Abstract
Objective
To review the incidence, predictors and prognosis of bladder cancer recurrence after management of upper tract urothelial carcinoma (UTUC).
Patients and methods
We retrospectively reviewed patients who were surgically treated for UTUC from 1983 to 2013. The tumours were categorised according to the 1997 Tumour-Node-Metastasis (TNM) staging and the three-tiered World Health Organization grading systems. The primary endpoint was the occurrence of any intravesical recurrence after treatment. We studied the possible risk factors that may contribute to development of intravesical recurrence, as well as the prognosis of the patients who had recurrence.
Results
In all, 297 patients were eligible for analysis. Recurrent bladder tumours occurred in 139 patients (46.8%). The mean (range) time to recurrence after surgery was 33 (6–300) months. Neither sex, past history of bladder tumours, concomitant bladder tumour, the side of the tumour, UTUC stage, grade, presence of carcinoma in situ or multicentricity at the time of diagnosis of UTUC, were significant predictors of intravesical tumour recurrence. Ureteric tumour was the only identified risk factor (P = 0.02). Post-treatment bladder recurrence was a significant predictor of later urethral recurrence (P = 0.002).
Conclusions
In our present series, bladder cancer recurrence of urothelial malignancy occurred in nearly half of the patients after surgical management of UTUC. Ureteric tumour was the only identifiable risk factor, thus patients with ureteric tumours may benefit from prophylactic intravesical chemoimmunotherapy. Bladder recurrence does not appear to affect the cancer-specific survival after surgical management of UTUC.
Abbreviations: CIS, carcinoma in situ; RNU, radical nephroureterectomy; UTUC, upper tract urothelial carcinoma
Keywords: Urological neoplasms, Transitional cell carcinoma, Ureteric neoplasms, Urinary bladder neoplasms, Intravesical recurrence
Introduction
Upper tract urothelial carcinoma (UTUC) arises from the urothelial lining of the urinary tract, i.e. from the renal calyces to the ureteric orifice. It comprises ∼10% of all renal tumours and ∼5% of all urothelial malignancies. Multiple anatomical locations in the urinary tract, being either synchronous or metachronous, is a common feature of UTUC [1].
Whilst synchronous bladder tumour can be identified at the time of evaluation of UTUC, recurrent bladder tumours remain a major concern, with a high incidence that varies considerably from 20% to 50% [2], [3] and its detection requires long-term surveillance.
Although the risk factors for the development of bladder tumour after surgical management of UTUC have been previously studied, there is considerable variation in the published literature. A history of bladder tumour prior to UTUC [4], [5], primary tumour location in the ureter [6], multifocality [5], tumour stage and surgical procedures [3], as well as sex and systemic chemotherapy [7], have all been reported as predictors of bladder cancer recurrence. Consequently, and because of a lack of consensus, all patients are still subjected to the same routine 3-monthly cystoscopy follow-up schedule.
In the present study, urologists reviewed their results from >300 consecutive patients treated over a 30-year period for UTUC, at one of the largest tertiary Urology centres in the region, and stratified patients with UTUC based on their risk factors. This was undertaken to determine whether low-risk patients could benefit from an extended follow-up schedule and whether those at high risk may benefit from prophylactic intravesical chemotherapy. The expectation being to decrease patient suffering, the overall cost of the treatment, and lower the incidence of progression, as well as tumour recurrence, thus improving patients’ cancer survival and quality of life.
Patients and methods
After Institutional Review Board approval, we retrospectively reviewed our ongoing database for patients who were surgically treated for UTUC from 1983 to 2013.
The preoperative evaluation included complete history, physical examination and standard routine laboratory measurements, as well as radiological investigations (CT and/or MRI). In most patients, cystoscopy and retrograde ureteropyelography and/or diagnostic ureteroscopy were done in a separate session; any concomitant bladder tumours were resected, and when it was feasible, upper tract tumours were biopsied.
