Significance
RNA interference (RNAi) is a natural process occurring in cells, and is used to silence genes. Typically, RNAi occurs via small RNA molecules generated in the cell nucleus, which are exported to the cytoplasm where they silence messenger RNA (mRNA) molecules. However, RNAi is thought to occur in the nucleus as well. To demonstrate that this process can occur in the nucleus and to determine its dynamics, we generated human cell systems that enabled us to image living cells and to track gene silencing as it transpired in real time. We found that the RNAi machinery can target the mRNA as it is being transcribed, and that silencing is mediated through modifications occurring on histone proteins bound to the DNA.
Keywords: transcription, nuclear RNAi, histone methylation, live-cell imaging, argonautes
Abstract
Nuclear RNA interference (RNAi) is mediated by the canonical RNAi machinery and can lead to transcriptional silencing, transcriptional activation, or modulation of alternative splicing patterns. These effects transpire through changes in histone and DNA modifications via RNAi-mediated recruitment of chromatin-modifying enzymes. To prove that nuclear RNAi occurs and modulates transcription in human cells, we used live-cell imaging to detect and track nuclear RNAi transcriptional repression in single living human cells. While employing reporter genes constructed with inducible promoters and cognate-inducible short hairpin RNA (shRNA) targeted against the reporter coding region, we have characterized the dynamics of the nuclear RNAi process in living human cells. We show that the silencing effect is mediated through the nascent mRNA, followed by activity of histone methylating enzymes, but not through DNA methylation.
RNA interference (RNAi) is a cellular mechanism for controlling gene expression, in which the cell uses small RNAs to achieve posttranscriptional specific gene silencing (1). The small RNAs that mediate sequence-specific mRNA degradation, or protein translation inhibition, are ∼21-nucleotide-long short-interfering RNA molecules (siRNA) generated from Drosha and Dicer cleavage of longer double-stranded RNA (dsRNA), or micro-RNAs (miRNA) generated from nuclear transcribed dsRNA (2). RNAi results in sequence‐specific gene silencing through a variety of mechanisms (3–5). RNAi is considered a cytoplasmic process controlling gene expression long after mRNA transcription has occurred in the nucleus. However, many of the proteins of the RNAi machinery as well as the small RNAs have been detected in the nucleus (6, 7), and therefore RNAi might have nuclear roles and the ability to act on transcripts immediately after their release from the parental DNA, or even during transcription.
The nuclear process of RNAi in nonmammalian cells was initially identified in plants (8), and later recognized in other organisms (9–12). In mammalian cells, nuclear RNAi was first described by the identification of small RNAs that shared sequence homology to promoter sequences, and silencing was attributed to changes in histone modifications and DNA methylation (13). While this was highly controversial at the time (6), currently, it is known that the RNAi machinery is present and active in the nucleus (14), and it is generally accepted that several mechanisms of mammalian nuclear RNAi do exist. These can lead to RNAi-mediated gene silencing, gene activation, or modulation of alternative splicing, via recruitment of various enzymes that yield changes in histone modifications (6, 7, 15, 16).
The small RNAs could act in the nucleus through binding to DNA, RNA, or proteins of the transcription machinery (17). Naturally, as in the cytoplasm, the nascent RNA that is complementary to the small RNA would be a reasonable candidate for nuclear RNAi targeting. Indeed, cotranscriptional siRNA binding and RNA processing were suggested in yeast cells (18), but, currently, there is little evidence for these mRNA:siRNA interactions in mammalian cells. For instance, short hairpin RNAs (shRNAs) designed to target a promoter region were shown to cause transcriptional activation of the gene by binding to long noncoding RNAs (lncRNAs) transcribed from the promoter, although cleavage of the lncRNAs was not observed (19). Recently, a study in fission yeast has demonstrated that small RNAs bind directly to the nascent pre-mRNA and yield epigenetic repression (20). Moreover, those authors show that transcription must occur above a minimal threshold in order for small RNA-directed heterochromatin to form.
In this study, we set out to detect and follow the kinetics of nuclear RNAi as it occurs in real time in single living human cells. We expressed shRNAs targeting a GFP coding sequence transcribed by genes with two different inducible promoters that can be tracked in living cells (21, 22). Transcription from the targeted genes was significantly reduced in a time window of several hours after induction of shRNA expression. The silencing effect took place when the location of the GFP sequence in the gene was at the 5′ or at the 3′ of the gene, and far from the promoter, showing that the pre-mRNA was being targeted. Specific inhibitors of histone methyltransferases (HMTs) but not DNA methylation reduced the silencing effect, as did knockdown of Argonaute (AGO) proteins and HP1γ, indicating that the RNAi-mediated transcriptional silencing was occurring on the gene via the recruitment of RNAi factors, histone modifying enzymes, and transient epigenetic control of transcription.
Results
Generation of an Inducible Gene Expression System and an Inducible shRNA System That Can Be Visualized in Living Cells.
To examine whether nuclear RNAi can be observed in human cells as it occurs in real time, we decided to target mRNAs transcribed from genes with inducible promoters, for which the mRNAs can be detected and tracked over time in single living human cells. The approach we describe here sets out to assess the levels of gene activity in the nucleus under RNAi conditions, by using RNA fluorescence in situ hybridization (FISH) with probes that will detect the active genes. The levels of gene activity will be determined by measuring the intensities of the active transcription sites.
The RNAi activity was designed to target GFP coding sequences of the transcribed mRNAs. To this end, we designed a gene construct with a Tet-inducible promoter that encodes a combined transcript of turboRed fluorescent protein (tRFP), which contains in its 3′UTR an shRNA sequence that targets the mRNA sequence of GFP (Fig. 1A). The red fluorescent protein serves as an indicator for shRNA expression, and the intensity of the red color should be proportional to the levels of the expressed shRNA in individual cells.
Fig. 1.
Cell systems used for inducible expression of shRNA and tagged mRNA for the detection of gene activity in fixed and living cells. (A) The shRNA-GFP expressing gene construct contains an inducible promoter (Tet-On + dox), a tRFP coding sequence fused in the 3′UTR to an shRNA module that targets GFP mRNA (sh-GFP). (B) The E6 gene construct containing an inducible promoter (Tet-On + dox) and a gene encoding an mRNA with the following modules: β-globin coding region fused to Cerulean fluorescent protein (CFP, cyan) followed by a 3′UTR containing 18× MS2 sequence repeats (gray), which allows the detection of the transcribed mRNA by YFP-MCP dimers (yellow) that bind the MS2 stem loops in the mRNA. The protein product is detected as CFP-labeled peroxisomes. (C) An example of an E6 cell induced with dox for 12 h, showing the transcribed E6 mRNA at the transcription site (arrow) and throughout the cell labeled with YFP-MCP (yellow), and the CFP-SKL protein product in cytoplasmic peroxisomes (cyan). DIC is in gray. (Scale bar, 10 μm.) (D) The GFP-Dys construct contains an inducible promoter [minimal heat shock promoter (mHSP), induced by PonA]. The GFP-Dys gene encodes an mRNA with the following modules: fluorescent protein (GFP, green) fused to dystrophin coding regions, followed by a 3′UTR containing 24× MS2 sequence repeats (gray). The transcribed GFP-Dys mRNA is coated with YFP-MCP proteins (yellow) for detection in live-cell imaging experiments. (E) RNA-FISH with probes to the 5′-GFP (green) and 3′-MS2 (red) regions of the transcribed mRNAs of the GFP-Dys cells induced by PonA. Arrows point to the active transcription sites. (Scale bar, 10 µm.) (F) The expected results if nuclear RNAi is at play. The target gene and the shRNA are both inducible. The target gene will begin transcribing and an active transcription site will be observed during the first hours of induction. As the levels of the tRFP protein increase, i.e., the levels of shRNA/siRNA increase, we expect a reduction in the number of nuclear mRNAs transcribed from the gene. If there is also an effect on transcription, then a change in the size of the active transcription site should be observed.
The Tet-inducible shRNA construct was coexpressed in two types of inducible gene expression systems. The first cell system is also a gene system with a Tet-inducible promoter termed E6, which contains a gene with six exons and five introns, and is based on the β-globin minigene (21) (Fig. 1B). The last exon encodes an in-frame cyan fluorescent protein (CFP) that comprises a peroxisome targeting signal (Ser-Lys-Leu, SKL tripeptide), such that the protein translated from this gene accumulates in peroxisomes. The 3′UTR contains 18 MS2 sequence repeats that enable [after the induction of transcription by doxycycline (dox)] the detection of the transcribed E6 mRNAs in the cells, by a coexpressed YFP-MS2 coat protein (YFP-MCP) that specifically binds to the MS2 loops formed in the transcribed E6 mRNAs (Fig. 1C).
The second inducible gene system is induced by Ponasterone A (PonA) and transcribes a GFP-fusion protein of dystrophin. The mRNA also contains MS2 sequence repeats in its 3′UTR (22) (Fig. 1 D and E). Both E6 and GFP-Dys genes are stably integrated in human U2OS cells. Since the gene constructs were integrated as tandem gene arrays (23), they formed a strong and detectable site of transcription, and therefore have been used for tracking the transcriptional activity of genes in individual cells (21, 22).
