Significance
Medicinal cannabis use is booming despite limited preclinical evidence and mechanistic insight. Recent clinical trials of cannabidiol (CBD) in Dravet syndrome (DS) support its clinical efficacy for reduction of seizure frequency and invite study of its benefits for additional DS symptoms. We demonstrate here that treatment with CBD is beneficial for seizure frequency, duration, and severity and for autistic-like social deficits in a mouse model of DS. CBD rescue of DS symptoms is associated with increased inhibitory neurotransmission, potentially mediated by antagonism of the lipid-activated G protein-coupled receptor GPR55. These studies lend critical support for treatment of seizures in DS with CBD, extend the scope of CBD treatment to autistic-like behaviors, and provide initial mechanistic insights into CBD’s therapeutic actions.
Keywords: cannabidiol, Dravet syndrome, epilepsy, autism, Scn1a
Abstract
Worldwide medicinal use of cannabis is rapidly escalating, despite limited evidence of its efficacy from preclinical and clinical studies. Here we show that cannabidiol (CBD) effectively reduced seizures and autistic-like social deficits in a well-validated mouse genetic model of Dravet syndrome (DS), a severe childhood epilepsy disorder caused by loss-of-function mutations in the brain voltage-gated sodium channel NaV1.1. The duration and severity of thermally induced seizures and the frequency of spontaneous seizures were substantially decreased. Treatment with lower doses of CBD also improved autistic-like social interaction deficits in DS mice. Phenotypic rescue was associated with restoration of the excitability of inhibitory interneurons in the hippocampal dentate gyrus, an important area for seizure propagation. Reduced excitability of dentate granule neurons in response to strong depolarizing stimuli was also observed. The beneficial effects of CBD on inhibitory neurotransmission were mimicked and occluded by an antagonist of GPR55, suggesting that therapeutic effects of CBD are mediated through this lipid-activated G protein-coupled receptor. Our results provide critical preclinical evidence supporting treatment of epilepsy and autistic-like behaviors linked to DS with CBD. We also introduce antagonism of GPR55 as a potential therapeutic approach by illustrating its beneficial effects in DS mice. Our study provides essential preclinical evidence needed to build a sound scientific basis for increased medicinal use of CBD.
Global use of medicinal cannabis is rapidly escalating (www.unodc.org/wdr2017/) (1). In particular, extracts rich in cannabidiol (CBD), an abundant bioactive but nonpsychotomimetic constituent of Cannabis sativa, are increasingly used to treat refractory epilepsy (2). However, there is only limited evidence for its efficacy in preclinical models (3, 4) and human studies (5–7). Recent clinical trials found that CBD reduced seizure frequency in patients with DS and other treatment-refractory epilepsies (6, 7). However, preclinical studies of CBD in validated animal models can provide quantitative measures of CBD’s efficacy in controlling seizure duration and severity in addition to frequency. Studies in animal models can also assess potential beneficial effects of CBD on the behavioral comorbidities of Dravet syndrome (DS) and probe the underlying physiological and molecular mechanisms mediating these therapeutic effects.
Heterozygous loss-of-function mutations in SCN1A cause DS, a severe childhood neuropsychiatric disorder characterized by treatment-refractory epilepsy, autism, other comorbidities, and frequent premature death (8–10). The uncertainty of seizure onset; the increasing frequency, duration, and intensity of seizures; and the risk of injury is an immense source of anxiety for parents (11, 12). Moreover, the ensuing social impairment associated with DS adds an additional burden of care and increases risk for accidents and premature death (9, 10). Thus, pharmacological rescue of both epilepsy and autism-related behaviors would greatly increase the quality of life for DS patients and their caregivers.
Scn1a+/− mice (DS mice) are a well-defined phenocopy of human DS (13, 14), as they exhibit both thermally induced and spontaneous seizures (13, 15) and autism-like social deficits (16, 17). Previous work showed that DS symptoms result from the loss-of-function of Nav1.1 channels, which selectively reduces sodium current and excitatory drive in many types of GABAergic interneurons (13, 14, 18–20). Accordingly, targeting the Scn1a mutation to Nav1.1 channels in forebrain GABAergic interneurons recapitulated the DS phenotype and established that hypoexcitability of these interneurons is sufficient to cause the epileptic phenotype (21) and autistic-like behaviors (16) observed in DS mice. In contrast, targeting the Scn1a mutation to excitatory neurons ameliorates DS in mice, which is opposite to the effects of gene disruption in inhibitory neurons (22). Additional effects of compensatory changes in gene expression, electrical excitability, and genetic background also have a strong influence on epileptic phenotypes of DS mice (SI Discussion). These results suggest that effective treatment of DS could potentially be achieved through enhancing GABAergic signaling by inhibitory neurons (16, 23) or selectively decreasing electrical excitability in excitatory neurons (24).
Given the promising results for CBD in treating seizures in DS, coupled with its low side-effect profile, we have analyzed its effects on epilepsy and autistic-like behaviors in DS mice. Here we report that CBD substantially reduces the frequency, duration, and severity of seizures and ameliorates the impaired social interaction behaviors of DS mice. These beneficial effects are correlated with enhanced GABAergic neurotransmission, decreased excitation/inhibition ratio, and decreased action potential firing of excitatory neurons in response to strong stimuli. These changes in GABAergic neurotransmission are mimicked and occluded by inhibition of the lipid-activated G protein-coupled receptor GPR55, suggesting that CBD’s effects are mediated, at least in part, by this signal transduction pathway. Our results strengthen basic science support for clinical use of CBD in DS and give initial clues to its physiological and molecular mechanisms of action.
SI Discussion
Compensatory Regulation and Genetic Background Effects in DS Mice.
In the initial studies of DS mice, it was noted that mice of the C57BL/6J strain were profoundly affected by the DS mutation, whereas mice of the 129SvJ strain were not (13). Compensatory up-regulation of sodium currents was observed in hippocampal interneurons and cerebellar Purkinje neurons (13, 41). More detailed studies showed that electrical excitability of interneurons and boosting of incoming synaptic signals by subthreshold sodium currents are less affected by DS mutations in 129SvJ and 129S6/SvEvTac mice than in C57BL/6J mice (20, 42). In addition to these changes in intrinsic electrical excitability, extensive gene-mapping studies revealed that the gene encoding the α2 subunit of the GABAA receptor is a strong candidate for a genetic modifier of DS in mice, consistent with a key role for inhibitory neurons in DS (43).
Although reduced sodium currents are observed widely among different types of interneurons in DS mice (13, 14, 18, 25, 41, 44), excitatory neurons in the hippocampus (13), cerebral cortex (18), and thalamus (19) are comparatively unaffected by DS mutations. However, strain-dependent differences in up-regulation of sodium currents in hippocampal pyramidal neurons were also noted. In pure-bred C57BL/6J DS mice, no changes in sodium currents or action potential firing were observed at postnatal days 14 or 21 (42), whereas an increase in sodium current was observed on postnatal day 21 but not on postnatal day 14 in mice with a 50:50 C57BL/6J:129S6/SvEvTac genetic background (20). As postnatal day 21 is near the onset of spontaneous seizures in DS mice, changes in electrical excitability of pyramidal neurons at this pivotal time may influence the subsequent evolution and severity of DS. Overall, these studies reveal that the phenotypes of DS mice are strongly affected by age and genotype through genetic background effects and through compensatory changes in intrinsic electrical excitability.
Specificity of CBD and CID16020046 for GPR55.
Our experiments implicate GPR55 as an important target for the therapeutic effects of CBD in DS. However, the strength of the connection between GPR55 and DS depends critically on the specificity of CBD and CID16020046 for GPR55. In addition to GPR55 (36, 37, 39), CBD has several potential molecular targets on which pharmacological effects have been reported, including Transient Receptor Potential (TRP) channels and potential allosteric sites on the CB1 receptor itself (35, 39, 45). Therefore, we do not consider CBD to be a specific antagonist of GPR55. However, we have ruled out effects on the CB1 receptor by blocking its activity with a specific CB1 antagonist (Fig. 5), and the effects on TRP channels require higher concentrations than we have used (39). The specificity of CID16020046 has been tested in several cellular systems. For example, Kargl et al. (39) found no effects on 33 selected molecular targets at concentrations higher than both of the concentrations that we have used. They found detectable effects at concentrations above 10 μM on two cyclic nucleotide phosphodiesterases, acetylcholinesterase, μ-opioid receptors, and the KV11.1 potassium channel (KCNH2; hERG), a notorious molecular target for nonspecific drug effects (39). It is unlikely that these molecular targets are involved in the effects of CID in DS mice at the concentrations (1 μM and 10 μM) that we tested, but we cannot explicitly exclude unexpected effects of CID16020046 on these or other unidentified molecular targets.
