Significance
Visceral pain is a debilitating type of pain that afflicts adults and children. Major challenges exist in developing analgesics due to the limited understanding of the mechanisms that mediate visceral nociception. Using a model of colitis, we have identified the granulocyte-colony–stimulating factor (G-CSF) as an essential mediator of central sensitization that leads to visceral hypersensitivity, even after the resolution of inflammation. We demonstrate that G-CSF acting on spinal microglia activates a signaling platform that causes hyperexcitability of sensory neurons. We found that ablating microglia or blocking the G-CSF receptor prevents visceral sensitization. Our work establishes a microglial signaling mechanism in the transition to chronic visceral pain and likely other forms of persistent pain associated with chronic inflammatory diseases.
Keywords: visceral pain, microglia, colitis, G-CSF, nociceptors
Abstract
Pain is a main symptom of inflammatory diseases and often persists beyond clinical remission. Although we have a good understanding of the mechanisms of sensitization at the periphery during inflammation, little is known about the mediators that drive central sensitization. Recent reports have identified hematopoietic colony-stimulating factors as important regulators of tumor- and nerve injury-associated pain. Using a mouse model of colitis, we identify the proinflammatory cytokine granulocyte-colony–stimulating factor (G-CSF or Csf-3) as a key mediator of visceral sensitization. We report that G-CSF is specifically up-regulated in the thoracolumbar spinal cord of colitis-affected mice. Our results show that resident spinal microglia express the G-CSF receptor and that G-CSF signaling mediates microglial activation following colitis. Furthermore, healthy mice subjected to intrathecal injection of G-CSF exhibit pronounced visceral hypersensitivity, an effect that is abolished by microglial depletion. Mechanistically, we demonstrate that G-CSF injection increases Cathepsin S activity in spinal cord tissues. When cocultured with microglia BV-2 cells exposed to G-CSF, dorsal root ganglion (DRG) nociceptors become hyperexcitable. Blocking CX3CR1 or nitric oxide production during G-CSF treatment reduces excitability and G-CSF–induced visceral pain in vivo. Finally, administration of G-CSF–neutralizing antibody can prevent the establishment of persistent visceral pain postcolitis. Overall, our work uncovers a DRG neuron–microglia interaction that responds to G-CSF by engaging Cathepsin S-CX3CR1-inducible NOS signaling. This interaction represents a central step in visceral sensitization following colonic inflammation, thereby identifying spinal G-CSF as a target for treating chronic abdominal pain.
Long-lasting changes in nociceptive circuits precipitate the transition from acute to persistent pain in chronic inflammatory diseases. While significant improvement has been made to reduce acute inflammation, peripheral and central sensitization often leads to debilitating persistent pain even after disease remission, thus suggesting a high level of plasticity in nociceptive pathways during inflammation or through active processes of resolution (1–6). We and others have shown that a single bout of colonic inflammation can cause subsequent visceral sensitization that persists long after the inflammation has resolved (7). This persistent postinflammatory sensitization mirrors what is seen in patients with inflammatory diseases (4, 7–10). While a number of mechanisms of peripheral sensitization have been characterized (5, 11, 12), there is a growing appreciation that, as observed at the periphery, neuro-immune interactions occurring in the spinal cord regulate pain sensitivity caused by tissue damage (8, 12–16). Microglia, the tissue-resident macrophages of the central nervous system, have been directly implicated in the initiation of mechanical hypersensitivity following peripheral nerve injury (17–19). In this pathological condition, activated microglia in the spinal cord release proinflammatory cytokines such as IL-1β and TNF-α, which enhance pain sensation by increasing the excitability of dorsal root ganglion (DRG) nociceptors to facilitate synaptic transmission in the spinal dorsal horn (20, 21). With regard to visceral sensitivity, microglia have recently been found to mediate central sensitization in a rat model of narcotic bowel-like syndrome (22). However, the importance of microglial activation and the spinal neuro-immune mechanisms eliciting inflammation-induced visceral hypersensitivity remain elusive. Using the dextran sulfate sodium (DSS)-induced colitis model, we have identified granulocyte-colony–stimulating factor (G-CSF or Csf-3), a blood–brain barrier-permeant cytokine, as a regulator of central sensitization. We found that G-CSF is increased in the spinal cord during colonic inflammation, which coincides with microglial activation. Our data describe the mechanism whereby G-CSF signaling, through its G-CSF Receptor (G-CSFR), also known as CD114, promotes visceral hypersensitivity. Overall, our work shows that central neuro-immune interactions involving G-CSF can precipitate the establishment of persistent pain following peripheral inflammation. Thus, G-CSF signaling may represent a therapeutic target for the treatment of pain associated with chronic inflammatory diseases.
Results
G-CSF and Its Receptor Are Up-Regulated in Spinal Cord Tissue During DSS-Induced Colitis.
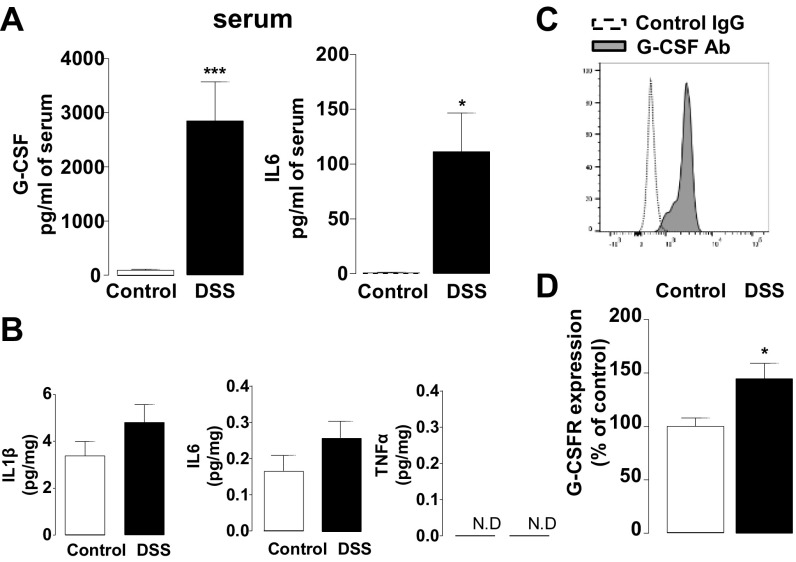
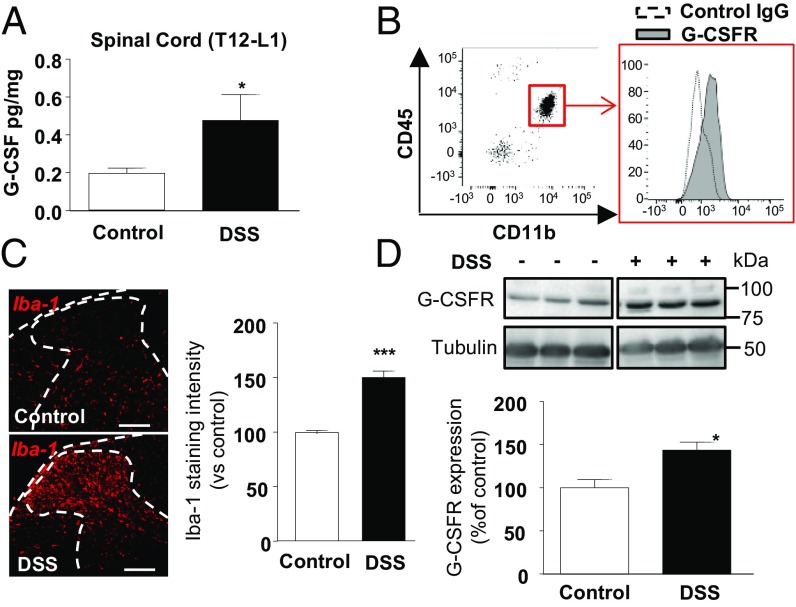
To examine inflammatory markers that could modulate central sensitization during colitis, we measured inflammatory cytokines in spinal cord tissues from mice treated with DSS for 7 d. As a surrogate marker of inflammation, both G-CSF and IL6 levels were high in the serum of colitis mice (Fig. S1A). Strikingly, we also found elevated G-CSF in gut-projecting thoracolumbar (T12–L1) spinal cord from DSS mice (Fig. 1A), whereas 29 other cytokines, including IL1β, IL6, and TNFα, remained unchanged (Fig. S1B and Table S1). Using flow cytometry, we isolated mononuclear cells from the spinal cord and found that microglia (CD45low CD11b+ cells) produced G-CSF (Fig. S1C) and expressed the G-CSFR (Fig. 1B). Accordingly, we observed an increase in Iba-1 immunopositive microglia in spinal cord sections of DSS mice (Fig. 1C), associated with an up-regulation in spinal G-CSFR at both mRNA (Fig. S1D) and protein levels (Fig. 1D).
Fig. S1.
Inflammatory state of spinal cord during acute colitis. (A) Measure of G-CSF and IL6 in the serum of control (white bar, n = 10) or DSS colitis (black bar, n = 7) mice. (B) Measure of IL1β, IL6, and TNFα levels from the thoracolumbar (T12–L1) spinal cord of control (white bar, n = 10) or DSS colitis (black bar, n = 7) mice. (C) Spinal cord mononuclear cells were isolated, and CD45low CD11b+ cells (microglia) were analyzed using flow cytometry. Expression of G-CSF was determined after in vitro stimulation with phorbol myristate acetate (PMA) and ionomycin for 3 h and is represented by increased binding of specific G-CSF antibody (gray histogram) compared with control IgG (dotted histogram). This is a representative result from three independent experiments. (D) Expression of G-CSFR mRNA was assessed by qPCR in the thoracolumbar spinal cord of control (white bar, n = 4) or colitis (black bar, n = 5) mice.
