Abstract
Helicobacter pylori γ-glutamyl transferase (gGT) is a key bacterial virulence factor that is not only important for bacterial gastric colonization but also related to the development of gastric pathology. Despite accumulating evidence for pathogenic and immunologic functions of H. pylori gGT, it is still unclear how it supports gastric colonization and how its specific effects on the host’s innate and adaptive immune responses contribute to colonization and pathology. We have compared mice showing similar bacterial load after infection with gGT-proficient or gGT-deficient H. pylori to analyse the specific role of the enzyme during infection. Our data indicate that H. pylori gGT supports initial colonization. Nevertheless, bacteria lacking gGT can still colonize and persist. We observed that the presence of gGT during infection favoured a proinflammatory innate and adaptive immune response. Notably, H. pylori gGT activity was linked to increased levels of IFNγ, which were attributed to a differential recruitment of CD8+ T cells to the stomach. Our data support an essential role for H. pylori gGT in gastric colonization and further suggest that gGT favours infiltration of CD8+ cells to the gastric mucosa, which might play an important and yet overlooked role in the pathogenesis of H. pylori.
Introduction
Over 50% of the world’s population persistently carries Helicobacter pylori, which is the main cause of gastric inflammation and more severe gastroduodenal diseases, such as gastric and duodenal ulcers, gastric cancer, or lymphoma1. Host, environmental and bacterial factors determine the inflammatory response towards the bacterium and thus the outcome of the disease.
H. pylori infection is associated with an increased influx of innate and adaptive immune cells into the stomach and production of proinflammatory cytokines2,3. During natural and experimental H. pylori infection in humans, gastric T lymphocytes are increased4,5 and T cell activation marker-positive (CD25+ and CD69+) cells are present at higher numbers compared to uninfected individuals6. Even though H. pylori is an extracellular pathogen, which are generally controlled by a Th2-driven antibody response, CD4+ T helper (Th) cells infiltrating the stomach rather display a Th1 and Th17 phenotype7–9]. Th cells produce a distinct set of cytokines. While IL-17 is the hallmark of a Th17 response, IFNγ is often used as a surrogate marker for a Th1 response. Studies with IFNγ-deficient mice demonstrated that IFNγ plays a major role in H. pylori-associated gastritis10,11. In addition, a regulatory T cell (CD3+/CD4+/FoxP3+) response is induced by H. pylori infection that is characterized by expression of IL-10 and TGFβ3,12,13.
Several virulence factors contribute to severity of H. pylori-associated pathology. Strong gastric inflammation and severe clinical manifestations in infected patients are, in part, attributed to the presence of cytotoxin-associated gene A (CagA) and vacuolating toxin A (VacA)14,15. These factors are directly - via a type 4 secretion system for CagA - or indirectly – by secretion and outer membrane vesicles for VacA - delivered to host cells and cause detrimental effects mainly on gastric epithelial cells16,17.
More recently, H. pylori γ-glutamyl transferase (gGT) has emerged as virulence factor that contributes to peptic ulcer disease and gastric cancer18,19. All H. pylori strains express gGT, stressing the fundamental function of this virulence factor. Thus, H. pylori-dependent glutaminase activity mediated by gGT, which results in the deprivation of glutamine and release of glutamate and ammonia into the periplasm as well as the surrounding environment, plays an important role in bacterial metabolism. For example, gGT-dependent production of ammonia has been shown to contribute to acid resistance of H. pylori 20. In addition, glutamate liberated by gGT can be taken up by H. pylori and is introduced into nitrogen and carbon metabolism21. Notably, gGT-dependent glutaminase activity not only contributes to bacterial metabolism, but also affects host’s epithelial and immune cells. Hence, glutamine deprivation, caused by H. pylori gGT activity, induces secretion of proinflammatory IL-8 in gastric epithelial cells19 and compromises T cell effector function22. Furthermore, H. pylori gGT activity drives dendritic cells towards a more tolerogenic phenotype favouring a regulatory T cell response, which might have consequences for immunopathology and persistence of the bacterium23,24.
Despite the fact that H. pylori gGT can directly affect host cells and at the same time is important for bacterial metabolism, the overall effect of this virulence factor during infection is not well understood. In this study, we have analysed the effect of gGT activity at different stages of infection in order to discriminate between the effects of the enzyme on the bacteria and the effects on host epithelial and immune cells. Moreover, we conducted a comprehensive analysis of the host’s immune response towards H. pylori infection in the absence and in the presence of gGT. Our results indicate that H. pylori gGT has a crucial role in gastric colonization and suggest that gGT enhances gastric inflammation and favours infiltration of CD8+ T cells to the gastric mucosa.
Results
H. pylori gGT activity contributes to the establishment of gastric colonization
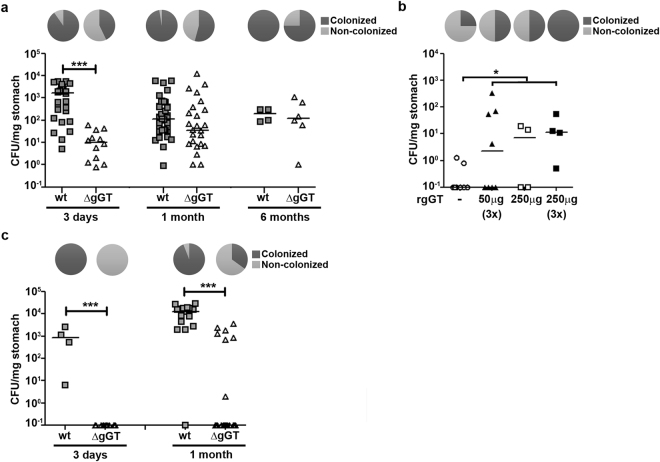
It has been suggested that H. pylori gGT is essential for colonization but it has also been argued that it is required for persistence24–26. To define the role of H. pylori gGT during different stages of murine infection, C57Bl/6 mice were infected with H. pylori PMSS1 wt (wild-type, gGT-positive) or ΔgGT (gGT-negative).Gastric colonization was analysed after three days, one and six months (Fig. 1a). The overall rates of H. pylori infection were significantly higher in gGT-positive compared to gGT-negative strains (94.7% [71/75] vs. 52.4% [44/84]; p < 0.0001). At day three, gGT-negative H. pylori failed to establish an infection in 57.1% of inoculated mice; in contrast the gGT-positive H. pylori colonized more than 90% (Table 1). At one and six months post infection, 54.2% and 75% of mice harboured gGT-deficient H. pylori, while wt bacteria were detected in all but one mice infected with H. pylori wt. To confirm that the bacteria found in the stomachs of H. pylori ΔgGT-infected mice were still gGT-deficient, isolates were plated on agar plates containing or lacking kanamycin (Supplementary Fig. S1a). No significant differences in colony count were found in the presence or in the absence of kanamycin, indicating that the resistance cassette interrupting the gGT gene was still present.
Figure 1.
H. pylori gGT supports initial colonization. (a) Mice were inoculated with H. pylori PMSS1 (2 × 109 CFU) wt or ΔgGT and sacrificed after three days, one month, or six months. Colonization level was determined by plating of serial dilutions. Mice from which bacteria could be grown at the time of analysis are shown. (b) C57Bl/6 mice were infected with H. pylori ΔgGT. 50 or 250 µg or recombinant H. pylori gGT were administered at the time of infection (1x) and on two consecutive days when indicated (3x). Data derived from one or two inoculations with four mice per group are shown. (c) Rag−/− mice were infected with H. pylori wt or ΔgGT for three days and one month. Colonization levels of mice are shown. Mann-Whitney U test. *p ≤ 0.05, ***p ≤ 0.001. Horizontal bars indicate medians. Pie graphs indicate % of colonized mice.