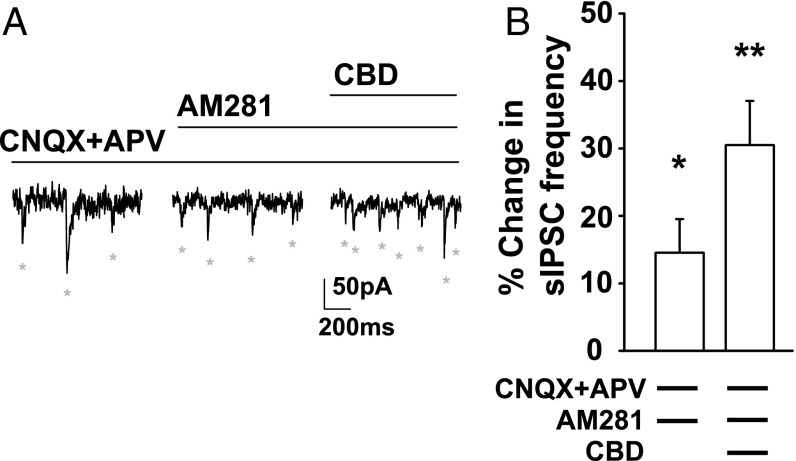
Fig. 5.
CBD enhancement of sIPSC frequency is independent of CB1 receptors. (A) Voltage-clamp recordings (Vh = −60 mV) of DGC sIPSCs (ECl = 0 mV; SI Materials and Methods) in the presence of CNQX (20 µM) and APV (50 µM), the CB1 receptor antagonist AM281 (1 µM), or AM281 and CBD (16 µM). (B) Percentage change in sIPSC frequency. AM281 enhanced sIPSC frequency from baseline [14.0 ± 4.4%, n = 6; t(5) = 2.94, P = 0.032, one-sample t test]. CBD further enhanced sIPSC frequency compared with AM281 alone [30.5 ± 6.52%, n = 6, t(5) = 4.67, P = 0.005]. *P < 0.05; **P < 0.01.
Results
CBD Protects Against Seizure in DS Mice.
The initial symptoms associated with DS are observed as early as 6–9 mo of age with the onset of febrile seizures, followed by spontaneous epileptic seizures with increasing severity and frequency (8, 9). We modeled febrile seizures by thermal induction in DS mice, in which core body temperature was slowly raised to 38 °C and held there for 30 min (15). Acute treatment with CBD (100 mg/kg or 200 mg/kg, i.p.) 1 h before thermal induction reduced the duration (Fig. 1A) and severity (Fig. 1B) of seizures. Such reduction in seizure severity to a Racine score of 3.5 or less is known to greatly reduce risk of sudden unexpected death in epilepsy in DS mice (21, 25). Because the half-life of CBD in rodents is much shorter than in humans [∼4.5 h in mice (26); ∼24 h in human (27)], it is difficult to precisely compare the 100–200 mg/kg effective i.p. dose range that we observed here with the 20–50 mg/kg oral dose range used in recent clinical trials (6, 7).
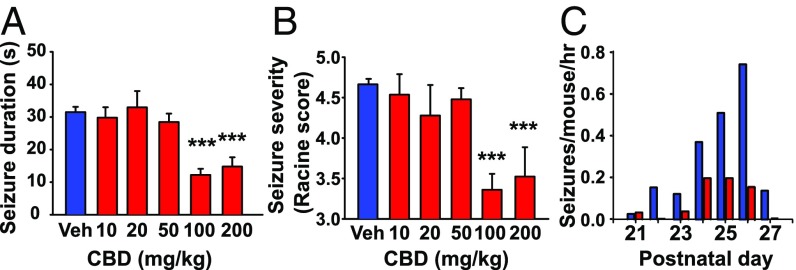
Fig. 1.
CBD reduced seizures in DS mice. (A) Dose dependence of CBD reduction in duration of thermally induced seizures (SI Materials and Methods): vehicle, 31.5 ± 1.6 s, n = 116 seizures; 100 mg/kg CBD, 12.2 ± 1.9 s, n = 36 seizures; 200 mg/kg CBD, 14.8 ± 2.9 s, n = 21 seizures). F(5,115) = 11.24, P < 0.001, one-way ANOVA; post hoc comparisons, P < 0.001. (B) Dose dependence of CBD reduction in seizure severity (SI Materials and Methods): vehicle, 4.66 ± 0.07; 100 mg/kg CBD, 3.36 ± 0.20; 200 mg/kg CBD, 3.52 ± 0.36. F(5,115) = 7.65, P < 0.001, one-way ANOVA; post hoc comparisons, P < 0.001. (C) Mean number of seizures/mouse/hour (bars) after twice-daily injection of vehicle (blue) or 100 mg/kg CBD (red) from postnatal day 21–27: vehicle, 0.31 ± 0.10 seizures/mouse/hour, n = 6; CBD, 0.09 ± 0.04, n = 7. F(1,13) = 7.99, P = 0.03, two-way ANOVA. ***P < 0.001.
To test if repeated CBD administration protects against spontaneous seizures, we administered CBD (100 mg/kg) twice daily during the period of maximum seizure risk, postnatal days 21–28 (13, 14, 20, 22, 25). We measured seizure frequency in the 4-h interval following treatment while the plasma drug concentration remained at >50% of peak (26). Consistent with previous reports, vehicle-treated DS mice exhibited spontaneous seizures at a rate of 0.31 seizures/mouse/hour. Treatment with CBD reduced the spontaneous seizure rate by 70% (Fig. 1C), further supporting the efficacy of CBD in treatment of epilepsy in DS mice. Together, these results show that CBD protects against both febrile seizures and spontaneous seizures in a validated mouse genetic model of DS.
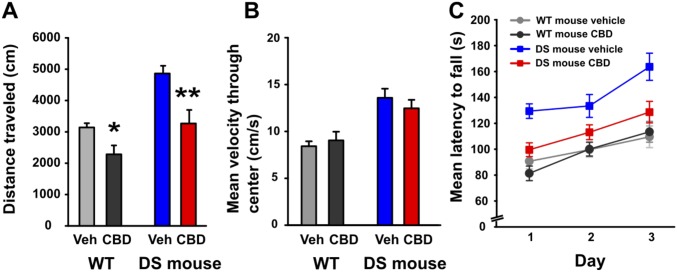
To test for potential adverse effects associated with 100 mg/kg CBD treatment, we measured the distance traveled and maximum velocity in the open field and the performance on the rotarod (28). It is known that DS mice are hyperactive compared with wild-type (WT) mice (16) (Fig. S1A). We found that treating DS mice with 100 mg/kg CBD significantly reduced their distance traveled (Fig. S1A), reaching a level comparable to control WT mice. By contrast, this treatment did not reduce the maximum velocity of movement of DS mice measured in the center of the open field (Fig. S1B). DS mice performed better on the rotorod than WT mice, and their performance was reduced to the WT level in the presence of 100 mg/kg CBD (Fig. S1C). One interpretation of these results is that the hyperactivity of DS mice improves their performance on the rotorod compared with WT due to their more frenetic efforts to stay on the rotatrod, and this advantage is lost upon CBD treatment because it reverses their hyperactivity. Together, these results suggest that 100 mg/kg CBD has a therapeutic effect to reverse hyperactivity of DS mice but does not have a significant adverse effect to impair motor function and coordination compared with untreated WT mice.
Fig. S1.
CBD does not impair locomotor activity in DS mice. (A) Summary plot showing the total distance traveled in the open field during a 10-min test session. CBD (100 mg/kg) reduced distance traveled in WT [t(12) = 2.90, P = 0.013; paired-t test] and DS mice [t(14) = 3.53, P = 0.003]. (B) Summary plot showing the mean velocity as the subject traversed through the center of the chamber. CBD had no effect on the mean velocity of mice of either genotype as they traveled through the center of the chamber. (C) Summary plot showing the mean latency to fall on the accelerating rotarod as an average of each of the 3 d. There was significant interaction between treatment and genotype [F(3,75) = 6.00, P = 0.017], revealing that CBD impaired performance in DS mice only, but DS mice treated with CBD still performed better than WT mice treated with either vehicle or CBD (all P < 0.05, post hoc comparisons). *P < 0.05; **P < 0.01.
CBD Rescues Autistic-Like Social Interaction Deficits in DS Mice.
