Abstract
Objective: We determined whether self-reported new or recurrent yeast infections were a risk factor for and/or consequence of vulvodynia and then determined the extent to which various levels of misclassification of self-reported yeast infections influenced these results.
Materials and Methods: In this case–control study we retrospectively assessed self-reported new and recurrent yeast infections prior and subsequent to first vulvar pain onset among 216 clinically confirmed cases and during a similar time period for 224 general population controls.
Results: A history of >10 yeast infections before vulvodynia onset was strongly but imprecisely associated with currently diagnosed vulvodynia after adjustment for age, age at first intercourse, and history of urinary tract infections [adjusted odds ratio = 5.5, 95% confidence interval (CI) 1.7–17.8]. Likewise, a history of vulvodynia was associated with a twofold risk of subsequent new or recurrent onset of yeast infections after adjustment for age, age at first intercourse, and history of yeast infections before vulvodynia onset (comparable time period among controls, 95% CI 1.5–2.9). Bias analyses showed that our observed associations were an underestimation of the true association when nondifferential misclassification of self-reported yeast infections and certain differential misclassification scenarios were present. However, if women with vulvodynia more frequently misreported having them when they truly did not, our observed associations were an overestimate of the truth.
Conclusions: There appears to be a positive relationship between yeast infections preceding and following the diagnosis of vulvodynia, but this relationship varies from strong to nonexistent depending on the relative accuracy of the recalled diagnosis of yeast infections among cases and controls. To better understand the bidirectional associations between yeast infections and vulvodynia, future validation studies are needed to determine the extent to which misclassification of self-reported yeast infections differs between women with and without vulvodynia.
Keywords: : case–control studies, vulvodynia, yeast infections, bias analyses
Introduction
Vulvodynia is highly prevalent in the general population,1 defined as debilitating vulvar discomfort due to burning pain or pain on contact that occurs in the absence of clinically visible pathological findings or identifiable disorders.2 Over the years, many studies have suggested an association between vulvodynia and vulvovaginal candidiasis (yeast) infections.3–5 Although several biological hypotheses have been suggested, including genetic susceptibility to Candida antigens, eliciting an altered immune response that results in chronic inflammation,6 or abnormal sensory processing as a result of repeated candidiasis infections,7 it is difficult to conclude that Candida infections are causally associated with new onset of vulvodynia. This is largely due to the inability of women to accurately recognize candidiasis infections, the poor reliability between telephone consultation with clinical providers and a true diagnosis, and the inadequate workup when women do seek care for their infections.8–10 Add to that the limitation of prior studies to elucidate the timing of the yeast infections in relation to the onset of vulvodynia symptoms and this association becomes very challenging to study.
However, it is difficult to ignore much of the biologically relevant evidence that suggests an important role for Candida in understanding vulvodynia pathogenesis. Evidence free of yeast exposure misclassification and the temporal relationship between yeast exposure and vulvar pain comes from a preclinical study of 15 mice where after 3 rounds of induced Candida infections, 6 experienced allodynia, based on hind paw sensitivity to von frey filament applications and visible signs of increased vulvar innervation, suggesting that multiple antecedent infections may be a potential risk factor for new onset vulvodynia.7 This is somewhat consistent with our earlier finding of a substantially increased risk of vulvodynia as a consequence of increasing numbers of self-reported past urogenital tract infections.11
Determining whether yeast infections (1) lead to the development of vulvar pain symptoms, or (2) can increase in frequency as a result of immunological or microbiota changes that might have occurred as a consequence of developing vulvodynia, is critical for understanding the biological mechanisms underlying this disorder. In our recently completed population-based study of women with and without clinically confirmed vulvodynia, we assessed self-reported yeast infections prior and subsequent to new and clinically confirmed vulvodynia onset to begin to sort out the temporal relationship between recurrent yeast infections and vulvodynia. Because self-reported candidiasis infections may be measured imperfectly, we used quantitative bias analysis to explore how these findings would change under plausible assumptions on the range of sensitivity and specificity of self-reported candidiasis infections under scenarios of nondifferential and differential misclassification.
Materials and Methods
The University of Minnesota Institutional Review Board approved this study and all participants provided written consent. Data for the present analyses were collected as part of a case–control study to explore etiological predictors of vulvodynia. Women 18–40 years of age, who were part of the administrative database of a large healthcare network that represents ∼27% of the population in the Minneapolis/Saint Paul metropolitan area, were initially recruited through self-administered surveys to examine the prevalence of vulvar pain. They had been seen for any reason in 1 of over 40 community health clinics within a 2-year window between March 2010 and October 2013; 30,676 screeners were received. This self-administered vulvar pain assessment has been previously described.12
Women likely to meet the International Society for the Study of Vulvovaginal Diseases criteria for vulvodynia based on their initial survey responses were invited to participate in a clinical visit to confirm the diagnosis.13 Of the 1,398 women invited, 350 completed their examination, and 234 were clinically confirmed. We compared screener questionnaire characteristics of those who agreed and did not agree to a clinical evaluation. In this assessment, we found no important differences in demographic characteristics (age, race, and marital status) or reproductive history (age at menarche, cycle regularity, and history of oral contraceptive use). Likewise, characteristics of the vulvar pain, such as limiting or preventing sexual intercourse, having never had a period of pain free intercourse versus secondary onset of pain that developed after a period of pain free intercourse, whether pain was provoked as opposed to continuous, and having sought care for their vulvar pain, were similar between those who did and did not agree to the clinical assessment.