Fig. 1.
G-CSF and its receptor, expressed on microglia, are increased in the spinal cord during acute DSS-induced colitis. Acute colitis was induced in mice with 2.5% DSS for 7 d. After blood removal, G-CSF levels were determined in the thoracolumbar (T12–L1) spinal cord by luminex technology in control (white bar, n = 10) or colitis (black bar, n = 7) mice (A). Mononuclear cells of the spinal cord were isolated, and CD45low CD11b+ cells (microglia) were analyzed using flow cytometry for their expression of G-CSFR represented by increased binding of specific G-CSFR antibody (gray histogram, representative of three independent experiments) compared with control IgG (dotted histogram) (B). (C, Left) Iba-1 expression was quantified by immunostaining in spinal cord sections of control and DSS colitis mice. (C, Right) Intensity of the immunostaining was measured using Image J on a total of 35 sections from three independent experiments. (D) G-CSFR expression level was determined by Western blot in spinal cord of control (white bar, n = 6) or colitis (black bar, n = 6) mice. Statistical analyses were performed using Mann–Whitney U test; *P < 0.05; ***P < 0.001. (Scale bar: 100 μm.)
Table S1.
List of the 29 other cytokines assessed in thoracolumbar spinal cord samples of DSS colitis mice
| Cytokine | Control (pg⋅mg⋅prot−1 ± SEM) | DSS (pg⋅mg⋅prot−1 ± SEM) | P value |
| Eotaxin | 15.51 ± 7.192 | 15.1 ± 3.687 | 0.9497 |
| GM-CSF | 0.2527 ± 0.1528 | 0.4766 ± 0.1670 | 0.1882 |
| IL1-α | 2.065 ± 0.9780 | 1.774 ± 0.7826 | 1 |
| IL1-β | 3.387 ± 0.6081 | 4.808 ± 0.7606 | 0.2008 |
| IL2 | 15.08 ± 3.002 | 11.16 ± 2.111 | 0.2810 |
| IL3 | 0.07104 ± 0.01948 | 0.1121 ± 0.02570 | 0.2972 |
| IL7 | 1.164 ± 0.4395 | 1.466 ± 0.4701 | 0.3282 |
| IL9 | 26.93 ± 8.364 | 14.81 ± 3.097 | 0.3969 |
| IL10 | 0.3893 ± 0.04860 | 0.4651 ± 0.05135 | 0.3846 |
| IL12p40 | 0.1428 ± 0.03515 | 0.2610 ± 0.1051 | 0.3541 |
| IL13 | 0.2693 ± 0.01220 | 0.1880 ± 0.03508 | 0.1 |
| IL15 | 7.122 ± 0.5335 | 7.477 ± 0.5723 | 0.4871 |
| IL17 | 0.4751 ± 0.03387 | 0.5165 ± 0.06526 | 0.2319 |
| IP10 | 2.077 ± 0.3551 | 2.478 ± 0.5123 | 0.6126 |
| KC | 0.6220 ± 0.09336 | 0.7188 ± 0.2028 | 0.9452 |
| MCP-1 | 2.396 ± 0.6789 | 0.3165 ± 0.06412 | 0.0023 |
| M-CSF | 0.7785 ± 0.04287 | 1.109 ± 0.2075 | 0.2319 |
| MIG | 2.533 ± 0.8734 | 1.855 ± 0.3666 | 0.9551 |
| MIP1α | 3.382 ± 0.5375 | 3.447 ± 0.4396 | 0.8665 |
| MIP2 | 3.799 ± 1.013 | 3.798 ± 1.137 | 0.7308 |
| RANTES | 0.1229 ± 0.04953 | 0.23 ± 0.1588 | 0.8548 |
| VEGF | 0.09536 ± 0.02448 | 0.09210 ± 0.01005 | 0.6228 |
| IL4 | 0.02267 ± 0.004177 | 0.02667 ± 0.002028 | 0.7 |
| IL12p70 | 0.587 ± 0.1039 | 0.5520 ± 0.3063 | 0.7 |
| TNFα | ND | ND | |
| IL5 | 0.05267 ± 0.008511 | 0.05633 ± 0.006009 | 1 |
| LIF | 0.08467 ± 0.01167 | 0.1013 ± 0.01477 | 0.4 |
| MIP1β | 1.825 ± 0.05341 | 1.864 ± 0.7349 | 0.7 |
| IL6 | 0.1715 ± 0.04381 | 0.2671 ± 0.04702 | 0.2319 |
Analysis of multiple cytokines in thoracolumbar spinal cord samples from control and DSS-induced experimental colitis. See also Materials and Methods. ND, not detectable.
G-CSF Signaling in Spinal Microglia Promotes Visceral Hypersensitivity.
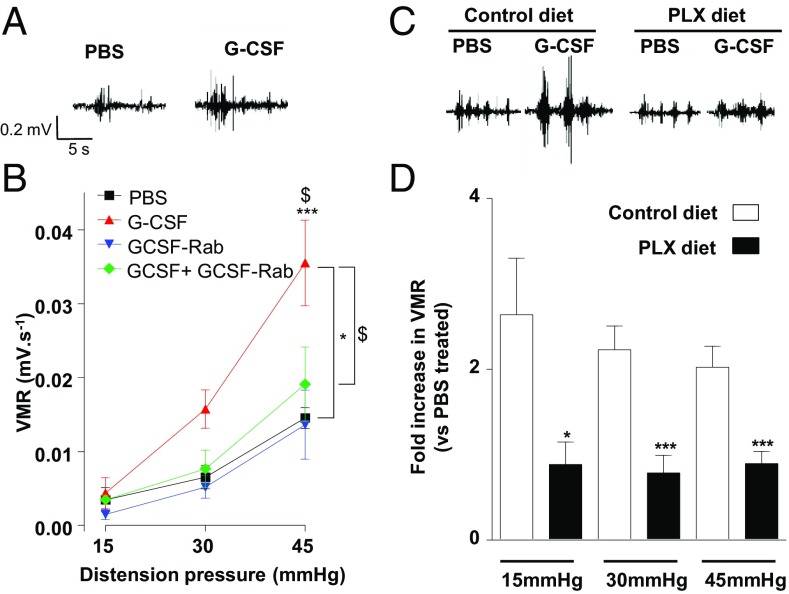
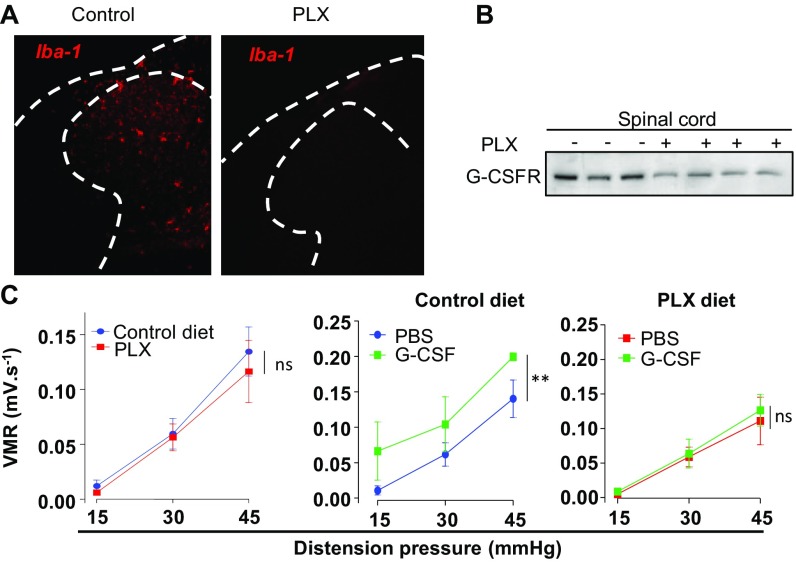
To investigate the effect of G-CSF on visceral sensitization, we assessed visceromotor responses (VMR) to colorectal distension 12 h after intrathecal administration of G-CSF (Fig. 2A). Compared with sham (PBS)-treated littermates, G-CSF–treated mice showed visceral hypersensitivity at mild and noxious distension pressures (Fig. 2B). This effect was mediated by the G-CSF receptor, as simultaneous administration of G-CSF with a G-CSF receptor-neutralizing antibody (G-CSF-Rab) completely prevented the development of visceral sensitivity. To determine the role of microglia in G-CSF–induced visceral hypersensitivity, we used chow containing PLX 5622, a CSF-1 receptor inhibitor known to deplete microglia in vivo without altering visceral sensitivity in basal conditions (Fig. S2) (23). Ablation of microglia with PLX prevented G-CSF–induced visceral hypersensitivity (Fig. 2C), compared with the twofold increase observed in mice receiving normal chow (Fig. 2D and Fig. S2C). Our data thus indicate that activation of the G-CSF/G-CSFR–signaling axis in the spinal cord triggers microglia-dependent visceral hypersensitivity.
Fig. 2.
G-CSF signaling in microglia induces visceral hypersensitivity. (A) Representative electromyogram recording elicited by 45 mmHg pressure at 12 h of intrathecal PBS or G-CSF (20 ng) injection. (B) VMR to colorectal distension after treatment with PBS (n = 4), G-CSF (20 ng; n = 8), anti–G-CSFR antibody (G-CSF-Rab 1 µg; n = 5; ***P < 0.001), or a combination of both (n = 5). (C) Representative electromyogram recording elicited by 45 mmHg pressure at 12 h of intrathecal PBS or G-CSF (20 ng) injection in mice that received control or the PLX 5622 diet for 2 wk. (D) VMR to colorectal distension after treatment with PBS (control diet: n = 15, PLX diet: n = 10) or G-CSF (control diet: n = 16, PLX diet: n = 11). Results are expressed as fold increase in VMR (±SEM) for control diet (white bar) or PLX diet (black bar) animals. Statistical analysis was performed using either repeated-measures two-way ANOVA and Bonferroni post-hoc test (B; *P < 0.05 G-CSF; ***P < 0.001 vs. PBS; $P < 0.05 G-CSF vs. G-CSF+G-CSF Receptor antibody) or the Mann–Whitney U test (D; *P < 0.05; ***P < 0.001).
