ABSTRACT
Ample evidence exists for the presence of infectious agents at the maternal-fetal interface, often with grave outcomes to the developing fetus (i.e., Zika virus, brucella, cytomegalovirus, and toxoplasma). While less studied, pregnancy-related transmissible spongiform encephalopathies (TSEs) have been implicated in several species, including humans. Our previous work has shown that prions can be transferred from mother to offspring, resulting in the development of clinical TSE disease in offspring born to muntjac dams infected with chronic wasting disease (CWD) (1). We further demonstrated protein misfolding cyclic amplification (PMCA)-competent prions within the female reproductive tract and in fetal tissues harvested from CWD experimentally and naturally exposed cervids (1, 2). To assess whether the PMCA-competent prions residing at the maternal-fetal interface were infectious and to determine if the real-time quaking-induced conversion (RT-QuIC) methodology may enhance our ability to detect amyloid fibrils within the pregnancy microenvironment, we employed a mouse bioassay and RT-QuIC. In this study, we have demonstrated RT-QuIC seeding activity in uterus, placentome, ovary, and amniotic fluid but not in allantoic fluids harvested from CWD-infected Reeves' muntjac dams showing clinical signs of infection (clinically CWD-infected) and in some placentomes from pre-clinically CWD-infected dams. Prion infectivity was confirmed within the uterus, amniotic fluid, and the placentome, the semipermeable interface that sustains the developing fetus, of CWD-infected dams. This is the first report of prion infectivity within the cervid pregnancy microenvironment, revealing a source of fetal CWD exposure prior to the birthing process, maternal grooming, or encounters with contaminated environments.
IMPORTANCE The facile dissemination of chronic wasting disease within captive and free-range cervid populations has led to questions regarding the transmission dynamics of this disease. Direct contact with infected animals and indirect contact with infectious prions in bodily fluids and contaminated environments are suspected to explain the majority of this transmission. A third mode of transmission, from mother to offspring, may be underappreciated. The presence of pregnancy-related prion infectivity within the uterus, amniotic fluid, and the placental structure reveals that the developing fetus is exposed to a source of prions long before exposure to the infectious agent during and after the birthing process or via contact with contaminated environments. These findings have impact on our current concept of CWD disease transmission.
KEYWORDS: CWD, maternal, pregnancy, prions
INTRODUCTION
Transmissible spongiform encephalopathies (TSEs), or prion diseases, are proteinaceous infectious neurodegenerative diseases affecting animals, including humans (3, 4). The disease is caused by the accumulation of an aberrant misfolded form (protease-resistant, PrPRES) of a normal cellular protein, the prion protein (PrPC) (5). A hallmark of all TSE-infected hosts is their ability to harbor prion infectivity in tissues and bodily fluids throughout the protracted asymptomatic, or silent-carrier, phase of disease, which can last years, decades, or perhaps an entire life span (6, 7).
The transmission of prions, while remaining poorly understood, has obvious animal and human public health significance. It is clear that TSEs are spread by horizontal means of transmission via animal to animal and by environmental contact with prions, as well as by medical means, including blood transfusion or iatrogenic exposure (reviewed in references 3 and 4). Less studied is the potential for prion transmission from mother to offspring. Although it is rare for pathogens to cross the placental barrier and infect the fetus, certain viruses, bacteria, and protists have demonstrated this ability (8). Therefore, it is not surprising that there are several reports of prions in the female reproductive tract (9, 10) and pregnancy-associated milieu (11–14). These reports, in conjunction with the recent demonstration of a silent variant Creutzfeldt-Jakob disease (vCJD) carrier state (1 in 2,000 people) in the United Kingdom (15) and with the first report of chronic wasting disease (CWD) in Europe (16), mandate a thorough investigation of the role maternal transmission plays in prion transmission and disease pathogenesis.
Few studies have been conducted to assess the presence of prions in female reproductive and pregnancy-related tissues and in fluids from women displaying clinical CJD. The studies that have been published provide conflicting results. Uterine and gestational tissues from a 41-year-old woman with clinical CJD did not reveal deposition of the abnormal prion protein (PrPRES) (17). Nor has there been a report of the manifestation of clinical TSE symptoms in children born to women with vCJD (18). Yet other studies demonstrate prions in tissues of the human reproductive system. Proteinase K (PK)-resistant prions were demonstrated in the uterus and ovaries of a nonpregnant 25-year-old woman diagnosed with vCJD (9, 19). Infectious prions were detected by mouse bioassay in blood, colostrum, and placental tissues from a 38-year-old woman diagnosed with CJD between her 20th and 30th weeks of pregnancy (14). Considerable evidence exists for maternal scrapie transmission resulting in TSE disease and perinatal trafficking of prions (10, 12, 13, 20–29). In addition, maternal transmission of feline spongiform encephalopathy (FSE) has been suspected (30). Vertical transmission of bovine spongiform encephalopathy (BSE) has not been realized (31–33), yet enhanced risk factors for maternal/vertical transmission have been reported (32, 34); vertical transmission has been evidenced in transgenic (Tg) mouse studies (35). Thus, the biological significance associated with gestational prion exposure remains unanswered.
Our development and use of a small polyestrous cervid host, the Reeves' muntjac deer, has permitted investigation of questions related to CWD mother-to-offspring prion transmission in the native cervid host (1). We have validated our muntjac system by demonstrating the presence of protein misfolding cyclic amplification (PMCA)-competent prions in in utero-harvested tissues from free-range naturally exposed elk (2). Building on these studies we have further assessed CWD prions (PrPCWD) at the maternal-fetal interface.
Using bioassay and real-time quaking-induced conversion (RT-QuIC), we undertook this study to determine the infectious nature and RT-QuIC seeding activity of (i) female reproductive tissues and fluids associated with the pregnancy microenvironment and (ii) the placentome, which is the semipermeable interface between mother and fetus throughout pregnancy.
RESULTS
Maternal reproductive tissue.
Our previous studies (1, 2) led to the discovery that PrPRES deposition in maternal tissues of the reproductive system was too low to detect by immunohistochemistry. This led us to the use of PMCA (1, 2) and now RT-QuIC and bioassay to investigate the seemingly low concentration of prions within these tissues and the pregnancy microenvironment. Here, we further investigated these tissues for the presence of the normal cellular form of the prion protein, PrPC. The detection of the normal cellular form of the prion protein in uterus, ovary, and placentome, i.e., pregnancy-related tissues (Fig. 1), suggests the presence of a substrate capable of fueling PrPC conversion. To this end, we detected RT-QuIC PrPC seeding activity (amyloid formation) in uterine (7/7 samples) and ovary (5/7 samples) tissue harvested from muntjac dams showing clinical signs of infection with CWD agent (clinically CWD-infected dams) (P < 0.0001). No amyloid formation was detected in the uterine (0/4) or ovary (0/3) tissue from pre-clinically CWD-infected muntjac dams (Table 1 and Fig. 2). We have further demonstrated the presence of infectious prions in uterine tissues harvested from a clinically ill CWD-infected muntjac dam (animal 45) by mouse bioassay: 8/8 mice inoculated with uterine tissue from dam 45 developed clinical signs consistent with TSE disease (ataxia, weight loss, and stiff tail) between 237 and 343 days postinfection (dpi). All 8 clinically ill mice revealed demonstrable PrPRES deposition in brain tissue as determined by Western blotting (Fig. 3). RT-QuIC seeding activity was demonstrated in brain tissue of all 8 mice and in spleen of 7/8. Note that 1/9 mice suffered intercurrent death (Table 2; Fig. 3). All mice inoculated with uterine tissue from a naive muntjac dam (animal 64) remained healthy for the same period of time and negative for PrPRES deposition and amyloid formation (Table 2 and Fig. 3).
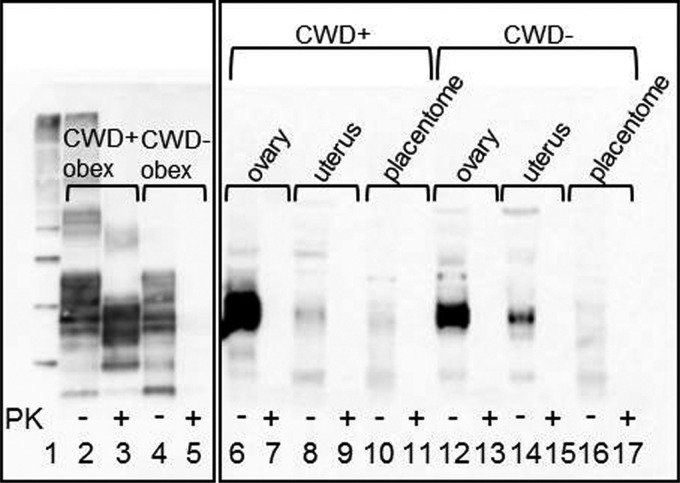
FIG 1.
Western blot demonstrating the presence of PrPC in muntjac reproductive tissues. Lane 3 demonstrates that PrPRES is present in a CWD-infected muntjac obex. Lane 5 shows complete proteinase K (PK) digestion of PrPC in a negative-control muntjac obex. Lanes 6, 8, 10, 12, 14, and 16 demonstrate PrPC detection in ovary, uterus, and placentome tissues from CWD-infected and negative-control muntjac. Lane 1, molecular weight marker. No PrPRES was detected in these reproductive tissues after PK digestion (2-mg tissue equivalents/lane).
TABLE 1.
