Abstract
Upon exposure to a ruthenium(0) catalyst, N-benzyl 3-hydroxy-2-oxindoles react with diverse alkynes to form products of C-H vinylation with complete control of regioselectivity and olefin geometry. This method contributes to a growing body of catalytic processes that enable direct conversion of lower alcohols to higher alcohols in the absence of stoichiometric organometallic reagents.
Graphical abstract

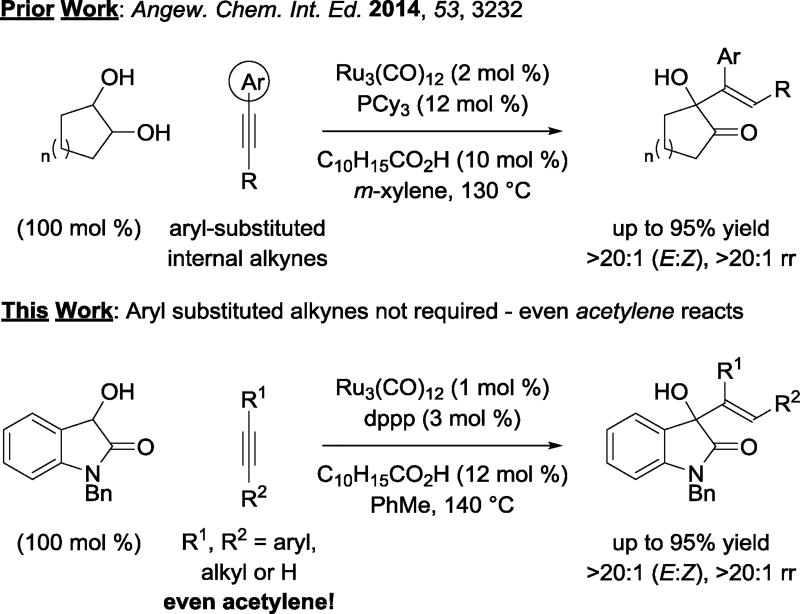
We have developed a broad, new class of catalytic C-C bond formations that merge the characteristics of carbonyl addition and transfer hydrogenation.1 The majority of these processes enable direct conversion of primary to secondary alcohols through mechanisms wherein alcohol oxidation is balanced by (a) reductive C-X bond cleavage2 or (b) π-bond hydrometalation3 to form transient aldehyde-organometal pairs that engage in carbonyl addition. Recently, we found that ruthenium(0) catalysts promote the conversion of activated secondary alcohols to tertiary alcohols.4 These processes proceed through a distinctly different mechanism wherein C=O/C=C oxidative coupling forms oxaruthenacycles,4, 5 which undergo transfer hydrogenolysis mediated by the secondary alcohol reactant to release product and regenerate the ketone required for oxidative coupling.
In the course of exploring ruthenium(0) catalyzed C-C couplings of secondary alcohols, it was found that 1,2-diols and other vicinally dioxygenated hydrocarbons (α-ketols, 1,2-diones) will react with alkynes to form products of carbinol C-H vinylation, that is, tertiary allylic alcohols in the form of α-hydroxy-β,γ-unsaturated ketones (Scheme 1).4e Although a variety of different diol partners could be employed, preparatively useful isolated yields were only achieved using aryl-substituted alkynes. Given the highly electrophilic nature of isatins, it was postulated that 3-hydroxy-2-oxindoles might serve as efficient partners for ruthenium(0) catalyzed C-C couplings with alkynes, potentially enabling broader scope with respect to the alkyne partner. Our interest was further motivated by the ubiquity of 3-substituted-3-hydroxy-2-oxindoles in naturally occurring compounds and as scaffolds in human medicine.6
Scheme 1.
Ruthenium(0) catalyzed couplings of activated secondary alcohols with alkynes.
