Abstract
Recent reports state that C-type lectin-like molecule-1 (CLL-1) in acute myeloid leukemia (AML) is expressed primarily on myeloid cells, but there is still no investigation about its prognostic significance on leukemic blast compartment. Hence, this study aimed to evaluate the prognostic value of CLL-1 in 123 patients with de novo CD34+ Non-M3 AML. Multiparameter flow cytometry was used to assess the expression of CLL-1 on immature compartment in AML and control groups. We found that CLL-1 expression level on blast compartment was closely linked to clinical characteristics, treatment response, and survival outcome of patients. Decreased expression of CLL-1 was observed on immature compartment from AML patients as compared with controls (62.6% vs. 86.5%, P < 0.05). Logistic model exhibited that CLL-1low independently predicted low complete remission rate with an odds ratio of 4.57 (2.53–6.61, P < 0.05). Additionally, CLL-1 expression level at diagnosis was inversely correlated to the residual blast cells (residual leukemia cell) after induction chemotherapy (r = −0.423, P < 0.05). Furthermore, multivariate Cox regression model demonstrated that CLL-1low was still an independent adverse predictor (P < 0.05 for event-free survival, P < 0.05 for overall survival). Notably, CLL-1low was able to discriminate poor survival patients from intermediate- and favorable-risk groups. Taken together, CLL-1 is a novel prognostic predictor that could be exploited to supplement the current AML prognostic risk stratification system, and potentially optimize the clinical management of AML.
Keywords: : low CLL-1 expression, a novel adverse predictor, acute myeloid leukemia
Introduction
Acute myeloid leukemia (AML) is a clonal malignant hematopoietic disease characterized by a block in differentiation, resulting in accumulation of immature myeloid cells [1,2]. Despite the ongoing optimization of clinical management and intensification of chemotherapy regimens, the 5-year survival rate is about 47.1% [3]. Many studies have reported that AML with CD34 positive (CD34+AML) accounts for majority of AML cases whereby its positivity in AML blast population indicated unfavorable survival [4–6]. Henceforth, it is necessary to find appropriate target markers in CD34+AML for better clinical management.
In recent years, there have been numerous advances in the next-generation sequencing and gene expression analysis technologies with more and more unique biomarkers of prognostic significance being discovered in AML. The 2016 NCCN guidelines is an updated version of the former set of AML prognostic risk stratification system guidelines with insight to classify risk on all AML cases with cytogenetics and molecular abnormities of FLT3-ITD, NPM1, and biallelic CEBPA mutation status [7,8]. The updated version in 2016 also included modifications such as normal cytogenetics with TP53 mutation and was classified into the poor-risk group, whereas c-KIT mutation was excluded from the intermediate-risk group. In the 2016 WHO classification [9], AML with recurrent genetic abnormalities was reclassified where RUNX1 was listed as independent AML subtype. However, there is around 50% of patients that are still being generally defined as “lacking characteristic feature of AML,” including patients with normal or undefined abnormal karyotype [10]. These patients actually display considerable heterogeneity.
Therefore, the quest for the common characteristics of these AML molecular markers and their correlation with disease prognosis has become a key focus for research. Some studies reported that C-type lectin-like molecule-1 (CLL-1) is a marker specifically expressed at different stages of differentiation in myeloid cells [11,12]; this suggested that CLL-1 could be a targeted marker potentially in AML [13,14]. As a member of a protein superfamily, CLL-1, also known as hMICL, DCAL-2, and KLRL-1, is a highly glycosylated type II transmembrane receptor, including one receptor recognition domain outside the cell, namely the C-type lectin-like domain (CTLD), a stem region, a transmembrane region, and an immunoreceptor tyrosine-based inhibition motif (ITIM) on the short cytoplasmic tail domain [15]. Apart from binding with ligands, the CLL-1 on the cell membrane is also involved in signal transduction, playing important roles in the immune system while maintaining a stable internal environment [16,17]. Recent studies reported that CLL-1 was mainly expressed in normal bone marrow (BM) granulocytes, monocytes, macrophages, dendritic cells, NK cells, and AML leukemia cells while it was not expressed in lymphocytes [18]. Therefore, it is necessary to explore the role of CLL-1 and its prognostic significance in AML. In this study, we used 10-color flow cytometry to investigate CLL-1 expression, focusing on the prognostic value of the latter in de novo CD34+AML.
