Significance
Each year, Streptococcus pneumoniae infections cause millions of deaths worldwide. The capsular polysaccharide (CPS) based glycoconjugate vaccine Prevnar13 prevents serious illness caused by 13 serotypes. S. pneumoniae serotype 5 (ST-5) is included in the vaccine; however, it suffers from production problems due to modifications or degradation during isolation and conjugation. A medicinal chemistry approach helped to understand the structural features of ST-5 CPS and design a stable semisynthetic oligosaccharide-based vaccine candidate. Oligosaccharide leads for immunological evaluations in vivo were identified employing glycan microarrays. The stable monovalent ST-5 oligosaccharide glycoconjugate vaccine candidate showed a superior immune response in rabbits when compared with the ST-5 CPS present in the multivalent vaccine Prevnar13.
Keywords: glycoconjugate, vaccine, S. pneumoniae, serotype 5, carbohydrate chemistry
Abstract
Glycoconjugate vaccines based on isolated capsular polysaccharide (CPS) save millions of lives annually by preventing invasive pneumococcal disease caused by Streptococcus pneumoniae. Some components of the S. pneumoniae glycoconjugate vaccine Prevnar13 that contains CPS antigens from 13 serotypes undergo modifications or degradation during isolation and conjugation, resulting in production problems and lower efficacy. We illustrate how stable, synthetic oligosaccharide analogs of labile CPS induce a specific protective immune response against native CPS using S. pneumoniae serotype 5 (ST-5), a problematic CPS component of Prevnar13. The rare aminosugar l-PneuNAc and a branched l-FucNAc present in the natural repeating unit (RU) are essential for antibody recognition and avidity. The epitope responsible for specificity differs from the part of the antigen that is stabilized by chemical modification. Glycoconjugates containing stable, monovalent synthetic oligosaccharide analogs of ST-5 CPS RU induced long-term memory and protective immune responses in rabbits superior to those elicited by the ST-5 CPS component in multivalent Prevnar13.
Pneumococcal infections continue to cause millions of fatalities among children and the elderly despite the widespread use of glycoconjugate vaccines (Synflorix, Prevnar13) (1). These vaccines aim at inducing an immune response against bacterial capsular polysaccharide (CPS) not present on human cells (2). Streptococcus pneumoniae is a Gram-positive human pathogen covered by CPS that is diverse and contains rare sugars (3, 4). All currently marketed pneumococcal vaccines (5) are manufactured using CPS isolated from the surface of S. pneumoniae.
S. pneumoniae serotype 5 (ST-5) is the fifth most prevalent among more than 90 S. pneumoniae serotypes with different CPS, causing invasive pneumococcal disease among young children globally (6, 7). Marketed glycoconjugate vaccines are not fully efficacious in preventing ST-5 infections (8). A change in the CPS glycan structure during antigen isolation and purification such that the ST-5 antigens no longer sufficiently resemble the native CPS may compromise vaccine efficacy (9, 10). Manufacturing glycoconjugate vaccines such as for ST-5 can be problematic when CPS contains labile groups (11–13).
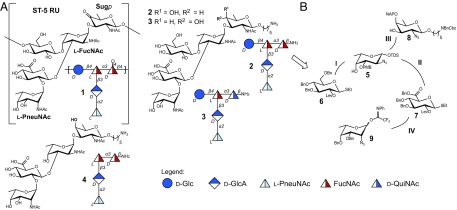
The ST-5 repeating unit (RU) structure was assigned in 1985 following the initial classification in 1929 (3, 11–14). The branched pentasaccharide ST-5 CPS RU 1 contains a central N-acetyl-l-fucosamine (l-FucNAc) amino sugar that is linked to d-glucose at C4 and to d-glucuronic acid at C3 (Fig. 1A). Two rare deoxyamino sugars, the ketoamino sugar 2-acetamido-2,6-dideoxy-d-xylose-hexos-4-ulose (Sugp) and N-acetyl-l-pneumosamine (l-PneuNAc), complete the RU.
Fig. 1.
ST-5 CPS RU (1) and synthetic antigen targets (2–4). (A) Structure of the ST-5 CPS RU and synthetic antigen targets. (B) Retrosynthetic analysis of ST-5 reduced RU 2.
Marketed glycoconjugate vaccines are manufactured from either native or depolymerized CPS (15) that is typically coupled to a carrier protein via reductive amination following isolation. The keto group present in the rare sugar Sugp is partially or fully reduced to form a mixture of ST-5 CPS components and degrades during ST-5 glycoconjugate production (10). The complex CPS thus generated is characterized by variable RUs, leading to manufacturing issues and decreased immunogenicity compared with the native ST-5 CPS (10).
Defined synthetic antigens are essential tools to identify protective glycan epitopes for the development of semisynthetic glycoconjugate vaccines (2). Employing synthetic ST-5 glycans based on the CPS RU provides valuable insights into how changes to the natural ST-5 CPS may influence antigen stability and immunogenicity. A flexible total synthesis approach had to be conceived to provide access not only to the natural keto containing RU 1 but also to the reduced forms of 1, particularly oligosaccharides 2, 3, and 4 (Fig. 1A). General aspects of vaccine design relating to the effect of branching, length, and the role of unique sugars like l-PneuNAc and Sugp on overall immunogenicity and protection in the context of the RU 1 had to be considered. A series of oligosaccharides related to the RU of ST-5 CPS equipped with a reducing end linker were synthesized to be fixed on glycan arrays and conjugated to the carrier protein diphtheria toxin mutant CRM197, currently used in the vaccine Prevnar13 (16).