Muntjac dam and fetal study animals, their disease and gestational stages, and reproductive tissue and fluid RT-QuIC results
| Dam disease status and identification no. (fetus) | Inoculum (amt and route)a | Dam disease stage (no. of mos. p.i.)b | Fetal gestational stage (no. of mos.)e | Tissue or fluidf | QuIC median ratef,g |
|---|---|---|---|---|---|
| CWD positive | |||||
| 11 | Brain (1 g p.o.) | 25c | NA | Uterus | 0.0470**** |
| Ovary | 0.0107 | ||||
| 15 | Brain (0.5 g s.c and 2 g p.o.) | 26c | Uterus | 0.0878**** | |
| Ovary | 0 | ||||
| 43 | Brain (0.35 g s.c. and 0.5 g p.o.) | 23c | Uterus | 0.1060**** | |
| Ovary | 0.0991**** | ||||
| 45 (58) | 22d | 4.8 | Uterus | 0.0700**** | |
| Ovary | 0.0799**** | ||||
| Placentome 1 | 0 | ||||
| Placentome 2 | 0.0598**** | ||||
| Placentome 3 | 0.0660**** | ||||
| Placentome 4 | 0.0980*** | ||||
| Placentome 5 | 0.0880**** | ||||
| Allantoic fluid | 0 | ||||
| 46 | 24c | NA | Uterus | 0.0793**** | |
| Ovary | 0.0714**** | ||||
| 47 (57) | 20d | >4.5 | Uterus | 0.0611**** | |
| Ovary | 0.0676**** | ||||
| Placentome 1 | 0.1200**** | ||||
| Placentome 2 | 0.1010**** | ||||
| Placentome 3 | 0.0176** | ||||
| Placentome 4 | 0.1092**** | ||||
| Placentome 5 | 0.1084**** | ||||
| Placentome 6 | 0 | ||||
| Amniotic fluid | 0.0527**** | ||||
| Allantoic fluid | 0 | ||||
| 50 (59) | 23c | 2 | Amniotic fluid | 0.0185** | |
| 78 | 22 | NA | Uterus | 0.0592**** | |
| Ovary | 0.0392**** | ||||
| 16 (115) | 7 | 3.5 | Uterus | 0 | |
| Ovary | 0 | ||||
| Placentome 1 | 0 | ||||
| Placentome 2 | 0 | ||||
| Placentome 3 | 0 | ||||
| Placentome 4 | 0 | ||||
| Placentome 5 | 0 | ||||
| Amniotic fluid | 0 | ||||
| Allantoic fluid | 0 | ||||
| 19 (116) | 7 | 3.5 | Uterus | 0 | |
| Placentome 1 | 0 | ||||
| Placentome 2 | 0.0194*** | ||||
| Placentome 3 | 0 | ||||
| Placentome 4 | 0.0172** | ||||
| Placentome 5 | 0 | ||||
| Amniotic fluid | 0 | ||||
| Allantoic fluid | 0 | ||||
| 73 (89) | 12 | 3 | Uterus | 0 | |
| Ovary | 0 | ||||
| Placentome 1 | 0.0086* | ||||
| Placentome 2 | 0.0283** | ||||
| Placentome 3 | 0 | ||||
| Placentome 4 | 0.0168* | ||||
| Placentome 5 | 0 | ||||
| Placentome 6 | 0.0086* | ||||
| 77 (90) | 12 | 3 | Uterus | 0 | |
| Ovary | 0 | ||||
| Placentome 2 | 0 | ||||
| Placentome 3 | 0 | ||||
| Placentome 4 | 0 | ||||
| Placentome 5 | 0 | ||||
| Placentome 6 | 0.0245*** | ||||
| Amniotic fluid | 0 | ||||
| 84 (98) | 5 | 7 | Placentome 1 | 0.0106* | |
| Placentome 2 | 0 | ||||
| Placentome 3 | 0 | ||||
| Placentome 4 | 0.0081* | ||||
| Amniotic fluid | 0 | ||||
| CWD negative | |||||
| 62 | Naive | None | NA | Uterus | 0 |
| Ovary | |||||
| 64 | Naive | 5 | Ovary | ||
| Placentome 1 | |||||
| Placentome 2 | |||||
| Amniotic fluid | |||||
| 75 | Brain (0.5 g s.c. and 0.5 g p.o.) | NA | Uterus | ||
| Ovary | |||||
| 86 | Naive | NA | Uterus | ||
| 107 (119) | Naive | 2 | Amniotic fluid |
s.c., subcutaneously; p.o., per os.
Terminal CWD clinical disease in experimentally inoculated muntjac dams manifests between 23 and 26 months postinfection (p.i.).
Euthanized due to terminal clinical CWD disease, with symptoms of hypersalivation, weight loss, and ataxia.
Early signs of clinical CWD (weight loss).
The full-term gestational period for Reeves' muntjac deer is 7 months. NA, not available (no fetus at necropsy).
Boldface indicates statistical significance (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ****, P < 0.0001).
Values represent 1/time to the ThT fluorescence threshold.
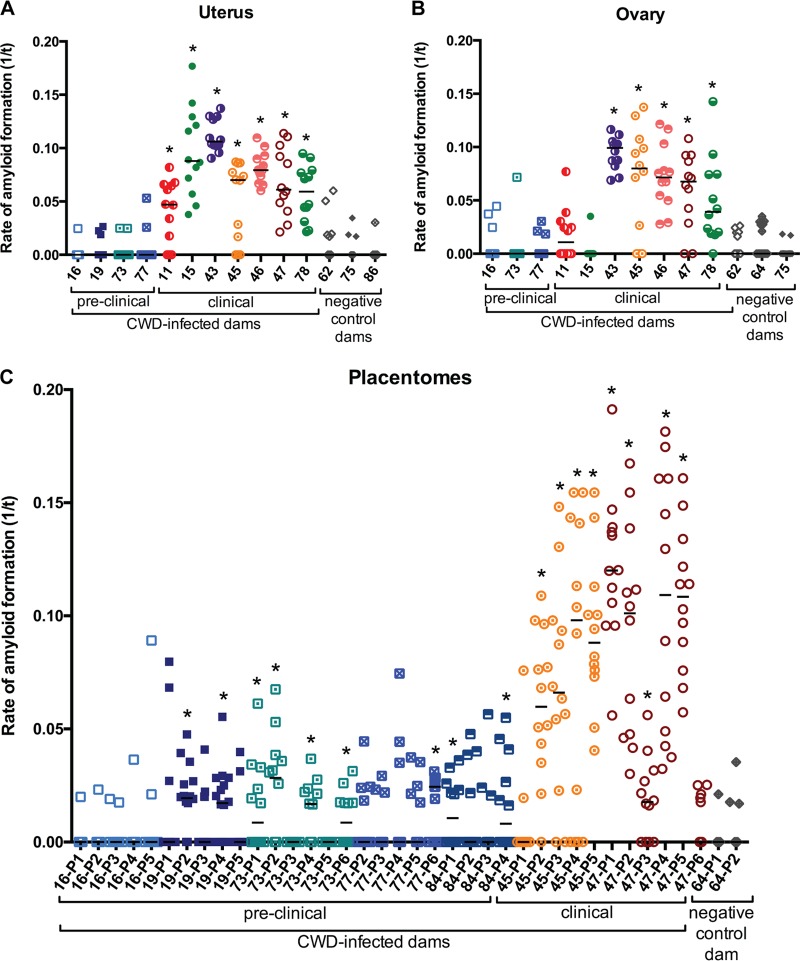
FIG 2.
RT-QuIC detection of amyloid formation in muntjac dam uterus, ovary, and placentomes. Detection of amyloid formation is displayed as reaction rates (1/time [t] to ThT fluorescence threshold). Square, pre-clinically CWD-infected dams; circles clinically CWD-infected dams; diamonds negative-control dams. Horizontal lines indicate the medians (n = 12 replicates for CWD-infected dams and n = 12 to 16 replicates for CWD-negative dams). Statistical significance is indicated above the sample replicates with an asterisk (P < 0.0001 for uterus and ovary; P ≤ 0.0476 for preclinical placentome; P ≤ 0.0018 for clinical placentome).
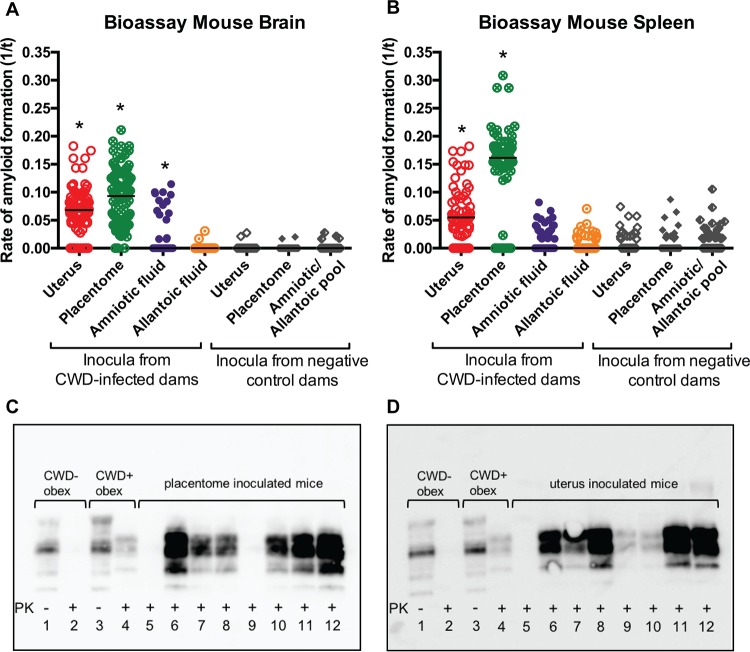
FIG 3.
