Significance
The mammalian gastrointestinal tract is home to diverse communities of bacteria that contribute to the metabolic health of their hosts. The epithelial lining of the intestine produces a diverse repertoire of antimicrobial proteins that limit the ability of these microorganisms to enter host tissues and cause disease. We have discovered that resistin-like molecule β (RELMβ) is a previously unknown member of the intestine's antibacterial arsenal. RELMβ is secreted from the intestinal surface and kills Gram-negative bacteria by damaging their membranes, thereby preventing these bacteria from coming into close contact with host tissues. Our findings reveal a new family of endogenous antibiotic proteins and contribute to the understanding of how mammals maintain mutually beneficial relationships with complex communities of intestinal bacteria.
Keywords: antibacterial protein, microbiota, innate immunity, intestinal epithelium
Abstract
The mammalian intestine is colonized by trillions of bacteria that perform essential metabolic functions for their hosts. The mutualistic nature of this relationship depends on maintaining spatial segregation between these bacteria and the intestinal epithelial surface. This segregation is achieved in part by the presence of a dense mucus layer at the epithelial surface and by the production of antimicrobial proteins that are secreted by epithelial cells into the mucus layer. Here, we show that resistin-like molecule β (RELMβ) is a bactericidal protein that limits contact between Gram-negative bacteria and the colonic epithelial surface. Mouse and human RELMβ selectively killed Gram-negative bacteria by forming size-selective pores that permeabilized bacterial membranes. In mice lacking RELMβ, Proteobacteria were present in the inner mucus layer and invaded mucosal tissues. Another RELM family member, human resistin, was also bactericidal, suggesting that bactericidal activity is a conserved function of the RELM family. Our findings thus identify the RELM family as a unique family of bactericidal proteins and show that RELMβ promotes host–bacterial mutualism by regulating the spatial segregation between the microbiota and the intestinal epithelium.
The trillions of microbes that colonize the mammalian gut are in a mutually beneficial nutrient-sharing relationship with their hosts (1). The intestinal epithelial barrier plays an essential role in ensuring that the mutualistic nature of this interaction is maintained. A key element of this barrier is a thick covering of mucus that overlies the epithelial surface and is organized into distinct inner and outer layers (2). Commensal microorganisms are abundant in the outer mucus layer but are largely excluded from the inner layer (3). This ensures that bacteria are spatially segregated from the intestinal epithelial surface, thus limiting their ability to invade host tissues and cause disease.
Antibacterial proteins are essential for enforcing this spatial segregation. For example, RegIIIγ is a bactericidal protein produced by intestinal epithelial cells that maintains physical separation between Gram-positive bacteria and the small intestinal epithelium (4). Other secreted proteins limit access of bacteria to the inner mucus layer of the colon, including Ly6/PLAUR domain containing 8 (Lypd8), which binds to flagellated bacteria (5), and zymogen granulae protein 16 (ZG16), which aggregates Gram-positive bacteria (6). The diversity of antimicrobial mechanisms required to maintain spatial segregation likely reflects the taxonomic complexity of the microbiota. Because of this complexity, we still have an incomplete understanding of the antimicrobial mechanisms that contribute to spatial segregation of microbiota and host.
Resistin-like molecule β (RELMβ) belongs to the RELM protein family, which also includes RELMα, RELMγ, and resistin. RELMβ is produced predominantly by colon goblet cells (7), is induced by the microbiota, and is markedly up-regulated during intestinal inflammation (8, 9). Initially, both RELMβ and resistin were characterized as hormones that modulate insulin action (10, 11). However, subsequent studies revealed that RELMβ also plays a role in several aspects of host defense, including protection against infection by parasitic nematodes and Citrobacter rodentium (7, 12). Although this has been attributed to cytokine-like activities of RELMβ, the mechanistic basis for RELMβ’s contributions to host defense remains unclear.
Here, we show that RELMβ kills Gram-negative bacteria. RELMβ binds to bacterial lipids and forms a membrane-permeabilizing pore that lyses the targeted bacterial cells. In mice lacking RELMβ, Proteobacteria are more abundant in the inner mucus layer of the colon, indicating that RELMβ is essential for maintaining spatial segregation of the intestinal microbiota. Human resistin can also disrupt microbial membranes and kill bacteria, suggesting that bactericidal activity is a conserved function of the RELM family. Thus, we identify RELM proteins as a previously unknown family of bactericidal proteins and provide essential insight into the mechanisms that separate the microbiota from the intestinal epithelium.
Results
RELMβ Is a Bactericidal Protein That Targets Gram-Negative Bacteria.
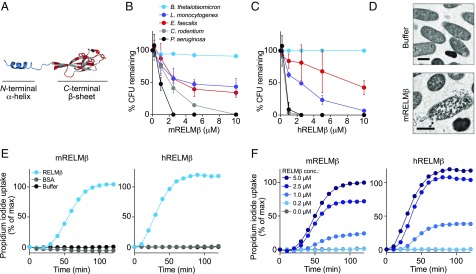
We began to consider whether RELMβ might be an antibacterial protein when we noted structural features that are consistent with an antimicrobial function. First, the predicted pI is 8.5 and therefore basic. This is a common characteristic of bactericidal proteins that target bacterial membranes, which are negatively charged (13). Second, the 3D structure of RELMβ (14) features a β-sheet–rich head region containing several aromatic residues that form a hydrophobic surface (Fig. 1A). Similar hydrophobic regions are a feature of several proteins that permeabilize membranes, such as RegIIIα (15), Bcl-2 (16), and Staphylococcus aureus α-toxin (17), where the hydrophobic residues are involved in contacting membrane lipids and driving membrane insertion.
Fig. 1.
RELMβ is a bactericidal protein that targets Gram-negative bacteria. (A) Crystal structure of mRELMβ (PDB ID code 1RH7) (14) showing the N-terminal α-helix and the C-terminal β-sheet with its aromatic residues. (B and C) RELMβ bactericidal activity. Purified recombinant mouse RELMβ (mRELMβ) (B) or human RELMβ (hRELMβ) (C) were added to midlogarithmic phase bacteria for 2 h, and numbers of surviving bacteria were quantified by dilution plating. Means ± SD are plotted. (D) Transmission electron microscopy of P. aeruginosa following a 2-h exposure to purified recombinant mRELMβ. (Scale bar: 0.5 μm.) (E) RELMβ permeabilizes bacterial membranes. C. rodentium was treated with 5 μM mRELMβ, hRELMβ, or BSA, and PI uptake was measured over 2 h. (F) PI uptake by C. rodentium in the presence of increasing concentrations of mRELMβ or hRELMβ. Assays were performed at least twice and repeated in triplicate within each experiment.
To test RELMβ for bactericidal activity, we produced recombinant mouse and human RELMβ in Escherichia coli and purified folded, monomeric protein (Fig. S1). We added the purified proteins to a panel of enteric bacteria that included both Gram-positive and Gram-negative species (Fig. 1 B and C). We observed a marked dose-dependent reduction in the viability of the Gram-negative bacteria Pseudomonas aeruginosa and Citrobacter rodentium (>99% decline in viability after a 2-h exposure to 10 μM RELMβ), but not Bacteroides thetaiotaomicron. The viability of the Gram-positive species Listeria monocytogenes and Enterococcus faecalis also declined, but less markedly (∼50% decline in viability after a 2-h exposure to 10 μM mouse RELMβ) (Fig. 1 B and C). Dimeric RELMβ retained bactericidal efficacy (Fig. S2 A and B), supporting the idea that the dimeric secreted form of RELMβ (8) is also bactericidal. Thus, mouse and human RELMβ have bactericidal activity against Gram-negative bacteria, and the effective antibacterial concentrations of both proteins are similar to those of other intestinal antimicrobial proteins (18–20).
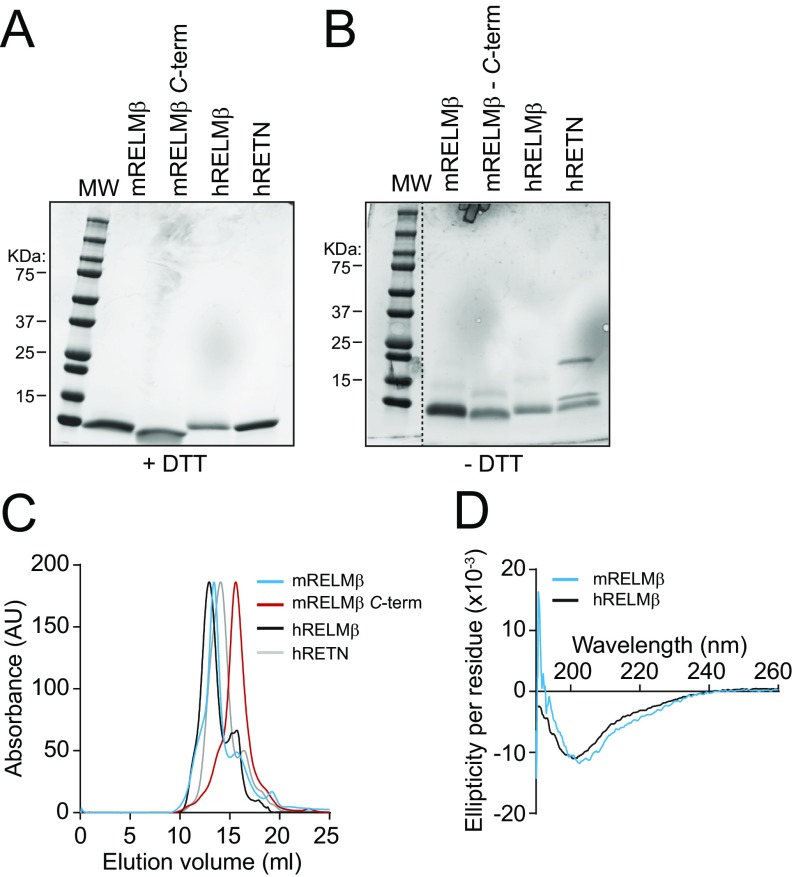
Fig. S1.
Expression and purification of recombinant RELM family proteins. (A and B) SDS/PAGE analysis of mRELMβ, mRELMβ C terminus, hRELMβ, and hRETN under reducing (A, +DTT) and nonreducing (B, −DTT) conditions. (C) Size exclusion chromatography of recombinant RELMs on a Superdex 75/300 column. (D) Circular dichroism (CD) spectroscopy of mRELMβ and hRELMβ. The CD spectra for both proteins exhibit maximal negative ellipticity in the range of 205–215 nm, indicating a predominantly β-sheet structure that is consistent with previously published spectra of members of this family (36) and with the RELMβ crystal structure (14). These results indicate that the proteins have acquired their expected secondary structures and are thus correctly refolded.