Design and Methods
Patients and sample
The primary screening criterion for the patients in this study was confirmed cases of de novo Non-M3 CD34+AML hospitalized in Rui Jin Hospital and Bei Zhan Hospital between April 2012 and February 2015, and 138 BM samples were collected from conforming patients. Eventually, of the 138 patients, only 123 subjects participated in the study (Supplementary Appendix A; Supplementary Data are available online at www.liebertpub.com/scd). On the other hand, to investigate the CLL-1 expression in control immature compartment, we collected immature compartment in BM from healthy control (Supplementary Fig. S1) and regenerating borrow marrow. All samples and specimens were obtained after signed and informed consent according to the Declaration of Helsinki.
Monoclonal antibodies and multiparameter flow cytometry (Navios)
Fresh BM samples were obtained from de novo AML patients and CD34+ AML samples were included for further CLL-1 investigation. The experimental procedure was conducted as previously described [19]. The Navios flow cytometer (3 lasers 10 colors) (Beckman Coulter Co. Ltd.) was used for the specimen investigation, whereas the Kaluza 1.2 software was used for analysis. Information about all monoclonal antibodies and their isotope control antibodies has been listed in the Supplementary Table S1.
Gating strategy and the cutoff value of CLL-1 expression
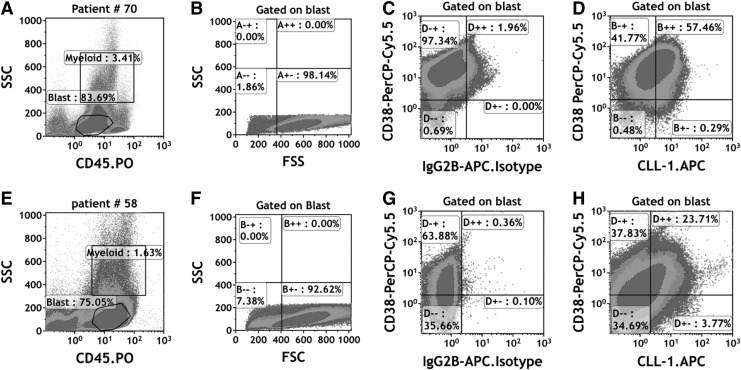
The gating strategy has been depicted in Fig. 1. First, the immature cells in AML were selected on the FSC/SSC scatter diagram and red blood cells and cell debris were simultaneously removed. Then, the bulk blast cell population was characterized by CD45 low or negative expression and low side scatter (CD45low/-/SSClow). The blast population cells were back-gated into a forward scatter (FSC)/SSC plot to ensure homogeneous scatter property; simultaneously isotope control antibodies were designed and produced for each corresponding antibody. The optimal cutoff values of CLL-1 expression in bulk blast were determined by means of receiver operating characteristic curve analysis (Supplementary Fig. S2). With the optimal cutoff value for CLL-1 expression set at 42.5%, the samples were separated into two groups: the CLL-1high group where the CLL-1 expression in bulk blast is ≥42.5% and the CLL-1low group where the CLL-1 expression is less than 42.5%. Of the 123 samples, there were 77 CLL-1high and 46 CLL-1low cases.
FIG. 1.