In both humans diagnosed with DS and in our mouse genetic model of DS, the frequency and severity of seizures abate following sexual maturity, while severe social deficits that are characteristic of autism persist (8–10, 16). Social/behavioral outcomes have not been measured in previous studies of CBD in DS (6, 7). To test CBD’s efficacy in treating autistic-like behaviors in DS mice, we used the Three-Chamber Test of social interaction (29) in which DS mice show profound deficits compared with WT littermates (16). We found that low doses of CBD (10 mg/kg or 20 mg/kg) administered i.p. 1 h before testing increased the proportion of time that DS mice spent interacting with the stranger mouse in the Three-Chamber Test, suggesting a rapid reversal of this autistic-like behavioral deficit (Fig. 2A). Importantly, treatment with these low doses of CBD did not impact locomotor activity in the open field (Fig. 2B). Surprisingly, we found that higher doses of CBD did not produce this beneficial effect (Fig. 2A), consistent with prior studies showing rescue of social deficits with low-dose benzodiazepine treatment in DS mice but not with higher doses (16). In contrast, there was no effect of CBD in WT mice when assessing this same behavior (Fig. 2A).
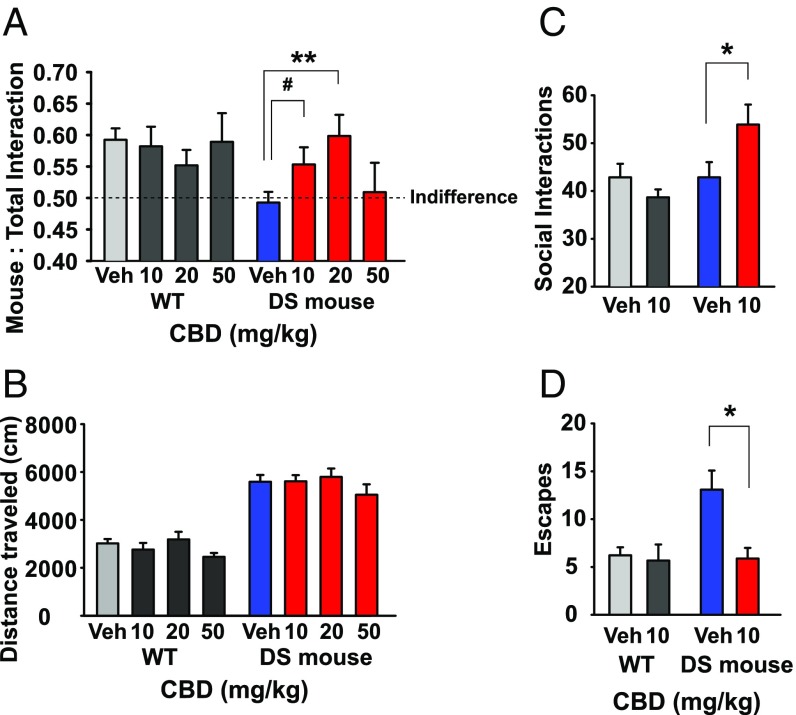
Fig. 2.
CBD improved social deficits in DS mice. (A) Effects of CBD in the Three-Chamber Test of Social Interaction. Preference ratio (PR) was measured as in SI Materials and Methods. WT with vehicle: PR = 0.59 ± 0.02, n = 47; t(46) = 5.09, P < 0.001, one-sample t test; DS with vehicle: PR = 0.49 ± 0.03, n = 53; t(52) = 0.43, P = 0.67. CBD improved PR in DS mice but not in WT: genotype by dose interaction, F(3,169) = 2.75, P = 0.045, two-way ANOVA. CBD showed a trend toward increased PR at 10 mg/kg: 0.55 ± 0.04, n = 11, P = 0.09, post hoc comparisons. CBD significantly increased the PR at 20 mg/kg: 0.60 ± 0.03, n = 11, P = 0.006. (B) Effect of CBD on locomotor activity in the Open Field Test (SI Materials and Methods). DS mice are hyperactive compared with WT [main effect of genotype, F(1,91) = 116.25, P < 0.001, two-way ANOVA]. CBD had no effect on distance traveled in either genotype [main effect of dose, F(3,91) = 1.19, P = 0.32]. (C and D) Effect of CBD on frequency of social interactions (C) and escape behaviors (D) in the Reciprocal Interaction Test (SI Materials and Methods) compared using independent-samples t-tests. (C) Social interactions. WT with vehicle, 42.9 ± 2.8, n = 14; DS with vehicle, 42.9 ± 3.2, n = 13; WT with 10 mg/kg CBD, 38.7 ± 1.7, n = 9; DS with 10 mg/kg CBD, 53.9 ± 4.2. n = 8, t(19) = 2.12, P = 0.048. (D) Defensive escapes: WT with vehicle, 6.21 ± 0.83, n = 14; DS with vehicle, 13.1 ± 2.0, n = 13; t(25) = 3.26, P = 0.003; WT with 10 mg/kg CBD, 5.67 ± 1.68, n = 9, P > 0.28; DS with 10 mg/kg CBD, 5.88 ± 1.11, n = 8, t(19) = 2.66, P = 0.015. #P = 0.09; *P < 0 05; **P < 0.01.
To extend this result, we tested CBD’s impact in the Reciprocal Interaction Test, which assesses behavior in an open field in the response to direct physical interaction with a stranger mouse. DS mice treated with vehicle engaged in a similar number of social interactions with a stranger mouse as did WT mice (Fig. 2C). However, DS mice made more frequent defensive escapes following the social interaction (Fig. 2D), which is characteristic of these mice. Acute treatment with CBD (10 mg/kg) increased the number of total social interactions of DS mice (Fig. 2C) and reduced the frequency of defensive escapes to WT levels (Fig. 2D). As in the Three-Chamber Test, there was no effect of CBD on WT mice when measuring similar behavior, suggesting that low doses of CBD significantly improve measures of social interaction only in the case of genetic deficit.
CBD Increases the Frequency of GABAergic Neurotransmission.
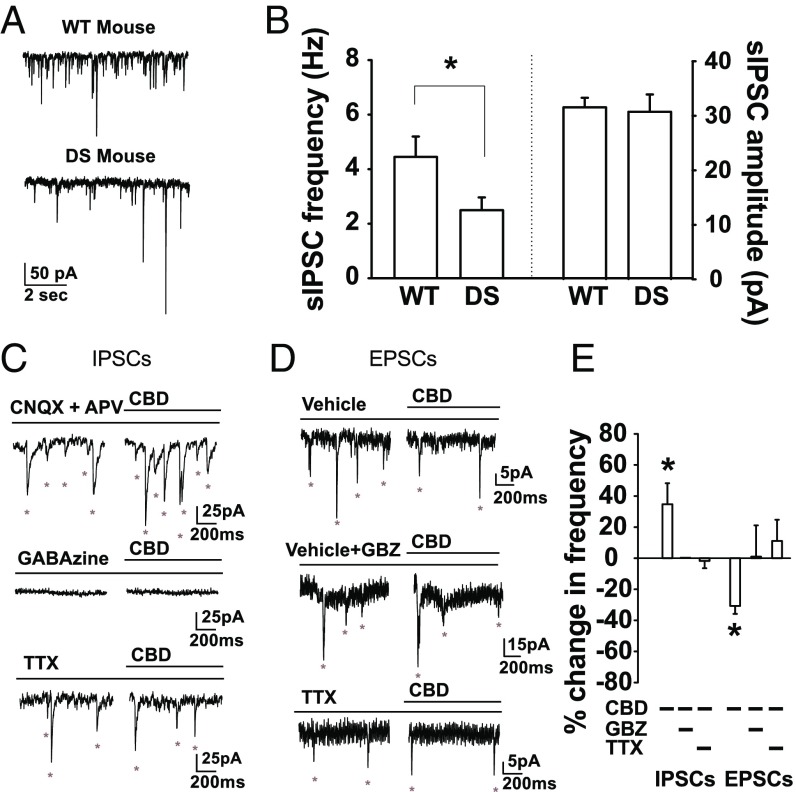
The beneficial effects of CBD on seizures and behavioral deficits in DS mice provide a unique opportunity to investigate the mechanistic basis for its efficacy. Scn1a+/− mutations cause a selective reduction in inhibitory interneuron excitability (13), which drives the DS phenotype (16, 21). We hypothesized that CBD reduces seizure frequency, duration, and severity and improves social behaviors through restoration of inhibitory interneuron excitability, and we tested this hypothesis in the dentate gyrus of the hippocampus. We focused on this well-characterized neural circuit because it protects against overexcitation in the hippocampus and enhanced excitatory output from this circuit leads to seizures in epileptic patients and animal models (30, 31). To assess the balance of excitatory vs. inhibitory neurotransmission, we performed voltage-clamp recordings (Vh = −60 mV) of DS mouse dentate granule cells (DGCs) in the presence of the ionotropic glutamate receptor antagonists 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) (20 µM) and 2-amino-5-phosphonovaleric acid (APV) (50 µM) to isolate effects on interneurons. We measured the amplitude and frequency of spontaneous, action potential (AP)-driven inhibitory postsynaptic currents [sIPSCs (ECl = 0 mV)]. The frequency of incoming sIPSCs was strikingly reduced in DGCs from DS mice compared with WT, whereas the amplitude was unchanged (16) (Fig. 3 A and B). Thus, DGCs in hippocampal slices from DS mice are a good model system to test effects of CBD on local circuit function.
