Significance
Sensing of the plant hormone jasmonate (JA) by the F-box protein CORONATINE INSENSITIVE 1 (COI1) triggers profound transcriptional changes that are regulated by the master regulator MYC2. However, it remains unclear how COI1 communicates with the general transcription machinery and chromatin. Here, we show that MED25, a subunit of the Mediator coactivator complex, physically and functionally interacts with COI1 on the promoters of MYC2 targets. MED25 also physically and functionally interacts with HISTONE ACETYLTRANSFERASE1 (HAC1), which selectively regulates histone (H) 3 lysine (K) 9 acetylation of MYC2 targets. Therefore, MED25 integrates regulatory signals that converge on the promoters of MYC2 targets. Our results reveal a fundamental mechanism by which Mediator coordinates the actions of both genetic and epigenetic regulators into a concerted transcriptional program.
Keywords: jasmonate, nuclear hormone receptor, MED25, COI1, MYC2
Abstract
Jasmonoyl-isoleucine (JA-Ile), the active form of the plant hormone jasmonate (JA), is sensed by the F-box protein CORONATINE INSENSITIVE 1 (COI1), a component of a functional Skp–Cullin–F-box E3 ubiquitin ligase complex. Sensing of JA-Ile by COI1 rapidly triggers genome-wide transcriptional changes that are largely regulated by the basic helix–loop–helix transcription factor MYC2. However, it remains unclear how the JA-Ile receptor protein COI1 relays hormone-specific regulatory signals to the RNA polymerase II general transcriptional machinery. Here, we report that the plant transcriptional coactivator complex Mediator directly links COI1 to the promoters of MYC2 target genes. MED25, a subunit of the Mediator complex, brings COI1 to MYC2 target promoters and facilitates COI1-dependent degradation of jasmonate–ZIM domain (JAZ) transcriptional repressors. MED25 and COI1 influence each other’s enrichment on MYC2 target promoters. Furthermore, MED25 physically and functionally interacts with HISTONE ACETYLTRANSFERASE1 (HAC1), which plays an important role in JA signaling by selectively regulating histone (H) 3 lysine (K) 9 (H3K9) acetylation of MYC2 target promoters. Moreover, the enrichment and function of HAC1 on MYC2 target promoters depend on COI1 and MED25. Therefore, the MED25 interface of Mediator links COI1 with HAC1-dependent H3K9 acetylation to activate MYC2-regulated transcription of JA-responsive genes. This study exemplifies how a single Mediator subunit integrates the actions of both genetic and epigenetic regulators into a concerted transcriptional program.
Jasmonate (JA) is an oxylipin-derived plant hormone that regulates diverse aspects of plant immunity and development (1, 2). Decades of studies in the model plant Arabidopsis thaliana have revealed a core JA signaling module consisting of the F-box protein CORONATINE INSENSITIVE 1 (COI1) (3), a group of jasmonate–ZIM domain (JAZ) proteins (4–6), and the basic helix–loop–helix transcription factor MYC2 (7, 8). COI1 forms a functional Skp–Cullin–F-box (SCF) E3 ubiquitin ligase SCFCOI1 along with Cullin1 and Skp1-like1 (ASK1) (9, 10), MYC2 acts as a master transcription factor that differentially regulates diverse aspects of JA responses (11–13), and the JAZ proteins are substrates of SCFCOI1 and serve as transcriptional repressors of MYC2 (4, 5, 14).
The identification of jasmonoyl-isoleucine (JA-Ile) as the receptor-active form of the hormone, along with the discovery that sensing of JA-Ile involves formation of the SCFCOI1–JAZs coreceptor complex (4, 15–17), represented a breakthrough in our mechanistic understanding of JA signaling. In the absence of the hormone, JAZ repressors interact with and repress the activity of MYC2. In response to internal or external cues that trigger JA-Ile synthesis, elevated JA-Ile levels promote SCFCOI1-dependent degradation of JAZ repressors, and thereby activate (de-repress) the MYC2-directed transcriptional program. These discoveries imply that sensing of the active hormone is tightly linked to transcription of JA-responsive genes throughout the genome. In this context, an important challenge in the study of JA signaling is unraveling the molecular determinants that enable the JA-Ile receptor to transmit hormone-specific regulatory signals to the RNA polymerase II (Pol II) general transcription machinery, which transcribes most protein-coding genes in eukaryotic cells (18).
The intimate association between sensing of JA-Ile and genome-wide transcriptional reprogramming implies that coordinated epigenetic regulatory events, such as histone modifications and chromatin remodeling, are an integral part of JA signaling. However, it remains unclear how plants integrate the actions of multiple epigenetic regulators and the aforementioned genetic regulators (i.e., COI1, MYC2, JAZs, etc.) into a concerted transcriptional program.
To investigate these closely related issues, we sought to identify COI1-interacting proteins, reasoning that the molecular determinants that bridge COI1 with the general transcription machinery and chromatin must interact physically with COI1. Among the COI1-interacting proteins we identified was the MED25 subunit of Arabidopsis Mediator (19–22), an evolutionarily conserved multisubunit coregulatory complex whose activity is essential for Pol II-dependent transcription in eukaryotic cells (23–29).
Here, we report that MED25 bridges COI1 to Pol II and chromatin during JA signaling. We found that MED25 physically interacts with COI1 on MYC2 target promoters and facilitates COI1-dependent degradation of JAZ proteins. MED25 also physically and functionally interacts with HISTONE ACETYLTRANSFERASE1 (HAC1), a histone modification enzyme that selectively regulates histone (H) 3 lysine (K) 9 acetylation (H3K9ac) of MYC2 target promoters during JA signaling. Moreover, MED25 cooperates with both COI1 and HAC1 on MYC2 target promoters. Therefore, MED25 directly links the JA-Ile receptor to transcriptionally active chromatin during hormone-elicited activation of MYC2.
Results
COI1 Is Enriched on the Promoters of JAZ8 and ERF1.
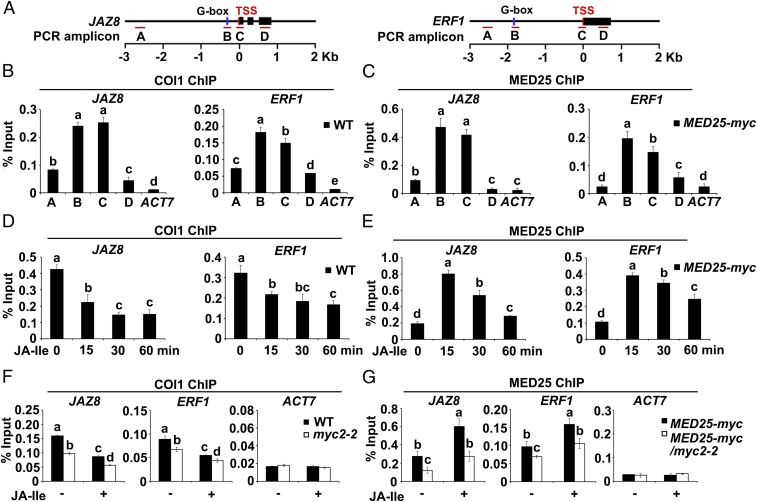
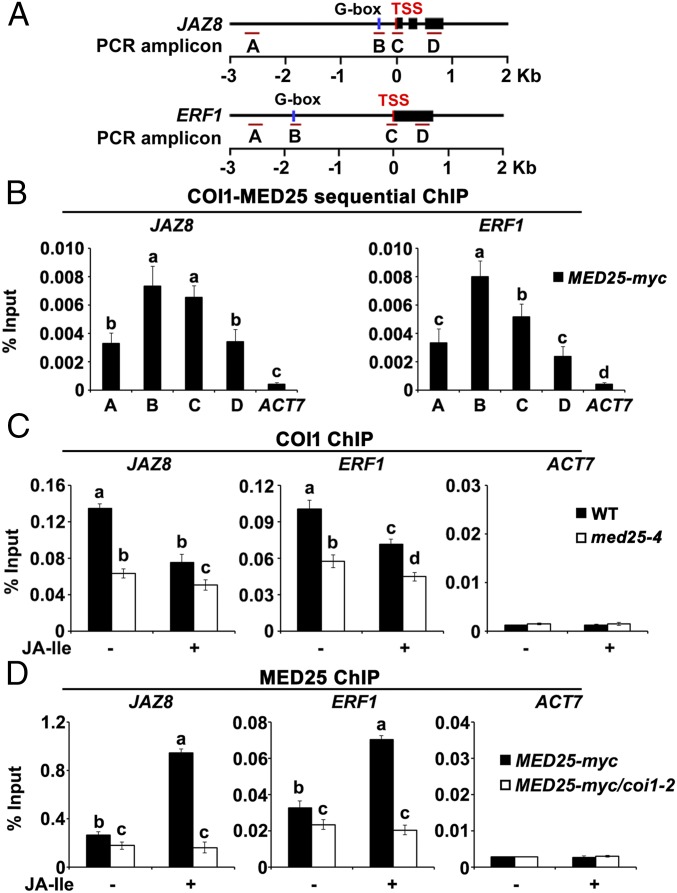
Given that COI1-dependent JA-Ile perception triggers rapid degradation of JAZ proteins, which interact with and repress MYC2 in the resting stage, we hypothesized that COI1 associates with promoter regions bound by MYC2. To test this possibility, we performed chromatin immunoprecipitation (ChIP)-quantitative PCR (qPCR) assays to measure the enrichment of COI1 on the chromatin of JAZ8 and ERF1, which are direct transcriptional targets of MYC2 (5, 11, 21) (Fig. 1A and Fig. S1). ChIP-qPCR assays of wild-type (WT) seedlings using an anti-COI1 antibody revealed that, without JA-Ile stimulation, COI1 was much more highly enriched on the G-box regions and transcription start sites (TSSs) of these genes than on the upstream promoter regions and gene bodies (Fig. 1 A and B), indicating that COI1 preferentially associates with the G-box and TSS regions of JAZ8 and ERF1.
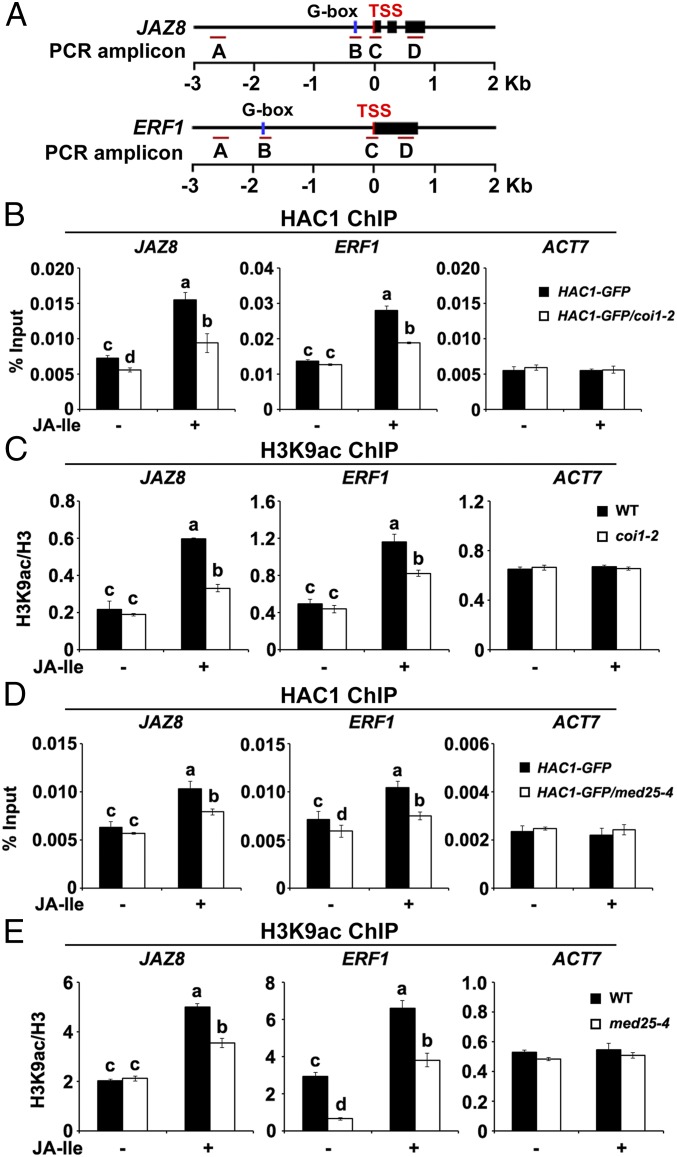
Fig. 1.
Enrichment of COI1 and MED25 on the promoters of JAZ8 and ERF1. (A) Schematic diagrams of JAZ8, ERF1, and PCR amplicons indicated as letters A–D used for ChIP-qPCR. (B) ChIP-qPCR showing the enrichment of COI1 on the chromatin of JAZ8 and ERF1. Chromatin of WT plants was immunoprecipitated using anti-COI1 antibody. (C) ChIP-qPCR showing enrichment of MED25 on the chromatin of JAZ8 and ERF1. Chromatin of MED25-myc plants was immunoprecipitated using anti-myc antibody. (D) ChIP-qPCR showing enrichment of COI1 on the TSS regions of JAZ8 and ERF1 upon JA-Ile stimulation. WT plants were treated with 30 μM JA-Ile for the indicated times before cross-linking, and chromatin from each sample was immunoprecipitated using anti-COI1 antibody. (E) ChIP-qPCR showing enrichment of MED25 on the TSS regions of JAZ8 and ERF1 upon JA-Ile stimulation. MED25-myc plants were treated with 30 μM JA-Ile for the indicated times before cross-linking. Chromatin of each sample was immunoprecipitated using anti-myc antibody. (F) ChIP-qPCR assays showing that myc2-2 impairs the enrichment of COI1 on the TSSs of JAZ8 and ERF1 before and after JA-Ile stimulation. WT and myc2-2 plants were treated with or without 30 μM JA-Ile for 15 min before cross-linking, and chromatin of each sample was immunoprecipitated using anti-COI1 antibody. (G) ChIP-qPCR assays showing that myc2-2 impairs the enrichment of MED25 on the TSSs of JAZ8 and ERF1 before and after JA-Ile stimulation. MED25-myc and MED25-myc/myc2-2 plants were treated with or without 30 μM JA-Ile for 15 min before cross-linking, and chromatin of each sample was immunoprecipitated using anti-myc antibody. For B–G, precipitated DNA was quantified by qPCR, and the DNA enrichment is shown as a percentage of input DNA. ACTIN7 (ACT7) was used as a nonspecific binding site. Error bars indicate SD of three independent experiments (n = 3). ANOVA was performed for statistical analysis; bars with different letters are significantly different from each other (P < 0.01).
Fig. S1.