A retrosynthetic analysis of the ST-5 RU revealed the need for five differentially protected monosaccharide building blocks (5–9). The assembly of reduced ST-5 RU glycans 2 and 3 (Fig. 1B) requires the procurement of the rare amino sugars l-pneumosamine (9) via a novel synthetic route, as little attention has been paid to this sugar (17–19). l- and d-fucosamine (5 and 8), the other two aminosugars, as well as d-glucose (6) and d-glucuronic acid (7) building blocks, will be synthesized via modified literature protocols (20, 21) (Fig. 1B). The synthetically challenging branched 3,4-substituted l-fucosamine has to be installed early in the oligosaccharide syntheses to avoid issues related to sterics and reactivity. d-Fucosamine and l-pneumosamine will be added sequentially at the end of the assembly process (Fig. 1B). Finally, reduction and acetylation of the azido groups followed by global deprotection will provide the desired glycans.
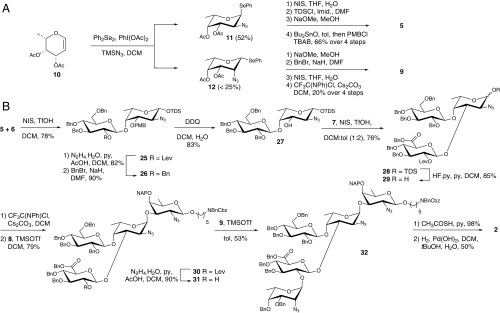
The synthesis commenced with the procurement of the differentially protected monosaccharide building blocks. While known synthetic approaches to l-fucosamine building blocks are sufficient (22–24), the few routes to l-pneumosamine suffer from low yields (17–19). The azido-phenylselenation reaction was selected as key to access mainly l-fucosamine (8) and less l-pneumosamine (9) from l-fucal (10) (22) (Scheme 1A). Protecting group manipulations of 11 and 12 provided nucleophile 5 and glycosylating agent 9, respectively (Scheme 1A). d-fucosamine 8 was derived from d-fucal using the same approach (SI Appendix, Scheme S1). The syntheses of differentially protected d-glucose 6 (20) and d-glucuronic acid 7 building blocks followed established procedures (SI Appendix, Scheme S2).
Scheme 1.
Synthesis of ST-5 reduced RU. (A) Synthesis of building blocks 5 and 9. (B) Synthesis of pentasaccharide 2. BnBr, benzyl bromide; DCM, dichloromethane; DDQ, 2,3-dichloro-5,6-dicyano-1,4-benzoquinone; Lev, levulinoyl group; NIS, N-iodosuccinimide, py, pyridine; TDS, thexyl-dimethylsilyl; TfOH, trifluoromethanesulfonic acid; TMSOTf, trimethylsilyl trifluoromethanesulfonate; Tol, toluene.
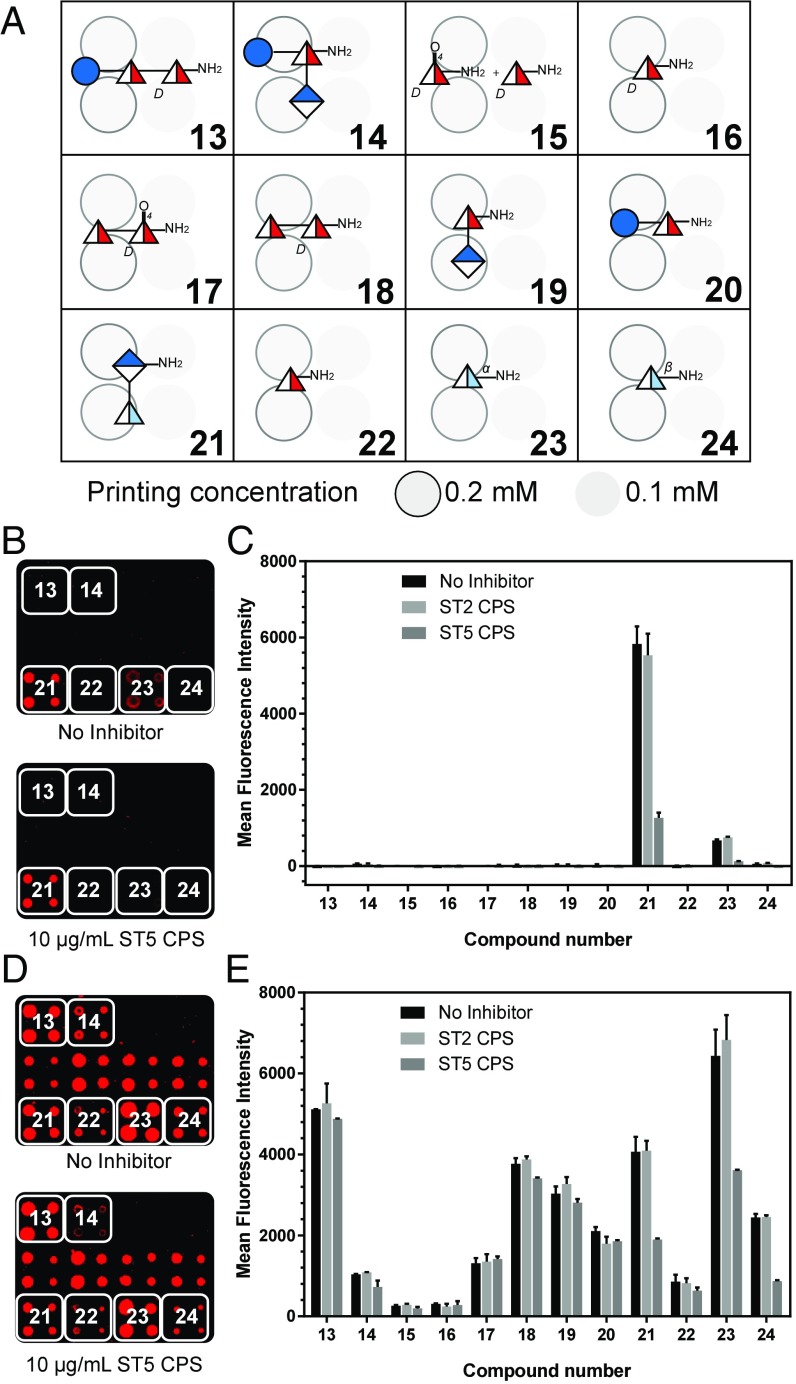
Using the building blocks that were designed for potential access to the complete RU including the Sugp residue, several saccharides were synthesized for microarray analyses to identify immunodominant fragments of the RU (Fig. 2A). While the synthetically challenging Sugp was included in this microarray analysis, the binding pattern seen after incubation with two different sera strongly suggested that the GlcA-PneuNAc branch is the most important substructure for tight antibody binding. A rabbit serum used to identify ST-5 strains in serotyping procedures showed strong reactivity to disaccharide 21 representing the branch, followed by the PneuNAc monosaccharide 23 in alpha configuration with already comparatively lower signals (Fig. 2 B and C).
Fig. 2.