Fig. 3.
CBD increased inhibitory neurotransmission to DGCs. (A) Voltage-clamp recordings (Vh = −60 mV) of sIPSCs in DGCs (ECl = 0 mV) in WT and DS mice (SI Materials and Methods). (B) sIPSC frequency: WT, 4.5 ± 0.75 Hz, n = 7; DS: 2.5 ± 0.5 Hz, n = 10; t(15) = 2.34, P = 0.034, independent-samples t test. sIPSC amplitude: WT: 31.5 ± 1.8 pA; DS: 30.7 ± 3.2 pA; P = 0.83. (C) Voltage-clamp recordings (Vh = −60 mV) of DGC sIPSCs (ECl = 0 mV) in CNQX (20 µM) and APV (50 µM), GABAzine (GBZ), or TTX (500 nM). (D) Voltage-clamp recordings (Vh = −60 mV) of DGC sEPSCs (ECl = −60 mV) alone, in GBZ, or in TTX. (E) Percentage change in frequency of sIPSCs and EPSCs. sIPSCs: CBD, 34.6 ± 13.6%; t(6) = 2.55, P = 0.043, n = 7, one-sample t test; CBD + GBZ, 0% change, n = 3; CBD + TTX, −1.8 ± 4.7%, n = 7. sEPSCs: CBD, −30.7 ± 5.1%; t(4) = 6.0, P = 0.004, n = 5, one-sample t test; CBD + GBZ, 0.92 ± 23.5%; t(4) = 0.05, P = 0.97, n = 5; CBD + TTX, 11.0 ± 13.6%, n = 6. Frequencies, amplitudes, and decay of miniature IPSCs and EPSCs (mIPSCs and mEPSCs) were unchanged by CBD. mIPSCs: frequency, −1.8 ± 4.7%, n = 7; amplitude, 5.7 ± 7.0%; decay, 10.0 ± 12.6%. mEPSCs: frequency, 11.0 ± 13.6%, n = 6; amplitude, 0.4 ± 4.1%; decay, −3.5 ± 7.1%; all *P > 0.05, one-sample t tests.
Treatment with CBD (16 µM), corresponding to the peak brain concentration following a 100 mg/kg i.p. injection in vivo (26), increased the frequency of GABAA receptor-mediated sIPSCs in hippocampal slices from DS mice (Fig. 3 C and E) without affecting the amplitude of the sIPSCs (Fig. 3, legend). Blocking GABAA receptors with the broad-spectrum antagonist GABAzine (10 μM) or blocking APs with tetrodotoxin (TTX; 500 nM) eliminated the effect of CBD (Fig. 3 C and E). These results indicate that CBD increases action potential generation in GABAergic interneurons, which in turn increases the frequency of sIPSPs recorded in DGCs.
Although increased GABAergic transmission to DGCs alone could account for the reduction in their output by CBD, reduced excitatory synaptic input could also be a contributing factor. Increased frequency of inhibitory neurotransmission would be expected to reduce the frequency of excitatory neurotransmission. Consistent with that expectation, CBD reduced the frequency of spontaneous excitatory postsynaptic currents (sEPSCs) recorded in DGCs [(ECl = −60 mV)], and this reduction was blocked by GABAzine and by TTX (Fig. 3 D and E). The amplitudes of EPSCs were unaffected, consistent with presynaptic effects of CBD (see legend for Fig. 3). Evidently, CBD directly enhances GABAA receptor-mediated inhibition and indirectly reduces excitatory transmission.
CBD Reduces Excitatory Output from the Dentate Gyrus.
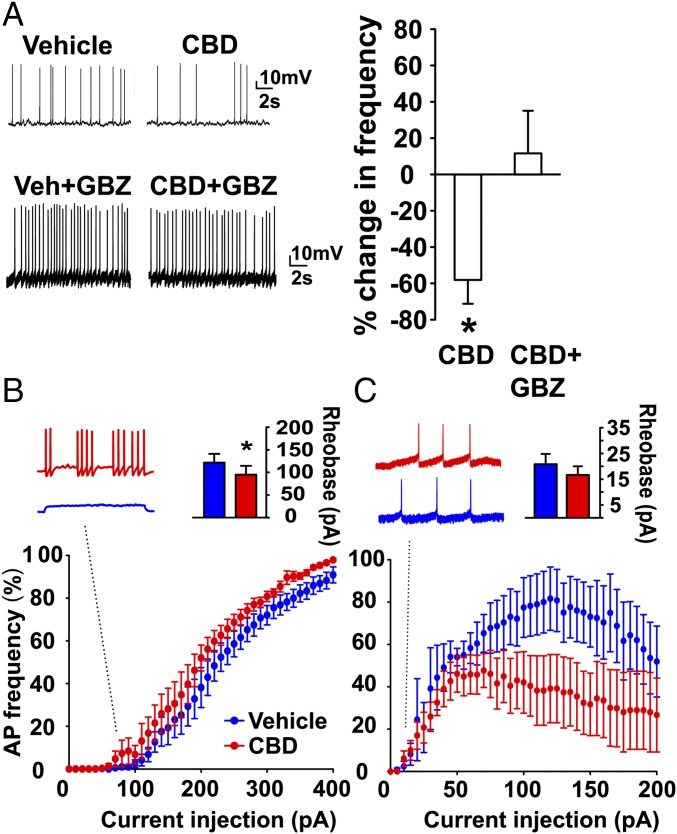
To directly assess the excitability of DGCs themselves, we recorded APs from DGCs in acutely prepared slices of DS mouse hippocampus using whole-cell patch-clamp recording techniques. During injection of subrheobase depolarizing current to elicit steady generation of APs, treatment with 16 µM CBD reduced the frequency of APs, and this effect was blocked by GABAzine (Fig. 4A). This result indicates that CBD reduces excitatory output from DGCs by enhancing GABAA receptor-mediated inhibition, as expected from the results of Fig. 3.
Fig. 4.
Differential effects of CBD on action potential firing in excitatory and inhibitory neurons. (A) Recordings of APs in DGCs (SI Materials and Methods) in the presence of subrheobase current (M = 30.83 ± 5.76 pA, n = 12) in vehicle, CBD (16 µM), or CBD + GBZ. Percent change in frequency: CBD, −58.2 ± 13.1%; t(4) = 4.44, P = 0.01, n = 5; CBD + GBZ, 11.6 ± 23.5%; t(6) = 0.50, P = 0.64, n = 7, one-sample t test). (B) Percentage maximum AP frequency in the presence of vehicle (blue) or 16 µM CBD (red) with increasing current injection in PV-positive interneurons identified with td-Tomato fluorescence (SI Materials and Methods). (Inset) Current-clamp recordings at 75 pA injection amplitude. Rheobase: DS + vehicle: 121.7 ± 19.4 pA, n = 6; DS + CBD: 95 ± 19.5 pA, n = 6; t(5) = 2.61, P = 0.048, paired-t test. CBD increased AP frequency at increasing current injection amplitudes [main effect of condition, F(1,200) = 7.87, P = 0.04, repeated-measures ANOVA]. (C) Percentage maximum AP frequency in vehicle (blue) or 16 µM CBD (red) at increasing current injection steps in DGCs. Rheobase: vehicle: 20.8 ± 4.0 pA; CBD: 16.7 ± 3.3 pA, n = 6, P > 0.4, CBD reduced. AP firing frequency [main effect of condition, F(1,120) = 12.65, P = 0.04, two-way repeated-measures ANOVA]. *P < 0.05.
To assess whether these effects of CBD are caused by directly increasing interneuron excitability, we measured CBD’s effects on AP firing in parvalbumin (PV)-positive fast-spiking interneurons because of their central role in suppressing DGC responses to cortical stimulation (32). CBD reduced rheobase by shifting the upswing of the frequency-stimulus curve to the left below 100 pA (Fig. 4B and Inset). CBD also increased the number of APs elicited across a wide range of current injection amplitudes (Fig. 4B), consistent with a significant increase in excitability of inhibitory interneurons.