Women from this same pool of screened women with no history of vulvar discomfort were randomly selected and invited to serve as controls. Of 2,287 women invited, 251 agreed and 234 were clinically confirmed as having no ongoing or past history of vulvar pain. These clinically confirmed, eligible, and enrolled controls were matched to a case and assigned a reference age identical to the age at first onset of vulvar pain in the matched case. Controls had to be older than the age at which their matched case was diagnosed with vulvodynia. On average controls were about 2 years older than cases. The interval length between actual age and age at onset of vulvodynia in cases, and actual age and reference age in controls, was no more than 2 years as well. This allowed for assessments of exposures in controls that were comparable to cases both prior and subsequent to onset of vulvodynia.
Additional eligibility criteria for both cases and controls included having no active genitourinary infections at the time of their clinical visit, and if parous, being at least 1-year postpartum. Women were asked to refrain from introducing anything into their vaginas for 48 hours before the clinical visit. Clinically confirmed, eligible, and enrolled cases self-reported the age at which they first experienced vulvar pain.
All cases and controls completed a background and medical history questionnaire by telephone that covered demographic characteristics, sexual and reproductive history, and personal hygiene practices. For cases, we attempted to collect all historical information within the temporal context of their age at first onset of vulvar pain (before and after) when appropriate. The same information was obtained for controls based on their assigned reference age. Thus, exposures were assessed using the same temporal context for both cases and controls.
We recorded the age of the woman at the time of her first self-reported yeast infection and whether she indicated the infection was clinically confirmed. We then asked the participant to indicate the category (0, 1–4, 5–10, 11–20, 21–30, >30) that represents the number of infections, subsequent to the initial infection, that occurred before vulvodynia onset (or reference age in controls) and then the number of infections subsequent to vulvodynia onset (or reference age in controls). We then obtained the following information for the aggregated category of number of yeast infections: proportion self-reported as clinically confirmed, frequency of use of over-the-counter medication, and treatment success. We also collected information on other urogynecological infections, including urinary tract infections, gonorrhea, genital warts, bacterial vaginosis, trichomoniasis, chlamydia, and genital herpes. All were temporally assessed in relation to first onset of vulvar pain in cases and reference age in controls.
Statistical analysis
The analyses were based on 216 cases and 224 controls in which all main exposure and primary covariates were successfully obtained. Thus, the reference age matching was not retained, but assessed as a covariate. We first assessed differences in key demographic, personal, and medical history characteristics and then differences in these same characteristics among those who did and did not self-report yeast infections.
Models were created to examine two distinct time periods–before the onset of vulvodynia or reference age (referred to as the antecedent model) and at or after the onset of vulvodynia or reference age (referred to as the post-onset model). The association between yeast infections and subsequent onset of vulvodynia is based on a case–control analysis, and odds ratios are presented. The post-vulvodynia onset (reference age in controls) yeast infection estimate is based on a retrospective cohort analysis and we therefore present relative risk estimates. For both analyses, potential confounders were selected from the literature and are described in the results in reference to Table 1. The models were refined using logistic regression with stepwise selection.
Table 1.
Demographic Characteristics of 216 Women with Chronic Vulvar Pain (Cases) and 224 Women with No Vulvar Pain History (Controls)
| Cases | Controls | |
|---|---|---|
| Characteristics | n = 216 | n = 224 |
| Current age, years, mean (SD) | 29.1 (±5.2) | 31.6 (±5.0) |
| Current age in categories, n (%) | ||
| 18–24 years | 37 (17.1) | 19 (8.5) |
| 25–29 years | 82 (38.0) | 60 (26.8) |
| 30+ years | 97 (44.9) | 145 (64.7) |
| Reference/onset age, years, mean (SD) | 21.0 (±5.7) | 21.1 (±5.8) |
| White, n (%) | 189 (87.9) | 199 (88.8) |
| Age at first sexual intercourse, years, mean (SD) | 18.1 (±3.6) | 17.8 (±3.8) |
| No. of sexual partners, mean (SD) | 8.1 (±9.2) | 9.3 (±11.1) |
| Family members with depression, mean (SD) | 1.6 (±1.7) | 1.2 (±1.6) |
| History of abuse,an (%) | ||
| None | 70 (32.4) | 82 (36.6) |
| Moderate | 28 (13.0) | 37 (16.5) |
| Severe | 67 (31.0) | 47 (21.0) |
| Not reported | 51 (23.6) | 58 (25.9) |
| Antecedentb urinary tract infection, n (%) | 98 (45.4) | 65 (29.0) |
| Antecedent bacterial vaginosis, n (%) | 27 (12.5) | 16 (7.1) |
| Antecedent chronic constipation, n (%) | 20 (9.3) | 4 (1.8) |
| Antecedent anxiety, n (%) | ||
| None | 125 (57.9) | 165 (73.7) |
| Yes, not diagnosed | 27 (12.5) | 28 (12.5) |
| Yes, diagnosed | 64 (29.6) | 31 (13.8) |
| Antecedent depression, n (%) | ||
| None | 135 (62.5) | 150 (67.0) |
| Yes, not diagnosed | 15 (6.9) | 21 (9.4) |
| Yes, diagnosed | 66 (30.6) | 53 (23.7) |
| Antecedent allergies to medications, n (%) | 79 (36.6) | 49 (21.9) |
| Antecedent hormonal contraceptive use, n (%) | 133 (61.6) | 125 (55.8) |
| Antecedent pain conditions,cn (%) | 59 (27.3) | 20 (8.9) |
Physical or sexual abuse through age 11.
