Significance
To secure their colonization and survival, pathogens have evolved tactics to undermine host immune responses. Most particularly, Mycobacterium tuberculosis inhibits the activation of macrophages, one of whose roles is to recognize and kill invading microorganisms. Here, we used a library of M. tuberculosis mutants to infect macrophages and uncover molecular mechanisms by which the pathogen modulates the function of these immune cells. We found that M. tuberculosis produces cell envelope glycolipids that are antagonists of a macrophage receptor, named TLR2, which is dedicated to the recognition of pathogens, thereby preventing its efficient recognition by the immune system.
Keywords: tuberculosis, innate immunity, pattern recognition receptor, glycolipid
Abstract
Mycobacterium tuberculosis is a major human pathogen that is able to survive inside host cells and resist immune clearance. Most particularly, it inhibits several arms of the innate immune response, including phagosome maturation or cytokine production. To better understand the molecular mechanisms by which M. tuberculosis circumvents host immune defenses, we used a transposon mutant library generated in a virulent clinical isolate of M. tuberculosis of the W/Beijing family to infect human macrophages, utilizing a cell line derivative of THP-1 cells expressing a reporter system for activation of the transcription factor NF-κB, a key regulator of innate immunity. We identified several M. tuberculosis mutants inducing a NF-κB activation stronger than that of the wild-type strain. One of these mutants was found to be deficient for the synthesis of cell envelope glycolipids, namely sulfoglycolipids, suggesting that the latter can interfere with innate immune responses. Using natural and synthetic molecular variants, we determined that sulfoglycolipids inhibit NF-κB activation and subsequent cytokine production or costimulatory molecule expression by acting as competitive antagonists of Toll-like receptor 2, thereby inhibiting the recognition of M. tuberculosis by this receptor. Our study reveals that producing glycolipid antagonists of pattern recognition receptors is a strategy used by M. tuberculosis to undermine innate immune defense. Sulfoglycolipids are major and specific lipids of M. tuberculosis, considered for decades as virulence factors of the bacilli. Our study uncovers a mechanism by which they may contribute to M. tuberculosis virulence.
A highly successful intracellular pathogen, Mycobacterium tuberculosis has evolved numerous strategies to evade clearance by the immune system and most particularly the innate immune system (1). Notably, M. tuberculosis has adapted to replicate within macrophages and to subvert their function. It is able to inhibit phagosome maturation, to evade autophagy, or to dampen the production of proinflammatory cytokines. However, the molecular mechanisms by which M. tuberculosis circumvents host defenses are not completely understood. Innate immune recognition is based on the detection of molecular structures that are unique to microorganisms, referred to as microbe-associated molecular patterns (MAMPs), by a limited number of germline-encoded pattern recognition receptors (PRRs), which trigger NF-κB–dependent and IFN regulatory factor (IRF)-dependent signaling pathways. M. tuberculosis employs two main escape strategies to restrict PRR signaling. A first one consists in limiting MAMPs’ accessibility to PRRs, by masking the former, for example with cell-surface–associated phthiocerol dimycocerosate (PDIM) lipids (2). In the absence of PDIM, PAMPs’ recognition by and signaling via Toll-like receptors (TLRs) is increased. A second strategy is to negatively modulate PRR signaling. For instance, direct extracellular interaction between the early secreted antigen ESAT-6 of M. tuberculosis and TLR2 inhibits activation of transcription factor NF-κB and IRFs, attenuating TLR signaling in general (3). Similarly, M. tuberculosis exposes a surface lipoglycan at its cell envelope, namely mannose-capped lipoarabinomannan (ManLAM), which inhibits the production of proinflammatory cytokines and increases the production of the antiinflammatory cytokine IL-10 by human dendritic cells (4–6). ManLAM binding to the C-type lectin DC-SIGN triggers a signaling pathway that results in the reorientation of NF-κB, initially dedicated to the transcription of proinflammatory cytokine-coding genes upon TLR activation, on antiinflammatory promoter targets (7). Some M. tuberculosis strains of the W-Beijing family express phenolic glycolipids that contribute to their hypervirulence and down-regulate the production of proinflammatory cytokines in infected macrophages (8).
Although the main evasion strategies used by M. tuberculosis have been uncovered, the underlying molecular mechanisms identified remain scarce. Moreover, they have been investigated by hypothesis-driven approaches, using most of the time purified M. tuberculosis cell envelope molecules. Here we aimed at identifying yet unknown mechanisms employed by M. tuberculosis to inhibit innate immune response, using an unbiased global approach involving infected macrophages. Monitoring the activation of the transcription factor NF-κB, which is a key regulator of innate immunity, was chosen as a readout of macrophage response. A transposon mutant library made in a virulent clinical isolate of M. tuberculosis of the W-Beijing family and containing over 11,000 mutants (9) was used to infect human THP-1 monocyte/macrophage cells, which naturally express most of the PPRs involved in M. tuberculosis sensing, using a cell line derivative stably transfected with a NF-κB–inducible reporter system. Here, we report the characterization of one selected mutant that induced an increased NF-κB activation. Interestingly, this mutant carried an insertion in the mmpL8 gene, already known to be required for M. tuberculosis virulence and synthesis of sulfoglycolipids (10, 11), suggesting that the latter, which are major and specific lipids of M. tuberculosis (12–14), can interfere with innate immune responses.
We show that sulfoglycolipids are able to inhibit NF-κB activation and subsequent cytokine production or costimulatory molecule expression, by acting as competitive antagonists of Toll-like receptor 2. Our study uncovers a strategy used by M. tuberculosis to undermine innate immunity, as well as a mechanism by which sulfoglycolipids may contribute to M. tuberculosis virulence.
Results
Identification of Transposon Mutants That Induce an Altered NF-κB Activation in Macrophages.
