blood pressure >130/85 mmHg; and fasting glucose level >110.[9] IR was estimated using the homeostatic model assessment-IR (HOMA-IR): (fasting insulin [u/mi] * fasting glucose [μm mol/l]/22.5).[10] A HOMA-IR valve >3.5 probably reflected severe IR because the HOMA-IR threshold of 3.9 was based on insulin concentration above the upper limit of normal after a 100 g oral glucose tolerance test as the standard test.[11]
Fatty liver was diagnosed by abdominal ultrasound using standardized criteria. Ultrasound was performed in all subjects with the same equipment (ATL5000 or PhilipsIU22 scanner) and all ultrasound examinations were performed by one of authors (RMP) who was unaware of the clinical and laboratory results. The presence of fatty liver was determined in a qualitative manner using accepted criteria including a bright hepatic echo pattern, (compared with the right kidney) a homogeneous or coarse echo pattern, increased attenuation of the US beam, and loss of intrahepatic architectural details.[12,13]
Statistical analysis
All data were analyzed using SPSS 23 for Windows (SPSS Inc., Chicago, IL, USA). Data are presented as means ± standard deviations for continuous variables and number (percent) for categorical variables. Statistical significance was recognized with a P < 0.05. Independent sample t-test and Chi-square test were used to compare continuous and categorical variables between groups, respectively. The univariate and multivariate logistic regression technique was performed to identify risk factors associated with the prevalence of NAFLD in women with PCOS. The model included all variables considered according to their statistical significance obtained in the univariate logistic regression for each factor, and factors with significant impact, P < 0.2, were implemented in multivariate logistic regression model.
Results
Table 1 shows the comparison of the prevalence of fatty liver and measurements of anthropometric, biochemical, and hormonal profile between women with or without PCOS. BMI, waist circumference, fasting glucose, and systolic blood pressure were not significantly different between women with PCOS and those without PCOS. The level of triglyceride, cholesterol, LDL, AST, ALP, diastolic blood pressure, fasting insulin, and HOMA index in women with PCOS were significantly higher than women without PCOS. HDL and ALT in women with PCOS were significantly lower. Of women with PCOS, 53.3% had IR and 29.3% of women without PCOS had IR (P = 0.003). The frequency of Met S in women with PCOS was significantly than women without PCOS (33.3% vs. 10.7%, respectively, P = 0.001). The prevalence of fatty liver in women with PCOS was 38.7%, where in women without PCOS, 18.7% had fatty liver (0.008), but the grade of fatty liver was similar between the two groups.
Table 1.
Anthropometric, biochemical, and hormonal profile in women with polycystic ovary syndrome compared with those without polycystic ovary syndrome
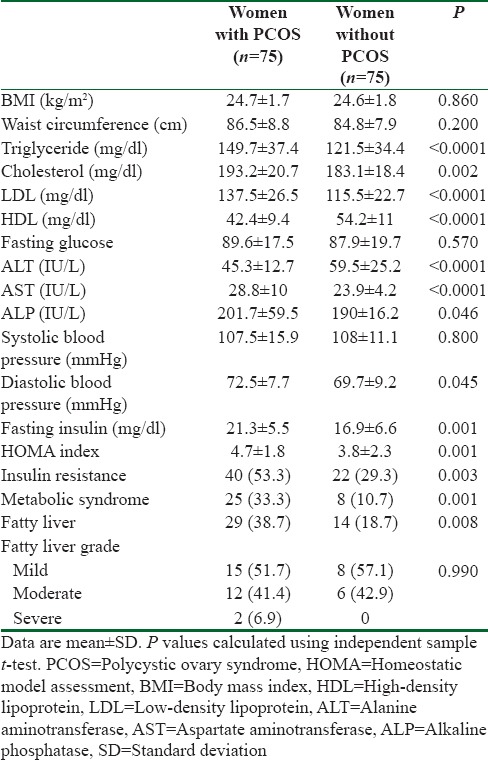
In 75 studied women, PCOS subgroups were found as follows; hyperandrogenism/PCO was detected in 25 (33.3%) of women, hyperandrogenism/menstrual irregularity in 24 (32%) of women, hyperandrogenism/PCO/menstrual irregularity in 12 (16%) of women, and menstrual irregularity/PCO was found in 14 (18.7%) of women.