First, we verified that the shRNA sequence targeting the GFP coding region was functional. Since GFP and CFP mRNA sequences are highly related, the shRNA to GFP was designed to target the GFP coding region in the GFP-Dys mRNA, as well as the CFP coding sequence in the E6 mRNA (Fig. S1). The potency of the shRNA was first tested by cotransfection of a noninducible shRNA and a GFP plasmid into HEK293T cells. A control shRNA to p53 (sh-p53) had no effect on the GFP fluorescence levels in the cells, whereas the shRNA to GFP (sh-GFP) significantly reduced GFP fluorescence levels (Fig. S2A). The same shRNA plasmids were also transfected into GFP-Dys cells, and the activity of the gene was examined using RNA FISH with probes that detect the MS2 region of the mRNAs. A dramatic reduction in the intensity of the active genes was observed (Fig. S2B). Next, the numbers of cytoplasmic and nuclear GFP-Dys mRNAs were counted using single-molecule RNA FISH. GFP-Dys mRNA levels were significantly reduced in both compartments by sh-GFP but not by sh-p53, as quantified (Fig. S2 C and D). In these experiments, the shRNA plasmids were cotransfected with either DsRed- or mCherry-encoding plasmids to identify cells that received the shRNA.
Fig. S1.
(A) The sequence of the sh-RNA to GFP. (B) The mRNA coding sequences of GFP, CFP, YFP, tRFP, and mCherry proteins. The region targeted by the shRNA is marked. The shRNA does not affect the tRFP and mCherry transcripts.
Fig. S2.
The shRNA sequence to GFP reduces GFP protein and mRNA levels. (A) HEK293T cells were transiently cotransfected with a GFP plasmid and a noninducible shRNA (sh-GFP or sh-p53) plasmid. Cells were also cotransfected with a pDsRed-N1 plasmid, and the GFP fluorescence measured values within the cells were normalized to DsRed to control for transfection efficiency; the experiment was repeated four times. P = 4.38E-9; ***P < 0.001. (B) GFP-Dys cells were cotransfected with the noninducible shRNA plasmid targeting p53 or GFP, while an mCherry plasmid (red) was used as an indicator of transfection efficiency. Twenty-four hours after transfection, cells were activated with Pon A for 24 h before fixation. RNA FISH to detect the mRNA was performed with a probe to the MS2 region in the transcripts (green). Hoechst DNA stain is in blue. (Scale bar, 10 μm.) (C and D) RNA FISH images were deconvolved and taken for further analysis for RNA counting. Average mRNA numbers (C) in the cytoplasm (n = 9 sh-GFP cells and 205 mRNAs, n = 11 sh-p53 cells and 618 mRNAs, P = 0.0023) and (D) in the nucleus (n = 12 sh-GFP cells and 225 mRNAs, n = 15 sh-p53 cells and 710 mRNAs, P = 2.146E-7; ***P < 0.001) were counted using the Imaris Spots tool.
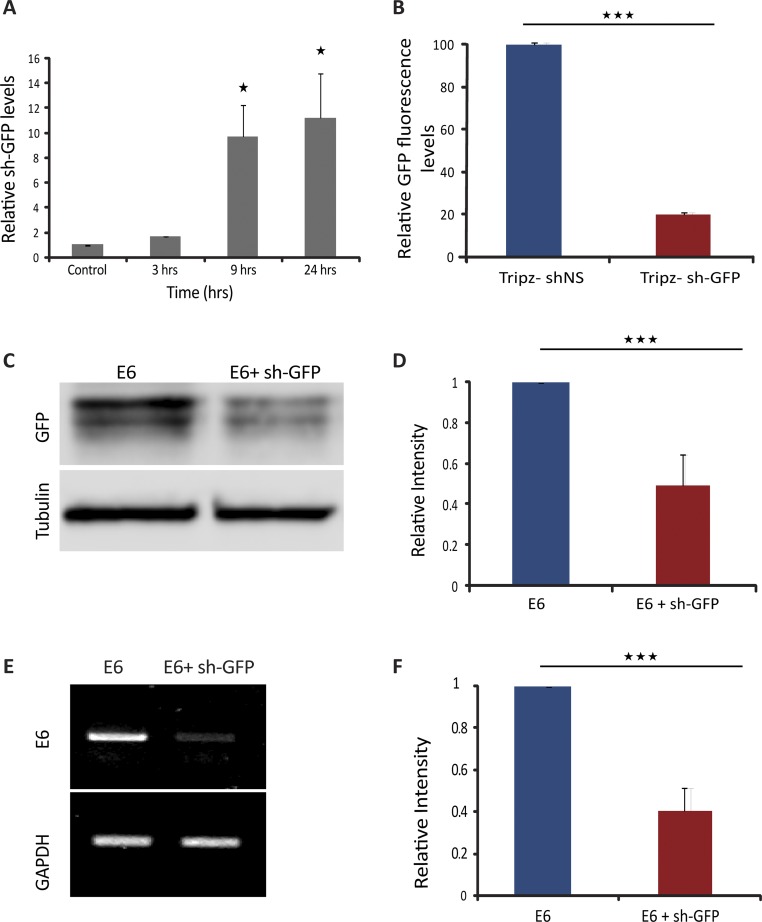
After confirming the silencing activity of the sh-GFP sequence, we used the Tet-inducible shRNA system (Fig. 1), which leads to the generation of a tRFP protein and a shRNA processed from the same transcript. To show that an siRNA was generated and that its levels increased over time after dox induction, we examined siRNA-GFP levels using a real-time RT-PCR approach that detects small RNAs (24). We observed a time-dependent increase in the siRNA levels (Fig. S3A). In this expression system, the RNAi process does not affect the levels of the red tRFP protein that is expressed as a marker for the expression of the shRNA, since there is no sequence compatibility between the shRNA and the tRFP mRNA sequences (Fig. S1). Notably, the shRNA was not targeted to the two different promoter regions of the genes. Rather, the shRNA targeted the GFP region of GFP-Dys mRNA located at the 5′ portion of the transcript, and the CFP region of the E6 mRNA located at the 3′ portion of the E6 transcript, allowing us to test if targeting the nascent mRNAs, rather than the promoter region, has a nuclear RNAi effect.
Fig. S3.
Reduction of E6 mRNA and protein levels after sh-GFP infection and expression. (A) RT-qPCR showing the increase in siRNA-GFP in E6 cells following dox induction for the indicated times (n = 3, *P < 0.05). Representative experiment out of three different RNA purifications from different days. (B) HEK293T cells were transiently cotransfected with a GFP plasmid and a Tet-on TRIPZ shRNA (TRIPZ-sh-GFP or TRIPZ-sh-NS) plasmid. GFP protein fluorescence measured values within the cells were normalized to TurboRFP protein fluorescence to control for transfection efficiency; this was repeated four times. P = 3.385E-6; ***P < 0.001. (C and D) E6 cells infected with the TRIPZ viral construct containing shRNA, were induced by dox for 96 h. Western blot of the E6 protein product (β-globin-CFP) in control cells and after sh-GFP expression. Anti-GFP was used for the detection of the proteins. Tubulin was used as a loading control. Blots are representative of three repeated experiments. The average quantification of three repeated experiments is presented in the plots below (mean ± SD). P = 0.00121. ***P < 0.001. (E and F) RT-PCR showing E6 mRNA levels in control and sh-GFP expressing cells. GAPDH was used as a control (P = 0.00078). ***P < 0.001.
As a control shRNA, we used a nonsilencing inducible shRNA (sh-NS). This construct had no effect on GFP fluorescence in HEK293T cells expressing a GFP construct, compared with sh-GFP that significantly reduced GFP fluorescence (Fig. S3B). Altogether, we expected to observe that the induction of the Tet-inducible shRNA gene, together with the transcriptional induction, should reduce the levels of cytoplasmic mRNAs and the protein products of the target gene, E6. This was confirmed by Western blotting (Fig. S3 C and D) and RT-PCR (Fig. S3 E and F). Importantly, if nuclear RNAi was indeed taking place, then we would expect to observe a reduction in the levels of the nuclear mRNAs transcribed from these genes. Moreover, if the action of the nuclear RNAi has an effect on gene activity per se, as suggested in some studies, then we would anticipate a change in the transcriptional activity levels of the transcribing genes, and this will be measured by RNA FISH (Fig. 1F).
RNAi Reduces the Levels of Gene Activity.
The tRFP/sh-GFP construct was transfected into E6 cells, and, upon dox addition, was expressed in parallel to the E6 transcript. The E6 mRNA was detected using RNA FISH with a probe to the MS2 region, which therefore detects both the cytoplasmic MS2-containing mRNAs and the nascent nuclear RNA at the transcription sites. We observed that cells containing high levels of the tRFP protein, i.e., expressing high levels of the shRNA to GFP, had significantly smaller transcription sites, compared with control untransfected cells and to cells that expressed a tRFP/sh-NS nonsilencing construct that generates a nontargeting control shRNA (Fig. 2 A and B). There was a correlation between the intensity levels of tRFP in the cells and the reduced levels in the mRNA intensity on the transcribed genes (Fig. 2 C and D). Using infection of the inducible shRNA constructs, we generated two stable E6 cell clones, expressing either the sh-GFP or the sh-NS. When we monitored these cells over time (from shRNA and E6 gene induction), we could see that transcriptional intensity on the E6 gene increased over time in the sh-NS-expressing cells as expected, but was significantly decreased in the sh-GFP-expressing cells (Fig. S4A).
Fig. 2.
Reduction of transcription site intensity in E6 cells after sh-GFP transfection and expression. (A) E6 cells that were transiently transfected with the TRIPZ viral construct containing shRNA (sh-NS/sh-GFP) were induced by dox for 24 h. RNA-FISH with a fluorescent probe to the MS2 sequence (yellow) shows a significant reduction in size and intensity of transcription sites in the E6 cells expressing sh-GFP (Bottom) but not in those cells expressing sh-NS (Top). The tRFP protein expression levels are in red. (Scale bar, 10 μm.) (B) Sum of intensity of all transcriptions sites was measured by ImageJ. The results show pronounced transcription sites in E6 (control, n = 341) or E6 sh-NS cells (n = 99), while sh-GFP (n = 75) expressing cells demonstrated a significant decrease. The average quantification of four repeated experiments (mean ± SD) (control-shGFP, P = 3.016E-7; shNS-shGFP, P = 3.9E-6). There is no statistical difference between the E6 cells and E6 expressing sh-NS. P = 0.7674; ***P < 0.001 (t test); n.s, not significant = P > 0.05. (C) Cells containing high levels of tRFP protein (red, expressing high levels of the sh-GFP) had significantly smaller transcription sites (white, RNA FISH) than control untransfected cells. (Scale bar, 10 μm.) (D) Measurements of tRFP and transcription site intensity for the cells numbered in C.