To identify M. tuberculosis effectors modulating NF-κB activation in macrophages, we screened a M. tuberculosis transposon mutant library in human THP-1 monocyte/macrophage cells expressing an NF-κB–inducible reporter system (secreted alkaline phosphatase). The 11,000 transposon mutants, which were generated in the M. tuberculosis W-Beijing GC1237 strain (9), were first harvested in individual wells of microplates, before addition of THP-1 cells. NF-κB activation induced after overnight incubation was monitored by reading optical density at 625 nm. Sixty-four hits with a Z score value of less than −1.45 or more than 2 were then selected for amplification and mapping of the transposon insertion site by ligation-mediated PCR. The 10 mutants for which the phenotype could be validated by a direct comparison with the wild-type strain, using at least three independent bacterial cultures, are reported in SI Appendix, Table S1. Mutant P118B01, which induced an increased NF-κB activation and carried an insertion in the mmpL8 gene, known to be required for M. tuberculosis virulence and synthesis of sulfoglycolipids (10, 11), was selected for further studies.
mmpL8 Mutant Induces a Stronger Innate Immune Response.
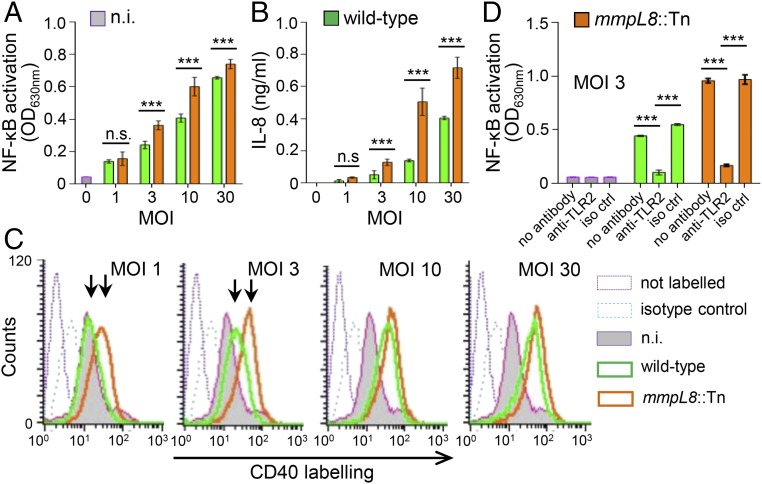
In agreement with published data (10, 11, 15), mmpL8::Tn mutant was impaired for surface-exposed tetraacylated sulfolipid (Ac4SGL) production and accumulated intracellular diacylated sulfolipid (Ac2SGL) (SI Appendix, Fig. S1). The mmpL8::Tn mutant was compared with the M. tuberculosis W-Beijing GC1237 wild-type strain (16) for its ability to activate the human monocyte/macrophage cell line THP-1. As expected from the screening, it induced an increased NF-κB activation (Fig. 1A). Moreover, it stimulated a stronger production of the cytokine IL-8 (Fig. 1B), as well as expression of the costimulatory molecule CD40 (Fig. 1C). Interestingly, NF-κB activation induced by both the wild-type and mmpL8::Tn mutant strains was found to be dependent on TLR2 (Fig. 1D), indicating that the mmpL8::Tn mutant triggers an increased TLR2 signaling.
Fig. 1.
mmpl8::Tn mutant induces a stronger innate immune response. THP-1 cells were infected by the M. tuberculosis GC1237 wild-type or mmpL8::Tn mutant strains at the indicated MOI. After 16 h, NF-κB activation (A and D), IL-8 production in the culture supernatant (B) and CD40 expression (C) were measured. (A and D) NF-κB activation was determined by measuring alkaline phosphatase activity in the culture supernatant and reading OD at 630 nm. TLR2 dependence was investigated by preincubating cells for 30 min at 37 °C with 5 µg/mL of anti-TLR2 or IgG1 isotype control antibodies. (B) IL-8 release in the culture supernatant was determined by sandwich ELISA. (C) CD40 expression was monitored by flow cytometry. Data show mean ± SEM ***P < 0.001; iso ctrl, isotype control; n.i., not induced; n.s., not significant.
We first hypothesized that mmpL8::Tn mutant might produce a higher amount of TLR2 ligands as a consequence of bacterial cell surface remodeling in response to the halted biosynthesis of a major lipid, such as Ac4SGL. Indeed, we previously observed that mmpL4b deletion in Mycobacterium abscessus, which leads to the specific impairment of glycopeptidolipid biosynthesis, was associated with an overexpression of TLR2-signaling lipoproteins in the mutant (17). TLR2 ligands produced by M. tuberculosis include lipoproteins as well as lipoglycans, namely, phosphatidyl-myo-inositol-mannosides (PIM), lipomannan (LM) and lipoarabinomannan (LAM) (18). However, biochemical analyses showed that lipoproteins or lipoglycans were not overproduced in the mutant strain (SI Appendix, Fig. S2 A and B). Moreover, lipoproteins and lipoglycans purified from the mutant showed the same capacity as those from the wild-type strain to induce NF-κB activation in THP-1 cells (SI Appendix, Fig. S2 C and D). Altogether, these data indicated that the mmpL8::Tn mutant does not produce a higher amount of, or more potent TLR2 ligands. Thus, we next hypothesized that the mutant had lost molecules that inhibit TLR2 signaling.
Ac4SGL Inhibits Activation of TLR2 Signaling.