Table 2 shows the univariate analysis of affecting variables on fatty liver in women with PCOS. Based on these analysis, among studied variables, higher level of BMI (odds ratio [OR] = 2.33; P = 0.0001), waist circumference (OR = 1.08; P = 0.012), ALT (OR = 1.22; P = 0.0001), AST (OR = 1.09; P = 0.005), fasting insulin (OR = 1.33; P = 0.0001), and IR (OR = 9; P = 0.0001) were significant factors associated with fatty liver in women with PCOS. Furthermore, based on these analysis, in addition to these significance factors, significant level for HDL and ALP was >0.2 and enter to the multivariate model of logistic regression [Table 3].
Table 2.
Univariate analyses of factor associated with fatty liver in women with in women with polycystic ovary syndrome
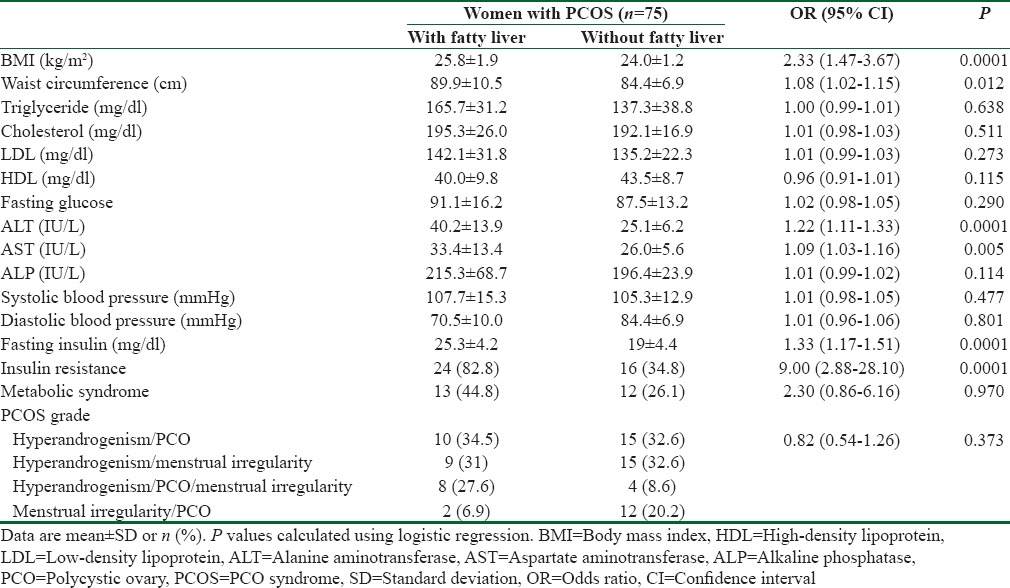
Table 3.
Multivariate analyses of factor associated with fatty liver in women with in women with polycystic ovary syndrome
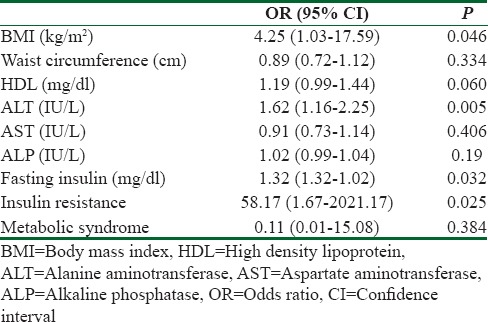
The result of the logistic regression multivariate analysis is presented in Table 3. In women with PCOS, BMI (OR = 4.25; P = 0.046), ALT (OR = 1.62; P = 0.005), fasting insulin (OR = 1.32; P = 0.032), and IR (OR = 58.17; P = 0.025) were associated with a higher fatty liver risk. The other variables entered in the multivariate analysis including waist circumference, HDL, AST, ALP, and Met S were not significantly associated with fatty liver.
Discussion
NAFLD is a chronic condition in patients who do not have a history of excessive alcohol consumption and is characterized by more than 5% of hepatocytes affected with lipid accumulation.[14,15,16] Caldwell et al. in 1999 suggested a relationship between NAFLD and cryptogenic cirrhosis, and several other studies have shown that NAFLD patients are at high risk of developing a wide range of progressive liver disease such as hepatocellular carcinoma.[5,14] Furthermore, NAFLD is considered as the hepatic manifestation of Met S since it worsens IR that predicts emergence of metabolic complications and increases the risk of cardiovascular events.[17]
We studied the frequency of NAFLD in different phenotypic subgroups of PCOS based on Rotterdam criteria in Iranian women. The overall frequency of NAFLD in these women was 38.7%. IR, BMI, ALT, and fasting insulin are associated risk factor with the prevalence of NAFLD in women with PCOS.