Antecedent to onset age (if case) or reference age (if control).
Pain conditions include Chronic Fatigue Syndrome, Temporomandibular Joint and Muscle Disorders, Irritable Bowel Syndrome, Fibromyalgia, and Interstitial Cyst.
SD, standard deviation.
After assessing all potential confounders and effect modifiers, our final antecedent model to estimate the influence of yeast infections on risk of vulvodynia included number of antecedent yeast infections (none, 1–4, 5–10, >10), current age, age at first sexual intercourse, and history of urinary tract infections.
The post-onset model, which assesses the risk of post-onset yeast infection among those with and without vulvodynia, used log-binomial models to estimate risk ratios (RRs). The post-onset model adjusted for current age, age at first intercourse, and history of yeast infections and categorized yeast infections as <5 or ≥5. A post-onset sensitivity analysis was conducted limited to the subset of women with no antecedent yeast infections. This allowed for the assessment of vulvodynia on the risk of new and post-onset only yeast infections. All analyses were conducted using SAS 9.3 statistical software (SAS Institute, Inc., Cary, NC).
To assess the impact of misclassification of yeast infection in both the antecedent infection and post-onset infection analyses, we performed a quantitative bias analysis.14 We investigated two distinct hypotheses about the misclassification, scenarios that applied to both the antecedent infection analysis and the post-onset analysis. In the first scenario, we considered it plausible that misreporting of yeast infections was the same regardless of whether a woman had vulvodynia or not (i.e., nondifferential misclassification). This scenario might occur if all women had difficulty remembering how many infections they had or there were errors in self-diagnosis, but with no distinct pattern (i.e., the rates of misclassification would be the same among those with and without vulvodynia). This would lead to an expected bias toward the null such that we would have underestimated the true association. However, the magnitude of that bias is unclear.
For the second scenario, we considered it plausible that women with vulvodynia would be less likely to underreport their number of yeast infections (both before and after diagnosis of vulvodynia) if they had ≥5 yeast infections but more likely to over report their number of yeast infections if they in fact had <5 yeast infections. This scenario might occur if women with vulvodynia spent more time trying to explain their pain by carefully recounting their yeast infections and potentially remembering more than there were, while women without vulvar pain would not have such stimulus to help them remember their infections. This would likely lead to our associations over estimating the truth, but again the magnitude is unclear.
In the antecedent infection model, we assessed the impact of misclassification of number of pre-onset yeast infections as the exposure at levels of specificity ranging from 0.85 to 1.0 among vulvodynia cases (the greatest range possible based on only 16.2% of cases reporting ≥5 yeast infections before the reference age). For controls, we assessed a specificity range from 0.93 to 1.0 (the greatest range possible based on only 8.5% of controls reporting ≥5 yeast infections after the reference age). For both cases and controls, we assessed sensitivity values of 0.50, 0.70, and 0.90.
In the post-onset models, we assessed the potential for misclassification of number of post-onset yeast infections as the outcome by varying specificity among those exposed (vulvodynia) from 0.70 to 1.0 (the greatest range possible based on 33.3% of those exposed reporting ≥5 new post-onset yeast infections) and unexposed from 0.85 to 1.0 (again, the greatest range based on plausible values from our observed data). We retained the same sensitivity values of 0.50, 0.70, and 0.90 as in the pre-vulvodynia onset models.
Results
Cases were younger than controls by 2 years, but the reference age assigned to controls was comparable to that of age at first onset of vulvodynia symptoms within a few months (Table 1). Relative to controls, women with vulvodynia were more likely to report a history of anxiety, prior urinary tract infections, a family history of depression, chronic constipation, allergies to medications, and chronic overlapping pain conditions. All the variables presented in Table 1 were assessed as potential confounders or modifiers of the associations between yeast infections and vulvodynia. It should be noted that only one case and eight controls self-reported a history of diabetes which, although associated with greater risk of yeast infections, had little impact on our results.
After adjustment for current age and age at first sexual intercourse, there was little difference in risk of vulvodynia by age at first onset of yeast infections. Among women who reported a history of yeast infections before onset of vulvodynia or assigned reference age, those whose first yeast infection was diagnosed by clinicians had about a 60% greater odds of vulvodynia than women whose first yeast infection was not diagnosed by a clinician (Table 2). A strong dose response was observed in the association between antecedent yeast infections and vulvodynia with a sevenfold increase in adjusted odds associated with reporting a history of more than 10 antecedent infections and a fivefold increased odds after further adjustment for history of urinary tract infections.
