Significance
Inherited retinal degenerations may result in blindness due to a progressive loss of photoreceptor cells. We assess subretinal delivery of human melanopsin using an adeno-associated viral vector to remaining retinal cells in a model of end-stage retinal degeneration. Human melanopsin, being already present in the eye, is unlikely to generate an immune response when introduced via gene therapy. Furthermore, this method of delivery has been proven to be safe in clinical trials and may be more effective at delivering vector in primates than the alternative method of intravitreal injection. We demonstrate long-term vector expression and restoration of visual function, indicating that this therapy could be stable and efficacious in the treatment of patients with end-stage retinal degenerations.
Keywords: human melanopsin, gene therapy, optogenetics
Abstract
Optogenetic strategies to restore vision in patients who are blind from end-stage retinal degenerations aim to render remaining retinal cells light sensitive once photoreceptors are lost. Here, we assessed long-term functional outcomes following subretinal delivery of the human melanopsin gene (OPN4) in the rd1 mouse model of retinal degeneration using an adeno-associated viral vector. Ectopic expression of OPN4 using a ubiquitous promoter resulted in cellular depolarization and ganglion cell action potential firing. Restoration of the pupil light reflex, behavioral light avoidance, and the ability to perform a task requiring basic image recognition were restored up to 13 mo following injection. These data suggest that melanopsin gene therapy via a subretinal route may be a viable and stable therapeutic option for the treatment of end-stage retinal degeneration in humans.
Inherited retinal degenerations such as retinitis pigmentosa (RP) affect 1 in 4,000 people (1), causing significant visual morbidity and blindness due to a progressive loss of photoreceptor cells. Even in end-stage disease, the remaining retinal layers and central visual projections remain structurally intact. Stimulation of these remaining cells is potentially sufficient to mimic visual responses and restore vision, and by this means the subretinal electronic implant has shown proof of principle for restoration of vision in patients after severe photoreceptor loss (2).
An alternative gene therapy strategy involves the expression of transgenes encoding photosensitive proteins in remaining retinal cells, making them directly light sensitive in the absence of rods and cones (3–7). A candidate protein for this purpose is melanopsin, the photopigment naturally present in a subset of ganglion cells that are intrinsically photosensitive [intrinsically photosensitive retinal ganglion cells (ipRGCs)] (8). Melanopsin is particularly suited to this purpose since it is native to the human eye (9) and therefore is less likely to be immunogenic. Melanopsin shows greater sensitivity to light than alternative microbial optogenetic tools such as channelrhodopsin-2 (3, 10, 11) or halorhodopsin (4), but has slower kinetics. Furthermore, the melanopsin transduction cascade involves the activation of ubiquitously expressed Gnaq/11-type G proteins (12), permitting signal amplification in multiple host cell types (13, 14).
Previous work used intravitreal delivery of an adeno-associated viral (AAV) vector to express mouse melanopsin in ganglion cells with restoration of visual responses (5). We investigated whether human melanopsin (OPN4) could be effectively delivered via an alternative subretinal approach, using a ubiquitous (CBA) promoter to drive expression in all remaining outer retinal cells for several reasons. Subretinal vector delivery is well established in human clinical trials (15, 16) but has not been assessed in combination with a CBA promoter as an optogenetic approach for vision restoration. Transduction of cells in the upstream retina maximizes the potential of retaining complex processing of the visual signal. Furthermore, increased availability of chromophore (retinal) in the outer retina may be required for effective photon capture in the absence of specialized outer segment discs. Other studies have used AAV vectors containing a mouse bipolar-cell–specific enhancer to target a melanopsin.mGluR6 chimera (17) or rhodopsin (6) to bipolar cells via intravitreal injection. However, there is variation in anatomy between primates and mouse models (18), and this may render the intravitreal approach less effective in humans. Virions delivered via intravitreal injection are diluted more in primates compared with mice due to the larger volume of the vitreous, reducing the concentration of vector reaching retinal cells. The inner limiting membrane on the retinal surface is also thicker in primates than in rodents (19), through which virions must pass to reach target cells. The increased risks of an inflammatory response following intravitreal AAV injection (20) may also limit the translational potential of this route of delivery. We therefore assessed transduction following subretinal delivery of OPN4 and whether this could support long-term restoration of light sensitivity and visual function in a mouse model of end-stage RP.
Results
Long-Term Expression of Human Melanopsin in Degenerate Retina Is Achieved Following Subretinal Delivery of an AAV Vector.
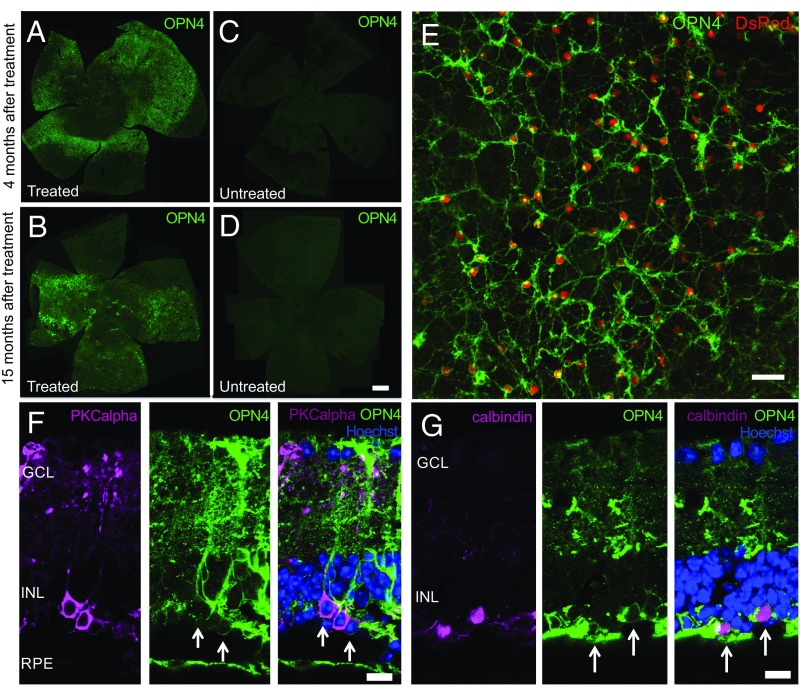
To model the extensive photoreceptor loss seen in end-stage RP, the rd1 mouse was used. These mice have a nonsense mutation in the Pde6b gene, which leads to rapid degeneration of rod photoreceptors followed by loss of cones (21). Retinal tropism was assessed 4 and 15 mo after subretinal delivery of a single capsid mutant AAV vector [rAAV2/8(Y733F) CBA-OPN4-IRES-DsRed] in 6- to 8-wk-old rd1 mice. This bicistronic vector included a DsRed fluorescent marker to permit visualization of transduced cells within the retina and ensure vector-driven expression. Retinal flatmounts stained with human OPN4-specific antibody showed widespread retinal transduction in treated eyes, which was sustained up to 15 mo postinjection (Fig. 1 A and B), but none in age-matched untreated controls (Fig. 1 C and D). Human melanopsin showed appropriate membrane localization (Figs. S1 and S2E), with the DsRed marker confirming vector-driven expression (Fig. 1E). Histological sections demonstrated robust and widespread OPN4 expression throughout the inner nuclear and inner plexiform layers of the degenerate retina in treated eyes, with immunohistochemistry using horizontal, bipolar (Fig. 1 F and G, and Fig. S1), and Müller cell (Fig. S2)-specific antibodies and cell morphology indicating widespread transduction of these cell types. There was no significant transduction of cell bodies in the ganglion cell layer following subretinal vector delivery, as assessed by cell morphology and ganglion cell-specific staining (Fig. S2).
Fig. 1.
Long-term expression of human melanopsin is achieved in the degenerate retina following subretinal delivery of an adeno-associated viral (AAV) vector. Flatmounts of the rd1 mouse retina assessed following immunolabeling for human OPN4 (green) at 4 mo (A) and 15 mo (B) after subretinal OPN4 vector delivery reveal widespread transduction. Labeling of human OPN4 was absent from untreated age-matched controls (C and D). (Scale bar, 500 μm.) Images A–D are composite fluorescence images each of a single mouse retina. Appropriate membrane localization of human melanopsin was evident demonstrating a network of transduced cells, with DsRed fluorescence confirming vector-driven OPN4 expression (E). (Scale bar, 25 μm.) Successful transduction of bipolar cells (labeled by PKCα, purple, F) and horizontal cells (labeled by calbindin, purple, G) using a ubiquitous promoter was evident following colabeling with OPN4 (green) and Hoechst nuclear stain (blue). (Scale bar, 25 μm.)
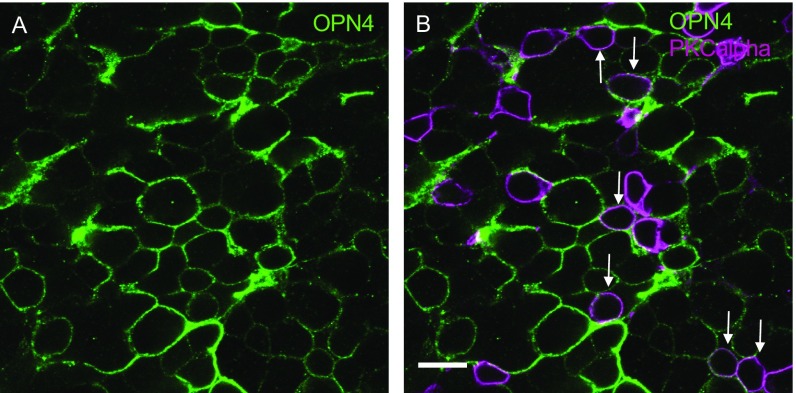
Fig. S1.
Subretinal vector delivery leads to appropriate membrane localization of human melanopsin and bipolar cell transduction in end-stage retinal degeneration. Staining of a retinal flatmount with OPN4 antibody (green) illustrates its location at the edges of INL cells, consistent with membrane localization of human melanopsin (A). Colabeling with PKCα antibody (purple) demonstrates successful transduction of a number of rod bipolar cells (arrows, B) following subretinal delivery of melanopsin vector. (Scale bar, 10 μm.)