Detection of amyloid formation in cervid transgenic mice [Tg(CerPrP-E226)5037+/−] inoculated with placentome and uterine tissues and birthing fluids from CWD-infected muntjac. (A and B) RT-QuIC detection of amyloid formation displayed as reaction rates (1/time to ThT fluorescence threshold). Replicates are shown as circles for mice inoculated with material from CWD-infected muntjac (uterus and placentome, n = 8 mice per cohort; amniotic and allantoic fluids, n = 9 mice per cohort; 12 replicates each) and as diamonds for mice inoculated with material from negative muntjac (uterus, n = 9 mice; placentome, n = 8 mice; amniotic and allantoic fluids, n = 7 mice per cohort; 12 replicates each). Horizontal lines indicate the medians. Statistical significance is indicated above the sample replicates with an asterisk. For panel A, P < 0.0001 for values from mouse brains [not NaPTA treated] tested from uterus and placentome bioassay and P = 0.0277 for values in the concentrated amniotic fluid bioassay compared to control values (values for the allantoic fluid were not significantly different). For panel B, P < 0.0001 for values from mouse spleens tested from the uterus and placentome bioassay compared to control values (values from the concentrated amniotic and allantoic fluid bioassay were not significantly different from control values). (C and D) Lanes 2 demonstrate complete proteinase K (PK) digestion of PrPC in a negative deer obex, and lanes 4 demonstrate PrPRES in a CWD-positive deer obex. In panel C, lanes 6 to 8 and 10 to 12 demonstrate PrPRES detection in the brains of 6/8 mice inoculated with placentome tissue from a CWD-infected muntjac. In panel D, lanes 6 to 12 demonstrate PrPRES detection in the brains of 7/8 mice inoculated with uterine tissue from a CWD-infected muntjac.
TABLE 2.
Cervid transgenic mouse bioassay: inocula and Western blotting and RT-QuIC analysis
| Muntjac dam disease status and identification no(s). | Inoculum |
Clinical TSE diseasea |
Western blotting of brainc | RT-QuIC assayc |
|||
|---|---|---|---|---|---|---|---|
| Tissue or fluid source (n) | Amt and route | No. of positive mice/total no. of mice | Time to disease (avg dpi ± SD) | Brain | Spleen | ||
| CWD positive | |||||||
| 45 | Uterus (8) | 30 μl i.c. of 10% homogenate | 8/8 | 302.6 ± 45.8 | 8/8 | 8/8 | 7/8 |
| Placentome 1 (6) | 30 μl i.c. of 10% homogenate | 0/6 | 500 | 0/6 | 0/6 | 1/6 | |
| 47 | Placentomes 1 and 5 (8) | 30 μl i.c. of 10% homogenate | 7/8 | 198.3 ± 45.4 | 6/8 | 8/8 | 8/8 |
| Amniotic fluid (9) | 30 μl i.c. | 1/9 | 320 | 0/9 | 1/9 | 2/9 | |
| Allantoic fluid (9) | 30 μl i.c. | 0/9 | 0/9 | 0/9 | 0/9 | ||
| CWD negative | |||||||
| 64 | Uterus (9) | 30 μl i.c. of 10% homogenate | 0/24b | 0/24 | 0/9 | 0/9 | |
| Placentome 1 (8) | 30 μl i.c. of 10% homogenate | 0/8 | 0/8 | ||||
| 64 and 107 | Amniotic and allantoic fluid (7) | 30 μl i.c. | 0/7 | 0/7 | |||
Clinical disease, demonstrated by lethargy, stiff tail, circling, and hind limb ataxia.
An age-matched negative-control mouse was harvested with each mouse euthanized with clinical TSE disease or at study termination at 343 days postinfection (dpi).
Values are the number of positive mice/total number of mice tested.
Maternal-fetal interface at the placentome.
We were not able to detect PrPRES deposition in placentomes by Western blot analysis or immunohistochemistry (data not shown), similar to other maternal reproductive tissues, yet we were able to demonstrate PMCA seeding activity in tissues at this intimate interface between mother and fetus (1, 2). Thus, RT-QuIC and bioassay studies were initiated. Significant RT-QuIC seeding activity was evident in 9/11 placentomes harvested from clinically CWD-infected dams (P ≤ 0.0018) (Table 1 and Fig. 2). Mice intracranially (i.c.) inoculated with homogenate of placentome sample 1 from clinically CWD-infected dam 45 (Fig. 2, 45-P1) remained healthy for 500 dpi and lacked PrPCWD deposition as determined by Western blotting or RT-QuIC seeding activity in brain or spleen tissues (Table 2). RT-QuIC analysis of dam 45-P1 suggested a lack of amyloid fibrils in this placentome (Fig. 2). Two placentomes with the highest RT-QuIC seeding activity rates (Fig. 2, 47-P1 and 47-P5) were assessed by bioassay. Prion infectivity was revealed, and 7/8 mice developed terminal TSE disease between 180 and 270 dpi (Table 2). PK-resistant prions were evident in brain tissue of 6/8 mice by Western blotting (Table 2 and Fig. 3), and upon RT-QuIC analysis all 8 mice were demonstrated to have RT-QuIC seeding activity in brain and spleen tissues. Note that 1/9 mice suffered intercurrent death (Table 2 and Fig. 3). RT-QuIC analysis of placentome tissue from pre-clinically CWD-infected dams (n = 5) indicated low to moderate levels of RT-QuIC seeding activity in 9/25 placentomes tested (P = 0.0004 to 0.0476); 4/5 pre-clinically infected dams had at least one RT-QuIC-positive placentome (Table 1 and Fig. 2). We initiated a mouse bioassay of these tissues to assess infectivity. RT-QuIC analysis of two placentomes and bioassay of one placentome from a naive muntjac dam (dam 64) showed neither amyloid formation nor infectivity (Tables 1 and 2; Fig. 2 and 3).
Fetal microenvironment.
Fluids collected from the fetal amniotic and allantoic sacs of the aforementioned muntjac pregnancies were concentrated through lyophilization, dialyzed, and assessed by RT-QuIC and/or bioassay (Table 2; Fig. 3 and 4). RT-QuIC seeding activity was present in amniotic fluid harvested from clinically ill CWD-infected dams (two, dams 59 and 47) in 79% of RT-QuIC replicates (n = 12/each) but was absent in allantoic fluid tested from the same pregnancies (Table 1; Fig. 4) and in amniotic and allantoic fluids harvested from pre-clinically CWD-infected dams (n = 4) (Table 1; Fig. 4). Mouse bioassay of amniotic fluid harvested from clinically ill CWD-infected dam 47 confirmed the presence of very low concentrations of infectious prions. One of nine (1/9) mice presented with signs consistent with TSE disease at 320 dpi and was euthanized. The remaining mice remained asymptomatic and were euthanized at 500 dpi. Although PrPCWD was not demonstrated in brain tissue of any of these mice by Western blotting, further analysis by RT-QuIC revealed prion seeding activity in brain (1/9 mice; the clinically ill mouse) and spleen (2/9 mice, including the clinically ill mouse) (Table 2; Fig. 3). None (0/9) of the mice inoculated with allantoic fluid from CWD-positive dams developed TSE disease or demonstrated PrPRES deposition or RT-QuIC seeding activity in brain or spleen tissues (Table 2; Fig. 3). RT-QuIC analysis and bioassay of amniotic and allantoic fluids harvested from naive muntjac dams showed neither amyloid formation nor infectivity (Table 2; Fig. 3).
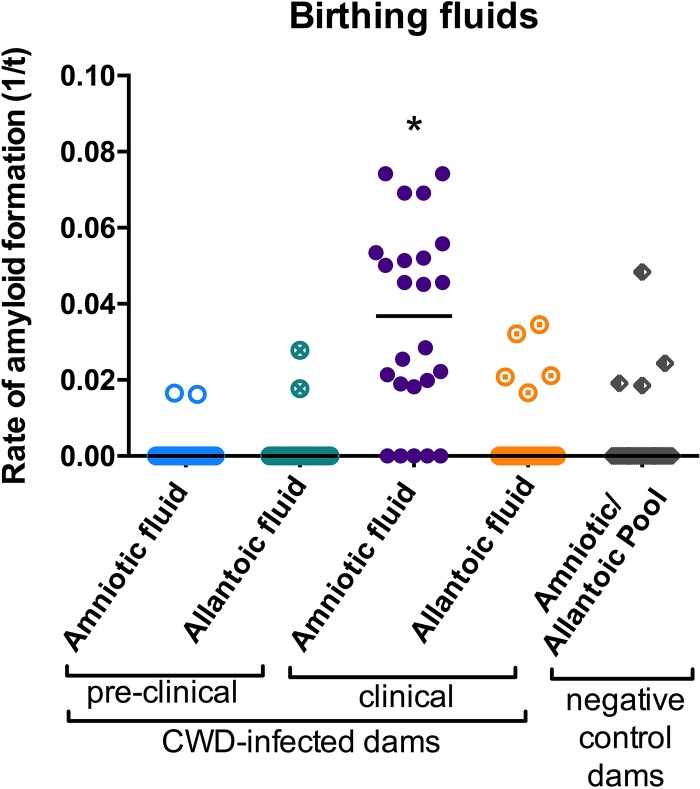
FIG 4.
RT-QuIC detection of amyloid formation in birthing fluids from clinically CWD-infected muntjac dams. For concentrated amniotic fluid from CWD-infected dams, values from preclinical specimens (4 dams) were not significantly different from control values, and values from clinical samples (2 dams) were significant at a P value of <0.0001. For allantoic fluid, values for both preclinical and clinical samples (2 dams each) from CWD-infected dams were not significantly different from values for negative dams (n = 2 dams). Number of replicates, 12 per dam per treatment group.
DISCUSSION
Our previous work demonstrates that prions can be transferred from mother to offspring, resulting in the development of clinical TSE disease in offspring born to CWD-infected muntjac dams (1). Because the offspring were born to and raised by their infected mothers, we were unable to parse the point source of infectivity, i.e., whether infection originated from contact with maternal prions during parturition or shortly thereafter or via in utero exposure. We addressed this question by revealing amplification-competent prions in in utero-harvested fetal tissues from CWD-infected muntjac dams regardless of disease or gestational stage (1) and in reproductive and fetal tissues harvested from free-range cervid dams naturally exposed to CWD but not presenting clinical signs of disease (preclinical dams) (2). What remained unanswered was whether the prions detected in in utero-harvested tissues were infectious. Here, we demonstrate, for the first time, prion infectivity within the maternal reproductive and pregnancy-related tissues and milieu of clinically CWD-infected dams. We further assessed tissues by an in vitro conversion assay, RT-QuIC, to determine if correlates could be drawn between the prion seeding activity detected by this assay and infectivity realized by animal bioassay. We found a direct correlation between RT-QuIC seeding activity and bioassay infectivity in samples harvested from clinically CWD-infected dams.