Table 2.
Relative Odds of Vulvodynia Among Those With Self-Reported Yeast Infections Before Onset of Vulvodynia or Comparable Time Period Among Controls, Compared to Those Without Yeast Infections
| Cases | Controls | Crude OR | Adjusted ORa | Adjusted ORb | |
|---|---|---|---|---|---|
| Yeast infections | n (%) | n (%) | 95% CI | 95% CI | 95% CI |
| Age at first onset, yearsc | |||||
| <17 | 40 (18.5) | 27 (12.1) | 1.7 (1.0–3.0) | 1.9 (1.1–3.4) | 1.6 (0.89–2.9) |
| 17–21 | 26 (12.0) | 38 (17.0) | 0.80 (0.46–1.4) | 1.0 (0.56–1.8) | 0.81 (0.45–1.5) |
| >21 | 28 (13.0) | 18 (8.0) | 1.8 (0.96–3.5) | 2.2 (1.2–4.4) | 1.8 (0.90–3.5) |
| No history of yeast infections | 120 (55.6) | 141 (63.0) | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) |
| MD Dx at first onsetd,e | |||||
| No | 19 (8.8) | 24 (10.7) | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) |
| Yes | 76 (35.2) | 59 (26.3) | 1.6 (0.82–3.2) | 1.6 (0.79–3.3) | 1.6 (0.78–3.3) |
| Antecedent infections | |||||
| None | 120 (55.6) | 141 (63.0) | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) |
| 1–4 | 61 (28.2) | 64 (28.6) | 1.1 (0.73–1.7) | 1.3 (0.80–2.0) | 1.1 (0.68–1.7) |
| 5–10 | 18 (8.3) | 15 (6.7) | 1.4 (0.68–2.9) | 1.9 (0.89–4.1) | 1.6 (0.76–3.5) |
| >10 | 17 (7.9) | 4 (1.8) | 5.0 (1.6–15.2) | 7.3 (2.3–23.0) | 5.5 (1.7–17.8) |
| Antecedent infections | |||||
| 0–4 | 181 (83.8) | 205 (91.5) | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) |
| ≥5 | 35 (16.2) | 19 (8.5) | 2.1 (1.2–3.8) | 2.8 (1.5–5.2) | 2.3 (1.2–4.4) |
Adjusted for current age and age of first sexual intercourse.
Model (a) plus adjustment for antecedent urinary tract infections.
Two cases missing age at onset.
One case missing MD Dx.
Excludes women who never reported yeast infections and those whose yeast infections only occurred after reference age (120 cases and 141 controls).
CI, confidence interval; MD Dx, Physician diagnosis at first onset; OR, odds ratio.
In Table 3 we estimated the extent to which misclassification of the number of self-reported prior yeast infections may have impacted our results when comparing 5 or more self-reported infections versus <5 yeast infections in women with vulvodynia versus controls (crude odds ratio = 2.1). Each cell in Table 3 represents an estimate of the association given the assumptions about the sensitivity and specificity of yeast infection misclassification. Each set of five rows represents the same assumptions about the sensitivity of the classification (probability of correctly reporting ≥5 infections when a woman truly had ≥5), but varies the assumptions about specificity (i.e., the probability of reporting <5 infections when a woman truly had <5). The cells shaded show the scenarios that resulted in our estimate of 2.1 being biased away from the null (a potential spurious inflation of the true association). Table 3 shows that under the scenario of nondifferential misclassification (shown as the bolded outlined cells), the bias was as expected toward the null (our observed estimate of 2.1 potentially underestimated the true effect). However, under nondifferential misclassification, the magnitude of effect would only increase dramatically if the probability of overreporting was as low as was plausible within the dataset (i.e., specificity of 93% among controls).
Table 3.
Estimates of the Effect (Odds Ratio) of Antecedent Infections (≥5 vs. 0–4) on Vulvodynia Corrected for Exposure Misclassification for Various Assumptions of Sensitivity (0.90, 0.70, 0.50) and Specificity (1.0, 0.95, 0.93, 0.85; Cases Only) of Classification of ≥5 Prior Yeast Infections
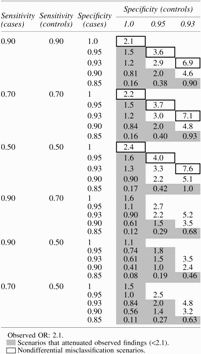 |
Under the more plausible scenario of recall bias (where sensitivity among cases is higher compared with controls and specificity is lower among cases compared with controls), the bias was such that our estimates may have overestimated the true effect. However, we overestimated the true association most often when the specificity among controls was perfect, a likely implausible scenario.
We then assessed the influence of vulvodynia on the risk of new or recurrent yeast infections (Table 4). Women with vulvodynia were twice as likely to self-report ≥5 yeast infections subsequent to their vulvodynia onset compared to controls after adjustment for current age, age at first intercourse, and yeast infections occurring before the onset of vulvodynia or reference age among controls. Furthermore, when we conducted a sensitivity analysis restricted only to women who's first and all subsequent yeast infections occurred after onset of vulvodynia or reference ages among controls, we observed the same twofold association (95% confidence interval 1.5–3.4) after adjustment for the same covariates above.