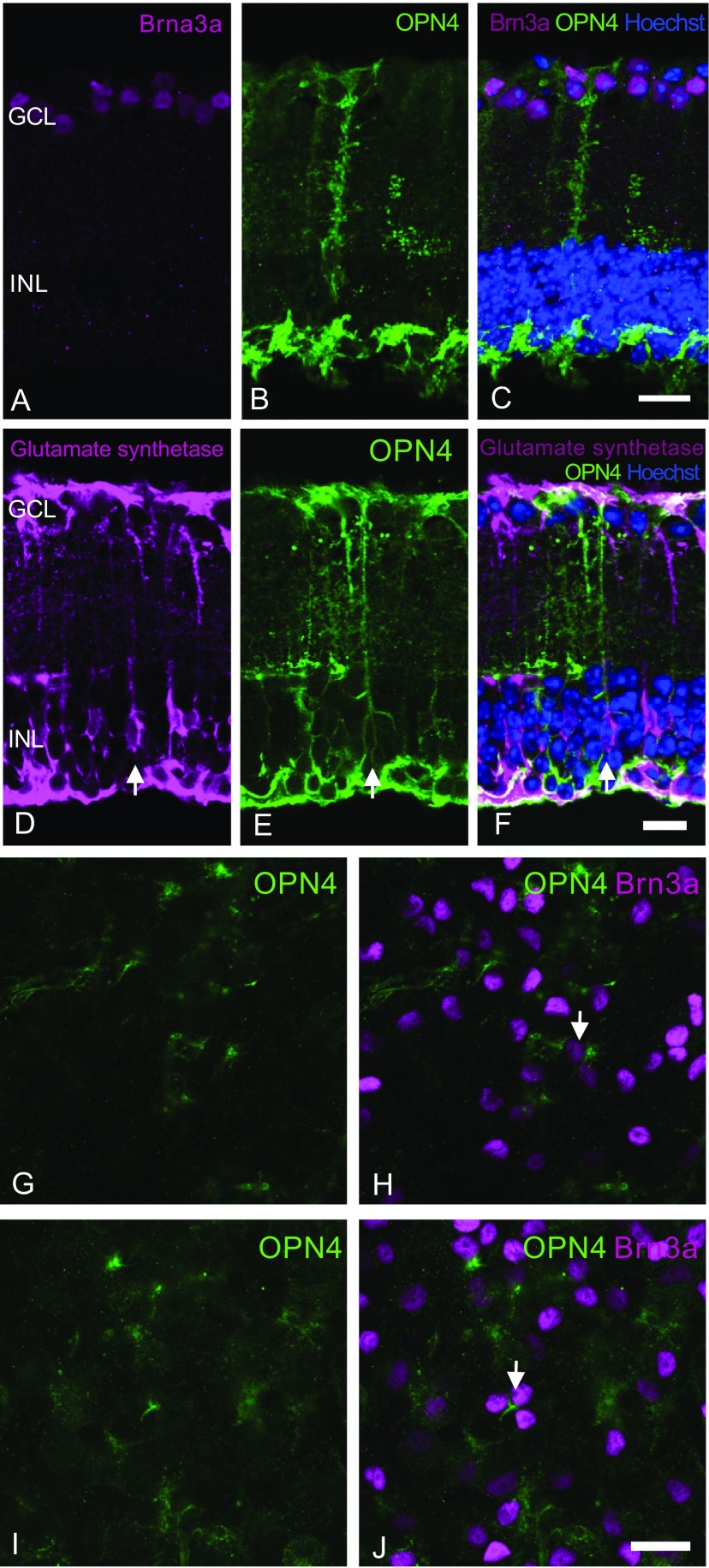
Fig. S2.
No significant transduction of cell bodies within the ganglion cell layer is seen following vector delivery. Retinal sections were stained with Brn3a (purple, A) and OPN4 (green, B) antibodies and overlaid with Hoechst nuclear stain (blue) to identify any ganglion cell transduction by the OPN4 vector (C). Examination of histological sections showed melanopsin staining within the ganglion cell layer, but this appeared to be due to labeling of Müller cell end feet [stained using glutamate synthetase antibody, purple, overlaid with Hoechst nuclear stain (blue), D–F]. Staining of retinal flatmounts (G–J) with Brn3a (purple) and OPN4 (green) antibodies did not show significant human melanopsin staining of cell bodies within the ganglion cell layer, suggesting that ganglion cells were not transduced by subretinal delivery of the OPN4 vector. Some human melanopsin-expressing axonal processes did appear to meet Brn3a-positive ganglion cells (arrows). These could be human melanopsin-expressing bipolar cells synapsing with ganglion cells, or Müller cell end feet surrounding them. (Scale bar, 25 μm.)
Human Melanopsin Expressed in the Degenerate Retina Is Able to Mediate a Functional Response to a Light Stimulus.
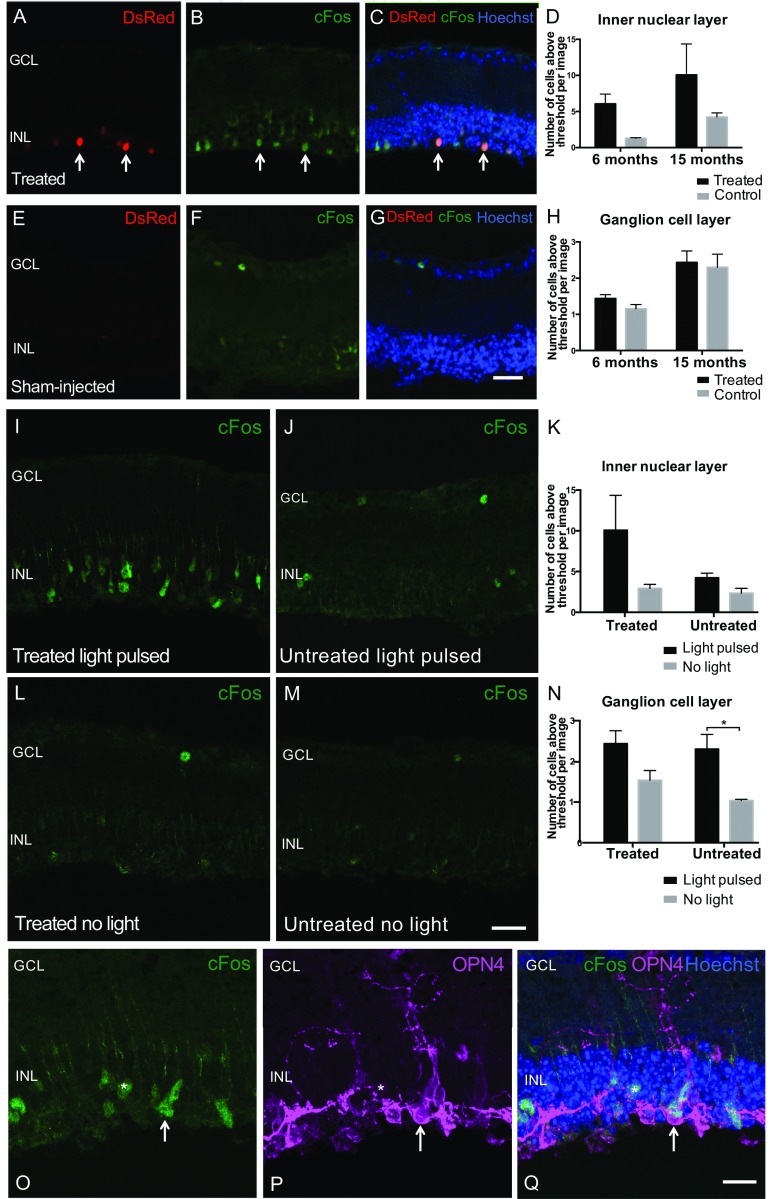
Expression of the “immediate early” gene c-Fos is a marker of cellular depolarization and has been widely used to monitor melanopsin-driven light responses in ipRGCs in the degenerate retina (22, 23). Comparison of light-induced c-Fos expression in the inner nuclear layer (INL) of vector-treated versus untreated retina showed a 2.5-fold increase in the number of c-Fos–positive cells in treated eyes (effect of treatment, P = 0.0197, two-way ANOVA; Fig. S3 A–G). High levels of colocalization were observed for both DsRed and c-Fos (Fig. S3 A–C), and human OPN4 and c-Fos (Fig. S3 O–Q), confirming light-induced depolarization of transduced cells in treated retina. In addition, some adjacent c-Fos–positive cells were observed that did not appear to express OPN4 (or DsRed), which might indicate depolarization of neighboring cells and cell-to-cell signaling resulting from ectopic expression of OPN4 in the degenerate retina.
Fig. S3.
Light-dependent c-Fos up-regulation in inner nuclear layer (INL) cells following subretinal OPN4 vector delivery indicates cell depolarization. To evaluate whether ectopic expression of OPN4 was able to mediate cell depolarization, c-Fos expression was assessed in treated rd1 mice versus age-matched controls at 6 and 15 mo after subretinal vector delivery. Mice were exposed to a 30-min white-light stimulus of 350-lx intensity. Mice were culled after a further 30 min in darkness and retinas collected and examined for c-Fos expression. INL: Areas of vector expression were identified by DsRed fluorescence (red) (A). Antibody staining for c-Fos (green, B) and composite images showing overlay with Hoechst nuclear labeling (blue, C) demonstrate increased c-Fos expression in the INL of treated retina (arrows indicate examples of cells expressing DsRed fluorescence that were labeled with c-Fos antibody). c-Fos expression was not widely seen in the INL of controls following a light pulse (E–G). (Scale bar, 25 μm.) There was a significant effect of treatment (P = 0.0197, two way ANOVA) but not of age (P = 0.103) on the number of c-Fos–positive cells in the INL following light pulsing (D; 6-mo-treated group, n = 303 c-Fos–positive cells, n = 5 retinas; control, n = 50 cells, n = 4 retinas; 15-mo-treated group, n = 302 cells, n = 3 retinas; control, n = 127 cells, n = 3 retinas). Ganglion cell layer (GCL): A few c-Fos–expressing cells were seen in the GCL in each group following light pulsing, but there was no effect of treatment (P = 0.3419) on the number of these cells (H; 6-mo-treated group, n = 72 c-Fos–positive cells, n = 5 retinas; control, n = 45 cells, n = 4 retinas; 15-mo-treated group, n = 73 cells, n = 3 retinas; control, n = 69 cells, n = 3 retinas). Previous work in wild-type mice has shown only 3–5% of ganglion cells express c-Fos in response to light (42) with the remainder of c-Fos–expressing cells in the GCL being amacrine and intrinsically photosensitive ganglion cells (43). The presence of only a small number of c-Fos–positive cells in the GCL of treated mice is consistent with this, and since the activation of amacrine cells is largely inhibitory they may not be depolarized by direct optogenetic activation of inner nuclear cells. To confirm that c-Fos expression was up-regulated by a light stimulus and not by other vector-related effects, retinas from mice subjected to a light pulse (I and J) were compared with those from mice handled in an identical manner but not exposed to light (L and M). There was a significant effect of light on the number of c-Fos–positive cells in the GCL following a light stimulus (N; P = 0.0038, two-way ANOVA; untreated group, P = 0.02, Bonferroni post hoc test), but the overall effect of light did not reach significance examining cells in the INL (K; P = 0.0739). *P < 0.05, Bonferroni post hoc test. To evaluate whether c-Fos–expressing cells in the INL expressed ectopic OPN4, retinas were colabeled with c-Fos antibody (green) and OPN4 (purple) along with Hoechst nuclear stain (blue). This demonstrated c-Fos expression in OPN4-expressing cells (arrow, O–Q). (Scale bar, 25 μm.) A few adjacent c-Fos–positive cells were identified that did not colocalize with OPN4 staining (*) suggestive of cell-to-cell signaling following a light stimulus.