Table 1.
Colonization of C57Bl/6 mice infected with gGT-positive (wt) and gGT-negative (ΔgGT) H. pylori.
| H. pylori straina | 3 days | 1 month | 6 months | |||
|---|---|---|---|---|---|---|
| wt | ΔgGT | wt | ΔgGT | wt | ΔgGT | |
| number of independent inoculations | 6 | 6 | 8 | 8 | 1 | 1 |
| % noCFU (non-colonized/all mice) | 9.7% (3/31) | 57.1% (16/28)*** | 2.5% (1/40) | 45.8% (22/48)*** | 0% (0/4) | 25% (2/8) |
| CFU/mg stomach (all mice)b | 661 (78.5–2947) | 0 (0–7.9)### | 107.5 (33.6–418) | 1 (0–52.5)### | 200 (89.5–304) | 77 (0.3–511) |
| CFU/mg stomach (colonized mice)b | 1693 (154–3134) | 10.4 (1.5–31)### | 111 (33.7–426) | 35 (7.2–314) | 200 (89.5–304) | 122 (44.5–746) |
| % colonization rate per inoculation (range) | 91.7% (75–100%) | 50% (0–56.25%) | 100% (100–100%) | 50% (40–84.4%) | 100% | 75% |
aC57Bl/6 mice were infected with 2 × 109 H. pylori PMSS1 and analysed at different times post infection.
bValues are medians with interquartile range in parentheses.
***p ≤ 0.001, significant difference compared to H. pylori wt-infected mice according to Chi-squared test.
###p ≤ 0.001, significant difference compared to H. pylori wt-infected mice according to Mann-Whitney U test.
When only mice that were colonized at the time of analysis were considered, the median CFU per mg stomach was still significantly lower (164-fold) in mice colonized with gGT-negative compared to gGT-positive H. pylori at day three post inoculation (Fig. 1a and Table 1). At one month, this trend was still observable (3.2-fold), although it was not statistically significant. No differences in bacterial load were detected in mice colonized with H. pylori wt or ΔgGT for six months.
These results suggest that H. pylori gGT supports initial colonization. Once colonization is established, the CFUs observed in the stomach of H. pylori ΔgGT-infected mice increase to a similar level as observed in mice infected with the wt strain.
To confirm the supporting role of gGT for initial colonization and exclude a possible effect of the host’s immune response that would preferentially clear gGT-deficient bacteria, we performed histological analysis of the gastric mucosa after three days post-infection. Infected mice did not present innate or adaptive immune cell infiltration at this early stage of infection (Supplementary Fig. S1b,c). This confirms that the presence of gGT supports colonization itself and might represent a metabolic advantage for the bacteria. We further substantiated this hypothesis by externally supplementing recombinant gGT protein during infection. Notably, recombinant gGT was able to complement the gGT-deficient strain to some extent and allowed infection of a higher percentage of mice (Fig. 1b). Increasing the dose (50 or 250 µg) and frequency of application (one or three doses) of the recombinant enzyme increased infection rate with H. pylori ΔgGT.
Since H. pylori is secreted into the periplasm and also found in the extracellular space26,27, we postulated that gGT produced by wt bacteria might support colonization of H. pylori ΔgGT. Mice were inoculated with either H. pylori wt, H. pylori ΔgGT, or a combination of both at a 1:1 ratio. However, the proportion of mice colonized with H. pylori ΔgGT was even lower after coinfection compared to inoculation of the gGT-deficient strain alone (0% [0/17] vs. 42.9% [9/21]; p < 0.0001), while colonization level and rate of H. pylori wt was unaffected by coinfection (Supplementary Fig. S1d). Also, an indirect effect mediated by tolerization of dendritic cells by gGT released from wild type bacteria does not seem to facilitate colonization with H. pylori ΔgGT. Thus, these findings suggest that lack of gGT represents an important metabolic disadvantage for H. pylori and thus bacteria deficient for gGT are outcompeted by the wt.
Moreover, we studied the colonization capacity of wt- and gGT-deficient H. pylori in the absence of adaptive immune responses. Thus, Rag−/− mice (lacking B and T cells) were infected with H. pylori wt or ΔgGT for three days or one month. After three days, no colony forming units were detected in the stomach of Rag−/− mice infected with gGT-deficient H. pylori (Table 2 and Fig. 1c). After one month, H. pylori ΔgGT failed to establish an infection in more than 50% of mice, as it was observed in wt mice. This indicates that the same initial hurdle constrains colonization in absence of gGT activity in Rag−/− mice as in wt mice. However, once the infection was established, gGT-negative H. pylori colonized Rag−/− mice at a significantly lower level compared to gGT-positive bacteria (Table 2). These results indicate that the equalization of CFU observed in the long term infection of wt mice in the absence of gGT depends on the host’s immune response.
Table 2.
H. pylori wt and ΔgGT colonization of immunodeficient Rag−/− mice.
| H. pylori straina | 3 days | 1 month | ||
|---|---|---|---|---|
| wt | ΔgGT | wt | ΔgGT | |
| number of independent inoculations | 1 | 3 | 4 | 4 |
| % noCFU (non-colonized/all mice) | 0% (0/4) | 100% (12/12) | 5.9% (1/17) | 65% (13/20) |
| CFU/mg stomach (all mice)b | 840 (139.5–2202) | 0 0*** | 13158 (3629–18093) | 0 (0–816)*** |
| % colonization rate per independent inoculation (range) | 100% (100%) | 0% (0) | 100% (85–100) | 38.3% (4.2–71–3) |
aMice were infected with 2 × 109 H. pylori PMSS1 and analysed at 3 days or 1 month post infection.
bValues are medians with interquartile range in parentheses.
***p ≤ 0.001, significant difference compared to H. pylori wt-infected mice according to Mann-Whitney U test.
A substantial proportion of gGT-deficient bacteria were able to establish an infection (Fig. 1a). This implies that H. pylori might be able to adapt to and compensate for absence of gGT activity. Therefore, the colonization capacity of H. pylori ΔgGT re-isolated from stomachs of mice that were colonized for different times (10 days and 1 month) and at different levels (high [558 CFU/mg] and low [22 CFU/mg]) was tested. C57Bl/6 mice were inoculated either with the H. pylori ΔgGT parental strain or with bacteria that had been isolated from H. pylori ΔgGT-colonized mice. Presence of kanamycin and absence of gGT activity in H. pylori ΔgGT isolates was confirmed by PCR and a gGT activity assay (Supplementary Fig. S1e,f). Interestingly, all mice were colonized at one-month post infection with the gGT-deficient isolates tested, while the gGT-negative parental strain colonized only three of four mice (Supplementary Fig. S1g). In addition, the bacterial burden was less variable within the groups of mice infected with the isolates compared to mice infected with the parental strain. This indicated that H. pylori was indeed able to adapt to a lack of gGT activity with respect to colonization capacity.
H. pylori gGT favours a proinflammatory innate immune response
We observed that H. pylori wt and ΔgGT established comparable levels of colonization at one month post infection (Fig. 1a). This allowed us to investigate gGT-dependent differences in the host’s immune response, since changes could be directly attributed to gGT activity and not to differences in bacterial burden.
