Abstract
Objective
This prospective study compared pre-surgical language localization with visual naming associated high-γ modulation (HGM) and conventional electrical cortical stimulation (ECS) in children with intracranial electrodes.
Methods
Drug-resistant epilepsy patients undergoing intracranial monitoring were included if able to name pictures. ECoG signals were recorded during picture naming (overt and covert) and quiet baseline. For each electrode the likelihood of high-γ (70–116 Hz) power modulation during naming task relative to the baseline was estimated. Electrodes with significant HGM were plotted on a 3D cortical surface model. Sensitivity, specificity, and accuracy were calculated compared to clinical ECS.
Results
Seventeen patients with mean age of 11.3 years (range: 4–19) were included. In patients with left hemisphere electrodes (n=10), HGM during overt naming showed high specificity (0.81, 95% CI 0.78–0.85), and accuracy (0.71, 95% CI 0.66–0.75, p<0.001), but modest sensitivity (0.47) when ECS interference with naming (aphasia or paraphasic errors) and/or oral-motor function was regarded as the gold standard. Similar results were reproduced by comparing covert naming associated HGM with ECS naming sites. With right hemisphere electrodes (n=7), no ECS naming deficits were seen without interference with oral motor function. HGM mapping showed a high specificity (0.81, 95% CI 0.78–0.84), and accuracy (0.76, 95% CI 0.71–0.81, p=0.006), but modest sensitivity (0.44) compared to ECS interference with oral motor function. Naming-associated ECoG HGM was consistently observed over Broca’s area (left posterior inferior frontal gyrus), bilateral oral/facial motor cortex, and sometimes over temporal pole.
Significance
This study supports the use of ECoG HGM mapping in children where adverse events preclude ECS, or as a screening method to prioritize electrodes for ECS testing.
Keywords: Language mapping, Functional brain mapping, High frequency oscillations, Epilepsy surgery
INTRODUCTION
Localization of language cortex and defining its anatomical relationship with seizure-onset zone is crucial for surgical decision making in patients with drug-resistant epilepsy (DRE). Despite advances in non-invasive technology, a substantial proportion of patients with DRE require implantation of intracranial electrodes for pre-surgical evaluation. In patients with subdural electrodes, the conventional method for language mapping is based on response inhibition during electrical cortical stimulation (ECS) performed sequentially between electrode-pairs. There have been long-standing concerns about safety and ecological validity of ECS, particularly in children. In young children, ECS thresholds for functional inhibition have been shown to be higher than those for after-discharges1. ECS is also time-intensive and involves risks of seizures, pain, and adverse effects from pre-medication2. An alternative approach for mapping language and other cortical functions has recently emerged, based on event-related spectral modulations in electrocorticographic (ECoG) recordings3. More pertinently, increases in ECoG broadband high-γ (60–150 Hz) activity has been reproducibly observed during several language tasks such as auditory discrimination and word production, and has correlated well with increased neural firing rates and blood-oxygen level dependent responses4; 5. Compared to lower frequency energy modulations (alpha/mu desynchronization), high-γ modulation (HGM) has shown better functional specificity, better consistency, and favorable spatial and temporal profiles for language tasks6. However, there is a paucity of data for clinical validation of spectral modulation based methods for language localization in pediatric DRE compared to the clinical standard of ECS. This prospective study compared the topography of ECoG HGM during overt and covert visual naming tasks with behavioral effects of clinical ECS on naming and oral motor function.
METHODS
Participants
All patients undergoing pre-surgical evaluation with intracranial electrodes at Cincinnati Children’s Hospital Medical Center since March 2012, with ability to overtly name pictures were eligible for inclusion. The subdural electrodes implanted in these patients for recording ECoG signals were 4.75 mm platinum/iridium discs embedded in silicone elastomer (1.5 mm exposed contacts) having 1 cm inter-electrode distance (Auragen, Integra Neurosciences, Plainsboro, NJ). A 2-contact subdural strip facing the dura away from the main recording arrays, served as reference and ground electrode. These patients were gradually weaned off their anti-epileptic drugs (AEDs) starting 1–2 weeks before, with the last dose often on the morning of admission. Demographic information, seizure history, and details of non-invasive (phase I) pre-surgical evaluation were obtained from the patients’ records for study purposes. The study was approved by the review board of the study institution (IRB #2012-0791).
Electrical cortical stimulation
Extra-operative bipolar ECS was performed with OCS2 Ojemann cortical stimulator (Integra Life Sciences, Plainsboro, NJ). The selection of electrode pairs, stimulation end-point, and the procedure of visual naming were at the discretion of the neurologist performing the ECS. The initial settings were: pulse frequency 50 Hz, pulse duration 500 µs, train duration 5 sec, and stimulus intensity 2 mA. Stimulus intensity was increased by 1–2 mA intervals until any of the 3 endpoints were reached: functional response (aphasia, paraphasic errors, dysarthria, or oral sensorimotor phenomena); after-discharges with evolution (in frequency, amplitude, or locus); or the instrument limit of 10 mA. In case of after-discharges, sometimes the stimulus intensity was reduced by 25%, pulse duration was increased to 1 ms and repeat stimulation was attempted7. For the last 6 patients, Nicolet cortical stimulator was used (Natus Medical Inc., San Carlos, CA). The procedure and initial settings were identical, except that the maximum current strength was 15 mA.
Experimental Protocol and Data Collection
ECoG recordings for study purposes were typically performed after adequate seizures had been captured but AEDs were still being withheld. A baseline period of ECoG signals was captured for each participant in an awake, relaxed, and silent state in a quiet environment for at least 5 minutes. Participants were then requested to name, first aloud (overt) and then silently (covert), a series of line drawings of common animate and inanimate objects presented on an electronic monitor. The pictures were shown for 3500 ms each, with 2500 ms inter-stimulus interval, repeatedly for 5 minutes (50 stimulations).
Covert naming is not routinely used for ECS mapping due to lack of verifiable behavioral responses, which prevents reliable ascertainment of the effect of ECS. ECS-induced naming deficits could be due to different mechanisms, including oral/facial sensorimotor phenomena (for example: involuntary muscle contractions, oro-glossal/facial sensations/pain, or paralysis), interference with cognitive processing of receptive and/or expressive language, and rarely, altered awareness (amnestic anomia). Hence, we used ECoG HGM during covert naming as an approximation of cortical sites where ECS would interfere with overt naming, without interfering with oral motor function.