Fig. S4.
Reduction of transcription site intensity in E6 and GFP-Dys cells after shRNA-GFP expression compared with sh-NS expression. (A) E6 cells stably expressing shRNA (sh-NS/sh-GFP) were induced by dox for 72 h. RNA FISH with a fluorescent probe to the MS2 region detected the active transcription sites. The signal is pseudocolored using the ImageJ Cyan Hot look-up table. Color scale: black, low intensity; white, high intensity. (B) GFP-Dys cells stably expressing shRNA (sh-NS/sh-GFP) were induced by dox for 16 h. RNA FISH with a fluorescent probe to the MS2 region (red) detected active transcription sites. From 9 h onward, there was a pronounced decrease in mRNA levels that was accompanied by a significant reduction in size and intensity of the active transcription sites (white arrows). GFP-Dys protein is seen in green. (C) IPO7-YFP U2OS cells in which one IPO7 allele contains an in-frame YFP coding region were transiently transfected with the sh-GFP/sh-NS inducible constructs. The shRNA was induced by dox for 24 h, and the active IPO7-YFP allele was detected with RNA FISH probes to the YFP region of the mRNA. Transcription sites of cells without shRNA expression (arrowheads) compared with cells with shRNA expression (arrows) are shown in the enlarged boxes. The boxed FISH signal was inverted and separately adjusted for the display of the transcription sites; tRFP protein is in red. (Scale bar, 10 μm.)
We tested this effect also in GFP-Dys tRFP/sh-GFP stably infected cells, in which we already observed a significant reduction in transcription site size (Fig. S2B). There are several advantages for using these cells in imaging experiments. The active transcription sites (tandem gene arrays) in the GFP-Dys cells are particularly large, the gene and shRNA are expressed from two different promoters, and the cell clone we used has several genomic integration loci. Therefore, we could observe three to four very large and highly transcriptionally active gene arrays in most cells, and could examine whether the nuclear RNAi activity was able to affect all of the active GFP-Dys genes. Indeed, a silencing effect was observed in GFP-Dys cells infected with sh-GFP and not with sh-NS (Fig. S4B). From 9 h onward after sh-GFP expression, there was a decrease in mRNA levels, which was accompanied by a significant reduction in the size and intensity of all of the transcription sites. The strong reduction in the intensity of the transcription sites (i.e., low fluorescence signal from the nascent transcripts being transcribed on these genes) suggested that there was a very powerful transcription silencing effect. Finally, we expressed the inducible sh-GFP and sh-NS constructs in U2OS cells containing an in-frame integration of the YFP coding region in one endogenous allele of the importin 7 (IPO7) gene (25). We reasoned that the nuclear RNAi effect should be detected also on an endogenous gene. Indeed, the intensity of the mRNAs transcribed on the active IPO7-YFP allele was significantly reduced when sh-GFP was expressed (Fig. S4C).
To quantify the nuclear RNAi effect, we used the stable tRFP/sh-GFP E6 cells, and compared the intensity of their transcription sites to control cells. Both the E6 gene and the sh-GFP gene were activated at the same time using dox. While, in control cells, the intensity of the transcription sites strongly increased over 9 h and then remained active even up to 72 h, the sh-GFP-infected cells, while accumulating tRFP protein, showed moderate transcriptional induction until ∼6 h, which then declined over time (Fig. 3A). The quantification of transcription site intensity showed that the 9-h time point demonstrated the largest difference between the control and shRNA-GFP-treated cells (Fig. 3B). The RNAi effect lasted for many hours but diminished after 48 h.
Fig. 3.
The shRNA directed to the GFP coding sequence reduces transcription site intensity. (A) Control E6 cells and E6 cells that were transfected with the TRIPZ viral construct encoding the sh-GFP sequence were induced by dox for 72 h. E6 mRNAs were detected using RNA FISH with a fluorescent probe that hybridizes to the MS2 region. From 9 h onward, there was a decrease in the E6 mRNA levels in the sh-GFP expressing cells, which was accompanied by a significant reduction in transcription size and intensity. The signal is pseudocolored using the ImageJ Cyan Hot look-up table and adjusted to make the weak transcription sites visible (Materials and Methods). Color scale is as follows: black, low intensity; white, high intensity. (Scale bar, 20 μm.) (B) Sums of intensity of all transcription sites were measured by ImageJ. The results show an increase in size and intensity of transcription sites in E6 cells over 72 h [n(3 h) = 115 cells, n(6 h) = 156 cells, n(9 h) = 140 cells, n(12 h) = 140 cells, n(24 h) = 166 cells, n(48 h) = 137 cells, and n(72 h) = 148 cells], while E6+sh-GFP cells demonstrated a significant decrease in both parameters [n(3 h) = 108 cells, n(6 h) = 112 cells, n(9 h) = 142 cells, n(12 h) = 143 cells, n(24 h) = 151cells, n(48 h) = 115 cells, and n(72 h) = 140 cells]. E6, blue; E6+sh-GFP, red; mean, diamond; median, horizontal line. P < 0.001.
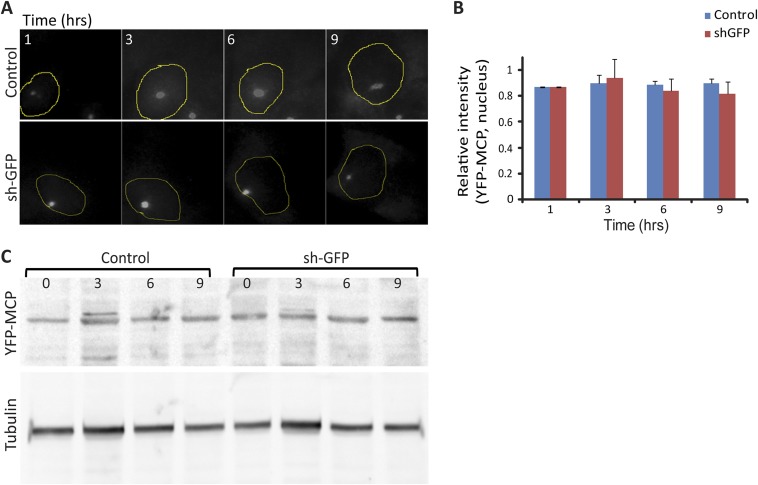
Taking advantage of the MS2 tag used for live-cell imaging of mRNA, we could follow the genes’ activity in real time, and observed a gradual decline in the transcription site size in cells expressing the sh-GFP, meaning that the silencing effect was not rapid but probably required a continuous flow of shRNA. The dynamics were similar to those observed in fixed cells, showing that the major drop in transcription site intensity was occurring around 9 h after dox induction (Fig. 4 and Movies S1–S5). Control cells that did not express the sh-GFP, even those imaged for 16 h, did not show a reduction in gene activity, implying that reduction in transcriptional activity was caused by the sh-GFP. It is important to note that the sh-GFP can potentially target the YFP sequence of the YFP-MCP mRNA. Therefore, we verified, by image quantification and by Western blotting, that the levels of YFP-MCP were not affected during shRNA induction (Fig. S5).
Fig. 4.
Tracking the shRNA-mediated silencing of transcription site activity in single living cells. (A) Average of E6 transcription site intensity of control (blue) and sh-GFP-treated (red) cells; n(control) = 9 cells, and n(sh-GFP) = 9 cells. *P < 0.05; ***P < 0.001 (t test). (B) The rate of change of the transcription site intensity over time following transcriptional induction, in control versus sh-GFP treated cells. (C) (Top Left) Control E6 cells stably expressing the YFP-MCP protein (green) were induced to transcribe by dox, and were followed using time-lapse imaging. Red denotes tRFP protein expression (indicator for shRNA expression). Frames are from Movie S1. (Bottom Left) E6 cells that were infected with the TRIPZ viral construct containing the sh-GFP sequence showed a gradual reduction in transcription sites size as well as weakened transcriptional intensity. Frames are from Movie S2. Images were acquired every 15 min for 8 h after 1 h of induction with dox. (Right) The gray plots show the complete set of plots from all of the cells imaged, and some intensity profiles are highlighted. (Scale bar, 10 μm.)
Fig. S5.
YFP-MCP levels were not affected during shRNA-GFP induction. (A) Live-cell imaging of YFP-MCP expressing E6 control and TRIPZ sh-GFP-infected cells that were induced with dox. Images from data collected and presented in Fig. 4 were taken for further analysis in ImageJ to measure the levels of YFP-MCP protein in the nucleus. Nuclear boundaries were depicted according to YCP-MCP nuclear fluorescence. (Magnification: 60×.) (B) No significant difference in YFP-MCP fluorescence levels was detected between the control and sh-GFP-infected cells (n = 9 control and for sh-GFP cells). (C) No significant difference was observed in protein levels of YFP-MCP.
Acquiring data from individual living cells showed that, under control conditions, the transcription site intensity tended to increase for many hours until reaching a plateau, whereas, under sh-GFP conditions, the transcription sites were reduced in size and in intensity (Fig. 5). The size-reduced transcription sites under sh-GFP treatment were active transcription sites, since the nascent mRNA transcripts (as seen by RNA FISH or YFP-MCP) were still associated with them. In addition, the transcription sites showed the continued presence of RNA polymerase II, although at lower levels, as seen by immunofluorescence of the endogenous polymerase (Fig. S6).