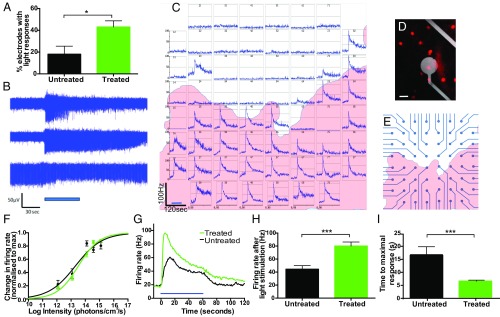
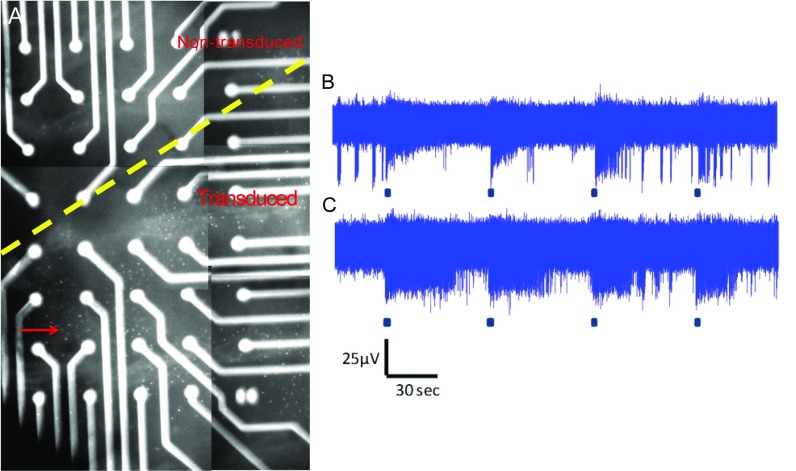
Multielectrode array (MEA) recordings from ex vivo retinal explants were performed 4 mo after subretinal vector delivery to assess whether expression of OPN4 in the INL was able to drive action potential firing in retinal ganglion cells, and therefore generate a signal that could be centrally transmitted. The percentage of electrodes showing a light-dependent increase in action potential firing in treated retinas (43.1 ± 5.6% electrodes, n = 6 retinas, n = 144 electrodes) was more than doubled compared with untreated age-matched controls (18.1 ± 7.3% electrodes, n = 9 retinas, n = 88 electrodes; P = 0.028, two-tailed unpaired t test; Fig. 2A). Responses observed in untreated retina were consistent with the activation of endogenous ipRGCs, whereas treated retinas include both these responses and those originating from ectopic expression of human OPN4 (Fig. 2C). In treated but not untreated retina, some electrodes showed a reduction in spike firing rate following light stimulation (Fig. 2B), which may add to the complexity of the visual signal generated following OPN4 expression in inner nuclear cells. Visualization of DsRed fluorescence using 540-nm light (Fig. 2D) confirmed that the location of responsive electrodes in treated retinas was highly correlated to areas transduced by the OPN4 vector (Fig. 2 C–E and Fig. S4A), indicative of these being responses from ectopically expressed OPN4.
Fig. 2.
Human melanopsin expressed in the degenerate retina is able to mediate a functional response to a light stimulus. Multielectrode array (MEA) recordings from ex vivo rd1 mouse retinas showed a higher percentage of electrodes demonstrating light-induced increases in action potential firing in treated retinas compared with untreated controls (A) (P = 0.028, two-tailed unpaired t test; treated, n = 6; untreated, n = 9). Examples of raw data obtained from individual electrodes following 60-s 480-nm light pulses are shown (B). Light responses recorded from electrodes from one treated retina are shown (C) [represented as mean spike firing rate (in hertz) measured in 1-s bins; data shown are 30 s of baseline recording followed by a 60-s 480-nm light stimulus at 3.99 × 1014 photons⋅cm−2⋅s−1; for original image of the retina showing location of DsRed-positive cells, see Fig. S4]. Visualization of DsRed fluorescence (dots) illustrates the area of the retina transduced by the human OPN4 vector relative to the position of MEA recording electrodes (D). (Scale bar, 15 μm.) A representation of DsRed expression (highlighted in red) is shown to indicate the transduced areas of the treated retina in C relative to the positioning of electrodes (E). In combination, this illustrates an increase in light-dependent action potential firing within regions of transduced retina. IRCs generated from responsive electrodes in treated (n = 51 electrodes, n = 3 retinas) and untreated (n = 22 electrodes, n = 4 retinas) retinas show a similar sensitivity of responses between groups (F), with action potential firing detected at the lowest light intensity assessed (1.20 × 1012 photons⋅cm−2⋅s−1). The mean response of all electrodes to a 3.99 × 1014 photons⋅cm−2⋅s−1 light stimulus is shown (G). Blue bars indicate duration of light stimuli (B, C, and G). There was a greater increase in spike firing rate in response to a light stimulus in treated versus untreated retinas (H); and time to maximal response was shorter in treated retinas compared with untreated controls (I) (***P < 0.001, two-tailed unpaired t test). For further description of response kinetics, see Fig. S4.
Fig. S4.
MEA recordings from treated retinas demonstrate a range of response kinetics. Four months after subretinal injection of OPN4 vector, multielectrode array (MEA) recordings were performed on ex vivo retinas of treated eyes and age-matched untreated controls. Following completion of recordings, DsRed fluorescence was visualized in treated eyes with the retina in situ over electrodes. This allowed areas of retina transduced by vector to be identified (A, the image shown is a composite illustrating the area covered by one treated retina overlying the MEA electrodes; arrow indicates dots that are DsRed-expressing cells). To examine duration of response, the time to half decay [time from maximal firing rate to half-response (t1/2)] of light responses recorded from treated retina following a 60-s light pulse of 1.3 × 1015 photons⋅cm−2⋅s−1 intensity was examined. This ranged from 6 to 147 s (mean, 51.1 ± 5.5 s; n = 54) and was not significantly different from values obtained from untreated controls where responses ranged from 14 to 144 s (mean, 64.8 ± 8.2 s; n = 25; P = 0.165, two-tailed unpaired t test). Time to half-decay was shorter following light pulses of 2-s duration, typically lasting 1–24 s in treated retina (mean, 6.9 ± 1.3 s; n = 16) and 1–12 s in untreated retina (mean, 6.4 ± 1.9 s; n = 5; P = 0.84, two-tailed unpaired t test). Responses with transient (B) and sustained kinetics (C) were also seen following a 2-s light stimulus. Examples of raw data obtained from individual electrodes recorded from a treated retina are shown following 480-nm light pulses of 2-s duration that were repeated every 60 s.
Irradiance response curves (IRCs) generated for responsive electrodes in retinas following vector delivery (n = 51 electrodes, n = 3 retinas) versus controls (n = 22 electrodes, n = 4 retinas) revealed similar overall light sensitivity between groups (Fig. 2F): with half-maximal responses (EC50) observed at 13.4 ± 0.11 log photons⋅cm−2⋅s−1 in electrodes from treated retina versus 13.2 ± 0.16 in controls (P = 0.26, two-tailed unpaired t test). However, at subsaturating levels of light (3.99 × 1014 photons⋅cm−2⋅s−1), the maximal spike firing rate was significantly higher on responsive electrodes from treated retinas (80.0 ± 6.2 Hz, n = 54) versus untreated controls (44.6 ± 5.4 Hz; n = 25; P = 0.0006, two-tailed unpaired t test; Fig. 2 G and H). A range of response kinetics was observed for both treated and untreated groups, including transient and sustained changes in action potential firing (Fig. 2B and Fig. S4 B and C). At subsaturating levels of light (3.99 × 1014 photons⋅cm−2⋅s−1), the time taken to reach maximal firing rate was significantly lower on electrodes from OPN4-treated retinas (6.63 ± 0.5 s; n = 54) compared with untreated controls (16.9 ± 2.9 s; n = 25; P < 0.0001, unpaired t test; Fig. 2I), indicating different response kinetics between ganglion cells firing in OPN4-treated retinas versus responses recorded from native ipRGCs in untreated controls.
Visual Function Restored by Human Melanopsin Expression in the Degenerate Retina Was Sustained at 13 mo.
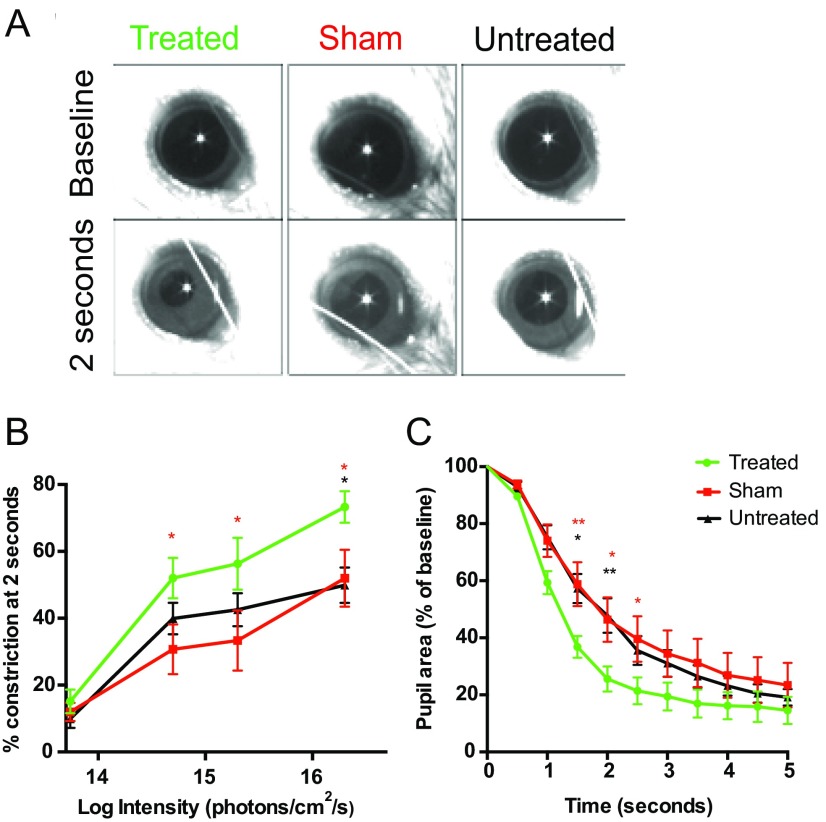
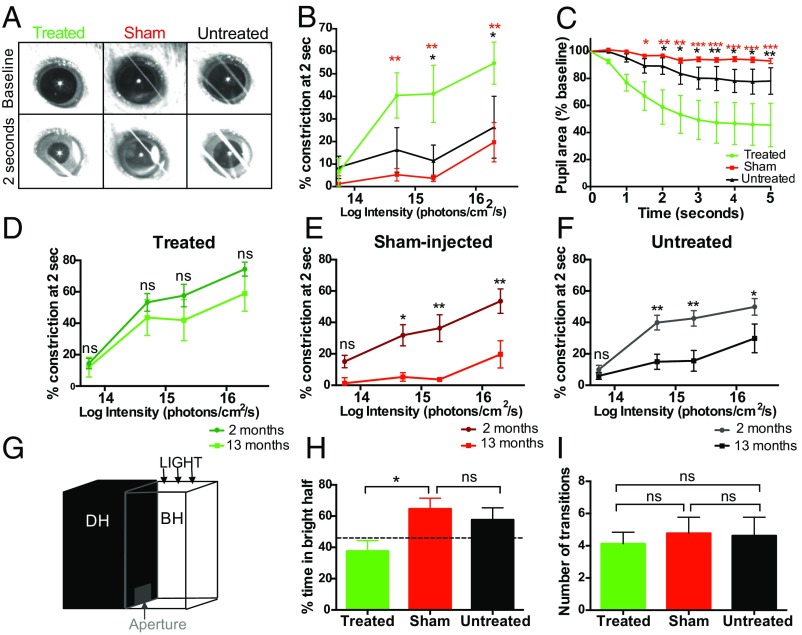
The pupil light reflex (PLR) was evaluated to determine whether light-dependent signals generated by ectopic OPN4 expression could affect central targets. Mice that received a unilateral subretinal injection of OPN4 vector were compared with age-matched mice that received a sham injection of PBS and also with untreated controls. The consensual PLR was assessed to avoid any surgical effect on pupil constriction in the treated eye. Two cohorts of mice were evaluated, one at 2 mo after injection (Fig. S5) and another at 13 mo to determine long-term functional improvement. Thirteen months after OPN4 vector delivery, pupil constriction was significantly greater in treated eyes compared with controls at multiple light intensities (overall treatment effect, P = 0.013, repeated-measures two-way ANOVA; Fig. 3 A and B). Pupil constriction was faster in the OPN4 vector group compared with sham-injected and untreated groups (interaction between treatment and time on pupil area: P < 0.0001, repeated-measures two-way ANOVA; Fig. 3C). Furthermore, pupil constriction was significantly reduced in older sham-injected (effect of age, P = 0.0018, repeated-measures two-way ANOVA) and untreated (P = 0.0067) control groups compared with their younger counterparts (Fig. 3 E and F). This effect was not seen in the OPN4 vector-treated group (P = 0.217; Fig. 3D), indicating a sustained treatment effect in the older cohort following OPN4 vector delivery.