Fig. S2.
Ablation of microglia by PLX 5622 has no effect on basal visceral sensitivity. Iba-1 expression was assessed by immunostaining (A), and G-CSFR expression was assessed by Western blot (B) in spinal cord of control-diet or PLX-fed mice. (C) Visceral sensitivity was measured in control (blue circle), G-CSF–treated (green square) or PLX mice (red square), and PLX mice treated with G-CSF (green square).
G-CSF Conditions Microglia to Sensitize DRG Neurons Through Cathepsin S-CX3CR1-Inducible NOS Signaling.
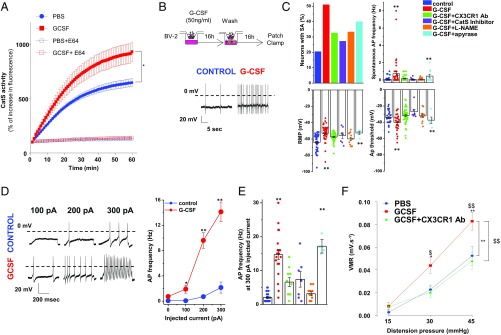
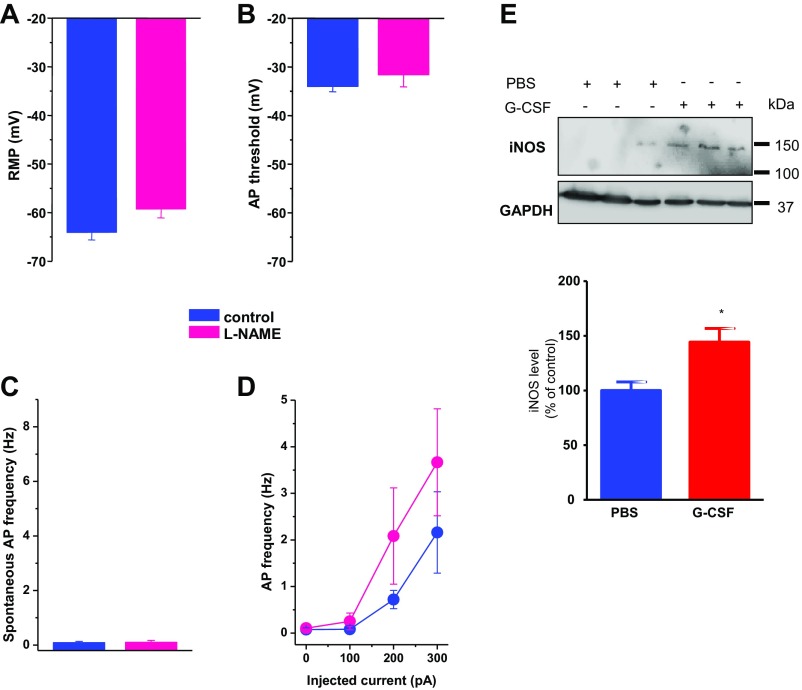
Intrathecal injection of colony-stimulating factor (Csf-1 also known as M-CSF) was recently reported to increase the expression of the cysteine proteinase Cathepsin S (Cat S) and CX3CR1 in the spinal cord (24). Based on the role of the Cat S/CX3CL1/CX3CR1-signaling axis in neuropathic pain (25), we examined the contribution of this pathway in driving G-CSF–induced visceral hypersensitivity. As shown in Fig. 3A, intrathecal injection of G-CSF increases Cat S activity in the spinal cord. To determine how G-CSF could engage microglia–DRG neuron interaction through Cat S activity, we developed a modified transwell coculture protocol (Materials and Methods). In this assay, BV-2 microglia cell monolayers seeded in the upper chamber were stimulated with G-CSF for 16 h (MicroG-CSF), washed, and then placed above acutely dissociated DRG neurons seeded into the lower chamber (Fig. 3B). DRG neurons were exposed to factors secreted from G-CSF–primed microglia cells for a further 16 h, after which both spontaneous and evoked neuronal activity was recorded by patch clamp electrophysiology (26, 27). As shown in Fig. 3, the percentage of capsaicin-responsive (TRPV1+) DRG neurons exhibiting spontaneous activity increased when cultured with MicroG-CSF compared with those cultured with “naive” microglia. Neurons exposed to MicroG-CSF displayed a more depolarized resting membrane potential and a lower action potential threshold, indicating overall that MicroG-CSF-secreted factor(s) modulate neuronal excitability in our coculture system (Fig. 3C). Moreover, the frequency of action potential (AP) evoked by an ascending ramp of injected current was greater in MicroG-CSF-cocultured neurons (Fig. 3 D and E). Both spontaneous and evoked electrical activities were partially blocked by treatment of cocultures with an anti-CX3CR1 antibody or a Cathepsin S inhibitor. To identify what factor(s) downstream of the microglial G-CSF receptor promote neuronal excitability, we examined the role of ATP and nitric oxide (NO), two known regulators of microglial activation and migration. While depleting microglial ATP with apyrase did not alter G-CSF–mediated hyperexcitability, blocking the NO pathway with the broad spectrum NOS inhibitor N(ω)-nitro-L arginine methyl esther (L-NAME) prevented it (Fig. 3 C and D). Of note, L-NAME alone had no effect on DRG neuron excitability (Fig. S3 A–D). These results correlated directly with an elevation of inducible NOS (iNOS) level in the spinal cord following G-CSF treatment in vivo (Fig. S3E) and therefore point to an excitatory effect of NO in response to the microglial CX3CR1-Cat S pathway. The anti-CX3CR1 antibody also blocked the ability of G-CSF to induce visceral hypersensitivity in vivo wherein the G-CSF–induced visceral hypersensitivity returned to basal levels upon G-CSF+CX3CR1 antibody administration (Fig. 3E). Our data indicate that G-CSF sensitizes DRG neurons through microglial stimulation and activation of Cat S-CX3CR1 signaling. This activation leads to an NO-driven neuronal hyperexcitability that underlies visceral hypersensitivity.
Fig. 3.
G-CSF–treated microglia increases excitability of DRG neurons through Cathepsin S-CX3CR1-iNOS signaling. (A) Mice were treated intrathecally with either PBS or G-CSF (20 ng) 12 h before evaluating Cathepsin S activity in thoracolumbar spinal cord lysate (n = 3 experiments). (B) Cartoon illustrating the coculture system experiment. BV2 microglia cells were plated into the upper chamber of a transwell and treated with G-CSF for 16 h. G-CSF was removed, and DRG neurons were plated in the lower chamber of the transwell for 16 h of coculture and then used for electrophysiological recordings. (Lower) A representative trace of spontaneous activity (SA) in a small-size TRPV1-sensitive DRG neuron cocultured with vehicle- or G-CSF–primed BV2 cells. (C) Exposure of BV2 microglia to G-CSF depolarizes the resting membrane potential (RMP) of DRG neurons and increases the percentage of neurons with SA (5/28 in control, 17/30 for G-CSF treated, 10/31 for G-CSF combined with CX3CR1 Ab-treated, 3/11 for G-CSF combined with Cat S inhibitor-treated, 5/15 for G-CSF combined with L-NAME, and 4/10 for G-CSF combined with Apyrase). (Right) G-CSF induces a decrease in the AP threshold and an increase in spontaneous AP discharge. These parameters are reversed by cotreatment with CX3CR1 Ab, Cathepsin S inhibitor, or L-NAME, but not apyrase. (D) Representative AP discharge evoked by 100-, 200-, and 300-pA current injections (1 s) in control (blue circle) and G-CSF (red circle) and for different conditions of treatment (E). (F) Measure of VMR to colorectal distension in mice injected with PBS (n = 9), G-CSF (20 ng; n = 12), or G-CSF + CX3CR1 Ab (5 µg; n = 9) 12 h before recording. Results indicate mean ± SEM (*P < 0.05; **P < 0.01). Statistical analysis was performed using one-way (C and E) or two-way ANOVA (A, D, and F) followed by Bonferroni post hoc test (*P < 0.05; **P < 0.01; $P < 0.05; $$P < 0.01).
Fig. S3.
Role of iNOS in G-CSF–induced hyperexcitability. Exposure to L-NAME (100 µM) alone has no effect on the resting membrane potential (A), the AP threshold (B), or spontaneous (C) and evoked (D) AP discharge. Results indicate mean ± SEM. Statistical analysis was performed using one-way ANOVA followed by Bonferroni post hoc test. (E) Western blot analysis of iNOS level from spinal cord samples (T12–L1) collected from G-CSF–treated mice. (Lower) Quantification of relative integrated density values from Western blots corrected from GAPDH and normalized to control PBS.
G-CSF Receptor Blockade in the Spinal Cord Prevents Persistent Visceral Hypersensitivity Postcolitis.