A standard radical nephroureterectomy (RNU) procedure was performed via an open approach in most of the patients; with one abdominal pararectal incision or two incisions, and a standard lumber and lower abdominal incision. In all, 24 cases were done laparoscopically and 13 were managed by open renal-sparing surgeries for solitary functioning renal units (seven with ileal ureter, four for distal ureterectomy/Boari flap, and two with ureteroureterostomy).
The tumour was staged according to the 1997 TNM classification. The three-tiered WHO grading system was used to determine the pathological grade by different pathologists [8]. The tumour location was divided into three groups: pelvicalyceal, ureteric, or both pelvicalyceal and ureteric. Multifocality was defined as the presence of two tumour foci in non-contiguous locations within the ipsilateral renal unit. None of our patients received neoadjuvant or adjuvant systemic chemotherapy.
In the first 2 years, cystoscopy was performed every 3 months and contrast-enhanced CT every 6 months. From the third to fifth year, cystoscopy was performed every 6 months and CT annually. Thereafter, urine analysis and cytology were completed annually during the clinical examination.
The primary endpoint of this study was the occurrence of initial intravesical recurrence and any subsequent recurrence thereafter. We studied the possible risk factors that may contribute to development of intravesical recurrence in terms of sex, past history or concomitant bladder tumour, surgical approach, tumour location, stage, grade, etc. Moreover, we also studied cancer survival, which was compared to those with no bladder recurrence.
As previous and/or concomitant bladder tumour may be a confounding factor, those patient were then eliminated and the analysis was repeated again leaving only patients with de novo bladder tumour after surgical management of UTUC, thus consolidating our results.
Frequency and percentage was used for nominal and categorical variables. The mean and standard deviation (SD) was used for normally distributed data; otherwise, the median and range was used. The chi-squared test was used for the analysis of nominal data and logistic regression analysis was used. Cancer-specific survival was estimated using the Kaplan–Meier method, with differences assessed using the log-rank test; survival time was calculated from the date of RNU. In all tests the P value was two-sided and significance was set at P < 0.05. Analysis was performed using the Statistical Package for the Social Sciences (SPSS®, version 16; SPSS Inc., IBM Corp., Armonk, NY, USA).
Results
Of 322 patients, 17 with incomplete files and eight patients with non-TCC at the final pathology were eliminated, leaving 297 patients eligible for review. The mean (SD, range) age was 59 (11, 26–85) years and the study included 262 men (88.2%). The median (range) follow-up period was 34 (6–300) months. The tumour was right sided in 135 cases (45.4%). In all, 49 patients (16.4%) had a history of bladder tumours and concomitant bladder tumours were found and resected in 78 (26.2%). Open RNU was performed in 260 patients (87.5%), renal-sparing surgeries in 13 (4.3%), and laparoscopic surgery in 24 (8%). The tumours were pelvicalyceal or ureteric in 40% of the patients and in both the pelvicalyceal system and ureter in 20% of the patients.
The tumour stage was T1 in 194 patients (65.6%), T2 in 43 (14.4%), T3 in 59 (19.8%), and T4 in 1 (0.33%). Most of the patients had Grade II tumours [184 (61.9%)], whilst 13 (4.3%) had Grade I and 100 (33.6%) had Grade III.