Fig. S5.
Pupil constriction is greater in rd1 mice treated with OPN4 vector 2 mo previously compared with controls. The consensual pupil light reflex (PLR) was assessed following stimulation of the left eye using a white-light stimulus of 2-s duration. Representative images of pupil size at baseline and at 2 s after light onset are shown for eyes treated by OPN4 vector, sham-injected, and untreated controls (A). Irradiance response curves were generated following analysis of pupil constriction at 2 s after light onset. This early time point was chosen to help distinguish any response seen from the contribution of ipRGCs to the PLR, the latter contributing most to the sustained pupil constriction that occurs following light offset. Irradiance response curves demonstrate greater pupil constriction in the OPN4 vector-treated group (green) compared with sham-injected (red) and untreated controls (black) at multiple light intensities [B; effect of treatment (P = 0.04) and light intensity (P < 0.0001) on pupil constriction, repeated-measures two-way ANOVA with Tukey post hoc test]. Pupil constriction at 2 × 1016 photons⋅cm−2⋅s−1 was also faster in the treated group compared with controls (C; P = 0.0009, interaction between treatment and time on pupil area, repeated-measures two-way ANOVA with Tukey post hoc test). Treated, n = 10; sham, n = 9; untreated, n = 12; *(red) denotes significance between treated and sham, and *(black) between treated and untreated; *P < 0.05, **P < 0.01.
Fig. 3.
Visual function restored by human melanopsin expression in the degenerate retina was sustained at 13 mo. Representative images show levels of pupil constriction observed 13 mo after human OPN4 vector delivery, compared with age-matched sham-injected and untreated controls (A). Significantly more pupil constriction was observed in mice treated with OPN4 vector compared with sham-injected (red*) and untreated controls (black*), at multiple light intensities (B) measured at 2 s after light onset (treated, n = 5; sham-injected, n = 8; untreated, n = 13). Time course of pupil constriction at 2 × 1015 photons⋅cm−2⋅s−1 demonstrated a faster response in the treated group (C). Pupil constriction was not significantly different in treated mice (D) at 2 and 13 mo, whereas the level of pupil constriction declined with age in sham-injected (E) and untreated mice (F) (2-mo cohort: treated, n = 10; sham-injected, n = 9; untreated, n = 12; ns, nonsignificant; *P < 0.05, **P < 0.01, repeated-measures two-way ANOVA with Tukey post hoc test). In the behavioral light–dark assay, mice could move freely between the bright half (BH) of the test chamber illuminated by a white light stimulus of 200 lx at ground level and the dark half (DH) (G). Mice treated with OPN4 vector 13 mo previously spent less time in the bright half of the chamber compared with sham-injected controls (H) (treated, n = 8; sham-injected, n = 9; untreated, n = 8; P = 0.03, one-way ANOVA with Tukey post hoc test). The number of transitions between compartments was similar across groups (I).
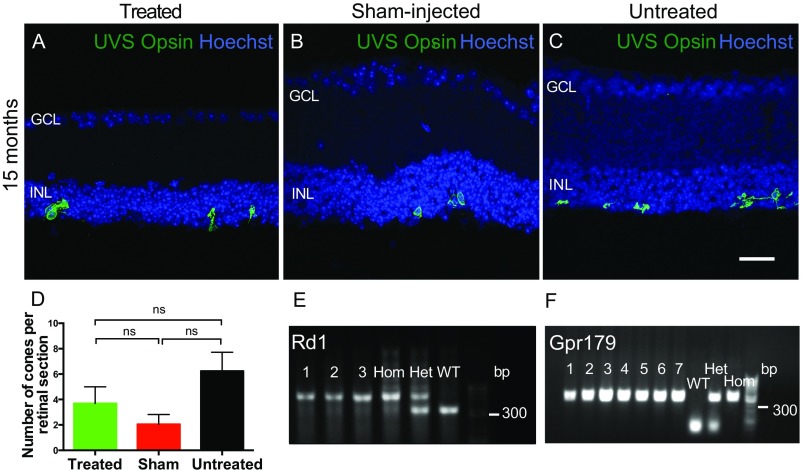
To assess whether information generated by ectopic expression of OPN4 in the retina could drive visually guided behavior, animals were assessed using a behavioral light avoidance assay (24, 25) based on the natural preference of mice to avoid brightly lit environments (Fig. 3G). Thirteen months after OPN4 vector delivery, there was a significant difference in the percentage of time spent in the brightly lit chamber between groups (treated, 37.65% ± 6.7; sham-injected, 64.66% ± 6.8; untreated, 57.65% ± 7.7; P = 0.03, one-way ANOVA; Fig. 3H), with the vector-treated group showing behavior closest to wild-type mice. This was not due to a difference in general or anxiety-related locomotor activity, since the number of transitions between light and dark chambers was similar between groups (P = 0.88, one-way ANOVA; Fig. 3I). Functional effects seen were unlikely to be mediated by residual cones in the rd1 mouse, since these cells were morphologically abnormal and there was no difference in numbers of remaining cells between treated, sham-injected, and untreated groups (Fig. S6 A–D; P = 0.123, one-way ANOVA). Furthermore, all rd1 mice selected were homozygous for the gpr179 mutation (Fig. S6 E and F), excluding any input from residual photoreceptors in mediating ON bipolar cell depolarization via the mGlur6 cascade in this mouse model (26).
Fig. S6.
Restoration of visual function is not mediated by residual cones. Degeneration of cones in the rd1 model is slower than that of rods, and to assess whether any functional differences seen between groups might be attributed to residual cones, retinal sections were immunolabeled with antibody for UV/S-cone (UVS) opsin (green) in OPN4 vector-treated (A), sham-injected (B), and untreated (C) eyes from mice 15 mo after injection. Images are overlaid with Hoechst labeling of nuclei (blue). Few cones remained in any group, with loss of outer segments and mislocalization of opsin to the cell body, indicating that these cells are unlikely to be functional. There were no significant differences in cone number between groups (D; P = 0.123, one way ANOVA), suggesting that these cells were not responsible for any group differences in behavioral or functional testing. Treated, n = 14; sham-injected, n = 8; untreated, n = 12. ns, nonsignificant. (Scale bar, 25 μm.) All mice were genotyped for the rd1 mutation before use using a duplex PCR protocol (E). Samples from several mice are shown (1–3), with homozygous, heterozygous, and wild-type controls indicating that all mice used were homozygous for the rd1 allele. C3H-derived mouse lines are known to carry a mutation in gpr179, a G-protein receptor required for bipolar cell depolarization in conjunction with the mGluR6 cascade to gate TRPM1 cation channels (26). All mice were genotyped and found to be homozygous for the gpr179 mutation (F). Therefore, residual photoreceptors in these mice would not have been able to drive ON bipolar cell depolarization, or any functional responses via this pathway.
Visual Responses Requiring Image-Forming Vision Are Generated Following Melanopsin Gene Therapy in the Degenerate Retina.
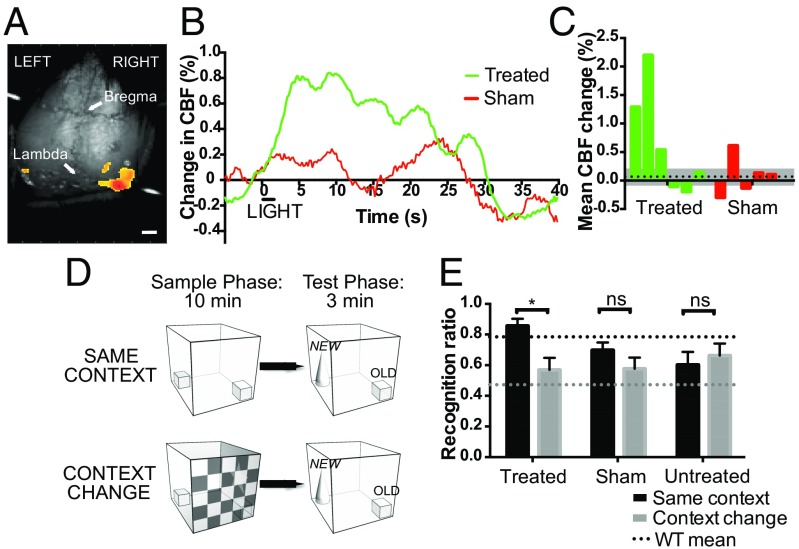
Pupillometry and behavioral light avoidance indicated that subretinal OPN4 delivery restored or improved light responses; however, signaling to the visual cortex is not necessary to mediate such effects. To study cortical responses, we examined light-induced changes in visual cortex blood flow using laser speckle contrast imaging (Fig. 4A). Six months after subretinal delivery of OPN4, both eyes were stimulated by 480-nm light of 2-s duration and cerebral blood flow (CBF) recorded over the visual cortices. Treated mice showed an increase in CBF with an initial peak at 5.4 s after light onset, consistent with the peak expected in wild-type animals (25) (Fig. 4 B and C and Fig. S7A). There was no clear corresponding initial peak in the sham-injected group (Fig. S7B).