In our experience, CWD-infected muntjac dams showing clinical signs of disease maintain fecundity, successfully breeding and producing full-term viable and nonviable offspring. Our findings (i) provide further evidence that CWD prions are present during gestation, placing the developing fetus at risk of TSE infection long before exposure to the infectious agent during the birthing process or maternal grooming behaviors shortly after parturition or via exposure to CWD-contaminated environments and (ii) suggest a correlation between RT-QuIC seeding activity and infectivity.
Maternal tissues: infectious prions are present in CWD-infected deer reproductive tissues.
We found PrPC, RT-QuIC seeding activity, and infectious CWD prions in the uterus of CWD-infected dams. Infectious agents can access reproductive tissues (i) via transfer from the abdominal cavity to fallopian tubes, (ii) from the oral cavity by means of the circulatory system, (iii) by direct hematogenous infection, and (iv) by ascending from the vagina to the female reproductive tract (36). Substantial evidence has been established for the presence of pathological prions in tissues of the oral (37–40) and abdominal cavities (41–43), as well as the blood of TSE-infected hosts (44–50), providing opportunity for reproductive tissue exposure to prions by the aforementioned routes of infection. Prions have been demonstrated in uterine tissues of vCJD-infected women (9) and scrapie-infected sheep (39). The normal cellular prion protein (PrPC), necessary for the conversion and maintenance of prion infection (51), has been found in high concentrations in reproductive tissues, including uterus (17, 52–55). It is therefore feasible that high levels of PrPC substrate within female reproductive tissues fuel amplification of infectious uterine prions, leading to sustained gestational exposure and subsequent in utero transmission to the developing embryo.
The role of the ovary in in utero prion exposure remains unclear. Here, we detected ample PrPC and RT-QuIC seeding activity in ovarian tissue harvested from clinically CWD-infected muntjac deer. The expression of PrPC in ovary tissues has been demonstrated in cervids, humans, sheep, and cattle (9, 10, 55, 56) and in ovarian follicles of cattle and mice (52, 57), providing a substrate to sustain prion infection. The prion protein may have a role in regulating embryonic stem cell pluripotency and differentiation in early embryonic developmental stages (58). PK-resistant prion protein has been detected in human ovary tissue (9). Embryo transfers of 6-day-old embryos recovered from scrapie-infected ewes and implanted into scrapie-free ewes resulted in the transmission of scrapie to the offspring (23). These data imply a role for the transmission of prions during the very early stages of embryogenesis or with the germ line. Studies to determine the infectious nature of ovaries harvested from CWD-infected cervids are ongoing.
Maternal-fetal interface: infectious prions are found within placentomes of CWD-infected dams.
Our bioassay study indicates that placentomes, i.e., the interface for metabolic exchange between dam and fetus during pregnancy, harbor infectious prions. The potential for prion trafficking across the placental structure has been evidenced by the identification of scrapie deposition within sheep placentomes (10, 20, 29, 59, 60) and in highly motile and phagocytic fetus-derived trophoblast cells (25) and by transmission of scrapie infectivity with placental tissues fed to sheep (12, 61). Prion infectivity has been demonstrated in the placenta of a woman showing clinical signs of CJD (14). A number of studies have established that infectious prions are present in the blood of symptomatic and asymptomatic TSE-infected hosts (45, 47–50, 62–64), and blood cells have been localized within trophoblast cells (65). Therefore, it is possible that infectious prions are trafficked via maternal blood to the fetal trophectoderm at the placentome.
Exposure of the fetal trophectoderm to maternal blood varies among species, dependent upon placental structure (66). A more intimate connection between mother and baby exists in human hemochorial placentation where the fetal trophectoderm is directly bathed in maternal blood (67). This placentation contrasts with that of the ruminant placentome, epitheliochorial placentation, where three to five tissue layers separate the maternal circulatory system from the offspring (67–69). Increased angiogenesis to ensure maintenance of the developing fetus is a hallmark of all pregnancies (70). Subchorionic hematoma, separation of the chorion from the endometrium, permits maternal blood leakage and pooling within the placenta/placentome during pregnancy (71, 72). In addition, the normal events associated with blastocyst implantation in hemochorial placentation, i.e., remodeling of the maternal decidua (67), directly expose the fetal trophectoderm to maternal blood. Thus, a breach in the uterine/placental membranes at implantation or during gestation may present an opportunity for infectious blood-borne or uterine-derived prions to enter the fetal environment. In this study, the detection of prion infectivity within uterine tissues of CWD-infected dams provides a local and perhaps persistent in utero prion source.
The muntjac placentome, densely interdigitated with both maternal and fetal tissues, is technically challenging to separate, ruling out contamination of one region with the other. Our bioassay inocula were therefore generated from tissue containing both maternal (caruncle) and fetal (cotyledon) portions of the placentome. Thus, we are unable to determine the degree to which infectivity is associated with fetal versus maternal placentome tissues. Nevertheless, our findings add to the body of evidence that maternal prions, whether maintained within the female reproductive microenvironment or hematogenously sourced, are present at the placental barrier.
Fetal microenvironment: infectious prions are detected in the amniotic fluid of CWD-infected deer.
The fetal microenvironment of a pregnant, clinically CWD-infected dam contained prion infectivity. Here, we have demonstrated low levels of prion infectivity in amniotic fluid but not in allantoic fluid harvested from a clinically CWD-infected dam. PrPRES has been detected in amniotic fluid of scrapie-infected sheep (60), yet the infectivity of this material has not been determined. The fetus is bathed in amniotic fluid throughout gestation. During pregnancy, to further the development of fetal respiratory and digestive systems, the fetus recirculates amniotic fluid (up to 500 ml/day) via oronasal ingestion (73–76). Amniotic fluid consists of maternal plasma and fetal urine, depending upon gestational stage (77–79). It is possible that blood-borne prions are trafficked with maternal plasma to amniotic fluid or that infectious prions are transported across the maternal-fetal interface via trophoblast cells from the uterine environment. In either case, susceptible fetal mucosal surfaces may be continuously bathed in, and thus exposed to, infectious prions throughout gestation.
Another potential reservoir for prion infectivity is the allantoic fluid, which consists of metabolic wastes produced by the fetus. Both PrPC (10) and PrPRES (29) have been detected in allantoic fluid collected from scrapie-infected ewes. This is not surprising because it is known that infectious prions are excreted in the shed by-products (urine and feces) of CWD- and scrapie-infected deer and sheep (80–83). However, in other studies, infectious prions were not found in fetal allantoic fluid (79, 84). If prion infectivity is present in fetal metabolic waste shed by the fetus (i.e., within allantoic fluid), the prions may be present at a concentration too low for detection, masked, or inactivated by pH or inhibiting properties present in these waste products, or the process of prion shedding may differ during gestation.
We are further investigating the ability of prions to traffic from the pregnancy and fetal microenvironment across the placental structure to establish infection in the developing fetus. To this end, bioassay studies to determine the infectivity of in utero-derived fetal tissues harvested from early- and late-stage experimental CWD-infected muntjac dams and free-range naturally infected elk dams are ongoing.
RT-QuIC and infectious prions.
RT-QuIC provides a rapid methodology for analysis of tissues and fluids containing minute quantities of prions (85, 86) and may have utility in quantitative and correlative investigations of early and carrier states of prion diseases and potentially other protein misfolding disorders (86–89). There is little to no evidence that RT-QuIC generates, or represents, the presence of prion infectivity. Yet, similar to PMCA (1, 2), RT-QuIC consistently detected the presence of prion seeding activity in the reproductive milieu of clinically CWD-infected dams that, in this study, correlated with bioassay infectivity. We further demonstrated the presence of RT-QuIC seeding activity in some placentomes from pre-clinically CWD-infected dams lacking prion seeding activity in uterine and ovary tissues. These results are intriguing in that they suggest the accumulation of amyloid fibrils at the maternal-fetal interface prior to that in maternal reproductive organs, suggesting the potential for hematogenous- rather than uterine-sourced prions at this semipermeable membrane between mother and baby. It is well established that prion peripheral distribution and accumulation increase as TSE disease progresses (90–93). Our ability to demonstrate RT-QuIC seeding activity and prion infectivity within the female reproductive and pregnancy-related milieu of clinically CWD-infected dams may reflect these findings. The demonstration of RT-QuIC seeding activity in tissues harvested from preclinical dams was much lower than that demonstrated in the same tissues harvested from clinically ill dams, and infectivity of these tissues has not yet been demonstrated. We have initiated bioassays of these tissues to further assess the biological significance of our RT-QuIC results.
Our previous studies have compared RT-QuIC seeding activity to CWD cervid brain in a 50% lethal dose (LD50) bioassay (88, 94). Because we used the same conditions and controls for this study, we applied this principle to our RT-QuIC data. We estimated an LD50 range from 18.7-fold less to 13.5-fold greater than that determined for CWD cervid brain (3.33 × 106 LD50s/g tissue) (88), suggesting a wide and variable range of brain equivalents in the reproductive milieu. More work is certainly needed to understand the significance of prion seeding activity and infectivity, as well as to discern a clearer picture of the isoforms detected by each methodology.
Implications associated with maternal CWD infections.
The facile dissemination of CWD within captive and free-range cervid populations has led to questions regarding the transmission dynamics of this TSE. Direct contact with infected animals (95, 96) and indirect contact with infectious prions in bodily fluids and contaminated environments (97, 98) are suspected to explain the majority of this transmission. The effects of maternal CWD infections and a gestational source of prions may be underappreciated.