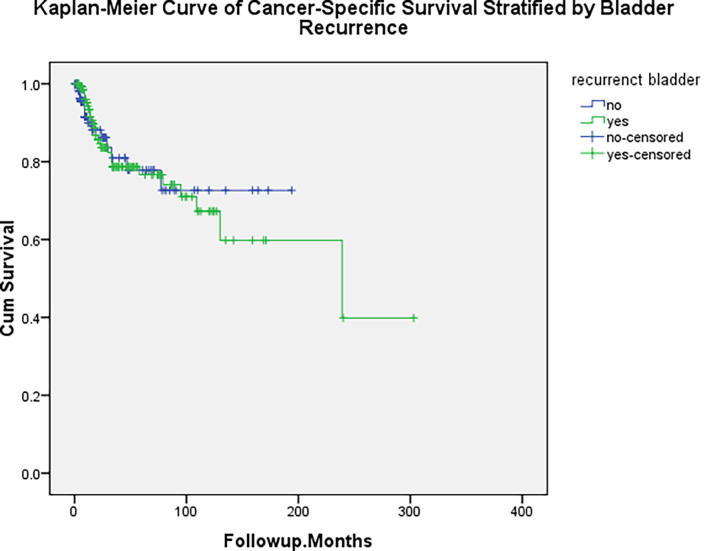
There was intravesical tumour recurrence after RNU in 139 (46.8%) patients after a median (range) follow-up of 33 (6–300) months. The details of the bladder tumour recurrences are shown in Table 1. All non-invasive bladder tumours were treated with endoscopic resection and intravesical chemoimmunotherapy as per the standard protocol. In all, 21/297 patients (7%) had invasive bladder tumours, 15 were fit for radical cystectomy and the rest received radiotherapy. We analysed possible risk factors for the development of bladder tumours after management of UTUC as shown in Table 2. It can be seen that sex, past history of bladder tumour, concomitant bladder tumour, surgical approach, side of the tumour, UTUC stage, grade, presence of carcinoma in situ (CIS) or multicentricity, at the time of diagnosis of UTUC were not significant predictors. Ureteric tumour was the only significant predictor for the development of bladder tumours after surgical management of UTUC (P = 0.04). After eliminating 106 patients with previous and/or concomitant bladder tumour, the ureteric tumour P value was 0.06 and the surgical approach was 0.014, as shown in Table 3. Post-treatment bladder recurrence was not a predictor for contralateral or local recurrence at the surgical site, whilst it was a significant predictor of urethral recurrence (P = 0.002), as shown in Table 4. In multivariate analysis, only a ureteric location sustained its significance [P = 0.044; Exp(B) (odds ratio) = 1.6]. Bladder recurrence did not affect the cancer-specific survival after surgical management of UTUC (log-rank test; P = 0.8), as shown in Fig. 1.
Table 1.
Recurrent bladder tumour characteristics (N = 139).
| Characteristics | N (%)* |
|---|---|
| History of previous bladder tumour | 25 (18) |
| Number of recurrences | |
| 1 | 78 (56) |
| 2 | 35 (25) |
| >2 | 26 (19) |
| Number of lesions in each episode | |
| Single bladder tumour | 66 (47) |
| Multicentric | 73 (53) |
| Stage of recurrent bladder tumour | |
| Ta | 12 (9) |
| Tis | 5 (4) |
| T1 | 101 (73) |
| Muscle invasive | 21 (15) |
| Grade of recurrent bladder tumour | |
| Grade I | 27 (19) |
| Grade II | 88 (63) |
| Grade III | 24 (17) |
Percentages rounded to whole numbers.
Table 2.
Univariate analysis of risk factors for development of bladder tumour after surgical management of UTUC.