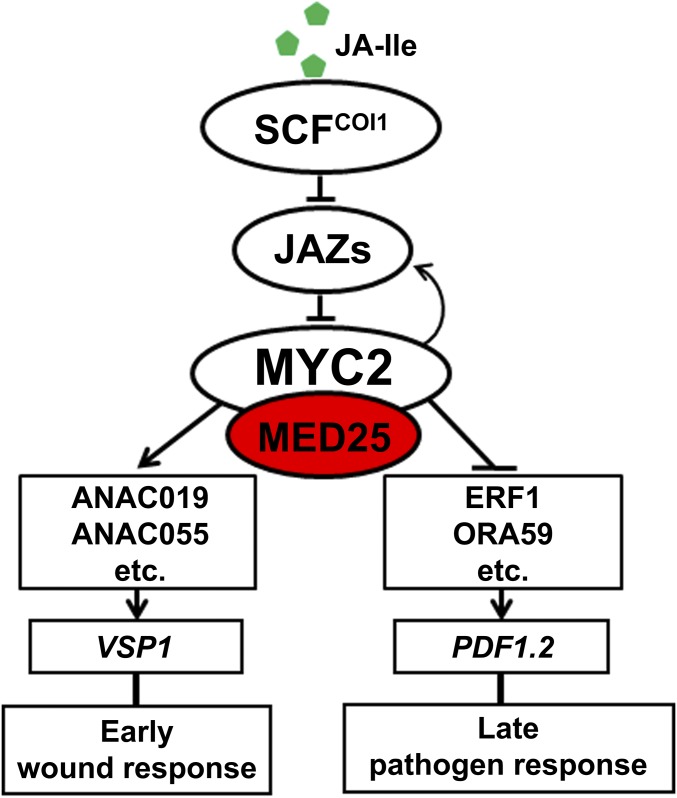
MYC2-dependent orchestration of different branches of JA responses. In response to JA-Ile, MYC2 positively regulates a group of intermediate transcription factors (TFs) (ANAC019, ANAC055, etc.), which, in turn, regulate the expression of downstream wound-responsive genes (VSP1 as a marker). MYC2 also negatively regulates a group of intermediate TFs (ERF1, ORA59, etc.), which, in turn, regulate the expression of downstream pathogen-responsive genes (PDF1.2 as a marker). Note that the positive regulation of wound response by MYC2 occurs relatively early (peaked expression occurs within 6 h after hormone elicitation), whereas the negative regulation of pathogen response by MYC2 occurs relatively late (peaked expression occurs after 48 h after JA application). MYC2 also directly regulates the expression of a group of immediate JA-responsive genes (peaked expression occurs within 1 h after hormone elicitation), including JAZs and JA biosynthetic genes.
To determine whether and how the promoter association of COI1 is regulated by JA signaling, we examined the JA-Ile–induced pattern of enrichment of COI1 on the TSSs of JAZ8 and ERF1 by ChIP-qPCR. In the absence of JA-Ile, COI1 enrichment levels on the TSSs of JAZ8 and ERF1 were relatively high, whereas following JA-Ile treatment, COI1 enrichment levels were reduced within 15 min and continued to decrease throughout the remainder of the experiment (Fig. 1D). These results reveal that COI1 is enriched on MYC2 target promoters and that this enrichment is down-regulated by hormone stimulation.
Further, we examined whether depletion of MYC2 affects the enrichment of COI1 on the promoters of JAZ8 and ERF1. In the absence of JA-Ile, COI1 enrichment levels on the TSSs of JAZ8 and ERF1 were significantly reduced in myc2-2 (8) in comparison to the WT (Fig. 1F). JA-Ile treatment decreased COI1 enrichment in both myc2-2 and the WT, and the level of COI1 enrichment was lower in myc2-2 than in the WT (Fig. 1F). These results indicate that the COI1 enrichment on MYC2 target promoters depends on the function of MYC2.
MED25 Interacts with COI1 and Facilitates COI1-Dependent Degradation of JAZ1.
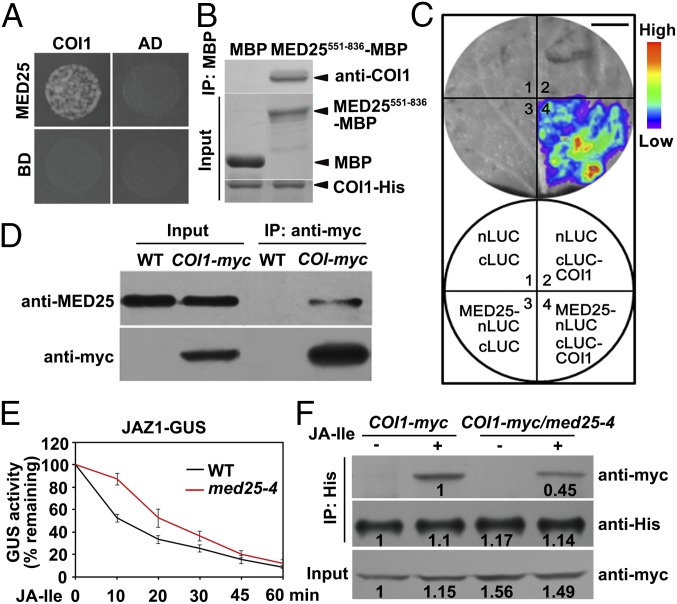
To identify the molecular determinants that link COI1 to MYC2 target promoters, we performed yeast two-hybrid (Y2H) assays to identify COI1-interacting proteins. Significantly, one of the COI1-interacting proteins we identified was the MED25 subunit of the plant Mediator coactivator complex (Fig. 2A). To confirm the physical interaction between MED25 and COI1, we performed in vitro pull-down experiments using a purified maltose-binding protein (MBP)–tagged MED25 fragment (MED25551-836-MBP) and histidine (His)-tagged COI1 (COI1-His). MED25551-836 could pull down COI1 (Fig. 2B), indicating that MED25 interacts with COI1 in vitro.
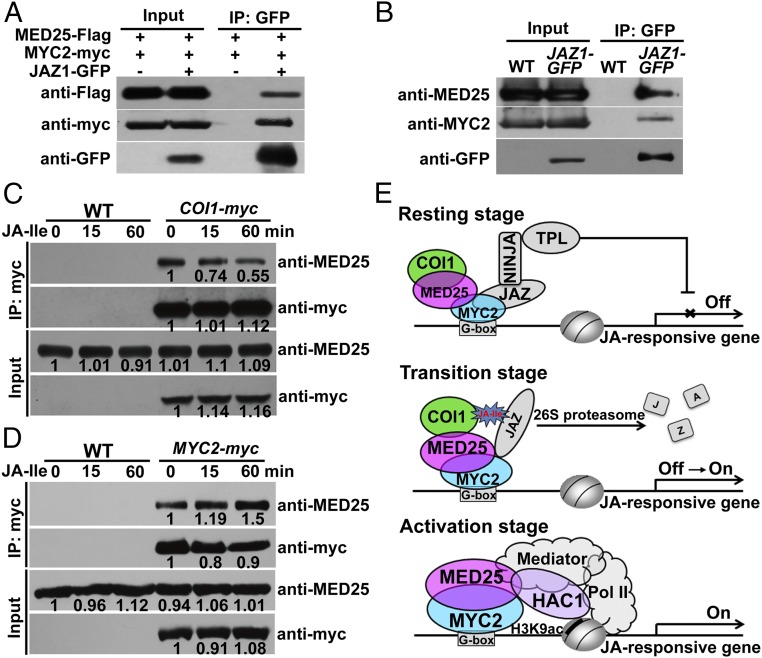
Fig. 2.
MED25 interacts with COI1 and facilitates COI1-dependent degradation of JAZ1. (A) Y2H assays showing that MED25 interacts with COI1. Transformed yeast strain was plated on SD medium lacking His, Leu, and Trp (SD/-3). (B) In vitro pull-down assays between MED25551-836-MBP and COI1-His. COI1-His was pulled down by MED25551-836-MBP immobilized on amylose resin. Protein bound to amylose resin was eluted and analyzed by immunoblotting using anti-COI1 antibody. (C) MED25 associates with COI1 in LCI assays. (Top) LUC images of N. benthamiana leaves coinfiltrated with various constructs are shown in the lower quadrant of the circle. The pseudocolor bar shows the range of luminescence intensity. (Scale bar, 1 cm.) (D) Co-IP assay between MED25 and COI1. Proteins extracted from WT and COI1-myc plants were immunoprecipitated using anti-myc antibody and immunoblotted using anti-MED25 antibody. (E) JA-Ile–triggered degradation of the JAZ1-GUS reporter in WT and med25-4 backgrounds. Seven-day-old seedlings were treated with 30 μM JA-Ile for the indicated durations before quantification of GUS activity. Error bars indicate SD of three independent experiments (n = 3). (F) Pull-down assays between JAZ1-His and COI1. Protein extracts from COI1-myc and COI1-myc/med25-4 seedlings were incubated with recombinant JAZ1-His protein in the presence or absence of 30 μM JA-Ile. COI1 was pulled down by JAZ1-His immobilized on nickel-nitrilotriacetic acid (Ni-NTA; Novagen) resin and eluted and analyzed by immunoblotting using anti-myc antibody. Bands were quantified using ImageJ.
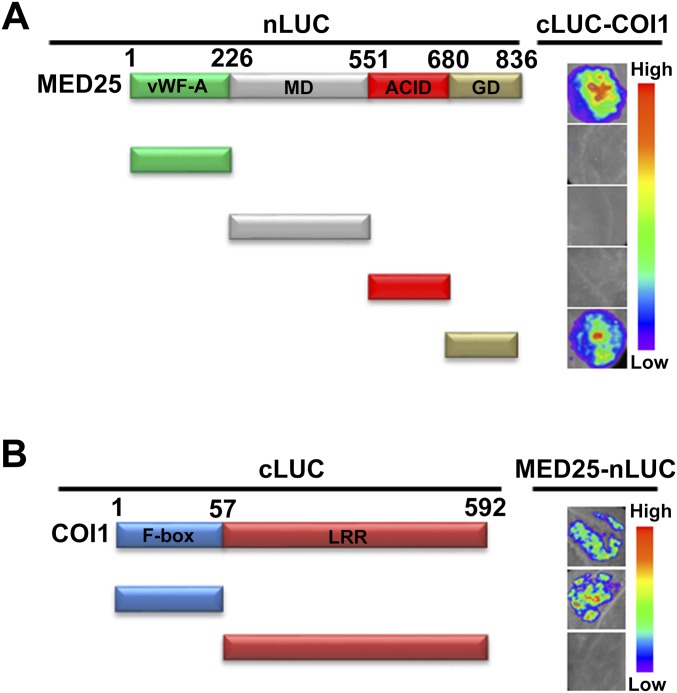
To determine whether MED25 interacts with COI1 in planta, we conducted firefly luciferase (LUC) complementation imaging (LCI) assays in Nicotiana benthamiana leaves (30). In these experiments, MED25 was fused to the N-terminal half of LUC (nLUC) to produce MED25-nLUC, whereas COI1 was fused to the C-terminal half of LUC (cLUC) to produce cLUC-COI1. N. benthamiana cells coexpressing MED25-nLUC and cLUC-COI1 displayed strong fluorescence signals, whereas those coexpressing nLUC and cLUC-COI1 or MED25-nLUC and cLUC displayed no signal (Fig. 2C), confirming that the MED25–COI1 interaction occurs in vivo. Domain mapping with LCI assays revealed that the MED25–COI1 interaction requires the glutamine-rich domain of MED25 and the F-box domain of COI1 (Fig. S2).
Fig. S2.
Mapping of the protein domains involved in MED25–COI1 interaction using LCI assays. (A) Based on the schematic protein structure of MED25, full-length MED25 or its derivatives (MED25-nLUC or MED25-nLUC derivatives) were tested for interactions with COI1 (cLUC-COI1). N. benthamiana leaves cotransformed with MED25-nLUC or MED25-nLUC derivatives and cLUC-COI1 were imaged 72 h after Agrobacterium infiltration. (B) Based on the schematic protein structure of COI1, full-length COI1 or its derivatives (cLUC-COI1 or cLUC-COI1 derivatives) were tested for interaction with MED25 (MED25-nLUC). N. benthamiana leaves cotransformed with cLUC-COI1 or cLUC-COI1 derivatives and MED25-nLUC were imaged 72 h after Agrobacterium infiltration. ACID, activator-interacting domain; GD, glutamine-rich domain; LRR, leucine-rich repeat; MD, middle domain; vWF-A, von Willebrand factor A domain. In A and B, the pseudocolor bar shows the range of luminescence intensity.
Next, we performed coimmunoprecipitation (co-IP) experiments using COI1-myc plants (9) and anti-MED25 antibody (21), and found that COI1-myc could pull down endogenous MED25 (Fig. 2D), corroborating that MED25 interacts with COI1 in planta.
To understand the functional relevance of MED25–COI1 interaction in JA signaling, we first investigated whether depletion of MED25 would affect the COI1-dependent degradation of JAZ proteins, a convenient reporter for assessing the in vivo JA response (4, 5). For this purpose, we introduced the JAZ1–β-glucuronidase (GUS) fusion protein (4) into the med25-4 mutant (21) background. As shown in Fig. 2E, JA-Ile–triggered degradation of JAZ1-GUS was considerably slower in med25-4 than in the WT, confirming that MED25 plays an important role in COI1-dependent degradation of JAZ1-GUS. We then performed an in vitro pull-down assay to test whether MED25 plays a role in JA-Ile–mediated promotion of COI1–JAZ1 interaction. In these experiments, protein extracts from COI1-myc/WT and COI1-myc/med25-4 seedlings were incubated with recombinant His-tagged JAZ1 protein (JAZ1-His) in the presence of JA-Ile, and protein bound to JAZ1-His was detected with an anti-myc antibody. The ability of JA-Ile to promote the COI1–JAZ1 interaction was reduced in med25-4 relative to the WT (Fig. 2F), suggesting that MED25 contributes to JA-Ile–mediated promotion of the COI1–JAZ1 interaction.
MED25 and COI1 Reciprocally Affect Each Other’s Enrichment on the Promoters of JAZ8 and ERF1.
Our observations that COI1 is enriched on MYC2 target promoter, and that MED25 interacts with COI1 in planta, suggested that the MED25–COI1 interaction occurs on the promoters of MYC2 targets. To test this possibility, we compared the JA-Ile–induced pattern of MED25 enrichment with that of COI1 on the chromatin of JAZ8 and ERF1. ChIP-qPCR assays using MED25-myc plants showed that, in the absence of JA-Ile treatment, MED25 was significantly more enriched on the G-box and TSS regions of JAZ8 and ERF1 than on the upstream promoter regions and gene bodies (Fig. 1 A and C), indicating that in the resting stage, MED25 exhibits an enrichment pattern similar to that of COI1 on MYC2 target promoters (Fig. 1 C vs. B). In response to JA-Ile treatment, however, MED25 enrichment levels on the TSSs of JAZ8 and ERF1 rapidly increased, reaching a maximum after 15 min (Fig. 1E), indicating that the JA-Ile–induced enrichment pattern of MED25 is distinct from that of COI1 (Fig. 1 E vs. D). Not surprisingly, MED25 enrichment on the TSS regions of JAZ8 and ERF1 was decreased in myc2-2 in comparison to the WT before and after JA-Ile treatment (Fig. 1G), indicating that the enrichment of MED25 on MYC2 target promoters depends on the function of MYC2.
The rapid reduction in enrichment of COI1 on MYC2 target promoters upon JA-Ile treatment, in contrast to the rapid increase in enrichment of MED25, prompted us to ask whether COI1 and MED25 are simultaneously associated with the promoters of MYC2 targets. To address this question, we performed sequential ChIP-qPCR assays using chromatin from MED25-myc seedlings, which was sequentially immunoprecipitated with anti-COI1 and anti-myc antibodies. The results revealed high enrichment of the TSSs of JAZ8 and ERF1 (Fig. 3 A and B), indicating that COI1 and MED25 are indeed enriched on the promoters of these genes at the same time.
Fig. 3.
COI1 and MED25 affect each other’s enrichment on the promoters of JAZ8 and ERF1. (A) Schematic diagrams of JAZ8, ERF1, and the PCR amplicons indicated as letters A–D used for ChIP-qPCR. (B) Sequential ChIP analysis showing that COI1 and MED25 co-occupy the promoters of JAZ8 and ERF1. Chromatin of MED25-myc plants was immunoprecipitated with anti-COI1 antibody, and then with anti-myc antibody. (C) ChIP-qPCR assays showing that med25-4 impairs the enrichment of COI1 on the TSSs of JAZ8 and ERF1 upon JA-Ile stimulation. WT and med25-4 plants were treated with or without 30 μM JA-Ile for 15 min before cross-linking, and chromatin of each sample was immunoprecipitated using anti-COI1 antibody. (D) ChIP-qPCR assays showing that coi1-2 impairs the enrichment of MED25 on the TSSs of JAZ8 and ERF1 upon JA-Ile stimulation. MED25-myc and MED25-myc/coi1-2 plants were treated with or without 30 μM JA-Ile for 15 min before cross-linking, and chromatin of each sample was immunoprecipitated using anti-myc antibody. For B–D, the precipitated DNA was quantified by qPCR, and DNA enrichment is displayed as a percentage of input DNA. ACT7 was used as a nonspecific binding site. Error bars indicate SD of three independent experiments (n = 3). ANOVA was performed for statistical analysis; bars with different letters are significantly different from each other (P < 0.01).