Microarray screening to identify ST-5 oligosaccharide fragments as hits for vaccine development. (A) Microarray printing pattern with ST-5 RU fragments. Printed arrays were incubated with (B) rabbit typing serum (dilution 1:1,800) or (D) human reference serum (007sp, dilution 1:40). The bound antibodies were detected using fluorescently labeled secondary antibodies. For the inhibition study, sera were preincubated with ST-5 CPS (10 µg/mL) and ST-2 CPS (10 µg/mL) as a control. ST-2 CPS was used as control because it resembles ST-5 CPS containing a branched linkage, a common GlcA moiety, and three deoxy sugars. Printed arrays were incubated with the above sera, and inhibition was analyzed by using fluorescently labeled secondary antibodies. Mean fluorescence intensities (MFI) of inhibition assay with (C) rabbit typing serum and (E) human reference serum (007sp) are shown. Data are represented as mean ± SD of duplicate determinations.
Importantly, an inhibition assay using native ST-5 CPS to capture ST-5-specific antibodies led to high degrees of signal suppression for all recognized oligosaccharides. The human reference serum pool 007sp (25) was used to confirm the findings of the rabbit serum but revealed a less clear picture (Fig. 2 D and E). Analogous to the rabbit serum, the PneuNAc containing glycans 21, 23, and 24 showed higher degrees of signal suppression compared with the other oligosaccharides, suggesting that the branch is also important for reactivity in humans, the ultimate recipients of vaccines. After identifying the branch as immunodominant, we focused the RU synthesis on the more accessible reduced glycans 2, 3, and 4 that were expected to be more stable than a Sugp containing compound.
The assembly of pentasaccharide 2 began with the synthesis of branched trisaccharide 28 (Scheme 1B). Glycosylation of the axial C4-hydroxy group in 5 with glycosylating agent 6 gave β-configured disaccharide 25. The participating levulinic ester was exchanged for a benzyl ether before p-methoxy benzyl (PMB) ether cleavage to furnish nucleophile 27. Glycosylation of 27 with thioglucoside 7 gave the desired trisaccharide 28 as a single anomer. The anomeric linkages of trisaccharide 28 were ascertained unambiguously by 1D z-filtered total correlation spectroscopy (zTOCSY) NMR measurements upon removal of the levulinic ester (SI Appendix, Fig. S1).
Trisaccharide imidate 29 was obtained from 28, by desilylation and formation of the glycosyl imidate. Glycosylation using d-FucNAc building block 8 furnished tetrasaccharide 30 (Scheme 1B). Cleavage of the temporary levulinic ester protecting group in 30 produced nucleophile 31. Glycosylation of 31 with l-pneumosazide 9 gave fully protected α-linked pentasaccharide 32. Branched pentasaccharide 2 was obtained by converting the azides in 32 to N-acetyl moieties using thioacetic acid followed by global deprotection via hydrogenolysis (Scheme 1B).
The synthesis of branched pentasaccharide 3 (Fig. 1A) containing a reducing end N-acetyl-d-quinovosamine (d-QuiNAc) began with d-FucNAc building block 8 (SI Appendix, Scheme S1). The d-QuiNAc acceptor 37 was engaged in the synthesis of pentasaccharide 3 in a manner analogous to nucleophile 8, following the reaction sequences detailed for the synthesis of 2 (SI Appendix, Scheme S3). To better understand the role played by the unique amino sugars and branching in the ST-5 RU, a series of related sequences, including the linear tetrasaccharide 4 (Fig. 1A), were synthesized (SI Appendix, Scheme S4). Attempts to synthesize the natural ketone-containing RU 1 did not meet with success, as the intermediate ketone proved highly unstable and could not be isolated in pure form.