Fig. 4.
Visual responses requiring image-forming vision are generated following melanopsin gene therapy in the degenerate retina. Laser speckle cortical imaging was used to measure changes in visual cortex blood flow following a 2-s, 480-nm light stimulus of 2,000-lx intensity (A). (Scale bar, 1 mm.) OPN4 vector-treated mice showed an appropriate light-dependent peak in cortical blood flow (CBF) compared with sham-injected controls (B; treated, n = 5; sham-injected, n = 4). The mean percentage change in CBF for each animal in the first 10 s after light onset is shown (C). The dashed line indicates mean change in sham-injected animals with SEM (gray). Visual context recognition testing (D): in the “same context” condition, the visual environment is identical in both phases. In the “Context Change” condition, there is a change in visual environment between phases, but all other factors are constant. The recognition ratio is calculated as N/(N + f), that is, time spent exploring the novel object (N) as a fraction of the total time spent exploring both the novel and a familiar (f) object. Thirteen months after OPN4 vector delivery, recognition ratios for the novel object were significantly different in treated mice when the visual environment was the same versus when it was changed, indicating a visual environment-dependent change in behavior (E) (P = 0.04, two-way repeated-measures ANOVA with Bonferroni post hoc test; dashed line, wild-type mean, *P < 0.05; treated, n = 9; sham-injected, n = 8; untreated, n = 11; ns, nonsignificant).
Fig. S7.
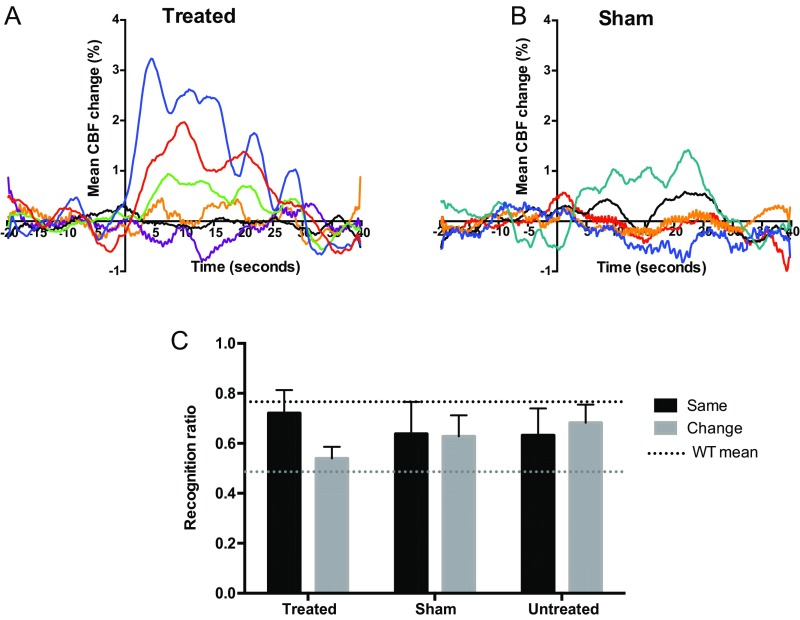
Melanopsin gene therapy leads to visual responses requiring image-forming vision. Blood flow changes in the visual cortex were measured for 20 s in darkness, and then a 2-s, 480-nm light stimulus was applied to both eyes of OPN4 vector-treated (n = 6) and sham-injected (n = 5) mice (light stimulus onset at 0 s). Examination of cortical blood flow responses detected using laser speckle cortical imaging from individual animals revealed three OPN4 vector-treated animals (A) with appropriate early peaks in blood flow following the light stimulus but no sham-injected control animals (B) showing characteristic responses. Visual context recognition testing was performed 2 mo after treatment (C). Recognition ratios for the novel object are shown when both sample and test phases were performed in the same visual environment (black bar) or when there was a change in environment (gray bar). There was a trend for treated mice to show visual context-dependent behavior (mean recognition ratio 0.72 ± 0.09 Same versus 0.54 ± 0.04 Context Change); however, when the younger cohort was analyzed alone, this did not reach statistical significance (two-way repeated-measures ANOVA with Bonferroni post hoc test). No such trend was seen in sham-injected or untreated mice: the level of performance was similar in the Same and Context Change conditions in these groups (sham-injected group mean recognition ratio 0.64 ± 0.13 Same versus 0.63 ± 0.08 Context Change; untreated group mean recognition ratio 0.63 ± 0.11 Same versus 0.68 ± 0.07 Context Change). Treated, n = 12; sham-injected, n = 10; untreated, n = 12; dashed line, wild-type mean. Post hoc power analyses revealed that the observed power of the visual restoration effect was lower in the younger cohort, suggesting that the likelihood of replicating the treatment effect is higher in older animals. However, when both younger and older cohorts were combined in a split-plot ANOVA, the effect of visual context on behavior in the treated group overall was highly significant (P = 0.003), with no significant interaction between treatment and age of the animals (P = 0.445) (Tables S1 and S2).
Finally, we assessed whether ectopic expression of OPN4 would aid image-forming vision. For this purpose, we used the one-trial spontaneous object recognition test (27, 28). In wild-type rodents with no retinal degeneration, a change in visual environment disrupts object recognition, indicating that these animals encode and remember the background visual environment in which an object is encountered (27, 28). By contrast, mice with visual deficits are not able to detect the visuospatial context of an object (29), indicating that they cannot encode visual information regarding their environment. Object recognition performance was analyzed to determine visual context recognition: this was determined by the ratio of time spent exploring a novel object relative to a previously encountered object. Two cohorts of mice were evaluated, one at 2 mo (Fig. S7C) and another at 13 mo after injection (Fig. 4D). Following OPN4 vector delivery 13 mo previously, treated mice showed a significant change in recognition ratio dependent on their visual environment (P = 0.04 for effect of visual context on object recognition performance; two-way ANOVA with Bonferroni post hoc test; Fig. 4E). Combining younger and older cohorts, the effect of visual context on behavior was highly significant in the treated group overall (P = 0.003, split-plot ANOVA; Tables S1 and S2). No significant changes were seen in sham-injected or untreated control groups, suggesting an inability to form and retrieve an association between the object and visual context in these mice. Melanopsin vector-treated mice showed a behavioral pattern similar to that observed in wild-type mice with functional rods and cones in a test requiring image-forming vision.
Table S1.
Additional analyses: Split-plot ANOVA with Age (Young versus Old) and Visual Context (Same Context versus Context Change) as factors
| Group | Main effect of Age | Main effect of Visual Context | Age × Visual Context interaction |
| Treated | F(1,19) = 1.140 | F(1,19) = 11.928 | F(1,19) = 0.609 |
| P = 0.299ns | P = 0.003** | P = 0.445ns | |
| Sham | F(1,16) < 0.005 | F(1,16) = 0.424 | F(1,16) = 0.299 |
| P = 0.986ns | P = 0.524ns | P = 0.592ns | |
| Untreated | F(1,21) = 0.103 | F(1,21) = 0.398 | F(1,21) = 0.003 |
| P = 0.751ns | P = 0.535ns | P = 0.954ns |
ns, nonsignificant. **P < 0.01 (statistically significant result highlighted in bold).
Table S2.
Additional analyses: A priori and post hoc power analyses in G*Power
| Type | Input parameters | Output | ||
| Assumed effect size of the treatment effect (partial η2) | α | Assumed power (1 − β) | ||
| (1) A priori power analysis | 0.542† | 0.05 | 0.95 | Total N required = 12 |
| (2) Post hoc power analysis in cohort | Observed effect size of the treatment effect (partial η2) | Total N | ||
| (a) Old | 0.185 | 0.05 | 28 | Achieved power = 0.911 |
| (b) Young | 0.053 | 0.05 | 34 | Achieved power = 0.377 |
Discussion
Data presented here demonstrate that a functional human melanopsin gene (OPN4) can be delivered to remaining retinal cells in a mouse model of end-stage retinal degeneration. This was achieved via subretinal injection of an AAV vector using a ubiquitous promoter, an approach currently validated in AAV gene therapy clinical trials (15, 16). Ectopically expressed OPN4 mediated depolarization of outer retinal cells and ultimately ganglion cell action potential firing, resulting in long-term restoration of the PLR and behavioral light avoidance up to at least 13 mo following injection. Finally, subretinal OPN4 expression led to light-induced changes in visual cortex blood flow and provided long-term improvements in a visually guided behavioral task that requires image-forming vision. In combination, these results suggest that this approach may be clinically useful in vision restoration in patients with end-stage RP.
In the interpretation of these data, a consideration is the mechanism by which visual responses were restored. We believe that responses detected arose from activation of retinal circuitry involved in image-forming vision rather than augmenting existing ipRGCs for several reasons. Human melanopsin was not detected by immunohistochemistry in ganglion cell membranes in transduced retinas. Similarly, light-induced c-Fos expression in vector-treated areas of retina was seen in multiple cells within the INL, whereas this pattern was not seen in the INL of controls. MEA recordings revealed a greater percentage of responsive electrodes in treated retinas compared with untreated controls, along with differences in firing rates and response kinetics, suggesting that a larger number of ganglion cells were generating light-induced action potentials in treated retinas.
To assess functional responses in vivo, a number of assessments were used including behavioral light avoidance since wild-type animals with functional rods and cones show aversion to bright light. Treated mice spent less time in the bright chamber compared with control mice, which showed an apparent preference for the bright chamber. This may be due to an inability of control mice to detect the difference in brightness between the two chambers resulting in exploration being guided primarily by nonvisual cues, for example, subtle differences in temperature, auditory, or olfactory cues. Interestingly, previous work including rodless/coneless mice has also demonstrated that mice have a preference for the front half of the chamber irrespective of whether the animal was placed there first or the test was performed in complete darkness (24).
Treated mice showed the ability to form and retrieve an association between an object and its visual environment in the visual context recognition task. In contrast, control groups were able to perform the object recognition task using nonvisual cues (since recognition ratios in these mice were above chance or 0.5), but performance did not vary according to visual environment. This test has been validated for the assessment of rod/cone-dependent image-forming visual responses (29). Previous work has also investigated the effect of changes in background irradiance on performance with wild-type mice requiring a substantial change in irradiance (e.g., an increase from 10 to 350 lx) to disrupt object recognition performance (29). Therefore, context-dependent behavior in treated mice is likely to be caused by the change in visual environment, rather than any changes in background irradiance caused by the different test arenas.
A potential concern is the effect of retinal remodeling seen in end-stage degeneration on restoration of visual function. Multiple changes within the remaining retina have been described in mouse models and human tissue (30–33), including neuronal morphological changes, cell death, network rewiring, and formation of gliosis between the retinal pigment epithelium and neural retina. Certain elements of remodeling such as the glial seal may potentially be overcome by subretinal injection, since the hydrostatic force generated could allow AAV to penetrate areas of gliosis. The process of remodeling does, however, demonstrate plasticity (30), and since we use a ubiquitous promoter to deliver melanopsin to the degenerate retina, it is possible that some of these abnormal connections from a variety of cells are used to restore visual responses. The clinical phenotype in human RP is of a rod–cone dystrophy in the majority of patients, which may also be variable with previous studies demonstrating differing degrees of degeneration even in the presence of the same genetic mutation and level of vision (34). Careful selection of potential patients for OPN4 optogenetic therapy would therefore be required since the ideal candidate would have severely affected vision yet grossly intact inner retinal structure (as visualized by ocular coherence tomography) and some remaining inner retinal function, detected for example using electrical phosphene testing (34, 35).