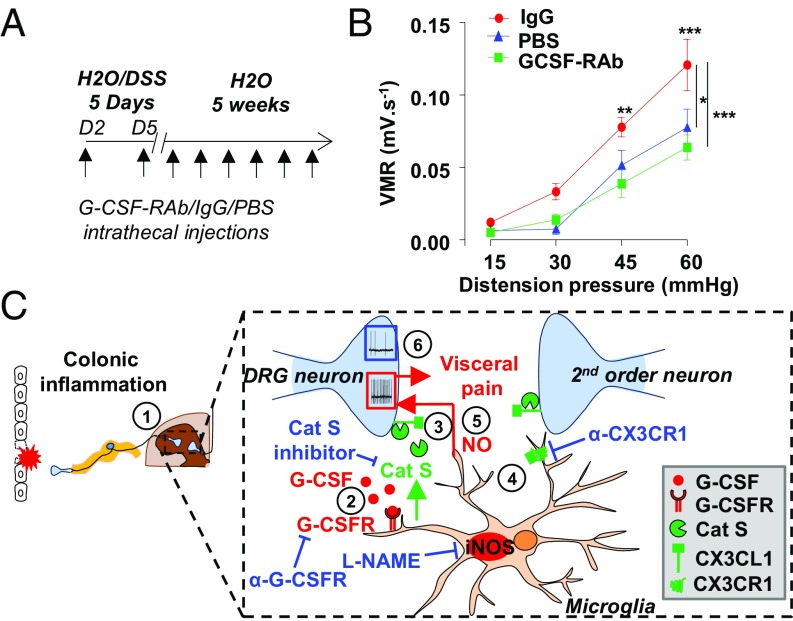
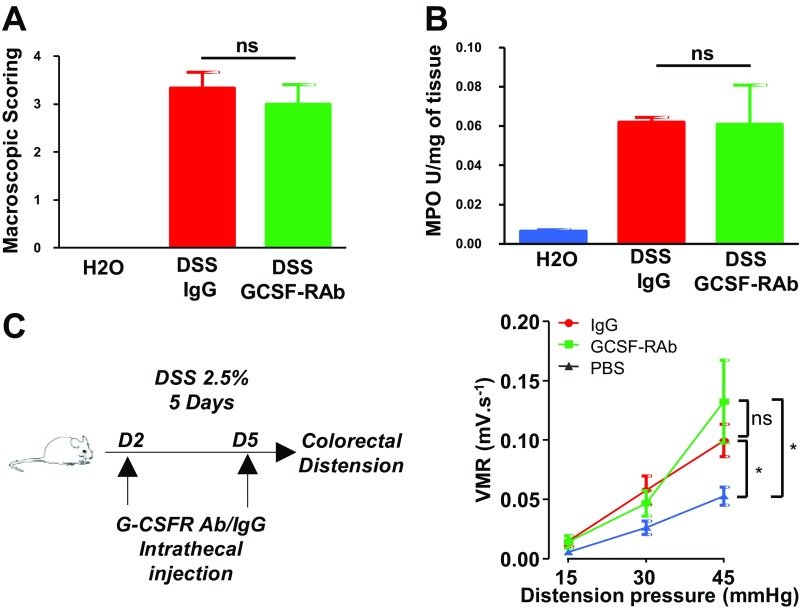
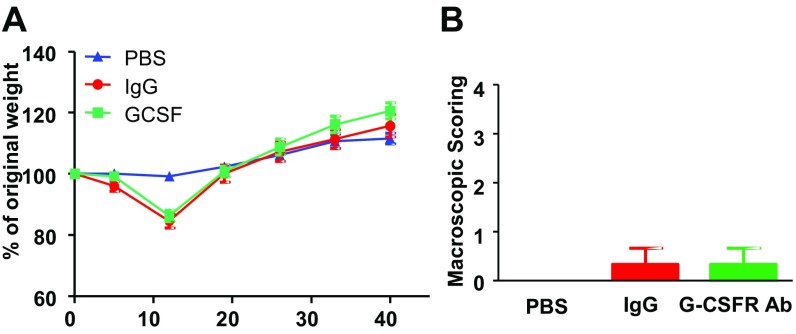
To test whether G-CSF could participate in the establishment of inflammation-induced chronic visceral pain, we blocked G-CSFR signaling in the spinal cord of DSS-treated animals that develop postcolitis pain (7). Briefly, mice were given 2.5% DSS for 5 d, after which they received control IgG or a G-CSFR–blocking antibody (GCSF-RAb) twice a week for a 5-wk recovery period (Fig. 4A). We previously reported that visceral hypersensitivity persists for at least 5 wk following acute colitis, despite the complete resolution of inflammation (7). Injection of GCSF-RAb intrathecally did not alter the acute inflammatory response measured by macroscopic scoring and myeloperoxidase (MPO) activity (Fig. S4 A and B) nor the resolution of inflammation following DSS discontinuation (Fig. S5). When we assessed VMR, postcolitis mice chronically treated with control IgG exhibited increased VMR to colorectal distension compared with healthy mice treated with saline. Strikingly, blockade of G-CSFR signaling completely abrogated visceral hypersensitivity in the postcolitis mice (Fig. 4B). In contrast, G-CSF-RAb did not have antinociceptive effects in the acute phase of colitis (Fig. S4C), thus indicating that G-CSF signaling in the spinal cord is responsible for the establishment of persistent visceral hypersensitivity post resolution but not during acute inflammation.
Fig. 4.
Blocking G-CSF receptor signaling in the spinal cord alleviates DSS-induced postinflammatory visceral hypersensitivity. (A) Colitis was induced with 2.5% DSS for 5 d, after which mice received only water for the next 5 wk. During the recovery period, mice were treated twice a week with either G-CSFR–blocking antibodies or control IgG. A healthy control group that did not receive DSS was intrathecally injected with PBS. (B) Visceromotor responses to colorectal distension post-DSS–induced colitis in G-CSF-Rab (green squares; n = 11) and control IgG (red circles; n = 6) treated mice compared with healthy mice injected with PBS (blue triangle; n = 4). Data are expressed as mean of VMR ± SEM. Statistical analysis was performed using repeated-measures two-way ANOVA and the Bonferroni post hoc test (*P < 0.05; **P < 0.01; ***P < 0.001). (C) Schematic representation of G-CSF–mediated visceral sensitization in the spinal cord. (1) Acute DSS colitis increases G-CSF in the spinal cord. (2) G-CSF binds to its receptor on microglia and (3) induces the secretion of Cathepsin S. (4) Cathepsin S triggers the release of soluble fractalkine that activates its receptor CX3CR1 at the microglial cell surface. (5) This drives a hyperexcitability of DRG neurons through a NO-dependent process and the establishment of visceral sensitization. (6) Inhibition of either G-CSFR or CX3CR1 prevents both DRG hyperexcitability and visceral hypersensitivity.
Fig. S4.
G-CSFR antibody does not alter disease recovery. Mice were treated for 7 d with 2.5% DSS and injected with either control IgG (n = 3) or G-CSF-RAb (n = 3) on days 2, 5, and 7 of DSS regimen. Macroscopic damages (A) and myeloperoxidase activity (B) were evaluated at day 7 of DSS. (C) Schematic representation of the experimental design to assess acute DSS-induced visceral hypersensitivity; colitis was induced by 2.5% DSS regimen and mice received intrathecal injection of either IgG (red circle n = 13) or G-CSF-RAb (green square n = 10). A control group receiving only water was injected with PBS IT (blue triangle n = 11) at similar time points. On day 5, visceral hypersensitivity was assessed by measuring visceromotor response to colorectal distension.
Fig. S5.
Chronic intrathecal treatment with G-CSF-RAb does not alter disease recovery post colitis. Colitis was induced by adding 2.5% DSS in the drinking water of mice. On day 5, DSS mice were on water regimen for 5 wk. During the recovery period, mice were treated twice a week with either G-CSFR–blocking antibody (blue square; n = 11) or control IgG (red circle; n = 6). A control group that was not exposed to DSS received intrathecal PBS (n = 4). Recovery of colitis was monitored by weighing mice weekly (A) and macroscopic scoring of colonic tissue damages (B) as previously described (46).
Discussion
Here, we report that G-CSF signaling drives persistent pain that lasts post resolution of inflammation via activating a Cathepsin S-CX3CR1-NO–signaling pathway between spinal microglia and DRG neurons (Fig. 4C). We demonstrate that (i) G-CSF is increased in the spinal cord of colitis mice and that intrathecal injection of G-CSF triggers visceral hypersensitivity through activation of the G-CSFR in microglia; (ii) G-CSF administration increases Cathepsin-S activity in the spinal cord; (iii) supernatants from G-CSF–treated microglia promote increased excitability of cocultured sensory neurons; and (iv) G-CSF–mediated hyperexcitability of DRG neurons involves the Cathepsin S-CX3CR1-NO pathway from microglia, which plays a major modulatory role in pain sensitization (28). Accordingly, injection of mice with a CX3CR1 antibody suppressed G-CSF–induced visceral hypersensitivity in vivo. Finally, we show that chronic treatment with a G-CSF-RAb alleviates postinflammatory visceral pain. Our data thus single out a role for G-CSF and its receptor in the establishment of postinflammatory pain that persists after tissue healing.