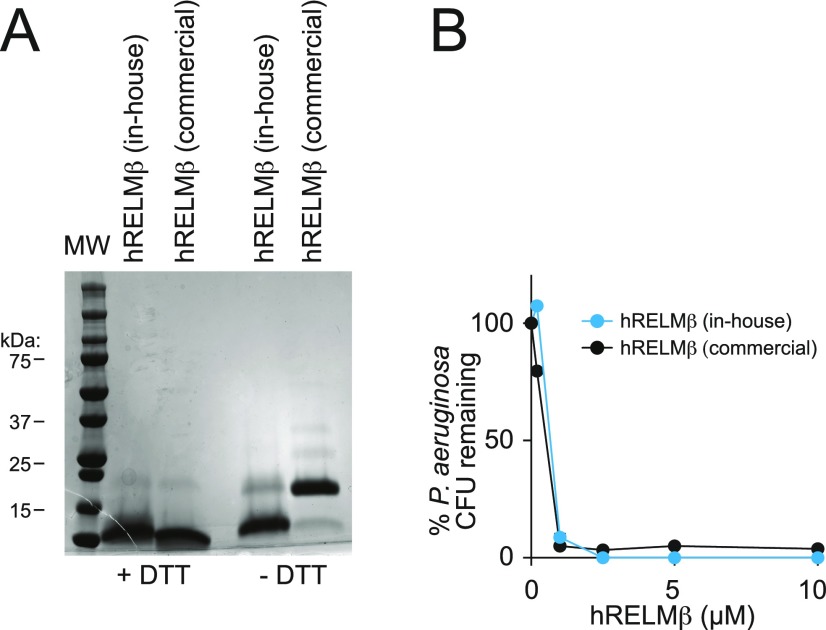
Fig. S2.
Dimeric hRELMβ has antibacterial activity. (A) SDS/PAGE analysis of hRELMβ produced in-house and commercially available hRELMβ (Shenandoah Biotechnology) under reducing (−DTT) and nonreducing (+DTT) conditions. Under nonreducing conditions, hRELMβ produced in-house migrates as a monomer while commercially available hRELMβ migrates as a dimer. (B) Antibacterial activity of monomeric hRELMβ and commercially available, dimeric hRELMβ against P. aeruginosa. Bactericidal assays were performed at least twice and repeated in triplicate within each experiment.
We used transmission electron microscopy to visualize morphological changes in P. aeruginosa cells after exposure to mouse RELMβ. The images showed evidence of cell wall damage and cytoplasmic leakage (Fig. 1D). These findings are similar to those obtained with the antibacterial proteins RegIIIγ (20) and human β-defensin-3 (18), which both kill bacteria by membrane permeabilization, suggesting that RELMβ might kill bacteria through a similar mechanism.
We assessed the capacity of RELMβ to permeabilize bacterial membranes by quantifying bacterial uptake of the membrane impermeant dye propidium iodide (PI). Mouse and human RELMβ increased PI uptake into C. rodentium in a dose-dependent manner, (Fig. 1 E and F). Thus, RELMβ permeabilizes the bacterial membrane, suggesting a mechanism for its bactericidal activity.
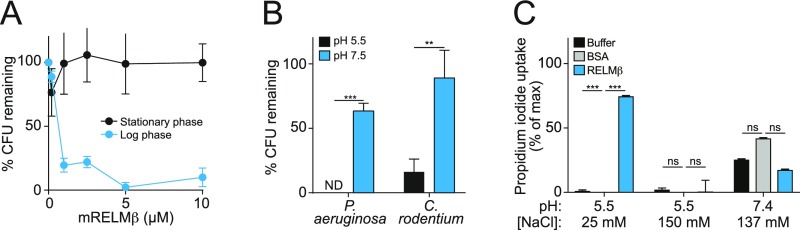
Interestingly, the bactericidal activity of RELMβ was dependent on the growth phase of the target bacteria, as C. rodentium grown to midlogarithmic phase was more readily killed than stationary-phase bacteria (Fig. S3A). Additionally, bactericidal activity and PI uptake required low salt concentrations and an acidic pH (Fig. S3 B and C). This is consistent with the salt and pH sensitivity of other antimicrobial peptides (21) and likely reflects the fact that such antibacterial proteins have evolved to function in the acidic, low-salt environment that is present in the mucus layer (22). The requirement for these specialized conditions might also explain why RELMβ was previously reported to lack antibacterial activity (12).
Fig. S3.
Characterization of mRELMβ antibacterial activity. (A) mRELMβ antibacterial activity is dependent on bacterial growth phase. C. rodentium was grown to either midlogarithmic phase or stationary phase before the addition of mRELMβ with incubation for 2 h at 37 °C, followed by dilution plating. (B) mRELMβ antibacterial activity is pH dependent, with higher activity at acidic pH (5.5) than at slightly basic pH (7.5). Bacteria were incubated with 5 μM mRELMβ. (C) Low pH (5.5) and a relatively low salt concentration (25 mM NaCl) are optimal for bacterial membrane permeabilization by mRELMβ. C. rodentium was treated with 5 μM mRELMβ, and PI uptake was measured over 2 h. Assays were performed at least twice and repeated in triplicate within each experiment. Means ± SD are plotted. Statistics were performed with Student’s t test; **P < 0.01; ***P < 0.001; ns, not significant.
RELMβ Binds to Negatively Charged Lipids and Forms a Multimeric Pore in Membranes.
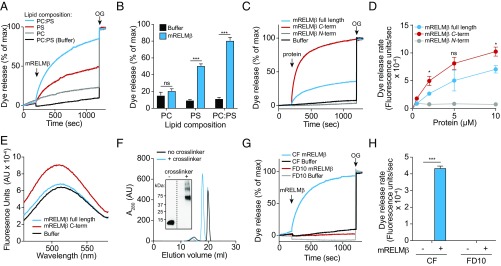
The ability of RELMβ to permeabilize bacterial membranes suggested that it might bind bacterial lipids. We tested this idea by performing an initial screen using membranes displaying various lipids. We found that RELMβ binds to lipids bearing negatively charged lipid head groups, but not to zwitterionic or neutral lipids (Fig. S4A). To determine whether lipid charge is important for RELMβ membrane permeabilization activity, we performed liposome disruption assays on liposomes having varying lipid composition. The liposomes encapsulated carboxyfluorescein (CF), a self-quenching dye that fluoresces upon dilution. RELMβ induced rapid dye efflux from liposomes composed of both phosphatidylcholine (PC), a zwitterionic phospholipid, and phosphatidylserine (PS), an acidic phospholipid (Fig. 2 A and B). The rate of efflux was reduced when PC-only liposomes were used (Fig. 2 A and B), indicating a preference for acidic phospholipids. Liposomes composed of PS alone also yielded a reduced rate of dye efflux, suggesting that charge density is an important factor for RELMβ membrane-disrupting activity, a characteristic shared with other cationic antimicrobial proteins (23, 24). Thus, RELMβ preferentially permeabilizes negatively charged lipid membranes, consistent with the salt sensitivity of RELMβ bactericidal activity (Fig. S3C), and with the acidic lipid content of bacterial membranes (13).
Fig. S4.
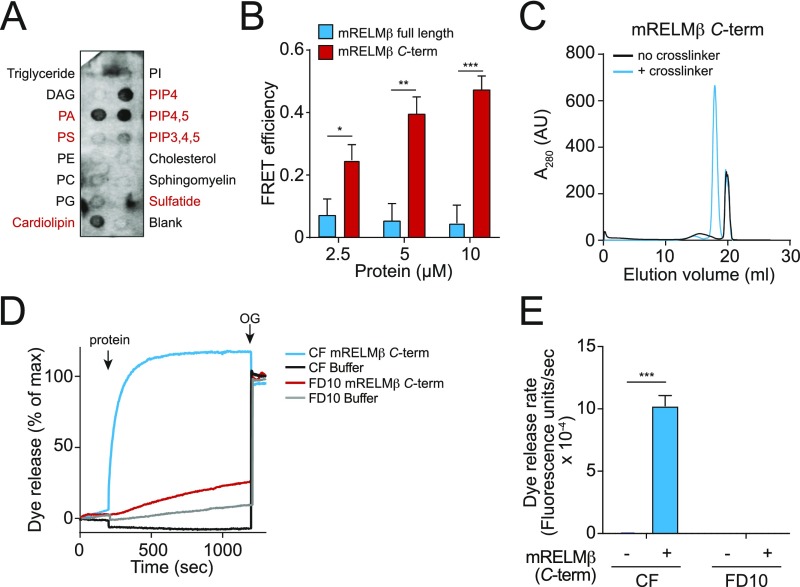
Characterization of mRELMβ lipid binding and membrane permeabilization activities. (A) mRELMβ binds to negatively charged lipids (indicated in red). Membranes displaying lipids were incubated with 1 μg/mL mRELMβ, followed by detection with anti-RELMβ antibody. (B) FRET efficiency as a function of mRELMβ full-length and mRELMβ C terminus concentration. Assays were performed in triplicate, and means ± SD are plotted. (C) The mRELMβ C terminus forms a multimer in the presence of liposomes. The mRELMβ C terminus was incubated with 100 mM PC:PS liposomes and cross-linked with bis(sulfosuccinimdyl) suberate. Cross-linked complexes were solubilized in detergent and resolved by size exclusion chromatography. (D) The mRELMβ C terminus forms size-selective pores in liposomes. The 10 μM full-length mRELMβ was added to 100 µM PC:PS liposomes loaded with carboxyfluorescein (CF) (∼10-Å Stokes diameter) or fluorescein isothiocyanate-dextran 10 (FD10) (∼44-Å Stokes diameter), and dye efflux was measured. The 1.0% octyl glucoside (OG) was added toward the end to disrupt remaining liposomes. Dye efflux is expressed as a percentage of maximal release by OG. (E) Means ± SD from three independent replicates of the experiment shown in D. Statistics were performed with Student’s t test; *P < 0.05; **P < 0.01; ***P < 0.001.
Fig. 2.