Fig. 5.
Live-cell imaging shows kinetics of RNAi activity in the nucleus. (A) E6 cells expressing YFP-MCP protein (green) were infected with the TRIPZ sh-GFP vector and then taken for live-cell imaging. Gene activity and sh-GFP expression were induced with dox for 16 h. Red denotes tRFP protein expression indicating sh-GFP levels. Arrows indicate cells that express sh-GFP. (B) Frames from time-lapse Movie S3 showing YFP-MCP protein levels on the transcribing genes. Arrows point to the two cells enlarged in C. White arrow, cell with gradual reduction in transcription site activity and high tRFP levels; yellow arrow, control cell with no tRFP expression. (C) Cell marked by a yellow arrow in B is shown in the first and second rows; cell marked by a white arrow in B is shown in the third and fourth rows. The YFP-MCP signal is pseudocolored using the ImageJ Grays look-up table; tRFP expression over time is in red. (Magnification: A, 60×; B and C, 40×.)
Fig. S6.
RNA polymerase (Pol) II is present at the active transcription sites during shRNA expression. RNA Pol II was recruited to transcription sites in E6 cells, and slightly less so in E6+sh-GFP cells, fixed 12 h after dox induction. Active transcription sites were detected using RNA-FISH. RNA Pol II was detected with an anti-Pol II antibody. Nuclei were stained using Hoechst. (Scale bar, 20 μm.)
Nuclear RNAi Transcriptional Repression Is Mediated Through HMTs.
Since we could detect the recruitment of RNA Pol II to the active transcription sites, we examined if any RNAi proteins might be accumulating to a detectable level on the genes. However, no particular accumulation was detected (Fig. S7). We next examined whether the nuclear RNAi effect might lead to methylation of promoter regions. Using bisulfite conversion, we examined whether methylation was occurring. We used a simpler HEK293 cell system that stably expressed a GFP gene driven by a CMV promoter to obtain high-quality mapping of DNA methylation sites. The cells were infected with the tRFP/shRNA construct. However, no change in the DNA methylation pattern was observed.
Fig. S7.
Proteins of the RNAi machinery are not enriched at the active transcription sites. Immunofluorescence with antibodies to endogenous (A) AGO1, (B) AGO2, (C) Drosha, and (D) Dicer, together with RNA FISH to the E6 transcription sites, observed with a probe that hybridizes to the MS2 sequences in the E6 mRNA (red). Insets show enlargement of boxed cells. Enlarged cells in A and D were adjusted so nuclear signal will be visible. DIC is in gray. (Scale bar, 10 μm.)
Next, we examined whether histone modifications might be involved in nuclear RNAi-induced transcriptional repression. Since it has been suggested that nuclear RNAi at active genes might lead to the recruitment of HMTs that generate methylations on H3K9, we treated the cells with specific inhibitors of HMTs. We used BIX01294, a potent, selective G9a and G9a-like protein histone lysine methyltransferase inhibitor; UNC0638, a potent, selective, and reversible G9 and G9a-like protein histone methyl transferase inhibitor; and Chaetocin, a nonselective histone lysine methyltransferase inhibitor. First, we verified that the HMT inhibitors indeed reduced the global levels of H3K9 methylation in cells (Fig. S8). Next, we added the HMT inhibitors 24 h before the dox induction of the gene and the shRNA (the inhibitor was present throughout the experiment). While the inhibitors did not change the levels of the transcription site intensity of the control cells, they all had an enhancing effect on transcription in the sh-GFP-treated cells, with Chaetocin having the largest effect (Fig. 6). This meant that histone methylation was induced by the sh-GFP expression, leading to transcriptional repression. To verify this, the cells were treated with 5-Aza-2′-deoxycytidine, which is a potent DNA methyltransferase 1 inhibitor, but no change was seen (Fig. S9 A and B), strengthening the results of the bisulfite conversion, and showing that methylation was not occurring at the DNA level, but rather on the histone level. Finally, when the sh-GFP was first induced for 24 h and then the inhibitors were added for another 24 h, we could still observe a relief in the transcriptional repression conferred by the shRNA-mediated nuclear RNAi (Fig. S9 C–F), implying that the repressive state is a transient one.
Fig. S8.
HMT inhibitors reduce the global levels of H3K9 methylation. Levels of H3K9me2 in control and HMT inhibitor treated cells (48 h) are shown. The immunofluorescence signal is pseudocolored using the ImageJ Fire look-up table. (Scale bar, 10 μm.)
Fig. 6.
Inhibitors of HMTs antagonize the shRNA-mediated transcriptional silencing. E6- and E6+sh-GFP-infected cells were treated with histone lysine methyltransferase inhibitors (A) BIX01294 and (C) Chaetocin for 24 h, and then induced by dox for another 24 h. Transcription sites were detected using RNA FISH with a probe that hybridizes to the MS2 region in the E6 mRNA. E6+sh-GFP infected cells showed a reduction in transcription site size and weakened intensity after dox induction. Treatment with BIX 01294 and Chaetocin for 24 h before dox induction caused an increase in size and intensity of transcription sites, compared with sh-GFP treated cells. In red, tRFP protein expression levels. (Scale bar, 10 μm.) (B) Quantitative analysis of transcription site intensities during BIX01294 treatment demonstrated an increase of 25% in the intensity of the transcription sites in treated cells compared with the nontreated E6+sh-GFP-infected cells; n(E6 cells) = 134, n(E6+Bix01294 cells) = 171, P = 0.338; n(E6+sh-GFP cells) = 136, n(E6+sh-GFP+Bix01294 cells) = 115, P = 6.54E-10. In the boxplots: mean, diamond; median, horizontal line. ***P < 0.001; n.s, nonsignificant = P > 0.05. (D) Quantitative analysis of Chaetocin treatment demonstrated an increase of 35% in the mean intensity of the transcription sites in E6+sh-GFP-infected cells n(E6 cells) = 41, n(E6+Chaetocin cells) = 48, P = 0.097; n(E6+sh-GFP cells) = 51, n(E6+sh-GFP+Chaetocin cells) = 51, P = 1.96E-7. In the boxplots: mean, diamond; median, horizontal line. ***P < 0.001; n.s = P > 0.05.
Fig. S9.
Examining the effects of inhibitors of DNA methylation and HMTs. (A) E6- and E6+sh-GFP-infected cells were treated with 5′-Aza for 24 h, and then by 5′-Aza and dox for another 24 h. Transcription sites were detected using RNA FISH with a probe that hybridizes to the MS2 region in the mRNA. (Top) E6+sh-GFP-infected cells showed a reduction in transcription site size and weakened intensity after dox induction. Treatment with 5′-Aza did not affect the shRNA treatment. (B) Quantitative analysis of size of E6 transcription sites under 5′-Aza treatment; n(E6 cells) = 35, n(E6+5′-Aza cells) = 23, P = 0.198; n(E6+shGFP cells) = 57, n(E6+shGFP+5′-Aza cells) = 25, P = 0.2. Mean, diamond; median, horizontal line. (C) E6 control cells and E6+sh-GFP-infected cells were induced with dox treated with Chaetocin for 24 h. (D) Quantitative analysis of E6 transcription sites under Chaetocin treatment; n(E6 cells) = 55, n(E6+Chaetocin cells) = 55, P = 0.26; n(E6+sh-GFP cells) = 78, n(E6+sh-GFP+Chaetocin cells) = 81, P = 8.49E-9. (E) E6+sh-GFP-infected cells were induced with dox to express the sh-GFP for 24 h, and then, together with control E6 cells, were treated with dox and UNC0638 for 24 h. (F) Quantitative analysis of E6 transcription sites under UNC0638 treatment; n(E6 cells) = 66, n(E6+UNC0638 cells) = 66, P = 0.94; n(E6+sh-GFP cells) = 71, n(E6+sh-GFP+UNC0638 cells) = 74, P = 9.46E-12. Plots show quantitative analysis of the treatments. Mean, diamond; median, horizontal line. ***P < 0.001; n.s, not significant = P > 0.05. (Scale bar, 10 μm.)