The prevalence of NAFLD in Cerda study was 41.5%, that is, similar to our results.[16] The results of this study shows that the level of lipid profile serum (10.6%), aminotransferase (57%), blood pressure (7%), and IR (53.3%) in patients with PCOS was higher than those of control group. The prevalence of Met S was higher than in the patient group (38.7%). Paradis et al. support that hyperglycemia and insulin are the key factors in the progression of liver injury, the mechanism of which is the upregulation of connective tissue growth factor.[18] Gambarin-Gelwan et al. found that presence of steatosis was associated with great BMI (obesity) and HOMA-IR (IR)[14] that was disagreement with our study that BMI was not different between two groups. In the published study by Setji et al.,15% of PCOS patients had aminotransferase elevations and six women had underwent liver biopsies. Histological examination of liver found evidence of nonalcoholic steatohepatitis with varying degree of fibrosis in all. The authors found that these patients had lower HDL and higher triglycerides, fasting insulin, and aminotransferase levels.[19] Similar to our study, Zhongyus study shows that the prevalence of NAFLD in Chinese women with PCOS was higher than control (32.9%vs. 18.5%) and was included 113 (57.1%) mild, 75 (37.3%) moderate, and 10 (5.1%) severe cases and NAFLD not significantly associated with hyperandrogenism.[20] In another research by Helen Jones, like our study, BMI or waist circumference was not difference between PCOS and control women (P > 0.05).[21] Our study has both strengths and limitations. Patients in this trial were not receiving any medicines such as oral contraceptive, metformin, and other insulin sensitizers. Furthermore, our study has been done with some limitations. Its small sample size as well as the low number of obese people can be considered are some of our limitations. Thus, a larger number of trials to evaluate NAFLD in phenotypically different PCOS are needed for the most appropriate screening method and having effective interventions. Another weakness point of the study is liver biopsy which was not used for recognition of NAFLD. Now, ultrasonography is the most widely used imaging method noninvasive, less time-taking feasible, and cost-effective for detecting fatty liver detection with 91% sensitivity and 93% specificity in the presence of >30%hepatic steatosis.[22] However, for grading of severity of hepatic steatosis and detection of inflammation and fibrosis liver, biopsy is required.
Conclusions
The findings of the present study show that the prevalence of NAFLD in women with PCOS is higher than the other women, and IR, BMI, ALT, and fasting insulin are associated with the prevalence of NAFLD in these women. Hence, it suggests that women with PCOS can be screened for liver disease given the potentially nature of the disease, and the possibility of early introduction of lifestyle changes that may improve NAFLD.
Financial support and sponsorship
The study was supported by the University of Esfahan.
Conflict of interest
There are no conflicts of interest.
Acknowledgments
We would like to thank Ferdous Mehrabian who assisted in this study.
References
- 1.Mehrabian F, Khani B, Kelishadi R, Ghanbari E. The prevalence of polycystic ovary syndrome in Iranian women based on different diagnostic criteria. Endokrynol Pol. 2011;62:238–42. [PubMed] [Google Scholar]
- 2.Chang RJ, Nakamura RM, Judd HL, Kaplan SA. Insulin resistance in non-obese patients with polycystic ovarian disease. J Clin Endocrinol Metab. 1983;57:356–9. doi: 10.1210/jcem-57-2-356. [DOI] [PubMed] [Google Scholar]
- 3.Dunaif A. Insulin resistance and the polycystic ovary syndrome: Mechanism and implications for pathogenesis. Endocr Rev. 1997;18:774–800. doi: 10.1210/edrv.18.6.0318. [DOI] [PubMed] [Google Scholar]
- 4.Mehrabian F, Khani B, Kelishadi R, Kermani N. The prevalence of metabolic syndrome and insulin resistance according to the phenotypic subgroups of polycystic ovary syndrome in a representative sample of Iranian females. J Res Med Sci. 2011;16:763–9. [PMC free article] [PubMed] [Google Scholar]