Reanalysis of the rabbit typing serum employing an extended glycan array (SI Appendix, Fig. S2) illustrated that the epitope recognized by anti–ST-5 CPS antibodies expanded beyond the disaccharide branch, with much stronger recognition of pentasaccharides 2 and 3 compared with the previous lead, disaccharide 21 (SI Appendix, Fig. S3). Tetrasaccharide 4 lacking the d-Glc connected to the FucNAc showed an intermediate signal. These larger oligosaccharides exhibited an encouraging intensity and inhibition pattern with human reference serum 007sp, thus suggesting a role of the larger glycan epitopes in antipneumococcal immunity against ST-5 strains in a human vaccine setting. Glycan array analyses of anti-Prevnar13 rabbit serum gave similar results (SI Appendix, Fig. S4).
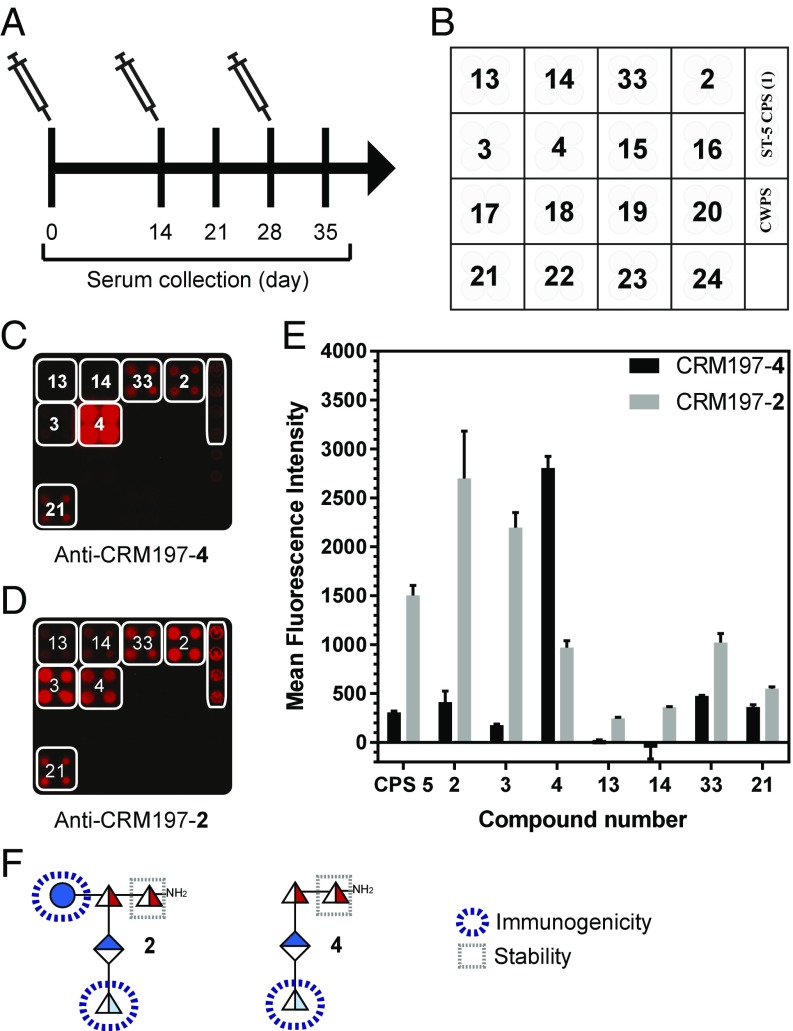
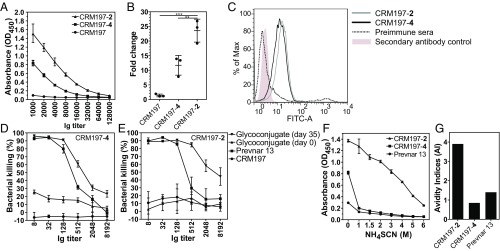
Based on insights concerning epitope size and the immunological response, pentasaccharide 2 and linear tetrasaccharide 4 were chosen for immunological evaluation, as they are significantly easier to synthesize than 3. Glycans 2 and 4 were conjugated to the carrier protein diphtheria toxin mutant CRM197 (SI Appendix, Fig. S5A) using the p-nitrophenyl adipate ester conjugation method (26) for single-point attachment and facile removal of excess coupling reagent, thus avoiding the formation of unwanted linker antibodies (27, 28). The glycoconjugates CRM197-2 and CRM197-4 contained 12 and 11 glycans per protein, respectively (SI Appendix, Fig. S5 B and C). Any new glycococonjugate vaccine lead compound against pneumococci has to induce a robust antibody response, as evidenced by a serological assay, antibody-mediated opsonic activity, and a long-term memory response laid out in the World Health Organization guidelines for pneumococcal glycoconjugate vaccines (29–31). Mindful of these requirements, the vaccine potential of glycoconjugates CRM197-2 and CRM197-4 was evaluated employing a multiarm study in rabbits, a reliable species for pneumococcal vaccination (32). CRM197-2 and CRM197-4 (10 µg of glycan per immunization) formulated with the adjuvant aluminum hydroxide were injected s.c. (Fig. 3A), and the immune response was assessed using glycan arrays (Fig. 3 B–E). CRM197-2 conjugate stimulated more cross-reactive antibodies than CRM197-4 against oligosaccharide 3 and the native ST-5 CPS. These results emphasize the importance of the d-glucose residue, present in 2 but not in 4, as vital for antibody recognition and cross-reactivity. The ST-5 epitope responsible for specificity (l-PneuNAc, d-Glc) differs from the reducing end sugar (d-FucNAc) modified to enhance stability (Fig. 3F). The branched pentasaccharide 2 is more immunogenic and a better mimic of the native ST-5 CPS, as conjugate CRM197-2 produced CPS-specific cross-reactive antibodies titers, in contrast to linear tetrasaccharide conjugate CRM197-4 (33, 34) (Fig. 4 A and B).