RELMβ binds to negatively charged lipids and forms a multimeric pore in membranes. (A) mRELMβ disrupts carboxyfluorescein (CF)-loaded unilamellar liposomes containing the negatively charged lipid phosphatidyl serine (PS), but not liposomes composed of the zwitterionic lipid phosphatidylcholine (PC). Liposomes were treated with 5 μM mRELMβ, and dye efflux was monitored over time. The 1.0% octyl glucoside (OG) was added toward the end to disrupt remaining liposomes. Dye efflux is expressed as a percentage of maximal release by OG. (B) Means ± SD from three independent replicates of the experiment shown in A. (C) mRELMβ membrane-disrupting activity is confined to the C terminus. PC:PS liposomes (100 µM) were incubated with 5 μM full-length mRELMβ or the mRELMβ N or C terminus. (D) Initial rate of liposome dye efflux as a function of mRELMβ concentration. Assays were done in triplicate, means ± SD are shown, and statistical significance was calculated relative to the mRELMβ C terminus. (E) The C-terminal portion of mRELMβ binds lipid. The 5 μM full-length mRELMβ or the mRELMβ N or C terminus was added to liposomes incorporating 5% dansyl-PE, and dansyl fluorescence was monitored as measure of binding. (F and G) mRELMβ forms a multimeric complex in the presence of liposomes. Full-length mRELMβ was incubated with 100 mM PC:PS liposomes and cross-linked with bis(sulfosuccinimdyl) suberate. Cross-linked complexes were solubilized in detergent, resolved by size exclusion chromatography (F), and analyzed by Western blotting with anti-RELMβ antibody (F, Inset). mRELMβ forms a complex of ∼60–70 kDa, or roughly six to eight protein units. (G) mRELMβ forms size-selective pores in liposomes. The 10 μM full-length mRELMβ was added to 100 μM PC:PS liposomes loaded with carboxyfluorescein (CF) (∼10-Å Stokes diameter) or fluorescein isothiocyanate-dextran 10 (FD10) (∼44-Å Stokes diameter). (H) Means ± SD from three independent replicates of the experiment shown in G. Statistics were performed with Student’s t test; *P < 0.05; ***P < 0.001; ns, not significant.
The crystal structure of mRELMβ reveals two distinct domains: an α-helix at the N terminus and a C-terminal β-sheet structure having a cluster of aromatic residues (14) (Fig. 1A). To determine which domain of mRELMβ drives membrane permeabilization, we synthesized a peptide representing the N-terminal α-helix and expressed a recombinant mRELMβ C terminus. When added to PC/PS liposomes, the mRELMβ C terminus yielded a dye efflux rate that exceeded that of full-length mRELMβ, while the mRELMβ N terminus resulted in virtually no dye release (Fig. 2 C and D). This finding was supported by measurements of mRELMβ lipid binding activity in which we measured fluorescence resonance energy transfer (FRET) between mRELMβ tryptophan residues and dansyl-labeled PC/PS liposomes (15). The mRELMβ C terminus produced greater FRET than full-length mRELMβ (Fig. 2E and Fig. S4B), supporting the idea that the C terminus drives mRELMβ–membrane interactions.
We next sought to gain insight into the mechanism by which RELMβ permeabilizes bacterial membranes. The intestinal bactericidal protein RegIIIα is a membrane-permeabilizing protein that forms a hexameric transmembrane pore (15). To determine whether mRELMβ also forms multimers in the presence of membranes, we added the purified monomeric protein to liposomes in the presence of the cross-linking agent bis(sulfosuccinimidyl)suberate. After solubilizing the products in detergent and separating them by size exclusion chromatography, we observed a product that migrated at a lower retention volume compared with the non–cross-linked monomer peak (Fig. 2F). The ability to form multimers was retained by the mRELMβ C terminus, supporting the importance of the C terminus in mediating interactions with lipid bilayers (Fig. S4C). Western blotting of the cross-linked protein showed a mobility of ∼60–70 kDa (Fig. 2F, Inset). Given the predicted molecular weight of monomeric RELMβ (8.8 kDa), this suggests that the multimeric membrane-associated mRELMβ assembly is composed of six to eight mRELMβ subunits.
To further define the functional properties of membrane-associated RELMβ, we loaded PC/PS liposomes with fluorescent dyes having different Stokes diameters. Both full-length mRELMβ and the mRELMβ C terminus triggered rapid dye efflux in liposomes loaded with CF (∼10-Å Stokes diameter), but not liposomes loaded with fluorescein isothiocyanate-dextran 10 (FD10) (∼44-Å Stokes diameter) (Fig. 2 G and H and Fig. S4 D and E). This indicates that mRELMβ forms size-selective transmembrane pores.
RELMβ Limits Entry of Gram-Negative Bacteria into the Colon Inner Mucus Layer.
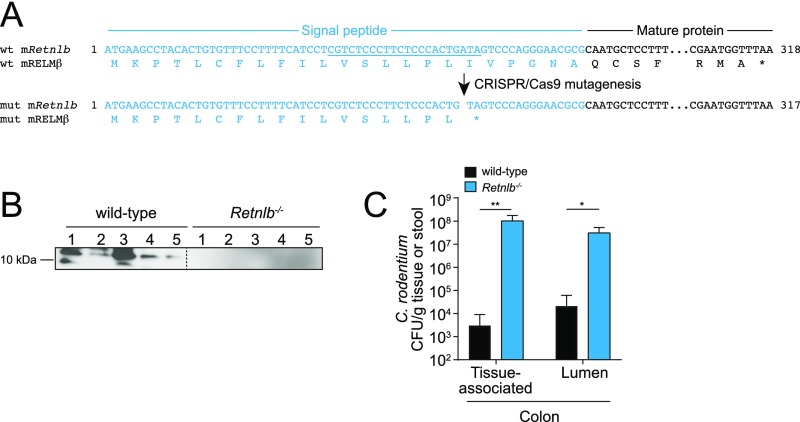
Our finding of a bactericidal function for RELMβ suggested that RELMβ might be involved in regulating microbiota composition and/or restricting host–bacterial contact in vivo. To test this idea, we used CRISPR/Cas9-mediated targeting to generate a frameshift mutation in the mouse Retnlb gene (encoding RELMβ) that produced a premature stop codon within the RELMβ signal sequence (Fig. S5A). We verified that mRELMβ was absent in the colons of Retnlb−/− mice (Fig. S5B) and showed that C. rodentium infection led to higher numbers of tissue-associated bacteria in the absence of RELMβ (Fig. S5C), as previously reported (12).
Fig. S5.
Generation of Retnlb−/− mice. (A) CRISPR/Cas mutagenesis was used to introduce edits into the mouse Retnlb exon 2 sequence encoding the RELMβ signal peptide (the guide RNA-targeted sequence is underlined). A mutation was selected that introduced a premature stop codon into the RELMβ signal peptide, and mice harboring this mutation were bred to homozygosity. (B) RELMβ is not expressed in colons from Retnlb−/− mice. Mice were orally infected with C. rodentium, and then colons were analyzed for RELMβ expression by Western blot. Each lane represents a different mouse. (C) Bacterial counts from C. rodentium-infected wild-type and Retnlb−/− colons. Mice were orally infected for 7 d. Assays were performed in triplicate, and means ± SD are shown. Statistics were performed with Student’s t test; *P < 0.05; **P < 0.01.
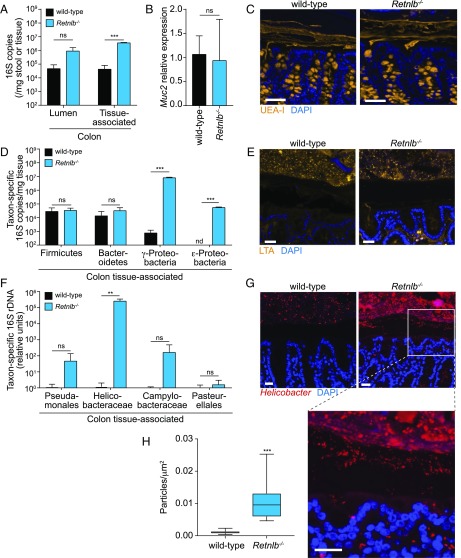
Other intestinal antibacterial proteins, including RegIIIγ, Lypd8, and ZG16, limit contact between intestinal bacteria and the intestinal epithelial surface, thus enforcing spatial segregation of microbiota and host (4–6). We therefore compared bacterial loads in the intestines of cocaged wild-type and Retnlb−/− mice by quantitative PCR (Q-PCR) determination of total 16S rRNA gene copy number. Bacterial loads in the colonic lumen trended higher in the Retnlb−/− mice, although the difference was not statistically significant. However, there was a significant two-log increase in the numbers of colonic tissue-associated bacteria in Retnlb−/− compared with wild-type mice (Fig. 3A). No significant differences were observed in either total luminal or tissue-associated bacteria in the small intestine (Fig. S6A), consistent with the lower abundance of RELMβ in the small intestine compared with the colon (11). The increase in colonic tissue-associated bacteria was unlikely to result from an altered mucus barrier, as Retnlb−/− mice did not show reduced expression of Muc2, which encodes a key mucus protein (3) (Fig. 3B), and the thickness of the mucus layer was not altered (Fig. 3C). Thus, RELMβ limits the association of bacteria with colonic tissues.
Fig. 3.
RELMβ limits entry of Gram-negative bacteria into the colon inner mucus layer. (A) Quantification of total colonic luminal and tissue-associated bacteria by Q-PCR determination of 16S rRNA gene copy number in cohoused wild-type and RELMβ-deficient (Retnlb−/−) mice. (B and C) MUC2 expression is not altered in RELMβ-deficient mice. (B) Q-PCR analysis of colonic Muc2 transcripts. (C) Immunofluorescence detection of the mucus layer in colons of wild-type and Retnlb−/− mice with Ulex europaeus agglutinin-I (UEA-I), which detects mucus glycans (34). (Scale bars: 50 µm.) (D) Q-PCR quantification of 16S gene copy number from specific bacterial groups. Bacteria were recovered from colonic tissue and analyzed using taxon-specific 16S rDNA primers. (E) Immunofluorescence detection of lipoteichoic acid (LTA) in colonic tissues indicates that spatial segregation of Gram-positive bacteria is not markedly impacted by RELMβ deficiency. (F) Q-PCR quantification of specific bacterial groups at the colonic mucosal surface. Values for each bacterial group are expressed relative to 16S rDNA levels in wild-type mice. (G) Immunofluorescence detection of Helicobacter species at the colon surface. (H) Helicobacter+ particles per square micrometer in the colon inner mucus layer. Quantification of particle density was performed using ImageJ from five fluorescent images from three mice of each genotype. For the 16S analyses, four mice per genotype were analyzed for each experiment, and Q-PCR assays were repeated in triplicate within each experiment. Means ± SD are plotted. Statistics were performed with Student’s t test; **P < 0.01; ***P < 0.001; ns, not significant. All tissues were counterstained with DAPI (blue), and antibody isotype controls are shown in Fig. S8. (Scale bars: 25 µm.)
Fig. S6.