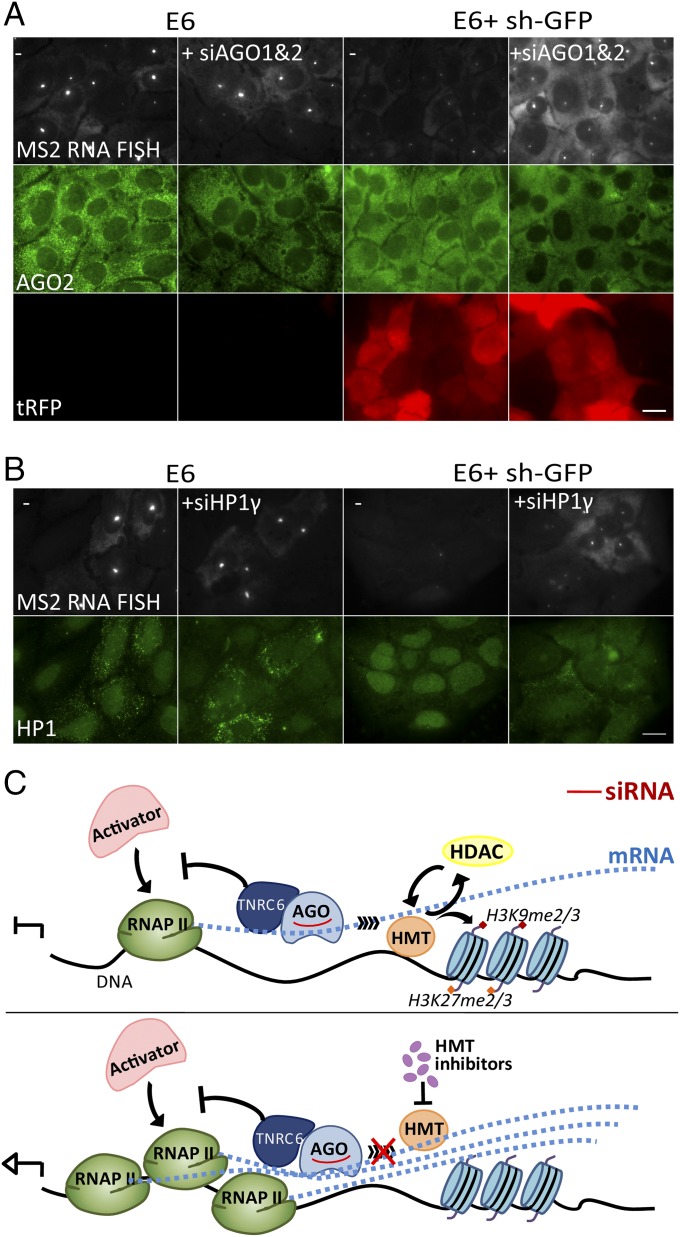
Finally, certain protein factors have been implicated in the binding of the mRNA and the small guide RNA, such as AGO1 and AGO2 (26). AGO proteins and heterochromatin protein 1 (HP1) have been associated with nuclear RNAi (27, 28), and AGO1 has been found to specifically bind a variety of gene sequences on a genome-wide level (29). We therefore examined whether knockdown of these factors (Fig. S10 A–F) would antagonize the nuclear RNAi effect. When we knocked down AGO1 and AGO2 together in E6 cells that also expressed the sh-GFP (Fig. 7A), the E6 gene continued to transcribe to high levels, meaning that the sh-GFP-mediated RNAi effect was less active (Fig. 7A). This was also seen when only AGO2 was knocked down (Fig. S10G). Knockdown of HP1γ also abolished the RNAi effect (Fig. 7B). Altogether, we have observed that the nuclear RNAi effect was mediated in part or in whole at the transcription level, when the shRNA was directed toward the coding region of the target gene.
Fig. S10.
Examining the effects of RNAi factors knockdown. The levels of AGO1, AGO2, and HP1γ mRNAs (A, C, and E) as quantified by RT-PCR [P(AGO) = 0.006, P(AGO2) = 0.005, P(HP1γ) = 0.001; *P < 0.05; **P < 0.01], or (B, D, and F) as seen in immunofluorescence, following siRNA treatment for 72 h. For HP1, a pan-HP1 antibody was used. In immunofluorescence, AGO1 KD was seen as cytoplasmic reduction; for AGO2, KD was mostly seen in the nucleus; and, for HP1γ, KD was seen in the nucleus (but reduction of signal cannot reach full levels, since the antibody is a pan-HP1 Ab). (G) E6 cells that express the sh-GFP were induced by dox for 24 h. A reduction in E6 transcription site intensity was observed by RNA FISH (gray) when the sh-GFP was induced, in comparison with no drop in intensity when AGO2 siRNA KD took place in parallel. Endogenous AGO2 protein levels are seen in green. (Scale bar, 20 μm.)
Fig. 7.
Knockdown of RNAi factors. (A) E6 control cells and the clone stably expressing sh-GFP were induced by dox for 24 h. A reduction in E6 transcription site intensity was observed by RNA FISH (gray) when the sh-GFP was induced, compared with no change in intensity in the control cells. Knockdown of AGO1 and AGO2 using siRNA abolished the sh-GFP effect even when the sh-GFP was expressed. Endogenous AGO2 protein levels are seen in green; tRFP levels are in red. (Scale bar, 20 μm.) (B) Knockdown of HP1γ with siRNA (green with pan-HP1 antibody), as above. Strong cytoplasmic dots in some cells are dox-induced CFP peroxisomes, since the CFP fluorescence leaks into the GFP channel. (Scale bar, 20 μm.) (C) Model of nuclear RNAi mediated by shRNA. (Top) Transcripts generated during RNA Pol II transcription are targeted by siRNA and might mediate the recruitment of HMTs that methylate histones along the gene and reduce the recruitment of more polymerases. AGO proteins with small guide RNA and TNRC6 will recruit the RNAi machinery. (Bottom) HMT inhibitors antagonize this effect and allow an increase in polymerase recruitment to the gene.
Discussion
Nuclear RNAi can modulate gene expression. Mechanisms of transcriptional activation, gene silencing, and RNA degradation are probably at play, but how exactly the RNAi message is conveyed to the gene is not completely understood. Hence, it is important to identify which elements of the transcription process are being targeted by the small RNAs (6). One option is that the small RNAs are targeting the nascent RNAs transcribed on the gene. This makes sense, since the core of cytoplasmic RNAi is based on the hybridization of mRNA:small-RNA complementary sequences, and therefore the general mechanism might be conserved in all cell compartments. Along these lines, significant attention has focused on regulatory RNAs transcribed from promoter regions (such as antisense lncRNAs) and the transcriptional effect of shRNAs/miRNAs that share homology with the promoter regions (13, 30–38). Indeed, the recruitment of AGO2 to promoter transcripts was also identified, although it seemed that cleavage of the promoter RNAs was not required for gene activation. This suggests that the RNA:small-RNA hybrid acts as a scaffold for small RNA-mediated recruitment of AGO2 and other RNAi factors, followed by chromatin modifying enzymes that modulate gene expression, without the need to induce RNA cleavage, as found for cytoplasmic RNAi. However, we cannot rule out the possibility that the well-accepted posttranscriptional gene silencing process occurring in the cytoplasm can elicit a feedback signal that reaches the nucleus, and modulate the levels of gene activity. Such indirect siRNA feedback mechanisms might occur (39) in conjunction with a direct shRNA effect, and require further research.
In this study, we examined the nuclear RNAi process in human cells and in real time. Importantly, the small RNAs generated were not targeted to the promoter region; rather, we used shRNAs targeting the coding region of two different genes. Particularly, we found that the shRNA could induce transcriptional repression even when the targeted sequence was far downstream from the promoter region. We focused mainly on two genes with different inducible promoters, stably transfected into human cells. Both genes encode fluorescent fusion proteins, and therefore the GFP/CFP region was the chosen target for the shRNA; in the E6 gene, the CFP coding region is located close to the 3′ end of the transcript, while, in the GFP-Dys gene, the GFP coding region is in the 5′ region. In both cases, a significant reduction in gene expression was observed after the induction of shRNA expression (Fig. 7C). Other shRNA sequences that did not target the particular genes had no effect on the transcriptional activity of these genes. The transcriptional silencing effect was quite potent, since the tested genes were integrated as tandem arrays and formed very large transcribed gene loci upon regular transcriptional induction, whereas the size of these active transcription sites was dramatically reduced upon shRNA induction. The RNAi effect could also be detected on an endogenous gene.
The levels of transcription of the genes were examined by live-cell imaging and by RNA FISH. Accordingly, the levels of the nascent mRNAs on the active transcription sites were very low after shRNA induction. We then tested whether the reduced transcription levels also affect RNA polymerase II levels. Indeed, the levels of RNA polymerase II were also reduced, suggesting that the RNAi effect was radiating from downstream areas of the gene to the promoter region, thus reducing promoter firing events. This also implied that the silencing effect was caused by the binding of the small RNAs to the nascent mRNAs. Studies on transcriptional modulation and heterochromatin assembly in yeast have shown that nuclear RNAi can assemble on nascent transcripts (18). Recently, it was demonstrated in Schizosaccharomyces pombe that the nuclear RNAi requires transcription of nascent mRNAs to achieve an epigenetic silencing effect (20). That study also showed that shutting down gene activity required a “transcriptional window” with conditions that are optimal for silencing. Those authors found that the heterochromatin formation induced by the siRNAs required transcription levels that were above a minimal threshold. Interestingly, we also found that silencing could only be observed after several hours and that the shRNA had to accumulate to high levels. Since the target gene and the shRNA were induced at the same time, we first observed regular transcriptional induction kinetics on the gene, and, only some hours later, when the shRNA was exported from the nucleus, processed, returned probably to the nucleus at high levels, and then recruited HMTs to the genes, could we see a silencing effect on the gene.
It is important to note that the live-cell imaging experiments demonstrate that the RNAi effect occurs during the life cycle of a cell and does not require passage through mitosis, since it might be assumed that the RNAi machinery reaches the nucleus only after cell division via the mixing of the nuclear and cytoplasmic compartments. This, however, is not the case, which means that there is entry of the small silencing RNAs from the cytoplasm to the nucleus, as shown for the shuttling of nuclear RNA-induced silencing complex from the cytoplasm to the nucleus (40). Inhibitors of the HMTs partially antagonized the silencing effect (Fig. 7C), showing that these enzymes function after the siRNA recruitment. This suggests that other modifications might be involved and/or that nuclear RNAi can cause some levels of RNA degradation. Importantly, knockdown of proteins involved in the RNAi activity such as AGO proteins and HP1 (27–29, 41) also antagonized the shRNA effect, corroborating their centrality in the nuclear RNAi process. Future experiments should elucidate which other factors mediate transcriptional silencing and are recruited by the nuclear RNAi machinery to modulate gene expression.
Materials and Methods
shRNA Plasmids.
The sh-GFP and sh-p53 lentivirus plasmids were a kind gift from Dr. Oded Singer, Weizmann Institute of Science, Rehovot, Israel. The sh-p53 plasmid encodes an anti-p53 shRNA, driven by the H1 Pol III promoter, while sh-GFP encodes an anti-GFP shRNA, driven by the U6 Pol III promoter (42).