Fig. 3.
Microarray with rabbit anti–CRM197-2 and anti–CRM197-4 conjugates sera. (A) Immunization and sera collection schedule. (B) Microarray slide printing pattern. Rabbits were immunized with CRM197-2 and CRM197-4 conjugates in a prime boost manner on days 0, 14, and 28. Preimmune and hyperimmune sera were collected at different time points from individual rabbits. (C and D) Microarray slides were incubated with rabbit sera (day 35) raised against CRM197-2 and CRM197-4 conjugates. (E) MFI of cross-reactive spots are plotted as mean ± SD in duplicates. (F) Representation of the monosaccharide moieties responsible for immunogenicity and stability within glycans 2 and 4.
Fig. 4.
ELISA and OPKA. (A) Pooled sera of rabbits (n = 3) before and after immunization with CRM197-2 and CRM197-4 conjugates were collected, the end point titer was analyzed by ELISA, and data were plotted as mean ± SD. (B) The antibody response of day 35 postimmune sera in relation to preimmune sera. Each dot represents an individual rabbit immune response, and data were analyzed by an unpaired t test. P values of <0.05 were considered statistically significant. (*P < 0.05; **P < 0.01; ***P < 0.001.) (C) UV inactivated pneumococci were incubated with anti–CRM197-2 and anti–CRM197-4 antibodies, and surface staining was analyzed by flow cytometry. (D) OPKAs were performed with differentiated HL-60 cells (N,N-dimethylformamide, 5 d) and incubated with pneumococci preopsonized with CRM197-4 conjugate-specific antibodies in the ratio of 1:400 (bacteria to effector cell) or with control sera. Bacterial survival was assessed after 45-min incubation. Percent killing of pneumococci was calculated based on viable pneumococcal colonies obtained relative to control sera. The experiment was repeated three times, and representative values of one out of three independent experiments were plotted. Prevnar13 and CRM197 sera were used as positive and negative controls, respectively. Data of all experiments were represented as mean ± SD values of triplicates. (E) OPKA as described for D with CRM197-2 conjugate-specific antibodies. (F) The avidity of antibodies was obtained using increasing concentrations (0 M to 4 M) of NH4SCN to perturb antigen−antibody interactions. (G) The AI values were calculated and plotted against the corresponding antigen labels. Data are represented as mean ± SD, triplicate determinations.
Antibodies produced in response to glycoconjugate vaccines are only protective when they bind to the native CPS on the pneumococcal surface. The phagocytic activity of anti-CPS antibodies is a major protective mechanism against pneumococci that is governed by fixing the complement on the surface of bacteria (35). To this end, UV-inactivated pneumococcal bacteria were incubated with antibodies raised against CRM197-2 (gray histogram, Fig. 4C) and CRM197-4 (black histogram) conjugates and were analyzed by flow cytometry (Fig. 4C).
Given the limited accessibility of an ST-5 animal challenge model, the functional relevance of antibodies induced in response to immunization with CRM197-2 or CRM197-4 was tested using the standard pneumococcal opsonophagocytic killing assay (OPKA) employing an HL-60 cell line as a phagocyte source (36). Both CRM197-2 and CRM197-4 conjugates generated opsonizing antibodies that promoted the killing of pneumococci (Fig. 4 D and E) in an antibody titer-dependent fashion (Fig. 4A). Antibodies induced by monovalent CRM197-2 and CRM197-4 were compared with anti-Prevnar13 antibodies to address the question whether semisynthetic vaccines based on synthetic glycan antigens can provide a more robust antibody response and protection than traditional CPS conjugates. Antibodies induced by both of these monovalent semisynthetic glyconjugates showed better opsonization than those raised against the CPS containing multivalent vaccine Prevnar13. CRM197-2 antibodies showed a significantly greater response than CRM197-4 antibodies (Fig. 4 D and E), thereby proving the potential of synthetic oligosaccharides to generate CPS-specific antibodies. Thus, the above OPKA data should correlate well with an ST-5 challenge model, based on similar observations in the literature (28, 37). Opsonic activities needed for protection are not governed by titers alone but also by antibody avidity, which describes an entropy-driven improvement of antibody binding due to multivalency (38). This effect can be probed by binding experiments in the presence of a chaotropic, avidity-disrupting salt such as ammonium thiocyanate (NH4SCN) (39) (Fig. 4F). To further validate the attributes of antibodies generated using synthetically modified glycans, the 50% avidity index (AI) was calculated for sera of animals immunized with glycoconjugates CRM197-2, CRM197-4, and Prevnar13 compared with the NH4SCN-free samples against ST-5 CPS (Fig. 4G). Antibodies raised against the CRM197-2 conjugate exhibited a relatively high avidity (3.9 M), while those raised against CRM197-4 conjugate and Prevnar13 displayed relatively low avidities (0.85 and 1.4 M, respectively) (36). These observations demonstrate that the monovalent semisynthetic CRM197-2 is a better glycoconjugate vaccine candidate than CRM197-4 and the ST-5 CPS glycoconjugate present in multivalent Prevnar13. Thus, synthetic ST-5 RU 2 was identified as a lead compound for glycoconjugate vaccine development in preclinical studies.