Human melanopsin as an optogenetic tool has significant advantages in its suitability for translation to patients. Being a native protein, OPN4 is unlikely to induce an immune response, and second, OPN4 shows greater light sensitivity than other optogenetic tools. We detected ganglion cell firing during MEA recordings at the lowest stimulus intensity tested (1.20 × 1012 photons⋅cm−2⋅s−1), whereas previous reports document a minimum stimulus of 1 × 1014 (36) or 1 × 1015photons⋅cm−2⋅s−1 (3) for detection of ganglion cell responses following channelrhodopsin gene therapy and 1 × 1016 for halorhodopsin (4). We also report restoration of visually guided behavior in the visual context recognition task at a light intensity of 50 lx, corresponding to low-level indoor lighting. The brightest light stimulus we used was 2 × 1016 photons⋅cm−2⋅s−1 during pupillometry (however, significant differences between groups were seen at lower intensities) or ≈13,000 lx (Table S3) equivalent to daylight conditions. The brighter stimulus intensity used for pupillometry in our experiments compared with previous studies using melanopsin likely reflects the use of different animal models (37) and our use of a 2-s white light stimulus (chosen to be a more useful stimulus for image-forming vision), whereas other studies used monochromatic light (37) and longer stimulus durations more suited for activating ipRGCs (5).
Table S3.
Light stimuli used for experiments
| Photon flux, photons⋅cm−2⋅s−1 | Irradiance, lx | ||||
| Experiment | Light source | Min | Max | Min | Max |
| MEA | 480-nm monochromatic | 1.2 × 1012 | 1.34 × 1015 | 0.5 | 561 |
| c-Fos | White LED | 3 × 1014 | 350 | ||
| Pupillometry | White xenon arc lamp | 5.5 × 1013 | 2.0 × 1016 | 13,000 | |
| Behavioral light avoidance | White fluorescent | 1.7 × 1014 | 200 | ||
| Laser speckle cortical imaging | Blue LED | 5 × 1015 | 2,000 | ||
| Object recognition | White fluorescent | 4.1 × 1013 | 50 | ||
MEA, multielectrode array recordings. Values in bold are those measured during experiments. Other values are calculated to allow comparison of light intensities between experiments using the rodent irradiance toolbox and are approximate (44).
The quality of vision that might be restored by OPN4 gene therapy is likely to be affected by its response kinetics. Although slower than classical (rod/cone) photopigments, the detection of transient light responses following melanopsin stimulation (Fig. S4B) as previously described (38) may be a useful input for image-forming vision. Furthermore, not all ganglion cell spikes are transmitted at the retinogeniculate synapse allowing for modification of the visual signal (39). Melanopsin gene therapy may provide functionally useful vision in a static visual environment such as in the visual context recognition test, which may be useful to patients in terms of aiding navigation. For more dynamic environments, modification of the visual input through devices such as image-processing glasses (40) may be required.
The use of human melanopsin delivered via subretinal injection using a CBA promoter for optogenetic restoration of vision has not previously been described. We demonstrate effective transduction of end-stage degenerate retina resulting in sustained restoration of visual function. The effects may be mediated by transduction of bipolar and horizontal cells. This approach has significant potential for translation to patients since, although technically more challenging, subretinal delivery has been established as safe in current clinical trials and provides the advantage of delivering a high concentration of vector to residual retinal cells, whereas the alternative method of intravitreal delivery may not be as effective in humans. Targeting the outermost surviving retinal layers will likely allow greater levels of signal processing to be performed by existing retinal circuitry, potentially resulting in restoration of more complex visual responses.
Materials and Methods
All animal experiments were conducted as part of a programme of work assessed by the Clinical Medicine Animal Welfare and Ethics Review board of the University of Oxford and legally approved by the U.K. Home Office. They were also conducted in accordance with Association for Research in Vision and Ophthalmology statements on care and use of animals in ophthalmic research. C3H/HeNHsd-Pde6brd1 (rd1) mice were 6–8 wk old at time of intraocular injection. Treated eyes were injected with a dose of 1.5 × 109 viral genomes (vg) per eye of AAV2/8(Y733F) CBA-OPN4-IRES-DsRed vector, with an equivalent volume of PBS injected in sham-treated eyes and age-matched untreated eyes also used as controls. Further experimental details are described in SI Materials and Methods, Tables S4 and S5.
Table S4.
Antibodies used for immunohistochemistry: Primary antibodies
| Staining | Antigen | Host | Source (product code) | Dilution |
| UVS opsin | N terminus of human OPN1SW | Goat | Santa Cruz (sc-14363) | 1:1,000 |
| Calbindin | Purified bovine kidney calbindin-D-28K | Mouse | Abcam (ab82812) | 1:500 |
| PKCα | C terminus of PKCα | Mouse | Abcam (ab11723) | 1:500 |
| Brn-3a | N terminus of human Brn-3a | Goat | Santa Cruz (sc-31984) | 1:250 |
| Human Opn4 | C terminus of human Opn4 | Rabbit | Santa Cruz (sc-32870) | 1:250 |
| c-Fos | Total human c-Fos protein | Rabbit | Cell Signaling Technology (2250S) | 1:200 |
PKCα, protein kinase Cα; Opn4, melanopsin. Abcam; Santa Cruz Biotechnology.
Table S5.
Antibodies used for immunohistochemistry: Secondary antibodies
| Reactivity | Host | Fluorescent label | Source (product code) | Dilution |
| Rabbit | Donkey | Alexa Fluor 488 | Invitrogen | 1:250 |
| Goat | Donkey | Alexa Fluor 555 | Invitrogen | 1:250 |
| Rabbit | Donkey | Alexa Fluor 633 | Invitrogen | 1:250 |
| Mouse | Donkey | Alexa Fluor 647 | Invitrogen | 1:250 |
SI Materials and Methods
Mice.
C3H/HeNHsd-Pde6brd1 (rd1) mice were purchased from Harlan Laboratories. By age 6–8 wk in this model, rod degeneration is virtually complete, with ongoing cone degeneration [the latter comprising only 3% of wild-type mouse photoreceptors, with a variable rate of degeneration in the rd1 mouse (21, 41)]. Wild-type mice of the same background strain without the rd mutation (non-rd C3H mice) were bred at the University of Oxford. Animals were kept under a 12-h light (<100 lx)/dark cycle, with no restriction on food and water.
For injection and laser speckle cortical imaging procedures, animals were anesthetized by i.p. injection of 1 mg/kg medetomidine (Dormitor, 1 mg/mL; Pfizer) and 60 mg/kg ketamine (Ketaset, 100 mg/mL; Fort Dodge). For procedures requiring pupil dilation, tropicamide (1%) and phenylephrine (2.5%) eye drops (both Bausch & Lomb) were used. All in vivo testing and tissue collection was performed during the day phase of the light/dark cycle, except for c-Fos induction.
Intraocular Injections.
Injections of mice aged 6–8 wk were performed tangentially through the sclera using a 10-mm 34-gauge needle (Hamilton AG) mounted on a 5-μL syringe (65 RN; Hamilton AG) under direct visual control using a surgical microscope (M620; Leica). A circular cover glass (Ø, 6 mm; VWR International) was placed on the cornea with a coupling gel (Viscotears; Novartis) to ensure good fundal view. A volume of 1.5 µL of viral vector solution (1.0 × 1012 vg/mL; i.e., dose of 1.5 × 109 vg) was injected into each eye. Age-matched mice had a sham injection of PBS to control for surgical effect of subretinal injection.
Viral Vectors.
Adeno-associated viral (AAV) vectors were produced using standard protocols. To produce the CMV.CBA-OPN4-IRES-DsRed construct, PCRs were performed to join the CMV enhancer/CBA promoter (isolated from a pUf6.1 plasmid; gift from Vince Chiodo, University of Florida, Gainesville, FL) to human OPN4. The IRES sequence from pIRES2-AcGFP plasmid (Clontech) was joined to DsRed (from pSIREN-DNR-DsRed-Express plasmid; Clontech) and the full construct created by a PCR in which MfeI and NotI restriction sites were inserted. The full CMV.CBA-OPN4-IRES-DsRed construct was inserted into an AAV production plasmid using MfeI and NotI restriction sites, containing wild-type AAV2 inverted terminal repeat (ITRs), a woodchuck hepatitis virus posttranscriptional regulatory element (WPRE) and bovine growth hormone polyadenylation signal, identical to sequences used in the ongoing choroideremia gene therapy clinical trial (16). After cloning, presence of the construct was confirmed by full-length sequencing (Source Bioscience) and ITR integrity by XmaI digestion. The vector was custom packaged into the single capsid mutant rAAV2/8(Y733F) vector by Genedetect. Viral titer determined by qPCR to a region within OPN4, was 1 × 1013 vg/mL.
Retinal c-Fos Expression.
Mice were placed in a light tight cabinet in open top cages and maintained on a 12-h light–dark cycle for 3 d. On the day of testing, 4 h after lights off (i.e., ZT 16), mice were pulsed with white light produced by LED light sources of 350-lx intensity measured at floor level for 30 min. Thirty minutes after the light stimulus ended, mice were killed and eyes fixed in 4% paraformaldehyde. Levels of retinal c-Fos expression were assessed using immunohistochemistry. For analysis, images of retinal sections were taken at 20× magnification using identical acquisition settings on a confocal microscope (LSM710; Zeiss) and cell counts and quantification of fluorescence intensity performed on 8-bit images using ImageJ software.
Multielectrode Array Recordings.
Following enucleation, retina were dissected under dim red light (>610 nm) at room temperature in Ames’ media bubbled with 95% O2/5% CO2 (pH 7.3) (Sigma-Aldrich). Retinas were then transferred to glass-bottomed multielectrode array (MEA) chambers (Multi Channel Systems), with ganglion cell side facing down and held in position using glass-coated metal harps (ALA Scientific Instruments). MEA chambers (containing 60 electrodes, each 30 μm in diameter and spaced 200 μm apart) were placed into the MEA recording device (MEA1060-Inv; Multi Channel Systems), fitted with a gas-permeable perfusion manifold (ALA Scientific Instruments), and positioned on the stage of an inverted Olympus IX71 microscope so that recording electrodes were located in the microscope light path. Retinas were perfused with Ames’ media bubbled with 95% O2/5% CO2 (pH 7.3) and maintained at 34 °C. This was done using a combination of a water bath heater (36 °C), in-line perfusion heater (35 °C), and base plate heater incorporated into the MEA system (34 °C) to minimize variations in temperature within the sample chamber. Recorded signals were collected, amplified, and digitized at 25 kHz using MC Rack software (Multi Channel Systems).