Table 4.
Relative Risk of ≥5 New or Recurrent Self-Reported Yeast Infections Subsequent to Vulvodynia Diagnosis Compared to Women Without Vulvodynia
| No. of post-onset yeast infections | Crude RR | Adjusted RRa | |||
|---|---|---|---|---|---|
| Exposure | Total | ≥5 | 0–4 | 95% CI | 95% CI |
| New or recurrent post-onset yeast infections | |||||
| Vulvodynia | 216 | 79 (36.6) | 137 (63.4) | 2.2 (1.5–3.0) | 2.1 (1.5–2.9) |
| No vulvodynia | 224 | 38 (17.0) | 186 (83.0) | 1.0 (referent) | 1.0 (referent) |
| New post-onset yeast infectionsb | |||||
| Vulvodynia | 120 | 40 (33.3) | 80 (66.6) | 1.9 (1.2–2.9) | 2.3 (1.5–3.4) |
| No vulvodynia | 141 | 25 (17.1) | 116 (82.3) | 1.0 (referent) | 1.0 (referent) |
Adjusted for current age, age of first sexual intercourse, and antecedent yeast infections (any, none).
Includes only women who had no antecedent yeast infections, Adjusted RRs in this category no longer adjust for antecedent yeast infections.
RR, risk ratio.
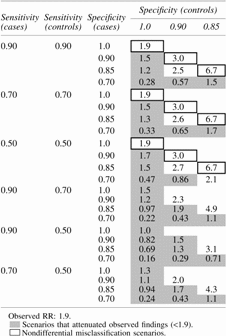
For the misclassification bias analysis, we estimated the extent to which our exposure (vulvodynia) was associated with 5 or more subsequent self-reported yeast infections (outcome) versus <5 (crude RR = 1.9) and how misclassification of the outcome (reporting of yeast infection) might affect this association. As with Table 3, each row in Table 5 shows a single RR corrected for the misclassification under the assumed values of sensitivity and specificity. As expected, when the misclassification is nondifferential (i.e., the same between cases and controls), our observed estimate of 1.9 may have been an underestimate of the true effect (as shown in the bolded outlined cells). The value of sensitivity (i.e., correctly reporting 5 or more yeast infections) plays very little role, while the value of specificity (i.e., correctly reporting fewer than 5 yeast infections) leads to a range of corrected estimates between 1.9 and 6.7. Under the more plausible situation of recall bias the corrected estimates are nearly or almost always toward the null (i.e., underestimates) suggesting our results may be an overestimate of the true effect.
Table 5.
Estimates of the Effect (RR) of Vulvodynia (Exposed) Versus No Vulvodynia (Unexposed) on Post-Onset Yeast Infections (≥5) Corrected for Outcome Misclassification for Various Estimates of Sensitivity (0.9, 0.7, 0.5) and Specificity (1.0, 0.90, 0.85, 0.7; Cases Only) Parameters
 |
Finally, we assessed differences in the diagnostic and treatment characteristics of yeast infections in women with and without vulvodynia, by analyzing infections that occurred prior and subsequent to vulvodynia onset or reference age among controls. The proportion of yeast infections always diagnosed by a clinician did not differ among those with and without vulvodynia before onset of vulvar pain (36.5% vs. 39.8%) or subsequent to onset of vulvar pain (31.7% vs. 30.5%). Women with vulvodynia used over-the-counter medications for yeast infections most or all of the time less frequently than controls (41.9% vs. 57.9% for antecedent infections and 33.1% vs. 50% for post-onset infections). Yet women with vulvodynia compared to those without vulvodynia reported a greater likelihood of failed Candida treatment success before onset of vulvodynia (15.8% vs. 4.9%) and subsequent to vulvodynia onset or reference age among controls (19.8% vs. 0.8%). Our data did not allow us to distinguish between treatment outcomes of using over-the-counter versus prescription medications.
Discussion
In an earlier study conducted in the Boston metropolitan area, women with a self-reported history of yeast infections had twice the odds of vulvodynia compared to those without after adjustment for a number of demographic and sexual history variables.11 In this study, we report a nearly sevenfold association of vulvodynia with 10 or more antecedent yeast infections. Furthermore, in the analyses presented in this study, we adjusted for urinary tract infections (UTIs), of particular importance, because the treatment of UTI's often involves therapeutic administration of antibiotics that may put women at greater risk of yeast infections due to disruptions in the normal microbial ecology of the vaginal epithelium.15 Although this adjustment did result in a substantial attenuation of the association from an odds ratio of 7.3 to an odds ratio of 5.5, it still remained highly elevated.
Our study is the first to assess the extent to which misclassification of self-reported yeast infections alters the association with vulvodynia and in which direction. In our bias analyses, our observed associations were most often present under the implausible scenario where specificity is perfect (or near perfect) among the controls, but imperfect among the cases.