Flow cytometric analysis of CLL-1 expression on AML blast and CD34+ leukemia cells in patient #70 and patient #58. (A) Patient #70, the bulk blast population in AML was characterized by low expression of CD45 and low side scatter (CD45dim/SSClow). Granulocytes were excluded based on SSC properties. The percentage of CLL-1 bulk blast is 85.11%. (B) The blast cells were back-gated into a forward scatter (FSC)/SSC plot to ensure homogeneous scatter property of the blast population. (C) Gate on blast population, IgG2B-APC isotope control was used as negative controls of CLL-1 expression in blast cells. (D) According to the gate of isotope controls in blast cells, percentage of CLL-1-positive leukemia cells are 57.75%, which is higher than 42.5% and classified into the CLL-1high group. Patient #58 dot plot (E, F) as the same description as the patient #70. (G) Gate on blast population, IgG2B-APC isotope control was used as negative controls of CLL-1 expression in blast cells. (H) According to the gate of isotope controls in blast cells, percentage of CLL-1-positive leukemia cells are 27.48%, which is less than 42.5% and classified into the CLL-1low group. AML, acute myeloid leukemia; CLL-1, C-type lectin-like molecule-1.
Statistical analyses
Chi-square test was used to compare the expression patterns of AML-associated antigen markers and CLL-1 between blast and myeloid cells at different time points (diagnosis, complete remission [CR], relapse, event-free survival [EFS], and the overall survival [OS]), defined as previously described [20]. The paired clinical data of patients at diagnosis and post-treatment were analyzed using the nonparametric Wilcoxon rank-sum test. The association between CLL-1 expression level and residual leukemia cells (RLC) after induction was assessed using the Spearman correlation assay. Furthermore, for prognosis analysis, the association of CLL-1 expression levels and OS/EFS were assayed using the Kaplan–Meier method followed by log-rank test. Multivariate logistic regression and multivariate Cox regression models were applied to the therapeutic response and survival data, and the prognostic value of CLL-1 was assessed after adjustment for well-known confounding factors. The proportional hazards assumption was checked for each variable before applying the Cox models.
Statistical analyses were performed using SPSS 16.0 software package (SPSS, Chicago, IL). All statistical tests were two-sided and P values <0.05 were considered as statistically significant.
Result
Enrolled patients of CLL-1low are closely correlated with unfavorable clinical characteristics
The median age for the 123 de novo Non-M3 CD34+AML cases was 46.5 years (16–66). The median white blood cell (WBC) count was 13.3 × 109/L (range 0.76 × 109/L to 265.00 × 109/L) and the median percentage of BM blasts was 65% (21%–95%). As seen in Table 1, we found that the CLL-1 expression has certain correlation with clinical and biological parameters: The CLL-1low group was significantly related to higher BM blast percentage (P < 0.05). More importantly, the CLL-1low group was closely related to the poor karyotype which was significantly higher than the CLL-1high group (19 of 46 cases, 41.30% vs. 16 of 73 cases, 21.91%) (P < 0.05). Biological data at diagnosis and chi-square analysis showed that favorable molecular markers (biallelic-CEBPA) in the CLL-1low group was significantly lower than in the CLL-1high group (1 of 38 cases, 2.63% versus 16 of 72 cases, 22.20%) (P < 0.05). However, there was no significance in other molecular markers such as c-KIT mutation (P = 0.13), FMS-like tyrosine kinase-3–internal tandem duplication (FLT3-ITD) (P = 0.59), as well as NPM1 mutations (P = 0.23) between CLL-1low and CLL-1high groups (Table 1). There was no significant relationship between CLL-1 levels and any of the following variables: age (P = 0.38), WBC (P = 0.45), gender (P = 0.17), French–American–British subtype (P = 0.47), and treatment protocol (P = 0.14) (Table 1).
Table 1.