The shRNA-encoding segments were designed to contain a sense passenger strand of 19 (p53) or 20 (GFP) nucleotides, a short spacer (TTCAAGAGA), the reverse complement guide strand, and five consecutive thymidines serving as an RNA polymerase III transcriptional stop signal.
sh-GFP (20 bp): GCAAGCTGACCCTGAAGTTC
sh-p53 (19 bp): GACTCCAGTGGTAATCTAC.
TRIPZ-shRNA Plasmids.
The Tet-inducible pTRIPZ lentiviral vector was purchased from Open Biosystems Inc. Cloned pTRIPZ derivatives (tRFP/sh) bearing shRNA targeting sequences were designed within a 97-bp PCR template oligonucleotide containing 22 bp of passenger/guide complementary sequences, a 19-bp hairpin, and miR-30-derived flanking sequences at both ends (43). PCR amplification of the 97-bp sequence was carried out using a Pfu DNA polymerase (Thermo Scientific) and two common primers flanking the 97-bp DNA ends containing restriction sites for XhoI and EcoRI
TRIPZ-XhoI: CAGAAGGCTCGAGAAGGTATATTGCTGTTGACAGTGAGCG
TRIPZ-EcoRI: CTAAAGTAGCCCCTTGAATTCCGAGGCAGTAGGCA
The shRNA-GFP mirRNA sequence in pTRIPZ:
TGCTGTTGACAGTGAGCGCGGCAAGCTGACCCTGAAGTTCTAGTGAAGCCACAGATGTAGAACTTCAGGGTCAGCTTGCCGTGCCTACTGCCTCGGA
The shRNA-NS mirRNA sequence in pTRIPZ:
TGCTGTTGACAGTGAGCGATCTCGCTTGGGCGAGAGTAAGTAGTGAAGCCACAGATGTACTTACTCTCGCCCAAGCGAGAGTGCCTACTGCCTCGGA.
PCR conditions were as follows: initial incubation at 95 °C for 1 min, 25 cycles of 95 °C for 30 s, 54 °C for 30 s, and 72 °C for 1 min, followed by a final incubation at 72 °C for 10 min. The length of the PCR product in each case was 137 bp.
Products were subsequently purified from the PCR by phenol−chloroform extractions. DNA was recovered by precipitation with ethanol and a glycogen carrier. Products were then digested with EcoRI/XhoI, and the ∼114-bp resulting shRNA-encoding stem−loop−stem-containing fragments were run on a 2% agarose gel, purified, and recovered using the “HiYieldTM Gel/PCR DNA Fragments Extraction Kit” (RBC). Following was ligation of the purified fragments into the EcoRI/XhoI-digested pTRIPZ vector. Sequences of shRNA generated in the pTRIPZ plasmids were verified by sequencing from both ends.
Cell Maintenance and Transfection.
HEK293T and HEK293 were cultured under standard conditions at 37 °C, 5% CO2, in DMEM supplemented with 10% FBS, 4 mM Glutamine, 100 IU/mL Penicillin, and 100 µg/mL Streptomycin (Biological Industries). Human U2OS cells were maintained in low-glucose DMEM containing 10% FBS, 100 IU/mL Penicillin, and 100 µg/mL Streptomycin (Biological Industries). E6 cells and GFP-Dys cells were previously described (21, 22). E6 cells were induced with 2 µg/mL dox (Sigma), and GFP-Dys cells were induced with 10 μg/mL PonA (Enzo).
Stable expression of YFP-MCP was obtained by cotransfection of cells with the YFP-MCP plasmid (10 µg) alongside a puromycin resistance plasmid (300 ng) using calcium phosphate transfection followed by simultaneous selection with Zeocin (50 µg/mL; Invivogen) and puromycin (1 μg/mL; Invivogen).
Stable expression of GFP for the genomic methylation study was performed by transfecting HEK293 cells with a CMV-promoter-driven peGFP-N1 plasmid (Clontech), followed by selection with 1 mg/mL G418 (A.G. Scientific). Individual cell clones expressing GFP under the CMV promoter were used for further manipulations.
Transient transfections of HEK293T or U2OS cells were carried out using Lipofectamine 2000 (Invitrogen) or DNA-In (MTI-GlobalStem) according to the manufacturer’s instructions. Cells were trypsinized and plated in 6-well or 24-well plates 24 h before transfection. Cells were cotransfected with mCherry or pDsRed-N1 (encoding DsRed-Express) and the shRNA plasmids.
Lentivirus Production.
Plasmids encoding shRNA from the Pol III promoter-driven constructs and the Tet-inducible pTRIPZ constructs were used to generate replication-incompetent lentivirus titers in HEK293T producer cells, by the BES-calcium phosphate transfection method, according to standard protocols. Second-generation helper plasmids psPAX2 and pMD2.G were used to produce the VSV-G pseudotyped virus (a kind gift of Didier Trono, École polytechnique fédérale de Lausanne, Lausanne, Switzerland). Viral Supernatants were concentrated by ultracentrifugation on a 20% sucrose cushion and suspended in a small volume of PBS.
Virus Infection and pTRIPZ Induction.
For generation of stable sh-GFP/sh-NS cells, 1 d before infection, E6 or GFP-Dys U2OS cells were seeded at 2 × 105 cells per well in a six-well plate, in DMEM containing 10% FBS and Penicillin/Streptomycin. On the day of infection, medium was changed to contain 8 µg/mL polybrene. Ultracentrifuge-concentrated shRNA-encoding virus was then added to each well. Cells were spun at 800 × g for 90 min at 32 °C, after which they were allowed to recover for 24 h. Thereafter, medium was changed daily. Induced wells were supplemented with 2 µg/mL dox.
Quantification of GFP Fluorescence Levels.
Cells were lysed with lysis buffer composed of 0.15 M NaCl, 10 mM Tris pH 7.4, 1 mM EDTA, 1 mM PMSF, and 2% CHAPS (Sigma). Net fluorescence intensity after negligible background subtraction of the lysis buffer fluorescence was determined using a Fluoroscan Ascent CF microplate reader with the 485/527 excitation/emission filters for GFP or 544/590 for DsRed.
Flow Cytometry.
Stable expression of tRFP/sh-GFP was performed by infecting the E6 cells with TRIPZ-sh-GFP and selection with puromycin (2 μg/mL). Cells with very high expression levels of tRFP were collected by FACS (FACSAria III; BD Biosciences).
Fluorescence in Situ Hybridization.
Cells were grown on coverslips coated with Cell-Tak (BD Biosciences) and fixed for 20 min in 4% paraformaldehyde, and overnight with 70% ethanol at 4 °C. The next day, cells were washed with 1× PBS and treated for 2.5 min with 0.5% Triton X-100. Cells were washed with 1× PBS and incubated for 10 min in 40% formamide (4% SSC; Sigma). Cells were hybridized overnight at 37 °C in 40% formamide with a specific fluorescently labeled Cy3 DNA probe (∼10-ng probe, 50-mer). The next day, cells were washed twice with 40% formamide for 15 min and then washed for 2 h with 1× PBS. Nuclei were counterstained with Hoechst 33342, and coverslips were mounted in mounting medium. The probe for the MS2 binding site was
CTAGGCAATTAGGTACCTTAGGATCTAATGAACCCGGGAATACTGCAGAC.
Stellaris (Biosearch Technologies) RNA FISH on the IPO7-YFP U2OS cells (25) transiently expressing sh-GFP/sh-NS was performed according to the manufacturer’s adherent cell protocol with a Cy5-labeled YFP probe set.
Immunofluorescence.
Cells were grown on coverslips coated with Cell-Tak (BD Biosciences), washed with PBS, and fixed for 20 min in 4% paraformaldehyde. Cells were then permeabilized with 0.5% Triton X-100 for 3 min. After blocking, cells were immunostained for 1 h with a primary antibody, and, after subsequent washes, the cells were incubated for 1 h with secondary fluorescent antibodies. Primary antibodies were goat anti-AGO1/EIF2C1, rat anti-AGO2 (Sigma), mouse anti-Dicer, rabbit anti-Drosha, rabbit anti-H3K9me2 (Abcam), rat anti-RNA Pol II antibody (Chromotek), and rabbit anti-pan-HP1 (Gene Tex). Secondary antibodies were Alexa 488-goat anti-rabbit, Alexa 647-chicken anti-rat, Alexa 594-goat anti-mouse (Invitrogen), and Alexa 488 anti-rat (Life Technologies). Nuclei were counterstained with Hoechst 33342 (Sigma), and coverslips were mounted in mounting medium.
siRNA Knockdowns.
E6 cells expressing sh-GFP or sh-NS were transfected with siRNA to AGO1 or AGO2 (Trifecta Kit; IDT) or siRNA to HP1γ as described in ref. 44, using Lipofectamine 2000. The mRNA expression levels of AGO1, AGO2, HP1γ, and GAPDH were examined by semiquantitative RT-PCR from total RNA using Tri Reagent (Sigma) 72 h after siRNA transfection. Primers used were as follows (for, forward; rev, reverse):
AGO1 for: CCAGCATTTCAAGCCTCAGA; rev: GATCTCCTTGGTGAAGCGAA
AGO2 for: TTCAAGCCTCCACCTAGACC; rev: GACGGACTGATGGAAGCCAAA
HP1γ for: GGCCTCCAACAAAACTACA; rev: GAGCTTCATCTTCTGGACA
GAPDH: for: TCTTCCAGGAGCGAGATCCCT;
rev: TGCAAATGAGCCCCAGCCTTCT.
Western Blotting.
SDS/PAGE and Western blotting were performed as previously described (45). Primary antibodies used were mouse anti-GFP (Roche), rabbit anti-MCP (#ABE76; Merck), and rabbit anti-tubulin (Abcam). The secondary antibody was an HRP-conjugated goat anti-rabbit (Millipore) and an HRP-conjugated goat anti-mouse (Jackson). Immunoreactive bands were detected by the Enhanced Chemiluminescence kit (ECL; Pierce). Experiments were performed three times.