Certainly, women suffering from vulvodynia are likely vigilant about identifying factors that may contribute to their vulvar pain. Our strongest association with vulvodynia was observed among those women reporting greater than 10 infections and thus we cannot rule out that some of this association might be due to hypervigilance of reporting on the part of women with vulvar pain. Nevertheless, the association with vulvodynia was stronger in those who reported clinically confirmed first onset of yeast infections versus those who reported that their first yeast infection was not clinically evaluated. This may suggest that the misclassification that exists with respect to self-reported yeast infections may be more nondifferential, and thus, as shown in Tables 3 and 5 stronger than what we actually observed. Furthermore, although our data limited our ability to assess misclassification of specificities lower than 0.85 among cases, we showed that as specificity decreased among controls, the risk of vulvodynia associated with yeast infections increased. However, it should also be noted that at all levels of specificity among controls, as specificity among cases decreased, the original estimate of association became more attenuated. We note that our lowest detectable values for specificity are higher than what has been reported in many studies. Unfortunately, lower values were not possible to assess within our dataset as they led to negative (i.e., impossible) corrected cells within our two-by-two tables.
To our knowledge, we are the first to use temporal analyses to suggest that vulvodynia may increase the risk of new or recurrent yeast infections and that post-vulvodynia onset infections are reported to be less successfully treated, compared to women with no history of vulvar pain. Biological evidence for mechanisms underlying such an association between vulvodynia and yeast infections is beginning to emerge. In a series of studies, Foster et al.16–18 have shown that vestibular fibroblasts isolated from vulvodynia patients produce higher levels of inflammatory cytokines, compared to fibroblasts isolated from controls, when challenged with yeast antigens in vitro. Most recently, this group of researchers has demonstrated that the production of high levels of prostaglandin E2 (PGE2) and interleukin-6 (IL-6) by vulvodynia-positive vestibular fibroblasts is regulated by Dectin-1 (a pattern recognition receptor that recognizes β-glucan on fungal cell walls) signaling through the transcription factor NFκB (nuclear factor kappa light chain enhancer of activated B cells).16 This is particularly interesting in light of the well-characterized increases in mast cell numbers and activation status observed in vulvar biopsies of vulvodynia patients.19,20 IL-6 is a survival factor for mast cells,21 and activated mast cells derived from patients with atopic eczema have shown exaggerated cytokine responses to the commensal skin yeast Malassezia sympodialis,22 suggesting that mast cell accumulation in tissue as is seen in vulvodynia patients can provoke dysregulated responses to yeast antigens thereby changing the frequency and nature of inflammatory responses to vulvovaginal Candida exposures after vulvodynia onset. Mast cells release nerve growth factor (NGF) and are increasingly recognized as neuromodulatory players in a variety of inflammatory and chronic pain disorders.23 PGE2 has been shown to enhance expression of NGF in rat hippocampal cultures24 and could therefore potentially directly contribute to the increased innervation seen in the vestibular tissue of vulvodynia patients. Since Foster et al. have demonstrated that as few as 100 Candida albicans colony forming units were needed to elicit this IL-6/PGE2 response in vitro by vestibular cells from vulvodynia patients,17 their findings suggest that vestibular fibroblast-mediated inflammation can be triggered by low levels of yeast exposure (i.e., nonclinically apparent infections that may be sufficient to maintain or trigger tissue changes associated with vulvar pain). One caveat is that these studies were performed in vitro, and therefore, the dynamics and kinetics of inflammatory cytokine production observed may differ from those occurring in vivo. However, these and other mechanistic studies of fibroblast-mediated tissue changes can be performed in preclinical models of Candida- or allergen-provoked vulvar pain that have been recently described.7,25 Such studies may also explain why post-vulvodynia yeast infections may be slower to resolve or more resistant to treatment by elucidating specific immune and neuroimmune characteristics of the altered vestibular tissue environment in which these infections take place.
Along with others, we have speculated that vulvodynia may develop as a consequence of exposures such as early life stressors,26,27 allergenic exposures,28 or repeated urogynecological infections,11 which affect immune response to vulvovaginal pathogens. Profound immunodeficiency certainly exacerbates susceptibility to Candidiasis in HIV+ individuals,29 while autoreactive immune cells activated in the context of Candida infection have been proposed to predispose women with susceptible genetic backgrounds to recurrent yeast infections.30 It is possible that, in a subset of women, the altered immune characteristics of affected vulvar tissue are associated with the development of autoimmunity that can promote recurrent yeast infections due to dysregulated local inflammation.
Given the specific temporal intersections of vulvar pain and yeast infection we discuss in this study, it is also interesting to consider that antecedent exposures and/or tissue changes accompanying the onset and maintenance of vulvar pain may alter the composition of the vaginal microbiota (dysbiosis) further dysregulating protective immune responses to Candida and other urogynecological pathogens.31 Bornstein et al. implicated heparanase-mediated degradation of the vestibular stroma and epithelial basement membrane in tissue damage associated with localized provoked vulvodynia.32 Exposure to pathogens, allergens, or injuries may all lead to such compromised mucosal barrier function that can perturb the balance of the vaginal microbiome by exposing commensals to the host immune system, inciting inappropriate inflammation and/or facilitating the colonization of noncommensal species. Across many chronic diseases, it is becoming universally recognized that persistent inflammation is accompanied by the shift of local tissue microbiomes from a “healthy” to “diseased” profile.33 Since the 1890s, lactobacilli have been recognized as the gatekeepers of a healthy vaginal ecosystem and it is likely that if this balance is disrupted, the vulvovaginal tissue will lack the ability to make balanced and appropriate responses to pathogens such as noncommensal yeasts leading to recurrent infections after the onset of vulvodynia.