| Variable | Development of bladder tumour, n/N (%) |
P | |
|---|---|---|---|
| No | Yes | ||
| Gender | |||
| Male | 138/262 (53) | 124/262 (47) | 0.6 |
| Female | 20/35 (57) | 15/35 (43) | |
| History of bladder tumour (preoperative) | |||
| No | 134/248 (54) | 114/248 (46) | 0.5 |
| Yes | 24/49 (49) | 25/49 (51) | |
| Concomitant bladder tumour | |||
| No | 111/219 (51) | 108/219 (49) | 0.4 |
| Yes | 47/78 (60) | 31/78 (40) | |
| Side of the tumour | |||
| Right | 65/135 (48) | 70/135 (52) | 0.1 |
| Left | 93/162 (57) | 69/162 (43) | |
| Diagnostic ureteroscopy | |||
| Not done | 90/173 (52) | 83/173 (48) | 0.6 |
| Done | 68/124 (55) | 56/124 (45) | |
| Surgical approach | |||
| Open RNU | 141/260 (54) | 119/260 (46) | 0.6 |
| Lap. RNU | 9/24 (37) | 15/24 (63) | |
| Conservative | 8/13 (62) | 5/13 (38) | |
| Site of the tumour | |||
| Kidney (pelvicalyceal) | 74/121 (61) | 47/121 (39) | 0.07 |
| Ureter | 57/121 (47) | 64/121 (53) | |
| Kidney and ureter | 27/55 (49) | 28/55 (51) | |
| Ureteric tumour | |||
| No | 73/121 (60) | 48/121 (40) | 0.04 |
| Yes | 85/176 (48) | 91/176 (52) | |
| Site of the ureteric tumours | |||
| No | 73/120 (61) | 47/120 (39) | 0.09 |
| Multicentric | 11/27 (41) | 16/27 (59) | |
| Lumbar | 15/24 (63) | 9/24 (37) | |
| Iliac | 7/18 (39) | 11/18 (61) | |
| Pelvic | 52/108 (48) | 56/108 (52) | |
| Positive bladder cuff for TCC | |||
| No | 148/280 (53) | 132/280 (47) | 0.6 |
| Yes | 10/17 (59) | 7/17 (41) | |
| Multifocality | |||
| No | 105/193 (54) | 88/193 (46) | 0.5 |
| Yes | 53/104 (51) | 51/104 (49) | |
| Presence of CIS | |||
| No | 148/282 (52) | 134/282 (48) | 0.2 |
| Yes | 10/15 (67) | 5/15 (33) | |
| Tumour grade | |||
| Grade I TCC | 8/13 (62) | 5/13 (38) | 0.2 |
| Grade II TCC | 91/184 (49) | 93/184 (51) | |
| Grade III TCC | 59/100 (59) | 41/100 (41) | |
| Tumour stage | |||
| Non muscle invasive | 96/194 (49) | 98/194 (51) | 0.07 |
| Muscle invasive. | 62/103 (60) | 41/103 (40) | |
Percentages were rounded to whole numbers.
Table 3.
Univariate analysis of risk factors for development of bladder tumour after surgical management of UTUC in patients after elimination of patients who had previous and/or concomitant bladder tumour.
| Variable | Development of bladder tumour, n/N (%) |
P | |
|---|---|---|---|
| No | Yes | ||
| Gender | |||
| Male | 88/168 (52) | 80/168 (48) | 0.9 |
| Female | 12/23 (52) | 11/23 (48) | |
| Side of the tumour | |||
| Right | 45/86 (52) | 41/86 (48) | 0.9 |
| Left | 55/105 (52) | 50/105 (48) | |
| Diagnostic ureteroscopy | |||
| Not done | 60/116 (52) | 56/116 (48) | 0.8 |
| Done | 40/75 (53) | 35/75 (47) | |
| Surgical approach | |||
| Open NU | 91/171 (53) | 80/171 (47) | 0.014 |
| Lap. NU | 4/15 (27) | 11/15 (73) | |
| Conservative | 5/5 (100) | – | |
| Site of the tumour | |||
| Kidney (pelvicalyceal) | 57/96 (59) | 39/96 (41) | 0.1 |
| Ureter | 32/67 (48) | 35/67 (52) | |
| Kidney and ureter | 11/28 (39) | 17/28 (61) | |
| Ureteric tumour | |||
| No | 57/97 (59) | 40/97 (41) | 0.06 |
| Yes | 43/94 (46) | 51/94 (54) | |
| Positive bladder cuff for TCC | |||
| No | 96/183 (52) | 87/183 (48) | 0.8 |
| Yes | 4/8 (50) | 4/8 (50) | |
| Multifocality | |||
| No | 66/120 (55) | 54/120 (45) | 0.3 |
| Yes | 34/71 (48) | 37/71 (52) | |
| Presence of CIS | |||
| No | 95/182 (52) | 87/182 (48) | 0.8 |
| Yes | 5/9 (56) | 4/9 (44) | |
| Tumour grade | |||
| Grade I TCC | 3/5 (60) | 2/5 (40) | 0.6 |
| Grade II TCC | 61/122 (50) | 61/122 (50) | |
| Grade III TCC | 36/64 (56) | 28/64 (44) | |
| Tumour stage | |||
| Non-muscle invasive | 64/127 (50) | 63/127 (50) | 0.4 |
| Muscle invasive | 36/64 (56) | 28/64 (44) | |
Percentages were rounded to whole numbers.