Summary of Clinical Cytogenetic and Molecular Features of 123 De Novo CD34+ Acute Myeloid Leukemia
| Diagnosis | CLL-1high | CLL-1low | P |
|---|---|---|---|
| Patients, n (%) | 77 (62.60) | 46 (36.58) | 0.34 |
| Male, n (%) | 44 (57.14) | 32 (69.56) | 0.17 |
| Age (years), median, (range) | 41 (15–78) | 46 (16–71) | 0.38 |
| BM blast count (%), median, (range) | 61.33 (13.5–95.00) | 50.00 (14.00–94.00) | 0.02 |
| WBC (109/L), median, (range) | 19.05 (1.12–252) | 15.80 (0.76–265) | 0.45 |
| FAB type (%) | 0.47 | ||
| M1 | 1/77 (1.30) | 0/46 (0.00) | 0.56 |
| M2 | 20/77 (25.97) | 11/46 (23.91) | 0.39 |
| M4 | 38/77 (49.35) | 21/46 (45.65) | 0.26 |
| M5 | 16/77 (20.78) | 7/46 (15.22) | 0.11 |
| M6 | 1/77 (1.30) | 1/46 (2.18) | 0.18 |
| Not classifieda | 1/77 (1.30) | 6/46 (13.04) | 0.18 |
| Cytogenetics (%) | |||
| Favorable risk | 19/73 (26.02) | 4/46 (8.70) | 0.01 |
| Intermediate risk | 38/73 (52.05) | 23/46 (50.00) | 0.83 |
| Poor risk | 16/73 (21.91) | 19/46 (41.30) | 0.02 |
| Gene mutationb (%) | |||
| FLT3-ITD | 16/72 (22.82) | 6/38 (15.78) | 0.59 |
| c-KIT | 6/72 (8.33) | 4/38 (10.53) | 0.13 |
| CEBPAc | 16/72 (22.22) | 1/38 (2.63) | 0.01 |
| NPM1 | 4/72 (5.50) | 2/38 (5.20) | 0.23 |
| Treatment protocolsd (%) | 0.14 | ||
| T1 | 70/77 (90.91) | 40/46 (86.96) | 0.28 |
| T2 | 7/77 (9.09) | 6/46 (14.04) | 0.12 |
| Transplantation | 12/77 (15.58) | 15/46 (32.60) | 0.21 |
| CR Status (%) | 59/77 (76.62) | 15/46 (32.60) | <0.01 |
P values were calculated by means of nonparametric test for continuous variables and χ2 test for categorical variables, respectively.
Not classified: AML patients without typical morphological characteristics defined in the FAB nomenclature.
Gene mutation data and treatment were available in 110/138 (79.71%) and 123/138 (89.13%) patients for analysis, respectively.
Double-allelic CEBPA mutations.
Treatment protocols: T1, idarubicin (10 mg/m2/day, days 1–3) or Daunorubicin (45 mg/m2/day, days 1–3) and cytarabine (Ara-C 100 mg/m2/day × 7). T2, homoharringtonin-based treatment.
AML, acute myeloid leukemia; BM, bone marrow; CLL-1, C-type lectin-like molecule-1; CR, complete remission; FAB, French–American–British; WBC, white blood cell.
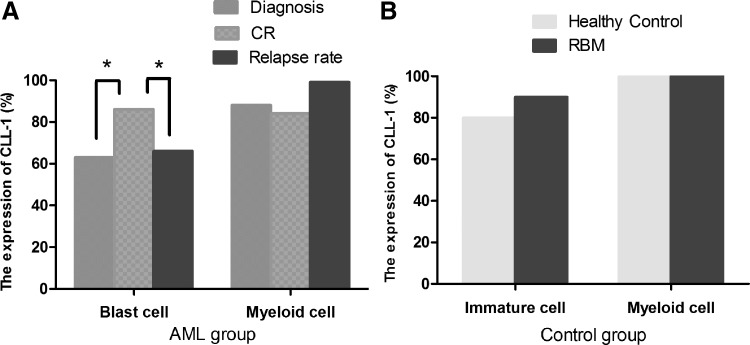
The expression rate of CLL-1 was median 62.6% (77/123) in de novo AML leukemia cells (Fig. 2A) and median 86.5% (20/23) in the control group (Fig. 2B), with the de novo AML group being significantly lower than the control group (P < 0.05). The expression level of CLL-1 was raised at CR (P < 0.05), whereas it was decreased at relapse (P < 0.05) (Fig. 2A). As far as the 123 cases de novo AML patients immunophenotype is concerned, the level of CD11B expression is higher in the CLL-1low group, whereas it is lower level in the CLL-1high group (12/46, 26.08% vs. 9/77, 11.68%, P < 0.05) (Supplementary Table S2).