- 5.Caldwell SH, Oelsner DH, Iezzoni JC, Hespenheide EE, Battle EH, Driscoll CJ, et al. Cryptogenic cirrhosis: Clinical characterization and risk factors for underlying disease. Hepatology. 1999;29:664–9. doi: 10.1002/hep.510290347. [DOI] [PubMed] [Google Scholar]
- 6.Caldwell SH, Crespo DM. The spectrum expanded: Cryptogenic cirrhosis and the natural history of non-alcoholic fatty liver disease. J Hepatol. 2004;40:578–84. doi: 10.1016/j.jhep.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Browning JD, Kumar KS, Saboorian MH, Thiele DL. Ethnic differences in the prevalence of cryptogenic cirrhosis. Am J Gastroenterol. 2004;99:292–8. doi: 10.1111/j.1572-0241.2004.04059.x. [DOI] [PubMed] [Google Scholar]
- 8.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 9.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 10.Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: Studies in subjects with various degrees of glucose characterized by an increased risk of hepatic steatosis compared to non hyptolerance and insulin sensitivity. Diabetes Care. 2000;23:57–63. doi: 10.2337/diacare.23.1.57. [DOI] [PubMed] [Google Scholar]
- 11.Fritz M, Speroff L. Clinical Gynecologic Endocrinology and Infertility. 8th ed. Lippincott Williams & Wilkins; 2011. pp. 491–531. [Google Scholar]
- 12.Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: Summary of AASLD Single Topic Conference. Hepatology. 2003;37:1202–19. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- 13.Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123:745–50. doi: 10.1053/gast.2002.35354. [DOI] [PubMed] [Google Scholar]
- 14.Gambarin-Gelwan M, Kinkhabwala SV, Schiano TD, Bodian C, Yeh HC, Futterweit W. Prevalence of nonalcoholic fatty liver disease in women with polycystic ovary syndrome. Clin Gastroenterol Hepatol. 2007;5:496–501. doi: 10.1016/j.cgh.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 15.Gutierrez-Grobe Y, Ponciano-Rodríguez G, Ramos MH, Uribe M, Méndez-Sánchez N. Prevalence of non alcoholic fatty liver disease in premenopausal, postmenopausal and polycystic ovary syndrome women. The role of estrogens. Ann Hepatol. 2010;9:402–9. [PubMed] [Google Scholar]
- 16.Cerda C. Nonalcoholic fatty liver disease in women with polycystic ovary syndrome. J Hepatol. 2007;47:412–7. doi: 10.1016/j.jhep.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 17.Karoli R, Fatima J, Chandra A, Gupta U, Islam FU, Singh G. Prevalence of hepatic steatosis in women with polycystic ovary syndrome. J Hum Reprod Sci. 2013;6:9–14. doi: 10.4103/0974-1208.112370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paradis V, Perlemuter G, Bonvoust F, Dargere D, Parfait B, Vidaud M, et al. High glucose and hyperinsulinemia stimulate connective tissue growth factor expression: A potential mechanism involved in progression to fibrosis in nonalcoholic steatohepatitis. Hepatology. 2001;34:738–44. doi: 10.1053/jhep.2001.28055. [DOI] [PubMed] [Google Scholar]
- 19.Setji TL, Holland ND, Sanders LL, Pereira KC, Diehl AM, Brown AJ. Nonalcoholic steatohepatitis and nonalcoholic Fatty Liver disease in young women with polycystic ovary syndrome. J Clin Endocrinol Meta. 2006;91:1741–7. doi: 10.1210/jc.2005-2774. [DOI] [PubMed] [Google Scholar]
- 20.Qu Z, Zhu Y, Jiang J, Shi Y, Chen Z. The clinical characteristics and etiological study of nonalcoholic fatty liver disease in Chinese women with PCOS. Iran J Reprod Med. 2013;11:725–32. [PMC free article] [PubMed] [Google Scholar]
- 21.Jones H. Polycystic ovary syndrome with hyperandrogenism iserandrogenic PCOS phenotypes and healthy controls, independent of obesity and healthy control, independent of obesity and insulin resistance. J Clin Endocrinol Metab. 2012;97:3722. doi: 10.1210/jc.2012-1382. [DOI] [PubMed] [Google Scholar]
- 22.Palmentieri B, de Sio I, La Mura V, Masarone M, Vecchione R, Bruno S, et al. The role of bright liver echo pattern on ultrasound B-mode examination in the diagnosis of liver steatosis. Dig Liver Dis. 2006;38:485–9. doi: 10.1016/j.dld.2006.03.021. [DOI] [PubMed] [Google Scholar]