Table 4.
Univariate analysis of patients with recurrent bladder tumour with post-management recurrence and distant metastasis.
| Characteristics | Intravesical recurrence, n/N (%) |
P | |
|---|---|---|---|
| No | Yes | ||
| Contralateral recurrence | |||
| No | 157/294 (53) | 137/294 (47) | 0.4 |
| Yes | 1/3 (33) | 2/3 (67) | |
| Urethral recurrence | |||
| No | 158/289 (54) | 131/289 (45) | 0.002 |
| Yes | 0/8 | 8/8 (100) | |
| Local recurrence | |||
| No | 151/281 (54) | 130/281 (46) | 0.4 |
| Yes | 7/16 (44) | 9/16 (56) | |
| Distant metastasis | |||
| No | 150/274 (55) | 124/274 (45) | 0.06 |
| Yes | 8/23 (35) | 15/23 (65) | |
Percentages were rounded to whole numbers.
Fig. 1.
Kaplan–Meier curve of cancer-specific survival stratified by bladder recurrence (P = 0.8).
Discussion
In the present investigation, we report our experience of a relatively large number of patients with UTUC from a single institute. Nearly half of these patients (46.8%) developed bladder tumour recurrence after a median (range) follow-up period of 35 (6–300) months. This incidence concurs with other published series experiences [3], [4], [9]. More than half of the patients (56%) developed one recurrence, a quarter had two recurrences, and the remaining patients had three or more recurrences; most of them were non-muscle invasive (Table 1). We reported 21/297 patients (7%) with invasive bladder cancer, which is similar to the 6.6% reported by Kim et al. [10] after RNU for UTUC. In that study, the incidence was doubled in patients with primary ureteric tumour location or a pathological stage ≥pT3 of the primary UTUC, and tripled with both risk factors.
To date, there has been no agreement in the literature about possible risk factors for bladder recurrence after UTUC. Koga et al. [7] suggested that three significant factors might share in the development of intravesical recurrence, including incomplete distal ureterectomy, postoperative chemotherapy, as well as female gender. However, the limited analysed numbers in that study, the non-standard surgical approach by excluding distal ureterectomy in some patients, as well as the non-routine use of systemic chemotherapy undermine the value of the study. Gender was not identified as a risk factor for tumour recurrence in our present study or other investigations [6], [11].
In the present study, ureteric tumour location was the only identified risk factor for intravesical recurrence after the management of UTUC as reported previously [6]. We even found a trend of increased incidence of bladder tumours in patients with distal rather than proximal ureteric tumours (Table 1). Park et al. [12] reported that renal ‘pelvis and ureteric TCC are not the same disease in terms of invasion and prognosis’. Ureteric TCC is associated with a higher local or distant failure rate than renal pelvis TCC. Moreover, ureteric tumour location was reported to be significantly associated with an increased risk of disease recurrence and cancer-specific death after surgery for UTUC compared with renal pelvis tumours [13].
Primary tumour stage and grade have provoked huge debate regarding their impact upon intravesical tumour recurrence. It was suggested that low pathological grade [13] and stage [14] are inversely correlated to the risk of tumour recurrence. Conversely, high pathological grade [6] and stage [11] were considered to be risk factors. However, our present study did not support the impact of either factor on future bladder recurrence. The debate also extends into tumour multiplicity. Multifocality is usually implicated in intravesical tumour recurrence [11], [14], [15]. On the other hand our present data, as well as that of Zigeuner et al. [6], excluded multicentricity as an independent risk factor.
Previous history of non-invasive bladder cancer in patients with UTUC was reported to be independently associated with later intravesical tumour recurrence [4], [16]. Conversely, Akdogan et al. [17] denied an independent impact of previous history of bladder tumours on such recurrence. To consolidate our present results, we repeated the same analysis after eliminating the confounders (previous and/or concomitant bladder tumour) leaving only patients who developed bladder tumour after surgical management of UTUC.