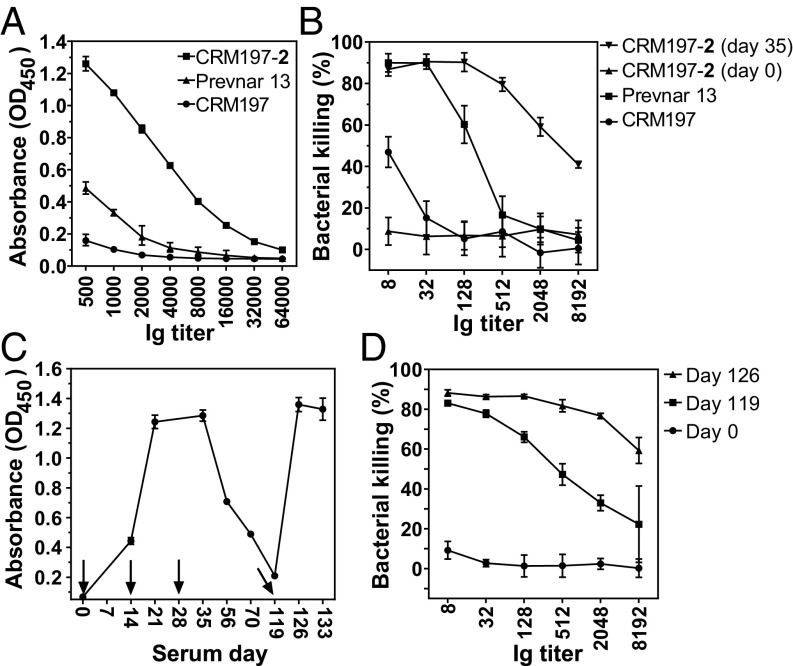
To compare ST-5 semisynthetic glycoconjugate CRM197-2 and ST-5 CPS glycoconjugate present in multivalent Prevnar13, rabbits were immunized with 2.2 µg of the glycan, a dose equivalent to that used in the Prevnar13. The ELISA results suggest that the CRM197-2 conjugate produces much higher antibody titers than Prevnar13 (Fig. 5A and SI Appendix, Fig. S6), with significantly higher opsonophagocytic killing properties (Fig. 5B). Prevnar13 containing glycoconjugates of multiple serotypes was utilized in this study as a reference, with the knowledge that the presence of additional serotypes can decrease the efficacy of individual serotypes in the vaccine (40, 41).
Fig. 5.
CRM197-2 conjugate produces very high opsonic antibody titer at 2.2 µg dose. (A) End point titer (total IgG) analysis performed on sera collected 1 wk after second booster immunization (day 35). (B) Antibodies promote pneumococcal killing by phagocytosis using differentiated HL-60 cells. (C) Antibody response analyzed by ELISA at day 119, confirming the long-term memory B-cell response. (D) Opsonophagocytosis performed with memory response sera and compared with preimmune sera (day 0) and after booster (day 126). Data are represented as mean ± SD, triplicate determinations.
Effective vaccines induce a long-term protective memory immune response that requires the presence of antibody-producing memory B cells. The memory B-cell response to CRM197-2 was determined over a period of 4 mo using the established prime boost regimen (Fig. 5C). Once antibody levels dropped to baseline, a CRM197-2 booster dose was administered on day 119. The additional dose recalled antibody levels to day 35 levels as assessed by analysis of CPS-specific antibodies by ELISA and OPKA. Clearly, the semisynthetic ST-5 glycoconjugate CRM197-2 induced a robust immunological memory (Fig. 5 C and D).
In summary, a medicinal chemistry approach to vaccine discovery yielded a promising candidate to protect from S. pneumoniae ST-5. While ST-5 CPS is contained in the multivalent vaccine Prevnar13, it suffers from serious production problems and varying efficacy that is caused by the presence of labile functional groups in the CPS. A series of oligosaccharides resembling the ST-5 RU were synthesized. Glycan array analyses of human reference sera and immunization experiments in rabbits identified the rare aminosugar l-PneuNAc, as well as branching, as key to antibody recognition and avidity. Oligosaccharide 2 containing a secondary alcohol in place of the labile ketone in native ST-5 CPS 1 resulted in improved antibody titers and opsonic activity compared with Prevnar13 that contains natural ST-5 CPS. The medicinal chemistry approach allowed for the identification of key epitopes and the replacement of nonessential, labile entities, that create production problems, with closely related, stable functional groups. Synthetic chemistry provides a solution to vaccine manufacturing problems associated with S. pneumoniae ST-5 vaccines by increasing stability, immunogenicity, and protection.
Materials and Methods
Oligosaccharide antigens were synthesized using standard protocols and conjugated to CRM197. Synthetic antigens were printed on NHS-activated microarray slides. Immunization (approved by the Landesamt für Landwirtschaft, Lebensmittelsicherheit und Fischerei Mecklenburg-Vorpommern) was carried out using Zikka rabbits, and the immune response was analyzed by microarrays and ELISA. The functional attribute of the immune response was monitored by OPKA using HL-60 cells. Detailed materials and methods can be found in SI Appendix.