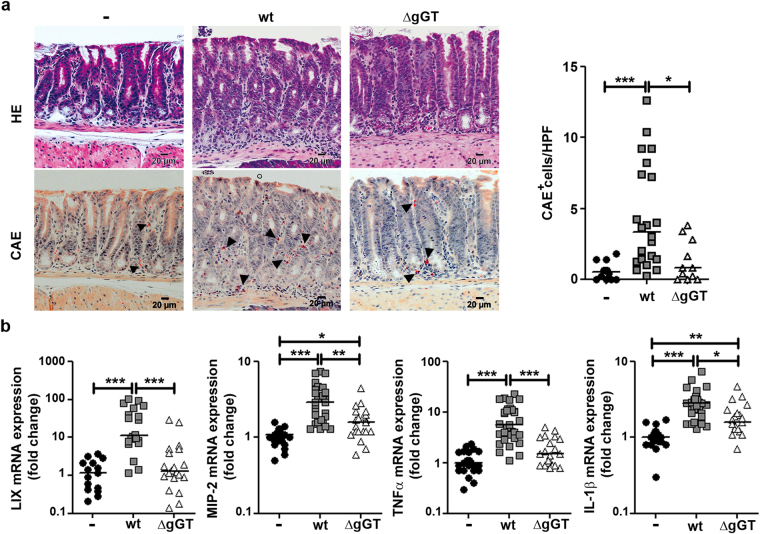
We first focused on innate immune responses and analysed infiltration of neutrophils into the stomach by chloroacetate esterase (CAE) staining of gastric tissue samples. H. pylori wt infection induced neutrophil recruitment to the stomach (Fig. 2a). Notably, presence of neutrophils was barely detected in the stomach of mice infected with H. pylori ΔgGT. After six months, no differences in gastric neutrophil infiltration were observed between mice infected with H. pylori wt and ΔgGT (Supplementary Fig. S2a,b).
Figure 2.
H. pylori gGT activity induces a strong innate immune response. C57Bl/6 mice were infected for one month. (a) Haematoxylin-eosin (HE) and chloroacetate esterase (CAE) staining of murine stomach sections. Images from representative mice of each group are shown. Number of CAE+ cells per high power field (HPF) was determined. Data are derived from three independent experiments. (b) mRNA expression levels of chemokines (LIX, MIP-2) and cytokines (TNFα, IL-1β) associated with the innate immune response determined by quantitative PCR. Results from four to six independent experiments are included. Kruskal-Wallis test followed by Dunn’s test for multiple comparisons. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001. Horizontal bars indicate medians.
Next, chemokines and cytokines expressed by innate immune cells and antigen-presenting cells including epithelial cells were assessed by quantitative PCR. In humans, CXCL5, also named epithelial-derived neutrophil-activating peptide 78 (ENA-78) since is mainly expressed by epithelial cells, is among the most strongly upregulated genes in human gastric mucosa upon infection with H. pylori 28,29. Interestingly, expression of the murine CXCL5 homologue, LIX, was strongly upregulated (11.3-fold) in mice colonized with H. pylori wt but not with H. pylori ΔgGT (Fig. 2b), despite of a similar bacterial burden. Another factor involved in neutrophil recruitment is IL-8. It is mainly produced by gastric epithelial cells upon infection with H. pylori 30,31. Thus, we analysed the expression of the murine IL-8 homologue, MIP-2. MIP-2 expression was higher in stomachs of mice infected with H. pylori wt compared to mice infected with H. pylori ΔgGT (2.9- vs. 1.6-fold) (Fig. 2b). We also analysed the expression of other cytokines involved in the innate immune response towards H. pylori such as TNFα and IL-1β. TNFα expression was induced 5.7-fold by colonization with H. pylori wt, while it was not induced by H. pylori ΔgGT (Fig. 2b). Thus, its expression was strongly gGT-dependent. Expression of IL-1β, mainly produced by monocytes and macrophages, was also significantly induced (2.8-fold) in mice colonized with H. pylori wt but at a lower level by colonization with H. pylori ΔgGT (Fig. 2b). Together these results indicate that the presence of gGT shapes innate immune responses against H. pylori towards a more proinflammatory phenotype.
Absence of gGT activity results in similar T helper cell responses, yet leads to reduction of H. pylori-induced IFNγ expression
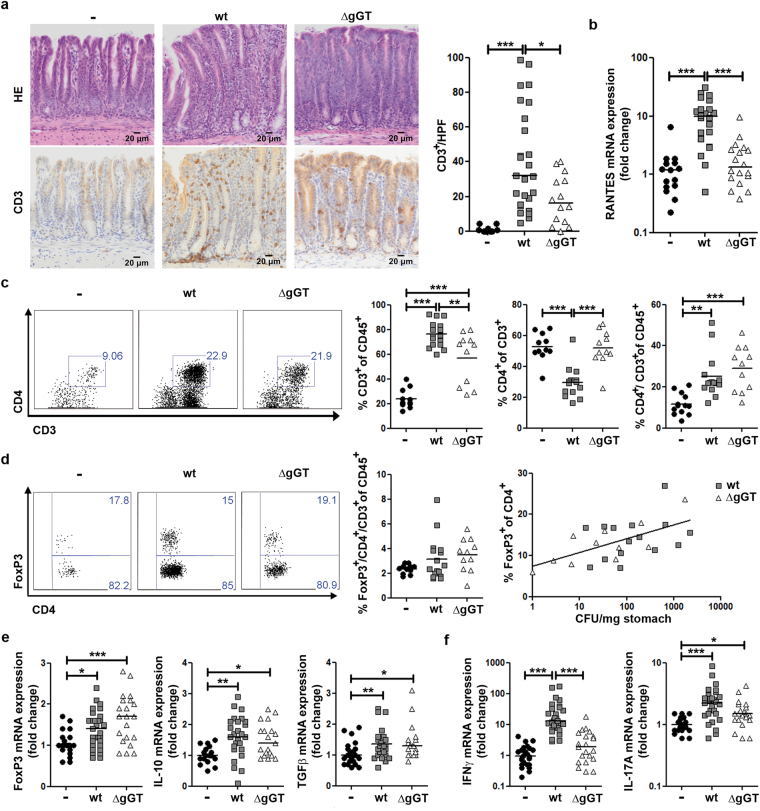
When analysing adaptive immune responses of mice infected for one month with H. pylori wt or ΔgGT, we observed that gGT-positive H. pylori induced significantly stronger infiltration of CD3+ T cells into the stomach mucosa compared to colonizing H. pylori devoid of gGT activity (Fig. 3a), even though T cell infiltration was induced by H. pylori ΔgGT as well. Thus, gGT contributed to enhance influx of T cells in the infected stomach. CD3+ T cells were mainly localized at the cardia and in antrum, whereas they were rarely found in the corpus. After six months, the effect of the presence of gGT activity on the recruitment of T cells was even more pronounced, since T cells accumulated over time during infection with H. pylori wt (Supplementary Fig. S2a,c).
Figure 3.
T cell response in mice infected with H. pylori wt or ΔgGT. Stomachs of mice were analysed one month post infection. (a) Representative images of Haematoxylin-eosin stain (HE) and CD3 expression in the stomach mucosa of mice from four independent experiments. Number of CD3+ cells per high power field (HPF) is shown. (b) mRNA levels of RANTES determined by quantitative PCR. Data depicted are derived from four independent experiments with four to eight mice per group. (c) Flow cytometry analysis of CD4+ T cell population in the gastric mucosa of mice. An exemplary dot plot of each group is depicted. (d) FoxP3-expressing CD4+ T cells. A representative dot plot of FoxP3 expression in CD4+ T cells is displayed. Proportion of FoxP3-expressing CD4+ T cells in the CD45+ leukocyte population and a positive non-linear correlation between the FoxP3-expressing CD4+ population and bacterial burden are shown (Spearman’s r = 0,7, p = 0.0204). (e) and (f) mRNA levels of cytokines associated with a T regulatory response FoxP3, IL-10, and TGFβ and expression of effector cytokines IFNγ and IL-17 A analysed by quantitative PCR are shown. Data presented are derived from five to six independent experiments with four to eight mice per group. Kruskal-Wallis test followed by Dunn’s test for multiple comparisons. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001. Horizontal lines represent medians.