In the second analysis, the significance of ureteric tumour was marginally decreased and just approaching significance (P = 0.06). In multivariate analysis, only ureteric location sustained its significance [P = 0.044; Exp(B) (odds ratio) = 1.6]. As the ureter is a narrow tunnel with continuous peristalsis, it is easy for detached tumour cells to settle in the bladder, and in particular in the case of ureteric tumours that are in close proximity to the bladder [5]. In an elegant study, this seeding theory was supported through the identical p53 gene mutation in both the UTUC and synchronous lower urothelial cancer [18]. However, others have denied such impact not only on intravesical recurrence [14], [15], but also on cancer-specific survival [19], [20] after management of UTUC.
Additionally, in the second analysis, patients who were operated upon laparoscopically had a higher incidence of bladder recurrence, which might be explained by the high pressure produced by laparoscopy that could result in the spread of some cancer cells down to the bladder. Similar observations have also been reported by Kume et al. [21] and Matsui et al. [14].
Our present laparoscopy cohort was small, with a total of 24 cases and only 15 in the second analysis. This cohort was in our early experience and was performed by different surgeons. Moreover, different methods were used for treating the bladder cuff; eight of 14 had recurrence following open bladder cuff excision vs seven of 10 in the laparoscopic excision arm. Accordingly, we cannot draw any conclusions on this specific issue.
Urethral recurrence has been reported to occur in ∼7% of men after cystectomy for bladder urothelial malignancy [22], [23]. We found a statistically significant increase in urethral recurrence rate (P = 0.002) after surgical management of UTUC in patients with intravesical recurrence. Notably, this was not associated with increased risk of either contralateral or local recurrence. Additionally, bladder recurrence may increase the chance of distant metastasis (P = 0.06). Prophylaxis against intravesical recurrence after RNU for UTUC has been suggested through maintenance therapy or even single intravesical dose instillation [24], [25]. A single postoperative dose of intravesical Mitomycin C was reported to reduce the absolute risk of intravesical recurrence by 11% and the relative risk by 40% [25].
We acknowledge that the lack of data on tumour size and the retrospective nature of the present study are limitations, but this may be acceptable in such a rare disease as UTUC. Also in our present study, we cannot explain the non-significance of past history of bladder tumour, UTUC stage, and multicentricity as significant factors for intravesical tumour recurrence – factors that have been implicated in other studies. However, our present series represents one of the largest series from a large single urology institute in the region.
Conclusion
From our present series, bladder cancer recurrence of urothelial malignancy occurred in nearly half of the patients after management of UTUC. Ureteric tumour was the only identifiable risk factor, thus such patients may benefit from prophylactic intravesical chemoimmunotherapy. Bladder recurrence did not affect cancer-specific survival after surgical management of UTUC.
Acknowledgements
None.
Conflict of interest
None.
Oncology/Reconstruction
Footnotes
Peer review under responsibility of Arab Association of Urology.
Contributor Information
Mohamed Mohamed Elawdy, Email: mmelawdy@gmail.com.
Yasser Osman, Email: y_osman99@yahoo.com.
Diaa Eldin Taha, Email: drdiaaeldin@gmail.com.
Mohamed H. Zahran, Email: zahranmha@yahoo.com.
Samer El-Halwagy, Email: samerhalwagy@yahoo.com.
Muftah El Garba, Email: elgarba_uro@yahoo.com.
Ahmed M. Harraz, Email: ahmed.harraz@hotmail.com.