ECoG signals were collected for clinical purposes using XLTek EMU128FS amplifier (Natus Medical Inc., San Carlos, CA), which included a break-out box to split signals to our research system, without interrupting the clinical data stream. The split ECoG signals were amplified and digitized with a g.USBamp (g.TEC Medical Engineering, Austria) amplifier at a sampling rate of 1.2 KHz. Vocal audio was captured using a cardioid lavalier wireless microphone (Shure Inc., Niles, IL) worn by the patient with appropriately adjusted audio gain level. This audio was recorded to a .wav file during the experiment using a BCI2000 module. The audio stream was also routed to a trigger box (g.TEC Medical Engineering, Austria), which converted the input signal to a transistor-transistor-logic pulse when the signal crossed the adjusted threshold. This digital pulse was recorded by the g.TEC amplifier synchronously with the ECoG data, allowing the onset of patient’s speech to be precisely measured2.
3D Cortical Model
A pre-surgical T1-weighted isotropic volumetric (1 mm slice thickness) brain MRI was co-registered with post-implantation computed tomographic (CT) scan of the head (0.5–1 mm slice thickness), with Curry 7 software (Neuroscan Inc., Charlotte, NC) for each participant, and segmented 3D cortical models with subdural electrode locations were exported into MATLAB format from Curry. A custom BCI2000 module based on Signal Modeling for Real-time Identification and Event Detection (SIGFRIED) was written to display the 3D model and activation projections8.
Data Analysis
ECoG signal processing approach was essentially similar to that in our previous publication9. Briefly, an autoregressive model for power features in 70–116 Hz frequency band was first fitted to the baseline data. During the picture naming tasks, the SIGFRIED algorithm was used to calculate real-time scores of the log-likelihood that the present sample block belonged to the baseline model for every electrode (thus each ECoG electrode had a unique SIGFRIED score). The SIGFRIED algorithm does not require prior information about the expected modulated frequency bands, and since any changes are encapsulated in a single score, no subjective assumptions about the data significance are required10. The data was processed in 500 ms blocks, and updated every 50 ms. Scores were measured and accumulated for the picture displays and the rest periods, separately for overt and covert naming, using vocal onsets/offsets as triggers for the overt condition, and image display onsets/offsets as triggers for the covert condition. The statistical significance of HGM at each electrode was determined by a Student’s t-test between the rest and naming scores with Bonferroni correction for the total number of channels. For plotting activations on the 3D cortical model, the negative logarithm of the p-values [−log10(p)] was used, which varies directly with cortical activation, is additive, and easy to interpret.
Comparisons
Electrodes where ECS interfered with language or oral motor functions were regarded ECS+, whereas other electrodes which were stimulated but did not show any such response were regarded as ECS−, irrespective of their lobar location. For this study, we defined language deficits during ECS as absent responses, and semantic or phonemic paraphasic errors; and oral motor deficits as dysarthria, visible oral/facial muscle contraction, or subjective (patient reported) oro-glossal sensorimotor phenomena. Although ECS is performed at electrode pairs, each electrode was counted separately to allow comparison with HGM mapping which is based on referential ECoG recordings. As in routine clinical interpretation of ECS, any electrode which was common to two or more tested pairs, was regarded as ECS+ only when it showed response inhibition in at least two paired stimulations which included the electrode. However, if the electrode was tested only in a single pair, it was scored the same as the including pair.
The electrodes which showed significant high-γ modulation relative to baseline (HGM+) were selected by taking the −log10p value between the language and baseline conditions, such that − log10(p) ≥ −log10(0.01/N), where N is the number of ECS electrodes. Sensitivity, specificity, and classification accuracy along with 95% confidence intervals were calculated for validation of HGM mapping against clinical ECS. Similar comparisons were also done with only electrodes that showed naming deficit during ECS being regarded as ECS+. Since each participant contributed multiple electrodes for the final comparisons, a Mantel-Haenszel chi-square test was performed with the null hypothesis being that the overall odds ratio for this diagnostic test comparison is one.
RESULTS
During the study period, 25 patients were eligible for inclusion, with exclusions due to inability (n=5) or unwillingness (n=1) to participate. Two of the initial patients were excluded due to technical limitations with ECoG signal acquisition. Seventeen patients (10 females) with median age of 11 years (range: 4–19) were included. There were 10 and 7 patients with left and right hemisphere electrodes respectively. Two patients were left handed and one was ambidextrous, all others being right handed. The age of onset of seizures varied from 4 months to 13 years (median 5 years, inter-quartile range [IQR] 8.6). Six patients had either normal brain MRI or non-specific findings which did not influence surgical decisions. Others had malformations of cortical development (n=3), tumors (n=2), perinatal brain injury (n=2), hippocampal sclerosis (n=1), Tuberous Sclerosis complex (n=1), and dual pathology (n=2). During phase I pre-surgical evaluation, functional MRI showed left lateralization of verb generation in 9 patients, was non-diagnostic in 4 patients, and was not done in the remaining ones (data not analyzed further for present study). The number of intracranial electrodes varied from 40 to 126 (median 90, IQR 36). Relevant summary of pre-surgical evaluation is provided in table 1.
Table 1.