The presence of prion infectivity at the maternal-fetal interface, i.e., within the uterus, placentome, and fetal microenvironments, would help explain the high rates of CWD among cervids. In our experience, CWD-infected muntjac dams successfully breed and produce viable and nonviable offspring throughout the course of CWD infection even when exhibiting clinical signs of TSE disease. In a natural setting, however, the day-to-day challenges encountered by cervid dams to nurse, care for, and protect their young are much greater than those in a laboratory setting. This would surely be compounded by CWD infection, particularly in the later stages of disease. This, in combination with the high nonviable birth rates observed in offspring born to CWD-infected muntjac dams (60%) (1), may result in increased offspring mortality leading to lower annual recruitment rates (99). Yet a portion of offspring born to CWD-infected dams that survive to weaning and reproductive age would enter the population as potential asymptomatic carriers.
Infectious CWD prions in the reproductive milieu of cervid dams may spread disease by the following: (i) placing each developing fetus in direct contact with infectious prions throughout gestation; (ii) passage of CWD prions with ova to each offspring, increasing the likelihood of multigenerational mother-to-offspring transmission; (iii) postpartum fetal and placental membranes contributing to environmental contamination; and (iv) availability of postpartum tissues for consumption by scavenger species, increasing the potential for cross-species transmission.
Here, using a native CWD-susceptible host, the Reeves' muntjac deer, we share the first report of prion infectivity within the pregnancy microenvironments and placental structure of CWD-infected cervid. These findings reveal a source of infectious gestational prions that expose the developing fetus to CWD and are likely shed during parturition, contributing to CWD environmental contamination, and may contribute to CWD asymptomatic carriers in cervid populations. These findings have impact on our current concept of CWD disease transmission.
MATERIALS AND METHODS
Reproductive tissue source and processing. (i) Tissue source.
Uterus, ovary, and placentome tissues and amniotic and allantoic fluids were harvested from muntjac dams as part of a previous CWD maternal transmission study performed at Colorado State University and approved by the International Animal and Care Use Committee (IACUC) (1). Dams received CWD-positive brain homogenate via oral and subcutaneous inoculation and were humanely euthanized for maternal and fetal tissue collections at time points prior to (preclinical) or after (clinical) presentation of clinical signs consistent with TSE disease. Reeves' muntjac dams show terminal clinical signs of TSE disease (hypersalivation, weight loss, and ataxia) at approximately 24 months postexposure (1). All dams were confirmed to be CWD positive (1). Negative-control dams were dosed in the same manner with naive brain, remained healthy throughout the observation period, and were confirmed to be free of CWD. Genotyping of the dams was performed and previously reported (1). Sample details are provided in Table 1.
(ii) Fluid and tissue collection and processing.
Great care was taken to minimize cross contamination between tissues and fluids as per previously published protocols (48, 49, 97). Amniotic and allantoic fluids were collected using single-use animal- and fluid-specific syringes and needles directly after each dam was euthanized and the uterus was removed for necropsy. New single-use, animal- and tissue-specific blades and forceps were used to harvest each tissue and fluid. Each ovary and placentome was frozen whole and bisected sagittally, and a midline section was taken for the homogenate. The uterus was bisected at necropsy, and the tissue was divided, with a portion stored at −80°C for bioassay, Western blotting, and RT-QuIC homogenates. A portion of each tissue was fixed in McLean's paraformaldehyde-lysine-periodate (PLP) solution for immunohistochemistry.
Western blotting.
Tissue homogenates were prepared from the obex region of the medulla oblongata at 10% (wt/vol) and from reproductive tissues (ovary, uterus, and placentome) at 20% (wt/vol) in Igepal buffer (10 mM Tris-HCl buffer, pH 7.5, 0.5% Igepal, 0.5% sodium deoxycholate) in an Omni Bead Ruptor 24 (Omni International, Inc.). Homogenates (2-mg tissue equivalent) were mixed with proteinase K (PK; Invitrogen) at a final concentration of 70 μg/ml and incubated at 37°C for 30 min with shaking. Samples were mixed with reducing agent (10×)-lithium dodecyl sulfate (LDS) sample buffer (4×) (Invitrogen) at a final concentration of 1×, heated to 95°C for 5 min, and then run through a NuPAGE 12% Bis-Tris gel at 125 V for 1.5 h. Proteins were transferred to a polyvinylidene fluoride (PVDF) membrane at 80 V for 1 h in transfer buffer (0.025 M Trizma base, 0.2 M glycine, 20% methanol, pH 8.3). The membrane was incubated with blocking buffer (5% nonfat dried milk in 1×Tris-buffered saline [TBS] with 0.1% Tween 20 [TBST]) for 20 min and then for 1 h with the primary antibody BAR224 (0.2-μg/ml final concentration; Cayman Chemical) diluted in TBST, followed by a 30-min wash with TBST. The membrane was incubated for 30 min with the secondary antibody, peroxidase-labeled goat anti-mouse IgG (0.05-μg/ml final concentration; KPL) diluted in TBST, followed by a 30-min wash with TBST. The membrane was developed with ECL Plus Western blotting detection reagents (Pierce) and viewed on a Luminescent Image Analyzer LAS-4000 (Fujifilm).
NaPTA precipitation.
Ten percent (10%; wt/vol) tissue homogenates made in 1× phosphate-buffered saline (PBS) (130 mM NaCl, 7 mM Na2HPO4·7H2O, 3 mM NaH2PO4·1H2O) in an Omni Bead Ruptor 24 (Omni International, Inc.) were diluted 1:10 in 0.1% SDS in 1× PBS for a total of 100 μl (0.1-mg tissue equivalent). This volume (100 μl) was subjected to protein precipitation (adapted from reference 100) by adding 7 μl of a freshly made solution containing 5% sodium phosphotungstate (NaPTA) octadecahydrate and 112 mM magnesium chloride hexahydrate prepared in sterile-filtered water (Sigma-Aldrich), followed by a 1-h incubation at 37°C and centrifugation for 30 min at 15,000 rpm. Supernatants were decanted, and the remaining pellet was resuspended in 10 μl of 0.1% SDS in 1× PBS for subsequent analysis by RT-QuIC.
RT-QuIC assay.
All real-time quaking-induced conversion (RT-QuIC) assays (adapted from references 85, 87, and 101) were performed blinded, whereby sample identities were not revealed until after the analysis was completed. CWD-positive and -negative spleen samples were treated alongside the test samples and were used as plate controls in every experiment (spleen was used in ovary and uterus experiments; uterus was used in placentome experiments). Reaction mixtures consisted of 2 μl of NaPTA-precipitated sample into 98 μl of RT-QuIC buffer (final concentrations in 1× PBS: 130 mM NaCl, 1 mM EDTA, 10 mM thioflavin T [ThT], 0.1 mg/ml truncated recombinant hamster PrPC substrate) loaded into wells of a 96-well black-bottom optic plate (Nalgene Nunc). Prepared plates containing quadruplicate wells per sample were placed in a BMG FLUOstar Omega microplate reader and subjected to 700-rpm double-orbital shaking for 1 min every other minute for 15 min. After each shaking cycle, ThT fluorescence was read at an excitation of 450 nm and emission of 480 nm. Gain was set at 1,700 and read using orbital averaging with 20 flashes per well with a 4-mm setting. Fluorescent readings were recorded for all sample reactions for a total time of 62 h at a temperature of 42°C. Three or four separate experiments were run for each sample in quadruplicate, totaling 12 to 16 replicates per sample. Sample replicates were considered positive if they crossed the plate threshold (5 standard deviations [SD] above the mean of the initial five readings). Amyloid formation rates for positive replicates were determined using the inverse of the time when each positive reaction exceeded a threshold value paraformaldehyde-lysine-periodate. Statistical analyses were run in GraphPad Prism. The QuIC median rates of replicates for a given tissue are listed in Table 1. A Mann-Whitney test was used to generate P values (Table 1) by comparing the sample rates to the rates of known negative-control tissues. The confidence interval considered for statistical significance is 95%.
Bioassay of cervid transgenic mice: inoculations and monitoring.
To further assess the presence of prion infectivity at the maternal-fetal interface, uterine tissue, placentomes, and amniotic and allantoic fluids harvested from CWD-infected and -negative muntjac dams were inoculated into Tg(CerPrP-E226)5037+/− mice for bioassay (102). Cohorts of 9 mice each were intracranially (i.c.) inoculated with 30 μl of a 10% (wt/vol) homogenate from clinically CWD-infected dam tissues (uterine, dam 45; placentome, dams 45 and 47 [3-mg tissue equivalent]) or 30 μl of concentrated amniotic (dam 47) and allantoic (dam 47) fluids (birthing fluids; (150-μl birthing fluid equivalent). The birthing fluids were concentrated by lyophilization to 5-fold less than their original volume and were dialyzed to remove the high salt concentrations. Biweekly weights and observations were taken until the mice were terminated due to disease or at 500 dpi, whichever occurred first. The negative-control mice received identical volumes of negative tissues and fluids and were followed similarly. Brain and spleen tissues harvested from these mice were assessed for the presence of prions by RT-QuIC and/or Western blotting.
ACKNOWLEDGMENTS
We thank Kaitlyn Miedema for her technical assistance, Amber Mayfield, Monica Brandhuber, and Richard Ruder for sample preparation, Kristen Davenport for analytical assistance, and Anca Selariu and Jeffrey Christiansen for critical review of the manuscript.
This work was supported by NIH-NIAID R01AI093634 and by the College of Veterinary Medicine and Biological Sciences College Research Council and the Department of Microbiology, Immunology and Pathology.