Supplementary Material
Acknowledgments
We thank Dr. S. Parameswarappa for helping with the conjugation chemistry; Dr. A. Calow for MALDI-TOF measurements; Prof. S. Hammerschmidt (University of Greifswald) for providing the S. pneumoniae ATCC6305 strain; Dr. B. Schumann, T. Monroe, and Dr. A. Berger for carefully revising the manuscript; and E. Settels and O. Niemeyer for technical support. We acknowledge financial support from the Max Planck Society and from grants from the German Research Foundation (SFB-TR 84, C3, and C6 to M.W. and C8 to P.H.S.) and from the German Federal Ministry of Education and Research (e:Med, CAPSys, FKZ 01ZX1304B/E to M.W.).
Footnotes
Conflict of interest statement: P.H.S. declares a significant financial interest in Vaxxilon, the company that commercializes the Streptococus pneumoniae vaccine candidate described here. There is, however, no conflict of interest, as this is a purely scientific manuscript that was not sponsored by the company.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1706875114/-/DCSupplemental.
References
- 1.Anonymous Closing the GAPP on pneumonia. Nat Rev Microbiol. 2009;7:838. doi: 10.1038/nrmicro2273. [DOI] [PubMed] [Google Scholar]
- 2.Schumann B, Anish C, Pereira CL, Seeberger PH. Biotherapeutics: Recent Developments using Chemical and Molecular Biology. R Soc Chem; London: 2013. Carbohydrate vaccines; pp. 68–104. [Google Scholar]
- 3.How MJ, Brimacombe JS, Stacey M. The pneumococcal polysaccharides. Adv Carbohydr Chem. 1964;19:303–358. doi: 10.1016/s0096-5332(08)60285-4. [DOI] [PubMed] [Google Scholar]
- 4.Kamerling JP. Pneumococcal polysaccharides: A chemical view. In: Liebert MA, editor. Streptococcus pneumoniae: Molecular Biology and Mechanisms of Disease. Larchmont; New York: 2000. pp. 81–114. [Google Scholar]
- 5.Feldman C, Anderson R. Review: Current and new generation pneumococcal vaccines. J Infect. 2014;69:309–325. doi: 10.1016/j.jinf.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Ginsburg AS, Alderson MR. New conjugate vaccines for the prevention of pneumococcal disease in developing countries. Drugs Today (Barc) 2011;47:207–214. doi: 10.1358/dot.2011.47.3.1556471. [DOI] [PubMed] [Google Scholar]
- 7.Johnson HL, et al. Systematic evaluation of serotypes causing invasive pneumococcal disease among children under five: The pneumococcal global serotype project. PLoS Med. 2010;7:e1000348. doi: 10.1371/journal.pmed.1000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cipollo JF. 2009 Review of Manufacturing Process –Prevnar13 [Pneumococcal 13-Valent Conjugate Vaccine (Diphteria CRM197 protein)] (Food Agric Org, Rome). Available at https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM570727.zip. Accessed September 22, 2017.
- 9.Sucher AJ, Chahine EB, Nelson M, Sucher BJ. Prevnar 13, the new 13-valent pneumococcal conjugate vaccine. Ann Pharmacother. 2011;45:1516–1524. doi: 10.1345/aph.1Q347. [DOI] [PubMed] [Google Scholar]
- 10.Mistretta N, Danve E, Moreau M. 2010. US patent 7,812,006 B2.
- 11.Heidelberger M, Macleod CM, Markowitz H, Roe AS. Improved methods for the preparation of the specific polysaccharides of pneumococcus. J Exp Med. 1950;91:341–349. doi: 10.1084/jem.91.4.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jansson P-E, Lindberg B, Lindquist U. Structural studies of the capsular polysaccharide from Streptococcus pneumoniae type 5. Carbohydr Res. 1985;140:101–110. doi: 10.1016/0008-6215(85)85053-9. [DOI] [PubMed] [Google Scholar]
- 13.Barker S, Bick SM, Brimacombe J, How M, Stacey M. Structural studies on the capsular polysaccharide of pneumococcus type V. Carbohydr Res. 1966;2:224–233. [Google Scholar]
- 14.Cooper G, Edwards M, Rosenstein C. The separation of types among the pneumococci hitherto called group IV and the development of therapeutic antiserums for these types. J Exp Med. 1929;49:461–474. doi: 10.1084/jem.49.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ravenscroft N, Costantino P, Talaga P, Rodriguez R, Egan W. Vaccine Analysis: Strategies, Principles, and Control. Springer; New York: 2015. Glycoconjugate vaccines; pp. 301–381. [Google Scholar]
- 16.Pichichero ME. Protein carriers of conjugate vaccines: Characteristics, development, and clinical trials. Hum Vaccin Immunother. 2013;9:2505–2523. doi: 10.4161/hv.26109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brimacombe JS, Cook MC. 510. Synthesis of 2-amino-2,6-dideoxy-L-mannose(L-rhamnosamine) J Chem Soc. 1964;1964:2663–2666. [Google Scholar]
- 18.Banaszek A, Karpiesiuk W. Studies of the stereoselective reduction of 2-hydroxyimino-hexopyranosides: LiBH4-Me3SiCl, a mild reducing agent of oximes to amines. Carbohydr Res. 1994;251:233–242. [Google Scholar]
- 19.Panek JS, Zhang J. Diastereoselectivity in the osmium tetraoxide promoted dihydroxylation of chiral (E)-crotylsilane: Asymmetric synthesis of silyl-functionalized γ-lactones. J Org Chem. 1993;58:294–296. [Google Scholar]