Non-invasive (phase I) pre-surgical evaluation and configuration of subdural electrodes
| Patient | Age (years), sex, hand |
Age of seizure onset |
Semiology | EEG | PET | SISCOM/ SPECT |
MEG | Pre-surgical etiology (MRI) | FSIQ | fMRI | SD electrodes |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Left hemisphere electrodes | |||||||||||
| L1 | 12, M, L | 14 m | LT, LF | LT, LFT | LT >LF | LT | L>R TFP | TSC (Multiple tubers (L>R), abnormal signal in LT WM) | 41 | ND | 104 (dl-F, lat-T, sub-T) |
| L2 | 9, f, R | 12 m | LF | LF | N | LF | LF, LP | Unknown | 45 | φ | 76 (dl-F, lat-T) |
| L3 | 19, f, R | 13 y | L | LT | LT | LT | LT | LF CD, PVNH | N/A | L (0.82) | 116 (dl-F, ant-P, lat-T, sub-T,TOJ) |
| L4 | 6, f, A | 4 y | L | LP | LP | LF, LP | LT, LP | TSC (mosaic) vs. CD (BL TP) | 81 | ND | 92 (dl-F, lat-T, sub-T, ant-P) |
| L5 | 11, f, R | 8 y | X | LT | LT | LT | LT | HS vs. CD | 60 | ND | 96 (dl-F, lat-T, ant-P, sub-T, sub-O) |
| L6 | 8, M, L | 4 m | T/Insula | LT | φ | φ | φ | Tumor | 101 | L (F:0.49, T:0.28) | 76 (lat-T, sub-T, dl-F, ant-P) |
| L7 | 4, M, R | 17 m | LF | LF, LT | LF, LT | LF, LT | LT | HS + CD (LT + Insula) | 60 | φ | 90 (dl-F, orb-F, lat-T, sub-T, ant-P) |
| L8 | 6, M, R | 5 y | L | LT | LT | φ | LCT | Tumor ± CD | 119 | φ | 40 (lat-T) |
| L9 | 12, f, R | 4.5 y | LF | BL | LT | LT | BL | Perinatal injury (LH volume loss, thalamic encephalomalacia) | 72 | L (F:0.48, T:-0.03) | 126 (dl-F, orb-F, lat-T, sub-T, ant-P) |
| L10 | 12, M, R | 10 y | LT | LT | LT | φ | LT >LF | Tumor (LSTG) | N/A | L (0.89) | 58 (lat-T, dl-F, sub-T, sub-O, inf-P) |
| Right hemisphere electrodes | |||||||||||
| R1 | 14, f, R | 11 y | R | RT | RT >RP | RT | RT | Unknown | 87 | L (F:0.93, T:0.74) | 100 (lat-T, dl-F, sub-T, TOJ) |
| R2 | 17, M, R | 12 y | F/T | RF, RT | RT> RF | φ | RT> RF | Unknown | 64 | ND | 68 (dl-F, FTPJ, TOJ, lat-T, lat-O) |
| R3 | 11, f, R | 9 y | R | RTP> BL | RP | RP | RP> RF | RP CD | 102 | L (F:0.84) | 88 (dl-F, ant-P, lat-T) |
| R4 | 17, M, R | 5 y | RF | RF | R | RF | RF | Unknown | 94 | L (F:0.92, T:0.94) | 120 (dl-F, orb-F, ant-P, lat-T, sub-T) |
| R5 | 9, f, R | 7 m | RF | RF | RF, RP | LT | RF | Perinatal injury | N/A | φ | 112 (dlt-F,orb-F,lat-P,dual-IH) |
| R6 | 8, f, R | 7 y | RP | RF, RP | N | RP | RF> RP | Unknown | 91 | L (0.83) | 64 (dl-F, lat-P, lat-T) |
| R7 | 17, f, R | 12 y | RT | R | RT | RT | RT | Unknown | 74 | L (F:0.78, T:1.0) | 58 (lat-T, sub-T, HC-D, dl-F, orb-F) |
(Abbreviations: φ Not performed, A Ambidextrous, ant Anterior, CD Cortical dysplasia, dl Dorsolateral, EEG Electroencephalograph, HC-D Hippocampal depth electrode, f Female, F Frontal, J Junction, L Left, lat Lateral, m months, M male, MEG Magetoencephalography, MRI Magnetic Resonance Imaging, N Normal, N/A Not available, ND Non-diagnostic, O Occipital, orb Orbital, R Right, P Parietal, PET Interictal fluorodeoxy-glucose positron emission tomography, PVNH Peri-ventricular nodular heterotopia, SD Subdural, SISCOM Subtraction ictal SPECT co-registered to MRI, SPECT Single photon emission computed tomography, STG Superior temporal gyrus, T Temporal, TSC Tuberous sclerosis complex, WM White matter)
Left hemisphere comparisons
For overt visual naming, when both language and oral motor ECS sites were used for comparison, HGM showed good specificity (0.81, 95% CI 0.78–0.85), with an accuracy of 0.71 (95% CI 0.66–0.75, p<0.001, table 2). However, the sensitivity (0.47, 95% CI 0.39–0.55) was modest. When only ECS electrodes with naming deficits were used for comparison, HGM showed specificity of 0.76 (95% CI 0.73–0.79), and accuracy of 0.68 (95% CI 0.64–0.73, p=0.012).
Table 2.