Recruitment of T cells is determined by chemokines and the corresponding receptors guiding activated T cells to the sites of infection. RANTES (CCL5), a chemokine attracting T cells, is also induced in gastric epithelial cells during H. pylori infection32. Expression of RANTES was induced 9.8-fold by colonization with H. pylori wt, while during colonization with H. pylori ΔgGT, its levels were comparable to those observed in uninfected animals (Fig. 3b). Lack of RANTES expression may, in part, account for reduced infiltration of T cells observed in absence of gGT.
As we observed that T cells were differentially recruited in absence of gGT activity, their phenotype was further analysed by flow cytometry of stomach homogenates. The proportion of CD3+ T cells within the CD45+ leukocyte population was increased upon colonization with H. pylori wt as well as ΔgGT (Fig. 3c). However, influx of CD3+ T cells was significantly enhanced in mice colonized with H. pylori wt compared to ΔgGT (Fig. 3c), confirming the results observed on tissue sections. Furthermore, we observed that CD4+ T cells were recruited to the stomach during infection with H. pylori. While the proportion of CD4+ T cells from the CD45+ leukocyte population was similar in mice colonized by H. pylori wt or ΔgGT, infiltration of non-CD4+ T cells - most likely CD8+ T cells - were more strongly induced by gGT-positive H. pylori (Fig. 3c).
Even though the proportion of CD4+ T cells with respect to the CD45+ population was similar in H. pylori wt and ΔgGT infected mice, the subtypes of the T helper cell population can differ. Since it had been suggested that H. pylori gGT activity promotes the establishment of a regulatory T cell (Treg) response24, FoxP3 expression and proportion of FoxP3-expressing CD4+ T cells in gastric tissue of infected mice were analysed. In mice colonized with H. pylori, infiltration of Foxp3 cells was slightly (not significantly) induced, independently of the gGT status. Interestingly, the proportion of FoxP3-expressing cells within the CD4+ T cell population correlated with CFU (Spearman’s r = 0.7; p = 0.0204) (Fig. 3d). This indicates that the abundance of other bacterial factors (not gGT) contributes to Treg induction. In accordance with this, FoxP3 mRNA expression was significantly induced also in mice colonized with H. pylori ΔgGT (Fig. 3e). In addition to FoxP3, we studied expression of cytokines that cooperate in Treg responses, specifically IL-10 and TGFβ. Both cytokines were slightly, yet significantly, induced, also in mice colonized with gGT-negative H. pylori (Fig. 3e). Thus, the Treg response was induced to a similar extent in mice colonized with H. pylori wt or ΔgGT.
We next analysed IFNγ and IL-17 levels as surrogates for Th1 and Th17 responses. Notably, H. pylori wt and ΔgGT-infected mice showed a distinct expression pattern of proinflammatory Th1 and Th17 effector cytokines. Infection with H. pylori wt caused a 13.1-fold and 2.2-fold increase in IFNγ and IL-17 A expression in the gastric mucosa, respectively (Fig. 3f). In the absence of gGT, IL-17 A expression but not IFNγ expression was induced by H. pylori. Thus, IFNγ but not IL-17 A expression was dependent on the presence of gGT. Taken together, these results demonstrate that H. pylori ΔgGT is not defective in eliciting a Treg response and it induces a similar CD4+ Th cell response compared to wt bacteria. However, IFNγ-expression is strongly dependent on gGT activity, suggesting that CD4+ T cells might not be the major source of IFNγ during infection.
H. pylori gGT induces strong gastric infiltration of CD8+ T cells
As the differential gastric expression of IFNγ between mice colonized by H. pylori wt bacteria and mice colonized by H. pylori ΔgGT could not be attributed to differences in infiltrating CD4+ T cells, we next studied the influx of CD8+ T cells. CD8+ T cells produce high levels of IFNγ and could account for IFNγ production during H. pylori infection. Indeed, we observed higher infiltration of CD8+ cells in the stomach of mice infected with H. pylori wt compared to animals infected with gGT-deficient bacteria (Fig. 4a and Supplementary Fig. S2a). This indicates that gGT favours recruitment of CD8+ cells to the stomach.
Figure 4.
Presence of H. pylori gGT induces CD8+ T cell infiltration in a glutamine-dependent manner. (a) Gastric tissue samples from mice infected with H. pylori wt or ΔgGT for one month were analysed for CD8+ cells by immunohistochemistry. Quantification of CD8+ cells per high power field (HPF) from three independent experiments was performed. (b) Infiltration of CD4+ and CD8α+ T cells in the stomach analysed by flow cytometry after one month of infection with H. pylori wt. One group of mice received a glutamine-enriched (20% [w/w]) diet compared to a group of mice that were fed a control diet (0%). Data are derived from two experiments with five mice per group. (c) IFNγ mRNA levels and (d) colony forming units (CFU) per mg of tissue in stomach samples of mice following a control (0%) or a glutamine-enriched (20%) diet. (e) Spearman’s correlation between gGT activity of freshly isolated H. pylori strains in vitro and CD8+ cells infiltrating corresponding human stomach samples. r = 0.599; p = 0.0236. (f) Representative CD8 staining of human gastric biopsies. Scale bar 100 µm. Mann-Whitney U test ([A]-[C]). *p ≤ 0.05, **p ≤ 0.01. Horizontal bars indicate medians.
We and others have shown that glutaminase activity of gGT may affect the viability of host cells. In fact, gGT-mediated glutamine deprivation in the gastric mucosa may compromise the epithelial barrier, induce proinflammatory NFκB signalling, and subsequently cause alterations in the migration pattern of immune cells. To compensate for glutamine deprivation induced by gGT activity, mice were fed a chow enriched with 20% glutamine or an isonitrous control diet during infection. Remarkably, supplementation of glutamine reduced infiltration of T cells and in particular of CD8+ T cells into the stomach, while CD4+ T cells were unaffected (Fig. 4b). Reduced CD8+ T cell infiltration correlated with reduced levels of IFNγ (Fig. 4c) suggesting that CD8+ cells are a major source of IFNγ during H. pylori infection. These effects could not be attributed to differences in bacterial burden since mice fed with the 20% glutamine enriched food presented similar colonization levels (Fig. 4d).
In order to study whether gGT activity was also linked to differential recruitment of T cells into the stomach in humans, we first analysed gGT activity of several clinical isolates. All clinical isolates tested showed gGT activity, but at different levels (Supplementary Fig. S3a). In parallel, we stained the corresponding gastric biopsies, from which the bacteria had been isolated, for CD3+, CD4+, and CD8+ cells. Although no differences in CD3+ cells infiltrating the gastric tissue were detected between samples showing high or low gGT activity (Supplementary Fig. S3b), we observed a significant correlation between CD8+ cells infiltrating the gastric tissue and gGT activity of the corresponding clinical isolates (Spearman’s r = 0.599; p = 0.0236) (Fig. 4e,f). Thus, clinical isolates showing higher gGT activity induced stronger recruitment of CD8+ cells into the gastric mucosa. In contrast, no correlation between gGT activity and infiltration of CD4+ cells was detected (Supplementary Fig. S3c). Taken together, this indicates that gGT activity favours the recruitment of CD8+ cells to the stomach of H. pylori infected individuals, as was observed in mice.