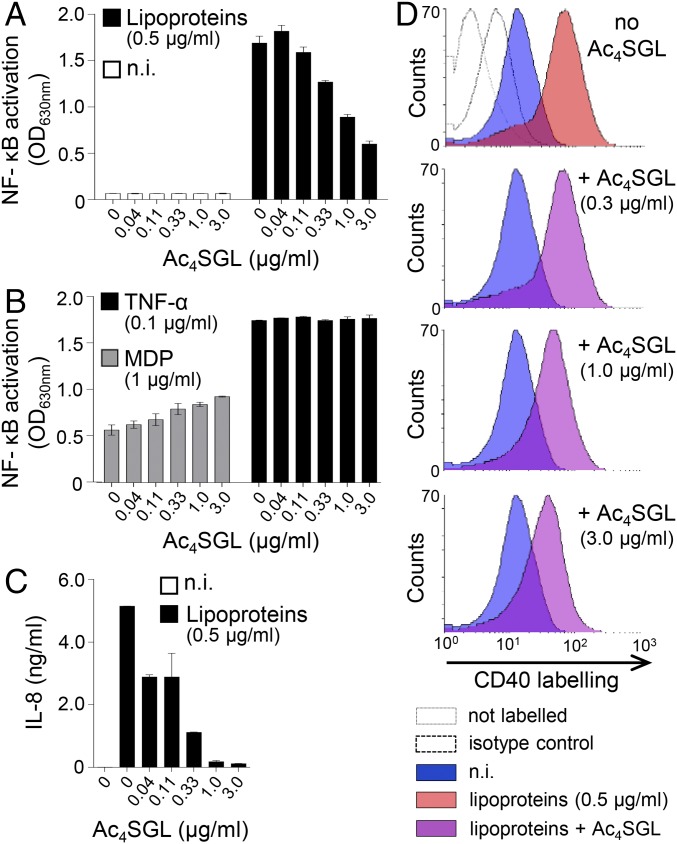
As the mmpL8::Tn mutant lacks Ac4SGL, we tested whether this glycolipid was able to inhibit TLR2 activation. Interestingly, we found that this glycolipid indeed inhibited in a dose-dependent fashion NF-κB activation in THP-1 cells stimulated by M. tuberculosis lipoproteins (Fig. 2A). However, Ac4SGL had no inhibitory effect on NF-κB activation induced by either muramyl dipeptide (MDP), a NOD2 ligand, or TNFα (Fig. 2B), indicating that Ac4SGL is not toxic to the cells at the doses tested; does not act on the components that are common to TLRs, NOD and TNFα receptor signaling pathways; and might thus specifically inhibit TLR2. It is noticeable that Ac4SGL slightly increased NF-κB activation triggered by MDP. However, the underlying mechanisms are unknown. As shown in Fig. 2 C and D, Ac4SGL also efficiently inhibited IL-8 production and CD40 expression induced by lipoproteins.
Fig. 2.
Ac4SGL inhibits activation of TLR2 signaling. THP-1 cells were stimulated with various stimuli in the presence or not of Ac4SGL at the indicated concentrations. After 16 h, NF-κB activation (A and B), IL-8 in the culture supernatant (C), and CD40 expression (D) were measured. (A and B) NF-κB activation was determined by measuring alkaline phosphatase activity in the culture supernatant and reading OD at 630 nm. (C) IL-8 release in the culture supernatant was determined by sandwich ELISA. (D) CD40 expression was monitored by flow cytometry. Data show mean ± SEM. MDP, muramyl dipeptide, a ligand of NOD2; n.i., not induced.
Structural Features Required for TLR2 Inhibition.
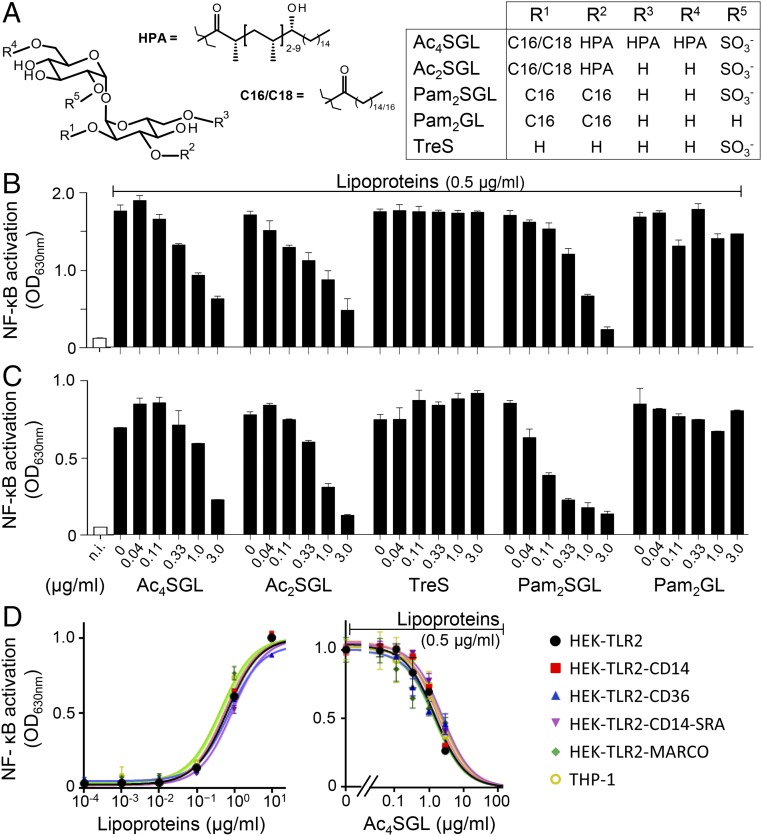
We next sought to determine the molecular bases of Ac4SGL inhibitory activity. Ac4SGL is composed of a trehalose core, sulfated in position 2′, and esterified by four fatty acids: one short chain (palmitic or stearic) fatty acid in position 2 and three polymethylated and hydroxylated long chain [hydroxy-phthioceranoic acid (HPA)] fatty acids in positions 3, 6, and 6′ (15, 19) (Fig. 3A). We first investigated the role played by fatty acids. Natural Ac2SGL, acylated by one short fatty acid on position 2 and one HPA on position 3 (Fig. 3A), showed the same inhibitory activity as Ac4SGL, whereas trehalose-2′-sulfate was inactive, indicating that fatty acids are required but two are sufficient (Fig. 3B). To determine whether the complex HPA was required, we chemically synthesized an Ac2SGL analog molecule esterified by two palmitic acids (20) (Pam2SGL; Fig. 3A) that is naturally produced in small amount by M. tuberculosis (15). Interestingly, Pam2SGL efficiently inhibited NF-κB activation (Fig. 3B and SI Appendix, Fig. S3A). However, inhibitory activity was lost upon removal of the 2′-sulfate anion (Pam2GL) (Fig. 3B). Altogether, these data showed that a simple Pam2SGL structure was sufficient to inhibit TLR2 signaling. Moreover, Pam2SGL, as Ac4SGL, efficiently inhibited IL-8 production (SI Appendix, Fig. S3C) and CD40 expression (SI Appendix, Fig. S3D) induced by lipoproteins, but had no effect on NF-κB activation induced by either MDP or TNFα (SI Appendix, Fig. S3B).