RT-PCR.
Total RNA was produced using Tri-Reagent (Sigma), and DNA was removed using Turbo-DNase free kit (Ambion). The cDNA (1 µg of RNA) was synthesized using the ReverseAid First Strand cDNA Synthesis Kit (Quanta). The cDNA was amplified by conventional PCR with primers to β-globin (E6) mRNAs (first exon and second exon regions).
Exon 1 for: TCTGACACAACTGTGTTCAC
Exon 2 rev: TCCACGTGCAGCTTGTCACA
GAPDH was used as a normalization control (same primers as above).
The reaction (25 µL total volume) contained 2× Gotaq green mix (Promega), 1 µL of gDNA dilution, 0.5 mM of each primer, and water. The reaction was performed using SensoQuest (Danyel Biotech) following the protocol: 2 min at 94 °C, followed by 35 cycles consisting of 60-s denaturation at 94 °C, 60-s annealing at 52 °C, and 60-s extension at 72 °C.
siRNA Quantification by RT-qPCR.
We followed the protocol for quantifying small RNA levels by RT-qPCR using poly(A) tailing and reverse transcription (RT) as described (24), and specific primers were designed using the miRprimer software (46). Total RNA was extracted (Tri Reagent) and used for cDNA synthesis: 100 ng of RNA in a final volume of 10 μL including 1 μL of 10× poly(A) polymerase (PAP) buffer, 0.1 mM of ATP, 1 μM of RT primer, 0.1 mM of each deoxynucleotide (dATP, dCTP, dGTP, and dTTP), 100 units of M-MuLV reverse transcriptase (New England Biolabs), and 1 unit of PAP (New England Biolabs) were incubated at 42 °C for 1 h followed by enzyme inactivation at 95 °C for 5 min. The sequence of the RT primer (IDT) was 5′-CAGGTCCAGTTTTTTTTTTTTTTTCG. The cDNA was amplified using the following primers:
sh-GFP primers: for – GCAGGAACTTCAGGGTCAG
rev – CAGTTTTTTTTTTTTTTTCGGCAA
miRNA (miR)-21 primers: for – TCAGTAGCTTATCAGACTGATG
rev – CGTCCAGTTTTTTTTTTTTTTTCAAC.
Real-time PCR (qPCR) was performed using the primers listed above, and PerfeCTa SYBR Green FastMix, ROX (Quanta Biosciences) according to the manufacturer’s protocol on a CFX-96 system (Bio-Rad). Analysis was performed with the software Bio-Rad CFX manager. Relative levels of RNA expression were measured as the ratio of comparative threshold cycle to miR-21 as an internal control. This miRNA is abundant in U2OS cells. All negative controls gave negative results. The controls were cDNA from dox-induced E6/sh-GFP RNA that did not undergo treatment with PAP, a reaction with dox-induced E6/sh-GFP RNA that did not undergo treatment with M-MuLV RT, a reaction with dox-induced E6/sh-GFP RNA that did not undergo treatment with both enzymes, and a reaction with both enzymes and without RNA.
Transcription Site Quantification.
Measuring the sum of intensity of RNA signals on the active genes was performed manually using ImageJ, and background was subtracted from all measurements. Intensity of the active transcription site (Is) was measured for each frame. Background from another location in the nucleus (In) was subtracted for each frame, and the final intensity was calculated using: I = Is(t) − In(t) and then normalized to the highest intensity. Quantifications were applied to experiments performed on different days.
Fluorescence Microscopy, Live-Cell Imaging, and Data Analysis.
Wide-field fluorescence images were obtained using the Cell^R system based on an Olympus IX81 fully motorized inverted microscope (60× PlanApo objective, 1.42 NA) fitted with an Orca-AG CCD camera (Hamamatsu) driven by the Cell^R software. Live-cell imaging was carried out using the Cell^R system with rapid wavelength switching. For time-lapse imaging, cells were seeded on glass-bottomed tissue culture plates (MatTek) coated with Cell-Tak (BD Biosciences) in medium containing 10% FBS at 37 °C. The microscope is equipped with an incubator that includes temperature and CO2 control (Life Imaging Services). For long-term imaging, several cell positions were chosen and recorded by a motorized stage (Scan IM; Märzhäuser). Cells expressing YFP-MCP were imaged in three dimensions (10-µm-wide stack per time point; 0.8 µm between planes) every 15 min, for 9 h. For presentation of the movies, the 4D image sequences were transformed into a time sequence using the maximum or sum projection options or manually selecting the in-focus plane using the ImageJ software. Time-lapse data were collected from single cells in several fields and on several days until reaching an appropriate sample size, and then all single-cell data were pooled and either averaged and presented as plots or presented as single-cell data. For displaying of cells with very small transcription sites (due to RNAi) versus cells with large transcription sites (normal transcription) in the same field or in the same figure, in some cases, full original images required adjustment such that the weak transcription sites or the tRFP would be visible to the reader. In some figures, the images were inverted or presented in pseudocolors using different look-up tables (LUTs in ImageJ: Cyan Hot, Grays, or Fire as noted) to generate better representation of the data.
Tracking and Data Analysis.
The intensity of the active transcription sites labeled with YFP-MCP in time-lapse 3D movies was analyzed using Imaris (Bitplane). The sum of intensity (Is) for each transcription site was measured using Imaris spot tracker. Mean intensity of the nucleus (In) was measured for each frame and was multiplied by the number of pixels covered by the transcription site. This value was subtracted from the sum of intensity at the transcription site. The final intensity was calculated using: I = Is(t) − In(t) and then normalized to the initial intensity. Corrected intensity of the bleaching was calculated using the average intensity of the nucleus (Iavg n),
Values of rate of change (ΔI/Δt) in the transcription site intensity over time were obtained by measuring the intensity difference (ΔI) between two consecutive time points divided by the time difference (Δt) between the two time points,
Experiments were performed on different days.
Statistical Analysis.
A two-tailed t test was performed in the following experiments: FISH quantification, RT-qPCR, and live-cell movie analysis.
Treatment of Cells with DNA Methyltransferase Inhibitors.
E6- and E6+sh-GFP-infected cells were seeded in a 24-well plate; 5-Aza-dC (Sigma) was dissolved in DMSO and stored at 4 °C. After 24 h of plating, cells were treated with 1 µM 5-Aza-dC for 48 h. Reagent and medium were changed every 24 h.
Treatment of Cells with Histone Lysine Methyltransferase Inhibitors.
E6- and E6+shGFP-infected cells were seeded in a 24-well plate and treated with various materials. Bix01294 (Cayman Chemical) was dissolved in DMSO and stored at 4 °C. After 24 h of plating, cells were treated with 106 nM Bix01294 for 24 h and then activated with dox (1 μg/mL) for another 24 h. Chaetocin (Cayman Chemical) was dissolved in DMSO and stored at 4 °C. After 24 h of plating, cells were treated with 10 nM Chaetocin for 24 h and then activated with dox (1 μg/mL) for another 24 h, or cells were treated with both Chaetocin and dox for 24 h. UNC0638 (Cayman Chemical) was dissolved in DMSO and stored at 4 °C. After 24 h of plating, cells were activated with dox (1 μg/mL) for 24 h and then treated with both 1 μM UNC0638 and dox for another 24 h.
DNA Methylation Analysis.
Genomic DNA from tRFP/sh-GFP targeted and control tRFP/sh-NS cells was purified using the Quick-gDNA Mini Prep kit (Zymo Research). Bisulphite conversion of the genomic DNA (0.5 μg) was carried out using the Lightning kit (Zymo Research). Bisulfite-converted DNA was then used to amplify two regions of the CMV minimal promoter and GFP coding region. Results were repeated with three individual clones expressing GFP. Primers for the CMV promoter were derived from ref. 47 (540-bp product),
For: GGGTTATTAGTTTATAGTTTATATATGG
Rev: GATTCACTAAACCAACTCTACTTA
Inner for: ATTAGTTTATAGTTTATATATGGAGTTTC
Inner rev: CAACTCTACTTATATAAACCTCCC
Primers for amplifying the bisulfite converted GFP coding region (168-bp product):
For: ATCGGTCGTTATTATGGTGAGTAAG
Rev: CCGATAATACAAATAAACTTCAAAATCAAC.
PCR amplification of genomic DNA after bisulphite conversion was carried out using Taq DNA Polymerase master mix (Lamda Biotech). Conditions were as follows: initial incubation at 94 °C for 1 min, 40 cycles of 94 °C for 30 s, 54 °C for 30 s, and 72 °C for 40 s. A common terminal incubation step at 72 °C for 2 min was applied for all amplifications. PCR products were gel-purified and sequenced on an ABI machine.
Supplementary Material
Acknowledgments
We thank Oded Singer (Weizmann Institute) and Didier Trono (EPFL) for reagents. We are grateful to Shulamit Michaeli, Vaibhav Chikne, and K. Shanmugha Rajan [Bar-Ilan University (BIU)] for assistance and advice on quantifying siRNA levels, and to Avi Jacob (BIU) for helping with image analysis. Y.S.-T. was supported by the Israel Science Foundation and the European Research Council. D.C. was supported by the Orgler Fund for Cancer Genetics Research.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1707440114/-/DCSupplemental.