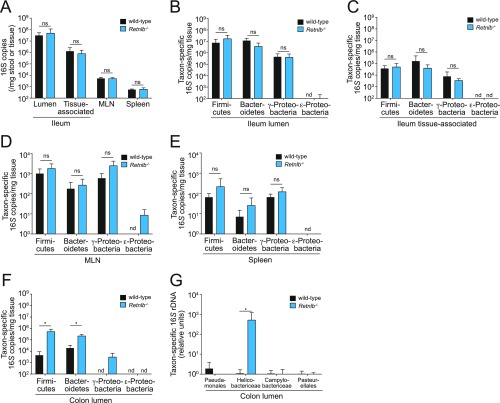
Determination of bacterial 16S copy number in small intestine, colon, MLN, and spleen from wild-type and Retnlb−/− mice. (A) Q-PCR determination of 16S rRNA gene copy number in the distal small intestinal (ileal) lumen, ileal tissue, MLN, and spleen of cohoused wild-type and Retnlb−/− mice. (B–F) Q-PCR analysis of 16S gene copy numbers from specific bacterial groups. Bacteria were recovered from the ileal lumen (B), ileal tissue (C), MLN (D), spleen (E), and colon lumen (F), and analyzed using taxon-specific 16S rDNA primers. (G) Q-PCR quantification of specific subgroups of Proteobacteria in the colon lumen. Values for each bacterial group are expressed relative to the 16S rDNA levels in wild-type mice. Four mice per genotype were analyzed for each experiment, and Q-PCR assays were repeated in triplicate within each experiment. Means ± SD are plotted. Statistics were performed with Student’s t test; *P < 0.05; ns, not significant; nd, not detected.
Fig. S8.
Isotype controls for immunofluorescence detection of bacteria. Colon tissues from wild-type and Retnlb−/− mice were detected with mouse IgG (isotype control for anti-LTA detection shown in Fig. 3E) and rabbit IgG (isotype control for anti-Helicobacter detection shown in Fig. 3G). (Scale bars: 25 µm.)
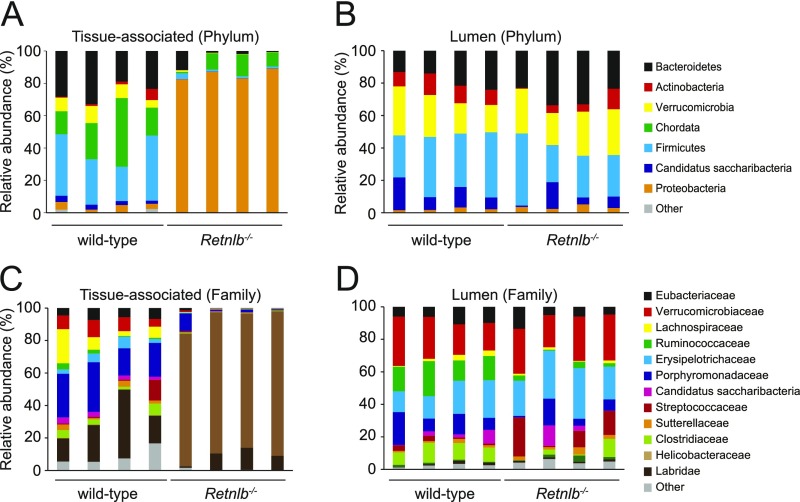
Because RELMβ preferentially kills Gram-negative bacteria, we predicted that Retnlb−/− mice would show an increased abundance of tissue-associated Gram-negative bacteria. We therefore compared the abundance of specific bacterial taxa in cocaged wild-type and Retnlb−/− mice by Q-PCR with 16S rRNA gene primers targeting specific bacterial groups. These included the Gram-positive Firmicutes, the Gram-negative Bacteroidetes, and the Gram-negative γ- and ε-Proteobacteria. While similar numbers of Firmicutes and Bacteroides were associated with colonic tissue, there was a marked increase in the numbers of γ- and ε-Proteobacteria in Retnlb−/− mice (Fig. 3D). These findings were supported by 16S rRNA deep sequencing, which revealed an increase in the abundance of tissue-associated Proteobacteria in Retnlb−/− mice, and minimal alterations in phylum-level abundances among luminal bacteria (Fig. S7 A and B).
Fig. S7.
Phylogenetic analysis of 16S rRNA from tissue-associated and lumen microbial communities. Operational taxonomic units with an average of 100 reads and populations greater than or equal to 1% were included in the graphical analysis. (A and B) Phylum-level analysis of tissue-associated (A) and luminal (B) colonic bacteria. (C and D) Family-level analysis of tissue-associated (C) and luminal (D) colonic bacteria.
We further analyzed specific subgroups of Proteobacteria, finding markedly elevated numbers of Helicobacter (ε-Proteobacteria) associated with Retnlb−/− colonic tissue (Fig. 3F) and in the colon lumen (Fig. S6G). The 16S rRNA Q-PCR analysis was supported by 16S deep sequencing (Fig. S7 C and D) and by visualization of bacteria within the colonic mucus layer of wild-type and Retnlb−/− mice. When we detected Gram-positive bacteria using an anti-lipoteichoic acid (LTA) antibody, the bacteria remained confined to the outer mucus layer in both wild-type and Retnlb−/− mice (Fig. 3E). In contrast, detection with an anti-Helicobacter antibody showed a marked increase in the numbers of bacteria in the inner mucus layer of Retnlb−/− mice, as well as within the epithelial layer (Fig. 3 G and H). Thus, RELMβ limits the numbers of Proteobacteria that associate with colon tissues.
Our in vivo findings are consistent with the potent bactericidal activity of RELMβ for P. aeruginosa and C. rodentium (both Proteobacteria) and diminished mRELMβ bactericidal activity toward B. thetaiotaomicron (belonging to the Bacteroidetes) and E. faecalis (belonging to the Firmicutes) (Fig. 1B). The abundances of lumen- and tissue-associated bacteria in the small intestine remained similar between wild-type and Retnlb−/− mice across all of the taxonomic groups (Figs. S6 B and C and S7), and we did not detect significantly altered numbers of bacteria translocating to mesenteric lymph nodes and spleen (Fig. S6 D and E). Despite there being no difference in the abundances of Firmicutes and Bacteroides associated with colon tissue, we did detect an increase in the abundances of these two groups in the colon lumen of Retnlb−/− mice (Fig. S6F). It is not yet clear how RELMβ deficiency causes these changes in luminal microbiota composition. However, we propose that they may arise as a consequence of the altered tissue-associated communities, which in turn could alter the luminal environment to promote blooms in certain taxonomic groups.
Human Resistin Is a Bactericidal Protein.
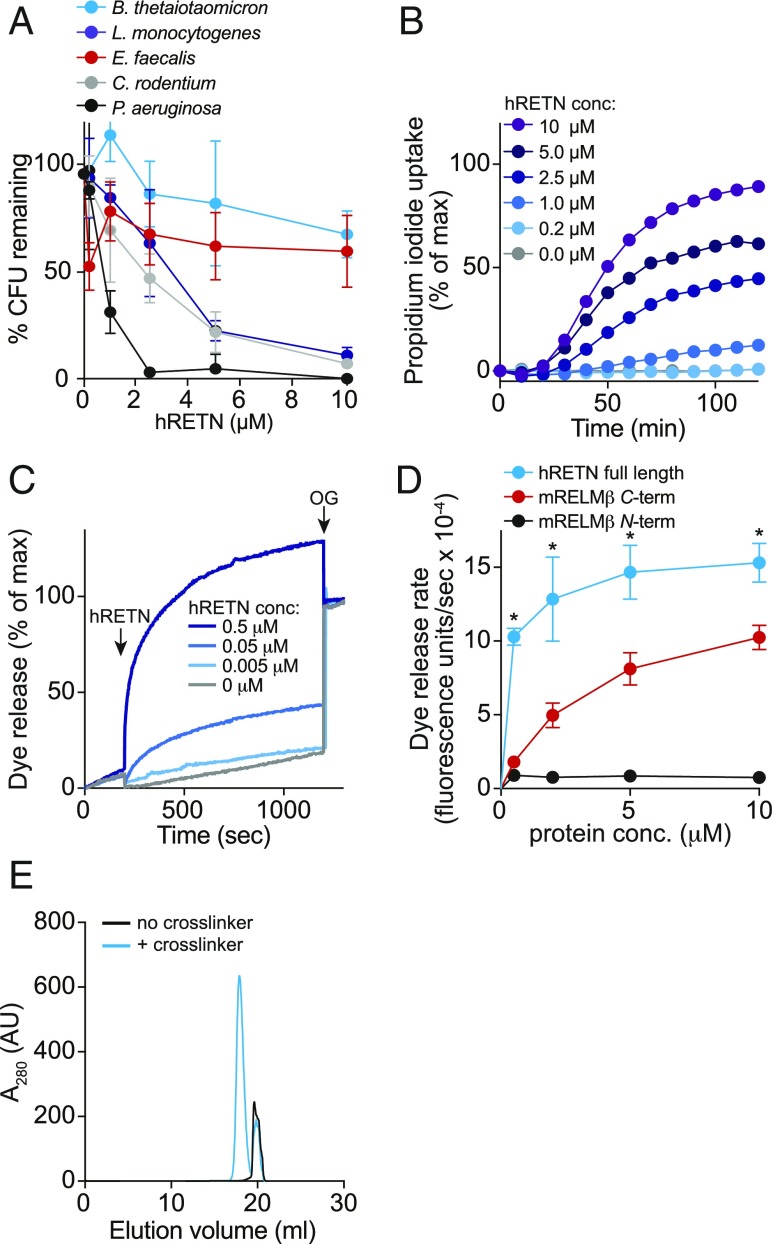
The RELM family member resistin (RETN) is produced by adipocytes and has been proposed to be a hormone that functions in metabolic regulation (25). However, human resistin (hRETN) is also expressed in monocytes and epithelial cells (26), suggesting a possible antimicrobial function. hRETN has a high degree of homology with hRELMβ (51% identity overall), particularly in the C terminus (60% amino acid identity), leading us to postulate that hRETN might also have bactericidal activity. Purified recombinant hRETN had potent bactericidal activity for the Gram-negative species P. aeruginosa (>99% decline in viability after a 2-h exposure to 2.5 μM hRETN) (Fig. 4A). The viability of C. rodentium and L. monocytogenes also declined (∼90% decline in viability after a 2-h exposure to 10 μM hRETN), while E. faecalis and B. thetaiotaomicron were mostly resistant to hRETN (Fig. 4A). hRETN permeabilized C. rodentium membranes (Fig. 4B) and induced rapid dye release from PC/PS liposomes (Fig. 4 C and D). Indeed, full-length hRETN induced dye release at a faster rate than the mRELMβ C terminus (Fig. 4D). Finally, hRETN formed multimers in association with PC/PS liposomes as revealed by cross-linking experiments (Fig. 4E). Thus, hRETN kills bacteria by forming membrane-permeabilizing pores, suggesting that bactericidal activity is a conserved function of the RELM family.