Our work supports data obtained over the past few years demonstrating a key role for the CNS in regulating pain sensitization and altered behavior following peripheral inflammation (29, 30). In the gastrointestinal tract, inflammation induced by either 2,4,6-trinitrobenzene sulfonic acid (TNBS) (31) or DSS (32), correlates with microglial activation in the hippocampus, which leads to increased susceptibility to seizures in mice (31). In addition, neonatal colon irritation is known to mediate microglial activation in the hippocampus and hence precipitate visceral hypersensitivity (33). To date, activation of microglia in the brain is linked to increases in hippocampal TNFα and IL1β, two cytokines thought to be responsible for behavioral changes (31, 33). In our new work, we did not observe any increase of IL1β in the spinal cord of acute DSS-treated mice, and no TNFα could be detected in either group, suggesting a different pattern of cytokines at play in the spinal cord compared with the ones identified in the hippocampus after peripheral inflammation. From a panel of 30 cytokines analyzed (Table S1), we found that only G-CSF, a hematopoietic stem cell factor that has been known to drive the differentiation of neutrophils from myeloid progenitors and trigger their release from the bone marrow (34), was significantly up-regulated. Although G-CSF is primarily known to be produced by monocytes/macrophages, fibroblasts, and endothelial and mesothelial cells upon stimulation, several studies have reported that hippocampal neurons (35) and astrocytes (36, 37), as well as microglia (38–40), can synthesize G-CSF in the central nervous system. Our data also show that peripheral inflammation can induce the production of this factor centrally in the spinal cord. Given that we observed activation of the microglia in the spinal cord during colitis, as attested to by increased Iba-1 expression, and based on our findings that microglia can produce G-CSF, we propose that microglia represent the source of G-CSF during DSS-induced colitis. Nevertheless, further studies using in situ hybridization, for example, would delineate whether other cells in the spinal cord are enabled to produce the cytokine. G-CSF, acting via its G-CSFR, stimulates several classes of cells in the nervous system such as neurons (35, 41), astrocytes, or microglia (42). G-CSFR–mediated signaling can also drive microglial proliferation (43) and activation in a model of amyotrophic lateral sclerosis (44). Accordingly, not only did we find that spinal cord microglia express G-CSFR, but its expression was increased in the spinal cord during colitis.
It has been shown that paw injection of hematopoietic growth factors such as G-CSF or granulocyte macrophage-CSF (GM-CSF) induces both thermal and mechanical hyperalgesia in mice and that locally secreted G-CSF is responsible for bone cancer-associated pain in mice (41). Also, recent work has reported that neutralizing G-CSFR with a monoclonal antibody could prevent and reverse arthritic pain in both adaptive and innate immune arthritis models, thus bringing forward the concept that G-CSF could be a key player in generating chronic pain associated with various inflammatory conditions (45). We and others have previously shown that, in a mouse model of DSS colitis, animals exhibit substantial visceral discomfort and pain both in the acute and the postresolution phase of the inflammation (7, 46, 47). Our data indicate that G-CSF could mediate this effect. Although we acknowledge that G-CSF may modulate pain sensation locally at the site of inflammation, G-CSF acting on microglia likely regulates the plasticity of spinal nociceptive circuits and therefore drives persistent visceral pain following colonic inflammation.
G-CSF was reported to activate nociceptors directly (41), inducing transcriptional changes concordant with an increase in nerve activity (48). Other researchers have proposed an indirect effect of G-CSF through prostanoid production (49) and were unable to detect G-CSFR expression in DRGs (45). Our model provides three major findings to suggest that G-CSF–promoted visceral hypersensitivity results from a direct action of G-CSF on microglia. We show that (i) G-CSFR is expressed by spinal cord microglia; (ii) G-CSF–induced visceral pain is abolished in mice depleted of microglia; and (iii) G-CSF-stimulated microglia can sensitize naive DRG neurons. Our results thus uncover a mechanism of G-CSF–induced visceral pain involving microglia. Along these lines, two recent papers concomitantly show that another hematopoietic growth factor, macrophage-CSF (M-CSF), secreted by injured nociceptors, leads to chronic pain caused by nerve injury. This effect of M-CSF, in keeping with our findings, is also mediated by activation of spinal cord microglial M-CSF receptors. Strikingly, spinal injection of M-CSF alone can induce mechanical allodynia, indicating that activation of microglia by the hematopoietic growth factor is sufficient to drive pain phenotypes (24, 50).
Although activation of microglia and their role in chronic hyperalgesic pain states is now well established in the settings of nerve injury (25), chronic pancreatitis (51), chronic stress (52, 53), or colonic inflammation (54), the mechanisms that underlie microglia-dependent pain sensitization are still unclear. While Cathepsin S was recently identified as a biased agonist of proteinase-activated receptor-2 (PAR2) (55), our results obtained using the anti-CX3CR1 antibody point to the contribution of the CX3CR1 agonist ligand, fractalkine/CX3CL1, known to promote neuropathic pain (56) or chronic pain associated with arthritis (28, 57). This pathway involves the microglial release of Cathepsin S, which then cleaves membrane-bound fractalkine on sensory neurons (58). Cleaved fractalkine in turn activates the CX3CR1 receptor on microglia, thus leading to the release of proinflammatory mediators, including NO, that strengthen synaptic activity in the spinal dorsal horn (25). Indeed, NO is a central factor of sensitization in the spinal dorsal horn, and targeting iNOS can attenuate inflammatory pain hypersensitivity (59–62). During colitis, this pathway is likely stimulated by spinal G-CSF. Collectively, our results agree with previous studies reporting that fractalkine can induce visceral hypersensitivity when injected intrathecally (53), that Cathepsin S activity is increased in the spinal cord during acute colitis (47), and that hematopoietic growth factor can induce Cathepsin S mRNA expression when injected in the spinal cord (24). Altogether, our findings demonstrate the importance of the proteinase-regulated pathway in our newly described G-CSF–mediated effect on visceral hypersensitivity in the context of colitis. Given that we observed only a partial inhibition of DRG neuron excitability using the CX3CR1 Ab in our in vitro setting, it is possible that other factors or mechanisms contribute to neuron sensitization. Indeed, G-CSF–treated BV.2 microglia also produce brain-derived neurotrophic factor (42), a factor broadly implicated in central sensitization processes (25). Nevertheless, the use of a CX3CR1 antibody was able to reverse hypersensitivity fully when administered intrathecally to mice, thus confirming that the Cathepsin S-CX3CR1– signaling axis plays a pivotal role in G-CSF–mediated central sensitization.
Central sensitization is often associated with chronic widespread pain states in inflammatory diseases such as inflammatory bowel disease (IBD) or arthritis. While anti-TNFα therapy, steroids, or nonsteroidal anti-inflammatory drugs can be effective at reducing acute inflammation in these conditions, many patients experience persistent pain after disease remission. Our work suggests that, in IBD, G-CSF signaling in microglia may contribute to this central sensitization process. We found that chronic spinal inhibition of G-CSF signaling by the GCSF-RAb prevented the maintenance of postinflammatory visceral pain in colitis. Interestingly, inhibition of G-CSF signaling did not affect peripheral inflammation, most likely because the antibody cannot cross the blood–brain barrier to reach the periphery (63) (Figs. S4 and S5).
Pharmacological inhibition of microglial activation in other mouse models of gastro-intestinal inflammation (TNBS) has been shown to reduce both acute (54) and chronic pain (51), suggesting that additional microglial mechanisms may be implicated during acute inflammation, whereas G-CSF acting on microglia may be required for maintaining persistent pain postresolution.
Of relevance, post-IBD irritable bowel syndrome patients with chronic pain symptoms represent a substantial clinical challenge due to the absence of treatment options (64). Our work identifying G-CSF as a central regulator of the transition to postinflammatory chronic visceral pain thus offers a potential therapeutic target to treat chronic abdominal pain in IBD and possibly in other pain conditions that persist after inflammatory disease remission.
Materials and Methods
Detailed methods, including reagents, mice strains, in vivo study design, induction of colitis, depletion of microglia with PLX diet, intrathecal injections and visceral pain assessment, colonic inflammation assessment, mRNA extraction, real-time PCR and Western blot detection of G-CSFR, immunocytochemical detection of Iba-1 expression in the mouse spinal cord, measurement of spinal cord Cathepsin S activity, measurements of spinal cord cytokine levels, coculture of BV-2 microglia and DRG neurons and in vitro study design, electrophysiological studies, and statistical analyses appear in SI Materials and Methods. All animal experiments were approved by the University of Calgary Animal Care Committee and were performed in accordance with the international guidelines for the ethical use of animals in research and guidelines of the Canadian Council on Animal Care.
SI Materials and Methods
Mice.
Six-week-old wild-type C57BL/6 mice were obtained from Charles River Laboratories. All mice were housed under standard conditions with drinking water and food available ad libitum. All experiments were conducted on aged-matched animals under protocols approved by the University of Calgary Animal Care Committee and in accordance with the international guidelines for the ethical use of animals in research and guidelines of the Canadian Council on Animal Care.
Depletion of Microglia.
Mice were fed with control or PLX 5622-containing diet (1,200 mg/kg, Plexxicon) for 2 wk. Microglia ablation was assessed by Iba-1 immunostaining in the spinal cord as previously described (23).
Induction of Colitis and Inflammation Assessment.
Acute colonic inflammation was induced by administration of 2.5% (wt/vol) DSS (MP Biochemicals) in drinking water for 7 d. Water consumption was monitored and found to be equal between groups. Chronic visceral hypersensitivity was induced as previously described (7). Briefly, colitis was induced by administration of 2.5% (wt/vol) DSS in the drinking water for 5 d. On day 5, DSS was removed and mice were allowed to recover for 5 wk. Weight changes were measured weekly in all experiments. Macroscopic damage was assessed and scored based on the following parameters: erythema (0, absent; 1, <1 cm; 2, >1 cm); edema (0, absent; 1, present); fecal blood (0, absent; 1, present); strictures (0, absent; 1, present); and adhesion (0, absent; 1, present). Colonic thickness was also measured at the time of death.
Cytokine Profiling.
After intracardiac blood puncture, mice were perfused with 10 mL cold PBS. Spinal cord was collected from the thoracolumbar T12–L1. Tissues were homogenized in RIPA buffer [1× PBS, 1% Igepal CA-630, 0.5% sodium deoxycholate, and 0.1% SDS (all from Sigma-Aldrich)] containing Complete-Mini protease inhibitor (Roche Diagnostics). Lysates were centrifuged at 10,000 × g for 10 min at 4 °C, and supernatants were collected. Sera were obtained by blood centrifugation at 10,000 × g for 10 min at 3 °C. Samples were processed using a multiplex assay with the MILLIPLEX Mouse Cytokine/Chemokine Array 30-Plex (EMD Millipore) on a Luminex xMAP multiplexing technology (Eve Technologies), as previously described (7). Protein concentration was quantified using a Bradford assay (Bio-Rad Laboratories) for normalization.