In a series of initial experiments (Table 1), N-benzyl 3-hydroxy-2-oxindole 1a (100 mol %) was exposed to 1-phenyl-1-butyne 2a (300 mol %) in the presence of Ru3(CO)12 (2 mol %) in toluene (2.0 M) at 140 °C. While in the absence of ligand no reaction occurred (Table 1, entry 1), in the presence of 1,3-bis(diphenylphosphino)propane (dppp), the product of C-C coupling 3a was formed in 54% yield as a single regioisomer (Table 1, entry 2). Carboxylic acid additives have been shown to enhance yield dramatically by co-catalyzing the transfer hydrogenolysis of oxaruthenacycles.7 Upon evaluation of a series of carboxylic acids (Table 1, entries 3–6), adamantane carboxylic acid was most effective, allowing adduct 3a to be obtained in 90% yield (Table 1, entry 6). Further variation of ligand (Table 1, entries 7–13) and temperature (Table 1, entries 14–16) did not enhance the isolated yield of 3a. Finally, an attempt was made to decrease the loading of the catalyst, ligand and alkyne 2a (Table 1, entries 17–20). Although reduced loadings of alkyne 2a were not possible, a 90% yield of 3a could be obtained using only Ru3(CO)12 (1 mol %) and dppp (3 mol %) (Table 1, entry 19).
Table 1.
Selected optimization experiments in the ruthenium(0) catalyzed coupling of 3-hydroxy-2-oxindole 1a with alkyne 2a to form adduct 3a.a

| ||||
|---|---|---|---|---|
|
| ||||
| entry | ligand | t °c | RCO2H | 3a yield (%) |
| 1 | --- | 140 | --- | trace |
| 2 | dppp | 140 | --- | 54 |
| 3 | dppp | 140 | PhCO2H | 60 |
| 4 | dppp | 140 | p-NO2C6H4CO2H | 26 |
| 5 | dppp | 140 | C6F5CO2H | 71 |
| 6 | dppp | 140 | C10H15CO2H | 90 |
| 7 | dppe | 140 | C10H15CO2H | 63 |
| 8 | dCype | 140 | C10H15CO2H | 39 |
| 9 | dppb | 140 | C10H15CO2H | 59 |
| 10 | dppf | 140 | C10H15CO2H | 45 |
| 11 | rac-BINAP | 140 | C10H15CO2H | 49 |
| 12 | BIPHEP | 140 | C10H15CO2H | 59 |
| 13 | PCy3 | 140 | C10H15CO2H | 53 |
| 14 | dppp | 120 | C10H15CO2H | 34 |
| 15 | dppp | 130 | C10H15CO2H | 54 |
| 16 | dppp | 150 | C10H15CO2H | 60 |
| 17b | dppp | 140 | C10H15CO2H | 47 |
| 18c | dppp | 140 | C10H15CO2H | 56 |
 19d
19d
|
dppp | 140 | C10H15CO2H | 90 |
| 20d,e | dppp | 140 | C10H15CO2H | 65 |
Cited yields are of material isolated by silica gel chromatography. C10H15CO2H refers to adamantane carboxylic acid. See Supporting Information for further experimental details.
2a (150 mol %).
Ru3(CO)12 (1 mol %), dppp (3 mol %), C10H15CO2H (6 mol %).
Ru3(CO)12 (1 mol %), dppp (3 mol %), C10H15CO2H (12 mol %).
2a (200 mol %).
To assess reaction scope, optimal conditions determined for the coupling of oxindole 1a with alkyne 2a were applied to alkynes 2b–2i (Table 2). Aryl-substituted alkynes 2a–2c delivered adducts 3a–3c in excellent yield. Heteroatoms are not tolerated at the propargylic position of the alkyne, however, as demonstrated by the formation of adduct 2d, the presence of heteroatoms at the homopropargylic position is not problematic. Encouraged by these results, the coupling of oxindole 1a with terminal alkynes 2e–2f was explored. Adducts 3e–3f were obtained in good yield as single regioisomers. The coupling of oxindole 1a with acetylene 2i (1 atm) also was attempted. Remarkably, the product of carbinol C-H vinylation 3i was obtained in 69% yield.
Table 2.
Ruthenium(0) catalyzed coupling of 3-hydroxy-2-oxindole 1a with alkynes 2a–2i to form adducts 3a–3i.a
Cited yields are of material isolated by silica gel chromatography. C10H15CO2H refers to adamantane carboxylic acid. See Supporting Information for further experimental details.