FIG. 2.
Comparing the expression rates of CLL-1 in different stage cells of AML (A) and control group (B). The expression rate of CLL-1 was 62.6% (77/123) in de novo AML leukemia cells and 86.5% (20/23) in the control group, with the de novo AML group being significantly lower than the control group (P < 0.05), whereas the difference between the myeloid cells from the two groups was not significant (P = 0.22). *P < 0.05
The low expression level of CLL-1 in bulk blast is an independent low CR rate predictor
To investigate the correlation between the CLL-1 expression and treatment response of 123 de novo Non-M3 CD34+ AML cases, 74 patients attained CR after 1 cycle of induction chemotherapy. The overall CR rate was 60.16% (74/123) with the CLL-1high group CR rate being 76.62% (59/77), whereas the CLL-1low group CR rate was 32.60% (15/46), the differences between the two groups were statistically significant (P < 0.05). To investigate whether CLL-1low was an independent non-remission factor from the other well-established factors, such as age, WBC, treatment protocol, cytogenetics, and gene mutation, we applied logistic model to investigate the relationship between the various well-established factors and CLL-1 level and found out that the low level of CLL-1 expression in bulk blast could be an independent marker in predicting low CR rate with an odds ratio of 4.57 (2.53–6.61, P < 0.01) (Table 2).
Table 2.
Multivariate Analysis of Clinical Parameters and C-Type Lectin-Like Molecule-1 Levels for Complete Remission, Event-Free Survival, and Overall Survival
| CR | EFS | OS | ||||
|---|---|---|---|---|---|---|
| Variables | P | 0R (95% CI)a | P | HR (95% CI)a | P | HR (95% CI)a |
| Ageb | 0.23 | 0.97 (0.94–1.01) | 0.98 | 1.00 (0.97–1.02) | 0.57 | 1.00 (0.98–1.02) |
| WBCb | 0.26 | 0.99 (0.98–1.00) | 0.17 | 1.00 (0.99–1.01) | 0.47 | 1.00 (0.99–1.01) |
| Risk status 1 vs. 2c | 0.85 | 0.85 (0.15–4.67) | 0.46 | 1.39 (0.58–3.32) | 0.37 | 1.59 (0.56–4.50) |
| Risk status 3 vs. 2c | 0.01 | 0.06 (0.007–0.63) | 0.47 | 1.49 (0.50–4.44) | 0.56 | 1.42 (0.42–4.79) |
| Transplantationd | <0.01 | 0.22 (0.09–0.49) | <0.01 | 0.21 (0.08–0.50) | ||
| Biallelic CEBPAe | 0.28 | 3.47 (0.35–33.72) | <0.01 | 0.12 (0.02–0.58) | 0.03 | 0.10 (0.01–0.89) |
| FLT3-ITDf | 0.14 | 5.75 (0.55–59.96) | 0.78 | 0.84 (0.26–2.73) | 0.80 | 0.84 (0.25–2.82) |
| c-KITf | 0.05 | 10.48 (0.94–116.71) | 0.04 | 0.34 (0.12–0.96) | 0.10 | 0.43 (0.16–1.20) |
| T1 vs. T2g | 0.16 | 0.29 (0.05–1.65) | 0.11 | 1.99 (0.87–4.54) | 0.28 | 1.56 (0.69–3.56) |
| CLL-1high vs. CLL-1low | 0.006 | 4.57 (2.53–6.61) | 0.01 | 0.44 (0.23–0.84) | 0.02 | 0.46 (0.24–0.89) |
| RLCb | <0.01 | 1.02 (1.00–1.04) | <0.01 | 1.04 (1.02–1.06) | ||
Odds ratio (OR) >1 correspond to an increased tendency of complete remission compared with the lower values of continuous variables or the reference group of categorical. Hazard ratios (HR) >1 correspond to an increased risk of death/relapse compared with the lower values of continuous variables or the reference group of categorical.