Intrathecal Injections and Visceromotor Response to Colorectal Distension.
Mice were subjected to a single 10 μL injection of sterile saline, 20 ng of G-CSF (Cedarlane), 1 μg of G-CSF receptor antibody (G-CSFR Ab) (clone H-176; Santa Cruz Biotechnology), or 5 µg of CX3CR1 antibody (Torrey Pines Scientific). VMR to colorectal distension was performed 12 h post injection as previously described (7). To mitigate and interpret VMR variability that can be observed among animal groups, we always run a control naive group for each condition tested. For chronic intrathecal treatment, mice were injected with 10 µL of either 1 µg of G-CSFR Ab or 1 µg rabbit IgG (Jackson Imunoresearch) twice a week during the 6 wk of the experiment. The final injection was done 12 h before performing the colorectal distension. No behavioral defect was observed in mice chronically treated. Mice were anesthetized with xylazine/ketamine and implanted with two electrodes in the abdominal external oblique muscle as previously described (46). The mice were allowed to recover for 3 d before intrathecal (IT) injections and visceral sensitivity assessment. At the time of treatment, mice were anesthetized with 3% isoflurane, and IT injection was performed between the L5 and L6 vertebrae. Tail flick was used as an indication of successful entry into the intradural space.
Cathepsin S Activity.
Thoracolumbar (T12–L1) spinal cord was harvested and ground using a bullet blender (Nextadvance) with SSB05 beads (Nextadvance) in 200 μL of Opti-MEM medium (w/o Phenol red, Gibco). After centrifugation (7 min; 8,000 × g) 20 μL of the supernatant was used to assess protease activity. Supernatants were first incubated with 10 mM of the broad spectrum cysteine protease inhibitor E64 for 20 min. Cathepsin S activity was assessed by incubating the samples with Z-VVR-AMC substrate (50 μM, Enzo Life Sciences) in 0.01 M potassium phosphate buffer and 5 mM disodium EDTA, 20 mM L-Cysteine (Sigma-Aldrich), pH 7.5. Increase in fluorescence over 60 min was read at 37 °C in a VictorX5 plate reader (Perkin-Elmer). Fluorescence was normalized to protein content (measured by Bradford assay) and expressed as the percentage of increase compared with the first time point.
Spinal Microglia Isolation and Flow Cytometry.
Spinal cord was harvested by hydraulic extrusion using an 18-gauge needle inserted at the base of the spinal canal. Pooled spinal cords from two mice were gently ground using a Potter tissue grinder and digested for 40 min at 37 °C in HBSS (Sigma) 0.5 mg/mL Collagenase D (Roche) and 0.025 U/mL DNase I. Mononuclear cells were then isolated at the interface of a 35%/70% Percoll gradient and washed in PBS with 1% FBS and 2 mM EDTA, and used for FACS staining.
For G-CSFR staining, microglia were first incubated for 15 min at 4 °C with Fc block (eBioscience) and then incubated with either 1 μg rabbit polyclonal anti–G-CSFR antibody (clone H-176; Santa Cruz Biotechnology) or 1 μg control rabbit IgG (Jackson ImmunoResearch Laboratories) for 16 h at 4 °C. Cells were then washed and stained with an anti–rabbit IgG-Alexa488 (Invitrogen), anti-mouse CD11b-PercP Cy5.5 (clone M1/70; eBioscience), and anti-mouse CD45-PE (clone 30-F11; eBioscience) for 1 h at 4 °C. Cells were then analyzed using a FACS Canto II (BD Bioscience).
For G-CSF staining, spinal microglia were plated in DMEM supplemented with 10% heat-inactivated FBS (HI-FBS), 100 μg/mL streptomycin, and 100 U/mL penicillin (Invitrogen) in a 24-well plate and stimulated for 3 h with 500 ng⋅ml−1 of Phorbol 12-myristate 13-acetate (Sigma) and 500 ng⋅ml−1 ionomycin (Sigma). Brefeldin A (eBioscience) was added to the medium after 1 h of stimulation. Cells were then harvested, washed in PBS with 1% FBS and 2 mM EDTA, and incubated for 15 min at 4 °C with Fc block. After washing, cells were incubated for 20 min with anti-mouse CD11b-PercP Cy5.5 and anti-mouse CD45-PE (both from eBioscience). After washing, cells were fixed for 10 min with 2% paraformaldehyde (PFA) and then permeabilized with 0.5% Triton X-100 for 15 min at 4 °C. Cells were then incubated overnight with 1 μg Rat IgG2a anti-mouse G-CSF-eFluor 660 (clone 9B4CSF; eBioscience) or 1 μg isotype-matched control (clone eBR2a; eBioscience) diluted in PBS with 1% FBS and 0.1% Triton X-100. Cells were then washed and analyzed using a FACS Canto II (BD Bioscience).
BV-2 Cell Culture and DRG Neuron Isolation.
The murine microglial (BV-2) cells were grown to ∼80% confluence at 37 °C (5% CO2) in DMEM supplemented with 10% HI-FBS, 100 μg/mL streptomycin, and 100 U/mL penicillin (Invitrogen). Cells were plated on transwell inserts [polyethylene terephtalate (PET) membrane, 3-µm pores, Greiner Bio-One]. After 8 h, the cells were treated with 50 ng/mL G-CSF (Cedarlane) or vehicle control for 16 h.
DRGs were excised from 6-wk-old mice and enzymatically dissociated in HBSS containing 2 mg/mL collagenase and 4 mg/mL dispase (Invitrogen) for 45 min at 37 °C (65). DRGs were rinsed twice in HBSS and once in the culture medium mentioned above supplemented with 100 ng/mL Nerve Growth Factor (Invitrogen). Individual neurons were dispersed by trituration through a fire-polished glass Pasteur pipette in 4 mL media and cultured on glass coverslips, previously treated with HBSS with 25% Poly-Ornithin and Laminin (both from Sigma). Coverslips with DRG neurons were placed at the bottom of the transwell plate seeded with BV-2 cells previously treated with G-CSF and washed. The plates were maintained for another 16 h at 37 °C (5% CO2).
Electrophysiological Measurements.
Current clamp experiments were performed using small-diameter mouse DRG neurons at room temperature in a 2-mL bath containing (in mM): 140 NaCl, 5 KCl, 1.5 CaCl2, 2 MgCl2, 10 Hepes, and 10 D-glucose (pH 7.4 adjusted with NaOH and osmolarity of 315 mOsm). Borosilicate glass (Harvard Apparatus) pipettes were pulled and polished to 3–4 MΩ resistance with a DMZ-Universal Puller (Zeitz Instruments). Pipettes were filled with an internal solution containing (in mM): 5 NaCl, 1 CaCl2, 1 MgCl2, 10 Hepes, 5 EGTA, 5 MgATP, and 0.5 GTP (pH 7.3 adjusted with KOH, 315 mOsm). Recordings were performed using an Axopatch 200B amplifier (Axon Instruments) (66). Current clamp protocols were applied using pClamp 10.5 software (Axon Instruments). Data were filtered at 1 kHz (8-pole Bessel) and digitized at 10 kHz with a Digidata 1440 A converter (Axon Instruments). Average cell capacitance for the DRG neurons was 13.46 ± 0.98 pF for the non-G-CSF–treated cells and 12.59 ± 0.83 pF for the G-CSF–treated ones. Only capsaicin-responsive cells that exhibited a stable voltage control throughout the recording were used for analysis. All of the drugs used [G-CSF 50 ng/mL (Cedarlane), CX3CR1 antibody 2 µg/mL (Torrey Pines Biolabs), Cathepsin S Inhibitor 1 µM (Calbiochem), L-NAME 100 µM (Sigma), apyrase 5 U/mL (Sigma), and capsaicin 1 µM (Sigma)] were diluted in the extracellular buffer from a stock solution to achieve final concentrations. All solutions were prepared and used at room temperature (22 ± 2 °C). The number of spontaneous APs was calculated as the number of APs per second during extracellular buffer application. The number of evoked APs was calculated as APs per second evoked by increasing injected currents.
Immunohistochemistry.
After intracardiac blood puncture, mice were perfused with 10 mL of cold PBS to wash out the remaining blood and then perfused with 4% PFA. Lumbar spinal cord was harvested and then postfixed with 4% PFA for 16 h and incubated in 30% sucrose at 4 °C for 1 d before being embedded in optimal cutting temperature solution (Thermo-Fisher Scientific). Frozen samples were stored at −20 °C until used. Embedded tissues were sliced (10-μm thickness) and mounted on Superfrost Plus slides (VWR International). Slides were washed twice in PBS, and nonspecific binding was blocked for 1 h at room temperature in PBS containing 0.3% Triton X-100 (Sigma-Aldrich) and 3% HI-FBS. Slides were incubated at 4 °C overnight with rabbit anti-mouse Iba-1 (1:1,000; Wako Chemicals). Slides were washed in PBS twice and incubated in Alexa 555-conjugated anti-rabbit antibodies (1:1,000; Invitrogen). After several washes, slides were mounted with Aqua PolyMount (Polysciences). Images were taken with a Zeiss LSM 510 Meta confocal microscope and AxioCam HRm camera and analyzed using a 20× objective. Sections of 10 µm with a 30-µm space between each section were imaged and analyzed using Image J. The three control and three DSS mice were analyzed through three separated experiments (DSS regimen, mouse fixation, and immunostaining). A total of 35 immunostained sections were used for statistical analysis.
mRNA Extraction and Quantitative PCR from Spinal Cord.