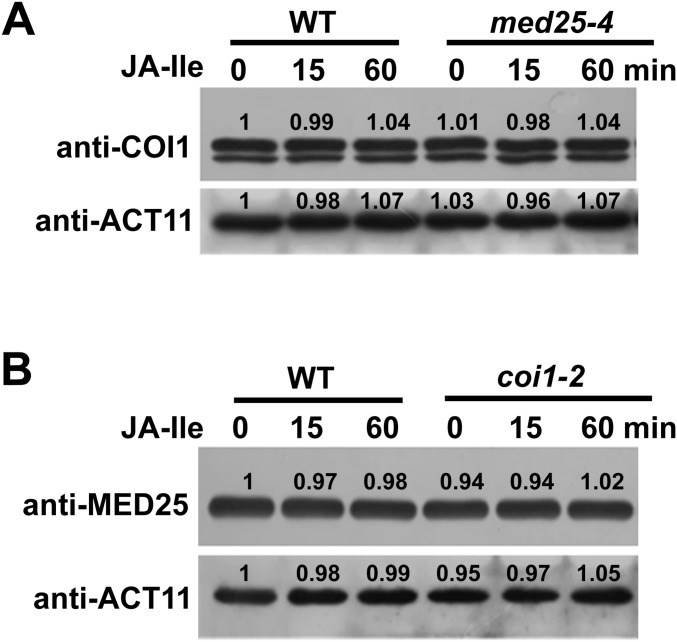
To further understand the significance of the MED25–COI1 interaction on MYC2 target promoters, we assessed whether depletion of MED25 affects the enrichment of COI1 on the TSSs of JAZ8 and ERF1. In the absence of JA-Ile, COI1 enrichment levels on the TSSs of JAZ8 and ERF1 were substantially reduced in med25-4 in comparison to the WT (Fig. 3C). JA-Ile treatment decreased COI1 enrichment in both med25-4 and the WT, and the level of COI1 enrichment was lower in med25-4 than in the WT (Fig. 3C). These results, together with the finding that the med25-4 mutation showed a negligible effect on COI1 protein levels before and after JA-Ile treatment (Fig. S3A), indicate that MED25 mainly affects the enrichment of COI1 on the promoters of JAZ8 and ERF1 before JA-Ile stimulation.
Fig. S3.
MED25 and COI1 do not affect the protein levels of each other. (A) Effect of the med25-4 mutation on the protein levels of COI1. WT and med25-4 plants were treated with or without 30 μM JA-Ile for indicated durations, and proteins were extracted for immunoblotting using anti-COI1 antibody. ACTIN11 (ACT11) was used as a loading control. (B) Effect of the coi1-2 mutation on the protein levels of MED25. WT and coi1-2 plants were treated with or without 30 μM JA-Ile for indicated durations, and proteins were extracted for immunoblotting using anti-MED25 antibody. ACT11 was used as a loading control.
In parallel, we performed ChIP-qPCR to investigate whether depletion of COI1 affects enrichment of MED25 on the TSSs of JAZ8 and ERF1. In the absence of JA-Ile, MED25 enrichment levels on the TSSs of JAZ8 and ERF1 were slightly reduced in coi1-2 in comparison to the WT (Fig. 3D). By contrast, in the presence of JA-Ile, MED25 enrichment levels on the TSSs of JAZ8 and ERF1 were greatly reduced in coi1-2 (Fig. 3D). These results, together with the finding that the coi1-2 mutation showed negligible effect on MED25 protein levels (Fig. S3B), indicate an important role for COI1 in JA-Ile–induced recruitment of MED25 to MYC2 target promoters.
Collectively, the above results reveal important functions of MED25 for linking COI1 to MYC2 target promoters in the resting stage, and thereby facilitating COI1-dependent degradation of JAZ repressors in response to hormone elicitation. These two aspects of mechanistically related functions favor the hormone-induced activation of MYC2.
MED25 Interacts with HAC1, Which Is also Enriched on the Promoters of JAZ8 and ERF1.
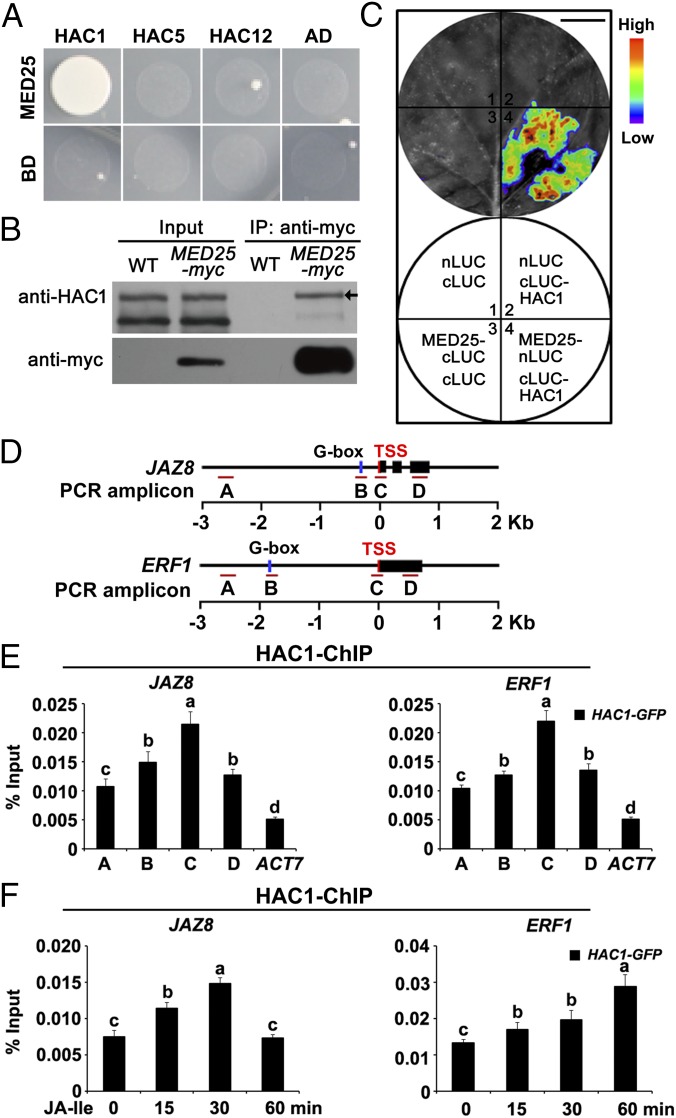
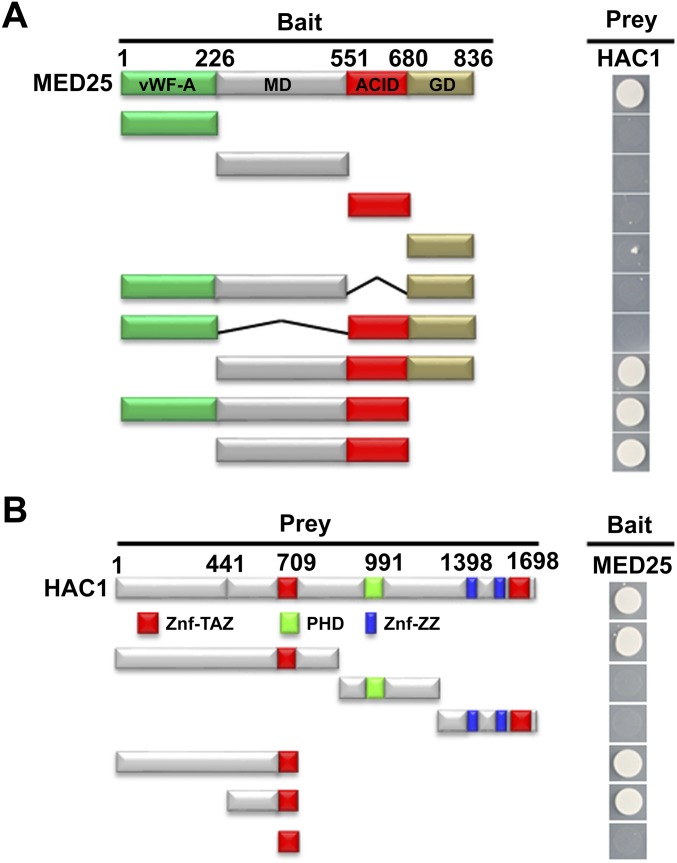
To further explore the significance of the MED25–COI1 interaction on MYC2 target promoters, we tested the possible interaction of MED25 with histone modification enzymes whose activities are associated with active transcription. Among the three Arabidopsis CREB-binding protein (CBP)–like proteins that exhibit histone acetyltransferase activity (31–33), MED25 preferentially interacted with HAC1, but not with HAC5 and HAC12, in Y2H assays (Fig. 4A). Domain mapping with Y2H assays indicated that the middle domain (MD), together with the activator-interacting domain (ACID) of MED25 (21) and the HAC1 fragment containing the transcription adaptor putative Zinc finger (TAZ)-type Zinc finger (Znf-TAZ) domain (31, 33), is involved in the MED25–HAC1 interaction (Fig. S4).
Fig. 4.
MED25 interacts with HAC1, which is recruited to the promoters of JAZ8 and ERF1. (A) Y2H assays showing that MED25 interacts with HAC1 but not HAC5 and HAC12. Transformed yeast strains were plated on SD medium lacking His, Ade, Leu, and Trp (SD/-4). (B) Co-IP assay of MED25 with HAC1. Proteins extracted from WT and MED25-myc plants were immunoprecipitated using anti-myc antibody and immunoblotted using anti-HAC1 antibody. The arrow indicates the position of HAC1. (C) LCI assays showing that MED25 interacts with HAC1. (Top) LUC images of N. benthamiana leaves coinfiltrated with the different construct combinations are shown in the lower quadrant of the circle. The pseudocolor bar shows the range of luminescence intensity. (Scale bar, 1 cm.) (D) Schematic diagrams of JAZ8, ERF1, and PCR amplicons indicated as letters A–D used for ChIP-qPCR. (E) ChIP-qPCR showing enrichment of HAC1 on the chromatin of JAZ8 and ERF1. Chromatin of HAC1-GFP plants was immunoprecipitated using anti-GFP antibody. (F) ChIP-qPCR showing enrichment of HAC1 on the TSSs of JAZ8 and ERF1 upon JA-Ile stimulation. HAC1-GFP plants were treated with 30 μM JA-Ile for the indicated durations before cross-linking, and the chromatin of each sample was immunoprecipitated using anti-GFP antibody. For E and F, the precipitated DNA was quantified by qPCR, and DNA enrichment is displayed as a percentage of input DNA. ACT7 was used as a nonspecific binding site. Error bars indicate the SD of three independent experiments (n = 3). ANOVA was performed for statistical analysis; bars with different letters are significantly different from each other (P < 0.01).
Fig. S4.
Mapping of the protein domains involved MED25–HAC1 interaction using Y2H assays. (A) Based on the schematic protein structure of MED25, full-length MED25 or its derivatives (pGBKT7-MED25 or pGBKT7-MED25 derivatives) were tested for interaction with HAC1 (pGADT7-HAC1). Yeast cells cotransformed with pGBKT7-MED25 or pGBKT7-MED25 derivatives (baits) and pGADT7-HAC1 (prey) were grown on selective media lacking Ade, His, Leu, and Trp (SD/-4) to test protein interaction. (B) Based on the schematic protein structure of HAC1, full-length HAC1 or its derivatives (pGADT7-HAC1 or pGADT7-HAC1 derivatives) were tested for interaction with MED25 (pGBKT7-MED25). Yeast cells cotransformed with pGADT7-HAC1 or pGADT7-HAC1 derivatives (prey) and pGBKT7-MED25 (bait) were grown on selective media lacking Ade, His, Leu, and Trp (SD/-4) to test protein interaction. ACID, activator-interacting domain; GD, glutamine-rich domain; LRR, leucine-rich repeat; MD, middle domain; PHD, plant homeodomain; vWF-A, von Willebrand factor A domain; Znf-TAZ, transcription adaptor putative Zinc finger (TAZ)-type Zinc finger; Znf-ZZ, two Zinc binding domain (ZZ)-type Zinc finger.
In co-IP assays using MED25-myc plants and an anti-HAC1 antibody, endogenous HAC1 could be pulled down by MED25-myc (Fig. 4B). In LCI assays, N. benthamiana cells cotransformed with MED25 fused to nLUC (MED25-nLUC) and HAC1 fused to cLUC (cLUC-HAC1) displayed strong fluorescence signals, whereas those cotransformed with MED25-nLUC and cLUC or cLUC-HAC1 and nLUC displayed no signal (Fig. 4C), confirming that the MED25–HAC1 interaction occurred in vivo.
To determine whether HAC1 is also recruited to the promoters of JAZ8 and ERF1, we performed ChIP-qPCR experiments with transgenic plants expressing functional HAC1-GFP fusion (Fig. S5) and an anti-GFP antibody. In the absence of JA-Ile treatment, HAC1 was mainly enriched on the TSSs of JAZ8 and ERF1 (Fig. 4 D and E). In response to JA-Ile treatment, HAC1 enrichment on the TSS of JAZ8 was obviously elevated at 15 min, reaching a peak at 30 min. By contrast, JA-Ile–triggered induction of HAC1 enrichment on the TSS of ERF1 occurred later (Fig. 4 D and F). These results indicate that, similar to MED25 and COI1, HAC1 is also enriched on the promoters of JAZ8 and ERF1, and this enrichment is regulated by JA-Ile stimulation.
Fig. S5.
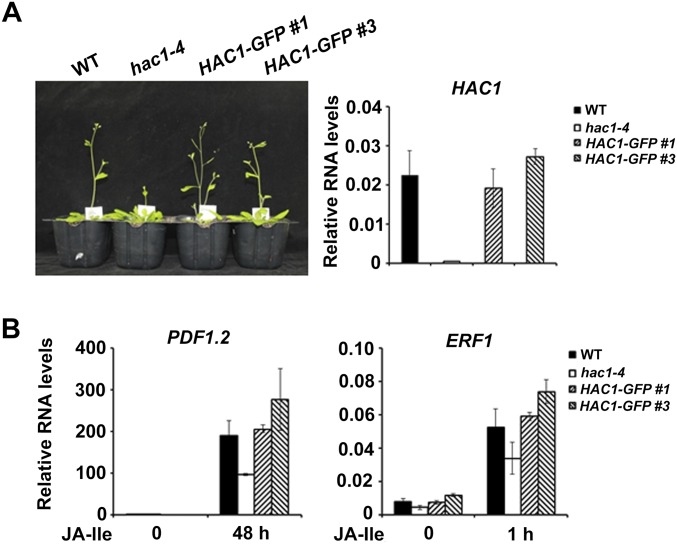
Phenotypic and molecular characterization of pHAC1:HAC1-GFP/hac1-4 (HAC1-GFP) transgenic plants. (A, Left) The hac1-4 mutants exhibited a late-flowering phenotype, whereas the flowering times of HAC1-GFP #1 and #3 were comparable to those of WT plants, indicating that HAC1-GFP protein retains the biological function as endogenous HAC1. (A, Right) Relative RNA levels of HAC1 are also shown. Plants were grown under long-day (LD, 16-h white light/8-h dark) conditions. (B) qRT-PCR shows that HAC1-GFP #1 and #3 rescue the phenotype of hac1-4 in terms of JA-Ile–induced gene expression. Ten-day-old seedlings were treated with or without 30 μM JA-Ile for the indicated times. Error bars indicate SD of three independent experiments (n = 3).