Fig. 3.
SGL structural features required for TLR2 inhibition. (A) Chemical structures of SGLs and derivatives. The main acyl forms of natural Ac4SGL and Ac2SGL, predominantly acylated by HPA, are depicted. Minor acyl forms containing a combination of HPA, phthioceranoic, stearic and/or palmitic acids also exist. (B and C) THP-1 (B) and HEK-TLR2 (C) cells were stimulated by M. tuberculosis lipoproteins in the presence or not of Ac4SGL, Ac2SGL, trehalose-2′-sulfate (TreS), Pam2SGL, or unsulfated Pam2SGL (Pam2GL) at the indicated concentrations. (D) HEK-TLR2 cells coexpressing various scavenger receptors (CD14, CD36, SRA, or MARCO) or THP-1 cells were stimulated by M. tuberculosis lipoproteins at 0.5 µg/mL in the presence of Ac4SGL at the indicated concentrations. After 16 h, NF-κB activation was determined by measuring alkaline phosphatase activity in the culture supernatant and reading OD at 630 nm. Data show mean ± SEM. n.i., not induced.
The structural analogy between Pam2SGL and the conserved S-diacylglyceryl moiety of lipoproteins/lipopeptides (SI Appendix, Fig. S4) recognized by the binding pocket of TLR2 (21) prompted us to hypothesize that sulfoglycolipids might compete for the binding of TLR2 agonists.
Sulfoglycolipids Are Competitive Inhibitors of TLR2.
In a first attempt to support this assumption, we investigated the capacity of sulfoglycolipids to inhibit TLR2 signaling in an artificial HEK cell line overexpressing TLR2, but no other coreceptor, and a reporter system for NF-κB activation (HEK-TLR2). Interestingly, sulfoglycolipids inhibited NF-κB activation in HEK-TLR2 cells (Fig. 3C) and the structural requirements for inhibitory activity were the same as for THP-1 cells (Fig. 3B). To test the possible involvement of coreceptors that would help binding and transferring sulfoglycolipids to TLR2, we investigated the inhibitory activity of Ac4SGL on HEK-TLR2 cells coexpressing the classical TLR2 accessory receptors CD14 or CD36, or two other scavenger receptors known to bind mycobacterial lipids bearing complex fatty acids, namely SRA (22) and MARCO (23). However, addition of either of these receptors did not improve the IC50 of Ac4SGL toward M. tuberculosis lipoproteins (Fig. 3D).
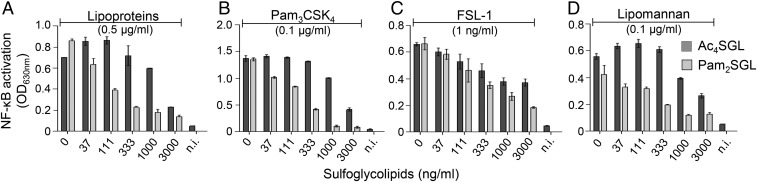
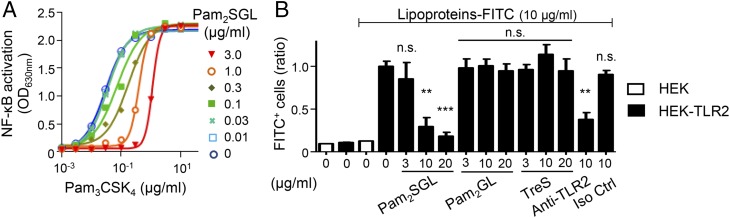
In competitive inhibition, ligands and inhibitors compete for binding to the same receptor site. Thus, a competitive inhibitor inhibits signaling induced by different agonists using the same binding site and, in its presence, the ligand’s EC50 increases but maximal effective concentration (ECmax) is not modified. We next checked whether sulfoglycolipids fulfilled these criteria. First, we investigated their ability to inhibit TLR2 signaling induced by different agonists binding to the same site as lipoproteins, but using different TLR heterodimers or being of different chemical families: the triacylated lipopeptide Pam3CSK4, a ligand of TLR2/TLR1 heterodimer, the diacylated lipopeptide FSL-1, a ligand of TLR2/TLR6 heterodimer, and mycobacterial lipomannan, a ligand of TLR2/TLR1 heterodimer, but harboring a mannosylphosphatidyl-myo-inositol lipid anchor instead of the acylated cysteinyl group found in lipopeptides (18) (SI Appendix, Fig. S4). Both Ac4SGL and Pam2SGL inhibited, in a dose-dependent fashion, NF-κB activation triggered by the different TLR2 agonists (Fig. 4). Pam2SGL appeared more efficient than Ac4SGL for inhibiting the TLR2/TLR1 agonist lipoproteins, Pam3CSK4 and lipomannan, whereas both compounds inhibited the TLR2/TLR6 agonist FSL-1 with the same efficiency. Interestingly, augmenting Pam2SGL concentration from 0 to 3 µg/mL resulted in a ∼30-fold increase of Pam3CSK4 EC50, from 30 ng/mL to 1 µg/mL (Fig. 5A). In contrast, Pam3CSK4 ECmax remained the same, strongly supporting the hypothesis that sulfoglycolipids are competitive inhibitors of TLR2. To definitely demonstrate it, we investigated whether sulfoglycolipids were able to compete for the binding of TLR2 ligands, using a binding assay of FITC-labeled lipoproteins (FITC-LPs) to HEK-TLR2 cells (24, 25). FITC-LPs bound HEK-TLR2, but not the parent HEK cells, and, as expected, their binding to HEK-TLR2 cells was blocked by an anti-TLR2 antibody (Fig. 5B). Interestingly, addition of Pam2SGL inhibited in a dose-dependent fashion FITC-LPs binding to TLR2, whereas Pam2GL and trehalose-2′-sulfate (TreS) had no effect (Fig. 5B). Altogether, our data demonstrate that sulfoglycolipids are competitive antagonists of TLR2.