REFERENCES
- 1.Nalls AV, McNulty E, Powers J, Seelig DM, Hoover C, Haley NJ, Hayes-Klug J, Anderson K, Stewart P, Goldmann W, Hoover EA, Mathiason CK. 2013. Mother to offspring transmission of chronic wasting disease in Reeves' muntjac deer. PLoS One 8:e71844. doi: 10.1371/journal.pone.0071844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Selariu A, Powers JG, Nalls A, Brandhuber M, Mayfield A, Fullaway S, Wyckoff CA, Goldmann W, Zabel MM, Wild MA, Hoover EA, Mathiason CK. 2015. In utero transmission and tissue distribution of chronic wasting disease-associated prions in free-ranging Rocky Mountain elk. J Gen Virol 96:3444–3455. doi: 10.1099/jgv.0.000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aguilar-Calvo P, Garcia C, Espinosa JC, Andreoletti O, Torres JM. 2015. Prion and prion-like diseases in animals. Virus Res 207:82–93. doi: 10.1016/j.virusres.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 4.Cohen ML. 2014. Human prion diseases, p 2045–2054. In McManus LM, Mitchell RN (ed), Pathobiology of human disease: a dynamic encyclopedia of disease mechanisms. Academic Press, Waltham, MA. [Google Scholar]
- 5.Prusiner SB. 1982. Novel proteinaceous infectious particles cause scrapie. Science 216:136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- 6.Dickinson AG, Fraser H, Outram GW. 1975. Scrapie incubation time can exceed natural lifespan. Nature 256:732–733. doi: 10.1038/256732a0. [DOI] [PubMed] [Google Scholar]
- 7.Dickinson AG, Outram GW. 1979. The scrapie replication-site hypothesis, p 13–21. In Prusiner SB, Hadlow WJ (ed), Slow transmissible diseases of the nervous system, vol 2 Academic Press, New York, NY. [Google Scholar]
- 8.Robbins JR, Bakardjiev AI. 2012. Pathogens and the placental fortress. Curr Opin Microbiol 15:36–43. doi: 10.1016/j.mib.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Notari S, Moleres FJ, Hunter SB, Belay ED, Schonberger LB, Cali I, Parchi P, Shieh WJ, Brown P, Zaki S, Zou WQ, Gambetti P. 2010. Multiorgan detection and characterization of protease-resistant prion protein in a case of variant CJD examined in the United States. PLoS One 5:e8765. doi: 10.1371/journal.pone.0008765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tuo W, Zhuang D, Knowles DP, Cheevers WP, Sy MS, O'Rourke KI. 2001. Prp-c and Prp-Sc at the fetal-maternal interface. J Biol Chem 276:18229–18234. doi: 10.1074/jbc.M008887200. [DOI] [PubMed] [Google Scholar]
- 11.Kimberlin RH. 1981. Scrapie. Br Vet J 137:105–112. [DOI] [PubMed] [Google Scholar]
- 12.Pattison IH, Hoare MN, Jebbett JN, Watson WA. 1972. Spread of scrapie to sheep and goats by oral dosing with foetal membranes from scrapie-affected sheep. Vet Rec 90:465–468. doi: 10.1136/vr.90.17.465. [DOI] [PubMed] [Google Scholar]
- 13.Spiropoulos J, Hawkins SA, Simmons MM, Bellworthy SJ. 2014. Evidence of in utero transmission of classical scrapie in sheep. J Virol 88:4591–4594. doi: 10.1128/JVI.03264-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tamai Y, Kojima H, Kitajima R, Taguchi F, Ohtani Y, Kawaguchi T, Miura S, Sato M, Ishihara Y. 1992. Demonstration of the transmissible agent in tissue from a pregnant woman with Creutzfeldt-Jakob disease. N Engl J Med 327:649. doi: 10.1056/NEJM199208273270918. [DOI] [PubMed] [Google Scholar]
- 15.Gill ON, Spencer Y, Richard-Loendt A, Kelly C, Dabaghian R, Boyes L, Linehan J, Simmons M, Webb P, Bellerby P, Andrews N, Hilton DA, Ironside JW, Beck J, Poulter M, Mead S, Brandner S. 2013. Prevalent abnormal prion protein in human appendixes after bovine spongiform encephalopathy epizootic: large scale survey. BMJ 347:f5675. doi: 10.1136/bmj.f5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benestad SL, Mitchell G, Simmons M, Ytrehus B, Vikoren T. 2016. First case of chronic wasting disease in Europe in a Norwegian free-ranging reindeer. Vet Res 47:88. doi: 10.1186/s13567-016-0375-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao X, Miravalle L, Yuan J, McGeehan J, Dong Z, Wyza R, MacLennan GT, Golichowski AM, Kneale G, King N, Kong Q, Spina S, Vidal R, Ghetti B, Roos K, Gambetti P, Zou WQ. 2009. Failure to detect the presence of prions in the uterine and gestational tissues from a gravida with Creutzfeldt-Jakob disease. Am J Pathol 174:1602–1608. doi: 10.2353/ajpath.2009.081045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murray K, Peters JJ, Stellitano LL, Winstone AM, Verity CC, Will RG. 2011. Is there evidence of vertical transmission of variant Creutzfeldt-Jakob disease? J Neurol Neurosurg Psychiatry 82:729–731. doi: 10.1136/jnnp.2009.172148. [DOI] [PubMed] [Google Scholar]
- 19.Belay ED, Sejvar JJ, Shieh WJ, Wiersma ST, Zou WQ, Gambetti P, Hunter S, Maddox RA, Crockett L, Zaki SR, Schonberger LB. 2005. Variant Creutzfeldt-Jakob disease death, United States. Emerg Infect Dis 11:1351–1354. doi: 10.3201/eid1109.050371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andreoletti O, Lacroux C, Chabert A, Monnereau L, Tabouret G, Lantier F, Berthon P, Eychenne F, Lafond-Benestad S, Elsen JM, Schelcher F. 2002. PrPSc accumulation in placentas of ewes exposed to natural scrapie: influence of foetal PrP genotype and effect on ewe-to-lamb transmission. J Gen Virol 83:2607–2616. doi: 10.1099/0022-1317-83-10-2607. [DOI] [PubMed] [Google Scholar]
- 21.Dickinson AG, Stamp JT, Renwick CC. 1974. Maternal and lateral transmission of scrapie in sheep. J Comp Pathol 84:19–25. doi: 10.1016/0021-9975(74)90023-1. [DOI] [PubMed] [Google Scholar]
- 22.Dickinson AG, Young GB, Stamp JT, Renwick CC. 1965. An analysis of natural scrapie in Suffolk sheep. Heredity 20:485–503. doi: 10.1038/hdy.1965.64. [DOI] [PubMed] [Google Scholar]
- 23.Foster JD, Goldmann W, Hunter N. 2013. Evidence in sheep for pre-natal transmission of scrapie to lambs from infected mothers. PLoS One 8:e79433. doi: 10.1371/journal.pone.0079433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hadlow WJ, Kennedy RC, Race RE. 1982. Natural infection of Suffolk sheep with scrapie virus. J Infect Dis 146:657–664. doi: 10.1093/infdis/146.5.657. [DOI] [PubMed] [Google Scholar]
- 25.Lacroux C, Corbiere F, Tabouret G, Lugan S, Costes P, Mathey J, Delmas JM, Weisbecker JL, Foucras G, Cassard H, Elsen JM, Schelcher F, Andreoletti O. 2007. Dynamics and genetics of PrPSc placental accumulation in sheep. J Gen Virol 88:1056–1061. doi: 10.1099/vir.0.82218-0. [DOI] [PubMed] [Google Scholar]
- 26.O'Rourke KI, Zhuang D, Truscott TC, Yan H, Schneider DA. 2011. Sparse PrP(Sc) accumulation in the placentas of goats with naturally acquired scrapie. BMC Vet Res 7:7. doi: 10.1186/1746-6148-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pattison I. 1972. Scrapie—a personal view. J Clin Pathol Suppl 6:110–114. [PMC free article] [PubMed] [Google Scholar]
- 28.Schneider DA, Madsen-Bouterse SA, Zhuang D, Truscott TC, Dassanayake RP, O'Rourke KI. 2015. The placenta shed from goats with classical scrapie is infectious to goat kids and lambs. J Gen Virol 96:2464–2469. doi: 10.1099/vir.0.000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tuo W, O'Rourke KI, Zhuang D, Cheevers WP, Spraker TR, Knowles DP. 2002. Pregnancy status and fetal prion genetics determine PrPSc accumulation in placentomes of scrapie-infected sheep. Proc Natl Acad Sci U S A 99:6310–6315. doi: 10.1073/pnas.072071199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bencsik A, Debeer S, Petit T, Baron T. 2009. Possible case of maternal transmission of feline spongiform encephalopathy in a captive cheetah. PLoS One 4:e6929. doi: 10.1371/journal.pone.0006929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilesmith JW, Ryan JB, Arnold ME, Stevenson MA, Burke PJ. 2010. Descriptive epidemiological features of cases of bovine spongiform encephalopathy born after July 31, 1996 in Great Britain. Vet Rec 167:279–286. doi: 10.1136/vr.c4552. [DOI] [PubMed] [Google Scholar]
- 32.Wilesmith JW, Wells GA, Ryan JB, Gavier-Widen D, Simmons MM. 1997. A cohort study to examine maternally-associated risk factors for bovine spongiform encephalopathy. Vet Rec 141:239–243. doi: 10.1136/vr.141.10.239. [DOI] [PubMed] [Google Scholar]
- 33.Wrathall AE, Holyoak GR, Parsonson IM, Simmons HA. 2008. Risks of transmitting ruminant spongiform encephalopathies (prion diseases) by semen and embryo transfer techniques. Theriogenology 70:725–745. doi: 10.1016/j.theriogenology.2008.05.049. [DOI] [PubMed] [Google Scholar]
- 34.Donnelly CA, Ferguson NM, Ghani AC, Wilesmith JW, Anderson RM. 1997. Analysis of dam-calf pairs of BSE cases: confirmation of a maternal risk enhancement. Proc Biol Sci 264:1647–1656. doi: 10.1098/rspb.1997.0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Castilla J, Brun A, Diaz-San Segundo F, Salguero FJ, Gutierrez-Adan A, Pintado B, Ramirez MA, del Riego L, Torres JM. 2005. Vertical transmission of bovine spongiform encephalopathy prions evaluated in a transgenic mouse model. J Virol 79:8665–8668. doi: 10.1128/JVI.79.13.8665-8668.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldenberg RL, Hauth JC, Andrews WW. 2000. Intrauterine infection and preterm delivery. N Engl J Med 342:1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 37.Casalone C, Corona C, Crescio MI, Martucci F, Mazza M, Ru G, Bozzetta E, Acutis PL, Caramelli M. 2005. Pathological prion protein in the tongues of sheep infected with naturally occurring scrapie. J Virol 79:5847–5849. doi: 10.1128/JVI.79.9.5847-5849.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeJoia C, Moreaux B, O'Connell K, Bessen RA. 2006. Prion infection of oral and nasal mucosa. J Virol 80:4546–4556. doi: 10.1128/JVI.80.9.4546-4556.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eklund CM, Kennedy RC, Hadlow WJ. 1967. Pathogenesis of scrapie virus infection in the mouse. J Infect Dis 117:15–22. doi: 10.1093/infdis/117.1.15. [DOI] [PubMed] [Google Scholar]