Diagnostic validation of language mapping with high-γ modulation (HGM) against conventional electrical cortical stimulation (ECS): Patients with left hemisphere electrodes
| Overt naming | ||||||||
|---|---|---|---|---|---|---|---|---|
| Patient ID | Both naming and oral motor sites as ECS+ | Only naming sites as ECS+ | ||||||
| ECS + | HGM + |
ECS + | HGM − |
ECS − | HGM + |
ECS − | HGM − |
ECS + | HGM + |
ECS + | HGM − |
ECS − | HGM + |
ECS − | HGM − |
|
| L1 | 2 | 4 | 11 | 35 | 0* | 0* | 13 | 39 |
| L2 | 4 | 4 | 1 | 14 | 4 | 4 | 1 | 14 |
| L3 | 18 | 24 | 0 | 13 | 9 | 22 | 9 | 15 |
| L4 | 3 | 7 | 5 | 31 | 1 | 1 | 7 | 37 |
| L5 | 1 | 2 | 2 | 8 | 1 | 2 | 2 | 8 |
| L6 | 5 | 2 | 10 | 33 | 4 | 1 | 11 | 34 |
| L7 | 1 | 6 | 0 | 8 | 1 | 6 | 0 | 8 |
| L8 | 1 | 1 | 5 | 12 | 1 | 1 | 5 | 12 |
| L9 | 6 | 2 | 5 | 12 | 1 | 1 | 10 | 13 |
| L10 | 6 | 1 | 2 | 13 | 4 | 1 | 4 | 13 |
| N = 10 | 47 | 53 | 41 | 179 | 26 | 39 | 62 | 193 |
| Summary statistics (95% Confidence limits) | ||||||||
| Sensitivity | 0.47 (0.39, 0.55) | 0.40 (0.29, 0.51) | ||||||
| Specificity | 0.81 (0.78, 0.85) | 0.76 (0.73, 0.79) | ||||||
| Accuracy | 0.71 (0.66, 0.75) | 0.68 (0.64, 0.73) | ||||||
| MH test | p <0.001 | p = 0.012 | ||||||
| Covert naming | ||||||||
| Patient ID | Both naming and oral motor sites as ECS+ | Only naming sites as ECS+ | ||||||
| ECS + | HGM + | ECS + | HGM − | ECS − | HGM + | ECS − | HGM − | ECS + | HGM + | ECS + | HGM − | ECS − | HGM + | ECS − | HGM − | |
| L3 | 2 | 40 | 0 | 13 | 0 | 31 | 2 | 22 |
| L4 | 2 | 8 | 9 | 27 | 0 | 2 | 11 | 33 |
| L5 | 0 | 3 | 0 | 10 | 0 | 3 | 0 | 10 |
| L6 | 2 | 5 | 3 | 40 | 2 | 3 | 3 | 42 |
| L7 | 1 | 6 | 0 | 8 | 1 | 6 | 0 | 8 |
| L8 | 0 | 2 | 1 | 16 | 0 | 2 | 1 | 16 |
| L9 | 5 | 3 | 2 | 15 | 0 | 2 | 7 | 16 |
| L10 | 0 | 7 | 0 | 15 | 0 | 5 | 0 | 17 |
| N = 8 | 12 | 74 | 15 | 144 | 3 | 54 | 24 | 164 |
| Summary statistics (95% Confidence limits) | ||||||||
| Sensitivity | 0.14 (0.08, 0.20) | 0.05 (0.01, 0.14) | ||||||
| Specificity | 0.91 (0.88, 0.94) | 0.87 (0.86, 0.90) | ||||||
| Accuracy | 0.64 (0.60, 0.68) | 0.68 (0.66, 0.72) | ||||||
| MH test | p = 0.282 | p = 0.114 | ||||||
(Notes: No naming sites found on ECS, MH Mantel-Haenszel test)
For covert naming, HGM again showed high specificity (0.91, 95% CI 0.88–0.94) compared to ECS naming and oral motor sites, but poor sensitivity, with overall accuracy of 0.64 (95% CI 0.60–0.68, p=0.282, table 2). Using only ECS naming sites for comparison, HGM had a specificity of 0.87 (95% CI 0.86–0.90), and accuracy of 0.68 (95% CI 0.66–0.72, p=0.114).
Right hemisphere comparisons
No electrodes with language deficits during ECS were identified in the right hemisphere. Also, none of these patients with right-sided electrodes had right lateralizing language on functional MRI (Table 1). Hence, electrodes showing ECS interference with oral motor function were used for comparison. On overt visual naming, HGM was noted to have high specificity (0.81, 95% CI 0.78–0.84), with accuracy of 0.76 (95% CI 0.71–0.81, p=0.006), but modest sensitivity (0.44, 95% CI 0.26–0.63, table 3). With covert naming, HGM had high specificity (0.96, 95% CI 0.94–0.98), with accuracy of 0.83 (95% CI 0.79–0.87, p=0.004), and poor sensitivity (0.20, 95% CI 0.08–0.32, table 3).
Table 3.
Diagnostic validation of language mapping with high-γ modulation (HGM) against conventional electrical cortical stimulation (ECS): Patients with right hemisphere electrodes
| Overt naming | ||||
|---|---|---|---|---|
| Patient ID | ECS + | HGM + | ECS + | HGM − | ECS − | HGM + | ECS − | HGM − |
| R1 | 2 | 2 | 3 | 9 |
| R2 | 6 | 0 | 3 | 10 |
| R3 | 0 | 0 | 6 | 16 |
| R4 | 1 | 11 | 3 | 29 |
| R5 | 0 | 0 | 6 | 21 |
| R6 | 0 | 0 | 0 | 16 |
| R7 | 2 | 1 | 7 | 19 |
| N = 7 | 11 | 14 | 28 | 120 |
| Summary statistics (95% CI) | ||||
| Sensitivity | 0.44 (0.26, 0.63) | |||
| Specificity | 0.81 (0.78, 0.84) | |||
| Accuracy | 0.76 (0.71, 0.81) | |||
| MH test | p = 0.006 | |||
| Covert naming | ||||
| Patient ID | ECS+ | HGM + | ECS + | HGM - | ECS - | HGM + | ECS - | HGM - |
| R1 | 2 | 2 | 1 | 11 |
| R2 | 0 | 6 | 0 | 13 |
| R3 | 0 | 0 | 0 | 22 |
| R4 | 1 | 11 | 4 | 28 |
| R6 | 0 | 0 | 0 | 16 |
| R7 | 2 | 1 | 0 | 26 |
| N = 6 | 5 | 20 | 5 | 116 |
| Summary statistics (95% CI) | ||||
| Sensitivity | 0.20 (0.08, 0.32) | |||
| Specificity | 0.96 (0.94, 0.98) | |||
| Accuracy | 0.83 (0.79, 0.87) | |||
| MH test | p = 0.004 | |||
(Notes: ECS+ represents only electrodes with oral motor dysfunction during stimulation (defined in the text) in this table, MH Mantel-Haenszel test)
Topography of ECoG high-γ modulation
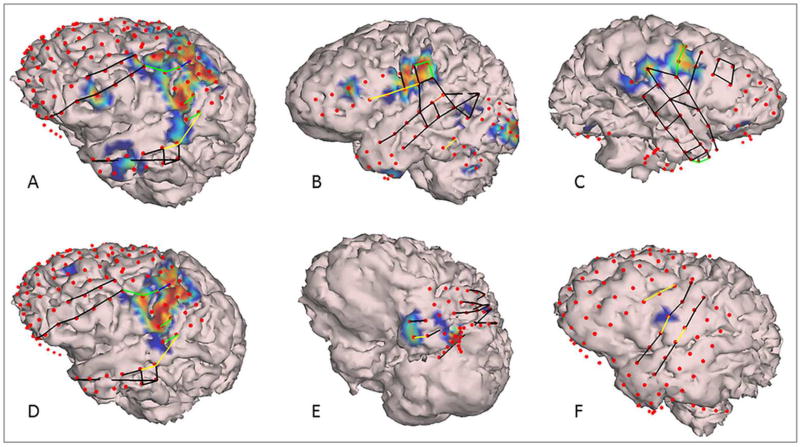