Fig. 4.
Human resistin (hRETN) is a bactericidal protein. (A) Human resistin (hRETN) bactericidal activity. Purified recombinant hRETN was added to midlogarithmic phase bacteria for 2 h, and numbers of surviving bacteria were quantified by dilution plating. Means ± SD are plotted. (B) hRETN permeabilizes bacterial membranes. C. rodentium was treated with increasing concentrations of hRETN, and PI uptake was measured over 2 h. The assay was performed twice and was repeated in triplicate within each experiment. (C) hRETN disrupts carboxyfluorescein (CF)-loaded PC:PS liposomes. Liposomes were treated with increasing concentrations of hRETN, and dye efflux was monitored over time. The 1.0% octyl glucoside (OG) was added at the end to disrupt remaining liposomes. Dye efflux is expressed as a percentage of maximal release by OG. (D) hRETN membrane-disrupting activity is superior to the membrane-disrupting activity of C terminus of mRELMβ. CF-loaded PC:PS liposomes (100 µM) were incubated with varying concentrations of full-length hRETN or the mRELMβ N or C terminus, and initial rates of liposome dye efflux as a function of hRETN concentrations are plotted. Assays were done in triplicate, and means ± SD are shown. (E) hRETN forms a multimeric complex in the presence of liposomes. The 10 μM full-length hRETN was incubated with 100 mM PC:PS liposomes and cross-linked with bis(sulfosuccinimdyl) suberate. Cross-linked complexes were solubilized in detergent and resolved by size exclusion chromatography. Statistics were performed with Student’s t test; *P < 0.05.
Discussion
We have identified a bactericidal function for members of the RELM family. RELMβ preferentially kills Gram-negative bacteria through a mechanism involving the formation of multimeric membrane-permeabilizing pores that lyse targeted bacterial cells. In mice, RELMβ restricts entry of Proteobacteria into the colon inner mucus layer and thus limits bacterial contact with the colonic mucosal surface. Human resistin is also bactericidal through the formation of multimeric membrane-permeabilizing pores, suggesting that membrane toxicity and bactericidal activity are conserved functions of the RELM family. Altogether, our findings identify RELM proteins as a previously unknown family of bactericidal proteins and enhance our understanding of how bacteria are kept physically separated from the intestinal epithelium.
The complexity of intestinal microbial communities suggests that multiple antimicrobial mechanisms are required to maintain spatial segregation of the intestinal microbiota. Accordingly, several distinct antimicrobial mechanisms have been identified that limit bacterial penetration of the inner mucus layer of the intestine. RegIIIγ is a bactericidal protein that specifically targets Gram-positive bacteria in the small intestine (4, 15, 20), Lypd8 binds to flagellin and thus reduces the motility of flagellated Gram-negative bacteria in the colon (5), and ZG16 binds and aggregates Gram-positive bacteria in the colon (6). RELMβ is mechanistically unique relative to these antimicrobial factors in that it is a bactericidal protein that selectively targets Gram-negative bacteria, thus reducing their penetration into the colonic inner mucus layer.
An interesting question is whether the bactericidal activity of RELMβ can help to explain the metabolic abnormalities that are observed in RELMβ-deficient mice (27). The composition of intestinal bacterial communities has a marked influence on metabolic outcomes such as susceptibility to obesity, glucose tolerance, and insulin resistance (28). Thus, it seems plausible that alteration of intestinal bacterial communities by RELMβ, particularly those that are most closely associated with host tissues, could have metabolic consequences. In particular, Proteobacteria (targeted by RELMβ) have been shown to contribute to changes in host metabolism (29, 30).
A related question is whether the bactericidal activity of RELMβ could in part account for the various ways in which RELMβ alters intestinal immune function in vivo. For example, RELMβ-deficient mice show enhanced colonic expression of TH2 cytokines and IL-17 (31), as well as decreased T cell recruitment during intestinal infection with C. rodentium and parasitic worms (7, 12). It will be important to determine whether these effects are secondary to RELMβ-dependent alterations in the composition or location of intestinal microbial communities, or arise from cytokine-like activities of RELMβ that may be independent of its bactericidal function.
Finally, our finding that human resistin has bactericidal activity suggests that this member of the RELM family may also function in host defense. Resistin is expressed in monocytes and epithelial cells (26), and thus could also be involved in host defense against pathogenic infections. The bactericidal activity of resistin and its expression in adipose tissue are also consistent with the known role of adipocytes in producing antimicrobial proteins that protect the host from bacterial infection (32).
Methods
Full methods are presented in SI Methods.
Mice.
Retnlb−/− mice were generated using CRISPR/Cas9 genome editing as described in SI Methods. Wild-type and Retnlb−/− mice were cocaged to ensure a shared microbiota. All mice were housed and bred in the specific pathogen-free facility at University of Texas (UT) Southwestern according to protocols approved by the Institutional Animal Care and Use Committees of UT Southwestern Medical Center.
Cloning, Expression, and Purification of Recombinant RELM Family Proteins.
cDNAs encoding mRELMβ, hRELMβ, and hRETN were PCR amplified from codon-optimized genes, using the primers listed in Table S1 (full details are available in SI Methods). The expression and purification of the RELM proteins were based on a previously published protocol (33) and are detailed in SI Methods.
Table S1.
PCR primers for cloning of genes encoding RELM family members
| Primer | Sequence, 5′→3′ |
| mRELMβ full-length forward | CCAGATCATATGCAATGCTCCTTTGAATCC |
| mRELMβ full-length reverse | CGTTGCTGTCGTATGGCGTAAGGATCCTACTAG |
| mRELMβ C terminus forward | GGATACCATATGCCGAAAACCATCAGTTGC |
| hRELMβ forward | GGATACCATATGCAGTGTTCACTGGATTCGGTC |
| hRELMβ reverse | GATGATGGATCCTTACGTCAGGTGACAGCAGCGGGC |
| hRETN forward | GGATACCATATGAGCTCTAAAACCCTGTGTTCC |
| hRETN reverse | GATGATGGATCCTTACGGTTGAACACGACAGCAACG |
Assays for Bactericidal Activity.
Bactericidal assays were performed as previously described (20). Briefly, purified proteins were added to logarithmic-phase bacteria and incubated for 2 h at 37 °C. Remaining live bacteria were quantified by dilution plating (Table S2). Surviving colonies were counted and calculated as a percentage of the colonies on the control plate.
Table S2.
Bacterial strains used in this study
| Strain | Media | Mid log OD600 |
| Citrobacter rodentium, DBS100 | LB | 0.4–0.6 |
| Pseudomonas aeruginosa, ATCC27853 | LB | 0.4–0.6 |
| Listeria monocytogenes, EGD-e | BHI (BD Biosciences; 237500) | 0.3–0.5 |
| Enterococcus faecalis, V583 | BHI | 0.4–0.6 |
| Bacteroides thetaiotaomicron, VPI5482 | TYG (broth)/BHI blood agar | 0.6–0.7 |
Dye Uptake Assays.
Midlogarithmic phase bacteria were diluted into assay buffer (10 mM Mes, pH 5.5, 25 mM NaCl) containing 5.5 µg/mL PI. Recombinant purified RELM proteins were added and fluorescence output was measured for 2 h using a Spectramax plate reader (Molecular Devices). Dye uptake was measured against the maximum fluorescence output from the positive control [0.05% (wt/vol) SDS].
Assays for Lipid Binding and Liposome Disruption.
Recombinant mRELMβ (1 mg/mL) was incubated with membrane lipid strips (Echelon) overnight at 4 °C, followed by washing and detection with rabbit anti-RELMβ antibody (raised against the purified recombinant mRELMβ). Liposome disruption assays were performed as previously described (15). The mRELMβ N-terminal peptide (QCSFESLVDQRIKEALSRQE) was synthesized by the Protein Chemistry Core at UT Southwestern and purified by HPLC. FRET assays were performed as previously described (15) on liposomes composed of 80% PC, 15% PS, and 5% dansyl-PE.
Real-Time Q-PCR.
RNA was isolated from tissue using the RNeasy Midi kit (Qiagen), and cDNA was synthesized using the MMLV kit (Thermo Fisher). Q-PCR analysis was performed using SYBR Green master mix (Thermo Fisher). Primer sequences are listed in Table S3, and gene expression was normalized to 18S rRNA.
Table S3.
Primers for Q-PCR gene expression analysis
| Gene | Sequence, 5′→3′ | Annealing temperature, °C |
| Muc2 | CTGACCAAGAGCGAACACAA | 56 |
| CATGACTGGAAGCAACTGGA | ||
| 18S | GTAACCCGTTGAACCCCATT | 56 |
| CCATCCAATCGGTAGTAGCG |
16S rRNA Sequencing.
Fecal and tissue DNAs were extracted as described (6). Two micrograms of DNA were amplified using primers specific for the 16S rRNA sequence (forward, 5′-AGAGTTTGATCMTGGCTCAG-3′, and reverse, 5′- CGGTTACCTTGTTACGACTT-3′) (6), yielding an amplicon that encompassed the entire 16S rRNA sequence (∼1,450 bp). Amplification reactions were carried out with the HotStarTaq polymerase kit (Qiagen) and then diluted 1:10 into H2O. The diluted DNA samples were then analyzed by Q-PCR using the SYBR Green kit (Thermo Fisher) and the primers found in Table S4. PCRs were quantified using standard curves generated from template controls for each primer set.
Table S4.
Primers for 16S Q-PCR taxonomic analysis
| Taxonomic group | Sequence, 5′→3′ |
| Universal (37) | ACTCCTACGGGAGGCAGCAGT |
| ATTACCGCGGCTGCTGGC | |
| Bacteroides (38) | GGTTCTGAGAGGAGGTCCC |
| CTGCCTCCCGTAGGAGT | |
| Firmicutes (37) | GGAGYATGTGGTTTAATTCGAAGCA |
| AGCTGACGACAACCATGCAC | |
| γ-Proteobacteria (39) | TCGTCAGCTCGTGTYGTGA |
| CGTAAGGGCCATGATG | |
| ε-Proteobacteria (40) | TAGGCTTGACATTGATAGAATC |
| CTTACGAAGGCAGTCTCCTTA | |
| Pseudomonales (41) | ACTTTAAGTTGGGAGGAAGGG |
| ACACAGGAAATTCCACCACCC | |
| Pasteurellales (42) | CGGGTTGTAAAGTTCTTTCGGT |
| GGAGTTAGCCGGTGCTTCTTC | |
| Helicobacteraceae (43) | TGGGAGAGGTAGGTGGAATTCT |
| GTCGCCTTCGCAATGAGTATTC | |
| Campylobacteraceae (43) | GGATGACACTTTTCGGAG |
| AATTCCATCTGCCTCTCC |
Immunofluorescence Detection and Electron Microscopy.