- 40.Mulcahy ER, Bartz JC, Kincaid AE, Bessen RA. 2004. Prion infection of skeletal muscle cells and papillae in the tongue. J Virol 78:6792–6798. doi: 10.1128/JVI.78.13.6792-6798.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Donaldson DS, Else KJ, Mabbott NA. 2015. The gut-associated lymphoid tissues in the small intestine, not the large intestine, play a major role in oral prion disease pathogenesis. J Virol 89:9532–9547. doi: 10.1128/JVI.01544-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kimberlin RH, Walker CA. 1990. Intraperitoneal infection with scrapie is established within minutes of injection and is non-specifically enhanced by a variety of different drugs. Arch Virol 112:103–114. doi: 10.1007/BF01348988. [DOI] [PubMed] [Google Scholar]
- 43.Pattison IH, Millson GC. 1962. Distribution of the scrapie agent in the tissues of experimentally inoculated goats. J Comp Pathol 72:233–244. doi: 10.1016/S0368-1742(62)80026-5. [DOI] [PubMed] [Google Scholar]
- 44.Clarke MC, Haig DA. 1967. Presence of the transmissible agent of scrapie in the serum of affected mice and rats. Vet Rec 80:504. doi: 10.1136/vr.80.16.504. [DOI] [PubMed] [Google Scholar]
- 45.Houston F, Foster JD, Chong A, Hunter N, Bostock CJ. 2000. Transmission of BSE by blood transfusion in sheep. Lancet 356:999–1000. doi: 10.1016/S0140-6736(00)02719-7. [DOI] [PubMed] [Google Scholar]
- 46.Hunter N, Foster J, Chong A, McCutcheon S, Parnham D, Eaton S, MacKenzie C, Houston F. 2002. Transmission of prion diseases by blood transfusion. J Gen Virol 83:2897–2905. doi: 10.1099/0022-1317-83-11-2897. [DOI] [PubMed] [Google Scholar]
- 47.Llewelyn CA, Hewitt PE, Knight RS, Amar K, Cousens S, Mackenzie J, Will RG. 2004. Possible transmission of variant Creutzfeldt-Jakob disease by blood transfusion. Lancet 363:417–421. doi: 10.1016/S0140-6736(04)15486-X. [DOI] [PubMed] [Google Scholar]
- 48.Mathiason CK, Hayes-Klug J, Hays SA, Powers J, Osborn DA, Dahmes SJ, Miller KV, Warren RJ, Mason GL, Telling GC, Young AJ, Hoover EA. 2010. B cells and platelets harbor prion infectivity in the blood of deer infected with chronic wasting disease. J Virol 84:5097–5107. doi: 10.1128/JVI.02169-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mathiason CK, Powers JG, Dahmes SJ, Osborn DA, Miller KV, Warren RJ, Mason GL, Hays SA, Hayes-Klug J, Seelig DM, Wild MA, Wolfe LL, Spraker TR, Miller MW, Sigurdson CJ, Telling GC, Hoover EA. 2006. Infectious prions in the saliva and blood of deer with chronic wasting disease. Science 314:133–136. doi: 10.1126/science.1132661. [DOI] [PubMed] [Google Scholar]
- 50.Peden AH, Head MW, Ritchie DL, Bell JE, Ironside JW. 2004. Preclinical vCJD after blood transfusion in a PRNP codon 129 heterozygous patient. Lancet 364:527–529. doi: 10.1016/S0140-6736(04)16811-6. [DOI] [PubMed] [Google Scholar]
- 51.Bueler H, Fischer M, Lang Y, Bluethmann H, Lipp HP, DeArmond SJ, Prusiner SB, Aguet M, Weissmann C. 1992. Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature 356:577–582. doi: 10.1038/356577a0. [DOI] [PubMed] [Google Scholar]
- 52.Forde N, Rogers M, Canty MJ, Lonergan P, Smith GW, Coussens PM, Ireland JJ, Evans AC. 2008. Association of the prion protein and its expression with ovarian follicle development in cattle. Mol Reprod Dev 75:243–249. doi: 10.1002/mrd.20807. [DOI] [PubMed] [Google Scholar]
- 53.Horiuchi M, Yamazaki N, Ikeda T, Ishiguro N, Shinagawa M. 1995. A cellular form of prion protein (PrPC) exists in many non-neuronal tissues of sheep. J Gen Virol 76:2583–2587. doi: 10.1099/0022-1317-76-10-2583. [DOI] [PubMed] [Google Scholar]
- 54.Johnson ML, Grazul-Bilska AT, Reynolds LP, Redmer DA. 2014. Prion (PrPC) expression in ovine uteroplacental tissues increases after estrogen treatment of ovariectomized ewes and during early pregnancy. Reproduction 148:1–10. doi: 10.1530/REP-13-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mathiason CK, Foos T, Eliason G, Sigurdson C, Miller M, Lewis-Weis L, Fishcer J, Langenberg J, LaFond K, Osborn D, Hoover EA. 2004. PrPc mapping in adult and fetal deer tissues, abstr 56. Proceedings of The Animal Prion Diseases and The Americas Conference, Ames, IA, 14 to 16 October 2004. [Google Scholar]
- 56.Thumdee P, Ponsuksili S, Murani E, Nganvongpanit K, Gehrig B, Tesfaye D, Gilles M, Hoelker M, Jennen D, Griese J, Schellander K, Wimmers K. 2007. Expression of the prion protein gene (PRNP) and cellular prion protein (PrPc) in cattle and sheep fetuses and maternal tissues during pregnancy. Gene Expr 13:283–297. doi: 10.3727/000000006780666984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fujisawa M, Kanai Y, Nam SY, Maeda S, Nakamuta N, Kano K, Kurohmaru M, Hayashi Y. 2004. Expression of Prnp mRNA (prion protein gene) in mouse spermatogenic cells. J Reprod Dev 50:565–570. doi: 10.1262/jrd.50.565. [DOI] [PubMed] [Google Scholar]
- 58.Miranda A, Pericuesta E, Ramirez MA, Gutierrez-Adan A. 2011. Prion protein expression regulates embryonic stem cell pluripotency and differentiation. PLoS One 6:e18422. doi: 10.1371/journal.pone.0018422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alverson J, O'Rourke KI, Baszler TV. 2006. PrPSc accumulation in fetal cotyledons of scrapie-resistant lambs is influenced by fetus location in the uterus. J Gen Virol 87:1035–1041. doi: 10.1099/vir.0.81418-0. [DOI] [PubMed] [Google Scholar]
- 60.Garza MC, Fernandez-Borges N, Boles R, Badiola JJ, Castilla J, Monleon E. 2011. Detection of PrPres in genetically susceptible fetuses from sheep with natural scrapie. PLoS One 6:e27525. doi: 10.1371/journal.pone.0027525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Onodera T, Ikeda T, Muramatsu Y, Shinagawa M. 1993. Isolation of scrapie agent from the placenta of sheep with natural scrapie in Japan. Microbiol Immunol 37:311–316. doi: 10.1111/j.1348-0421.1993.tb03215.x. [DOI] [PubMed] [Google Scholar]
- 62.Andreoletti O, Litaise C, Simmons H, Corbiere F, Lugan S, Costes P, Schelcher F, Vilette D, Grassi J, Lacroux C. 2012. Highly efficient prion transmission by blood transfusion. PLoS Pathog 8:e1002782. doi: 10.1371/journal.ppat.1002782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McCutcheon S, Alejo Blanco AR, Houston EF, de Wolf C, Tan BC, Smith A, Groshup MH, Hunter N, Hornsey VS, MacGregor IR, Prowse CV, Turner M, Manson JC. 2011. All clinically relevant blood components transmit prion disease following a single blood transfusion: a sheep model of vCJD. PLoS One 6:e23169. doi: 10.1371/journal.pone.0023169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wroe SJ, Pal S, Siddique D, Hyare H, Macfarlane R, Joiner S, Linehan JM, Brandner S, Wadsworth JDF, Hewitt P, Collinge J. 2006. Clinical presentation and pre-mortem diagnosis of variant Creutzfeldt-Jakob disease associated with blood transfusion: a case report. Lancet 368:2061–2067. doi: 10.1016/S0140-6736(06)69835-8. [DOI] [PubMed] [Google Scholar]
- 65.Myagkaya G, Schellens JP. 1981. Final stages of erythrophagocytosis in the sheep placenta. Cell Tissue Res 214:501–518. doi: 10.1007/BF00233491. [DOI] [PubMed] [Google Scholar]
- 66.Maltepe E, Bakardjiev AI, Fisher SJ. 2010. The placenta: transcriptional, epigenetic, and physiological integration during development. J Clin Invest 120:1016–1025. doi: 10.1172/JCI41211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hafez S. 2017. Comparative placental anatomy: divergent structures serving a common purpose. Prog Mol Biol Transl 145:1–28. doi: 10.1016/bs.pmbts.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 68.Wooding FB, Flint AP. 1994. Placentation, 4:235–289. In Lamming GE. (ed), Marshall's physiology of reproduction, 4th ed, vol 3, Pregnancy and lactation Churchill Livingstone, London, United Kingdom. [Google Scholar]
- 69.Wooding FB, Morgan G, Adam CL. 1997. Structure and function in the rumiant synepithelialchorial placenta: central role of the trophoblast binucleate cell in deer. Microsc Res Tech 38:88–99. [DOI] [PubMed] [Google Scholar]
- 70.Reynolds LP, Redmer DA. 2001. Angiogenesis in the placenta. Biol Reprod 64:1033–1040. doi: 10.1095/biolreprod64.4.1033. [DOI] [PubMed] [Google Scholar]
- 71.Abu-Yousef MM, Bleicher JJ, Williamson RA, Weiner CP. 1987. Subchorionic hemorrhage: sonographic diagnosis and clinical significance. Am J Roentgenol 149:737–740. doi: 10.2214/ajr.149.4.737. [DOI] [PubMed] [Google Scholar]
- 72.Benirschke K. 19 January 2012. Comparative placentation. http://placentation.ucsd.edu/tenrecfs.htm.