Fig. 4.
SGLs inhibit NF-κB activation induced by various TLR2 agonists. HEK-TLR2 cells were stimulated by M. tuberculosis lipoproteins (A), Pam3CSK4 (B), FSL-1 (C), or M. tuberculosis lipomannan (D) in the presence or not of Ac4SGL or Pam2SGL at the indicated concentrations. After 16 h, NF-κB activation was determined by measuring alkaline phosphatase activity in the culture supernatant and reading OD at 630 nm. Data show mean ± SEM. n.i., not induced.
Fig. 5.
SGLs increase TLR2 agonist EC50 (A) and compete for the binding of M. tuberculosis lipoprotein to TLR2 (B). (A) HEK-TLR2 cells were coincubated with Pam3CSK4, at a concentration ranging from 0.1 ng to 10 µg/mL, and Pam2SGL, at a concentration ranging from 10 ng to 3 µg/mL. After 16 h, NF-κB activation was determined by measuring alkaline phosphatase activity in the culture supernatant and reading OD at 630 nm. (B) HEK-TLR2 cells were incubated with FITC-labeled M. tuberculosis lipoproteins and preincubated or not for 30 min at 37 °C with Pam2SGL, Pam2GL, TreS, anti-TLR2 antibody, or isotype control (Iso Ctrl) at the indicated concentrations. The percentage of FITC+ cells was determined by flow cytometry analysis. Results for each condition are expressed relative to the value obtained with HEK-TLR2 cells incubated with FITC-labeled M. tuberculosis lipoproteins alone and set to 1. Data show mean ± SEM. **P < 0.01; ***P < 0.001; n.s., not significant. P value is given vs. lipoproteins–FITC alone on HEK-TLR2 cells.
Discussion
To successfully colonize and survive inside its host, M. tuberculosis has evolved evasion strategies to undermine host innate immune response, in particular by restricting PRR signaling. Using an unbiased global approach involving infected macrophages and NF-κB activation as a readout, we have identified a mechanism employed by M. tuberculosis to limit MAMPs recognition by PRRs. Indeed, in addition to the previously uncovered strategy consisting of decreasing MAMPs’ accessibility to bacilli cell surface (2), we show that M. tuberculosis can directly inhibit MAMPs’ binding to PRRs via the production of antagonist glycolipids. The mmpL8::Tn mutant, which is impaired in the production of surface-exposed Ac4SGL, was found to trigger an increased TLR2 signaling in macrophages, associated with higher cytokine production and costimulatory molecule expression, compared with the wild-type strain. This result is in agreement with previous data by Domenech et al. (11) showing that a MmpL8 mutant strain less efficiently suppressed key indicators of a Th1-type immune response, suggesting an immunomodulatory role for SGLs in the pathogenesis of tuberculosis. Indeed, as stated by the authors (11), the simplest interpretation of these data would be a direct effect of SGLs on the immune system.
Here, we demonstrate that SGLs are indeed competitive inhibitors of either TLR2/TLR1 or TLR2/TLR6 heterodimers, and therefore undermine the cellular activation, such as NF-κB activation, cytokine production, or costimulatory molecule expression, induced by M. tuberculosis TLR2 agonists. Accordingly, the mmpL8::Tn mutant, which lacks surface-exposed Ac4SGL, induces a stronger TLR2-dependent activation of macrophage cells. Structure–function relationships analyses indicate that a diacylated SGL structure is sufficient to inhibit the signaling triggered by TLR2 agonists. Indeed, natural M. tuberculosis Ac2SGLs are as inhibitory as Ac4SGLs. Although produced in low amount and localized in the cell wall, presumably inserted in the plasma membrane (10, 11), Ac2SGLs might also play a role in the modulation of host immune response in vivo via their delivery from infected macrophages, through exosomes or apoptotic vesicles, to bystander cells, as shown for other M. tuberculosis lipids (26, 27). Surprisingly, the complex and M. tuberculosis-specific hydroxy-phthioceranoic acids were not required for inhibitory activity since an Ac2SGL analog molecule esterified by two palmitic acids (Pam2SGL) was as efficient as, or even more efficient than, the natural molecule. The role of these complex fatty acids, only found in SGLs, remains unclear (11), although it is worth noting that the fine structure of fatty acids governs the recognition of Ac2SGL by CD1-restricted T cells (20, 28). Interestingly, Pam2SGL is a fully synthetic molecule that efficiently blocks TLR2 activation in vitro and that should be further tested in different pathological models in vivo where TLR2 is involved, to determine its therapeutic potential (29).