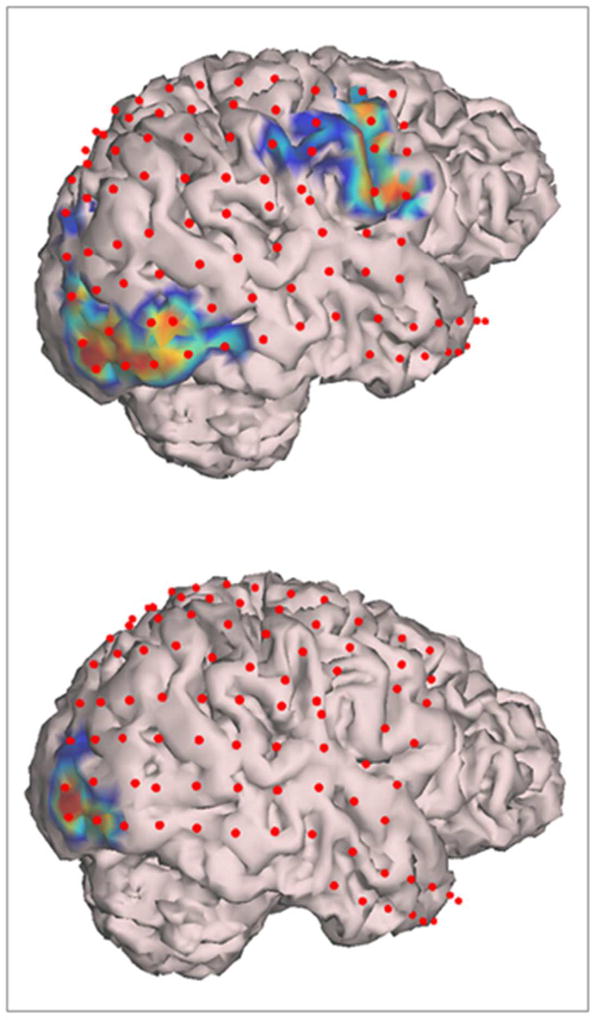
In patients with left hemisphere electrodes, significant HGM was seen with overt naming over the peri-Rolandic cortex having a wider extent over posterior parts of middle and inferior frontal gyri (IFG), particularly over Broca’s area (pars triangularis and pars opercularis of IFG), and oral-facial motor representation (Figure 1 A,B). HGM was also noted in premotor cortex, orbital-frontal cortex and temporal pole. In patients with right hemisphere electrodes, somewhat spatially restricted HGM was seen in inferior peri-Rolandic cortex representing oral/facial motor cortex (Figure 1C). With covert naming, HGM was seen in the region of Broca’s area, and rarely at the left temporal pole (Figure 1 D–F). Activation of the oral-facial motor cortex on the right side was clearly seen during overt but not during covert naming (Figure 2). In participants with occipital electrodes, HGM over the visual cortex was also noted (Figures 1, 2).
Figure 1.
Segmented cortical models derived from patient’s brain MRI with superimposed subdural electrodes (red dots), electrical cortical stimulation (ECS) results (green lines: oral motor, yellow lines: language deficits, black lines: stimulated but no language/oral motor deficits), and high-γ modulations (HGM) for overt (top panel) and covert (bottom panel) visual naming. HGM are plotted according to −log10(p) values for comparison of SIGFRIED scores between naming and baseline (see text for details and also for definitions of ECS deficits). The smallest score displayed is −log10(p)=4 corresponding to p=0.0001. Representative examples for patients L9 (A, D), L10 (B), R7 (C), L8 (E), and L7 (F).
Figure 2.
Segmented cortical model derived from patient R3’s brain MRI with superimposed subdural electrodes (red dots), and high-γ modulations (HGM) for overt (top panel) and covert (bottom panel) visual naming. Note the activation of oral/facial motor cortex with overt (top panel) but not covert (top panel) naming. Also note the activation of visual cortex in occipital lobe in both conditions.
Since ECoG HGM was predominantly localized to frontal lobes, a lobe wise subgroup analysis to compare language mapping by HGM and ECS was performed (Table e1). This revealed similar specificity (0.77, 95% CI 0.70, 0.83) and accuracy (0.70, 95% CI 0.62, 0.77), but improved sensitivity (0.59, 95% CI 0.49, 0.69) in left frontal lobe compared to overall dataset (p<0.001). In left temporal lobe, all 3 diagnostic indices were lower than the overall dataset and the comparison with ECS was not statistically significant. Only one of the patients with right hemisphere electrodes had temporal lobe coverage, so the subgroup analysis could not be done.
DISCUSSION
This study shows the feasibility of pre-surgical language mapping with ECoG high-γ spectral modulation in pediatric DRE. Though widely regarded as a clinical gold standard, ECS is associated with risks of pain, after-discharges, and seizures, which can preclude functional mapping or undermine its results11. ECoG HGM associated with overt visual naming was highly specific (0.76–0.81) and fairly sensitive (0.40–0.47) for localization of ECS+ electrodes in patients with left hemisphere implants, whether only language (p=0.012) or both language and oral motor ECS sites (p<0.001) were used for comparison (Table 2). Similarly, in patients with right hemisphere electrodes, HGM mapping was highly specific (0.81–0.96) for localization of ECS electrodes with oral motor dysfunction, using either overt (p=0.006) or covert (0.004) naming (Table 3). We investigated HGM associated with both overt and covert naming, in an attempt to delineate the cognitive and motor aspects of expressive language. However, when anomia is observed during ECS it may be challenging to differentiate the relative contributions of aphasia versus severe dysarthria, and the distinction is often based on neuroanatomy12. Hence, probably the most ecologically valid comparisons are those with electrodes where ECS interfered with language and/or oral motor function. Based on our finding of overall accuracy of about 70% in pediatric DRE lateralized to the left hemisphere, HGM mapping can potentially be used as a surrogate for ECS when adverse events preclude ECS mapping. HGM mapping can potentially also be used to prioritize electrodes for ECS, particularly in patients with right hemisphere electrodes. Given the high specificity of HGM mapping, HGM+ electrodes can be predicted to be ECS+, and may not require ECS unless within the margins of the proposed resection.