To determine whether the reduction in sEPSCs might be caused by a combination of enhanced GABAegic signaling plus reduced intrinsic excitability of excitatory neurons, we measured the effect of CBD on DGC APs using a similar experimental paradigm (Fig. 4C). CBD had no effect on rheobase but reduced AP frequency at stimulus levels above 75 pA, more than 20-fold higher than rheobase (Fig. 4C and Inset). This effect may result from direct CBD inhibition of Nav1.6 channels, as these channels are abundant in excitatory neurons and are inhibited by CBD (33). Treatment with CBD has a selective effect on resurgent sodium currents generated by NaV1.6, which are thought to drive repetitive action potential firing (33). Recent studies with the atypical sodium channel blocker GS967 show that sodium channel inhibition can be an effective treatment for DS in mice (24). Together, these results suggest that CBD reduces DGC output through three mechanisms. At low stimulus levels, CBD reduces rheobase in inhibitory neurons, which would increase their probability of firing trains of APs in response to small depolarizations. Above rheobase, CBD increases frequency of AP generation during trains in interneurons in response to a wide range of stimulus intensities, from rheobase to 400 pA. Finally, CBD decreases the frequency of AP generation of excitatory neurons during periods of high excitatory stimulation at levels (75 pA–400 pA) that are far above rheobase.
CBD Effects Are Independent of CB1 Receptors.
CBD is thought to act independently of the canonical cannabinoid receptor CB1, which mediates the functional effects of brain endocannabinoids and the psychotropic effects of Δ9-tetrahydrocannabinol (34). Consistent with this expectation, blocking CB1 receptors with the antagonist, AM281 (1 µM) did not prevent the increase in sIPSCs induced by CBD treatment (Fig. 5). These results suggest that CBD increased sIPSC frequency by increasing AP-dependent GABA release from synaptic vesicles through a CB1-independent mechanism.
CBD Increases Inhibitory Neurotransmission by Blocking GPR55.
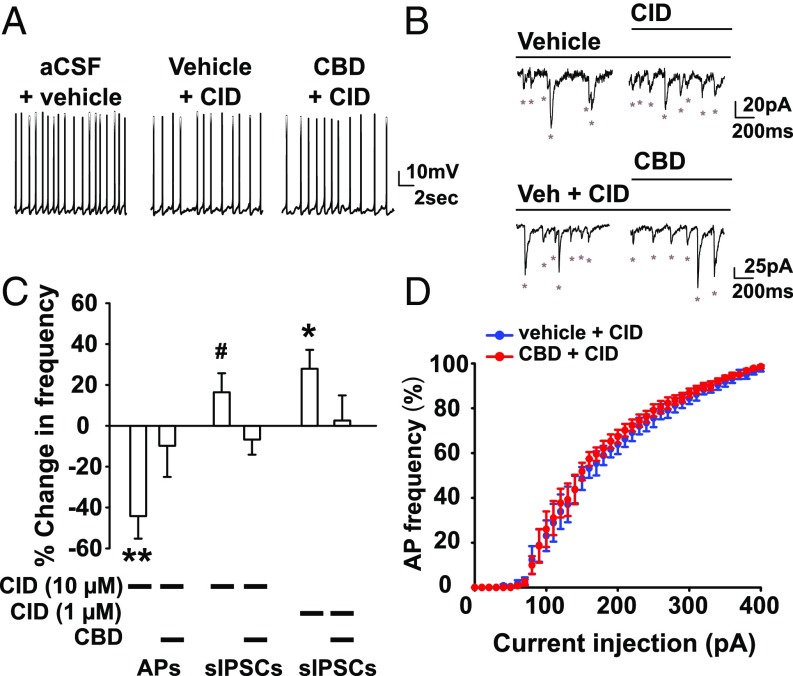
Given that CBD’s most robust effects are on inhibitory neurons, we next sought to identify the molecular target through which CBD enhances inhibitory signaling. Numerous CBD targets in the brain have been identified (35), including the lipid-activated G protein-coupled receptor GPR55 (36, 37), which is expressed in both interneurons and excitatory neurons in the dentate gyrus and other regions of the hippocampus and modulates hippocampal synaptic plasticity [Allen Brain Atlas (38)]. To determine if CBD antagonism of GPR55 contributed to our observed CBD-induced enhancement of inhibitory signaling, we bath-applied the GPR55 antagonist CID16020046 (CID, 10 µM) (39) and measured the effect of CBD on DGC output and inhibitory neuron signaling. CID (10 µM) alone mimicked the effect of CBD, reducing the frequency of APs generated during injection of subthreshold stimulating current (Fig. 6 A and C). Consistent with CBD antagonism of GPR55, treatment with CBD had no further effect when added in combination with CID, indicating that blockade of GPR55 by this antagonist occluded the reduction in AP generation by CBD (Fig. 6 A and C). Similarly, the enhancement in sIPSC frequency (Fig. 6 B and C) and excitability of PV-positive interneurons (Fig. 6D) was also occluded in the presence of CID. To further analyze the specificity of the effects of CID, we tested a concentration of 1 µM on spontaneous IPSCs. Even at this much lower concentration, CID substantially increased the frequency of sIPSCs and occluded the effects of CBD (Fig. 6C). CID is selective for inhibition of GPR55 at 1 µM and 10 µM compared with many other potential cellular targets (SI Discussion). Together, these mechanistic studies reveal that CBD increases inhibitory neuron excitability in substantial part through antagonism of GPR55.
Fig. 6.
Role of GPR55 in the CBD-induced increase in inhibitory transmission. (A) Current-clamp recordings of DGCs (SI Materials and Methods) in the presence of subrheobase-stimulating current (M = 28.0 ± 10.0 pA, n = 10). (B) Voltage-clamp recordings (Vh = −60 mV) of DGC sIPSCs (ECl = 0 mV; SI Materials and Methods) in CID16020064 (CID, 10 µM) or CBD (16 µM) in the presence of CID, the AMPA receptor antagonist CNQX (20 µM), and the NMDA receptor antagonist APV (50 µM). Asterisks indicate sIPSC. (C) Percentage change in AP frequency: CID 10 µM, −44.18 ± 11.1%, t(9) = 4.00, P = 0.003, n = 10; CID (10 µM) + CBD, −9.8 ± 15.2%, n = 9, t(8) = 0.64, P = 0.64, one-sample t test. Percentage change in IPSC frequency: 10 µM CID, 16.4 ± 9.3%, n = 9, t(8) = 1.77, P = 0.1. CID 10 µM blocked the CBD-induced enhancement of sIPSC frequency [−6.74 ± 7.42%, n = 9, t(8) = 0.91, P = 0.39, one-sample t test]. CID (1 µM), 27.9 ± 9.3%, n = 9, t(8) = 3.00, P = 0.02. CID (1 µM) also blocked the CBD-induced enhancement of sIPSC frequency [2.54 ± 12.4%, n = 11, t(10) = 0.21, P = 0.84]. (D) Percentage maximum AP frequency in 10 µM CID (blue) or 16 µM CBD (red) at increasing current injection steps in PV-positive interneurons identified with td-Tomato fluorescence. Rheobase: vehicle, 64.0 ± 9.27 pA, n = 5; CBD: 62.0 ± 5.83, n = 5; t(4) = 0.54, P = 0.62, paired t test. CID blocked the effect of CBD on AP frequency [main effect of condition, F(1,160) = 2.31, P = 0.20, repeated-measures ANOVA]. #P = 0.07; *P < 0.05; **P < 0.01.
Discussion
With increasing access to medicinal cannabis, many will seek CBD-rich extracts for treating DS and other debilitating epilepsies, despite limited scientific support of their efficacy. Parents of children with DS have moved their families to states in the United States where medical cannabis is available to provide treatment (2). Recent clinical trials support CBD’s treatment potential against seizures in patients with DS (6, 7), showing efficacy of CBD in reducing seizure frequency in DS. These results are encouraging, but considering the diversity of patient age, background, and standard-of-care medications, these clinical studies do not yet provide quantitative measures of CBD’s effects on seizure generation or other comorbid symptoms of DS in the absence of potentially confounding variables.
In our experiments, the subject mice are genetically identical; they have no standard-of-care treatments; and seizure duration, frequency, and severity are measured in a blinded, objective manner. Therefore, our study provides strong preclinical evidence for CBD’s efficacy in reducing seizure frequency, as observed in DS patients (6, 7), and extends these findings by providing quantitative evidence of CBD efficacy on seizure duration and severity. These results provide further strong impetus for exploring the therapeutic benefit of CBD on the core behavioral symptoms of DS and other medically refractory epilepsy syndromes.