References
- 1.Jemal A., Siegel R., Ward E., Murray T., Xu J., Thun M.J. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Kirkali Z., Tuzel E. Transitional cell carcinoma of the ureter and renal pelvis. Crit Rev Oncol Hematol. 2003;47:155–169. doi: 10.1016/s1040-8428(03)00079-9. [DOI] [PubMed] [Google Scholar]
- 3.Hall M.C., Womack S., Sagalowsky A.I., Carmody T., Erickstad M.D., Roehrborn C.G. Prognostic factors, recurrence, and survival in transitional cell carcinoma of the upper urinary tract: a 30-year experience in 252 patients. Urology. 1998;52:594–601. doi: 10.1016/s0090-4295(98)00295-7. [DOI] [PubMed] [Google Scholar]
- 4.Raman J.D., Ng C.K., Boorjian S.A., Vaughan E.D., Jr, Sosa R.E., Scherr D.S. Bladder cancer after managing upper urinary tract transitional cell carcinoma: predictive factors and pathology. BJU Int. 2005;96:1031–1035. doi: 10.1111/j.1464-410X.2005.05804.x. [DOI] [PubMed] [Google Scholar]
- 5.Azemar M.D., Comperat E., Richard F., Cussenot O., Roupret M. Bladder recurrence after surgery for upper urinary tract urothelial cell carcinoma: frequency, risk factors, and surveillance. Urol Oncol. 2011;29:130–136. doi: 10.1016/j.urolonc.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Zigeuner R.E., Hutterer G., Chromecki T., Rehak P., Langner C. Bladder tumour development after urothelial carcinoma of the upper urinary tract is related to primary tumour location. BJU Int. 2006;98:1181–1186. doi: 10.1111/j.1464-410X.2006.06519.x. [DOI] [PubMed] [Google Scholar]
- 7.Koga F., Nagamatsu H., Ishimaru H., Mizuo T., Yoshida K. Risk factors for the development of bladder transitional cell carcinoma following surgery for transitional cell carcinoma of the upper urinary tract. Urol Int. 2001;67:135–141. doi: 10.1159/000050969. [DOI] [PubMed] [Google Scholar]
- 8.Lughezzani G., Burger M., Margulis V., Matin S.F., Novara G., Roupret M. Prognostic factors in upper urinary tract urothelial carcinomas: a comprehensive review of the current literature. Eur Urol. 2012;62:100–114. doi: 10.1016/j.eururo.2012.02.030. [DOI] [PubMed] [Google Scholar]
- 9.Mukamel E., Simon D., Edelman A., Konichezky M., Hadar H., Servadio C. Metachronous bladder tumors in patients with upper urinary tract transitional cell carcinoma. J Surg Oncol. 1994;57:187–190. doi: 10.1002/jso.2930570310. [DOI] [PubMed] [Google Scholar]
- 10.Kim K., You D., Jeong I., Hong J., Ahn H., Kim C. Muscle-invasive bladder cancer developing after nephroureterectomy for upper urinary tract urothelial carcinoma. Urol Oncol. 2013;31:1643–1649. doi: 10.1016/j.urolonc.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 11.Hisataki T., Miyao N., Masumori N., Takahashi A., Sasai M., Yanase M. Risk factors for the development of bladder cancer after upper tract urothelial cancer. Urology. 2000;55:663–667. doi: 10.1016/s0090-4295(99)00563-4. [DOI] [PubMed] [Google Scholar]
- 12.Park S., Hong B., Kim C.S., Ahn H. The impact of tumor location on prognosis of transitional cell carcinoma of the upper urinary tract. J Urol. 2004;171:621–625. doi: 10.1097/01.ju.0000107767.56680.f7. [DOI] [PubMed] [Google Scholar]
- 13.Yafi F.A., Novara G., Shariat S.F., Gupta A., Matsumoto K., Walton T.J. Impact of tumour location versus multifocality in patients with upper tract urothelial carcinoma treated with nephroureterectomy and bladder cuff excision: a homogeneous series without perioperative chemotherapy. BJU Int. 2012;110:E7–13. doi: 10.1111/j.1464-410X.2011.10792.x. [DOI] [PubMed] [Google Scholar]