Depletion of HAC1 Affects JA-Responsive Gene Expression and Impairs H3K9ac Levels of JAZ8 and ERF1 Promoters.
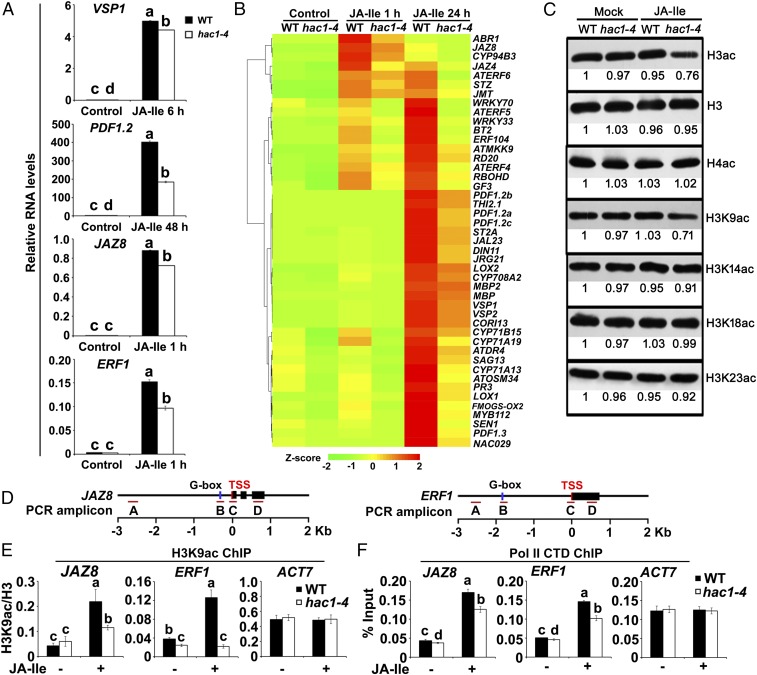
To determine the biological significance of the MED25–HAC1 interaction, we compared JA-responsive gene expression between the WT and the hac1-4 mutant (34). JA-Ile–induced expression of VEGETATIVE STORAGE PROTEIN 1 (VSP1), a marker gene for the JA-regulated wound response (35) (Fig. S1), exhibited a slight yet significant reduction in hac1-4 expression (Fig. 5A). By contrast, the JA-Ile–induced expression of the plant defensin gene PDF1.2, a marker gene for the JA-regulated pathogen response (36) (Fig. S1), was markedly reduced in this mutant (Fig. 5A). These results suggest that HAC1 preferentially affects pathogen-responsive genes. Not surprisingly, JA-Ile–induced expression of MYC2 direct target genes, including JAZ8 and ERF1 (Fig. S1), was significantly reduced in hac1-4 (Fig. 5A).
Fig. 5.
Depletion of HAC1 impairs JA-responsive gene expression and reduces H3K9ac accumulation on the promoters of JAZ8 and ERF1. (A) qRT-PCR showing JA-Ile–induced expression of indicated genes in WT and hac1-4. WT and hac1-4 plants were treated with or without 30 μM JA-Ile for the indicated durations. Error bars indicate SD of three independent experiments (n = 3). ANOVA was performed for statistical analysis; bars with different letters are significantly different from each other (P < 0.01). (B) Hierarchical clustering of the selected JA-Ile–responsive genes showing reduced expression in hac1-4 plants at the indicated time points. (C) Protein gel analyses showing global H3K9ac levels in the WT and hac1-4 in response to JA-Ile. WT and hac1-4 plants were treated with or without 30 μM JA-Ile for 30 min before extraction of nuclear proteins for immunoblotting using the indicated antibodies. Bands were quantified using ImageJ. (D) Schematic diagrams of JAZ8, ERF1, and PCR amplicons indicated as letters A–D used for ChIP-qPCR. (E) ChIP-qPCR assays showing that hac1-4 impairs the enrichment of H3K9ac on the TSS regions of JAZ8 and ERF1 in response to JA-Ile. WT and hac1-4 plants were treated with or without 30 μM JA-Ile for 30 min before cross-linking, and chromatin of each sample was immunoprecipitated using anti-H3 and anti-H3K9ac antibodies. Precipitated DNA was quantified by qPCR, and H3K9ac levels are normalized to H3. (F) ChIP-qPCR assays showing that hac1-4 impairs the enrichment of Pol II C-terminal domain (CTD) on the TSS regions of JAZ8 and ERF1 in response to JA-Ile stimulation. WT and hac1-4 plants were treated with or without 30 μM JA-Ile for 30 min before cross-linking, and chromatin of each sample was then immunoprecipitated using anti-Pol II CTD antibody. Precipitated DNA was quantified by qPCR, and DNA enrichment is displayed as a percentage of input DNA. For E and F, ACT7 was used as a nonspecific binding site. Error bars indicate SD of three independent experiments (n = 3). ANOVA was performed for statistical analysis; bars with different letters are significantly different from each other (P < 0.01).
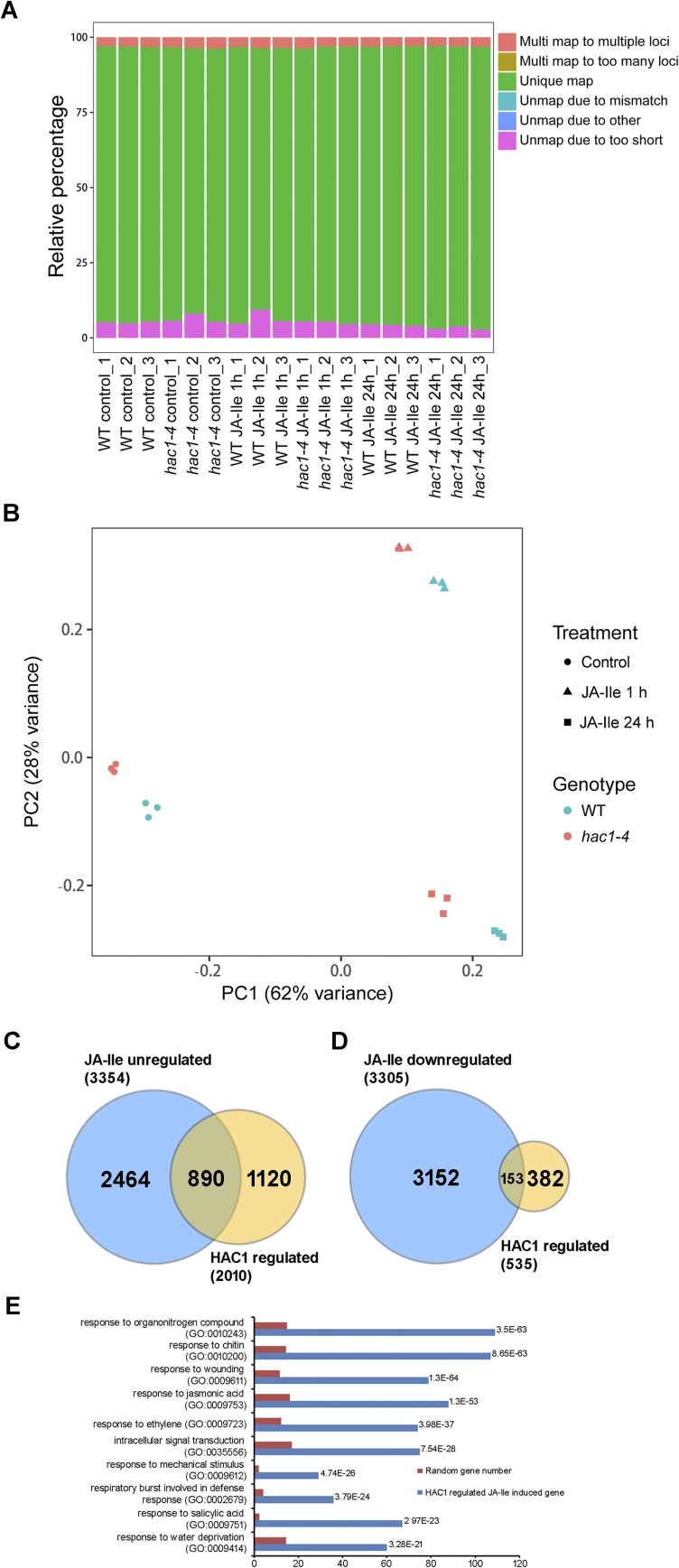
To evaluate the impact of HAC1 on JA-regulated gene expression on a genome-wide scale, we performed RNA-sequencing (RNA-seq) experiments to compare the transcriptome profiles between WT and hac1-4 seedlings treated with or without JA-Ile (Materials and Methods). Quality assessment of the RNA-seq data is shown in Fig. S6 A and B. We identified 3,354 genes that were up-regulated by JA-Ile at any time point (1 or 24 h) in WT [fold change > 1.5, false discovery rate (FDR)-adjusted P < 0.05). We also identified 2,010 genes whose expression was significantly reduced in hac1-4 compared with the WT at any time point after JA-Ile treatment (fold change > 1.5, FDR-adjusted P < 0.05) (Fig. S6C and Dataset S1). Comparison of these two sets of genes led to the identification of 890 genes showing significantly reduced expression in JA-Ile–treated hac1-4 seedlings compared with JA-Ile–treated WT seedlings (fold change > 1.5, FDR-adjusted P < 0.05) (Fig. S6C and Dataset S2). Thus, HAC1 is involved in the activation of around 26.5% (i.e., 890 of 3,354) of the JA-Ile–up-regulated genes. These 890 genes were defined as HAC1– and JA-Ile–co–up-regulated genes. Gene ontology (GO) analysis indicated that these genes are enriched in pathways related to JA response, wounding response, and other defense responses (Dataset S2). The top 10 enriched biological processes are shown in Fig. S6E. Not surprisingly, many well-characterized JA-inducible genes were identified as HAC1– and JA-Ile–co–up-regulated genes (Fig. 5B and Dataset S2). This list includes genes involved in JA metabolism, JA signaling, and JA-induced defense responses (Fig. 5B).
Fig. S6.
Summary of the RNA-seq analysis. (A) Overview of RNA-seq data from JA-Ile–treated WT and hac1-4 plants. Relative percentages of multiple (Multi) mapped reads, unique mapped reads, and unmapped reads are shown. (B) Principal component (PC) analysis showing the relatedness among the gene expression patterns of samples used for RNA-seq analysis. Colors represent different genotypes, and symbols represent treatments. (C) Venn diagrams of JA–up-regulated genes (up-regulated genes in WT after JA-Ile treatment for 1 or 24 h; fold change > 1.5, FDR-adjusted P < 0.05) and HAC1-regulated genes (down-regulated genes in hac1-4 compared with WT after JA-Ile treatment for 1 or 24 h; fold change > 1.5, FDR-adjusted P < 0.05). Genes coregulated by JA-Ile and HAC1 are shown in the overlapping region. The number of differentially expressed genes with significant differential expression (FDR-adjusted P < 0.05) is shown. (D) Venn diagrams of JA–down-regulated genes (down-regulated genes in WT after JA-Ile treatment for 1 or 24 h; fold change > 1.5, FDR-adjusted P < 0.05) and HAC1-regulated genes (up-regulated genes in hac1-4 compared with WT after JA-Ile treatment for 1 or 24 h; fold change > 1.5, FDR-adjusted P < 0.05). Genes coregulated by JA-Ile and HAC1 are shown in the overlapping region. The number of differentially expressed genes with significant differential expression (fold change > 1.5, FDR-adjusted P < 0.05) is shown. (E) GO analysis of HAC1-regulated JA-Ile–induced genes. GO terms (biological processes) enriched in the set of genes identified as JA–up-regulated genes that were down-regulated in hac1-4 compared with WT are shown. The P value was expressed in exponential notation, replacing part of the number with E + n, where E multiplies the preceding number by 10 to the nth power.
In parallel, we identified 3,305 genes that were down-regulated by JA-Ile in WT (fold change > 1.5, FDR-adjusted P < 0.05). We also identified 535 genes whose expression was significantly higher in hac1-4 compared with the WT at any time point after JA-Ile treatment (fold change > 1.5, FDR-adjusted P < 0.05) (Fig. S6D and Dataset S3). Comparison of these two sets of genes led to the identification of 153 genes that were less repressed in JA-Ile–treated hac1-4 seedlings compared with JA-Ile–treated WT seedlings (fold change > 1.5, FDR-adjusted P < 0.05) (Fig. S6D and Dataset S4). Thus, HAC1 is involved in the repression of around 4.6% (i.e., 153 of 3,305) of the JA-Ile–down-regulated genes. These 153 genes were defined as HAC1– and JA-Ile–co–down-regulated genes. GO analysis indicated that this group of genes does not show significant enrichment in any biological processes (Dataset S4). Taken together, our RNA-seq experiments indicated that HAC1 mainly acts as a coactivator of JA-induced gene expression.
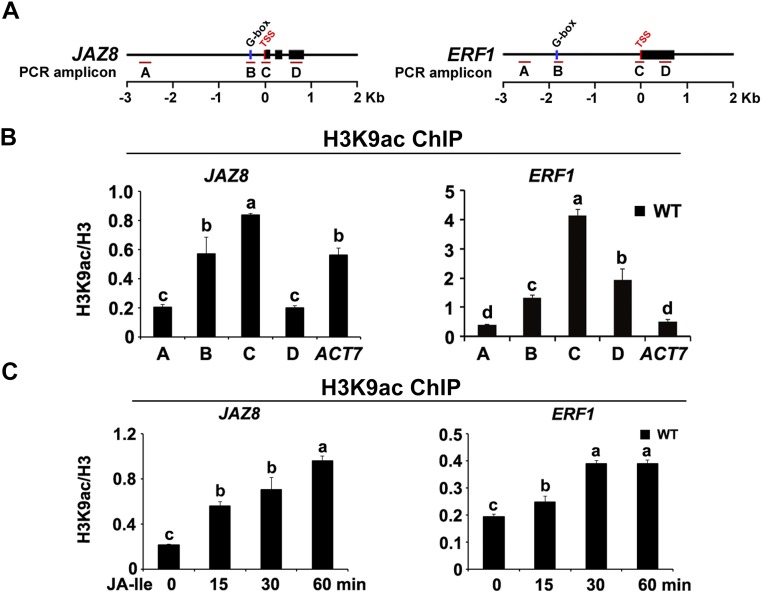
To determine the substrate specificity of HAC1 for histone modifications, we compared the histone acetylation profiles of the WT and hac1-4. In response to JA-Ile treatment, global levels of H3ac, but not H4ac, were reduced in hac1-4 to a greater extent than in the WT (Fig. 5C), indicating that HAC1 mainly affects H3ac. Next, we examined the site specificity of HAC1 for H3ac and found that, in response to JA-Ile treatment, global H3K9ac levels were reduced in hac1-4 to a greater extent than in the WT (Fig. 5C), indicating that HAC1 mainly affects H3K9ac, a chromatin mark that is typically associated with actively transcribed genes (32). ChIP-qPCR assays revealed that, in WT plants, H3K9ac was primarily enriched on the TSSs of JAZ8 and ERF1 (Fig. S7 A and B), and that H3K9ac enrichment on these TSSs was increased by JA-Ile treatment (Fig. S7 A and C), indicating that the H3K9ac enrichment pattern on MYC2 target promoters is similar to that of HAC1 and MED25. In hac1-4 mutants, JA-Ile–induced H3K9ac levels on the TSSs of JAZ8 and ERF1 were dramatically reduced in comparison to the WT (Fig. 5 D and E), confirming that the defective JA-responsive gene expression in hac1-4 is linked to impaired H3K9ac of these MYC2 target promoters. Consistently, JA-Ile–induced enrichment of the conserved C-terminal domain of Pol II on the TSSs of JAZ8 and ERF1 was obviously reduced in hac1-4 in comparison to the WT (Fig. 5 D and F), indicating that HAC1 function is required for the recruitment of Pol II to MYC2 target promoters.