Segments of unflushed colons from each mouse were fixed in methacarn (60% methanol, 30% chloroform, and 10% glacial acetic acid) for at least 4 h at room temperature and further prepared as described in SI Methods. Tissues were detected with Ulex europaeus agglutinin I (EY Labs) or antibodies against lipoteichoic acid (Thermo Fisher) and Helicobacter species (Abcam) and imaged by fluorescence microscopy. mRELMβ-treated Pseudomonas aeruginosa was prepared for transmission electron microscopy as described in SI Methods.
Statistical Analysis.
All statistical analyses were performed using two-tailed Student’s t test. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; and ns, P > 0.05.
SI Methods
Mice.
Retnlb−/− mice were generated using CRISPR/Cas9 genome editing with a guide RNA targeting exon 2 of the Retnlb gene (targeted sequence: 5′-CGTTCCCTGGGACTATCAGTGG-3′). Guide RNAs were injected into fertilized C57BL/6J embryos along with in vitro-transcribed Cas9 mRNA by the UT Southwestern Transgenic Core facility. Healthy blastocysts were implanted into pseudopregnant mice, the resulting litters were screened by genomic sequencing to detect the presence of Retnlb edits, and the edited allele was bred to homozygosity. Wild-type and Retnlb−/− mice were cocaged to ensure a shared microbiota. All mice were housed and bred in the specific pathogen-free facility at UT Southwestern according to protocols approved by the Institutional Animal Care and Use Committees of UT Southwestern Medical Center.
Construction of RELM Family Expression Plasmids.
Sequences encoding mRELMβ, hRELMβ, and hRETN RELMs were PCR amplified from codon-optimized genes (GenScript; sequences listed below) using the primers shown in Table S1 and the KOD Hot Start Polymerase kit (EMD Millipore; 71086). PCR-amplified products were purified using the Quik PCR purification kit (Qiagen; 28104) and digested with NdeI and BamHI (New England Biolabs). The amplicons were ligated to digested pET28a vector (EMD Millipore; 69864), transformed into One Shot TOP10 competent cells (Thermo Fisher; C404010), and plated for ampicillin-resistant clones. Plasmid DNA was purified using the QIAprep Spin Miniprep kit (Qiagen; 27106) and sequenced by the UT Southwestern Sequencing Core Facility.
Codon-optimized genes were as follows:
Mouse Retnlb: ATGAAACCGACGCTGTGCTTCCTGTTTATCCTGGTCTCACTGCTGCCGCTGATCGTGCCGGGTAACGCGCAATGCTCCTTTGAATCCCTGGTCGATCAGCGTATTAAAGAAGCACTGTCTCGCCAAGAACCGAAAACCATCAGTTGCACCTCCGTTACGAGCTCTGGTCGTCTGGCGTCATGTCCGGCCGGTATGGTGGTTACCGGTTGCGCATGTGGCTATGGTTGCGGCTCGTGGGACATTCGCAACGGCAATACGTGTCATTGTCAGTGTTCGGTGATGGATTGGGCGTCCGCTCGTTGCTGTCGTATGGCGTAA
Human RETNLB:
CAGTGTTCACTGGATTCGGTCATGGACAAGAAAATTAAAGATGTGCTGAACTCCCTGGAATATAGTCCGTCCCCGATCAGCAAAAAACTGAGCTGTGCATCTGTGAAAAGTCAAGGTCGTCCGAGCTCTTGTCCGGCCGGTATGGCAGTTACCGGTTGCGCTTGTGGCTACGGTTGCGGCTCTTGGGATGTCCAGCTGGAAACCACGTGCCATTGTCAATGCAGCGTGGTTGACTGGACCACGGCCCGCTGCTGTCACCTGACG
Human RETN:
AGCTCTAAAACCCTGTGTTCCATGGAAGAAGCAATTAACGAACGTATCCAGGAAGTCGCGGGTAGCCTGATTTTTCGCGCCATTAGTTCCATCGGCCTGGAATGTCAATCAGTGACCTCGCGTGGTGATCTGGCAACCTGTCCGCGCGGCTTCGCTGTGACCGGTTGCACGTGTGGTAGCGCATGCGGCTCTTGGGATGTTCGTGCTGAAACCACGTGCCATTGTCAGTGTGCCGGTATGGACTGGACCGGTGCACGTTGCTGTCGTGTTCAACCG
Expression and Purification of Recombinant RELM Family Members.
The expression and purification of mRELMβ, hRELMβ, and hRETN are based on a previously published protocol (33) with significant changes detailed below. RELM-encoding expression plasmids were transformed into chemically competent BL21 DE3 RIL (Agilent; 230245) cells and plated on LB agar (Sigma; L7533) with chloramphenicol (Cam) (30 µg/mL) and kanamycin sulfate (Kan) (50 µg/mL) plates. A 10-mL overnight culture was used to inoculate 1 L of LB (Sigma; L7658) containing Cam and Kan. The culture was grown to midlogarithmic phase (OD600, ∼0.5–0.7), induced with 0.4 mM isopropyl-β-d-thiogalactoside (GoldBio Technology; I2481), and grown for 16–20 h at 20 °C with shaking. Bacterial cells were pelleted, resuspended in 75 mL of lysis buffer (20 mM Tris, pH 7.5, 1% Triton X-100, and 2 mM PMSF), and lysed by sonication (Misonix Ultrasonic Cell Disruptor). Lysed cells were centrifuged, and the pellets were resuspended in 40 mL of inclusion body buffer (100 mM Tris, pH 9.0, 7 M guanidine hydrochloride) followed by the addition of 100 mM sodium sulfite and 20 mM sodium tetrathionate. This mixture was stirred overnight at room temperature. The solubilized inclusion bodies were then passed over a Ni-NTA column (Qiagen; 30210) equilibrated with wash buffer (25 mM Tris, pH 7.5, 20 mM glycine, and 6 M urea). The column was then washed, and bound protein was eluted with wash buffer containing 250 mM imidazole. Fractions containing protein were pooled and diluted to 0.1 mg/mL in prechilled refolding buffer (100 mM Tris, pH 8.5, 20 mM glycine, 300 mM NaCl, 5 mM EDTA, and 2 M urea) at 4 °C. After the solution became homogeneous, cysteine (5 mM final concentration) was added and mixed until dissolved. Once dissolved, solution was removed from stirrer and left at 4 °C for 72 h. The protein solution was then dialyzed [at least 20:1 (vol/vol)] against 25 mM Mes, pH 6.0, 100 mM NaCl, with three buffer changes over 8–14 h at 4 °C. Proteins were concentrated in Amicon Ultra centrifugal filters (Millipore; UFC900324) to ∼2 mL, thrombin (0.5 U/mg protein; Sigma; 10602400001) was added, and the solution was mixed for 12–16 h at 4 °C. Proteins were then separated by size exclusion chromatography on Superdex 75/300 (GE Healthcare; 17-5174-01), and fractions containing monomeric protein were pooled, concentrated, and flash frozen in liquid N2.
Generation of Anti-RELMβ Antiserum.
Purified recombinant mouse RELMβ was submitted to Cocalico Biologicals for polyclonal antibody generation in rabbits.
Assays for Antimicrobial Activity.
Antimicrobial assays were performed as previously described (20). Briefly, liquid bacterial cultures were inoculated from an overnight colony and were grown in strain-specific media (Table S2) to an OD600 that represents midlogarithmic phase, depending on the bacterial strain (listed in Table S2). After reaching midlogarithmic phase, 10 mL of bacteria were pelleted. The supernatant was discarded, and the pellet was resuspended in 10 mL of assay buffer (10 mM Mes, pH 5.5, 25 mM NaCl), pelleted again, and resuspended in assay buffer. Purified RELM proteins were added in varying concentrations to ∼5 × 106 cells per mL bacteria and were incubated at 37 °C for 2 h. After 2 h, samples were plated onto appropriate agar plates and incubated overnight at 37 °C. Surviving colonies were counted and calculated as a percentage of the remaining colonies on the control (no-protein) sample. Commercially available, dimeric hRELMβ was purchased from Shenandoah Biotechnology (100-160).
PI Uptake Assay.
Bacteria were grown to midlogarithmic phase, washed with assay buffer (10 mM Mes, pH 5.5, 25 mM NaCl), and diluted into assay buffer to 5.5 × 108 CFU/mL containing 5.5 µg/mL PI (Thermo Fisher; P3566). Bacterial samples (90 µL each) were added to black 96-well Costar plates (Fisher; 07-200-567) and placed into a Spectramax plate reader (Molecular Devices) that was preequilibrated to 37 °C. After an initial reading (T0, 0 s), 10 µL of recombinant RELM protein at varying concentrations was added, and the reaction continued for 2 h with fluorescence readings [excitation (Ex), 535 nm; emission (Em), 617 nm] every 10 min. For data analysis, T0 readings were subtracted from each well from each time point. A negative control, BSA (Boval; LY0081), was included. Membrane permeabilization activity was measured against the maximum fluorescence output from the positive control [0.05% (wt/vol) SDS]. For the permeability assays that measured the effects of salt and pH, the following buffers were used: (i) Mes, low salt (same as assay buffer) (10 mM Mes, 25 mM NaCl, pH 5.5); (ii) Mes, high salt: 10 mM Mes, 150 mM NaCl, pH 5.5; and (iii) PBS: 1× PBS (10 mM sodium phosphate, 154 mM NaCl, pH 7.4; Sigma; P5493).
Lipid Strip Assay.
Membrane lipid strips (Echelon; P-6002) were stored and used according to the manufacturer’s instructions. The lipid strips were blocked with hybridization buffer (10 mM Mes, 25 mM NaCl, pH 5.5, 2% BSA, and 0.05% Tween 20) for 1 h at room temperature. Recombinant mRELMβ was diluted to 1 µg/mL in hybridization buffer and incubated with the lipid strip overnight at 4 °C. The lipid strip was then washed five times for 5 min with wash buffer (10 mM Mes, 25 mM NaCl, 0.05% Tween 20). Anti-RELMβ IgG polyclonal antibody was diluted to 1 µg/mL in hybridization buffer and incubated at room temperature for 1 h. The blot was then washed five times for 5 min with wash buffer, and then secondary antibody (goat α-rabbit HRP; 1:5,000; Santa Cruz Biotechnology; sc-2004) was diluted into hybridization buffer and incubated at room temperature for 1 h. The blot was then washed five times for 5 min with wash buffer and HRP was detected with ECL reagent (Bio-Rad; 1705060) using the ChemiDoc Imaging System (Bio-Rad).