SGLs are major and specific lipids of M. tuberculosis (12, 14), and five decades of in vivo and in vitro studies have led to consider them as virulence factors of the bacilli. They have been shown to exhibit several immunomodulatory activities, either suppressive or stimulatory, in vitro in immune cells (30–37). For example, purified sulfolipid-I (SL-I), the most abundant tetraacylated sulfoglycolipid acyl form (38), has been found to prevent phagosome–lysosome fusion in cultured mouse peritoneal macrophages (30), to suppress the production of reactive oxygen species by LPS-, IFNγ-, IL1β-, TNFα- or MDP-primed human monocytes (31, 32), to inhibit the production of IL1β, the activity of protein kinase C, and alter the pattern of protein phosphorylation in LPS-stimulated human monocytes (31, 32). In contrast, purified SL-I has also been proposed to activate the production of reactive oxygen species by human neutrophils (36, 37) and primed monocytes (32, 37), as well as the cytokines IL1β and TNFα by the latter cells (32). Reasons for these somehow conflicting data are unclear, but may result from differences in SL-I preparations, including the possible presence of impurities or differences in the immune cells used, such as cell type, host species, and culturing or priming conditions (35). In a recent study, Gilmore et al. (35) reported that purified SL-I, as well as a fully synthetic analog (devoid of any trace levels of bioactive contaminants), in contrast to Pam3CSK4, did not induce a pattern of proinflammatory gene expression in human monocyte-derived dendritic cells (hDCs). In addition, they observed, in agreement with our data, that neither of the two compounds stimulated the production of the proinflammatory cytokines TNFα in a variety of cell types, including hDCs, human primary macrophages, THP-1 cells, or the murine macrophage RAW cell line. Although some toxic or immunosuppressive properties of sulfoglycolipids, such as disruption of mitochondrial oxidative phosphorylation (39) or accumulation in the lysosomes (30), may result from their ability to directly interact with host cell membranes, some others might well be determined by their binding to host cell receptors, including TLR2. Indeed, signals from TLRs, including TLR2, are required for phagosome maturation (40), and activation of TLR2 has been shown to reduce the viability of M. tuberculosis in macrophages (41). So, blocking TLR2 signaling might be, at least in part, responsible for purified sulfoglycolipid’s ability to prevent phagosome–lysosome fusion in macrophages (30). Similarly, induction of an increased TLR2 signaling is likely to explain the intracellular growth defect of sulfoglycolipid-deficient mutants in hDCs (34), although the phenotype of the mutants seems to be immune cell- and host species-specific, and reflective of the balance between different antimycobacterial effector mechanisms (35). LPS, if not specifically repurified to eliminate low concentrations of highly bioactive lipoproteins, activates TLR2 in addition to TLR4 (42, 43). The reported immunosuppressive activities of purified sulfolipids on LPS-primed monocytes (31, 32) might thus be in part due their capacity to act as TLR2 antagonists.
In vivo, early studies have reported a correlation between virulence of different strains of M. tuberculosis in guinea pigs and the amount of SGLs produced by these strains grown in vitro in culture broth (13, 44). However, more recent studies using isogenic strains have led to contrasting results. ΔmmpL8 mutant strains, lacking Ac4SGL, showed an attenuated virulence in mice, in terms of bacilli burden (10) or mice survival (11). In contrast, Δpks2 mutant strains, defective for SGLs production, were not impaired for growth in mice or guinea pigs (10, 45). Again, the reasons for these discrepancies are not fully understood (10, 11, 35). Nevertheless, a recent work using multiple deletions in the polyketide synthase gene repertoire of M. tuberculosis revealed functional overlap of cell envelope lipids in host–pathogen interactions (46). Indeed, PDIM clearly appeared as dominant lipid virulence factors masking the function of other lipid families, such as SGLs. However, interestingly, the loss of SGLs increased the attenuated phenotype of a PDIM-less mutant in mice and affected bacilli growth in human macrophages (46).
In conclusion, our findings reveal that producing glycolipid antagonists of PRRs, such as TLR2, is a strategy used by M. tuberculosis to undermine innate immune clearance. Moreover, we uncover a mechanism by which SGLs may contribute to M. tuberculosis virulence.
Material and Methods
Mutant Library Screening.
Three-week-old M. tuberculosis Beijing GC1237 mutant individual culture (20 µL) from the previously used library (9) was transferred into a 384-well assay plate. THP-1 Blue cells (2 × 104 per well; Invivogen) were distributed in 30 µL of HEK Blue Detection medium (Invivogen). After 16 h, OD was read at 625 nm. Details are provided in SI Appendix, SI Materials and Methods.
THP-1 and HEK-TLR2 Cell Experiments.
The different stimuli and competitors, obtained as described in SI Appendix, SI Materials and Methods, were added in 96-well plates at multiplicity of infection (MOI) or concentrations indicated in the figures. Cells (105 per well for THP-1; 5 × 104 per well for HEK-TLR2), maintained as described in SI Appendix, SI Materials and Methods, were then distributed in 200 µL of appropriate culture medium and stimulated for 18 h. NF-κB activation was measured by mixing 20 µL of the culture supernatant and 180 µL of Quanti-Blue (InvivoGen) and reading OD at 630 nm. IL-8 was assayed in the culture supernatant by sandwich ELISA using commercially available kits (eBioscience). CD40 expression was monitored by flow cytometry using a CD40 PE-conjugated antibody (Beckman Coulter).
TLR2 Binding Assay.
HEK parent or HEK-TLR2 cells (2.5 × 105 per well) were distributed in 96-well plates, washed with PBS/Ca2+ (Gibco) containing 0.5% BSA (buffer A) and incubated with 10 µg/mL of FITC-labeled lipoproteins for 30 min at 37 °C in buffer A. In competition experiments, cells were preincubated for 30 min at 37 °C with 10 µg/mL of anti-TLR2 monoclonal antibody (clone T2.5, InvivoGen) or isotype control (IgG1, eBioscience), or 3, 10, or 20 μg/mL of Pam2SGL, Pam2GL, or TreS in buffer A. Cells were then washed with buffer A. Cell integrity was determined by treatment with 2 µg/mL of 7-aminoactinomycin D (eBioscience) for 5 min at 4 °C in buffer A. The percentage of FITC+ cells was determined by flow cytometry analysis on a LSRII apparatus (BD Biosciences). The percentage of FITC+ cells in the presence of FITC-labeled lipoproteins was set to the value of 1. Results for each condition are expressed relative to this value and are the mean of three independent experiments.
Supplementary Material
Acknowledgments
We thank Camille Robert, Pierre-Henri Degoy, Adrien Vaquié, Yannick Poquet, and Florence Levillain (Institut de Pharmacologie et de Biologie Structurale, Toulouse) for helpful assistance. This article is dedicated to the memory of Dr. Gérard Tiraby (1941–2017), professor of Microbiology at the Université Paul Sabatier (Toulouse) and founder of InvivoGen. This work was supported by the Centre National de la Recherche Scientifique and Université Paul Sabatier (Toulouse, France), the Inserm Avenir Program, and the European Community (TB-VIR no. 200973, ERC-STG INTRACELLTB no. 260901). L.B. was the recipient of a PhD fellowship from Invivogen and the Conseil Régional Midi-Pyrénées.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1707840114/-/DCSupplemental.