Fig. S7.
Enrichment of H3K9ac on the chromatin of JAZ8 and ERF1. (A) Schematic diagrams of JAZ8, ERF1, and the PCR amplicons indicated as letters A–D used for ChIP-qPCR. (B) ChIP-qPCR shows the enrichment of H3K9ac on the chromatin of JAZ8 and ERF1. The chromatin of WT plants was immunoprecipitated using anti-H3 and anti-H3K9ac antibodies, respectively. (C) ChIP-qPCR shows the enrichment of H3K9ac on the TSS regions of JAZ8 and ERF1 in response to JA-Ile elicitation. WT plants were treated with 30 μM JA-Ile for indicated durations before cross-linking, and the chromatin of each sample was immunoprecipitated using anti-H3 and anti-H3K9ac antibodies, respectively. For (B) and (C), the precipitated DNA was quantified by qPCR, and H3K9ac levels are normalized to H3. ACT7 was used as a nonspecific binding site. Error bars indicate the SD of three independent experiments (n = 3). ANOVA was performed for statistical analysis; bars with different letters are significantly different from each other (P < 0.01).
Enrichment and Function of HAC1 on the Promoters of JAZ8 and ERF1 Depend on the Function of COI1 and MED25.
To understand how HAC1, a general histone modification enzyme, selectively regulates H3K9ac of JA-responsive genes, we asked whether depletion of COI1 affects the enrichment levels of HAC1 itself and HAC-dependent H3K9ac on the promoters of JAZ8 and ERF1. ChIP-qPCR assays revealed that, in coi1-2, JA-Ile–induced enrichment of HAC1-GFP on the TSSs of JAZ8 and ERF1 was substantially lower than in the WT (Fig. 6 A and B). Consistent with this, H3K9ac levels on these TSSs were also dramatically reduced in coi1-2 (Fig. 6 A and C). Given that COI1 did not affect HAC1 protein levels (Fig. S8), these results demonstrate that the enrichment and function of HAC1 on MYC2 target promoters depend on COI1.
Fig. 6.
Depletion of COI1 or MED25 impairs the function of HAC1 on the promoters of JAZ8 and ERF1. (A) Schematic diagrams of JAZ8, ERF1, and PCR amplicons indicated as letters A–D used for ChIP-qPCR. (B) ChIP-qPCR assays showing that coi1-2 impairs the enrichment of HAC1 on the TSS regions of JAZ8 and ERF1 in response to JA-Ile. HAC1-GFP and HAC1-GFP/coi1-2 plants were treated with or without 30 μM JA-Ile for 30 min before cross-linking, and chromatin of each sample was then immunoprecipitated using anti-GFP antibody. Precipitated DNA was quantified by qPCR, and DNA enrichment is displayed as a percentage of input DNA. (C) ChIP-qPCR assays showing that coi1-2 impairs the enrichment of H3K9ac on the TSS regions of JAZ8 and ERF1 in response to JA-Ile. WT and coi1-2 plants were treated with or without 30 μM JA-Ile for 30 min before cross-linking, and chromatin of each sample was then immunoprecipitated using anti-H3 and anti-H3K9ac antibodies. Precipitated DNA was quantified by qPCR, and H3K9ac levels are normalized to H3. (D) ChIP-qPCR assays showing that med25-4 impairs the enrichment of HAC1 on the TSS regions of JAZ8 and ERF1 in response to JA-Ile. HAC1-GFP and HAC1-GFP/med25-4 plants were treated with or without 30 μM JA-Ile for 30 min before cross-linking, and chromatin of each sample was then immunoprecipitated using anti-GFP antibody. Precipitated DNA was quantified by qPCR, and DNA enrichment is displayed as a percentage of input DNA. (E) ChIP-qPCR assays showing that med25-4 impairs the enrichment of H3K9ac on the TSS regions of JAZ8 and ERF1 in response to JA-Ile. WT and med25-4 plants were treated with or without 30 μM JA-Ile for 30 min before cross-linking, and chromatin of each sample was then immunoprecipitated using anti-H3 and anti-H3K9ac antibodies. Precipitated DNA was quantified by qPCR, and H3K9ac levels are normalized to H3. For B–E, ACT7 was used as a nonspecific binding site. Error bars indicate SD of three independent experiments (n = 3). ANOVA was performed for statistical analysis; bars with different letters are significantly different from each other (P < 0.01).
Fig. S8.
Effect of COI1 and MED25 on the protein levels of HAC1. WT, coi1-2, and med25-4 plants were treated with or without 30 μM JA-Ile for indicated durations, and proteins were extracted for immunoblotting using anti-HAC1 antibody. The arrowhead indicates the HAC1-specific band. ACT11 was used as a loading control.
Parallel ChIP-qPCR assays revealed that, in med25-4, JA-Ile–induced enrichment of HAC1-GFP (Fig. 6 A and D) and HAC-dependent H3K9ac (Fig. 6 A and E) on the TSSs of JAZ8 and ERF1 was greatly reduced in comparison to the WT. Considering that MED25 does not affect the protein levels of HAC1 (Fig. S8), our results demonstrate that the enrichment and function of HAC1 on MYC2 target promoters also depend on MED25.
MED25 and JAZ1 Simultaneously Interact with MYC2 in the Resting Stage, and Hormone Elicitation Shows Differential Effects on the MED25–COI1 Interaction and the MED25–MYC2 Interaction.
The above results, together with our recent finding that MED25 physically and functionally interacts with MYC2 (21), raise a possibility that MED25 and JAZ proteins could be bound simultaneously to MYC2 in the resting stage. To test this, we performed co-IP experiments by transiently expressing combinations of MYC2-myc, Flag epitope (Flag)-tagged MED25 (MED25-Flag), and JAZ1-GFP in N. benthamiana leaves. As expected, both MED25-Flag and MYC2-myc were coimmunoprecipitated by JAZ1-GFP (Fig. 7A), indicating that MED25-Flag and JAZ1-GFP could be bound simultaneously to MYC2-myc in the resting stage.
Fig. 7.
MED25 cooperates with both genetic and epigenetic regulators in regulating hormone-induced activation of MYC2. (A) Co-IP assays of MED25, MYC2, and JAZ1 in N. benthamiana. MED25-Flag and MYC2-myc were transiently coexpressed with or without JAZ1-GFP in N. benthamiana leaves. Protein extracts were immunoprecipitated using anti-GFP antibody and analyzed by immunoblotting with anti-Flag, anti-myc, and anti-GFP antibodies. (B) Co-IP assay of MED25, MYC2, and JAZ1 in Arabidopsis. Proteins extracted from WT and JAZ1-GFP plants were immunoprecipitated using anti-GFP antibody and immunoblotted using anti-MED25 and anti-MYC2 antibodies. (C) Co-IP assay between MED25 and COI1. WT and COI1-myc plants were treated with or without 30 μM JA-Ile for the indicated times. Protein from each sample was immunoprecipitated using anti-myc antibody and immunoblotted using anti-MED25 antibody. Bands were quantified using ImageJ. (D) Co-IP assay between MED25 and MYC2. WT and MYC2-myc plants were treated with or without 30 μM JA-Ile for the indicated times. Protein from each sample was immunoprecipitated using anti-myc antibody and immunoblotted using anti-MED25 antibody. Bands were quantified using ImageJ. (E) Proposed working model for the mechanistic roles of MED25 in regulating JA-Ile–induced activation of MYC2. In the resting stage, the MED25–COI1 interaction is relatively strong, whereas the MED25–MYC2 interaction is relatively weak because JAZ repressors compete with MED25 for interaction with MYC transcription factors. Basal levels of MED25 bring COI1 to MYC2 target promoters through physical interaction. In the hormone-mediated transition stage, JA-Ile acts as molecular glue to promote the formation of the COI1–JAZ coreceptor complex, which leads to proteasome-dependent degradation of JAZ repressors. During this stage, the MED25–COI1 interaction is weakened in a hormone-dependent manner, whereas the MED25–MYC2 interaction is enhanced in a hormone-dependent manner. Upon degradation of JAZ repressors, MED25 interacts with MYC2 and recruits HAC1 as well as Pol II to the promoters of MYC2 target genes, and thereby activate their expression. NINJA, Novel Interactor of JAZ; TPL, TOPLESS.
To substantiate these observations, we conducted co-IP experiments with transgenic Arabidopsis plants expressing JAZ1-GFP (Fig. S9) and anti-MYC2 or anti-MED25 (21) antibody. Again, endogenous MYC2 and MED25 could be coimmunoprecipitated by JAZ1-GFP (Fig. 7B). Together, these results demonstrate that the MED25 coactivator and JAZ repressors could be bound simultaneously to the master transcription factor MYC2 and suggest the existence of a JAZ–MYC2–MED25 ternary complex. In this context, our study reveals two aspects of closely related functions for MED25 in regulating JA signaling. First, in the resting stage, it brings COI1 to MYC2 target promoters through physical interaction. Second, upon JA-Ile elicitation, it cooperates with both genetic and epigenetic regulators to activate MYC2-dependent gene transcription. In this perspective, we speculate that JA-Ile elicitation might affect the MED25–COI1 interaction as well as the MED25–MYC2 interaction.
Fig. S9.
Molecular characterization of p35S:JAZ1-GFP (JAZ1-GFP) transgenic plants. WT and JAZ1-GFP plants were treated with or without 30 μM JA-Ile for 15 min, and proteins were extracted for immunoblotting using anti-GFP antibody. ACT11 was used as a loading control.
To determine whether JA-Ile treatment affects the MED25–COI1 interaction in planta, we treated COI1-myc plants with JA-Ile and examined the ability of COI1-myc to pull down native MED25. Upon JA-Ile treatment, the ability of COI1-myc to pull down MED25 was slightly reduced at 15 min and dramatically reduced at 60 min (Fig. 7C), suggesting that the MED25–COI1 interaction is weakened upon JA-Ile elicitation. This observation is consistent with the above results that the enrichment of COI1 on MYC2 target promoters is decreased upon JA-Ile elicitation (Fig. 1D).
Similarly, we treated MYC2-myc plants (37) with JA-Ile and examined the ability of MYC2-myc to pull down native MED25. Upon JA-Ile treatment, the ability of MYC2-myc to pull down MED25 was slightly increased at 15 min and obviously increased at 60 min (Fig. 7D), suggesting that, in contrast to the above MED25–COI1 interaction case, the MED25–MYC2 interaction is enhanced upon JA-Ile elicitation. This observation is consistent with the above results that the enrichment of MED25 on MYC2 target promoters is increased upon JA-Ile elicitation (Fig. 1E) and the recent finding that JAZ repressors compete with MED25 for interaction with MYC transcription factors (22).
In summary, we propose a working model (Fig. 7E). In the absence of JA-Ile, MED25 and JAZ proteins could be bound to MYC2, and therefore form a JAZ–MYC2–MED25 ternary complex. At this stage, JAZ proteins do not interact with COI1 but interact with MYC2, and thereby function as transcription repressors. It is noteworthy that, at this stage, the MED25–COI1 interaction is relatively strong, whereas the MED25–MYC2 interaction is relatively weak because JAZ repressors interfere with the interaction of MED25 with MYC transcription factors (22). Basal levels of MED25 bring COI1 to MYC2 target promoters through physical interaction. In response to stress or developmental cues, plants produce JA-Ile, which acts as molecular glue to promote the formation of the COI1–JAZ coreceptor complex (17). During this stage, JAZ proteins transiently switch the repressor function into a coreceptor function in a JA-Ile–dependent manner (22). Coincidently, the MED25–COI1 interaction is weakened, whereas the MED25–MYC2 interaction is enhanced. The formation of the COI1–JAZ coreceptor complex will eventually lead to proteasome-dependent degradation of JAZ proteins. Upon JAZ degradation, MED25 shows enhanced interaction with MYC2 and recruits HAC1 as well as Pol II to the promoters of MYC2 target genes, and thereby activates their expression. This model highlights the mechanistic function of MED25 in transmitting the hormone-specific signals from the JA-Ile receptor protein COI1 to activate MYC2-regulated gene transcription.
Discussion
The recent biochemical isolation of the plant Mediator complex from Arabidopsis (19) significantly facilitated the characterization of the diverse functions of individual plant Mediator subunits (38–40). However, a thorough mechanistic understanding of plant Mediator function remains elusive. In particular, it is unclear how specific Mediator subunits integrate regulatory signals from diverse internal or external cues, as well as how they convey these signals to the Pol II general transcription machinery to regulate the expression of specific target genes.
In this work, we show that MYC2 forms a ternary complex together with the MED25 coactivator and the JAZ repressors in the resting stage and reveal functions of MED25 in regulating JA signaling. First, in the resting stage, MED25 brings COI1 to MYC2 target promoters through physical interaction, and thereby facilitates COI1-dependent degradation of JAZ repressors. Second, upon hormone elicitation, MED25 brings the coactivator HAC1 to MYC2 target promoters and thereby regulates H3K9ac, which favors gene activation. These findings, together with our previous observation that MED25 bridges MYC2 and Pol II for preinitiation complex assembly during JA-regulated gene transcription (21), advance the overall understanding about how the JA signals are transmitted to activate gene expression.
However, several aspects of the molecular details by which the multitalented MED25 executes these versatile functions remain to be further explored. An important aspect concerns the functional significance of MED25–COI1 interaction for JA signaling. The COI1 enrichment on MYC2 target promoters is relatively high in the resting stage and exhibits a rapid reduction upon JA-Ile treatment (Fig. 1D). The findings that the MED25 enrichment on MYC2 target promoters is relatively low in the resting stage and exhibits a rapid induction upon JA-Ile treatment (Fig. 1E) and that depletion of MED25 reduces COI1 enrichment on MYC2 target promoters in the resting stage and impairs COI1-dependent degradation of JAZ proteins in response to JA-Ile treatment (Figs. 2E and 3C) support the notion that the major functional relevance of MED25–COI1 interaction in JA signaling is to bring COI1 to MYC2 target promoters in the resting stage and to facilitate COI1-dependent degradation of JAZ proteins upon JA-Ile elicitation. We reasoned that JA-Ile–triggered reduction of the COI1 enrichment on MYC2 target promoters could be due to the finding that JA-Ile stimulates the formation of the COI1–JAZ coreceptor complex, and thereby promotes JAZ degradation, since JAZ degradation might lead to disassociation of COI1 from MYC2 target promoters.
Recent structural studies of the COI1–JAZ coreceptor complex (17) and the MYC–JAZ repression complex (22) have revealed that hormone-mediated protein interaction is a major strategy governing JA-Ile perception and JAZ repression of MYC transcription factors. Here, we show that, in the resting stage, there exists a JAZ–MYC2–MED25 ternary complex and that hormone elicitation triggers extensive changes of protein interactions involving MED25 and the major components of the core JA signaling module. Specifically, the MED25–COI1 interaction is relatively strong in the resting stage and tends to be weakened upon JA-Ile elicitation (Fig. 7C). In contrast, the MED25–MYC2 interaction is relatively weak in the resting stage and tends to be enhanced upon JA-Ile elicitation (Fig. 7D). These results suggest that hormone elicitation exerts differential effects on the MED25–COI1 interaction and the MED25–MYC2 interaction. In light of the observations that JAZ proteins undergo pronounced conformational changes (through the Jas motif) before and after hormone elicitation, and therefore switch their interactions with MYC transcription factors (in the absence of JA-Ile) or with COI1 (in the presence of JA-Ile) (17, 22), it is reasonable to speculate that the JA-Ile–dependent alterations of the MED25–COI1 interaction or the MED25–MYC2 interaction may be coupled with conformational changes of the COI1-JAZ coreceptor and/or their interacting MYC2 and MED25. Future structural studies should provide insight into the mechanism by which MED25 changes its interaction with the hormone receptor COI1 or with the master transcription factor MYC2. Similarly, structural studies promise to provide insight into the mechanism by which MED25 facilitates JA-Ile–induced degradation of JAZ proteins by enhancing COI1–JAZ interaction.