Spinal cord from control or colitis mice were collected in RNA Later (Invitrogen), incubated at 4 °C overnight, and stored at −80 °C until used. mRNA extraction and quantitative PCR were performed as previously described. Briefly, tissue samples were homogenized in RLT buffer (Qiagen) using 0.5-mm stainless steel beads and a Bullet blender. Total RNA was extracted using an RNeasy Mini kit (Qiagen), according to the manufacturer’s instructions, including on-column DNase digestion. The quality and quantity of RNA were determined using a Nanodrop 2000c spectrophotometer (Thermo-Fisher Scientific). Relative G-CSFR gene expression (normalized to GAPDH) was determined by qPCR using Quantitect SYBR Green PCR Master Mix (Qiagen) and a StepOnePlus real-time PCR detection system (Applied Biosystems). The following primers were used: G-CSFR—TATGCTAGGGTCCAGCGAGT and GGGAGGCTCCAATTTCACA; GAPDH—GATGCTGGTGCTGAGTATGTCG and GTGGTGCAGGATGCATTGCTGA.
Western Blot.
Spinal cord (T12–L1) and DRGs (T12–L1) were harvested, and tissues were homogenized in RIPA buffer [1× PBS, 1% Igepal CA-630, 0.5% sodium deoxycholate, and 0.1% SDS (all from Sigma-Aldrich)] containing Complete-Mini protease inhibitor (Roche Diagnostics). Lysates were centrifuged at 10,000 × g for 10 min at 4 °C, supernatants were collected, and protein concentration was quantified and normalized using a Bradford assay (Bio-Rad Laboratories). Total lysates were separated by SDS/PAGE (7–10%) and transferred onto nitrocellulose membranes (Sigma-Aldrich). Membranes were blocked in 5% nonfat dry milk for 1 h at room temperature and then probed with anti–G-CSFR antibody (1/200 dilution in 5% milk; H-176; Santa Cruz Biotechnology) or anti-iNOS antibody (1/200 dilution in 5% milk; ab15323, Abcam) at 4 °C overnight. Membranes were then washed three times with Tris-buffered saline-Tween 20 and incubated with horseradish peroxidase (HRP)-conjugated anti-mouse or anti-rabbit antibodies (1:1,000; GE Healthcare) for 1 h at room temperature. Bands were visualized using the Immobilon Western chemiluminescent HRP Substrate (Millipore), and band density was calculated using Image J. Intensity of rabbit anti-Beta II tubulin antibody (1/1,000 dilution in 5% milk; T2200; Sigma-Aldrich) or mouse anti-GAPDH antibody (1/1,000 dilution in 5% milk; 1D4; Santa Cruz Biotechnology) band was used for normalization between samples.
Statistical Analysis.
Numeric values were expressed as means ± SEM. Mann–Whitney U test was used to assess statistical significance when comparing two means, whereas two-way ANOVA followed by the Bonferroni post hoc test was used to compare three or more groups for colorectal distension. Statistical significance was established at P ≤ 0.05. Statistical analyses were performed using Prism 6 (GraphPad).
For electrophysiology data, analysis was completed using Clampfit 10.5 software (Axon Instruments). Graphs and statistical analyses were performed using Origin 7.0 analysis software (OriginLab). All averaged data are plotted as mean ± SEM, and numbers in parentheses reflect the number of cells (n). One-way analysis of variance (ANOVA) followed by the Bonferroni post hoc test was used for multiple comparisons.
Acknowledgments
We thank Plexxikon for providing the PLX 5622 diet; Dr. Celine Deraison for technical assistance with the Cathepsin S assay; Laurie Alston for the MPO assay; Dr. Karen K. H. Poon and the Nicole Perkins Microbial Communities Core Labs for technical assistance with FACS acquisition; and Dr. Nathalie Vergnolle for providing critical comments. This work was supported by operating grants from the Canadian Institutes of Health Research (to C.A., D.M.K., and M.D.H.) and by the Vi Riddell Child Pain Program of the Alberta Children’s Hospital Research Institute (C.A.). C.A. holds a Canada Research Chair in Inflammatory Pain (Tier2). L.B. holds an “Eyes High” fellowship from the University of Calgary.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1706053114/-/DCSupplemental.
References
- 1.Basso L, Bourreille A, Dietrich G. Intestinal inflammation and pain management. Curr Opin Pharmacol. 2015;25:50–55. doi: 10.1016/j.coph.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Farrokhyar F, Marshall JK, Easterbrook B, Irvine EJ. Functional gastrointestinal disorders and mood disorders in patients with inactive inflammatory bowel disease: Prevalence and impact on health. Inflamm Bowel Dis. 2006;12:38–46. doi: 10.1097/01.mib.0000195391.49762.89. [DOI] [PubMed] [Google Scholar]
- 3.Teruel C, Garrido E, Mesonero F. Diagnosis and management of functional symptoms in inflammatory bowel disease in remission. World J Gastrointest Pharmacol Ther. 2016;7:78–90. doi: 10.4292/wjgpt.v7.i1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young Casey C, Greenberg MA, Nicassio PM, Harpin RE, Hubbard D. Transition from acute to chronic pain and disability: A model including cognitive, affective, and trauma factors. Pain. 2008;134:69–79. doi: 10.1016/j.pain.2007.03.032. [DOI] [PubMed] [Google Scholar]
- 5.Woolf CJ. What is this thing called pain? J Clin Invest. 2010;120:3742–3744. doi: 10.1172/JCI45178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bourinet E, et al. Calcium-permeable ion channels in pain signaling. Physiol Rev. 2014;94:81–140. doi: 10.1152/physrev.00023.2013. [DOI] [PubMed] [Google Scholar]
- 7.Lapointe TK, et al. TRPV1 sensitization mediates postinflammatory visceral pain following acute colitis. Am J Physiol Gastrointest Liver Physiol. 2015;309:G87–G99. doi: 10.1152/ajpgi.00421.2014. [DOI] [PubMed] [Google Scholar]
- 8.Deiteren A, et al. Histamine H4 and H1 receptors contribute to postinflammatory visceral hypersensitivity. Gut. 2014;63:1873–1882. doi: 10.1136/gutjnl-2013-305870. [DOI] [PubMed] [Google Scholar]
- 9.Zhou Q, Price DD, Caudle RM, Verne GN. Visceral and somatic hypersensitivity in a subset of rats following TNBS-induced colitis. Pain. 2008;134:9–15. doi: 10.1016/j.pain.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Long MD, Drossman DA. Inflammatory bowel disease, irritable bowel syndrome, or what?: A challenge to the functional-organic dichotomy. Am J Gastroenterol. 2010;105:1796–1798. doi: 10.1038/ajg.2010.162. [DOI] [PubMed] [Google Scholar]
- 11.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brierley SM, Linden DR. Neuroplasticity and dysfunction after gastrointestinal inflammation. Nat Rev Gastroenterol Hepatol. 2014;11:611–627. doi: 10.1038/nrgastro.2014.103. [DOI] [PubMed] [Google Scholar]
- 13.Grace PM, Hutchinson MR, Maier SF, Watkins LR. Pathological pain and the neuroimmune interface. Nat Rev Immunol. 2014;14:217–231. doi: 10.1038/nri3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nam Y, et al. Reversible induction of pain hypersensitivity following optogenetic stimulation of spinal astrocytes. Cell Rep. 2016;17:3049–3061. doi: 10.1016/j.celrep.2016.11.043. [DOI] [PubMed] [Google Scholar]
- 15.Noor S, et al. Prenatal alcohol exposure potentiates chronic neuropathic pain, spinal glial and immune cell activation and alters sciatic nerve and DRG cytokine levels. Brain Behav Immun. 2017;61:80–95. doi: 10.1016/j.bbi.2016.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu X, et al. Sustained elevated adenosine via ADORA2B promotes chronic pain through neuro-immune interaction. Cell Rep. 2016;16:106–119. doi: 10.1016/j.celrep.2016.05.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beggs S, Trang T, Salter MW. P2X4R+ microglia drive neuropathic pain. Nat Neurosci. 2012;15:1068–1073. doi: 10.1038/nn.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nayak D, Roth TL, McGavern DB. Microglia development and function. Annu Rev Immunol. 2014;32:367–402. doi: 10.1146/annurev-immunol-032713-120240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsuda M, et al. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424:778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- 20.Old EA, Malcangio M. Chemokine mediated neuron-glia communication and aberrant signalling in neuropathic pain states. Curr Opin Pharmacol. 2012;12:67–73. doi: 10.1016/j.coph.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 21.Burke NN, Fan CY, Trang T. Microglia in health and pain: Impact of noxious early life events. Exp Physiol. 2016;101:1003–1021. doi: 10.1113/EP085714. [DOI] [PubMed] [Google Scholar]
- 22.Agostini S, et al. Evidence of central and peripheral sensitization in a rat model of narcotic bowel-like syndrome. Gastroenterology. 2010;139:553–563, 563.e1-5. doi: 10.1053/j.gastro.2010.03.046. [DOI] [PubMed] [Google Scholar]
- 23.Acharya MM, et al. Elimination of microglia improves cognitive function following cranial irradiation. Sci Rep. 2016;6:31545. doi: 10.1038/srep31545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guan Z, et al. Injured sensory neuron-derived CSF1 induces microglial proliferation and DAP12-dependent pain. Nat Neurosci. 2016;19:94–101. doi: 10.1038/nn.4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malcangio M. Microglia and chronic pain. Pain. 2016;157:1002–1003. doi: 10.1097/j.pain.0000000000000353. [DOI] [PubMed] [Google Scholar]