- 14.Matsui Y., Utsunomiya N., Ichioka K., Ueda N., Yoshimura K., Terai A. Risk factors for subsequent development of bladder cancer after primary transitional cell carcinoma of the upper urinary tract. Urology. 2005;65:279–283. doi: 10.1016/j.urology.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 15.Terakawa T., Miyake H., Muramaki M., Takenaka A., Hara I., Fujisawa M. Risk factors for intravesical recurrence after surgical management of transitional cell carcinoma of the upper urinary tract. Urology. 2008;71:123–127. doi: 10.1016/j.urology.2007.08.054. [DOI] [PubMed] [Google Scholar]
- 16.Milojevic B., Djokic M., Sipetic-Grujicic S., Grozdic Milojevic I., Vuksanovic A., Nikic P. Prognostic significance of non-muscle-invasive bladder tumor history in patients with upper urinary tract urothelial carcinoma. Urol Oncol. 2013;31:1615–1620. doi: 10.1016/j.urolonc.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Akdogan B., Dogan H.S., Eskicorapci S.Y., Sahin A., Erkan I., Ozen H. Prognostic significance of bladder tumor history and tumor location in upper tract transitional cell carcinoma. J Urol. 2006;176:48–52. doi: 10.1016/S0022-5347(06)00511-8. [DOI] [PubMed] [Google Scholar]
- 18.Habuchi T., Takahashi R., Yamada H., Kakehi Y., Sugiyama T., Yoshida O. Metachronous multifocal development of urothelial cancers by intraluminal seeding. Lancet. 1993:3421087–3421088. doi: 10.1016/0140-6736(93)92066-3. [DOI] [PubMed] [Google Scholar]
- 19.Favaretto R.L., Shariat S.F., Chade D.C., Godoy G., Adamy A., Kaag M. The effect of tumor location on prognosis in patients treated with radical nephroureterectomy at Memorial Sloan-Kettering Cancer Center. Eur Urol. 2010;58:574–580. doi: 10.1016/j.eururo.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raman J.D., Ng C.K., Scherr D.S., Margulis V., Lotan Y., Bensalah K. Impact of tumor location on prognosis for patients with upper tract urothelial carcinoma managed by radical nephroureterectomy. Eur Urol. 2010;57:1072–1079. doi: 10.1016/j.eururo.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Kume H., Teramoto S., Tomita K., Nishimatsu H., Takahashi S., Takeuchi T. Bladder recurrence of upper urinary tract cancer after laparoscopic surgery. J Surg Oncol. 2006;93:318–322. doi: 10.1002/jso.20459. [DOI] [PubMed] [Google Scholar]
- 22.Stein J.P., Clark P., Mirranda G., Cai J., Groshen S., Skinner D.G. Urethral tumor recurrence following cystectomy and urinary diversion: clinincal and pathological characteristics in 768 male patients. J Urol. 2005;173:1163–1168. doi: 10.1097/01.ju.0000149679.56884.0f. [DOI] [PubMed] [Google Scholar]
- 23.Boorjian S., Kim S.P., Weight C.J., Cheville J.C., Thapa P., Frank I. Risk factors and outcomes of urethral recurrences following radical cystectomy. Eur Urol. 2011;60:1266–1272. doi: 10.1016/j.eururo.2011.08.030. [DOI] [PubMed] [Google Scholar]
- 24.Sakamoto N., Naito S., Kumazawa J., Ariyoshi A., Osada Y., Omoto T. Prophylactic intravesical instillation of mitomycin C and cytosine arabinoside for prevention of recurrent bladder tumors following surgery for upper urinary tract tumors: a prospective randomized study. Int J Urol. 2001;8:212–216. doi: 10.1046/j.1442-2042.2001.00286.x. [DOI] [PubMed] [Google Scholar]
- 25.O'Brien T., Ray E., Singh R., Coker B., Beard R. Prevention of bladder tumours after nephroureterectomy for primary upper urinary tract urothelial carcinoma: a prospective, multicentre, randomised clinical trial of a single postoperative intravesical dose of mitomycin C (the ODMIT-C Trial) Eur Urol. 2011;60:703–710. doi: 10.1016/j.eururo.2011.05.064. [DOI] [PubMed] [Google Scholar]