In addition to the JA-Ile receptor protein COI1, we found that MED25 physically and functionally interacts with the evolutionarily conserved coactivator HAC1 (31–33). Our results support a scenario by which, in response to JA-Ile elicitation, MED25 recruits HAC1 to MYC2 target promoters, and thereby regulates H3K9ac. Notably, we found that JA-Ile treatment led to obvious reduction of H3ac and H3K9ac levels in hac1-4 but not in the WT (Fig. 5C). The distinct action modes of JA-Ile on H3ac and H3K9ac levels between hac1-4 and the WT can be explained by previous observations that JA could activate the expression of HISTONE DEACETYLASE6 (HDA6) and HDA19, two histone deacetylase genes that affect H3ac levels (41, 42). Considering that histone acetylation and deacetylation play an important role in the regulation of gene expression, it is reasonable to speculate that plants have evolved a mechanism to keep a dynamic balance between histone acetyltransferase and histone deacetylase activities. Under this scenario, JA-Ile may simultaneously up-regulate the activities of histone acetyltransferases and histone deacetylases, thereby maintaining a steady-state level of H3ac and H3K9ac in the WT. In hac1-4 mutants, the JA-Ile–induced up-regulation of H3ac or H3K9ac through HAC1 is blocked, but JA-Ile still reduces H3ac and H3K9ac through activating HDA6 and HDA19. These effects likely lead to reduced H3ac and H3K9ac levels of hac1-4 in response to JA-Ile treatment.
Our study suggests two related directions for future exploration. First, recent advances have led to the surprising discovery that, in addition to JA, the main receptors for several other plant hormones, including auxin (43), gibberellin (44), abscisic acid (45), and salicylic acid (46), are localized in the nucleus and directly linked to hormone-regulated gene transcription. An important future direction will be to determine whether the signaling paradigm described herein can be extended to other hormones whose receptors are localized in the nucleus. If this is the case, it will be critical to study the principles that govern the general and/or context-specific functions of Mediator, and especially to determine how a single multiprotein complex can perform so many diverse tasks.
Moreover, this study reveals several aspects of striking analogy between plant and animal nuclear hormone receptor (NR) systems. First, the overall protein domain composition of the plant MED25 is largely similar to that of its animal counterpart (19, 21). Second, in addition to interacting with MYC2, the plant MED25 interacts with the JA-Ile receptor protein COI1; in an analogous manner, the human MED25 engages in a ligand-dependent interaction with retinoic acid receptor (RAR) and several other NRs, which are themselves transcription factors (26, 28, 29, 47). Thus, in term of their interaction with MED25, the JA-Ile coreceptor complex (SCFCOI1–JAZs), together with the master transcription factor MYC2, resembles the NR system of metazoans (26, 28, 29, 47). Third, we show here that the plant MED25 cooperates with HAC1, an Arabidopsis ortholog of the well-studied animal histone acetyltransferase CBP, for JA-Ile–triggered activation of MYC2; in a similar manner, the animal MED25 also cooperates with CBP for RAR activation (48). Thus, the plant MED25 and its animal counterpart cooperate with similar epigenetic regulators in distinct signaling pathways. Animal Mediator was first biochemically isolated as a thyroid hormone receptor-associated protein (TRAP) complex (47), and has been shown to be an indispensable NR-interacting coactivator (26, 28, 29, 47). These previous observations, together with our findings, support a scenario in which plants and animals have evolved distinct, but nonetheless largely similar, mechanisms for NR activation at the level of transcriptional regulation. Future studies aimed at elucidating the roles of plant Mediator in integrating different plant hormone responses should provide deeper insight into these mechanisms.
Materials and Methods
Plant Materials and Growth Conditions.
A. thaliana ecotype Columbia was used as the WT. The following plant materials used in this study were previously described: coi1-2 (9), med25-4 (21), hac1-4 (34), COI1-myc (9), MED25-myc (21), MYC2-myc (37), and JAZ1-GUS (4). Further details can be found in SI Materials and Methods.
Plasmid Construction and Plant Transformation.
To construct pHAC1:HAC1-GFP (HAC1-GFP), the coding sequence of GFP was amplified and cloned into pCAMBIA1300 to obtain pCAMBIA1300-GFP, and the promoter and coding sequence of HAC1 were amplified and subsequently cloned into pCAMBIA1300-GFP to obtain pHAC1:HAC1-GFP. Primers used for plasmid construction are listed in Table S1. Further details can be found in SI Materials and Methods.
Table S1.
Oligonucleotide primers used in this work
| Assays | Destination products | Template | Primer name | Primer sequence (5′-3′) |
| Y2H | AD-MED25 | MED25 | AD-MED25 F | CGGAATTCATGTCGTCGGAGGTGAAACAGCTGATCGT |
| AD-MED25 R | TTGGATCCCTCCCATGAAGCCAGCTCCAGGCATGTTA | |||
| BD-MED25 | MED25 | BD-MED25 F | CGGAATTCATGTCGTCGGAGGTGAAACAGCTGATCGT | |
| BD-MED25 R | TTGGATCCcTCCCATGAAGCCAGCTCCAGGCATGTTA | |||
| AD-COI1 | COI1 | AD-COI1 F | GGAATTCCATATGATGGAGGATCCTGATATCAAGA | |
| AD-COI1 R | TCCCCCCGGGTCATATTGGCTCCTTCAGGAC | |||
| AD-HAC1 | HAC1 | AD-HAC1 F | CCGGCCATGGAGGCCATGAATGTTCAGGCTCACATGT | |
| AD-HAC1 R | TCCCCCGGGGGACCTGAGCCCCCAGCGACTTC | |||
| AD-HAC5 | HAC5 | AD-HAC5 F | GGAATTCCATATGATGGCTCAGGGGCAGAATAGG | |
| AD-HAC5 R | CGCGGATCCGTCATTCAGGAGTGGAGGCCG | |||
| AD-HAC12 | HAC12 | AD-HAC12 F | CCGGCCATGGAGGCCATGAATGTTCAGGCTCAC | |
| AD-HAC12 R | TCCCCCGGGGGTCAACCCGAGGTTCCAGCGAC | |||
| AD-HAC11-894 | HAC1 | AD-HAC11-894 F | CCGGCCATGGAGGCCATGAATGTTCAGGCTCACATGT | |
| AD-HAC11-894 R | TCCCCCGGGGGTGTATGTTCAGTAGACTCTGCCA | |||
| AD-HAC1887-1,200 | HAC1 | AD-HAC1887-1,200 F | CCGGCCATGGAGGCCCTGGCAGAGTCTACTGAACATACA | |
| AD-HAC1887-1,200 R | TCCCCCGGGGGATAAACTCGGCGTTGATTGGGA | |||
| AD-HAC11,200-1,698 | HAC1 | AD-HAC11,200-1,698 F | CCGGCCATGGAGGCCCTCTCGTATTTGGATTCTG | |
| AD-HAC11,200-1,698 R | TCCCCCGGGGGACCTGAGCCCCCAGCGACTTC | |||
| AD-HAC11-710 | HAC1 | AD-HAC11-710 F | CCGGCCATGGAGGCCATGAATGTTCAGGCTCACATGT | |
| AD-HAC11-710 R | TCCCCCGGGGGCTTCACAGGAATACAGACAGG | |||
| AD-HAC1260-710 | HAC1 | AD-HAC1260-710 F | CCGGCCATGGAGGCCCCCATGATGGTACCTCAG | |
| AD-HAC1260-710 R | TCCCCCGGGGGCTTCACAGGAATACAGACAGG | |||
| AD-HAC1630-710 | HAC1 | AD-HAC1630-710 F | CCGGCCATGGAGGCCGATCCGAGATTCAAAAATC | |
| AD-HAC1630-710 R | TCCCCCGGGGGCACAGGAATACAGACAGGGCAG | |||
| LCI | MED25-nLUC | MED25 | MED25-nLUC F | CGGGATCCATGTCGTCGGAGGTGAAACAGCTGATCGT |
| MED25-nLUC R | ACGCGTCGACTCCCATGAAGCCAGCTCCAGGCATGTTA | |||
| MED25vWF-A-nLUC | MED25 | MED25-nLUC F | CGGGATCCATGTCGTCGGAGGTGAAACAGCTGATCGT | |
| MED25vWF-A-nLUC R | ACGCGTCGACCTCCGAGATCAGGACAAGATAGA | |||
| MED25MD-nLUC | MED25 | MED25MD-nLUC F | CGGGATCCATGAATTTTGTGGAGGCATGTGCTGCCT | |
| MED25MD-nLUC R | ACGCGTCGACGCCATCATCGGGGGCTATGCAG | |||
| MED25ACID-nLUC | MED25 | MED25ACID-nLUC F | CGGGATCCATGACTTCACAATCCAAATATGTGAA | |
| MED25ACID-nLUC R | ACGCGTCGACATTTGGAATTTGTGGTTTAAACA | |||
| MED25GD-nLUC | MED25 | MED25GD-nLUC F | CGGGATCCATGCAGCAACAGCAGCAGCAACAACAA | |
| MED25-nLUC R | ACGCGTCGACTCCCATGAAGCCAGCTCCAGGCATGTTA | |||
| cLUC-COI1 | COI1 | cLUC-COI1 F | CGGGGTACCATGGAGGATCCTGATATCAAGA | |
| cLUC-COI1 R | ACGCGTCGACTCATATTGGCTCCTTCAGGAC | |||
| cLUC-COI1F-box | COI1 | cLUC-COI1 F | CGGGGTACCATGGAGGATCCTGATATCAAGA | |
| cLUC-COI1F-box R | ACGCGTCGACCATAGTCACATGCTCTCTCGTCTC | |||
| cLUC-COI1LRR | COI1 | cLUC-COI1LRR F | CGGGGTACCGCGCTTTGCTACACTGCGACGCC | |
| cLUC-COI1 R | ACGCGTCGACTCATATTGGCTCCTTCAGGAC | |||
| cLUC-HAC1 | HAC1 | cLUC-HAC1 F | TCCCCCGGGATAATGTTCAGGCTCACATG | |
| cLUC-HAC1 R | ACGCGTCGACTTAACCTGAGCCCCCAGCGAC | |||
| Pull down | pMAL-MED25551-836 | MED25 | pMAL-MED25551-836 F | CGGGATCCATGACTTCACAATCCAAATATGTGAA |
| pMAL-MED25551-836 R | GAAGTCGACTTAGTGGTGGTGGTGGTGGTGTCCCAT | |||
| GAAGCCAGCTCCAGGCATGTTA | ||||
| pMAL-JAZ1 | JAZ1 | pMAL-JAZ1 F | CGGAATTCATGTCGAGTTCTATGGAATGTTCTG | |
| pMAL-JAZ1 R | CGGGATCCGTGGTGGTGGTGGTGGTGTATTTCAGCT | |||
| GCTAAACCGAGC | ||||
| pET28a-COI1 | COI1 | pET28a-COI1 F | GGAATTCCATATGATGGAGGATCCTGATATCAAGA | |
| pET28a-COI1 R | ACGCGTCGACTCATATTGGCTCCTTCAGGAC | |||
| Antibody | pET28a-HAC1 | HAC1 | pET28a-HAC1 F | GCGTCGACGTAATGTTCAGGCTCACATGTCG |
| pET28a-HAC1 R | ATAAGAATGCGGCCGCTACCTGAGCCCCCAGCGAC | |||
| pMAL-MYC2 | MYC2 | pMAL-MYC2 F | GCTAGCATGACTGATTACCGGCTACAAC | |
| pMAL-MYC2 R | CTGCAGACCGATTTTTGAAATCAAACTTGC | |||
| Transformation | pHAC1:HAC1-GFP | HAC1 promoter | HAC1 promoter F | AAACTGCAGGTGAGTTTTGAGATCGATATG |
| HAC1 promoter R | ACGCGTCGACAGCTACCTCTTCAGTCTC | |||
| HAC1 | HAC1 CDS F | ACGCGTCGACATGAATGTTCAGGCTCAC | ||
| HAC1 CDS R | TCCCCCGGGGACCTGAGCCCCCAGCGAC | |||
| GFP | GFP F | GAAGGTACCATGGTGAGCAAGGGCGAGG | ||
| GFP R | GAATTCCTTGTACAGCTCGTCCATGCCGA | |||
| p35S:JAZ1-GFP | JAZ1 | JAZ1 F | CACCATGTCGAGTTCTATGGAATG | |
| JAZ1 R | TATTTCAGCTGCTAAACCGAGCC | |||
| p35S:MED25-Flag | MED25 | MED25 F | CACCATGTCGTCGGAGGTGAAACAGCTGATCGT | |
| MED25 R | TCCCATGAAGCCAGCTCCAGGCATGTTA | |||
| p35S:MYC2-Flag | MYC2 | MYC2 F | CACCATGACTGATTACCGGCTACA | |
| MYC2 R | ACCGATTTTTGAAATCAAACTTGC | |||
| ChIP | JAZ8 | JAZ8-A F | GTTGTCGAAGTTTGTTGGTAGG | |
| JAZ8-A R | CTCGGTTGCAATTGTATCAAC | |||
| JAZ8-B F | AAAACCTTAAAGTTGCACAAATG | |||
| JAZ8-B R | TTTACAACGGCGGACTCTTAG | |||
| JAZ8-C F | TGTCCTCTCTCTCCTCACAAATAG | |||
| JAZ8-C R | ATAAGAAGTGGGAAAAAGACGAAG | |||
| JAZ8-D F | GATCGCAAGCAGAGAAATGA | |||
| JAZ8-D R | TATCGTCGTGAATGGTACGG | |||
| ERF1 | ERF1-A F | TATCGTATCAAAGGTCTCTCCCAA | ||
| ERF1-A R | GCAACATTTTCTGTCGTTCGGA | |||
| ERF1-B F | AAGAGATACAATGTCCAGGTTT | |||
| ERF1-B R | ATTAGAACACTACACATCATCACA | |||
| ERF1-C F | TCAATCCACTAACGATCCCTAA | |||
| ERF1-C R | TTAGAAGAAGAGGATGAGAAAGAA | |||
| ERF1-D F | GGCGGAGAGAGTTCAAGAGT | |||
| ERF1-D R | TCCGTCTCATCGAGTGTTTC | |||
| ACTIN7 | ACT7 F | CCATAGCATTGTCTCTCCCA | ||
| ACT7 R | CGGCAGCAGCTAACACTAAG | |||
| qRT-PCR | VSP1 | VSP1 F | CCTCGAATCGAACACCATCT | |
| VSP1 R | GGCACCGTGTCGAAGTTTAT | |||
| PDF1.2 | PDF1.2 F | CGCACCGGCAATGGTGGAAG | ||
| PDF1.2 R | CACACGATTTAGCACCAAAG | |||
| JAZ8 | JAZ8 F | TCGTCTTTTTCCCACTTCTTATGA | ||
| JAZ8 R | AAGTCATCCTCAAACGGGTCG | |||
| ERF1 | ERF1 F | GGAAATTCGCGGCGGAGATAAGA | ||
| ERF1 R | CAACGCCACAACCGGAGAACAAC | |||
| HAC1 | HAC1 F | CCCGATGAAGGAGGACTTTA | ||
| HAC1 R | TCCCTGTCTTCACGTCTCTG | |||
| MED25 | MED25 F | GTCAAATGTCGCTTCAAACC | ||
| MED25 R | CTGCCACCAAGTTGTTGTATT | |||
| ACTIN7 | ACTIN7 F | CCATTCAGGCCGTTCTTTC | ||
| ACTIN7 R | CGTTCTGCGGTAGTGGTGA |
ACID, activator-interacting domain; F, forward; GD, glutamine-rich domain; MD, middle domain; R, reverse.