Discussion
H. pylori is a highly effective pathogen colonizing the hostile niche of the stomach in every second human worldwide. For that, the bacteria employ a panel of sophisticated virulence traits not only to establish an acute infection but also to achieve persistent colonization. While the importance of certain bacterial adhesins is well appreciated in this process, much less is known about metabolic bacterial factors. In this study, we investigated H. pylori gGT, an enzyme with highly conserved function among H. pylori strains. All clinical isolates seem to harbour and express active gGT, suggesting an important role for this bacterial factor during infection. While studies of H. pylori in humans are generally limited to subjects with established infection, murine infection models offer the great advantage that infection in presence and absence of gGT can be studied. Previous studies in mice were not conclusive regarding the question of whether H. pylori gGT is mainly required for initial colonization or for the establishment of a persistent infection24–26. Differences among these studies regarding colonization capacity of H. pylori ΔgGT are likely due to different strains used and the variability in colonization rate from independent inoculations.
By analysing bacterial load at day three and manipulating the presence of enzymatic activity, we found evidence that gGT mainly determines colonization capacity during initial colonization. The study of colonization levels for up to six months revealed that H. pylori ΔgGT was capable to colonize persistently in mice once it had established an infection. While further metabolomic analyses are necessary to explore what discriminates those gGT-deficient bacteria that were able to colonize from those that failed to establish an infection, our results indicate that H. pylori gGT activity provides an advantage to the bacterium favouring initial colonization. Several functional roles of H. pylori gGT by which the enzyme may support the bacteria have been suggested. gGT provides nitrogen and carbon sources for bacterial metabolism and contributes to acid resistance through release of ammonium20,21,33. Considering that H. pylori ΔgGT is not growth-deficient in vitro, the stomach environment seems to pose a specific hurdle for colonization that is overcome with aid of gGT-activity. H. pylori gGT may modify the availability of amino acids or produce neutralizing ammonia. In humans, H. pylori seems to depend even more on gGT activity as no gGT-deficient human isolate has been described. This indicates that a stronger selective pressure, such as higher acidity or fewer alternative nutrients, is encountered in the stomach of humans compared to mice. The notion that H. pylori gGT was important to establish an infection in the first place is supported by the absence of signs for an innate or adaptive immune defence at three days post infection. In addition, a similar initial hurdle was also observed in Rag−/− mice, where adaptive immune cells are absent.
Direct effects of H. pylori gGT on gastric epithelial and immune cells had been proposed from previous studies. On the one hand, toxic effects on gastric epithelial cells due to oxidative stress and ammonia production promoting proinflammatory pathways have been observed19,34,35. On the other hand, H. pylori gGT has been demonstrated to drive dendritic cells towards a tolerogenic phenotype23,24 and to suppress effector T cell activity in a glutamine-dependent manner22. The contributions of these effects during infection are still elusive.
Given the finding that mice were colonized with H. pylori wt or ΔgGT at a comparable level at one and six months in this study, changes in the immune response could be attributed to gGT activity (rather than colonization density). At a similar bacterial burden, other bacterial factors known to influence the immune response, e.g. VacA and CagA, are assumed to be present at similar levels. In humans, IL-8, but not ENA-78/CXCL5 expression, has been shown to be associated with CagA status28. Here, we demonstrate that the induction of the murine CXCL5 homologue, LIX, is strongly dependent on the presence of gGT. CXCL5 is involved in the regulation of CXCR2-dependent neutrophil trafficking and has been associated with the pathology of inflammatory bowel disease in human and DSS-induced colitis in mice36,37. Thus, we hypothesize that induction of CXCL5 may be one mechanism by which gGT contributes to gastric inflammation.
Surprisingly, T helper cell responses were not altered in mice colonized with the gGT-deficient H. pylori strain, since CD4+ T cell infiltration, IL-17 A expression, and Treg markers were comparable to mice infected with the wt strain. However, IFNγ expression and infiltration of CD3+ T cells (to a lesser extend) were induced stronger in mice colonized with gGT-proficient bacteria. IFNγ plays a major role in H. pylori-associated gastritis, as previous studies with IFNγ-deficient mice have shown10,11. Since infiltration of CD4+ T cells was not changed, we assume that IFNγ might originate from CD8+ T cells. Interestingly, Ruiz et al. reported a dominance of CD8+ T cells over CD4+ T cells during H. pylori infection and that a higher proportion of CD8+ T cells compared to CD4+T cells isolated from the stomach of H. pylori PMSS1-infected mice produced IFNγ upon CD3/CD28 and PMA/Ionomycin stimulation38. This concurs with results presented in this study, as we found a greater proportion of CD8+ T cells compared to CD4+T cells in the stomach of H. pylori-infected mice, which was accompanied by strong expression of IFNγ. Notably, infiltration of CD8+ T cells into the gastric mucosa of infected mice as well as IFNγ expression was highly dependent on the presence of gGT. This was corroborated by our findings in human tissue revealing that infiltration of CD8+ T cells correlated with higher gGT activity. Therefore, we sought to determine how gGT activity may influence CD8+ T cell levels in the stomach. Interestingly, gGT-induced effects on gastric epithelial cells have been attributed to glutamine deprivation and toxic ammonia19,35. To counteract gGT-induced glutamine deprivation in the gastric mucosa, we supplemented glutamine during infection with H. pylori. It has been reported before that glutamine administration protects rodents against H. pylori-induced pathology39,40, even though it had not been linked to gGT activity. Remarkably, gGT-induced effects were reverted by administration of glutamine, as infiltration of CD8+T cells was strongly reduced, while bacterial load was unaffected. This suggests that gGT-dependent glutamine deprivation plays a pivotal role in promoting H. pylori-induced mucosal inflammation and differential recruitment of T cells into the stomach. Glutamine deprivation was shown to stimulate mTOR-JNK-dependent chemokine secretion41, to trigger an endoplasmic reticulum (ER) stress response, and to induce NFκB activation - all culminating in the induction of the proinflammatory chemokine IL-842. Notably, a subset of CD8+ T cells bearing the CXC chemokine receptor 1 (CXCR1) was described. These cells responded to IL-8 and expressed perforin, granzyme B, and IFNγ, and had high cytotoxic potential43.
In human gastric tissue, CD4+ as well as CD8+ T cells are increased upon H. pylori infection5,8,44. Moreover, CD8+ T cells isolated from H. pylori-infected patients produced more IFNγ than CD4+ T cells on a per cell basis upon antigen restimulation45. In experimental H. pylori infection of mice, CD8+ as well as CD4+ T cells contribute to control of colonization since absence of either T cell subtype, due to MHC class I or II deficiency, led to a similar increase in bacterial burden compared to wt mice46. However, most research focused on CD4+ T cells and neglected possible clinically relevant roles of CD8+ T cells. Our results suggest a significant role for CD8+ T cells in the course of H. pylori infection, which has been overlooked so far and merits further characterization.
Taken together, our results provide evidence that the enzymatic activity of gGT is not only important for initial colonization by the bacterium, but that it profoundly changes the stomach milieu instructing differential recruitment of immune cells into the gastric mucosa, which can have a major impact in the final outcome of the infection.