Age, WBC, RLC were analyzed as continuous variables.
Risk status 1, 2, 3 stand for risk stratification of favorable, intermediate, and poor risk, respectively, RLC.
Stands for transplantation as consolidation treatment.
Biallelic CEBPA mutant versus monoallelic CEBPA mutants/WT.
Mutant versus WT.
T1, T2 as previously reported.
CI, confidence interval; EFS, event-free survival; OS, overall survival; RLC, residual leukemia cell; WT, wild type.
The rate of minimal residual disease negativity is low in the CLL-1low group and the CLL-1 expression level is significantly inversely related to the proportion of residual blast cells
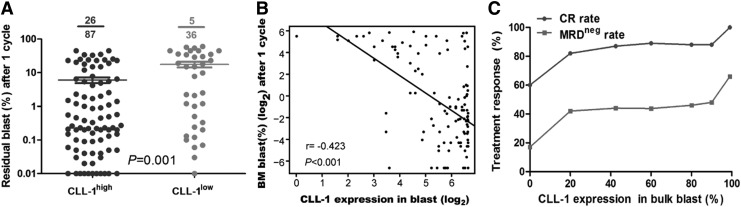
It is well known that multiparameter flow cytometry is fast, with a high degree of sensitivity and wide range coverage. It has been used as a standard and routine technique to track minimal residual disease (MRD) in AML [21]. There is significant correlation between MRD and prognosis and in AML, the threshold value is approximately 0.1%. MRD value below 0.1% is considered as MRD negative (MRDneg), which could indicate early treatment response [22,23]. Of the 123 patients, Leukemia-associated phenotype could be detected in 102 cases. Of the 74 patients who achieved morphological CR, 31 patients stayed MRD negative: the proportion of MRD-negative patients was significantly lower in the CLL-1low group (10.86% (5/46)) than the CLL-1high group (33.76% [26/77], P < 0.05) (Fig. 3A). When Spearman's Rho correlation analysis was used to analyze the correlation between the CLL-1 expression level and residual blast after induction chemotherapy, the association was significantly negatively related (r = −0.423, P < 0.01) (Fig. 3B). More importantly, CLL-1 expression level was closely associated with treatment response: the lower the CLL-1 level, the lower the response rate (Fig. 3C), further indicating that lower CLL-1 expression level in blast is a novel adverse predictor.
FIG. 3.
The correlation between the treatment response and the CLL-1 expression levels. (A) The Bubble chart showed the significant difference of the residual blast between CLL-1high and CLL-1low after induction chemotherapy. (B) Correlation analysis indicated that CLL-1 expression was negatively correlated with residual blast after induction chemotherapy (r = −0.423, P < 0.001). (C) The rates of CR and MRD after complete remission was negativity associated with distinct CLL-1 expression levels: the lower of the CLL-1 levels, the less of the response rates. CR, complete remission; MRD, minimal residual disease.