- 73.Grassi R, Farina R, Floriani I, Amodio F, Romano S. 2005. Assessment of fetal swallowing with gray-scale and color Doppler sonography. Am J Roentgenol 185:1322–1327. doi: 10.2214/AJR.04.1114. [DOI] [PubMed] [Google Scholar]
- 74.Harding R, Bocking AD, Sigger JN, Wickham PJ. 1984. Composition and volume of fluid swallowed by fetal sheep. Q J Exp Physiol 69:487–495. doi: 10.1113/expphysiol.1984.sp002835. [DOI] [PubMed] [Google Scholar]
- 75.Ross MG, Nijland MJ. 1998. Development of ingestive behavior. Am J Physiol 274:R879–R893. [DOI] [PubMed] [Google Scholar]
- 76.Wintour EM, Shandley L. 1993. Effects of fetal fluid balance on amniotic fluid volume. Semin Perinatol 17:158–172. [PubMed] [Google Scholar]
- 77.Cho CK, Shan SJ, Winsor EJ, Diamandis EP. 2007. Proteomics analysis of human amniotic fluid. Mol Cell Proteomics 6:1406–1415. doi: 10.1074/mcp.M700090-MCP200. [DOI] [PubMed] [Google Scholar]
- 78.Underwood MA, Gilbert WM, Sherman MP. 2005. Amniotic fluid: not just fetal urine anymore. J Perinatol 25:341–348. doi: 10.1038/sj.jp.7211290. [DOI] [PubMed] [Google Scholar]
- 79.Wintour EM, Laurence BM, Lingwood BE. 1986. Anatomy, physiology and pathology of the amniotic and allantoic compartments in the sheep and cow. Aust Vet J 63:216–221. doi: 10.1111/j.1751-0813.1986.tb02999.x. [DOI] [PubMed] [Google Scholar]
- 80.Haley NJ, Seelig DM, Zabel MD, Telling GC, Hoover EA. 2009. Detection of CWD prions in urine and saliva of deer by transgenic mouse bioassay. PLoS One 4:e4848. doi: 10.1371/journal.pone.0004848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Safar JG, Lessard P, Tamguney G, Freyman Y, Deering C, Letessier F, Dearmond SJ, Prusiner SB. 2008. Transmission and detection of prions in feces. J Infect Dis 198:81–89. doi: 10.1086/588193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tamguney G, Miller MW, Wolfe LL, Sirochman TM, Glidden DV, Palmer C, Lemus A, DeArmond SJ, Prusiner SB. 2009. Asymptomatic deer excrete infectious prions in faeces. Nature 461:529–532. doi: 10.1038/nature08289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Terry LA, Howells L, Bishop K, Baker CA, Everest S, Thorne L, Maddison BC, Gough KC. 2011. Detection of prions in the faeces of sheep naturally infected with classical scrapie. Vet Res 42:65. doi: 10.1186/1297-9716-42-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Murugavel K, Abdul Salam D, Barathiraja S, Thanislass J, Antoine D, Raju MS. 2014. Biochemical constituents of amniotic and allantoic fluids in chital deer (Axis axis). Indian J Anim Reprod 35:31–33. [Google Scholar]
- 85.Atarashi R, Moore RA, Sim VL, Hughson AG, Dorward DW, Onwubiko HA, Priola SA, Caughey B. 2007. Ultrasensitive detection of scrapie prion protein using seeded conversion of recombinant prion protein. Nat Methods 4:645–650. doi: 10.1038/nmeth1066. [DOI] [PubMed] [Google Scholar]
- 86.Orru CD, Groveman BR, Hughson AG, Zanusso G, Coulthart MB, Caughey B. 2015. Rapid and sensitive RT-QuIC detection of human Creutzfeldt-Jakob disease using cerebrospinal fluid. mBio 6:e02451-14. doi: 10.1128/mBio.02451-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Henderson DM, Denkers ND, Hoover CE, Garbino N, Mathiason CK, Hoover EA. 2015. Longitudinal detection of prion shedding in saliva and urine by chronic wasting disease-infected deer by real-time quaking-induced conversion. J Virol 89:9338–9347. doi: 10.1128/JVI.01118-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hoover CE, Davenport KA, Henderson DM, Pulscher LA, Mathiason CK, Zabel MD, Hoover EA. 2016. Detection and quantification of CWD prions in fixed paraffin embedded tissues by real-time quaking-induced conversion. Sci Rep 6:25098. doi: 10.1038/srep25098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Orru CD, Favole A, Corona C, Mazza M, Manca M, Groveman BR, Hughson AG, Acutis PL, Caramelli M, Zanusso G, Casalone C, Caughey B. 2015. Detection and discrimination of classical and atypical L-type bovine spongiform encephalopathy by real-time quaking-induced conversion. J Clin Microbiol 53:1115–1120. doi: 10.1128/JCM.02906-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Beekes M, McBride PA. 2007. The spread of prions through the body in naturally acquired transmissible spongiform encephalopathies. FEBS J 274:588–605. doi: 10.1111/j.1742-4658.2007.05631.x. [DOI] [PubMed] [Google Scholar]
- 91.Fox KA, Jewell JE, William ES, Miller MW. 2006. Patterns of PrPCWD accumulation during the course of chronic wasting disease infection in orally inoculated mule deer (Odocoileus hemionus). J Gen Virol 87:3451–3461. doi: 10.1099/vir.0.81999-0. [DOI] [PubMed] [Google Scholar]
- 92.Seelig DM, Mason GL, Telling GC, Hoover EA. 2010. Pathogenesis of chronic wasting disease in cervidized transgenic mice. Am J Pathol 176:2785–2797. doi: 10.2353/ajpath.2010.090710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sigurdson CJ, Spraker TR, Miller MW, Oesch B, Hoover EA. 2001. PrP(CWD) in the myenteric plexus, vagosympathetic trunk and endocrine glands of deer with chronic wasting disease. J Gen Virol 82:2327–2334. doi: 10.1099/0022-1317-82-10-2327. [DOI] [PubMed] [Google Scholar]
- 94.Henderson DM, Davenport KA, Haley NJ, Denkers ND, Mathiason CK, Hoover EA. 2015. Quantitative assessment of prion infectivity in tissues and body fluids by real-time quaking-induced conversion. J Gen Virol 96:210–219. doi: 10.1099/vir.0.069906-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Miller MW, Wild MA, Williams ES. 1998. Epidemiology of chronic wasting disease in captive Rocky Mountain elk. J Wildl Dis 34:532–538. doi: 10.7589/0090-3558-34.3.532. [DOI] [PubMed] [Google Scholar]
- 96.Williams ES, Young S. 1992. Spongiform encephalopathies in Cervidae. Rev Sci Tech 11:551–567. doi: 10.20506/rst.11.2.611. [DOI] [PubMed] [Google Scholar]
- 97.Mathiason CK, Hays SA, Powers J, Hayes-Klug J, Langenberg J, Dahmes SJ, Osborn DA, Miller KV, Warren RJ, Mason GL, Hoover EA. 2009. Infectious prions in pre-clinical deer and transmission of chronic wasting disease solely by environmental exposure. PLoS One 4:e5916. doi: 10.1371/journal.pone.0005916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Miller MW, Williams ES, Hobbs NT, Wolfe LL. 2004. Environmental sources of prion transmission in mule deer. Emerg Infect Dis 10:1003–1006. doi: 10.3201/eid1006.040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Blanchong JA, Grear DA, Weckworth BV, Keane DP, Scribner KT, Samuel MD. 2012. Effects of chronic wasting disease on reproduction and fawn harvest vulnerability in Wisconsin white-tailed deer. J Wildl Dis 48:361–370. doi: 10.7589/0090-3558-48.2.361. [DOI] [PubMed] [Google Scholar]
- 100.Wadsworth JD, Joiner S, Hill AF, Campbell TA, Desbruslais M, Luthert PJ, Collinge J. 2001. Tissue distribution of protease resistant prion protein in variant Creutzfeldt-Jakob disease using a highly sensitive immunoblotting assay. Lancet 358:171–180. doi: 10.1016/S0140-6736(01)05403-4. [DOI] [PubMed] [Google Scholar]
- 101.Wilham JM, Orru CD, Bessen RA, Atarashi R, Sano K, Race B, Meade-White KD, Taubner LM, Timmes A, Caughey B. 2010. Rapid end-point quantitation of prion seeding activity with sensitivity comparable to bioassays. PLoS Pathog 6:e1001217. doi: 10.1371/journal.ppat.1001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Browning SR, Mason GL, Seward T, Green M, Eliason GA, Mathiason C, Miller MW, Williams ES, Hoover E, Telling GC. 2004. Transmission of prions from mule deer and elk with chronic wasting disease to transgenic mice expressing cervid PrP. J Virol 78:13345–13350. doi: 10.1128/JVI.78.23.13345-13350.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]