To probe reaction scope further, a set of substituted N-benzyl 3-hydroxy-2-oxindoles 1a–1f (100 mol %) were exposed to phenyl-1-propyne 2b (300 mol %) under optimal conditions determined for the coupling of oxindole 1a with alkyne 2a (Table 3). Uniformly high isolated yields of the respective coupling products 3b, 3j–3n were obtained, and in each case, a single geometrical isomer of the trisubstituted olefin was observed by 1H NMR. Finally, reductive coupling of N-benzyl isatin dehydro-1a (100 mol %) with 1-phenyl-1-butyne 2a (300 mol %) mediated by 2-propanol (200 mol %) was attempted under otherwise standard conditions (eq 1). The adduct 3a was obtained in 65% yield as a single regioisomer with complete control of olefin geometry.
 |
(1) |
Table 3.
Ruthenium(0) catalyzed coupling of 3-hydroxy-2-oxindole 1a–1f with alkyne 2b to form adducts 3b, 3j–3n.a
Cited yields are of material isolated by silica gel chromatography. C10H15CO2H refers to adamantane carboxylic acid. See Supporting Information for further experimental details.
2-PrOH (200 mol %)
To illustrate how the coupling products may be used as building blocks in chemical synthesis, adduct 3i was converted to the corresponding acrylic ester, which was subjected to ring-closing metathesis8 to deliver the spirooxindole 4a (eq 2). Alternatively, conversion of adduct 3i to the corresponding α-diazo ester using p-acetamidobenzenesulfonyl azide (p-ABSA)9 followed by exposure to rhodium acetate results in diastereoselective intramolecular cyclopropanation to form the spirooxindole 4b (eq 3).10 The stereochemistry of 4b was assigned on the basis of nOe studies, as described in the Supporting Information.
 |
(2) |
 |
(3) |
In summary, by harnessing the reducing power of alcohols, one may avoid the use of premetalated reagents in carbonyl addition.1 Here, under the conditions of ruthenium(0) catalyzed C-C bond-forming transfer hydrogenation, N-benzyl 3-hydroxy-2-oxindole reacts with internal or terminal alkynes – even acetylene – to form products of hydrohydroxyalkylation. Alternatively, as demonstrated by the coupling of isatin dehydro-1a with 1-phenyl-1-butyne 2a, 2-propanol-mediated alkyne-carbonyl reductive coupling also is possible. Future studies will focus on the use of α-olefins11 and related chemical feedstocks as pronucleophiles in redox-triggered carbonyl addition via alcohol-mediated hydrogen transfer.
Supplementary Material
Acknowledgments
Acknowledgment is made to the Robert A. Welch Foundation (F-0038), the NIH-NIGMS (RO1-GM069445), the UT Austin Center for Green Chemistry and Catalysis and the China Scholarship Council (S.C. fellowship) for partial support of this research.
Footnotes
The authors declare no competing financial interest.
Supporting Information Available. Spectral data for all new compounds (1H NMR, 13C NMR, IR, HRMS). Single crystal X-ray diffraction data for compound 3c. This material is available free of charge via the internet at http://pubs.acs.org.
References
- 1.For selected reviews on the catalytic C-C coupling of alcohols via transfer hydrogenation, see: Ketcham JM, Shin I, Montgomery TP, Krische MJ. Angew. Chem. Int. Ed. 2014;53:9142. doi: 10.1002/anie.201403873.Dechert-Schmitt A-MR, Schmitt DC, Gao X, Itoh T, Krische MJ. Nat. Prod. Rep. 2014;31:504. doi: 10.1039/c3np70076c.Perez F, Oda S, Geary LM, Krische MJ. Top. Curr. Chem. 2016;374:365. doi: 10.1007/s41061-016-0028-0.
- 2.For selected examples, see: Kim IS, Ngai M-Y, Krische MJ. J. Am. Chem. Soc. 2008;130:14891. doi: 10.1021/ja805722e.Woo SK, Geary LM, Krische MJ. Angew. Chem. Int. Ed. 2012;51:7830. doi: 10.1002/anie.201203334.Dechert-Schmitt A-MR, Schmitt DC, Krische MJ. Angew. Chem. Int. Ed. 2013;52:3195. doi: 10.1002/anie.201209863.