References
- 1.Cambier CJ, Falkow S, Ramakrishnan L. Host evasion and exploitation schemes of Mycobacterium tuberculosis. Cell. 2014;159:1497–1509. doi: 10.1016/j.cell.2014.11.024. [DOI] [PubMed] [Google Scholar]
- 2.Cambier CJ, et al. Mycobacteria manipulate macrophage recruitment through coordinated use of membrane lipids. Nature. 2014;505:218–222. doi: 10.1038/nature12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pathak SK, et al. Direct extracellular interaction between the early secreted antigen ESAT-6 of Mycobacterium tuberculosis and TLR2 inhibits TLR signaling in macrophages. Nat Immunol. 2007;8:610–618. doi: 10.1038/ni1468. [DOI] [PubMed] [Google Scholar]
- 4.Nigou J, Zelle-Rieser C, Gilleron M, Thurnher M, Puzo G. Mannosylated lipoarabinomannans inhibit IL-12 production by human dendritic cells: Evidence for a negative signal delivered through the mannose receptor. J Immunol. 2001;166:7477–7485. doi: 10.4049/jimmunol.166.12.7477. [DOI] [PubMed] [Google Scholar]
- 5.Geijtenbeek TB, et al. Mycobacteria target DC-SIGN to suppress dendritic cell function. J Exp Med. 2003;197:7–17. doi: 10.1084/jem.20021229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vergne I, Gilleron M, Nigou J. Manipulation of the endocytic pathway and phagocyte functions by Mycobacterium tuberculosis lipoarabinomannan. Front Cell Infect Microbiol. 2015;4:187. doi: 10.3389/fcimb.2014.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gringhuis SI, et al. C-type lectin DC-SIGN modulates toll-like receptor signaling via Raf-1 kinase-dependent acetylation of transcription factor NF-kappaB. Immunity. 2007;26:605–616. doi: 10.1016/j.immuni.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Reed MB, et al. A glycolipid of hypervirulent tuberculosis strains that inhibits the innate immune response. Nature. 2004;431:84–87. doi: 10.1038/nature02837. [DOI] [PubMed] [Google Scholar]
- 9.Brodin P, et al. High content phenotypic cell-based visual screen identifies Mycobacterium tuberculosis acyltrehalose-containing glycolipids involved in phagosome remodeling. PLoS Pathog. 2010;6:e1001100. doi: 10.1371/journal.ppat.1001100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Converse SE, et al. MmpL8 is required for sulfolipid-1 biosynthesis and Mycobacterium tuberculosis virulence. Proc Natl Acad Sci USA. 2003;100:6121–6126. doi: 10.1073/pnas.1030024100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Domenech P, et al. The role of MmpL8 in sulfatide biogenesis and virulence of Mycobacterium tuberculosis. J Biol Chem. 2004;279:21257–21265. doi: 10.1074/jbc.M400324200. [DOI] [PubMed] [Google Scholar]
- 12.Middlebrook G, Coleman CM, Schaefer WB. Sulfolipid from virulent tubercle bacilli. Proc Natl Acad Sci USA. 1959;45:1801–1804. doi: 10.1073/pnas.45.12.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goren MB, Brokl O, Schaefer WB. Lipids of putative relevance to virulence in Mycobacterium tuberculosis: Correlation of virulence with elaboration of sulfatides and strongly acidic lipids. Infect Immun. 1974;9:142–149. doi: 10.1128/iai.9.1.142-149.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bertozzi CR, Schelle MW. Sulfates metabolites from Mycobacterium tuberculosis: Sulfolipid-1 and beyond. In: Daffé M, Reyrat J-M, editors. The Mycobacterial Cell Envelope. ASM Press; Washington, DC: 2008. pp. 291–304. [Google Scholar]
- 15.Layre E, et al. Deciphering sulfoglycolipids of Mycobacterium tuberculosis. J Lipid Res. 2011;52:1098–1110. doi: 10.1194/jlr.M013482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caminero JA, et al. Epidemiological evidence of the spread of a Mycobacterium tuberculosis strain of the Beijing genotype on Gran Canaria Island. Am J Respir Crit Care Med. 2001;164:1165–1170. doi: 10.1164/ajrccm.164.7.2101031. [DOI] [PubMed] [Google Scholar]
- 17.Roux AL, et al. Overexpression of proinflammatory TLR-2-signalling lipoproteins in hypervirulent mycobacterial variants. Cell Microbiol. 2011;13:692–704. doi: 10.1111/j.1462-5822.2010.01565.x. [DOI] [PubMed] [Google Scholar]
- 18.Ray A, Cot M, Puzo G, Gilleron M, Nigou J. Bacterial cell wall macroamphiphiles: Pathogen-/microbe-associated molecular patterns detected by mammalian innate immune system. Biochimie. 2013;95:33–42. doi: 10.1016/j.biochi.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 19.Goren MB. Mycobacterial fatty acid esters of sugars and sulfosugars. In: Hd J, editor. Handbook of Lipid Research. Vol 6. Plenum Press; New York: 1990. pp. 363–461. [Google Scholar]
- 20.Gilleron M, et al. Diacylated sulfoglycolipids are novel mycobacterial antigens stimulating CD1-restricted T cells during infection with Mycobacterium tuberculosis. J Exp Med. 2004;199:649–659. doi: 10.1084/jem.20031097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin MS, et al. Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell. 2007;130:1071–1082. doi: 10.1016/j.cell.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Ernst JD. Macrophage receptors for Mycobacterium tuberculosis. Infect Immun. 1998;66:1277–1281. doi: 10.1128/iai.66.4.1277-1281.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bowdish DM, et al. MARCO, TLR2, and CD14 are required for macrophage cytokine responses to mycobacterial trehalose dimycolate and Mycobacterium tuberculosis. PLoS Pathog. 2009;5:e1000474. doi: 10.1371/journal.ppat.1000474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nigou J, et al. Mannan chain length controls lipoglycans signaling via and binding to TLR2. J Immunol. 2008;180:6696–6702. doi: 10.4049/jimmunol.180.10.6696. [DOI] [PubMed] [Google Scholar]