It remains to be determined whether recurrent yeast infections themselves, or the self-initiated treatments for the infections, play a role in predisposing toward vulvodynia or recurrent onset of yeast infections as a consequence of the development of vulvodynia. However, both are biologically plausible. Other limitations in our data, beyond that of self-reported nondifferential and differential misclassification which we have tried to address in this report, may be the impact of recall between cases and controls irrespective of whether they recall accurately their yeast infection history. To some extent this is taken into account in our bias analyses. In the post-onset models, cases had on average 2 years less follow-up time to report the development of yeast infections compared to controls (women without vulvodynia), which may have led to an underestimation of this association. Furthermore, our study is restricted to women with and without vulvodynia with no active infections at the time of the clinical examination. We excluded 17 potential cases and 4 potential controls due to an active yeast infection at the time of the clinical examination. However, given that women with, compared to women without, vulvodynia are likely to experience a greater number of yeast infections, excluding women at the time of the clinical examination due to active infections may have preferentially skewed our cases toward those less likely to suffer from yeast infections. This would then have underestimated our observed associations. We also conducted our bias analysis using values from the literature, but were restricted based on the limitations imposed by the size of our study regarding the lowest possible sensitivities and specificities that could be explored. We note that the values for specificity, in particular, are higher than what has been published in the literature. We also note that these values may be changing over time since diagnosis within our cohort, which is something we were not able to model.
Conclusions
We have shown that a history of recurrent yeast infections is associated with risk of first onset vulvodynia. We have further shown that once women receive a diagnosis of vulvodynia, they are more likely to report subsequent recurrent yeast infections. However, we have also shown that there are scenarios by which misclassification of self-reported yeast infections can attenuate these findings. New research that focuses on both sorting out the potential misclassification and also understanding the complex inflammatory changes that may underlie tissue sensitivity and the effects of altered vaginal microbiota may help to clarify these epidemiological findings. Clinicians caring for women with vulvodynia should be aware of this potential risk and structure their clinical care and maintenance to treat each condition accurately and effectively to help reduce the risk of subsequent yeast infections.
Acknowledgments
The authors thank Dr. David Foster from the University of Rochester and Dr. Paul Nyirjesy from Drexel University for their peer review and advice on this article. No fee for this service was incurred. This research was supported by NIH-NICHD R01 HD058608.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Harlow BL, Kunitz CG, Nguyen RH, Rydell SA, Turner RM, MacLehose RF. Prevalence of symptoms consistent with a diagnosis of vulvodynia: Population-based estimates from 2 geographic regions. Am J Obstet Gynecol 2014;210:40.e1–40.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moyal-Barracco M, Lynch PJ. 2003 ISSVD terminology and classification of vulvodynia: A historical perspective. J Reprod Med 2004;49:772–777 [PubMed] [Google Scholar]
- 3.Arnold LD, Bachmann GA, Rosen R, Rhoads GG. Assessment of vulvodynia symptoms in a sample of US women: A prevalence survey with a nested case control study. Am J Obstet Gynecol 2007;196:128.e1–128.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith EM, Ritchie JM, Galask R, Pugh EE, Jia J, Ricks-McGillan J. Case-control study of vulvar vestibulitis risk associated with genital infections. Infect Dis Obstet Gynecol 2002;10:193–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bohm-Starke N. Medical and physical predictors of localized provoked vulvodynia. Acta Obstet Gynecol Scand 2010;89:1504–1510 [DOI] [PubMed] [Google Scholar]
- 6.Sarma AV, Foxman B, Bayirli B, Haefner H, Sobel JD. Epidemiology of vulvar vestibulitis syndrome: An exploratory case-control study. Sex Transm Infect 1999;75:320–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farmer MA, Taylor AM, Bailey AL, et al. Repeated vulvovaginal fungal infections cause persistent pain in a mouse model of vulvodynia. Sci Transl Med 2011;3:101ra91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allen-Davis JT, Beck A, Parker R, Ellis JL, Polley D. Assessment of vulvovaginal complaints: Accuracy of telephone triage and in-office diagnosis. Obstet Gynecol 2002;99:18–22 [DOI] [PubMed] [Google Scholar]