- 3.For selected examples, see: Bower JF, Skucas E, Patman RL, Krische MJ. J. Am. Chem. Soc. 2007;129:15134. doi: 10.1021/ja077389b.Shibahara F, Bower JF, Krische MJ. J. Am. Chem. Soc. 2008;130:6338. doi: 10.1021/ja801213x.Zbieg JR, Yamaguchi E, McInturff EL, Krische MJ. Science. 2012;336:324. doi: 10.1126/science.1219274.Geary LM, Woo SK, Leung JC, Krische MJ. Angew. Chem. Int. Ed. 2012;51:2972. doi: 10.1002/anie.201200239.Nguyen KD, Herkommer D, Krische MJ. J. Am. Chem. Soc. 2016;138:14210. doi: 10.1021/jacs.6b09333.
- 4.For selected examples, see: Leung JC, Geary LM, Chen T-Y, Zbieg JR, Krische MJ. J. Am. Chem. Soc. 2012;134:15700. doi: 10.1021/ja3075049.Yamaguchi E, Mowat J, Luong T, Krische MJ. Angew. Chem. Int. Ed. 2013;52:8428. doi: 10.1002/anie.201303552.Park BY, Montgomery TP, Garza VJ, Krische MJ. J. Am. Chem. Soc. 2013;135:16320. doi: 10.1021/ja4087193.McInturff EL, Mowat J, Waldeck AR, Krische MJ. J. Am. Chem. Soc. 2013;135:17230. doi: 10.1021/ja410533y.McInturff EL, Nguyen KD, Krische MJ. Angew. Chem. Int. Ed. 2014;53:3232. doi: 10.1002/anie.201311130.Park BY, Luong T, Sato H, Krische MJ. J. Am. Chem. Soc. 2015;137:7652. doi: 10.1021/jacs.5b04688.
- 5.For C=O/C=C oxidative coupling in ruthenium(0) catalyzed Pauson-Khand type reactions, see: Chatani N, Tobisu M, Asaumi T, Fukumoto Y, Murai S. J. Am. Chem. Soc. 1999;121:7160.Tobisu M, Chatani N, Asaumi T, Amako K, Ie Y, Fukumoto Y, Murai S. J. Am. Chem. Soc. 2000;122:12663.
- 6.For selected reviews, see: Tsukano C, Takemoto Y. Heterocycles. 2014;89:2271.Santos MMM. Tetrahedron. 2014;70:9735.Macaev FZ, Sucman NS, Boldescu VV. Russ. Chem. Bull. 2014;63:15.Mohammadi Ziarani G, Moradi R, Lashgari N. Tetrahedron: Asymm. 2015;26:517.Yu B, Yu D-Q, Liu H-M. Eur. J. Med. Chem. 2015;97:673. doi: 10.1016/j.ejmech.2014.06.056.Kaur M, Singh M, Chadha N, Silakari O. Eur. J. Med. Chem. 2016;123:858. doi: 10.1016/j.ejmech.2016.08.011.
- 7.A mechanistic rationale can be found in references 4b, 4d and 4e and literature cited therein.
- 8.For a related ring-closing metathesis, see: Alcaide B, Almendros P, Rodriguez-Acebes R. J. Org. Chem. 2006;71:2346. doi: 10.1021/jo0525027.
- 9.Baum JS, Shook DA, Davies HML, Smith HD. Synth. Commun. 1987;17:1709. [Google Scholar]
- 10.For a related intramolecular cyclopropanation of a phenyl-substituted α-diazo ester, see: Doyle MP, Davies SB, Hu W. Org. Lett. 2000;2:1145. doi: 10.1021/ol005730q.
- 11.For a review on the metal catalyzed reductive coupling of olefin-derived nucleophiles, see: Nguyen KD, Park BY, Luong T, Sato H, Garza VJ, Krische MJ. Science. 2016;354:300. doi: 10.1126/science.aah5133.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.