Methods
Bacterial infection of C57Bl/6 mice with H. pylori
Six- to eight-week old female C57Bl/6 wild-type (wt) mice were obtained from Harlan. C57Bl/6 Rag−/− mice were derived from in-house breeding. Mice were housed under specific pathogen-free conditions and maintained under microisolator cages with chow and water ad libitum. Experiments were conducted in compliance with European guidelines for the care and use of laboratory animals and were approved by the local authorities (Regierung von Oberbayeren [AZ 55.2.1-54-2532-147-12]). Prior to infection, mice were fasted for four to six hours. H. pylori bacteria (2 × 109 CFU) were administered to mice once by oral gavage in 200 µl Brucella broth containing 10% FCS. The H. pylori PMSS1 strain and its isogenic mutant hp1118::kan (ΔgGT) was used47. Mice were sacrificed after three days, one month, or six months post infection, and gastric tissue was collected for histologic examination, quantitative bacterial culture, mRNA expression analysis, and flow cytometry.
Experimental design, administration of recombinant gGT
Mice inoculated with H. pylori ΔgGT, as described above, were administered recombinant H. pylori gGT (rgGT)22. Groups of four mice orogastrically received 50 µg or 250 µg rgGT (150 µl in PBS) at the time of infection and on the two consecutive days (3x). One group that had received the high dose (250 µg) was only treated once. A control group was only given bacteria. Colonization level was analysed three days after infection.
Experimental design, supplementation of glutamine
For supplementation of glutamine, mice infected with H. pylori wt or ΔgGT received research diets enriched with 20% glutamine or an isonitrous control diet (Ssniff Research Diet). Chow was provided ad libitum during the infection period. Mice were infected with H. pylori wt or ΔgGT for one month. Control groups received the respective diets but were not given bacteria.
Assessment of bacterial colonization
For colonization levels, longitudinal gastric tissue sections were weighted and homogenised in Brucella broth containing 10% FCS. Then, number of colony forming units (CFU) was determined by plating serial dilutions on WC-dent agar plates supplemented with bacitracin (200 µg/ml), nalidixic acid (10 µg/ml), and polymyxin B (3 µg/ml).
Histologic examination of gastric tissue
For histology of murine stomachs, a longitudinal strip was cut from the oesophagus to the duodenum, along the lesser curvature, and back to the oesophagus along the longer curvature. Gastric tissue was fixed with formalin and embedded in paraffin. Human gastric tissue samples were obtained from the Institut für Pathologie, Klinikum München-Bogenhausen, after approval of the ethical committee of the Klinikum rechts der Isar. Informed consent was obtained from all subjects undergoing gastric endoscopy. All experiments were performed in accordance with relevant guidelines and regulations.
For histologic evaluation haematoxylin and eosin (HE), chloroacetate esterase staining (CAE) and immunohistochemistry were performed. Pathology score was determined according to the updated Sydney score system48.
For immunohistochemistry, sections were dewaxed in xylene and gradually rehydrated 50-100% ethanol. Heat-induced antigen retrieval was performed in 0.01 M sodium citrate pH 6 and slides were blocked in 5% goat serum for 1 h at room temperature. A primary monoclonal CD3 antibody was purchased from Neomarkers LabVision. A CD8 antibody from Thermo Scientific was used to stain human samples while a CD8 antibody from Dianova was used on murine samples. Gastric sections were incubated with primary antibodies overnight at 4 °C following manufacturer’s instructions. HRP-conjugated secondary antibody was applied for 1 h and samples were developed using SignalStain DAB substrate (Cell Signaling). Murine sections were stained on an automated staining machine (Bondmax Rxm) with 15 min incubation of the primary antibody, a secondary rabbit-anti-rat antibody (Vector Laboratories) and the Polymer Refine Detections system (Leica). Sections were counterstained with haematoxylin. Automated image acquisition was performed using the Virtual Slide Scanning System VS120 (Olympus). Five high power fields (20x magnification) were randomly scored for each sample.
Flow cytometry
Gastric samples were placed in RPMI (Gibco) containing 10% FCS and processed using DNase (1 mg/ml, Roche Diagnostics) and Collagenase IV (200 µg/ml, Sigma-Aldrich) digestion for 30 min at 37 °C under rapid shaking to isolate immune cells. Cells were treated with ethidium monoazide for live/dead discrimination. Then, surface antigens were stained using following fluorochrome-conjugated antibodies (eBioscience): CD45-APC, CD3-PECy7, CD4-eF450, and CD8α-APC-Cy7. For flow-cytometric detection of FoxP3, cells were stained for cell surface markers, fixed, permeabilized, and stained with a PE-conjugated FoxP3 antibody (eBioscience). After incubation, cells were fixed with 0.5% paraformaldehyde. Data were acquired by a CyAN ADP 9 color analyzer (Beckman Coulter) and analysed using Flow Jo software (Tree Star).
Real-time quantitative PCR
Total RNA was isolated from homogenized gastric tissue using the RNeasy Mini Kit (Qiagen) according to the manufacturer’s instructions including on-column DNase digestion. RNA integrity was confirmed by agarose gel electrophoresis. RNA was converted to cDNA using a combination of random hexamer primers and M-MLV reverse transcriptase (Promega). Subsequently, cDNAs were amplified using a CFX384 qPCR cycler (Bio-Rad). The sequences of the gene-specific primers used were as follows: GAPDH sense primer, 5′-GCCTTCTCCATGGTGGTGAA-3′; GAPDH antisense primer, 5′-GCACAGTCAAGGCCGAGAAT-3′; IFNγ sense primer, 5′-TCAAGTGGCATAGATGTGGAAGAA-3′; IFNγ antisense primer, 5′-TGGCTCTGCAGGATTTTCATG-3′; IL-17 A sense primer, 5′-GCTCCAGAAGGCCCTCAGA-3′; IL-17 A antisense primer, 5′-AGCTTTCCCTCCGCATTGA-3′; TNFα sense primer, 5′-CGATGGGTTGTACCTTGTC-3′; TNFα antisense primer, 5′-CGGACTCCGCAAAGTCTAAG-3′; LIX sense primer, 5′-GGTCCACAGTGCCCTACG-3′; LIX antisense primer, 5′-GCGAGTGCATTCCGCTTA-3′; MIP-2 sense primer, 5′-AGTGAACTGCGCTGTCAATGC-3′; MIP-2 antisense primer, 5′-AGGCAAACTTTTTGACCGCC-3′; IL-1β sense primer, 5′-CAACCAACAAGTGATATTCTCCATG-3′; IL-1β antisense primer, 5′-GATCCACACTCTCCAGCTGCA-3′; FoxP3 sense primer, 5′-AGGAGCCGCAAGCTAAAAGC-3′; FoxP3 antisense primer, 5′-TGCCTTCGTGCCCACTGT-3′; TGFβ sense primer, 5′-ATCCTGTCCAAACTAAGGCTCG-3′; TGFβ antisense primer, 5′-ACCTCTTTAGCATAGTAGTCCGC-3′; IL-10 sense primer, 5′-CTAGAGCTGCGGACTGCCTTC-3′; IL-10 antisense primer, 5′-CCTGCTCCACTGCCTTGCTCTTAT-3′; RANTES sense primer, 5′-CACCACTCCCTGCTGCTT-3′; RANTES antisense primer, 5′-ACACTTGGCGGTTCCTTC-3′. GAPDH was used as an endogenous control to normalise the target gene expression. Then, fold change was calculated using the 2−ΔΔCT method.
gGT activity assay
H. pylori gGT activity was measured using a colorimetric assay previously described27. 2 × 108 bacteria were resuspended in 1 ml of PBS and incubated at 37 °C (5% CO2, 110 rpm) for 2 h. After centrifugation (13.000 rpm, 10 min, RT), 50 µl of supernatant per well were applied to a 96-well plate. Each well was filled up to a total volume of 200 µl per well with reaction buffer consisting of 5 mM L-gamma-glutamyl-p-nitroanilide (L-gGpNA) as substrate and 100 mM glycylglycine (Gly-Gly) as acceptor in 0.1 M Tris buffer, pH 8. Experiments were conducted in duplicates and performed three independent times. Absorption was monitored at 405 nm using a Mithras LB 940 Multimode Microplate Reader (Berthold Technologies) after 1 h of incubation at 37 °C.