- 9.Ferris DG, Nyirjesy P, Sobel JD, Soper D, Pavletic A, Litaker MS. Over-the-counter antifungal drug misuse associated with patient-diagnosed vulvovaginal candidiasis. Obstet Gynecol 2002;99:419–425 [DOI] [PubMed] [Google Scholar]
- 10.Hoffstetter S, Barr S, LeFevre C, Gavard JA. Telephone triage: Diagnosis of candidiasis based upon self-reported vulvovaginal symptoms. J Low Genit Tract Dis 2012;16:251–255 [DOI] [PubMed] [Google Scholar]
- 11.Nguyen RH, Swanson D, Harlow BL. Urogenital infections in relation to the occurrence of vulvodynia. J Reprod Med 2009;54:385–392 [PubMed] [Google Scholar]
- 12.Nguyen RH, Turner RM, Rydell SA, Maclehose RF, Harlow BL. Perceived stereotyping and seeking care for chronic vulvar pain. Pain Med 2013;14:1461–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bornstein J, Goldstein AT, Stockdale CK, et al. 2015 ISSVD, ISSWSH, and IPPS consensus terminology and classification of persistent vulvar pain and vulvodynia. J Lower Gen Tract Dis 2016;20:1–5 [DOI] [PubMed] [Google Scholar]
- 14.Lash TL, Fox MP, Fink AK, SpringerLink (Online Service). Applying quantitative bias analysis to observational epidemiologic research. Statistics for biology and health. New York, NY: Springer Science+Business Media, LLC, 2009 [Google Scholar]
- 15.Borges S, Silva J, Teixeira P. The role of lactobacilli and probiotics in maintaining vaginal health. Arch Gynecol Obstet 2014;289:479–489 [DOI] [PubMed] [Google Scholar]
- 16.Falsetta ML, Foster DC, Woeller CF, et al. Identification of novel mechanisms involved in generating localized vulvodynia pain. Am J Obstet Gynecol 2015;213:38.e1–38.e12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foster DC, Falsetta ML, Woeller CF, et al. Site-specific mesenchymal control of inflammatory pain to yeast challenge in vulvodynia-afflicted and pain-free women. Pain 2015;156:386–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foster DC, Piekarz KH, Murant TI, LaPoint R, Haidaris CG, Phipps RP. Enhanced synthesis of proinflammatory cytokines by vulvar vestibular fibroblasts: Implications for vulvar vestibulitis. Am J Obstet Gynecol 2007;196:346.e1–346.e8 [DOI] [PubMed] [Google Scholar]
- 19.Bornstein J, Goldschmid N, Sabo E. Hyperinnervation and mast cell activation may be used as histopathologic diagnostic criteria for vulvar vestibulitis. Gynecol Obstet Invest 2004;58:171–178 [DOI] [PubMed] [Google Scholar]
- 20.Goetsch MF, Morgan TK, Korcheva VB, Li H, Peters D, Leclair CM. Histologic and receptor analysis of primary and secondary vestibulodynia and controls: A prospective study. Am J Obstet Gynecol 2010;202:614.e1–614.e8 [DOI] [PubMed] [Google Scholar]
- 21.Oskeritzian CA, Zhao W, Pozez AL, Cohen NM, Grimes M, Schwartz LB. Neutralizing endogenous IL-6 renders mast cells of the MCT type from lung, but not the MCTC type from skin and lung, susceptible to human recombinant IL-4-induced apoptosis. J Immunol 2004;172:593–600 [DOI] [PubMed] [Google Scholar]
- 22.Ribbing C, Engblom C, Lappalainen J, et al. Mast cells generated from patients with atopic eczema have enhanced levels of granule mediators and an impaired Dectin-1 expression. Allergy 2011;66:110–119 [DOI] [PubMed] [Google Scholar]
- 23.Theoharides TC, Alysandratos KD, Angelidou A, et al. Mast cells and inflammation. Biochim Biophys Acta 2012;1822:21–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedman WJ, Larkfors L, Ayer-LeLievre C, Ebendal T, Olson L, Persson H. Regulation of beta-nerve growth factor expression by inflammatory mediators in hippocampal cultures. J Neurosci Res 1990;27:374–382 [DOI] [PubMed] [Google Scholar]
- 25.Martinov T, Glenn-Finer R, Burley S, et al. Contact hypersensitivity to oxazolone provokes vulvar mechanical hyperalgesia in mice. PLoS One 2013;8:e78673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harlow BL, Stewart EG. Adult-onset vulvodynia in relation to childhood violence victimization. Am J Epidemiol 2005;161:871–880 [DOI] [PubMed] [Google Scholar]
- 27.Khandker M, Brady SS, Stewart EG, Harlow BL. Is chronic stress during childhood associated with adult-onset vulvodynia? J Womens Health (Larchmt) 2014;23:649–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harlow BL, He W, Nguyen RH. Allergic reactions and risk of vulvodynia. Ann Epidemiol 2009;19:771–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mayer FL, Wilson D, Hube B. Candida albicans pathogenicity mechanisms. Virulence 2013;4:119–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ashman RB, Ott AK. Autoimmunity as a factor in recurrent vaginal candidosis and the minor vestibular gland syndrome. J Reprod Med 1989;34:264–266 [PubMed] [Google Scholar]
- 31.Thaiss CA, Levy M, Suez J, Elinav E. The interplay between the innate immune system and the microbiota. Curr Opin Immunol 2014;26:41–48 [DOI] [PubMed] [Google Scholar]
- 32.Bornstein J, Cohen Y, Zarfati D, Sela S, Ophir E. Involvement of heparanase in the pathogenesis of localized vulvodynia. Int J Gynecol Pathol 2008;27:136–141 [DOI] [PubMed] [Google Scholar]
- 33.Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell 2014;157:121–141 [DOI] [PMC free article] [PubMed] [Google Scholar]