Unilamellar Liposome Preparation.
Lipids were purchased from Avanti Polar Lipids: 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine or POPC (PC) (850457C), 1,2-dioleoyl-sn-glycero-3-phospho-l-serine or DOPS (PS) (840035C), and 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(5-dimethylamino-1-naphthalenesulfonyl) or dansyl PE (810330C). The lipids were prepared as previously described (15). Lipids dissolved in chloroform were mixed in defined molar ratios in glass tubes and then dried under a stream of N2, followed by drying under vacuum overnight to ensure complete removal of organic solvents. 5(6)-Carboxyfluorescein (CF) (100 mM; Sigma; 21877) or with fluorescein-dextran 10 (FD10) (100 mg/mL; TDB Consultancy; FD10) was added to the lipids and vortexed for 5 min. Lipids were then transferred to 2-mL cryotubes and subjected to five freeze–thaw cycles in liquid N2 and stored at −80 °C. Lipids were then thawed and extruded through 100-nm pore membranes using a miniextruder kit (Avanti Polar Lipids; 610000). Liposomes were passaged through the membrane filter 30 times, and sizes were confirmed using dynamic light scattering (Protein Solutions). Liposomes loaded with CF were purified on a PD-10 column to remove excess dye. Liposomes loaded with FD10 were purified by size exclusion chromatography on a Sephadex 200 10/300 column (GE Healthcare; 17517501).
Liposome Disruption Assay.
Samples were read on a QuantaMaster 300 fluorometer (Photon Technology International) as before (3). Fluorescein-loaded liposomes were diluted to a final concentration of 100 µM into assay buffer (10 mM Mes, pH 5.5, 25 mM NaCl), and fluorescence readings (Ex, 490 nm; Em, 516 nm) were taken for 200 s. Reading was momentarily suspended while protein or buffer was added and mixed, and then commenced for 1,200 s. The detergent n-octyl glucoside (OG) (Anatrace; O311) was added to a final concentration of 1% (vol/vol) to completely disrupt the liposomes. Each reading was calibrated to the beginning fluorescence output and normalized to the total fluorescence output observed from the addition of 1% OG. The mRELMβ N-terminal peptide (QCSFESLVDQRIKEALSRQE) was synthesized by the Protein Chemistry Core at UT Southwestern and purified by HPLC.
Dansyl Liposome–Tryptophan FRET Assay.
RELMβ binding to liposomes was measured by FRET between protein tryptophan residues and dansyl-PE, a fluorescent lipid. Liposomes were prepared as detailed above, but the lipid composition was changed to 80% PC, 15% PS, and 5% dansyl-PE, and the settings on the fluorometer were adjusted accordingly (Ex, 295 nm; Em, 450–560 nm). Liposomes incorporating dansyl-PE were diluted to 100 µM in assay buffer (10 mM Mes, pH 5.5, 25 mM NaCl), and protein was added and allowed to stand for 15 min at room temperature before reading. After subtracting out protein-only and liposome-only controls, FRET efficiency (15) was calculated as follows:
Protein Cross-Linking.
Liposomes were prepared as described as above but without added fluorophore. Liposomes were diluted in assay buffer (10 mM Mes, pH 5.5, 25 mM NaCl; 400 µM lipid in 400 µL), followed by the addition of protein (40 µM final concentration). The mixture was incubated at room temperature for 20 min. After 20 min, bis(sulfosuccinimdyl) suberate (10 µM final concentration, BS3; Thermo Fisher; 21580) was added, and reactions were mixed at room temperature for 1 h. OG was then added to a final concentration of 4% (vol/vol), and samples were mixed at room temperature overnight. The next day, samples were separated by size exclusion chromatography on a Superdex 75/300 column in assay buffer, and fractions were analyzed by SDS/PAGE and Western blot.
Citrobacter rodentium Infections.
Wild-type and Retnlb−/− mice were cohoused for 2 wk before infection to ensure a shared microbiota. An overnight culture of C. rodentium containing nalidixic acid (Nal) (100 µg/mL) was used to inoculate a 100-mL culture that was grown overnight in a shaking incubator at 37 °C. Bacteria were pelleted and washed with PBS, and then pelleted and resuspended in PBS. Each mouse was infected with ∼7.5 × 109 CFU by intragastric gavage. After 7 d, mice were killed, and samples were either processed for RNA isolation (RNeasy Midi kit; Qiagen; 75142) or for colony counting. For colony counting, tissues and fecal contents were added to 2 mL of PBS, homogenized by rotor and stator (TH Tissue Homogenizer; OMNI; TH01), and plated onto LB-Nal agar plates.
Q-PCR.
cDNA was synthesized from extracted RNA (2 µg per sample) using the MMLV kit (Thermo Fisher; 28025013) according to the manufacturer’s instructions. Q-PCR analysis was performed using SYBR Green master mix (Thermo Fisher; 4309155). Primers (10 µM final concentration) can be found in Table S3, and gene expression was normalized to 18S rRNA.
DNA Extraction for 16S rRNA Analysis.
For isolation of luminal contents from the colon, a section of distal colon was cut open longitudinally, and whole fecal pellets were extracted and weighed before processing. For analysis of tissue-associated bacteria, the same tissue samples that were used for analysis of luminal contents were washed in PBS until no visible fecal material was attached, and then the whole tissue was weighed before processing. For ileal lumen samples, contents were flushed with 1 mL of PBS into a sterile Eppendorf tube. The contents were pelleted at 16,000 × g for 10 min, and supernatants were removed, and then transferred to DNA extraction tubes as described below. For analysis of ileal tissue-associated bacteria, the flushed tissues were cut open longitudinally, washed in PBS until visibly clean, and the tissue was weighed before processing.
DNA was extracted as previously described (6). Briefly, samples were added to Lysine Matrix E tubes (MP Biomedicals; 116914050), and then 1 mL of lysis buffer [1× Tris-EDTA buffer (Sigma; 93283) with 0.5% SDS] was added. Tubes were then added to a bead beating homogenizer (Bullet Blender; Next Advance) with five 1-min pulses, with 1 min on ice in-between pulses. After homogenization, the lysates were centrifuged for 10 min at 4 °C at 16,000 × g. The supernatants were then transferred to clean tubes, and DNA was extracted with phenol:chloroform (35) and precipitated with sodium acetate and ethanol. DNA pellets were resuspended in 1× TE and stored at −20 °C.
16S Taxonomic Analysis.
Two micrograms of DNA were amplified using primers specific for the 16S rRNA sequence (forward, 5′-AGAGTTTGATCMTGGCTCAG-3′, and reverse, 5′- CGGTTACCTTGTTACGACTT-3′) (6), yielding an amplicon that encompassed the whole 16S rRNA sequence (∼1,450 bp). Amplification reactions were carried out with the HotStarTaq polymerase kit (Qiagen) and then diluted 1:10 into H2O. The diluted DNA samples were then analyzed by Q-PCR using the SYBR Green kit (Thermo Fisher; 4309155) and the primers found in Table S4. PCR control reactions containing water were included to identify possible contamination. PCRs were quantified using standard curves generated from template controls for each primer set. Deep sequencing of the V3–V4 region of 16S rRNA was performed by Molecular Research DNA (MrDNA) with Illumina MiSeq. For phylogenetic analysis, populations that averaged above 100 reads and greater than 1% of the relative population were included.
Tissue Fixation and Immunofluorescence Detection.
Segments of unflushed colons from each mouse were fixed in methacarn (60% methanol, 30% chloroform, and 10% glacial acetic acid) for at least 4 h at room temperature. Tissues were washed twice in absolute methanol and twice in absolute ethanol. Samples were embedded in paraffin and cut onto slides by the UT Southwestern Pathology Core. To prepare samples for staining, we dewaxed and cleaned the slides in the following solutions for 10 min each: two times in xylene, two times in 100% ethanol, two times in 95% ethanol, two times in 90% ethanol, and one time in H2O. Slides were blocked in hybridization buffer (2% BSA and 0.05% Tween 20 in PBS) for 1 h at room temperature and then washed three times in PBS-T. After drying, biotinylated UEA-I (20 µg/mL; EY Labs; BA-2201-2), anti-lipoteichoic acid (α-LTA) (1:100 dilution; Thermo Fisher; MA1-7402), and anti-Helicobacter (1:100 dilution; Abcam; ab20459) antibodies were added to the slides, which were then covered and incubated overnight at 4 °C. The next day, slides were washed five times in PBS-T, and then incubated with streptavidin-Cy3 (GeneTex; GTX85902), goat anti-mouse IgG Alexa Fluor 594 (1:100; Abcam; ab20459), or goat anti-rabbit IgG Alexa Fluor 647 (1:100; Abcam; ab150079), respectively, for 5 h at 4 °C. The slides were then washed five times in PBS-T, dried, and mounted with DAPI Fluormount-G (Southern Biotech; 0100-20). Mouse IgG isotype control (Abcam; 37355) and rabbit IgG isotype control (Abcam; ab199376) were used at the same dilution. Samples were left to set for at least 24 h at room temperature before imaging on Zeiss AxioImager M1 Microscope.
Fluorescence Image Analysis.
Fluorescence images were analyzed using ImageJ (NIH, Bethesda; https://imagej.nih.gov/ij/). Distances were measured using the scale bar as a reference distance. For particle analysis, the inner mucus was encircled and measured for area, and then the particles present in the area were counted.
Electron Microscopy.