Statistical analysis
Data are presented as median and nonparametric tests were used to determine statistical significance using Prism 5 software (GraphPad). Data from two experimental groups were compared by the Mann-Whitney U test. Data from more than two groups were analysed using the Kruskal-Wallis test followed by Dunn’s multiple post hoc test for multiple comparisons. Chi-Squared test was used to compare the number of infected and uninfected mice. Correlations were established using Spearman’s rank correlation coefficient. Statistical significance of differences was established when p ≤ 0.05.
Electronic supplementary material
Acknowledgements
Authors thank Veit Buchholz for helpful comments and discussion and Raphaela Semper for assistance with mouse experiments. This work was supported by the German Research Council (SFB576) and the Elite Network of Bavaria.
Author Contributions
S.W., F.A., C.S., A.W. and R.M.-L. performed experiments and interpreted the data. K.S., A.N., and M.V. contributed to histological staining and evaluation. S.W., M.G. and R.M.-L. wrote the manuscript. R.M.-L. and M.G. planned and supervised the study. All authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Raquel Mejías-Luque and Markus Gerhard contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-14028-1.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Raquel Mejías-Luque, Email: raquel.mejias-luque@tum.de.
Markus Gerhard, Email: markus.gerhard@tum.de.
References
- 1.Kusters JG, van Vliet AHM, Kuipers EJ. Pathogenesis of Helicobacter pylori infection. Clinical microbiology reviews. 2006;19:449–490. doi: 10.1128/CMR.00054-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lindholm C, Quiding-Järbrink M, Lönroth H, Hamlet A, Svennerholm AM. Local cytokine response in Helicobacter pylori-infected subjects. Infection and immunity. 1998;66:5964–5971. doi: 10.1128/iai.66.12.5964-5971.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goll R, et al. Helicobacter pylori stimulates a mixed adaptive immune response with a strong T-regulatory component in human gastric mucosa. Helicobacter. 2007;12:185–192. doi: 10.1111/j.1523-5378.2007.00495.x. [DOI] [PubMed] [Google Scholar]
- 4.Bontems P, Robert F, van Gossum A, Cadranel S, Mascart F. Helicobacter pylori modulation of gastric and duodenal mucosal T cell cytokine secretions in children compared with adults. Helicobacter. 2003;8:216–226. doi: 10.1046/j.1523-5378.2003.00147.x. [DOI] [PubMed] [Google Scholar]
- 5.Nurgalieva ZZ, et al. B-cell and T-cell immune responses to experimental Helicobacter pylori infection in humans. Infection and immunity. 2005;73:2999–3006. doi: 10.1128/IAI.73.5.2999-3006.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strömberg E, et al. Increased frequency of activated T-cells in the Helicobacter pylori-infected antrum and duodenum. FEMS immunology and medical microbiology. 2003;36:159–168. doi: 10.1016/S0928-8244(03)00026-9. [DOI] [PubMed] [Google Scholar]
- 7.Karttunen R, Karttunen T, Ekre HP, MacDonald TT. Interferon gamma and interleukin 4 secreting cells in the gastric antrum in Helicobacter pylori positive and negative gastritis. Gut. 1995;36:341–345. doi: 10.1136/gut.36.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bamford KB, et al. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology. 1998;114:482–492. doi: 10.1016/S0016-5085(98)70531-1. [DOI] [PubMed] [Google Scholar]
- 9.Luzza F, et al. Up-regulation of IL-17 is associated with bioactive IL-8 expression in Helicobacter pylori-infected human gastric mucosa. Journal of immunology Baltimore, Md. 2000;165:5332–5337. doi: 10.4049/jimmunol.165.9.5332. [DOI] [PubMed] [Google Scholar]
- 10.Sawai N, et al. Role of gamma interferon in Helicobacter pylori-induced gastric inflammatory responses in a mouse model. Infection and immunity. 1999;67:279–285. doi: 10.1128/iai.67.1.279-285.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smythies LE, et al. Helicobacter pylori-induced mucosal inflammation is Th1 mediated and exacerbated in IL-4, but not IFN-gamma, gene-deficient mice. Journal of immunology Baltimore, Md. 2000;165:1022–1029. doi: 10.4049/jimmunol.165.2.1022. [DOI] [PubMed] [Google Scholar]
- 12.Amedei A, et al. The neutrophil-activating protein of Helicobacter pylori promotes Th1 immune responses. The Journal of clinical investigation. 2006;116:1092–1101. doi: 10.1172/JCI27177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kandulski A, et al. Naturally occurring regulatory T cells (CD4+, CD25high, FOXP3+) in the antrum and cardia are associated with higher H. pylori colonization and increased gene expression of TGF-beta1. Helicobacter. 2008;13:295–303. doi: 10.1111/j.1523-5378.2008.00612.x. [DOI] [PubMed] [Google Scholar]
- 14.Gunn MC, Stephens JC, Stewart JA, Rathbone BJ, West KP. The significance of cagA and vacA subtypes of Helicobacter pylori in the pathogenesis of inflammation and peptic ulceration. Journal of clinical pathology. 1998;51:761–764. doi: 10.1136/jcp.51.10.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuipers EJ, Lee A, Klinkenberg-Knol EC, Meuwissen SG. Review article: the development of atrophic gastritis‐Helicobacter pylori and the effects of acid suppressive therapy. Alimentary pharmacology & therapeutics. 1995;9:331–340. doi: 10.1111/j.1365-2036.1995.tb00391.x. [DOI] [PubMed] [Google Scholar]
- 16.Backert S, et al. Translocation of the Helicobacter pylori CagA protein in gastric epithelial cells by a type IV secretion apparatus. Cellular microbiology. 2000;2:155–164. doi: 10.1046/j.1462-5822.2000.00043.x. [DOI] [PubMed] [Google Scholar]
- 17.Fiocca R, et al. Release of Helicobacter pylori vacuolating cytotoxin by both a specific secretion pathway and budding of outer membrane vesicles. Uptake of released toxin and vesicles by gastric epithelium. The Journal of pathology. 1999;188:220–226. doi: 10.1002/(SICI)1096-9896(199906)188:2<220::AID-PATH307>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 18.Gong M, Ling SS, Lui SY, Yeoh KG, Ho B. Helicobacter pylori gamma-glutamyl transpeptidase is a pathogenic factor in the development of peptic ulcer disease. Gastroenterology. 2010;139:564–573. doi: 10.1053/j.gastro.2010.03.050. [DOI] [PubMed] [Google Scholar]
- 19.Rimbara E, Mori S, Kim H, Shibayama K. Role of γ-glutamyltranspeptidase in the pathogenesis of Helicobacter pylori infection. Microbiology and immunology. 2013;57:665–673. doi: 10.1111/1348-0421.12089. [DOI] [PubMed] [Google Scholar]
- 20.Miller EF, Maier RJ. Ammonium metabolism enzymes aid Helicobacter pylori acid resistance. Journal of bacteriology. 2014;196:3074–3081. doi: 10.1128/JB.01423-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leduc D, Gallaud J, Stingl K. & Reuse, H. d. Coupled amino acid deamidase-transport systems essential for Helicobacter pylori colonization. Infection and immunity. 2010;78:2782–2792. doi: 10.1128/IAI.00149-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wüstner S, et al. Helicobacter pylori γ-glutamyltranspeptidase impairs T-lymphocyte function by compromising metabolic adaption through inhibition of cMyc and IRF4 expression. Cellular microbiology. 2015;17:51–61. doi: 10.1111/cmi.12335. [DOI] [PubMed] [Google Scholar]