Our results agree with studies of HGM mapping in adults. A study including 13 patients aged 16–47 years, using naming of line drawings, found 84% specificity and 43% sensitivity, when both language and oral motor electrodes were regarded as true positive13. This study defined high-γ frequency band as 80–100 Hz and included only patients with left hemisphere electrodes having a full-scale IQ above 80. Although an important validation of HGM mapping, the high-γ activation thresholds were arbitrarily varied across subjects and 12 electrodes with highest activation were empirically chosen for every patient in this study13. Another study including 4 native Dutch speakers aged 24–49 years found specificity of 0.90 and sensitivity of 0.21 for naming-associated activation in 65–95 Hz band14. These investigators primarily tested whether inclusion of spontaneous conversation related HGM along with task-based activation, improves sensitivity and/or specificity of HGM mapping compared to ECS, and they inferred that this is not the case. Contrariwise, we have recently shown HGM associated with spontaneous conversation to have a high sensitivity (88.9%) and fair specificity (63.6%) compared to ECS9. Although the present study was not designed to compare HGM during natural conversation with structured language tasks, together with our previous data, it suggests that a combination of tasks may further improve accuracy of HGM mapping compared to ECS, an attractive hypothesis for further investigation. Another study in 9 patients, aged 15–37 years, showed 100% sensitivity and 85% specificity for 50–119 Hz HGM and ECS comparison15. However, this study compared the task and baseline power features in 20 Hz clusters, and counted electrodes showing a significant difference from baseline in even a single cluster as HGM+. Further, these investigators regarded an electrode to be true positive for ECoG HGM if a neighboring electrode was ECS positive: the so-called next-neighbor approach15. While the specificity found in this study is comparable to ours, their rather liberal criteria for defining true positive electrodes is likely responsible for higher sensitivity. The next-neighbor approach for defining ECS+ electrodes does not represent the usual clinical practice, though sometimes used in research1; 12. Recently, a study in 7 adults found 80.5% specificity and 63.4% sensitivity for visual naming, using an online system for spatial-temporal analysis of task-associated spectral modulation in 70–110 Hz high-γ band16.
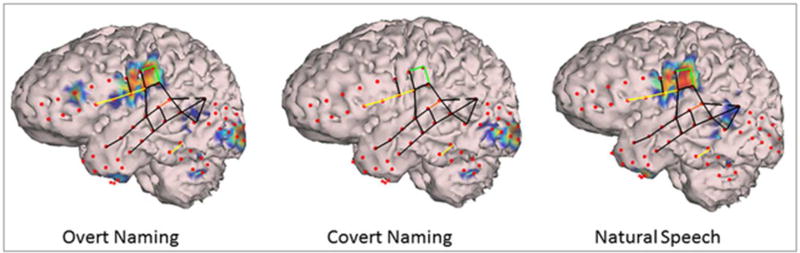
The topography of ECoG HGM seen in our study is consistent with previous studies showing HGM to be an index of overall neural firing rates that can be used to study cortical function with excellent spatial and temporal resolution5. We observed HGM in oral/facial motor cortex in both hemispheres, Broca’s area, and occasionally in left orbital frontal, left pre-motor, and bilateral temporal polar cortices (Figures 1, 2). In children with occipital coverage, activation of primary visual cortex and temporal-occipital junction was also seen (Figures 1, 2). Similar topography of broadband (50–120 Hz) HGM has been shown in 54 patients aged 4–56 years in modality-specific sensory cortices during stimulus presentation and inferior Rolandic regions during responses17. Specifically, HGM was seen in bilateral occipital and left middle-temporal, inferior parietal, and frontal lobes in association with object naming. However, this study grouped electrodes according to empiric anatomic regions, and assigned language dominant hemisphere based on handedness and etiology of epilepsy. Together with our observations, these results suggest that neural information processing in high-γ band may not be modality specific, which is consistent with previous observations of HGM during functional activation in sensory, motor, and association cortices3; 18. For example, in one of our patients with left hemisphere electrodes, HGM was observed in both visual and inferior precentral cortices during overt naming, with only visual activation being seen with covert naming, which indicated processing of visual information but raising doubts about patient participation with covert naming. Further, selective activation of inferior precentral cortex including Broca’s and oral motor cortex was seen during natural speech with absence of occipital activation in the same patient, consistent with a lack of visual stimulation during spontaneous conversation9 (Figure 3). These results also agree with primate data on the role of ventral visual stream in object recognition, identification, and feedforward to language areas in posterior temporal lobe19; 20.
Figure 3.
Segmented cortical model derived from patient L10’s brain MRI with superimposed subdural electrodes (red dots); high-γ modulations (HGM) for overt naming, covert naming, and natural conversation; and electrical cortical stimulation (ECS) findings (green lines: oral motor, yellow lines: language deficits, black lines: stimulated but no language/oral motor deficits, see text for definitions). Note HGM in posterior inferior frontal gyrus encroaching on middle frontal gyrus (Broca’s area and oral/facial motor cortex), premotor cortex, and temporal pole during overt but not during covert naming. Visual cortex activation in seen during both of these conditions. During natural conversation, HGM is limited to Broca’s area, oral motor representation, with some activation in posterior superior temporal gyrus (Wernicke’s area), but not in the visual cortex.