In DS, convulsive seizures may continue in adulthood, although their frequency and severity decreases and they become more responsive to pharmacological treatments (9). Unfortunately, DS patients in their teenage years and beyond suffer from severe cognitive deficits and autistic-like behaviors (9, 10). There are no reports of CBD’s efficacy in treating autism; however, a human clinical trial is currently underway (Identifier: NCT02956226). We found that CBD increased time spent in social interaction using two separate social behavior paradigms and reduced the frequency of defensive escape behavior. Thus, our results provide an initial indication that CBD treatment might be beneficial for treating autism-like social deficits caused by mutations in sodium channels.
A high dose of CBD (100 mg/kg) is needed to protect against seizures, while low doses (∼10–20 mg/kg) improve social behaviors in DS mice, and these beneficial effects are lost at higher doses. These findings present a conundrum for designing treatments of DS that both control seizures and improve social behavior. We found a similar discrepancy between the high doses of clonazepam required for seizure control and the low doses that are necessary for improved cognition and social behavior in DS mice and another mouse model of autism (16, 18). In that case, preferential drug effects on GABAA receptors having α2 and/or α3 subunits may be beneficial for treatment of cognitive and social interaction deficits, whereas effects on α1 subunit-containing GABAA receptors prevent seizures but induce substantial sedation. It is conceivable that the effects of low-dose CBD and high-dose CBD may also reflect actions on different molecular mechanisms. Our studies point to GPR55 as the molecular target for high doses of CBD that are effective in control of seizures.
A major benefit of studies of CBD in a well-validated animal model is the ability to probe its mechanism of action. We have taken two important steps in that direction here. First, we find that CBD rebalances the ratio of excitation to inhibition in the hippocampus of DS mice. The decrease in interneuron rheobase for generation of trains of APs may be the dominant effect because it would provide more immediate and effective response of the inhibitory neurons to increases in excitatory input. These results suggest that CBD acts on the core deficit in DS, which is failure of firing of GABAergic interneurons (13, 21). Furthermore, we find that pharmacological control over impaired inhibitory signaling can be achieved by CBD antagonism of GPR55. It has been shown that CBD antagonizes GPR55 activity in transfected cells (36), and our results extend these findings to intact neurons in brain slices. We show that antagonism of GPR55 occludes CBD’s actions and mimics CBD’s enhancement of inhibitory transmission to DGCs and reduction of spontaneous APs of DGCs. Since the reduction in excitatory transmission by CBD was blocked by the broad-spectrum GABAA antagonist GABAzine, antagonism of GPR55 by CBD may dampen spontaneous excitatory transmission by increasing inhibition. Therefore, CBD antagonism of GPR55 in the dentate gyrus likely affects inhibitory transmission directly. This mechanism of action holds much promise for future drug development aimed at GPR55 and its downstream signaling pathways. Unfortunately, the GPR55 antagonist used here (CID16020046) does not enter the central nervous system efficiently in vivo, and future studies are therefore required to assess the effect of GPR55 antagonists injected in vivo.
In addition to rescue of GABAergic signaling in DS mice, we find that CBD reduces the excitability of DGCs following high-current injection amplitudes well above rheobase, consistent with stimulus levels that may be observed during seizures. The combination of CBD’s antipodal effects on interneuron and DGC excitability (i.e., enhancement vs. reduction, respectively) may provide a mechanistic explanation for improvement in seizure characteristics by CBD treatment. By enhancing GABAergic inhibition of DGCs, CBD may reduce the likelihood of seizures occurring. However, when excitatory transmission increases during thermal induction and seizure occurrence is inevitable, CBD reduction in DGC excitability may dampen the severity of the seizure and shorten its duration. These dual actions of CBD make it a promising antiepileptic strategy in an otherwise medically resistant disorder. Coupled with its relatively low side-effect profile compared with other antiepileptic drugs (5–7), the emerging data supporting efficacy of CBD in treatment-resistant epilepsies holds great promise for improved therapy of these devastating diseases.
Materials and Methods
All procedures conform to the regulations detailed in the National Institute of Health’s Guide for the Care and Use of Laboratory Animals (40) and were approved by the Institutional Animal Care and Use Committee at the University of Washington. Scn1a mutant mice were generated (13), seizures were thermally induced (15), spontaneous seizures were measured (25), and social interaction tests were carried out (16) using previously described methods that are presented in SI Materials and Methods. Hippocampal slices were prepared and electrophysiological recordings were made using previously described methods (16, 18) that are presented in SI Materials and Methods. CBD (GW Pharmaceuticals) was dissolved in a vehicle solution (1% ethanol:1% cremophore:18% saline) and used for up to 1 wk. The SR 95531 hydrobromide (GABAzine), tetrodotoxin, and CID16020046 were from purchased from Tocris Bioscience.
SI Materials and Methods
Animals.
Scn1a mutant mice were generated by targeted deletion of exon 26 encoding domain IV from the S3 to S6 segment and the entire C-terminal tail of Nav1.1 channels on a congenic 129/SvJ background backcrossed to the C57BL/6J background as described previously (13). Heterozygous mice were crossed with C57BL/6J WT mice to yield WT and heterozygote mutant mice at a 1:1 ratio. PV Cre+/+; td-Tomato+/+ mice were generated for visual identification of PV-expressing interneurons by crossing the PV Cre mouse line (stock #008069; Jackson Labs) with a td-Tomato reporter mouse (stock #007914; Jackson Labs) line in which td-Tomato is expressed when bred to mice that express Cre recombinase.
Thermal Induction of Seizures.
Seizures were induced as previously described (15) with slight modifications. Briefly, body temperature was measured continuously with a rectal temperature probe and controlled to ±0.3 °C with a feedback temperature controller (TCAT2DF; Physitemp) and heat lamp. Male and female mice (P21–P28) were injected with CBD or vehicle 1 h before thermal induction. Mouse body temperature was held at 36.5 °C during this 1-h period while CBD reached maximum brain concentration (26) and was then elevated by 0.5 °C every 2 min until 38 °C and held for 30 min. For each mouse, the duration and severity of each seizure was determined from video recordings. Seizure severity was assessed based on the Racine Scale scoring system (46): (1) mouth and facial movements; (2) head nodding; (3) forelimb clonus, usually one limb; (4) forelimb clonus with rearing; and (5) Generalized tonic-clonic seizure, rearing, clonus, and falling. Because seizures with a severity below 3 cannot be reliably assessed in mice, we limited our seizure severity assessment to seizures with a Racine score of 3–5.
Spontaneous Seizures.
Male and female mice were injected with CBD (100 mg/kg i.p.) or vehicle at 1 PM and 5 PM daily from postnatal day 21 through 27. Spontaneous seizures were assessed from 2 PM through 9 PM. We injected mice twice daily to limit the number of stressful injection events during this vulnerable period in DS mice. We injected at 4-h intervals to sustain drug concentration in plasma at >50% of peak level for 8 h. Continuous digital videos of mice in their home cages were collected using high resolution, infrared equipment, and digital video cameras (D-link) connected to a PC workstation for data storage. The resulting video files were reviewed at 8× speed. Suspected seizures were reviewed in real time.
Social Interaction Tests.
Four- to 6-mo-old male WT mice and heterozygote littermates were used. Mice were individually housed for a minimum of 4 d before testing and maintained on a 12-h light–dark cycle (on: 7 AM, off: 7 PM). Mice were injected i.p. with vehicle or the indicated doses of CBD 1 h before testing and held in their home cage. Tests were performed as previously described in ref. 16 with minor modifications.
Open field.
Each mouse was placed near the bottom-left wall of a 40- × 40-cm open-field arena. The movement of the mouse was recorded by USB webcam (LifeCam HD-6000; Microsoft) and PC-based video capture software (WinAVI Video Capture; ZJMedia Digital Technology) for 10 min. The recorded video file was analyzed by off-line video tracking software (EthoVision XT 8.5; Noldus Technology). Total distance traveled was measured. Mean velocity through the center of the chamber was assessed by centering an analysis square within 25% of the area of the chamber and limiting velocity measurement to this region using the tracking software. The open-field arena was cleaned with 70% ethanol and wiped with paper towels between each trial.
Three-chamber test of social preference.