- 26.Bavencoffe A, et al. Persistent electrical activity in primary nociceptors after spinal cord injury is maintained by scaffolded adenylyl cyclase and protein kinase A and is associated with altered adenylyl cyclase regulation. J Neurosci. 2016;36:1660–1668. doi: 10.1523/JNEUROSCI.0895-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Voisin T, Bourinet E, Lory P. Genetic alteration of the metal/redox modulation of Cav3.2 T-type calcium channel reveals its role in neuronal excitability. J Physiol. 2016;594:3561–3574. doi: 10.1113/JP271925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nieto FR, et al. Neuron-immune mechanisms contribute to pain in early stages of arthritis. J Neuroinflammation. 2016;13:96. doi: 10.1186/s12974-016-0556-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farrell KE, Keely S, Graham BA, Callister R, Callister RJ. A systematic review of the evidence for central nervous system plasticity in animal models of inflammatory-mediated gastrointestinal pain. Inflamm Bowel Dis. 2014;20:176–195. doi: 10.1097/01.MIB.0000437499.52922.b1. [DOI] [PubMed] [Google Scholar]
- 30.Chesnokova V, Pechnick RN, Wawrowsky K. Chronic peripheral inflammation, hippocampal neurogenesis, and behavior. Brain Behav Immun. 2016;58:1–8. doi: 10.1016/j.bbi.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riazi K, et al. Microglial activation and TNFalpha production mediate altered CNS excitability following peripheral inflammation. Proc Natl Acad Sci USA. 2008;105:17151–17156. doi: 10.1073/pnas.0806682105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zonis S, et al. Chronic intestinal inflammation alters hippocampal neurogenesis. J Neuroinflammation. 2015;12:65. doi: 10.1186/s12974-015-0281-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang G, et al. Hippocampal microglial activation and glucocorticoid receptor down-regulation precipitate visceral hypersensitivity induced by colorectal distension in rats. Neuropharmacology. 2016;102:295–303. doi: 10.1016/j.neuropharm.2015.11.028. [DOI] [PubMed] [Google Scholar]
- 34.Hamilton JA, Cook AD, Tak PP. Anti-colony-stimulating factor therapies for inflammatory and autoimmune diseases. Nat Rev Drug Discov. 2016;16:53–70. doi: 10.1038/nrd.2016.231. [DOI] [PubMed] [Google Scholar]
- 35.Schneider A, et al. The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. J Clin Invest. 2005;115:2083–2098. doi: 10.1172/JCI23559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aloisi F, et al. Production of hemolymphopoietic cytokines (IL-6, IL-8, colony-stimulating factors) by normal human astrocytes in response to IL-1 beta and tumor necrosis factor-alpha. J Immunol. 1992;149:2358–2366. [PubMed] [Google Scholar]
- 37.Ding J, et al. Rho kinase inhibitor Fasudil induces neuroprotection and neurogenesis partially through astrocyte-derived G-CSF. Brain Behav Immun. 2009;23:1083–1088. doi: 10.1016/j.bbi.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 38.Mazzio EA, et al. Natural product HTP screening for antibacterial (E. coli 0157:H7) and anti-inflammatory agents in (LPS from E. coli O111:B4) activated macrophages and microglial cells; focus on sepsis. BMC Complement Altern Med. 2016;16:467. doi: 10.1186/s12906-016-1429-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strecker JK, et al. Monocyte chemoattractant protein-1-deficiency impairs the expression of IL-6, IL-1β and G-CSF after transient focal ischemia in mice. PLoS One. 2011;6:e25863. doi: 10.1371/journal.pone.0025863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Veroni C, et al. Activation of TNF receptor 2 in microglia promotes induction of anti-inflammatory pathways. Mol Cell Neurosci. 2010;45:234–244. doi: 10.1016/j.mcn.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 41.Schweizerhof M, et al. Hematopoietic colony-stimulating factors mediate tumor-nerve interactions and bone cancer pain. Nat Med. 2009;15:802–807. doi: 10.1038/nm.1976. [DOI] [PubMed] [Google Scholar]
- 42.Guo Y, et al. Granulocyte colony-stimulating factor improves alternative activation of microglia under microenvironment of spinal cord injury. Neuroscience. 2013;238:1–10. doi: 10.1016/j.neuroscience.2013.01.047. [DOI] [PubMed] [Google Scholar]
- 43.Chen CH, et al. Intrathecal granulocyte colony-stimulating factor modulate glial cell line-derived neurotrophic factor and vascular endothelial growth factor A expression in glial cells after experimental spinal cord ischemia. Neuroscience. 2013;242:39–52. doi: 10.1016/j.neuroscience.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 44.Yamasaki R, et al. Restoration of microglial function by granulocyte-colony stimulating factor in ALS model mice. J Neuroimmunol. 2010;229:51–62. doi: 10.1016/j.jneuroim.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 45.Lee MC, et al. G-CSF receptor blockade ameliorates arthritic pain and disease. J Immunol. 2017;198:3565–3575. doi: 10.4049/jimmunol.1602127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boué J, et al. Endogenous regulation of visceral pain via production of opioids by colitogenic CD4(+) T cells in mice. Gastroenterology. 2014;146:166–175. doi: 10.1053/j.gastro.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 47.Cattaruzza F, et al. Cathepsin S is activated during colitis and causes visceral hyperalgesia by a PAR2-dependent mechanism in mice. Gastroenterology. 2011;141:1864–1874.e1-3. doi: 10.1053/j.gastro.2011.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bali KK, et al. Transcriptional mechanisms underlying sensitization of peripheral sensory neurons by granulocyte-/granulocyte-macrophage colony stimulating factors. Mol Pain. 2013;9:48. doi: 10.1186/1744-8069-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carvalho TT, et al. Granulocyte-colony stimulating factor (G-CSF)-induced mechanical hyperalgesia in mice: Role for peripheral TNFα, IL-1β and IL-10. Eur J Pharmacol. 2015;749:62–72. doi: 10.1016/j.ejphar.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 50.Okubo M, et al. Macrophage-colony stimulating factor derived from injured primary afferent induces proliferation of spinal microglia and neuropathic pain in rats. PLoS One. 2016;11:e0153375. doi: 10.1371/journal.pone.0153375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu PY, et al. Spinal microglia initiate and maintain hyperalgesia in a rat model of chronic pancreatitis. Gastroenterology. 2012;142:165–173.e2. doi: 10.1053/j.gastro.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 52.Bradesi S, et al. Role of spinal microglia in visceral hyperalgesia and NK1R up-regulation in a rat model of chronic stress. Gastroenterology. 2009;136:1339–1348, e1-2. doi: 10.1053/j.gastro.2008.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saab CY, Wang J, Gu C, Garner KN, Al-Chaer ED. Microglia: A newly discovered role in visceral hypersensitivity? Neuron Glia Biol. 2006;2:271–277. doi: 10.1017/S1740925X07000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kannampalli P, et al. Analgesic effect of minocycline in rat model of inflammation-induced visceral pain. Eur J Pharmacol. 2014;727:87–98. doi: 10.1016/j.ejphar.2014.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao P, et al. Cathepsin S causes inflammatory pain via biased agonism of PAR2 and TRPV4. J Biol Chem. 2014;289:27215–27234. doi: 10.1074/jbc.M114.599712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clark AK, et al. Inhibition of spinal microglial cathepsin S for the reversal of neuropathic pain. Proc Natl Acad Sci USA. 2007;104:10655–10660. doi: 10.1073/pnas.0610811104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Clark AK, Grist J, Al-Kashi A, Perretti M, Malcangio M. Spinal cathepsin S and fractalkine contribute to chronic pain in the collagen-induced arthritis model. Arthritis Rheum. 2012;64:2038–2047. doi: 10.1002/art.34351. [DOI] [PubMed] [Google Scholar]
- 58.Clark AK, Yip PK, Malcangio M. The liberation of fractalkine in the dorsal horn requires microglial cathepsin S. J Neurosci. 2009;29:6945–6954. doi: 10.1523/JNEUROSCI.0828-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pedersen LM, Jacobsen LM, Mollerup S, Gjerstad J. Spinal cord long-term potentiation (LTP) is associated with increased dorsal horn gene expression of IL-1beta, GDNF and iNOS. Eur J Pain. 2010;14:255–260. doi: 10.1016/j.ejpain.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 60.Heine S, et al. CNGA3: A target of spinal nitric oxide/cGMP signaling and modulator of inflammatory pain hypersensitivity. J Neurosci. 2011;31:11184–11192. doi: 10.1523/JNEUROSCI.6159-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carr FB, Géranton SM, Hunt SP. Descending controls modulate inflammatory joint pain and regulate CXC chemokine and iNOS expression in the dorsal horn. Mol Pain. 2014;10:39. doi: 10.1186/1744-8069-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gühring H, et al. Suppressed injury-induced rise in spinal prostaglandin E2 production and reduced early thermal hyperalgesia in iNOS-deficient mice. J Neurosci. 2000;20:6714–6720. doi: 10.1523/JNEUROSCI.20-17-06714.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Iwasaki A. Immune regulation of antibody access to neuronal tissues. Trends Mol Med. 2017;23:227–245. doi: 10.1016/j.molmed.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Siegel CA, MacDermott RP. Is chronic pain an extraintestinal manifestation of IBD? Inflamm Bowel Dis. 2009;15:769–771. doi: 10.1002/ibd.20844. [DOI] [PubMed] [Google Scholar]
- 65.Flynn R, et al. Targeting the transient receptor potential vanilloid type 1 (TRPV1) assembly domain attenuates inflammation-induced hypersensitivity. J Biol Chem. 2014;289:16675–16687. doi: 10.1074/jbc.M114.558668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Iftinca M, et al. The stress protein heat shock cognate 70 (Hsc70) inhibits the transient receptor potential vanilloid type 1 (TRPV1) channel. Mol Pain. 2016;12:pii: 1744806916663945. doi: 10.1177/1744806916663945. [DOI] [PMC free article] [PubMed] [Google Scholar]