- 20.Watt GM, Boons G-J. A convergent strategy for the preparation of N-glycan core di-, tri-, and pentasaccharide thioaldoses for the site-specific glycosylation of peptides and proteins bearing free cysteines. Carbohydr Res. 2004;339:181–193. doi: 10.1016/j.carres.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 21.Veselý J, et al. Preparation of ethyl 2-azido-2-deoxy-1-thio-β-d-mannopyranosides, and their rearrangement to 2-S-ethyl-2-thio-β-d-mannopyranosylamines. Synthesis. 2006;2006:699–705. [Google Scholar]
- 22.Pereira CL, Geissner A, Anish C, Seeberger PH. Chemical synthesis elucidates the immunological importance of a pyruvate modification in the capsular polysaccharide of Streptococcus pneumoniae serotype 4. Angew Chem Int Ed Engl. 2015;54:10016–10019. doi: 10.1002/anie.201504847. [DOI] [PubMed] [Google Scholar]
- 23.Danieli E, et al. Synthesis of Staphylococcus aureus type 5 capsular polysaccharide repeating unit using novel L-FucNAc and D-FucNAc synthons and immunochemical evaluation. Bioorg Med Chem. 2012;20:6403–6415. doi: 10.1016/j.bmc.2012.08.048. [DOI] [PubMed] [Google Scholar]
- 24.Gagarinov IA, Fang T, Liu L, Srivastava AD, Boons G-J. Synthesis of Staphylococcus aureus type 5 trisaccharide repeating unit: Solving the problem of lactamization. Org Lett. 2015;17:928–931. doi: 10.1021/acs.orglett.5b00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldblatt D, et al. Establishment of a new human pneumococcal standard reference serum, 007sp. Clin Vaccine Immunol. 2011;18:1728–1736. doi: 10.1128/CVI.05252-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu X, Ling C-C, Bundle DR. A new homobifunctional p-nitro phenyl ester coupling reagent for the preparation of neoglycoproteins. Org Lett. 2004;6:4407–4410. doi: 10.1021/ol048614m. [DOI] [PubMed] [Google Scholar]
- 27.Möginger U, et al. Cross reactive material 197 glycoconjugate vaccines contain privileged conjugation sites. Sci Rep. 2016;6:20488. doi: 10.1038/srep20488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schumann B, et al. A semisynthetic Streptococcus pneumoniae serotype 8 glycoconjugate vaccine. Sci Transl Med. 2017;9:eaaf5347. doi: 10.1126/scitranslmed.aaf5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization . Meeting Report. WHO Workshop on Standardization of Pneumococcal Opsonophagocytic Assay. World Health Org; Geneva: 2007. [Google Scholar]
- 30.World Health Organization 2013. Recommendations to assure the quality, safety and efficacy of pneumococcal conjugate vaccines. WHO Expert Committee on Biological Standardization. Sixtieth Report (World Health Org, Geneva), WHO Tech Rep Ser, No 977, Annex 3, pp 93−151.
- 31.World Health Organization 2005. Guidelines on Nonclinical Evaluation of Vaccines (World Health Org, Geneva), WHO Tech Rep Ser, No 927, Annex 1.
- 32.Caulfield MJ, Ahl PL, Blue JT, Cannon JL. 2012. US Patent 8,192,746 B2.
- 33.Phalipon A, et al. Characterization of functional oligosaccharide mimics of the Shigella flexneri serotype 2a O-antigen: Implications for the development of a chemically defined glycoconjugate vaccine. J Immunol. 2006;176:1686–1694. doi: 10.4049/jimmunol.176.3.1686. [DOI] [PubMed] [Google Scholar]
- 34.Safari D, et al. Identification of the smallest structure capable of evoking opsonophagocytic antibodies against Streptococcus pneumoniae type 14. Infect Immun. 2008;76:4615–4623. doi: 10.1128/IAI.00472-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khan N, Qadri RA, Sehgal D. Correlation between in vitro complement deposition and passive mouse protection of anti-pneumococcal surface protein A monoclonal antibodies. Clin Vaccine Immunol. 2015;22:99–107. doi: 10.1128/CVI.00001-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Birnie GD. The HL60 cell line: A model system for studying human myeloid cell differentiation. Br J Cancer Suppl. 1988;9:41–45. [PMC free article] [PubMed] [Google Scholar]
- 37.Parameswarappa SG, et al. A semi-synthetic oligosaccharide conjugate vaccine candidate confers protection against Streptococcus pneumoniae serotype 3 infection. Cell Chem Biol. 2016;23:1407–1416. doi: 10.1016/j.chembiol.2016.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Usinger WR, Lucas AH. Avidity as a determinant of the protective efficacy of human antibodies to pneumococcal capsular polysaccharides. Infect Immun. 1999;67:2366–2370. doi: 10.1128/iai.67.5.2366-2370.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Romero-Steiner S, et al. Avidity determinations for Haemophilus influenzae type b anti-polyribosylribitol phosphate antibodies. Clin Diagn Lab Immunol. 2005;12:1029–1035. doi: 10.1128/CDLI.12.9.1029-1035.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khatun F, Stephenson RJ, Toth I. An overview of structural features of antibacterial glycoconjugate vaccines that influence their immunogenicity. Chemistry. 2017;23:4233–4254. doi: 10.1002/chem.201603599. [DOI] [PubMed] [Google Scholar]
- 41.Dagan R, Poolman J, Siegrist C-A. Glycoconjugate vaccines and immune interference: A review. Vaccine. 2010;28:5513–5523. doi: 10.1016/j.vaccine.2010.06.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.