The apparatus is a nontransparent Plexiglas box (58 × 30 cm) with two partitions that make left, center, and right chambers (30 × 19.3 cm). Each partition has a square opening (5 × 5 cm) in the bottom center. Inverted cylindrical wire cages (10.5-cm diameter; Galaxy Pencil Cup; Spectrum Diversified Designs) were placed in opposite corners of the chamber (top left and top right) and were used as an inanimate object or to cage the stranger mouse. Cylindrical bottles filled with water were placed on top of the wire cups to prevent the test mouse from climbing on top of the cups. The wire cups and chamber were cleaned with 70% ethanol and wiped with paper towels between each test mouse. In the habituation phase, a test mouse was placed in the center of the chamber without wire cups and allowed to freely explore the three chambers for 10 min. The test mouse was then returned briefly to its home cage. For the test phase, a stranger age- and sex-matched C57BL/6J mouse was placed in one of the two wire cups; the opposite wire cup was empty. The test mouse was then returned to the center of the chamber and allowed to freely explore for 10 min. The side of the chamber with the stranger mouse was counterbalanced between cohorts. The movement of the mouse was recorded by a USB webcam and analyzed by EthoVision XT 8.5 as described above. Time spent in each chamber and time spent within a 5-cm radius proximal to each wire cage were measured.
Reciprocal interaction in the open field.
A test mouse and an age- and sex-matched stimulus mouse (identified by a black mark on tail using permanent marker) were introduced simultaneously to the open-field chamber and allowed to explore the chamber or interact for 10 min. Behavior was recorded by USB webcam and analyzed with EthoVision XT 8.5 as described above. The frequency of nose–nose contacts and anogenital sniffing was assessed manually using the event-recording function in the video-tracking software by a researcher blind to the genotype of the mice and added to obtain the total number of interactions. Escape behavior was defined as a defensive dart to the chamber wall following a social interaction that reached a minimum velocity of 8 cm/s and was not followed by a subsequent interaction for a minimum of 2 s. These behaviors were identified by the video-tracking software and confirmed by a researcher blind to the genotype of the mice.
Accelerating rotarod.
WT and DS mice were injected with either 100 mg/kg CBD or vehicle 1 h before the first test session of each of the three subsequent testing days. Subjects were placed on an accelerating rotarod (rotarod series 8; IITC Life Science), which started at 1 rpm and increased linearly to 40 rpm over 300 s. The time spent on the rotarod before falling was recorded. Each mouse was tested in three trials/day spaced 15 min apart.
Preparation of Brain Slices.
Hippocampal slices were prepared acutely each day of experiments. Male and female mice (25–40 d old) were maintained on a standard 12-h light–dark cycle. Animals were anesthetized with isoflurane and killed by decapitation. Whole brain was isolated and immersed in sucrose-based cutting solution (2°) containing the following (in mM): 87 NaCl, 1.25 NaH2PO4, 2.5 KCl, 7 MgCl2, 25 NaHCO3, 0.5 CaCl2, 25 D-glucose, 75 sucrose, and bubbled with 95% O2/5% CO2, pH 7.4. Transverse slices (400 µm) were made with a vibrating tissue slicer (Vibratome). Slices were incubated for 1 h in warmed artificial cerebrospinal fluid (aCSF) (33 ± 1 °C) containing the following (in mM): 125 NaCl, 1.25 NaH2PO4, 26 NaHCO3, 3 KCl, 10 D-glucose, 2 MgCl2, 2 CaCl2, and bubbled with 95% O2/5% CO2, pH 7.4. Kynurenic acid (1 mM) was included in the dissection, incubation, and holding solutions, but was omitted from the experimental solutions. After 1 h, slices were held in aCSF at 22–23 °C until used.
Electrophysiology.
Slices were placed in a submersion chamber on an upright microscope and viewed with an Olympus 40× (0.9 N.A.; BX51WI) water-immersion objective with differential interference contrast and infrared optics and were perfused with oxygenated aCSF (2 mL/min). DGCs were distinguished by their location, appearance, and physiological properties. PV-positive interneurons were identified by td-Tomato fluorescence. All recorded PV-positive interneurons displayed typical fast-spiking patterns. Whole-cell recordings were made using patch pipettes constructed from thick-walled borosilicate glass capillaries and filled with internal solutions optimized for current- or voltage-clamp recordings of sIPSCs or sEPSCs, as described below. Filled patch pipettes had resistances ranging between 3 and 5 MΩ. The internal solution for all current-clamp experiments was as follows (in mM): 132.3 K-gluconate, 7.7 KCI, 4 NaCl, 0.5 CaCl2, 10 Hepes, 5 EGTA free acid, 4 ATP Mg2+ salt, 0.5 GTP Na+ salt, pH buffered to 7.2–7.3 with KOH. The internal solution for measuring sIPSCs (Vh = −60 mV; ECl = 0 mV) in voltage clamp was as follows (in mM): 130 CsCl, 4 NaCl, 0.5 CaCl2, 10 Hepes, 5 EGTA, 4 ATP Mg2+ salt, 0.5 GTP Na+ salt, 5 QX-314, pH buffered to 7.2–7.3 with CsOH. sIPSCs were measured in the presence of ionotropic glutamate receptor antagonists, CNQX (20 µM) and APV (50 µM). The internal solution for measuring sEPSCs (Vh = −60 mV; ECl = −60 mV) in voltage clamp was as follows (in mM): 145 Cs-Gluconate, 2 MgCl2, 10 Hepes, 0.5 EGTA, 2 ATP-Tris, 0.2 GTP Na+ salt, pH buffered to 7.2–7.3 with CsOH. Recordings were obtained through a multiclamp 700A amplifier (Molecular Devices) by pCLAMP 8.0 software (Molecular Devices). Drugs were dissolved in aCSF and bath-applied for a minimum of 7 min before analysis. Data from electrophysiology experiments were analyzed using Clampfit 9.0 (Molecular Devices) software. Access resistance was continuously monitored for each cell. Cells were discarded if access resistance changed by >15%. Only one cell was recorded from each brain slice.
Statistics.
For assessment of electrophysiological and behavioral experiments, all data are expressed as mean ± SEM. Single-sample t tests and independent-sample t tests were used where indicated. For within-subjects assessments, paired t tests and repeated-measures ANOVA were used when appropriate. In the cases when ANOVA revealed significant main effects or interactions, we conducted post hoc comparisons using the Student–Newman–Keuls method. In all cases, statistical comparisons were two-tailed, and the significance threshold was set at P < 0.05. For figures, *P < 0.05; **P < 0.01; ***P < 0.001.
Acknowledgments
We thank Dr. Ben Whalley (University of Reading), Dr. Orrin Devinsky (New York University), and scientists at GW Pharmaceuticals for valuable discussions that led to our development of this research project; GW Pharmaceuticals for a generous supply of CBD; Jeremy Bazinet (University of Washington) for early experiments on thermal induction of seizures; and Kate Swinney (University of Washington) for help with CBD supply and recordkeeping. We acknowledge generous research support from the Alcohol and Drug Abuse Institute at the University of Washington (Grant ADAI-1016-12 to J.S.K.); Citizens United for Cure of Epilepsy (CURE) (R.E.W.); the NIH National Institute of Neurological Disorders and Stroke (Grant R01 NS025704 to W.A.C.); and the NIH National Institute on Drug Abuse (Grant R01 DA026430 to N.S.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1711351114/-/DCSupplemental.