- 23.Kabisch R, Semper RP, Wustner S, Gerhard M, Mejias-Luque R. Helicobacter pylori gamma-Glutamyltranspeptidase Induces Tolerogenic Human Dendritic Cells by Activation of Glutamate Receptors. Journal of immunology. 2016;196:4246–4252. doi: 10.4049/jimmunol.1501062. [DOI] [PubMed] [Google Scholar]
- 24.Oertli M, et al. Helicobacter pylori gamma-glutamyl transpeptidase and vacuolating cytotoxin promote gastric persistence and immune tolerance. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:3047–3052. doi: 10.1073/pnas.1211248110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGovern KJ, et al. gamma-Glutamyltransferase is a Helicobacter pylori virulence factor but is not essential for colonization. Infection and immunity. 2001;69:4168–4173. doi: 10.1128/IAI.69.6.4168-4173.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chevalier C, Thiberge JM, Ferrero RL, Labigne A. Essential role of Helicobacter pylori gamma-glutamyltranspeptidase for the colonization of the gastric mucosa of mice. Molecular microbiology. 1999;31:1359–1372. doi: 10.1046/j.1365-2958.1999.01271.x. [DOI] [PubMed] [Google Scholar]
- 27.Schmees C, et al. Inhibition of T-cell proliferation by Helicobacter pylori gamma-glutamyl transpeptidase. Gastroenterology. 2007;132:1820–1833. doi: 10.1053/j.gastro.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 28.Rieder G, et al. Comparison of CXC chemokines ENA-78 and interleukin-8 expression in Helicobacter pylori-associated gastritis. Infection and immunity. 2001;69:81–88. doi: 10.1128/IAI.69.1.81-88.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wen S, et al. Inflammatory gene profiles in gastric mucosa during Helicobacter pylori infection in humans. Journal of immunology (Baltimore, Md. 2004;172:2595–2606. doi: 10.4049/jimmunol.172.4.2595. [DOI] [PubMed] [Google Scholar]
- 30.Crabtree JE, et al. Helicobacter pylori induced interleukin-8 expression in gastric epithelial cells is associated with CagA positive phenotype. Journal of clinical pathology. 1995;48:41–45. doi: 10.1136/jcp.48.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crabtree JE, Lindley IJ. Mucosal interleukin-8 and Helicobacter pylori-associated gastroduodenal disease. European journal of gastroenterology & hepatology. 1994;6(Suppl 1):S33–38. [PubMed] [Google Scholar]
- 32.Mori N, et al. Helicobacter pylori induces RANTES through activation of NF-kappa B. Infection and immunity. 2003;71:3748–3756. doi: 10.1128/IAI.71.7.3748-3756.2003. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Shibayama K, et al. Metabolism of glutamine and glutathione via gamma-glutamyltranspeptidase and glutamate transport in Helicobacter pylori: possible significance in the pathophysiology of the organism. Molecular microbiology. 2007;64:396–406. doi: 10.1111/j.1365-2958.2007.05661.x. [DOI] [PubMed] [Google Scholar]
- 34.Shibayama K, et al. A novel apoptosis-inducing protein from Helicobacter pylori. Molecular microbiology. 2003;47:443–451. doi: 10.1046/j.1365-2958.2003.03305.x. [DOI] [PubMed] [Google Scholar]
- 35.Ling SSM, Khoo LHB, Hwang L-A, Yeoh KG, Ho B. Instrumental Role of Helicobacter pylori γ-Glutamyl Transpeptidase in VacA-Dependent Vacuolation in Gastric Epithelial Cells. PloS one. 2015;10:e0131460. doi: 10.1371/journal.pone.0131460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Z’Graggen K, Walz A, Mazzucchelli L, Strieter RM, Mueller C. The C-X-C chemokine ENA-78 is preferentially expressed in intestinal epithelium in inflammatory bowel disease. Gastroenterology. 1997;113:808–816. doi: 10.1016/S0016-5085(97)70175-6. [DOI] [PubMed] [Google Scholar]
- 37.Kwon JH, et al. Topical antisense oligonucleotide therapy against LIX, an enterocyte-expressed CXC chemokine, reduces murine colitis. American journal of physiology. Gastrointestinal and liver physiology. 2005;289:G1075–1083. doi: 10.1152/ajpgi.00073.2005. [DOI] [PubMed] [Google Scholar]
- 38.Ruiz VE, Sachdev M, Zhang S, Wen S, Moss SF. Isolating, immunophenotyping and ex vivo stimulation of CD4+and CD8+gastric lymphocytes during murine Helicobacter pylori infection. Journal of immunological methods. 2012;384:157–163. doi: 10.1016/j.jim.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hagen SJ, et al. Inflammation and foveolar hyperplasia are reduced by supplemental dietary glutamine during Helicobacter pylori infection in mice. The Journal of nutrition. 2009;139:912–918. doi: 10.3945/jn.108.097790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amagase K, et al. New frontiers in gut nutrient sensor research: prophylactic effect of glutamine against Helicobacter pylori-induced gastric diseases in Mongolian gerbils. Journal of pharmacological sciences. 2010;112:25–32. doi: 10.1254/jphs.09R11FM. [DOI] [PubMed] [Google Scholar]
- 41.Shanware NP, et al. Glutamine deprivation stimulates mTOR-JNK-dependent chemokine secretion. Nature communications. 2014;5:4900. doi: 10.1038/ncomms5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liboni KC, Li N, Scumpia PO, Neu J. Glutamine modulates LPS-induced IL-8 production through IkappaB/NF-kappaB in human fetal and adult intestinal epithelium. The Journal of nutrition. 2005;135:245–251. doi: 10.1093/jn/135.2.245. [DOI] [PubMed] [Google Scholar]
- 43.Hess C, et al. IL-8 responsiveness defines a subset of CD8 T cells poised to kill. Blood. 2004;104:3463–3471. doi: 10.1182/blood-2004-03-1067. [DOI] [PubMed] [Google Scholar]
- 44.Fan XJ, et al. Gastric T lymphocyte responses to Helicobacter pylori in patients with H pylori colonisation. Gut. 1994;35:1379–1384. doi: 10.1136/gut.35.10.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quiding-Järbrink M, Lundin BS, Lönroth H, Svennerholm AM. CD4+and CD8+T cell responses in Helicobacter pylori-infected individuals. Clinical and experimental immunology. 2001;123:81–87. doi: 10.1046/j.1365-2249.2001.01427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pappo J, et al. Helicobacter pylori infection in immunized mice lacking major histocompatibility complex class I and class II functions. Infection and immunity. 1999;67:337–341. doi: 10.1128/iai.67.1.337-341.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oertli M, et al. Helicobacter pylori gamma-glutamyl transpeptidase and vacuolating cytotoxin promote gastric persistence and immune tolerance. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:3047–3052. doi: 10.1073/pnas.1211248110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. The American journal of surgical pathology. 1996;20:1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.