Though ECoG offers the advantage of high signal-to-noise ratio and being relatively free of artifact, it is spatially filtered to begin with. Comparison with MEG as a whole-head modality for frequency-specific energy modulations reveals ECoG HGM to have better localization. In a study of 11 healthy subjects, using block design for picture naming (10 drawings shown, 30 s task, 21 s rest), task-related modulation in 7–12 Hz and 17–22 Hz were observed over a widespread region including occipital, temporo-parieto-occipital junction (TPOJ), peri-Rolandic, and lateral temporal cortices21. MEG language studies have particularly looked at temporal aspects of task-related activations. A study of 8 Dutch speakers analyzed dipole sources in pre-specified time windows after stimulus presentation, supposedly representing sub-processes involved in naming. The sources localized to angular and supra-marginal gyri at 150–275 ms (lemma selection), posterior peri-Sylvian cortex at 275–400 ms (phonological encoding) and peri-Rolandic region at 400–600 ms (phonetic and articulatory processing)22. However, sources were constrained to peri-Rolandic and peri-Sylvian cortices. This issue was also evident with another MEG study of 32 patients having epilepsy or brain tumors, where source imaging of an object naming task led to widespread statistically significant localization including TPOJ and even periventricular white matter23. However, the authors constrained source localization to regions of interest in Broca’s area and posterior superior temporal gyrus. Another MEG study looking at differences between “naming” and “request” tasks also constrained source localization to regions known to be involved in speech processing24. Other than the clinical necessity of spatially limited intracranial electrode coverage, no further presumptions about localization of ECoG HGM were made in our study.
Like other studies on ECoG spectral modulation in humans13; 14; 16, we found imperfect agreement between ECS and HGM mapping. There are several important reasons for this, not unexpected, finding. Perhaps most importantly, HGM mapping is based on functional activation compared to ECS which is based on behavioral effects of a transient lesion. Moreover, there are other reasons for ECS not being an ideal comparator for HGM mapping. The biophysics of spread of current applied to in vivo brain tissue during ECS is poorly understood and is probably a function of inter-individual variability in functional anatomy11. Given the high prevalence of after-discharges with ECS (up to 70%), it is likely that ECS may also deactivate remote cortex with strong functional connectivity to the site of stimulation3; 25. Further, ECS does not always reliably predict postoperative language deficits. For example, ECS of anterior fusiform gyrus and anterior inferior temporal gyrus has been shown to interfere with language tasks, but corresponding deficits have not been consistently reported after anterior temporal resection26; 27. In addition to these potential shortcomings of ECS, there are limitations with the current methodology of HGM mapping as well. Given the low amplitude and high spatial granularity of high-γ responses, recordings with conventional subdural electrodes may underestimate task-associated activation16. The amplitudes of field potential oscillations decrease logarithmically with increasing frequency, implying that higher frequency oscillations are probably generated by smaller or more dispersed neuronal ensembles, which may be insufficiently sampled by conventional macro-electrodes13; 28. Whereas posterior temporal lobe ECS can also cause naming deficits, naming-associated HGM predominantly localized to the frontal lobes. On subgroup analyses, HGM had similar specificity but improved sensitivity for detection of ECS+ electrodes in left frontal lobe (Table e1). This may suggest better validity of HGM mapping for Broca’s area compared to posterior language sites. However, we believe that including all ECS+ sites irrespective of their brain location provides most conservative validation of HGM mapping as an alternative for ECS.
The variability in sensitivities and specificities reported by different investigators may also be due to methodological differences in the computation of ECoG HGM. Our study used a modification of SIGFRIED algorithm10, which calculates the likelihood of the distribution of HGM during blocks of task performance being different from the distribution during rest blocks. Other studies have computed time-resolved magnitude and significance of event-related HGM during accumulating trials of task performance13; 15; 17. This latter approach demands greater behavioral control, which can be challenging for young children. Finally, different investigators have used somewhat arbitrary and dissimilar, albeit overlapping, frequency bands within the broad range of high-γ frequencies (60–150 Hz). Hence, additional studies are needed to determine which frequency bands are best predictive of ECS results and help prevent postoperative language deficits, which is the ultimate goal of pre-surgical language mapping.
Supplementary Material
KEY POINTS.
-
▪
Though clinical gold standard, language mapping with electrical cortical stimulation is challenging in children
-
▪
ECoG high-y modulation during visual naming showed high specificity and accuracy compared to clinical standard of cortical stimulation mapping
-
▪
High-y modulation during visual naming was consistently observed over Broca’s area and bilateral oral/facial motor cortex
-
▪
Pre-surgical language mapping using ECoG high-y modulation may be used where adverse events preclude electrical cortical stimulation
-
▪
It may also help prioritize selection of electrodes for testing with electrical stimulation
Acknowledgments
RA wishes to acknowledge support from Susan T. Herman, M.D. through American Epilepsy Society’s EpiPORT program.
Funding:
The study was supported by Research Innovation Project Grant from Cincinnati Children’s Research Foundation, awarded to JAW and RA, and the Division of Neurology at Cincinnati Children’s Hospital Medical Center. The funding source had no role in the study design; collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Disclosures:
RA receives research support from Pediatric Epilepsy Research Foundation (Co-I); KDH received funding from the following NIH grants: R01 NS062756 (PI), R01NS062806 (Co-I), and R01NS065020 (Co-I). None of the other authors has any pertinent disclosure. Findings from this work were presented partially at the 2016 annual meeting of American Epilepsy Society.
Footnotes
Authors’ Contributions:
Study concept was developed by JAW, DFR and KDH. RA, JAW, AWB, and DFR designed the study. Data was collected by RA and JAW with instrumentation support from JB and LR. FTM performed all the surgical procedures. Computational methods were developed by JAW with help in segmenting 3D cortical models from HF. Data was analyzed by JAW, RA, GM and PSH with support from BE, AM, and NEC. RA wrote the first draft of the manuscript which was critically reviewed by all authors. All authors approve of the final manuscript.