- 25.Vasselon T, Detmers PA, Charron D, Haziot A. TLR2 recognizes a bacterial lipopeptide through direct binding. J Immunol. 2004;173:7401–7405. doi: 10.4049/jimmunol.173.12.7401. [DOI] [PubMed] [Google Scholar]
- 26.Beatty WL, et al. Trafficking and release of mycobacterial lipids from infected macrophages. Traffic. 2000;1:235–247. doi: 10.1034/j.1600-0854.2000.010306.x. [DOI] [PubMed] [Google Scholar]
- 27.Schaible UE, et al. Apoptosis facilitates antigen presentation to T lymphocytes through MHC-I and CD1 in tuberculosis. Nat Med. 2003;9:1039–1046. doi: 10.1038/nm906. [DOI] [PubMed] [Google Scholar]
- 28.Guiard J, et al. Fatty acyl structures of mycobacterium tuberculosis sulfoglycolipid govern T cell response. J Immunol. 2009;182:7030–7037. doi: 10.4049/jimmunol.0804044. [DOI] [PubMed] [Google Scholar]
- 29.Savva A, Roger T. Targeting toll-like receptors: Promising therapeutic strategies for the management of sepsis-associated pathology and infectious diseases. Front Immunol. 2013;4:387. doi: 10.3389/fimmu.2013.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goren MB, D’Arcy Hart P, Young MR, Armstrong JA. Prevention of phagosome-lysosome fusion in cultured macrophages by sulfatides of Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1976;73:2510–2514. doi: 10.1073/pnas.73.7.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pabst MJ, Gross JM, Brozna JP, Goren MB. Inhibition of macrophage priming by sulfatide from Mycobacterium tuberculosis. J Immunol. 1988;140:634–640. [PubMed] [Google Scholar]
- 32.Brozna JP, Horan M, Rademacher JM, Pabst KM, Pabst MJ. Monocyte responses to sulfatide from Mycobacterium tuberculosis: Inhibition of priming for enhanced release of superoxide, associated with increased secretion of interleukin-1 and tumor necrosis factor alpha, and altered protein phosphorylation. Infect Immun. 1991;59:2542–2548. doi: 10.1128/iai.59.8.2542-2548.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okamoto Y, et al. Mycobacterial sulfolipid shows a virulence by inhibiting cord factor induced granuloma formation and TNF-alpha release. Microb Pathog. 2006;40:245–253. doi: 10.1016/j.micpath.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Mendum TA, Wu H, Kierzek AM, Stewart GR. Lipid metabolism and Type VII secretion systems dominate the genome scale virulence profile of Mycobacterium tuberculosis in human dendritic cells. BMC Genomics. 2015;16:372. doi: 10.1186/s12864-015-1569-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gilmore SA, et al. Sulfolipid-1 biosynthesis restricts Mycobacterium tuberculosis growth in human macrophages. ACS Chem Biol. 2012;7:863–870. doi: 10.1021/cb200311s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang L, English D, Andersen BR. Activation of human neutrophils by Mycobacterium tuberculosis-derived sulfolipid-1. J Immunol. 1991;146:2730–2736. [PubMed] [Google Scholar]
- 37.Zhang L, Goren MB, Holzer TJ, Andersen BR. Effect of Mycobacterium tuberculosis-derived sulfolipid I on human phagocytic cells. Infect Immun. 1988;56:2876–2883. doi: 10.1128/iai.56.11.2876-2883.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goren MB. Sulfolipid I of Mycobacterium tuberculosis, strain H37Rv. I. Purification and properties. Biochim Biophys Acta. 1970;210:116–126. doi: 10.1016/0005-2760(70)90067-6. [DOI] [PubMed] [Google Scholar]
- 39.Kato M, Goren MB. Synergistic action of cord factor and mycobacterial sulfatides on mitochondria. Infect Immun. 1974;10:733–741. doi: 10.1128/iai.10.4.733-741.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blander JM, Medzhitov R. Regulation of phagosome maturation by signals from toll-like receptors. Science. 2004;304:1014–1018. doi: 10.1126/science.1096158. [DOI] [PubMed] [Google Scholar]
- 41.Thoma-Uszynski S, et al. Induction of direct antimicrobial activity through mammalian toll-like receptors. Science. 2001;291:1544–1547. doi: 10.1126/science.291.5508.1544. [DOI] [PubMed] [Google Scholar]
- 42.Hirschfeld M, Ma Y, Weis JH, Vogel SN, Weis JJ. Cutting edge: Repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J Immunol. 2000;165:618–622. doi: 10.4049/jimmunol.165.2.618. [DOI] [PubMed] [Google Scholar]
- 43.Tapping RI, Akashi S, Miyake K, Godowski PJ, Tobias PS. Toll-like receptor 4, but not toll-like receptor 2, is a signaling receptor for Escherichia and Salmonella lipopolysaccharides. J Immunol. 2000;165:5780–5787. doi: 10.4049/jimmunol.165.10.5780. [DOI] [PubMed] [Google Scholar]
- 44.Gangadharam PRJ, Cohn ML, Middlebrook G. Infectivity, pathogenicity and sulpholipid fraction of some Indian and British strains of tubercle bacilli. Tubercle. 1963;44:452–455. doi: 10.1016/s0041-3879(63)80087-2. [DOI] [PubMed] [Google Scholar]
- 45.Rousseau C, et al. Sulfolipid deficiency does not affect the virulence of Mycobacterium tuberculosis H37Rv in mice and guinea pigs. Infect Immun. 2003;71:4684–4690. doi: 10.1128/IAI.71.8.4684-4690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Passemar C, et al. Multiple deletions in the polyketide synthase gene repertoire of Mycobacterium tuberculosis reveal functional overlap of cell envelope lipids in host-pathogen interactions. Cell Microbiol. 2014;16:195–213. doi: 10.1111/cmi.12214. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.