Details of additional experimental procedures, such as Y2H assay, antibody generation, protein expression and in vitro pull-down assays, co-IP assays, nuclear protein extraction and global histone acetylation analysis, ChIP-qPCR assays, sequential ChIP, qRT-PCR assay, RNA-seq and data analysis, LCI assay, and quantitative GUS activity assay, can be found in SI Materials and Methods.
SI Materials and Methods
Plant Materials and Growth Conditions.
Arabidopsis thaliana ecotype Columbia was used as the WT. The following plant materials used in this study were previously described: coi1-2 (9), med25-4 (21), hac1-4 (34), COI1-myc (9), MED25-myc (21), MYC2-myc (37), and JAZ1-GUS (4). COI1-myc was introduced into the med25-4 background, MED25-myc into the myc2-2 (8) or coi1-2 background, JAZ1-GUS into the med25-4 background, and HAC1-GFP into the coi1-2 or med25-4 background by crossing. Homozygous plants were selected by genotyping. All Arabidopsis plants were grown at 22 °C under long-day conditions (16 h light/8 h dark).
Plasmid Construction and Plant Transformation.
To construct pHAC1:HAC1-GFP (HAC1-GFP), the coding sequence of GFP was amplified and cloned into pCAMBIA1300 to obtain pCAMBIA1300-GFP, and the promoter and coding sequence of HAC1 were amplified and subsequently cloned into pCAMBIA1300-GFP to obtain pHAC1:HAC1-GFP. To construct p35S:JAZ1-GFP (JAZ1-GFP), the coding sequence of JAZ1 was amplified and subsequently cloned into pGWB5. Primers used for plasmid construction are listed in Table S1. The constructs were then transformed into Agrobacterium tumefaciens strain GV3101, which was used to transform Arabidopsis plants by floral dip. Transformants were selected based on their resistance to hygromycin. T3 or T4 homozygous lines were used for further experiments.
Y2H Assay.
The coding sequence of COI1 was amplified with the primers listed in Table S1 and cloned into vector pGBKT7 for the Y2H screening of a pGADT7-based Arabidopsis cDNA library constructed with mRNAs isolated from Arabidopsis seedlings. Y2H assays were based on Matchmaker GAL4 two-hybrid systems (Clontech). Yeast transformants were selected on SD/-Ade/-His/-Leu/-Trp/X–α-Gal (4 mg/mL) medium. Putative COI1-interacting clones were characterized and sequenced. The screen identified MED25. To verify the interaction between MED25 and COI1, the coding sequence of MED25 was fused with the BD domain in pGBKT7 and the coding sequence of COI1 was fused with the AD domain in pGADT7. To test the interaction of MED25 with HAC1 and other HACs, the coding sequences of HAC1, HAC5, HAC12, and HAC1 derivatives were fused with the AD domain in pGADT7. Primers used are listed in Table S1. Constructs for expression of the MED25 derivatives used in this study were described previously (21). Constructs for testing interactions were cotransformed into Saccharomyces cerevisiae strain AH109. The presence of transgenes was confirmed by growth on SD/-Leu/-Trp plates. To assess protein interactions of MED25 with HACs, the transformed yeasts were suspended in liquid SD/-Leu/-Trp to OD = 1.0. Five microliters of suspended yeast was spread on plates containing SD/-Ade/-His/-Leu/-Trp medium (Mairuida). To assess the protein interaction of MED25 with COI1, the transformed yeasts were suspended in liquid SD/-Leu/-Trp to OD = 1.0. Five microliters of suspended yeast was spread on SD/-His/-Leu/-Trp plates. Interactions were observed after 3 d of incubation at 30 °C.
Antibody Generation.
The N-terminal region of HAC1 (amino acids 1–710, HAC11-710) was PCR-amplified from WT cDNA using gene-specific primers (Table S1). The resultant PCR product was cloned into vector pET28a (Novagen) to express the His-HAC11-710 protein fusion in Escherichia coli BL21 (DE3). The recombinant fusion protein was purified with nickel-nitrilotriacetic acid (Ni-NTA) His•Bind Resin (Novagen) and used to raise polyclonal antibodies in mouse. Anti-HAC1 antibodies were used in protein gel blotting at a final dilution of 1:2,000. The coding sequence of MYC2 was amplified and cloned into vector pMAL-c2 (New England Biolabs) to express MYC2-MBP protein fusion in E. coli BL21 (DE3). Primers used for plasmid construction are listed in Table S1. The recombinant fusion protein was purified with amylose resin (New England Biolabs) and used to raise polyclonal antibodies in mice. Anti-MYC2 antibodies were used in immunoblotting at a final dilution of 1:2,000. COI1 monoclonal antibody was produced from a mouse hybridoma using purified COI1 protein as the immunogen (Abmart). The antibody was used in immunoblotting at a final dilution of 1:2,000 and in ChIP assays at a final dilution of 1:100.
Protein Expression and in Vitro Pull-Down Assays.
To produce COI1-His protein, the full-length coding sequence of COI1 was amplified and cloned into vector pET28a (Novagen). To produce His-MBP–tagged MED25551-836 protein (referred to as MED25551-836-MBP), MED25 fragment containing amino acids 551–836, C-terminally fused to the 6× His tag, was amplified and cloned into vector pMAL-c2 (New England Biolabs). To produce JAZ1-His-MBP protein (referred to as JAZ1-His), the full-length coding sequence of JAZ1 fused to the 6× His tag was amplified and cloned into vector pMAL-c2. Protein expressed in E. coli BL21 (DE3) was purified using either Ni-NTA resin (Novagen) or amylose resin (New England Biolabs). One microgram of MED25551-836-MBP was bound to the amylose resin, incubated with COI1-His in 1× IP buffer [50 mM Tris⋅HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA] at 4 °C for 1 h, and washed three times with elution buffer [50 mM Tris⋅HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA, 0.2% Nonidet P-40]. The washed amylose resin was resuspended in SDS/PAGE loading buffer containing 10 mM maltose. Following boiling for 5 min, the samples were detected by immunoblotting using an anti-COI1 antibody.
COI1–JAZ1 pull-down experiments were performed as described (4) with minor modifications. Assays contained total protein extract obtained from 10-d-old COI1-myc/WT or COI1-myc/med25-4 seedlings using extraction buffer [50 mM Tris⋅HCl (pH 7.5), 150 mM NaCl, 0.1% Triton X-100, 0.2% Nonidet P-40, 0.6 mM PMSF, 20 μΜ MG132, with Roche protease inhibitor mixture] (1 mg of total protein) and 1 μg of recombinant His-MBP–tagged JAZ1 in a volume of 300 μL, with or without 30 μM JA-Ile. Ni-NTA resin was used to bind JAZ1-His. After washing and elution with imidazole, proteins were detected by immunoblotting using anti-myc and anti-His antibodies.
Co-IP Assays.
Ten-day-old COI1-myc or MYC2-myc seedlings were treated with or without 30 μM JA-Ile for the indicated times. Each sample was collected and ground in liquid N2. Total proteins were extracted in extraction buffer [50 mM Tris⋅HCl (pH 7.5), 150 mM NaCl, 0.1% Triton X-100, 0.2% Nonidet P-40, 0.6 mM PMSF, 20 μΜ MG132, with Roche protease inhibitor mixture]. After protein extraction, 20 μL of protein G plus agarose (Santa Cruz Biotechnology) was added to extract (2 mg of protein) to reduce nonspecific Ig binding. After 1 h of incubation, the supernatant was transferred to a new tube. Myc antibody-bound agarose beads (Santa Cruz Biotechnology) were then added to the samples and incubated for 4 h at 4 °C with gentle rocking. WT seedlings were used as negative controls. The precipitated samples were washed at least four times using protein extraction buffer, and bound proteins were eluted by heating the beads in SDS protein loading buffer at 95 °C for 10 min. MED25 protein was detected by Western blotting using anti-MED25 antibody (21). To test the in vivo interaction of HAC1 with MED25, co-IP assays were performed in a similar manner, using MED25-myc plants and anti-HAC1 antibody.
For transient expression of MED25-Flag, MYC2-myc, and JAZ1-GFP in Nicotiana benthamiana leaves, the coding sequences of MED25, MYC2, and JAZ1 were cloned into pGWB11, pGWB17, and pGWB5 vectors, respectively, to create p35S:MED25-Flag (MED25-Flag), p35S:MYC2-myc (MYC2-myc), and p35S:JAZ1-GFP (JAZ1-GFP) fusion constructs. Agrobacterial strains carrying different construct combinations as well as p19 genes were coinfiltrated into N. benthamiana leaves. The infiltrated parts of N. benthamiana leaves were harvested, and the leaf tissue was then ground in liquid nitrogen and resuspended in extraction buffer [50 mM Tris⋅HCl (pH 7.5), 150 mM NaCl, 0.1% Triton X-100, 0.2% Nonidet P-40, 0.6 mM PMSF, 20 mΜ MG132, with Roche protease inhibitor mixture]. Co-IP assays were performed as described above.
Nuclear Protein Extraction and Global Histone Acetylation Analysis.
Ten-day-old WT and hac1-4 seedlings were treated with or without 30 μM JA-Ile for 30 min, and nuclei were extracted in nuclei isolation buffer [0.25 M sucrose, 15 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (pH 6.8), 5 mM MgCl2, 60 mM KCl, 15 mM NaCl, 1 mM CaCl2, 0.9% Triton X-100, 1 mM PMSF, with 1× Roche protease inhibitor mixture]. The solution was filtered through two layers of Miracloth and centrifuged. To obtain nuclear proteins, the resultant pellet was resuspended in nuclei lysis buffer (50 mM Hepes [pH 7.5], 150 mM NaCl, 1 mM EDTA, 1% SDS, 1% Triton X-100, 0.1% sodium deoxycholate, 1 mM PMSF, with 1 × Roche protease inhibitor mixture). To determine the substrate and site specificity of HAC1, SDS sample buffer was added to nuclear protein extracts. Protein samples were boiled for 5 min, separated on SDS/PAGE gels, and transferred to polyvinylidene fluoride membranes. To visualize nuclear proteins, the membranes were probed with antibodies against the following proteins: H3, H3ac, H4ac, H3K9ac, H3K14ac, H3K18ac, and H3K23ac (all from Abcam).
ChIP-qPCR Assays.
Ten-day-old seedlings of WT, coi1-2, myc2-2, med25-4, hac1-4, MED25-myc, MED25-myc/myc2-2, MED25-myc/coi1-2, HAC1-GFP, HAC1-GFP/coi1-2, and HAC1-GFP/med25-4 were treated with or without 30 μM JA-Ile for the indicated times, and 2 g of each sample was then harvested and cross-linked in 1% formaldehyde at room temperature for 10 min, followed by neutralization with 0.125 M glycine. The chromatin complex was isolated, resuspended in lysis buffer [50 mM Hepes (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1% SDS, 1% Triton X-100, 0.1% sodium deoxycholate, 1 mM PMSF, with 1× Roche protease inhibitor mixture], and sheared by sonication to reduce the average DNA fragment size to around 500 bp. The sheared chromatin was precleared with Protein A salmon sperm-coupled agarose (Millipore), and 10 μL of the precleared chromatin was removed for use as an input control. An equal amount of chromatin was immunoprecipitated overnight at 4 °C with specific antibodies, including anti-COI1, anti-myc (Millipore), anti-GFP (Abcam), anti-H3 (Abcam), and anti-H3K9ac (Abcam). The immunoprecipitated chromatin complex was incubated with protein A salmon sperm-coupled agarose (Millipore) and then washed with low-salt buffer [20 mM Tris⋅HCl (pH 8.0), 2 mM EDTA, 150 mM NaCl, 0.5% Triton X-100, 0.2% SDS], high-salt buffer [20 mM Tris⋅HCl (pH 8.0), 2 mM EDTA, 500 mM NaCl, 0.5% Triton X-100, 0.2% SDS], LiCl buffer [10 mM Tris⋅HCl (pH 8.0), 1 mM EDTA, 0.25 M LiCl, 0.5% Nonidet P-40, 0.5% sodium deoxycholate], and TE buffer [10 mM Tris⋅HCl (pH 8.0), 1 mM EDTA]. After washing, the immunoprecipitated chromatin was eluted with elution buffer (1% SDS, 0.1 M NaHCO3). Protein–DNA cross-linking was reversed by incubating the immunoprecipitated complexes at 65 °C overnight. DNA was recovered using a QIAquick PCR Purification Kit (Qiagen) and analyzed by real-time qPCR. Enrichment of DNA is shown as the percentage (%) input, which was calculated by determining the apparent IP efficiency at the JAZ8 and ERF1 loci as the ratio of the amount of immunoprecipitated DNA to the normalized amount of starting material (% of input DNA). H3K9ac levels are shown as the ratio of the amount of immunoprecipitated DNA assembled with H3K9ac to the amount of immunoprecipitated DNA assembled with H3. The TSS region of ACTIN 7 (At5g09810) was used as a nonspecific target gene locus. Primers for qPCR are listed in Table S1.
Sequential ChIP.
Sequential ChIP assays were carried out as described previously (49). Chromatin was prepared from the indicated plants as described for standard ChIP. For the first round of IP, chromatin was immunoprecipitated overnight using the indicated antibodies and washed as in standard ChIP. However, elution was conducted in buffer containing 10 mM DTT and 1% SDS, and the samples were incubated for 15 min at 65 °C. Eluted chromatin was diluted 20-fold in ChIP dilution buffer [50 mM Hepes (pH 7.5), 150 mM NaCl, 1 mM EDTA (pH 8.0), 1% Triton X-100, 1 mM PMSF, protease inhibitors] and incubated with the indicated secondary antibody. The second elution was conducted identical to standard ChIP. After the second ChIP and reverse cross-linking, the DNA was purified using a PCR purification kit (Qiagen) and quantified by qPCR.
RNA Extraction, Reverse Transcription, and Quantitative RT-PCR.
For quantitative reverse-transcription PCR (qRT-PCR) analysis of JA-responsive genes, total RNA was extracted from 10-d-old seedlings treated with 30 μM JA-Ile as indicated using the TRIzol reagent (Invitrogen). cDNA was prepared from 2 μg of total RNA with SuperScript III reverse transcriptase (Invitrogen) and quantified on a Roche 480 cycler with the SYBR Green kit (Takara). Expression levels of target genes were normalized against ACT7. Statistical significance was evaluated by Student’s t test. Primers are listed in Table S1.
RNA-Seq and Data Analysis.
Ten-day-old WT and hac1-4 seedlings were treated with 30 μM JA-Ile. At 1 or 24 h after treatment, seedlings from three biological replicates were collected for RNA extraction. Untreated seedlings were used as a control. Total RNA of each sample was extracted using an RNeasy plant Mini Kit (Qiagen) and treated with DNase I. The quality of the total RNA was assessed using a NanoDrop spectrophotometer and an Agilent 2100 Bioanalyzer. For each sample, 3 μg of total RNA was used to construct the Illumina sequencing libraries according to the manufacturer’s instructions. The libraries were sequenced using the Illumina HiSeq 2500 platform (Berry Genomics) to generate high-quality paired-end reads of 150 nt.