Retinas were perfused for 30 min in darkness before recording responses. A light stimulus of 60-s duration was used unless stated, and retinas were dark adapted for 20 min between recordings. A 480-nm (bandwidth, 20 nm) monochromatic light stimulus was generated by a xenon arc light source with a slit monochromator (Cairn Optoscan). This was delivered via 10× microscope objective beneath the MEA chamber. The duration and wavelength of light stimuli were controlled by Metafluor software (Molecular Devices). Neutral density filters (0–4 log units; Thor Labs) were used to control the intensity of light stimuli and were controlled via an automated filter wheel in the light path (Prior Scientific). Power of light stimuli (in microwatts per square centimeter per second) was measured at the sample focal plane using an in-line power meter (PM160T; Thor Labs).
Data were analyzed using MC Rack software (Multi Channel Systems). Action potentials were counted as electrical activity greater than 3 SDs of baseline activity and number of action potentials/second plotted for analysis. A light response was defined as change in firing rate of 5 Hz or greater between 10 s before light onset compared with 10 s after light onset. Electrical activity was not seen in every electrode since the retinal explant may not have covered it or made good contact; therefore, the percentage of electrodes that showed electrical activity is stated per experiment. Five light intensities (1.20 × 1012, 1.33 × 1013, 1.37 × 1014, 3.99 × 1014, and 1.34 × 1015 photons⋅cm−2⋅s−1) were used to generate irradiance response curves for each electrode, which were fitted with sigmoidal dose–response function (variable slope and EC50).
Laser Speckle Cortical Imaging.
Mice were dark adapted for 2 h before imaging, which took place in a dark room. The temperature of the mouse was monitored following anesthesia and maintained via a rectal temperature probe and heat pad. Recordings were only performed when core body temperature was 36–37 °C to ensure optimal conditions for recording physiological blood flow changes. Pupil dilation was administered as above, the head fixed in a stereotaxic frame, and a midline scalp incision made to reveal the cranium. Cranial landmarks could be visualized following hemostasis, enabling the area anterior to lambda, that is, the location of the visual cortex to be identified. The light stimulus comprised two blue (480-nm) LEDs positioned on either side of the mouse’s head of total intensity of 2,000 lx. A trial run was done without the mouse in situ to ensure that this light stimulus did not affect image capture.
Both eyes of treated mice (n = 6) and sham-injected mice (n = 5) were stimulated simultaneously by a 2-s light pulse with a 1-min interstimulus interval. Ten stimuli were administered to each animal and signal averaged to maximize signal-to-noise ratio. Blood flow changes were captured using the Speckle Contrast Imager (moorFLPI 2; Moor Instruments; 785-nm laser with 50-mW laser power) with 580 × 752-pixel images acquired at 5 frames/s. Data were processed using moorFLPI Review software and analyzed with MATLAB R2012a, version 7.14.0.739 (MathWorks), and Origin Pro8.6 (OriginLab). Time series of CBF changes were extracted from anatomically defined regions of interest corresponding to bilateral visual cortices. Percentage change in CBF was calculated for each trial with respect to prestimulus baseline values.
Pupillometry.
Experiments were done during the light phase of the light–dark cycle. Following dark adaptation for 2 h, an unanesthetized mouse was held with its left eye in front of a Ganzfeld sphere connected to a 100-W xenon arc lamp (LOT-Oriel) via a light pipe and right eye in front of an infrared digital camera (Cohu) to record the consensual pupil response. A 2-s white light stimulus was used with neutral density filters to generate four different light intensities: 5.5 × 1013, 5.0 × 1014, 2.0 × 1015, and 2.0 × 1016 photons⋅cm−2⋅s−1. The dimmest was tested first with a minimum interval of 20 min between stimuli during which the animal was kept in darkness. Pupil area was measured from images using ImageJ at 2 s after light onset and normalized to baseline size for generation of irradiance response curves. For time course curves, pupil area was measured at 500-ms intervals and normalized to baseline.
Behavioral Light–Dark Avoidance.
Mice were tested in a 26 × 26 × 26-cm box, containing equally sized light and dark chambers connected by a 4 × 5-cm opening via which animals could move freely. The bright half of the box was illuminated from above by white fluorescent light with intensity of 200 lx measured at floor level. Mice were light adapted and tested during the light phase of the light–dark cycle. Animals were placed in the bright half facing away from the opening to the dark half, the lid of the chamber closed, and movement recorded (Logitech HD webcam; Logitech). A trial lasted 3 min, and then testing apparatus was dismantled and cleaned with 70% ethanol. Videos were analyzed using ANY-maze tracking software and validated by comparison with manual analysis. Time spent in the bright half and number of four-paw transitions between chambers were recorded. Mice that did not transition between chambers during the trial were excluded from analysis.
Object Recognition Testing.
This task assessed whether mice could detect a change in visual environment independent of ambient light levels. The basis of this is an object recognition task, where initially a mouse is allowed to explore two identical replicates of an object (sample phase). After a short delay, the mouse is placed into a testing arena with one replicate of the object previously encountered and another novel and distinct object (test phase). Animals often spend more time exploring the novel object, and this preference is evidence that the other object has been recognized as familiar. A visual context recognition task can be incorporated into this test since animals detect the background context in which they encounter an object. For example, when a familiar object is encountered in an unfamiliar environment with all other factors being constant, animals tend to perceive the object as novel and spend more time exploring it than if encountered in its original context (27–29). However, if an animal is unable to form an association between the object and its visual context, the task will be performed using nonvisual cues and will not be influenced by visual environment.
Animals were tested in the light phase of the light–dark cycle. The sample phase was carried out either in (i) a 30 × 30-cm box made of white acrylic (“Same” condition) or (ii) a box with a checkerboard pattern with alternating 4 × 4-cm black-and-white squares on one wall and a white symmetrical five-point star (drawn within a notational circle with a diameter of 14 cm) on a black background on another wall (“Change” condition). The sample and test arenas were illuminated by a white fluorescent light of 50-lx intensity measured at floor level in the center of the arena. This level of illumination was chosen to be consistent with previous studies since bright-light stimuli disrupt object recognition performance in wild-type mice (29). When the lux meter was placed in the corner of the two patterned walls in the “Context Change” test arena, the reading varied by only 5–10 lx. Previous published data (29) showed that a change in performance level in wild-type mice was found only when there was a substantial increase in background irradiance (e.g., from 10 to 350 lx); therefore, this relatively subtle change in brightness (5–10 lx at the corner of the arena) would not significantly affect performance.
Each box contained two identical copies of an object placed in each corner, and the mouse was allowed to freely explore these objects for the duration of the sample phase (detailed descriptions of the test arenas and objects used can be found in ref. 29). Movement was recorded using a camera suspended from above (Sentient Mininight vision CCTV camera; Maplin). The mouse was then placed in its home cage for 5 min (delay phase) and subsequently placed in an identical copy of the 30 × 30-cm white acrylic box, with a new copy of the previously encountered object in one corner and a completely novel object in the other corner. For half the animals, the novel object was placed in the top corner of the box, and for the others the novel object was placed at the bottom corner to avoid place preference. The mouse was allowed to explore the objects for 3 min (test phase) and its movement recorded. For half of each group tested, the Same condition was assessed first (i.e., white box for both phases), and for the other half, the Change condition (i.e., patterned box for sample phase) was done first.
Videos of behavior during the test were analyzed using ANY-maze software. The outcome measure assessed was recognition ratio, N/(N + f), where f and N represent the average time per minute spent in contact with the familiar versus new objects (27). A preference for the novel object would give a ratio of >0.5, and preference for a familiar object would give a ratio <0.5. A ratio of 0.5 would suggest no differentiation between familiar and novel objects.
Tissue Collection and Processing.
For histological analysis, mice were killed 4 or 15 mo after subretinal injection. After enucleation, retinas were dissected using an operating microscope in 4% paraformaldehyde (PFA) (Thermo Fisher) in PBS. Following overnight fixation, eyecups were cryoprotected using a 10–30% sucrose gradient, embedded in optimal cutting temperature (OCT) compound (Tissue-Tek; Sakura Finetek), frozen on dry ice, and stored at −80 °C. Eyecups were cryosectioned to a thickness of 16 μm and affixed to poly-l-lysine–coated slides (Polysine; Thermo Scientific) and stored at −20 °C until processing.
Immunocytochemistry and Immunohistochemistry.
Retinal sections.
Following hydration and three washes each of 5-min duration in 0.01 M PBS, PBS plus 0.1% Triton X-100 plus 10% donkey serum was applied to block retinal sections for 1 h at room temperature. After 3 × 5-min washes, sections were incubated with primary antibody at 4 °C overnight, and subsequently with species-appropriate secondary antibody for 2 h at room temperature (Tables S4 and S5), both in PBS plus 0.1% Triton X-100 plus 1% serum. After each step, sections were rinsed for 2 × 5 min in PBS plus 0.1% Triton X, followed by 1 × 5 min in PBS alone. Sections were counterstained with Hoechst 33342 (Invitrogen) (1:5,000) and mounted (Prolong Gold; Invitrogen).
Retinal flatmounts.
Following fixation overnight in 4% PFA, retinas were dissected and placed in 30% sucrose overnight. Following 3 × 10-min washes in 0.01 M PBS plus 1% Triton X-100, retinas were blocked in PBS plus 1% Triton X-100 plus 10% donkey serum for 2 h at room temperature. Subsequently, retinas were incubated in primary antibody diluted in PBS plus 1% Triton X-100 plus 2.5% donkey serum at 4 °C for 3 d. Flatmounts were rinsed 3 × 30 min each in PBS plus 0.2% Triton X-100 and incubated in secondary antibody diluted in PBS plus 1% Triton X-100 plus 2.5% donkey serum overnight at 4 °C. Following 3 × 30-min washes in PBS plus 0.2% Triton X-100, and counterstaining with Hoechst 33342 (1:5,000), retinas were mounted.
For immunohistochemistry performed using two primary antibodies.
If both antibodies were raised in different species, they were applied simultaneously as per the protocol above, with species-specific secondary antibodies subsequently being applied together. Staining for c-Fos and human melanopsin involved labeling with two primary antibodies raised in rabbit. Staining with c-Fos antibody was carried out as above, and then two further blocking steps were applied to saturate unbound sites on the secondary antibody (rabbit anti-goat blocking antibody, 1:200, for 2 h at room temperature, and then donkey anti-rabbit monovalent fragments, 1:100, overnight at 4 °C). Sections were then incubated with human melanopsin antibody and the appropriate secondary antibody.
Confocal Microscopy.
Retinal sections were viewed on a confocal microscope (LSM710; Zeiss). Fluorescent cells were located using fluorescence illumination before taking a series of 0.5-μm thickness overlapping XY optical sections. Fluorescence of Hoechst, DsRed, and Alexa 555, 568, 633, or 647 were sequentially excited and a stack built. Image processing was performed using Volocity (Perkin-Elmer) and ImageJ (version 1.43; National Institutes of Health, https://imagej.nih.gov/ij).
Statistical Analysis.