References
- 1.Hannon GJ. RNA interference. Nature. 2002;418:244–251. doi: 10.1038/418244a. [DOI] [PubMed] [Google Scholar]
- 2.Zamore PD, Tuschl T, Sharp PA, Bartel DP. RNAi: Double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell. 2000;101:25–33. doi: 10.1016/S0092-8674(00)80620-0. [DOI] [PubMed] [Google Scholar]
- 3.Carthew RW, Sontheimer EJ. Origins and mechanisms of miRNAs and siRNAs. Cell. 2009;136:642–655. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014;15:509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 5.Wilson RC, Doudna JA. Molecular mechanisms of RNA interference. Annu Rev Biophys. 2013;42:217–239. doi: 10.1146/annurev-biophys-083012-130404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalantari R, Chiang CM, Corey DR. Regulation of mammalian transcription and splicing by nuclear RNAi. Nucleic Acids Res. 2016;44:524–537. doi: 10.1093/nar/gkv1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burger K, Gullerova M. Swiss army knives: Non-canonical functions of nuclear Drosha and Dicer. Nat Rev Mol Cell Biol. 2015;16:417–430. doi: 10.1038/nrm3994. [DOI] [PubMed] [Google Scholar]
- 8.Wassenegger M, Heimes S, Riedel L, Sänger HL. RNA-directed de novo methylation of genomic sequences in plants. Cell. 1994;76:567–576. doi: 10.1016/0092-8674(94)90119-8. [DOI] [PubMed] [Google Scholar]
- 9.Castel SE, Martienssen RA. RNA interference in the nucleus: Roles for small RNAs in transcription, epigenetics and beyond. Nat Rev Genet. 2013;14:100–112. doi: 10.1038/nrg3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cecere G, Grishok A. A nuclear perspective on RNAi pathways in metazoans. Biochim Biophys Acta. 2014;1839:223–233. doi: 10.1016/j.bbagrm.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Creamer KM, Partridge JF. RITS-connecting transcription, RNA interference, and heterochromatin assembly in fission yeast. Wiley Interdiscip Rev RNA. 2011;2:632–646. doi: 10.1002/wrna.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang XH, Liu Q, Michaeli S. Small nucleolar RNA interference induced by antisense or double-stranded RNA in trypanosomatids. Proc Natl Acad Sci USA. 2003;100:7521–7526. doi: 10.1073/pnas.1332001100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morris KV, Chan SW, Jacobsen SE, Looney DJ. Small interfering RNA-induced transcriptional gene silencing in human cells. Science. 2004;305:1289–1292. doi: 10.1126/science.1101372. [DOI] [PubMed] [Google Scholar]
- 14.Gagnon KT, Li L, Chu Y, Janowski BA, Corey DR. RNAi factors are present and active in human cell nuclei. Cell Rep. 2014;6:211–221. doi: 10.1016/j.celrep.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bühler M, Moazed D. Transcription and RNAi in heterochromatic gene silencing. Nat Struct Mol Biol. 2007;14:1041–1048. doi: 10.1038/nsmb1315. [DOI] [PubMed] [Google Scholar]
- 16.Alló M, et al. Control of alternative splicing through siRNA-mediated transcriptional gene silencing. Nat Struct Mol Biol. 2009;16:717–724. doi: 10.1038/nsmb.1620. [DOI] [PubMed] [Google Scholar]
- 17.Grewal SI, Moazed D. Heterochromatin and epigenetic control of gene expression. Science. 2003;301:798–802. doi: 10.1126/science.1086887. [DOI] [PubMed] [Google Scholar]
- 18.Bühler M, Verdel A, Moazed D. Tethering RITS to a nascent transcript initiates RNAi- and heterochromatin-dependent gene silencing. Cell. 2006;125:873–886. doi: 10.1016/j.cell.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X, Li H, Burnett JC, Rossi JJ. The role of antisense long noncoding RNA in small RNA-triggered gene activation. RNA. 2014;20:1916–1928. doi: 10.1261/rna.043968.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimada Y, Mohn F, Bühler M. The RNA-induced transcriptional silencing complex targets chromatin exclusively via interacting with nascent transcripts. Genes Dev. 2016;30:2571–2580. doi: 10.1101/gad.292599.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brody Y, et al. The in vivo kinetics of RNA polymerase II elongation during co-transcriptional splicing. PLoS Biol. 2011;9:e1000573. doi: 10.1371/journal.pbio.1000573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mor A, et al. Dynamics of single mRNP nucleocytoplasmic transport and export through the nuclear pore in living cells. Nat Cell Biol. 2010;12:543–552. doi: 10.1038/ncb2056. [DOI] [PubMed] [Google Scholar]
- 23.Darzacq X, Singer RH, Shav-Tal Y. Dynamics of transcription and mRNA export. Curr Opin Cell Biol. 2005;17:332–339. doi: 10.1016/j.ceb.2005.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balcells I, Cirera S, Busk PK. Specific and sensitive quantitative RT-PCR of miRNAs with DNA primers. BMC Biotechnol. 2011;11:70. doi: 10.1186/1472-6750-11-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheinberger J, et al. CD-tagging-MS2: Detecting allelic expression of endogenous mRNAs and their protein products in single cells. Biol Methods Protoc. 2017;2:bpx004. doi: 10.1093/biomethods/bpx004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weinberg MS, Morris KV. Transcriptional gene silencing in humans. Nucleic Acids Res. 2016;44:6505–6517. doi: 10.1093/nar/gkw139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agirre E, et al. A chromatin code for alternative splicing involving a putative association between CTCF and HP1α proteins. BMC Biol. 2015;13:31. doi: 10.1186/s12915-015-0141-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ameyar-Zazoua M, et al. Argonaute proteins couple chromatin silencing to alternative splicing. Nat Struct Mol Biol. 2012;19:998–1004. doi: 10.1038/nsmb.2373. [DOI] [PubMed] [Google Scholar]
- 29.Alló M, et al. Argonaute-1 binds transcriptional enhancers and controls constitutive and alternative splicing in human cells. Proc Natl Acad Sci USA. 2014;111:15622–15629. doi: 10.1073/pnas.1416858111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahlenstiel CL, et al. Direct evidence of nuclear Argonaute distribution during transcriptional silencing links the actin cytoskeleton to nuclear RNAi machinery in human cells. Nucleic Acids Res. 2012;40:1579–1595. doi: 10.1093/nar/gkr891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castanotto D, et al. Short hairpin RNA-directed cytosine (CpG) methylation of the RASSF1A gene promoter in HeLa cells. Mol Ther. 2005;12:179–183. doi: 10.1016/j.ymthe.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 32.Janowski BA, et al. Inhibiting gene expression at transcription start sites in chromosomal DNA with antigene RNAs. Nat Chem Biol. 2005;1:216–222. doi: 10.1038/nchembio725. [DOI] [PubMed] [Google Scholar]
- 33.Cho S, Park JS, Kang YK. AGO2 and SETDB1 cooperate in promoter-targeted transcriptional silencing of the androgen receptor gene. Nucleic Acids Res. 2014;42:13545–13556. doi: 10.1093/nar/gku788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Napoli S, Pastori C, Magistri M, Carbone GM, Catapano CV. Promoter-specific transcriptional interference and c-myc gene silencing by siRNAs in human cells. EMBO J. 2009;28:1708–1719. doi: 10.1038/emboj.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsui M, et al. Promoter RNA links transcriptional regulation of inflammatory pathway genes. Nucleic Acids Res. 2013;41:10086–10109. doi: 10.1093/nar/gkt777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hawkins PG, Santoso S, Adams C, Anest V, Morris KV. Promoter targeted small RNAs induce long-term transcriptional gene silencing in human cells. Nucleic Acids Res. 2009;37:2984–2995. doi: 10.1093/nar/gkp127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weinberg MS, et al. The antisense strand of small interfering RNAs directs histone methylation and transcriptional gene silencing in human cells. RNA. 2006;12:256–262. doi: 10.1261/rna.2235106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han J, Kim D, Morris KV. Promoter-associated RNA is required for RNA-directed transcriptional gene silencing in human cells. Proc Natl Acad Sci USA. 2007;104:12422–12427. doi: 10.1073/pnas.0701635104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kalo A, et al. Cellular levels of signaling factors are sensed by β-actin alleles to modulate transcriptional pulse intensity. Cell Rep. 2015;11:419–432. doi: 10.1016/j.celrep.2015.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohrt T, et al. Fluorescence correlation spectroscopy and fluorescence cross-correlation spectroscopy reveal the cytoplasmic origination of loaded nuclear RISC in vivo in human cells. Nucleic Acids Res. 2008;36:6439–6449. doi: 10.1093/nar/gkn693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hicks JA, et al. Human GW182 paralogs are the central organizers for RNA-mediated control of transcription. Cell Rep. 2017;20:1543–1552. doi: 10.1016/j.celrep.2017.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tiscornia G, Singer O, Verma IM. Design and cloning of lentiviral vectors expressing small interfering RNAs. Nat Protoc. 2006;1:234–240. doi: 10.1038/nprot.2006.36. [DOI] [PubMed] [Google Scholar]
- 43.Paddison PJ, et al. Cloning of short hairpin RNAs for gene knockdown in mammalian cells. Nat Methods. 2004;1:163–167. doi: 10.1038/nmeth1104-163. [DOI] [PubMed] [Google Scholar]
- 44.Saint-André V, Batsché E, Rachez C, Muchardt C. Histone H3 lysine 9 trimethylation and HP1γ favor inclusion of alternative exons. Nat Struct Mol Biol. 2011;18:337–344. doi: 10.1038/nsmb.1995. [DOI] [PubMed] [Google Scholar]
- 45.Aizer A, et al. The dynamics of mammalian P body transport, assembly, and disassembly in vivo. Mol Biol Cell. 2008;19:4154–4166. doi: 10.1091/mbc.E08-05-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Busk PK. A tool for design of primers for microRNA-specific quantitative RT-qPCR. BMC Bioinformatics. 2014;15:29. doi: 10.1186/1471-2105-15-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tian Y, et al. Expression of 2A peptide mediated tri-fluorescent protein genes were regulated by epigenetics in transgenic sheep. Biochem Biophys Res Commun. 2013;434:681–687. doi: 10.1016/j.bbrc.2013.04.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.