References
- 1.Fairman BJ. Trends in registered medical marijuana participation across 13 US states and District of Columbia. Drug Alcohol Depend. 2016;159:72–79. doi: 10.1016/j.drugalcdep.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whiting PF, et al. Cannabinoids for medical use: A systematic review and meta-analysis. JAMA. 2015;313:2456–2473. doi: 10.1001/jama.2015.6358. [DOI] [PubMed] [Google Scholar]
- 3.Jones NA, et al. Cannabidiol exerts anti-convulsant effects in animal models of temporal lobe and partial seizures. Seizure. 2012;21:344–352. doi: 10.1016/j.seizure.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Hill TD, et al. Cannabidivarin-rich cannabis extracts are anticonvulsant in mouse and rat via a CB1 receptor-independent mechanism. Br J Pharmacol. 2013;170:679–692. doi: 10.1111/bph.12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devinsky O, et al. Cannabidiol: Pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia. 2014;55:791–802. doi: 10.1111/epi.12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Devinsky O, et al. Cannabidiol in patients with treatment-resistant epilepsy: An open-label interventional trial. Lancet Neurol. 2016;15:270–278. doi: 10.1016/S1474-4422(15)00379-8. [DOI] [PubMed] [Google Scholar]
- 7.Devinsky O, et al. Cannabidiol in Dravet Syndrome Study Group Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med. 2017;376:2011–2020. doi: 10.1056/NEJMoa1611618. [DOI] [PubMed] [Google Scholar]
- 8.Dravet C. The core Dravet syndrome phenotype. Epilepsia. 2011;52:3–9. doi: 10.1111/j.1528-1167.2011.02994.x. [DOI] [PubMed] [Google Scholar]
- 9.Genton P, Velizarova R, Dravet C. Dravet syndrome: The long-term outcome. Epilepsia. 2011;52:44–49. doi: 10.1111/j.1528-1167.2011.03001.x. [DOI] [PubMed] [Google Scholar]
- 10.Berkvens JJ, et al. Autism and behavior in adult patients with Dravet syndrome (DS) Epilepsy Behav. 2015;47:11–16. doi: 10.1016/j.yebeh.2015.04.057. [DOI] [PubMed] [Google Scholar]
- 11.Barahmand U, Haji A. The impact of intolerance of uncertainty, worry and irritability on quality of life in persons with epilepsy: Irritability as mediator. Epilepsy Res. 2014;108:1335–1344. doi: 10.1016/j.eplepsyres.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Gallop K, Wild D, Nixon A, Verdian L, Cramer JA. Impact of Lennox-Gastaut Syndrome (LGS) on health-related quality of life (HRQL) of patients and caregivers: Literature review. Seizure. 2009;18:554–558. doi: 10.1016/j.seizure.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Yu FH, et al. Reduced sodium current in GABAergic interneurons in a mouse model of severe myoclonic epilepsy in infancy. Nat Neurosci. 2006;9:1142–1149. doi: 10.1038/nn1754. [DOI] [PubMed] [Google Scholar]
- 14.Ogiwara I, et al. Nav1.1 localizes to axons of parvalbumin-positive inhibitory interneurons: A circuit basis for epileptic seizures in mice carrying an Scn1a gene mutation. J Neurosci. 2007;27:5903–5914. doi: 10.1523/JNEUROSCI.5270-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oakley JC, Kalume F, Yu FH, Scheuer T, Catterall WA. Temperature- and age-dependent seizures in a mouse model of severe myoclonic epilepsy in infancy. Proc Natl Acad Sci USA. 2009;106:3994–3999. doi: 10.1073/pnas.0813330106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han S, et al. Autistic-like behaviour in Scn1a+/- mice and rescue by enhanced GABA-mediated neurotransmission. Nature. 2012;489:385–390. doi: 10.1038/nature11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ito S, et al. Mouse with Nav1.1 haploinsufficiency, a model for Dravet syndrome, exhibits lowered sociability and learning impairment. Neurobiol Dis. 2013;49:29–40. doi: 10.1016/j.nbd.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Han S, Tai C, Jones CJ, Scheuer T, Catterall WA. Enhancement of inhibitory neurotransmission by GABAA receptors having α2,3-subunits ameliorates behavioral deficits in a mouse model of autism. Neuron. 2014;81:1282–1289. doi: 10.1016/j.neuron.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalume F, et al. Sleep impairment and reduced interneuron excitability in a mouse model of Dravet Syndrome. Neurobiol Dis. 2015;77:141–154. doi: 10.1016/j.nbd.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mistry AM, et al. Strain- and age-dependent hippocampal neuron sodium currents correlate with epilepsy severity in Dravet syndrome mice. Neurobiol Dis. 2014;65:1–11. doi: 10.1016/j.nbd.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheah CS, et al. Specific deletion of NaV1.1 sodium channels in inhibitory interneurons causes seizures and premature death in a mouse model of Dravet syndrome. Proc Natl Acad Sci USA. 2012;109:14646–14651. doi: 10.1073/pnas.1211591109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogiwara I, et al. Nav1.1 haploinsufficiency in excitatory neurons ameliorates seizure-associated sudden death in a mouse model of Dravet syndrome. Hum Mol Genet. 2013;22:4784–4804. doi: 10.1093/hmg/ddt331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oakley JC, Cho AR, Cheah CS, Scheuer T, Catterall WA. Synergistic GABA-enhancing therapy against seizures in a mouse model of Dravet syndrome. J Pharmacol Exp Ther. 2013;345:215–224. doi: 10.1124/jpet.113.203331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson LL, Hawkins NA, Thompson CH, Kearney JA, George AL., Jr Unexpected efficacy of a novel sodium channel modulator in Dravet syndrome. Sci Rep. 2017;7:1682. doi: 10.1038/s41598-017-01851-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalume F, et al. Sudden unexpected death in a mouse model of Dravet syndrome. J Clin Invest. 2013;123:1798–1808. doi: 10.1172/JCI66220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deiana S, et al. Plasma and brain pharmacokinetic profile of cannabidiol (CBD), cannabidivarine (CBDV), Δ9-tetrahydrocannabivarin (THCV) and cannabigerol (CBG) in rats and mice following oral and intraperitoneal administration and CBD action on obsessive-compulsive behaviour. Psychopharmacology (Berl) 2012;219:859–873. doi: 10.1007/s00213-011-2415-0. [DOI] [PubMed] [Google Scholar]
- 27.Ohlsson A, et al. Single-dose kinetics of deuterium-labelled cannabidiol in man after smoking and intravenous administration. Biomed Environ Mass Spectrom. 1986;13:77–83. doi: 10.1002/bms.1200130206. [DOI] [PubMed] [Google Scholar]
- 28.Mandillo S, et al. Reliability, robustness, and reproducibility in mouse behavioral phenotyping: A cross-laboratory study. Physiol Genomics. 2008;34:243–255. doi: 10.1152/physiolgenomics.90207.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crawley JN. Translational animal models of autism and neurodevelopmental disorders. Dialogues Clin Neurosci. 2012;14:293–305. doi: 10.31887/DCNS.2012.14.3/jcrawley. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krook-Magnuson E, et al. In vivo evaluation of the dentate gate theory in epilepsy. J Physiol. 2015;593:2379–2388. doi: 10.1113/JP270056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dengler CG, Coulter DA. Normal and epilepsy-associated pathologic function of the dentate gyrus. Prog Brain Res. 2016;226:155–178. doi: 10.1016/bs.pbr.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee CT, et al. Causal evidence for the role of specific GABAergic interneuron types in entorhinal recruitment of dentate granule cells. Sci Rep. 2016;6:36885. doi: 10.1038/srep36885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel RR, Barbosa C, Brustovetsky T, Brustovetsky N, Cummins TR. Aberrant epilepsy-associated mutant Nav1.6 sodium channel activity can be targeted with cannabidiol. Brain. 2016;139:2164–2181. doi: 10.1093/brain/aww129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mechoulam R, Fride E, Di Marzo V. Endocannabinoids. Eur J Pharmacol. 1998;359:1–18. doi: 10.1016/s0014-2999(98)00649-9. [DOI] [PubMed] [Google Scholar]
- 35.Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199–215. doi: 10.1038/sj.bjp.0707442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ryberg E, et al. The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol. 2007;152:1092–1101. doi: 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sylantyev S, Jensen TP, Ross RA, Rusakov DA. Cannabinoid- and lysophosphatidylinositol-sensitive receptor GPR55 boosts neurotransmitter release at central synapses. Proc Natl Acad Sci USA. 2013;110:5193–5198. doi: 10.1073/pnas.1211204110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hurst K, et al. A putative lysophosphatidylinositol receptor GPR55 modulates hippocampal synaptic plasticity. Hippocampus. 2017;27:985–998. doi: 10.1002/hipo.22747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kargl J, et al. A selective antagonist reveals a potential role of G protein-coupled receptor 55 in platelet and endothelial cell function. J Pharmacol Exp Ther. 2013;346:54–66. doi: 10.1124/jpet.113.204180. [DOI] [PubMed] [Google Scholar]
- 40.National Research Council . Guide for the Care and Use of Laboratory Animals. 8th Ed National Academies Press; Washington, DC: 2011. [Google Scholar]
- 41.Kalume F, Yu FH, Westenbroek RE, Scheuer T, Catterall WA. Reduced sodium current in Purkinje neurons from Nav1.1 mutant mice: Implications for ataxia in severe myoclonic epilepsy in infancy. J Neurosci. 2007;27:11065–11074. doi: 10.1523/JNEUROSCI.2162-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rubinstein M, et al. Genetic background modulates impaired excitability of inhibitory neurons in a mouse model of Dravet syndrome. Neurobiol Dis. 2015;73:106–117. doi: 10.1016/j.nbd.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hawkins NA, Zachwieja NJ, Miller AR, Anderson LL, Kearney JA. Fine mapping of a Dravet syndrome modifier locus on mouse chromosome 5 and candidate gene analysis by RNA-seq. PLoS Genet. 2016;12:e1006398. doi: 10.1371/journal.pgen.1006398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rubinstein M, et al. Dissecting the phenotypes of Dravet syndrome by gene deletion. Brain. 2015;138:2219–2233. doi: 10.1093/brain/awv142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Straiker A, Mitjavila J, Yin D, Gibson A, Mackie K. Aiming for allosterism: Evaluation of allosteric modulators of CB1 in a neuronal model. Pharmacol Res. 2015;99:370–376. doi: 10.1016/j.phrs.2015.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]