A. thaliana genome sequences and annotated gene models were downloaded from TAIR10 (The Arabidopsis Information Resource, version 10; www.arabidopsis.org/). Raw sequencing reads were first processed to remove adaptors and low-quality bases, and then aligned to the genome sequences using STAR (2.5.2b), with the maximum allowed intron size set as 5,000 nt. The mapping quality and saturation analysis were performed using RSeQC (50). Differentially expressed genes were identified using DESeq2 (51) with an absolute fold change > 1.5 and FDR-adjusted P < 0.05. Genes with more than 1.5-fold change of expression (FDR-adjusted P < 0.05) between any time after JA-Ile treatment (1 or 24 h) and untreated control were defined as JA-Ile–regulated genes in different genotypes. Genes with more than 1.5-fold change of expression (FDR-adjusted P < 0.05) at any time after JA-Ile treatment (1 or 24 h) between WT and hac1-4 were defined as HAC1-regulated genes. JA-Ile– and HAC1-coregulated genes were selected for further investigation. GO enrichment analyses were performed using clusterProfiler (52). GO term enrichment is shown by the most specific subclass in the enrichment analysis. Comparison analysis and plots were performed using in-house transcripts based on Python and R.
LCI Assays.
LCI assays were carried out as described previously (30). Coding sequences of COI1, MED25, and their derivatives were cloned into vector pCAMBIA1300-nLUC, and the coding sequences of HAC1, COI1, and their derivatives were cloned into vector pCAMBIA1300-cLUC. Primers are summarized in Table S1. A. tumefaciens GV3101 carrying the indicated constructs was incubated in Luria–Bertani (LB) medium at 28 °C overnight, and then transferred to new LB medium containing 10 mM 2-(N-morpholine)-ethanesulfonic acid (pH 5.6) and 40 μM acetosyringone [1:100 ratio (vol/vol)] for 16 h. The culture was pelleted and resuspended in 10 mM MgCl2 containing 0.2 mM acetosyringone to a final concentration of OD600 = 1.5. The bacteria were kept at room temperature for at least 3 h without shaking. For coinfiltration, equal volumes of Agrobacterium suspensions carrying the indicated constructs were infiltrated into N. benthamiana leaves. After infiltration, plants were incubated at 23 °C for 72 h under 16-h light/8-h dark conditions before LUC activity was measured. A low-light-cooled CCD imaging apparatus (NightOWL II LB983 with Indigo software) was used to capture the LUC image. The leaves were sprayed with 0.5 mM luciferin and placed in darkness for 3 min before detection of luminescence.
Quantitative GUS Activity Assay of Plant Extracts.
Quantification of GUS activity from JAZ1-GUS seedlings was carried out essentially as described (53). Briefly, JAZ1-GUS fusion protein was collected from 7-d-old JAZ1-GUS seedlings using GUS extraction buffer [50 mM sodium phosphate (pH 7.0), 10 mM EDTA, 10 mM β-mercaptoethanol, 0.1% (wt/vol) SDS, 0.1% (vol/vol) Triton X-100]. To assay GUS activity, 10 μL of extract was mixed with 500 μL of 1 mM 4-methylumbelliferyl-d-glucuronide in extraction buffer prewarmed to 37 °C. The mixture was incubated at 37 °C for 10, 30, or 60 min; at each time point, 50 μL of the mixture was mixed with 450 μL of stop solution (0.2 M sodium carbonate). The resultant fluorescence was measured on a BioTek Synergy Mx multilabel counter, with excitation and emission at 365 nm and 455 nm, respectively. Protein content was determined according to the Bradford method to normalize GUS activity.
Supplementary Material
Acknowledgments
We thank Daoxin Xie for providing materials; Jiayang Li, Jian-Min Zhou, and Jianru Zuo for critical reading of the manuscript; and Chang Li for English language editing. This work was supported by Ministry of Science and Technology of China Grant 2015CB942900; Strategic Priority Research Program of the Chinese Academy of Sciences Grant XDB11030200; National Natural Science Foundation of China Grants 91317039, 31320103910, 31371223, and 31370311; the Chinese Academy of Sciences Youth Innovation Promotion Association Grant 2014082, and the Tai-Shan Scholar Program from the Shandong Provincial Government.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The raw sequence data reported in this paper have been deposited in the Genome Sequence Archive at the Beijing Institute of Genomics (BIG) Data Center, Chinese Academy of Sciences, bigd.big.ac.cn/gsa (accession no. CRA000278).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1710885114/-/DCSupplemental.
References
- 1.Browse J. Jasmonate passes muster: A receptor and targets for the defense hormone. Annu Rev Plant Biol. 2009;60:183–205. doi: 10.1146/annurev.arplant.043008.092007. [DOI] [PubMed] [Google Scholar]
- 2.Wasternack C, Hause B. Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann Bot. 2013;111:1021–1058. doi: 10.1093/aob/mct067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xie DX, Feys BF, James S, Nieto-Rostro M, Turner JG. COI1: An Arabidopsis gene required for jasmonate-regulated defense and fertility. Science. 1998;280:1091–1094. doi: 10.1126/science.280.5366.1091. [DOI] [PubMed] [Google Scholar]
- 4.Thines B, et al. JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature. 2007;448:661–665. doi: 10.1038/nature05960. [DOI] [PubMed] [Google Scholar]
- 5.Chini A, et al. The JAZ family of repressors is the missing link in jasmonate signalling. Nature. 2007;448:666–671. doi: 10.1038/nature06006. [DOI] [PubMed] [Google Scholar]
- 6.Yan Y, et al. A downstream mediator in the growth repression limb of the jasmonate pathway. Plant Cell. 2007;19:2470–2483. doi: 10.1105/tpc.107.050708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lorenzo O, Chico JM, Sánchez-Serrano JJ, Solano R. JASMONATE-INSENSITIVE1 encodes a MYC transcription factor essential to discriminate between different jasmonate-regulated defense responses in Arabidopsis. Plant Cell. 2004;16:1938–1950. doi: 10.1105/tpc.022319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boter M, Ruíz-Rivero O, Abdeen A, Prat S. Conserved MYC transcription factors play a key role in jasmonate signaling both in tomato and Arabidopsis. Genes Dev. 2004;18:1577–1591. doi: 10.1101/gad.297704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu L, et al. The SCFCOI1 ubiquitin-ligase complexes are required for jasmonate response in Arabidopsis. Plant Cell. 2002;14:1919–1935. doi: 10.1105/tpc.003368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devoto A, et al. COI1 links jasmonate signalling and fertility to the SCF ubiquitin-ligase complex in Arabidopsis. Plant J. 2002;32:457–466. doi: 10.1046/j.1365-313x.2002.01432.x. [DOI] [PubMed] [Google Scholar]
- 11.Dombrecht B, et al. MYC2 differentially modulates diverse jasmonate-dependent functions in Arabidopsis. Plant Cell. 2007;19:2225–2245. doi: 10.1105/tpc.106.048017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kazan K, Manners JM. MYC2: The master in action. Mol Plant. 2013;6:686–703. doi: 10.1093/mp/sss128. [DOI] [PubMed] [Google Scholar]
- 13.Zhai Q, et al. Phosphorylation-coupled proteolysis of the transcription factor MYC2 is important for jasmonate-signaled plant immunity. PLoS Genet. 2013;9:e1003422. doi: 10.1371/journal.pgen.1003422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pauwels L, et al. NINJA connects the co-repressor TOPLESS to jasmonate signalling. Nature. 2010;464:788–791. doi: 10.1038/nature08854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fonseca S, et al. (+)-7-iso-Jasmonoyl-L-isoleucine is the endogenous bioactive jasmonate. Nat Chem Biol. 2009;5:344–350. doi: 10.1038/nchembio.161. [DOI] [PubMed] [Google Scholar]
- 16.Yan J, et al. The Arabidopsis CORONATINE INSENSITIVE1 protein is a jasmonate receptor. Plant Cell. 2009;21:2220–2236. doi: 10.1105/tpc.109.065730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheard LB, et al. Jasmonate perception by inositol-phosphate-potentiated COI1-JAZ co-receptor. Nature. 2010;468:400–405. doi: 10.1038/nature09430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kornberg RD. The molecular basis of eukaryotic transcription. Proc Natl Acad Sci USA. 2007;104:12955–12961. doi: 10.1073/pnas.0704138104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bäckström S, Elfving N, Nilsson R, Wingsle G, Björklund S. Purification of a plant mediator from Arabidopsis thaliana identifies PFT1 as the Med25 subunit. Mol Cell. 2007;26:717–729. doi: 10.1016/j.molcel.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 20.Kidd BN, et al. The mediator complex subunit PFT1 is a key regulator of jasmonate-dependent defense in Arabidopsis. Plant Cell. 2009;21:2237–2252. doi: 10.1105/tpc.109.066910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen R, et al. The Arabidopsis mediator subunit MED25 differentially regulates jasmonate and abscisic acid signaling through interacting with the MYC2 and ABI5 transcription factors. Plant Cell. 2012;24:2898–2916. doi: 10.1105/tpc.112.098277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang F, et al. Structural basis of JAZ repression of MYC transcription factors in jasmonate signalling. Nature. 2015;525:269–273. doi: 10.1038/nature14661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelleher RJ, 3rd, Flanagan PM, Kornberg RD. A novel mediator between activator proteins and the RNA polymerase II transcription apparatus. Cell. 1990;61:1209–1215. doi: 10.1016/0092-8674(90)90685-8. [DOI] [PubMed] [Google Scholar]
- 24.Kornberg RD. Mediator and the mechanism of transcriptional activation. Trends Biochem Sci. 2005;30:235–239. doi: 10.1016/j.tibs.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 25.Malik S, Roeder RG. Dynamic regulation of pol II transcription by the mammalian mediator complex. Trends Biochem Sci. 2005;30:256–263. doi: 10.1016/j.tibs.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 26.Malik S, Roeder RG. The metazoan mediator co-activator complex as an integrative hub for transcriptional regulation. Nat Rev Genet. 2010;11:761–772. doi: 10.1038/nrg2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soutourina J, Wydau S, Ambroise Y, Boschiero C, Werner M. Direct interaction of RNA polymerase II and mediator required for transcription in vivo. Science. 2011;331:1451–1454. doi: 10.1126/science.1200188. [DOI] [PubMed] [Google Scholar]
- 28.Poss ZC, Ebmeier CC, Taatjes DJ. The mediator complex and transcription regulation. Crit Rev Biochem Mol Biol. 2013;48:575–608. doi: 10.3109/10409238.2013.840259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allen BL, Taatjes DJ. The mediator complex: A central integrator of transcription. Nat Rev Mol Cell Biol. 2015;16:155–166. doi: 10.1038/nrm3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen H, et al. Firefly luciferase complementation imaging assay for protein-protein interactions in plants. Plant Physiol. 2008;146:368–376. doi: 10.1104/pp.107.111740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bordoli L, Netsch M, Lüthi U, Lutz W, Eckner R. Plant orthologs of p300/CBP: Conservation of a core domain in metazoan p300/CBP acetyltransferase-related proteins. Nucleic Acids Res. 2001;29:589–597. doi: 10.1093/nar/29.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Earley KW, Shook MS, Brower-Toland B, Hicks L, Pikaard CS. In vitro specificities of Arabidopsis co-activator histone acetyltransferases: Implications for histone hyperacetylation in gene activation. Plant J. 2007;52:615–626. doi: 10.1111/j.1365-313X.2007.03264.x. [DOI] [PubMed] [Google Scholar]
- 33.Pandey R, et al. Analysis of histone acetyltransferase and histone deacetylase families of Arabidopsis thaliana suggests functional diversification of chromatin modification among multicellular eukaryotes. Nucleic Acids Res. 2002;30:5036–5055. doi: 10.1093/nar/gkf660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deng W, et al. Involvement of the histone acetyltransferase AtHAC1 in the regulation of flowering time via repression of FLOWERING LOCUS C in Arabidopsis. Plant Physiol. 2007;143:1660–1668. doi: 10.1104/pp.106.095521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berger S, Bell E, Sadka A, Mullet JE. Arabidopsis thaliana Atvsp is homologous to soybean VspA and VspB, genes encoding vegetative storage protein acid phosphatases, and is regulated similarly by methyl jasmonate, wounding, sugars, light and phosphate. Plant Mol Biol. 1995;27:933–942. doi: 10.1007/BF00037021. [DOI] [PubMed] [Google Scholar]
- 36.Penninckx IA, et al. Pathogen-induced systemic activation of a plant defensin gene in Arabidopsis follows a salicylic acid-independent pathway. Plant Cell. 1996;8:2309–2323. doi: 10.1105/tpc.8.12.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Q, et al. The basic helix-loop-helix transcription factor MYC2 directly represses PLETHORA expression during jasmonate-mediated modulation of the root stem cell niche in Arabidopsis. Plant Cell. 2011;23:3335–3352. doi: 10.1105/tpc.111.089870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kidd BN, Cahill DM, Manners JM, Schenk PM, Kazan K. Diverse roles of the mediator complex in plants. Semin Cell Dev Biol. 2011;22:741–748. doi: 10.1016/j.semcdb.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 39.Samanta S, Thakur JK. Importance of mediator complex in the regulation and integration of diverse signaling pathways in plants. Front Plant Sci. 2015;6:757. doi: 10.3389/fpls.2015.00757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonawitz ND, et al. Disruption of mediator rescues the stunted growth of a lignin-deficient Arabidopsis mutant. Nature. 2014;509:376–380. doi: 10.1038/nature13084. [DOI] [PubMed] [Google Scholar]
- 41.Wu K, Zhang L, Zhou C, Yu CW, Chaikam V. HDA6 is required for jasmonate response, senescence and flowering in Arabidopsis. J Exp Bot. 2008;59:225–234. doi: 10.1093/jxb/erm300. [DOI] [PubMed] [Google Scholar]
- 42.Zhou C, Zhang L, Duan J, Miki B, Wu K. HISTONE DEACETYLASE19 is involved in jasmonic acid and ethylene signaling of pathogen response in Arabidopsis. Plant Cell. 2005;17:1196–1204. doi: 10.1105/tpc.104.028514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dharmasiri N, Dharmasiri S, Estelle M. The F-box protein TIR1 is an auxin receptor. Nature. 2005;435:441–445. doi: 10.1038/nature03543. [DOI] [PubMed] [Google Scholar]
- 44.Shimada A, et al. Structural basis for gibberellin recognition by its receptor GID1. Nature. 2008;456:520–523. doi: 10.1038/nature07546. [DOI] [PubMed] [Google Scholar]
- 45.Park SY, et al. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science. 2009;324:1068–1071. doi: 10.1126/science.1173041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fu ZQ, et al. NPR3 and NPR4 are receptors for the immune signal salicylic acid in plants. Nature. 2012;486:228–232. doi: 10.1038/nature11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fondell JD. The mediator complex in thyroid hormone receptor action. Biochim Biophys Acta. 2013;1830:3867–3875. doi: 10.1016/j.bbagen.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 48.Lee HK, Park UH, Kim EJ, Um SJ. MED25 is distinct from TRAP220/MED1 in cooperating with CBP for retinoid receptor activation. EMBO J. 2007;26:3545–3557. doi: 10.1038/sj.emboj.7601797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Furlan-Magaril M, Rincón-Arano H, Recillas-Targa F. Sequential chromatin immunoprecipitation protocol: ChIP-reChIP. Methods Mol Biol. 2009;543:253–266. doi: 10.1007/978-1-60327-015-1_17. [DOI] [PubMed] [Google Scholar]
- 50.Wang L, Wang S, Li W. RSeQC: Quality control of RNA-seq experiments. Bioinformatics. 2012;28:2184–2185. doi: 10.1093/bioinformatics/bts356. [DOI] [PubMed] [Google Scholar]
- 51.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu G, Wang LG, Han Y, He QY. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS. 2012;16:284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blazquez M. 2007. Quantitative GUS activity assay of plant extracts. CSH Protoc, pdb prot4690.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.