Pseudomonas aeruginosa was grown to midlogarithmic phase as described above. Ten milliliters of bacteria were washed and resuspended in a final volume of 1 mL of assay buffer (10 mM Mes, pH 5.5, 25 mM NaCl). The 100 μM mRELMβ was added to 3 × 109 cells in a total volume of 300 µL of assay buffer with incubation for 2 h at 37 °C. Bacteria were centrifuged for 10 min at 16,000 × g, washed once in assay buffer, resuspended in cross-linking reagent (4% paraformaldehyde and 4% glutaraldehyde in 0.1 M phosphate buffer, pH 7.4), and incubated overnight at 4 °C. After three washes with 0.1 M sodium phosphate buffer, cell pellets were embedded in 3% agarose and sliced into small blocks (1 mm3), rinsed with the same buffer three times, and postfixed with 1% osmium tetroxide and 0.8% potassium ferricyanide in 0.1 M sodium phosphate buffer for 1.5 h at room temperature. Cells were rinsed with water and stained en bloc with 1% uranyl acetate in water for 1 h. Cells were dehydrated with increasing concentrations of ethanol, transitioned into propylene oxide, infiltrated with Embed-812 resin, and polymerized in a 60 °C oven overnight. Blocks were sectioned with a diamond knife (Diatome) on a Leica Ultracut 7 μLtramicrotome (Leica Microsystems), collected onto copper grids, and poststained with 2% aqueous uranyl acetate and lead citrate. Images were acquired on a Tecnai G2 spirit transmission electron microscope (FEI) equipped with a LaB6 source using a voltage of 120 kV.
Acknowledgments
This work was supported by NIH Grant R01 DK070855 (to L.V.H.), Welch Foundation Grant I-1874 (to L.V.H.), Cancer Prevention and Research Institute of Texas Grant RP130166 (to L.V.H.), Burroughs Wellcome Foundation New Investigators in the Pathogenesis of Infectious Diseases Award (to L.V.H.), the Walter M. and Helen D. Bader Center for Research on Arthritis and Autoimmune Diseases (L.V.H.), and the Howard Hughes Medical Institute (L.V.H.). D.C.P. was supported by NIH Grants T32-AI007520 and F32 DK100074, and T.A.H. was supported by the Burroughs Wellcome Foundation Minority Enrichment Program and the Dermatology Foundation.
Footnotes
The authors declare no conflict of interest.
Data deposition: The 16S rRNA gene-sequencing data reported in this paper have been deposited in the National Center for Biotechnology Information Sequence Read Archive (Bioproject SRP116327).
See Profile on page 11003.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1711395114/-/DCSupplemental.
References
- 1.Sonnenburg JL, Bäckhed F. Diet-microbiota interactions as moderators of human metabolism. Nature. 2016;535:56–64. doi: 10.1038/nature18846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johansson MEV, et al. Composition and functional role of the mucus layers in the intestine. Cell Mol Life Sci. 2011;68:3635–3641. doi: 10.1007/s00018-011-0822-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johansson ME, et al. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci USA. 2008;105:15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaishnava S, et al. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science. 2011;334:255–258. doi: 10.1126/science.1209791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okumura R, et al. Lypd8 promotes the segregation of flagellated microbiota and colonic epithelia. Nature. 2016;532:117–121. doi: 10.1038/nature17406. [DOI] [PubMed] [Google Scholar]
- 6.Bergström JH, et al. Gram-positive bacteria are held at a distance in the colon mucus by the lectin-like protein ZG16. Proc Natl Acad Sci USA. 2016;113:13833–13838. doi: 10.1073/pnas.1611400113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Artis D, et al. RELMbeta/FIZZ2 is a goblet cell-specific immune-effector molecule in the gastrointestinal tract. Proc Natl Acad Sci USA. 2004;101:13596–13600. doi: 10.1073/pnas.0404034101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He W, et al. Bacterial colonization leads to the colonic secretion of RELMbeta/FIZZ2, a novel goblet cell-specific protein. Gastroenterology. 2003;125:1388–1397. doi: 10.1016/j.gastro.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 9.Vaishnava S, Behrendt CL, Ismail AS, Eckmann L, Hooper LV. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc Natl Acad Sci USA. 2008;105:20858–20863. doi: 10.1073/pnas.0808723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajala MW, Obici S, Scherer PE, Rossetti L. Adipose-derived resistin and gut-derived resistin-like molecule-beta selectively impair insulin action on glucose production. J Clin Invest. 2003;111:225–230. doi: 10.1172/JCI16521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steppan CM, et al. A family of tissue-specific resistin-like molecules. Proc Natl Acad Sci USA. 2001;98:502–506. doi: 10.1073/pnas.98.2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bergstrom KSB, et al. Goblet cell derived RELMβ recruits CD4+ T cells during infectious colitis to promote protective intestinal epithelial cell proliferation. PLoS Pathog. 2015;11:e1005108. doi: 10.1371/journal.ppat.1005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 14.Patel SD, Rajala MW, Rossetti L, Scherer PE, Shapiro L. Disulfide-dependent multimeric assembly of resistin family hormones. Science. 2004;304:1154–1158. doi: 10.1126/science.1093466. [DOI] [PubMed] [Google Scholar]
- 15.Mukherjee S, et al. Antibacterial membrane attack by a pore-forming intestinal C-type lectin. Nature. 2014;505:103–107. doi: 10.1038/nature12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muchmore SW, et al. X-ray and NMR structure of human Bcl-xL, an inhibitor of programmed cell death. Nature. 1996;381:335–341. doi: 10.1038/381335a0. [DOI] [PubMed] [Google Scholar]
- 17.Menestrina G, Serra MD, Prévost G. Mode of action of β-barrel pore-forming toxins of the staphylococcal α-hemolysin family. Toxicon. 2001;39:1661–1672. doi: 10.1016/s0041-0101(01)00153-2. [DOI] [PubMed] [Google Scholar]
- 18.Harder J, Bartels J, Christophers E, Schroder J-M. Isolation and characterization of human β-defensin-3, a novel human inducible peptide antibiotic. J Biol Chem. 2001;276:5707–5713. doi: 10.1074/jbc.M008557200. [DOI] [PubMed] [Google Scholar]
- 19.Porter EM, van Dam E, Valore EV, Ganz T. Broad-spectrum antimicrobial activity of human intestinal defensin 5. Infect Immun. 1997;65:2396–2401. doi: 10.1128/iai.65.6.2396-2401.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cash HL, Whitham CV, Behrendt CL, Hooper LV. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313:1126–1130. doi: 10.1126/science.1127119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malik E, Dennison SR, Harris F, Phoenix DA. pH dependent antimicrobial peptides and proteins, their mechanisms of action and potential as therapeutic agents. Pharmaceuticals (Basel) 2016;9:67. doi: 10.3390/ph9040067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pelaseyed T, et al. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol Rev. 2014;260:8–20. doi: 10.1111/imr.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russell AL, et al. The effect of the placement and total charge of the basic amino acid clusters on antibacterial organism selectivity and potency. Bioorg Med Chem. 2011;19:7008–7022. doi: 10.1016/j.bmc.2011.10.033. [DOI] [PubMed] [Google Scholar]
- 24.Moniruzzaman M, Alam JM, Dohra H, Yamazaki M. Antimicrobial peptide Lactoferrin B-induced rapid leakage of internal contents from single giant unilamellar vesicles. Biochemistry. 2015;54:5802–5814. doi: 10.1021/acs.biochem.5b00594. [DOI] [PubMed] [Google Scholar]
- 25.Steppan CM, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 26.Jang JC, et al. Macrophage-derived human resistin is induced in multiple helminth infections and promotes inflammatory monocytes and increased parasite burden. PLoS Pathog. 2015;11:e1004579. doi: 10.1371/journal.ppat.1004579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hildebrandt MA, et al. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology. 2009;137:1716–1724.e2. doi: 10.1053/j.gastro.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 29.Shin N-R, Whon TW, Bae J-W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015;33:496–503. doi: 10.1016/j.tibtech.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 30.Fei N, Zhao L. An opportunistic pathogen isolated from the gut of an obese human causes obesity in germfree mice. ISME J. 2013;7:880–884. doi: 10.1038/ismej.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wernstedt Asterholm I, et al. Pathological type-2 immune response, enhanced tumor growth, and glucose intolerance in Retnlß (RELMβ) null mice: A model of intestinal immune system dysfunction in disease susceptibility. Am J Pathol. 2016;186:2404–2416. doi: 10.1016/j.ajpath.2016.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang LJ, et al. Innate immunity. Dermal adipocytes protect against invasive Staphylococcus aureus skin infection. Science. 2015;347:67–71. doi: 10.1126/science.1260972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holcomb IN, et al. FIZZ1, a novel cysteine-rich secreted protein associated with pulmonary inflammation, defines a new gene family. EMBO J. 2000;19:4046–4055. doi: 10.1093/emboj/19.15.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sakamoto K, et al. Quantitative study of changes in intestinal morphology and mucus gel on total parenteral nutrition in rats. J Surg Res. 2000;94:99–106. doi: 10.1006/jsre.2000.5937. [DOI] [PubMed] [Google Scholar]
- 35.Zoetendal EG, et al. Isolation of DNA from bacterial samples of the human gastrointestinal tract. Nat Protoc. 2006;1:870–873. doi: 10.1038/nprot.2006.142. [DOI] [PubMed] [Google Scholar]
- 36.Juan C-C, et al. Production and characterization of bioactive recombinant resistin in Escherichia coli. J Biotechnol. 2003;103:113–117. doi: 10.1016/s0168-1656(03)00099-3. [DOI] [PubMed] [Google Scholar]
- 37.Rehman A, et al. Nod2 is essential for temporal development of intestinal microbial communities. Gut. 2011;60:1354–1362. doi: 10.1136/gut.2010.216259. [DOI] [PubMed] [Google Scholar]
- 38.Salzman NH, et al. Enteric defensins are essential regulators of intestinal microbial ecology. Nat Immunol. 2010;11:76–83. doi: 10.1038/ni.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bacchetti De Gregoris T, Aldred N, Clare AS, Burgess JG. Improvement of phylum- and class-specific primers for real-time PCR quantification of bacterial taxa. J Microbiol Methods. 2011;86:351–356. doi: 10.1016/j.mimet.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 40.Yang Y-W, et al. Use of 16S rRNA gene-targeted group-specific primers for real time PCR analysis of predominant bacteria in mouse feces. Appl Environ Microbiol. 2015;81:6749–6756. doi: 10.1128/AEM.01906-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bergmark L, et al. Assessment of the specificity of Burkholderia and Pseudomonas qPCR assays for detection of these genera in soil using 454 pyrosequencing. FEMS Microbiol Lett. 2012;333:77–84. doi: 10.1111/j.1574-6968.2012.02601.x. [DOI] [PubMed] [Google Scholar]
- 42.Miller M, Zorn J, Brielmeier M. High-resolution melting curve analysis for identification of Pasteurellaceae species in experimental animal facilities. PLoS One. 2015;10:e0142560. doi: 10.1371/journal.pone.0142560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rinttilä T, Kassinen A, Malinen E, Krogius L, Palva A. Development of an extensive set of 16S rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. J Appl Microbiol. 2004;97:1166–1177. doi: 10.1111/j.1365-2672.2004.02409.x. [DOI] [PubMed] [Google Scholar]