Data are presented as mean ± SEM with significance level set as 0.05. For normally distributed data, a paired or unpaired t test or one- or two-way ANOVA was used as appropriate. Otherwise, a Mann–Whitney or Kruskal–Wallis test was performed. Post hoc testing was done as specified. Statistical analysis was done using Prism 6 for Mac OS X, SPSS (IBM), and G*Power.
Acknowledgments
Funding was provided by Wellcome Trust, National Institute for Health Research Biomedical Research Centres of Oxford and Moorfields, Medical Research Council, Biotechnology and Biological Sciences Research Council, and Royal College of Surgeons of Edinburgh.
Footnotes
Conflict of interest statement: S.R.D.S., A.R.B., M.W.H., and R.E.M. are listed as inventors on a patent submitted by the University of Oxford relevant to this work. R.E.M. is a founding director of Nightstarx Ltd., a retinal gene therapy company established by the University of Oxford and owned by the Wellcome Trust. M.W.H. is listed as an inventor on a patent owned by Imperial College London relating to restoring light responses by ectopic expression of melanopsin.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1701589114/-/DCSupplemental.
References
- 1.Bessant DA, Ali RR, Bhattacharya SS. Molecular genetics and prospects for therapy of the inherited retinal dystrophies. Curr Opin Genet Dev. 2001;11:307–316. doi: 10.1016/s0959-437x(00)00195-7. [DOI] [PubMed] [Google Scholar]
- 2.Stingl K, et al. Artificial vision with wirelessly powered subretinal electronic implant alpha-IMS. Proc Biol Sci. 2013;280:20130077. doi: 10.1098/rspb.2013.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lagali PS, et al. Light-activated channels targeted to ON bipolar cells restore visual function in retinal degeneration. Nat Neurosci. 2008;11:667–675. doi: 10.1038/nn.2117. [DOI] [PubMed] [Google Scholar]
- 4.Busskamp V, et al. Genetic reactivation of cone photoreceptors restores visual responses in retinitis pigmentosa. Science. 2010;329:413–417. doi: 10.1126/science.1190897. [DOI] [PubMed] [Google Scholar]
- 5.Lin B, Koizumi A, Tanaka N, Panda S, Masland RH. Restoration of visual function in retinal degeneration mice by ectopic expression of melanopsin. Proc Natl Acad Sci USA. 2008;105:16009–16014. doi: 10.1073/pnas.0806114105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cehajic-Kapetanovic J, et al. Restoration of vision with ectopic expression of human rod opsin. Curr Biol. 2015;25:2111–2122. doi: 10.1016/j.cub.2015.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bi A, et al. Ectopic expression of a microbial-type rhodopsin restores visual responses in mice with photoreceptor degeneration. Neuron. 2006;50:23–33. doi: 10.1016/j.neuron.2006.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Provencio I, Jiang G, De Grip WJ, Hayes WP, Rollag MD. Melanopsin: An opsin in melanophores, brain, and eye. Proc Natl Acad Sci USA. 1998;95:340–345. doi: 10.1073/pnas.95.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Provencio I, et al. A novel human opsin in the inner retina. J Neurosci. 2000;20:600–605. doi: 10.1523/JNEUROSCI.20-02-00600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doroudchi MM, et al. Virally delivered channelrhodopsin-2 safely and effectively restores visual function in multiple mouse models of blindness. Mol Ther. 2011;19:1220–1229. doi: 10.1038/mt.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cronin T, et al. Efficient transduction and optogenetic stimulation of retinal bipolar cells by a synthetic adeno-associated virus capsid and promoter. EMBO Mol Med. 2014;6:1175–1190. doi: 10.15252/emmm.201404077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hughes S, et al. Using siRNA to define functional interactions between melanopsin and multiple G protein partners. Cell Mol Life Sci. 2015;72:165–179. doi: 10.1007/s00018-014-1664-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Panda S, et al. Illumination of the melanopsin signaling pathway. Science. 2005;307:600–604. doi: 10.1126/science.1105121. [DOI] [PubMed] [Google Scholar]
- 14.Melyan Z, Tarttelin EE, Bellingham J, Lucas RJ, Hankins MW. Addition of human melanopsin renders mammalian cells photoresponsive. Nature. 2005;433:741–745. doi: 10.1038/nature03344. [DOI] [PubMed] [Google Scholar]
- 15.Cideciyan AV, et al. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc Natl Acad Sci USA. 2008;105:15112–15117. doi: 10.1073/pnas.0807027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacLaren RE, et al. Retinal gene therapy in patients with choroideremia: Initial findings from a phase 1/2 clinical trial. Lancet. 2014;383:1129–1137. doi: 10.1016/S0140-6736(13)62117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Wyk M, Pielecka-Fortuna J, Löwel S, Kleinlogel S. Restoring the ON switch in blind retinas: Opto-mGluR6, a next-generation, cell-tailored optogenetic tool. PLoS Biol. 2015;13:e1002143. doi: 10.1371/journal.pbio.1002143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dalkara D, et al. In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Sci Transl Med. 2013;5:189ra76. doi: 10.1126/scitranslmed.3005708. [DOI] [PubMed] [Google Scholar]
- 19.Dalkara D, et al. Inner limiting membrane barriers to AAV-mediated retinal transduction from the vitreous. Mol Ther. 2009;17:2096–2102. doi: 10.1038/mt.2009.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kotterman MA, et al. Antibody neutralization poses a barrier to intravitreal adeno-associated viral vector gene delivery to non-human primates. Gene Ther. 2015;22:116–126. doi: 10.1038/gt.2014.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carter-Dawson LD, LaVail MM, Sidman RL. Differential effect of the rd mutation on rods and cones in the mouse retina. Invest Ophthalmol Vis Sci. 1978;17:489–498. [PubMed] [Google Scholar]
- 22.Sheng M, McFadden G, Greenberg ME. Membrane depolarization and calcium induce c-fos transcription via phosphorylation of transcription factor CREB. Neuron. 1990;4:571–582. doi: 10.1016/0896-6273(90)90115-v. [DOI] [PubMed] [Google Scholar]
- 23.Semo M, Lupi D, Peirson SN, Butler JN, Foster RG. Light-induced c-fos in melanopsin retinal ganglion cells of young and aged rodless/coneless (rd/rd cl) mice. Eur J Neurosci. 2003;18:3007–3017. doi: 10.1111/j.1460-9568.2003.03061.x. [DOI] [PubMed] [Google Scholar]
- 24.Semo M, et al. Dissecting a role for melanopsin in behavioural light aversion reveals a response independent of conventional photoreception. PLoS One. 2010;5:e15009. doi: 10.1371/journal.pone.0015009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh MS, et al. Reversal of end-stage retinal degeneration and restoration of visual function by photoreceptor transplantation. Proc Natl Acad Sci USA. 2013;110:1101–1106. doi: 10.1073/pnas.1119416110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ray TA, et al. GPR179 is required for high sensitivity of the mGluR6 signaling cascade in depolarizing bipolar cells. J Neurosci. 2014;34:6334–6343. doi: 10.1523/JNEUROSCI.4044-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: Memory for objects, places, and contexts. Learn Mem. 2002;9:49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dix SL, Aggleton JP. Extending the spontaneous preference test of recognition: Evidence of object-location and object-context recognition. Behav Brain Res. 1999;99:191–200. doi: 10.1016/s0166-4328(98)00079-5. [DOI] [PubMed] [Google Scholar]
- 29.Tam SK, et al. Modulation of recognition memory performance by light requires both melanopsin and classical photoreceptors. Proc Biol Sci. 2016;283:20162275. doi: 10.1098/rspb.2016.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones BW, et al. Retinal remodeling triggered by photoreceptor degenerations. J Comp Neurol. 2003;464:1–16. doi: 10.1002/cne.10703. [DOI] [PubMed] [Google Scholar]
- 31.Marc RE, Jones BW. Retinal remodeling in inherited photoreceptor degenerations. Mol Neurobiol. 2003;28:139–147. doi: 10.1385/MN:28:2:139. [DOI] [PubMed] [Google Scholar]
- 32.Jones BW, et al. Retinal remodeling in human retinitis pigmentosa. Exp Eye Res. 2016;150:149–165. doi: 10.1016/j.exer.2016.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Milam AH, Li ZY, Fariss RN. Histopathology of the human retina in retinitis pigmentosa. Prog Retin Eye Res. 1998;17:175–205. doi: 10.1016/s1350-9462(97)00012-8. [DOI] [PubMed] [Google Scholar]
- 34.Jacobson SG, Sumaroka A, Luo X, Cideciyan AV. Retinal optogenetic therapies: Clinical criteria for candidacy. Clin Genet. 2013;84:175–182. doi: 10.1111/cge.12165. [DOI] [PubMed] [Google Scholar]
- 35.Naycheva L, et al. Phosphene thresholds elicited by transcorneal electrical stimulation in healthy subjects and patients with retinal diseases. Invest Ophthalmol Vis Sci. 2012;53:7440–7448. doi: 10.1167/iovs.12-9612. [DOI] [PubMed] [Google Scholar]
- 36.Macé E, et al. Targeting channelrhodopsin-2 to ON-bipolar cells with vitreally administered AAV restores ON and OFF visual responses in blind mice. Mol Ther. 2015;23:7–16. doi: 10.1038/mt.2014.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lucas RJ, Douglas RH, Foster RG. Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nat Neurosci. 2001;4:621–626. doi: 10.1038/88443. [DOI] [PubMed] [Google Scholar]
- 38.Sekaran S, Foster RG, Lucas RJ, Hankins MW. Calcium imaging reveals a network of intrinsically light-sensitive inner-retinal neurons. Curr Biol. 2003;13:1290–1298. doi: 10.1016/s0960-9822(03)00510-4. [DOI] [PubMed] [Google Scholar]
- 39.Guido W, Lu SM. Cellular bases for the control of retinogeniculate signal transmission. Int J Neurosci. 1995;80:41–63. doi: 10.3109/00207459508986093. [DOI] [PubMed] [Google Scholar]
- 40.Hicks SL, et al. A depth-based head-mounted visual display to aid navigation in partially sighted individuals. PLoS One. 2013;8:e67695. doi: 10.1371/journal.pone.0067695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.LaVail MM, Matthes MT, Yasumura D, Steinberg RH. Variability in rate of cone degeneration in the retinal degeneration (rd/rd) mouse. Exp Eye Res. 1997;65:45–50. doi: 10.1006/exer.1997.0308. [DOI] [PubMed] [Google Scholar]
- 42.Koistinaho J, Sagar SM. Light-induced c-fos expression in amacrine cells in the rabbit retina. Brain Res Mol Brain Res. 1995;29:53–63. doi: 10.1016/0169-328x(94)00218-4. [DOI] [PubMed] [Google Scholar]
- 43.Hughes S, et al. Characterisation of light responses in the retina of mice lacking principle components of rod, cone and melanopsin phototransduction signalling pathways. Sci Rep. 2016;6:28086. doi: 10.1038/srep28086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lucas RJ, et al. Measuring and using light in the melanopsin age. Trends Neurosci. 2014;37:1–9. doi: 10.1016/j.tins.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]