Ethical publication statement:
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Jayakar P, Lesser RP. Extraoperative functional mapping. In: Engel JJ, Pedley TA, editors. Epilepsy: A comprehensive textbook. Lippincott Williams & Wilkins; Philadelphia, PA: 2008. [Google Scholar]
- 2.Arya R, Wilson JA. Real-Time Mapping of Natural Speech in Children with Drug-Resistant Epilepsy. In: Guger C, Müller Putz G, Allison B, editors. Brain-Computer Interface Research: A State-of-the-art Summary. Springer International Publishing; 2015. pp. 9–17. [Google Scholar]
- 3.Crone NE, Sinai A, Korzeniewska A. High-frequency gamma oscillations and human brain mapping with electrocorticography. Prog Brain Res. 2006;159:275–295. doi: 10.1016/S0079-6123(06)59019-3. [DOI] [PubMed] [Google Scholar]
- 4.Crone NE, Hao L, Hart J, Jr, et al. Electrocorticographic gamma activity during word production in spoken and sign language. Neurology. 2001;57:2045–2053. doi: 10.1212/wnl.57.11.2045. [DOI] [PubMed] [Google Scholar]
- 5.Lachaux JP, Axmacher N, Mormann F, et al. High-frequency neural activity and human cognition: past, present and possible future of intracranial EEG research. Prog Neurobiol. 2012;98:279–301. doi: 10.1016/j.pneurobio.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crone NE, Miglioretti DL, Gordon B, et al. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. II. Event-related synchronization in the gamma band. Brain. 1998;121(Pt 12):2301–2315. doi: 10.1093/brain/121.12.2301. [DOI] [PubMed] [Google Scholar]
- 7.Jayakar P, Alvarez LA, Duchowny MS, et al. A safe and effective paradigm to functionally map the cortex in childhood. J Clin Neurophysiol. 1992;9:288–293. doi: 10.1097/00004691-199204010-00009. [DOI] [PubMed] [Google Scholar]
- 8.Schalk G, McFarland DJ, Hinterberger T, et al. BCI2000: A general-purpose, brain-computer interface (BCI) system. Ieee Transactions on Biomedical Engineering. 2004;51:1034–1043. doi: 10.1109/TBME.2004.827072. [DOI] [PubMed] [Google Scholar]
- 9.Arya R, Wilson JA, Vannest J, et al. Electrocorticographic language mapping in children by high-gamma synchronization during spontaneous conversation: Comparison with conventional electrical cortical stimulation. Epilepsy Res. 2015;110:78–87. doi: 10.1016/j.eplepsyres.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 10.Brunner P, Ritaccio AL, Lynch TM, et al. A practical procedure for real-time functional mapping of eloquent cortex using electrocorticographic signals in humans. Epilepsy Behav. 2009;15:278–286. doi: 10.1016/j.yebeh.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lesser R, Gordon B, Uematsu S. Electrical stimulation and language. J Clin Neurophysiol. 1994;11:191–204. doi: 10.1097/00004691-199403000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Lesser RP, Luders H, Klem G, et al. Extraoperative cortical functional localization in patients with epilepsy. J Clin Neurophysiol. 1987;4:27–53. doi: 10.1097/00004691-198701000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Sinai A, Bowers CW, Crainiceanu CM, et al. Electrocorticographic high gamma activity versus electrical cortical stimulation mapping of naming. Brain. 2005;128:1556–1570. doi: 10.1093/brain/awh491. [DOI] [PubMed] [Google Scholar]
- 14.Bauer PR, Vansteensel MJ, Bleichner MG, et al. Mismatch between electrocortical stimulation and electrocorticography frequency mapping of language. Brain Stimul. 2013;6:524–531. doi: 10.1016/j.brs.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Babajani-Feremi A, Narayana S, Rezaie R, et al. Language mapping using high gamma electrocorticography, fMRI, and TMS versus electrocortical stimulation. Clin Neurophysiol. 2016;127:1822–1836. doi: 10.1016/j.clinph.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Fifer MS, Flinker A, et al. Spatial-temporal functional mapping of language at the bedside with electrocorticography. Neurology. 2016;86:1181–1189. doi: 10.1212/WNL.0000000000002525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kojima K, Brown EC, Matsuzaki N, et al. Gamma activity modulated by picture and auditory naming tasks: Intracranial recording in patients with focal epilepsy. Clin Neurophysiol. 2013;124:1737–1744. doi: 10.1016/j.clinph.2013.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neuper C, Wortz M, Pfurtscheller G. ERD/ERS patterns reflecting sensorimotor activation and deactivation. Prog Brain Res. 2006;159:211–222. doi: 10.1016/S0079-6123(06)59014-4. [DOI] [PubMed] [Google Scholar]
- 19.Mishkin M, Ungerleider LG. Contribution of striate inputs to the visuospatial functions of parieto-preoccipital cortex in monkeys. Behav Brain Res. 1982;6:57–77. doi: 10.1016/0166-4328(82)90081-x. [DOI] [PubMed] [Google Scholar]
- 20.Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends Neurosci. 1992;15:20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- 21.Laaksonen H, Kujala J, Hulten A, et al. MEG evoked responses and rhythmic activity provide spatiotemporally complementary measures of neural activity in language production. Neuroimage. 2012;60:29–36. doi: 10.1016/j.neuroimage.2011.11.087. [DOI] [PubMed] [Google Scholar]
- 22.Levelt WJ, Praamstra P, Meyer AS, et al. An MEG study of picture naming. J Cogn Neurosci. 1998;10:553–567. doi: 10.1162/089892998562960. [DOI] [PubMed] [Google Scholar]
- 23.Huang CW, Huang MX, Ji Z, et al. High-resolution MEG source imaging approach to accurately localize Broca’s area in patients with brain tumor or epilepsy. Clin Neurophysiol. 2016;127:2308–2316. doi: 10.1016/j.clinph.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 24.Egorova N, Pulvermuller F, Shtyrov Y. Neural dynamics of speech act comprehension: an MEG study of naming and requesting. Brain Topogr. 2014;27:375–392. doi: 10.1007/s10548-013-0329-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pouratian N, Cannestra AF, Bookheimer SY, et al. Variability of intraoperative electrocortical stimulation mapping parameters across and within individuals. J Neurosurg. 2004;101:458–466. doi: 10.3171/jns.2004.101.3.0458. [DOI] [PubMed] [Google Scholar]
- 26.Luders H, Lesser RP, Hahn J, et al. Basal temporal language area. Brain. 1991;114(Pt 2):743–754. doi: 10.1093/brain/114.2.743. [DOI] [PubMed] [Google Scholar]
- 27.Krauss GL, Fisher R, Plate C, et al. Cognitive effects of resecting basal temporal language areas. Epilepsia. 1996;37:476–483. doi: 10.1111/j.1528-1157.1996.tb00594.x. [DOI] [PubMed] [Google Scholar]
- 28.Menon V, Freeman WJ, Cutillo BA, et al. Spatio-temporal correlations in human gamma band electrocorticograms. Electroencephalogr Clin Neurophysiol. 1996;98:89–102. doi: 10.1016/0013-4694(95)00206-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.