The low CLL-1 expression level is correlated with poor survival outcome
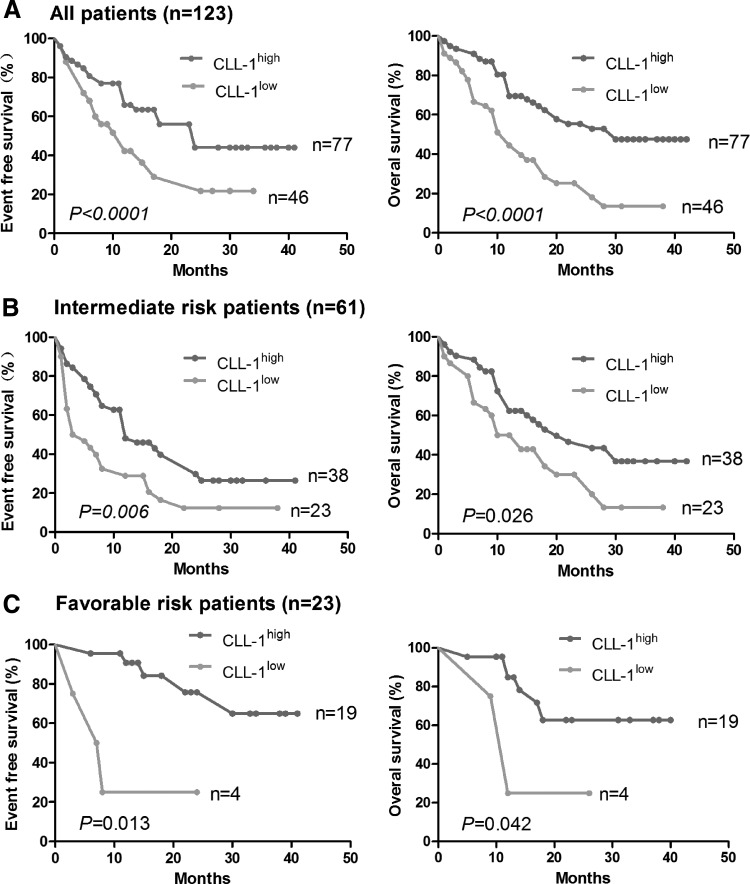
The complete follow-up data of 123 patients is available, and the follow-up time was from April 2012 to January 2016 (median follow-up time of 23 months). Median OS was 20 months (95% confidence interval [CI] 14.32–25.67) and the median EFS were 12 months (95% CI 7.99–16.01). The estimated 2-year OS was 47.1% (95% CI 26%–57%), whereas the estimated 2-year EFS was 35.8% (95% CI 14%–46%) (Fig. 4A).
FIG. 4.
Kaplan–Meier survival analysis followed with log-rank test of de novo AML patients classified as CLL-1high and CLL-1low groups. (A) Comparison of the event-free survival and the OS between CLL-1low and CLL-1high groups of all patients. (B) Comparison of the event-free survival and the OS between CLL-1low and CLL-1high groups of the intermediate-risk patients. (C) Comparison of the event-free survival and the OS between CLL-1low and CLL-1high groups of the favorable-risk patients. OS, overall survival.
As a result of the expression characteristic of CLL-1, the marker was both suitable for dichotomous or continuation variable analysis in the de novo CD34+AML (Supplementary Table S3). The univariate analysis showed that age, WBC, cytogenetic risk, transplantations, and genetic markers were statistically associated with CLL-1low for EFS or OS (Supplementary Table S4), whereas CLL-1low indicated poor prognosis (P < 0.01 for EFS, P < 0.01 for OS). We further verified whether the relationship between the poor outcome and low CLL-1 expression was confounded or modified by these factors, and multivariate Cox regression model analysis showed that CLL-1low was still independent from other well-established factors (karyotype, age, WBC, transplantations, gene mutation, and RLD) (P < 0.02 for EFS, P < 0.05 for OS). However, the differences in age (P = 0.98 for EFS, P = 0.57 for OS), WBC (P = 0.17 for EFS, P = 0.47 for OS), and FLT3-ITD mutation (P = 0.78 for EFS, P = 0.80 for OS) were not statistically significant. Nevertheless, after removing RLC and transplantation, WBC and age were found to be statistically significant (Supplementary Table S5).
When comparing the risk status of the intermediate- (Fig. 4B) and favorable-risk patients (Fig. 4C), the EFS of the CLL-1low (n = 23) and CLL-1high (n = 38) from the intermediate-risk patients was 11 and 19 months, respectively (P < 0.01), whereas their respective OS was 17 and 23 months (P < 0.05). On the other hand, the EFS of the CLL-1low (n = 4) and CLL-1high (n = 19) from the favorable-risk patients was 10 and 30 months, respectively (P < 0.05), whereas their respective OS was 14 and 37 months (P < 0.05). Hence, out of these 84 intermediate- and favorable-risk patients, CLL-1 could identify and differentiate 27 cases with poor prognosis, accounting for 32.14% (27/84) of the patients.
Since AML is a highly heterogeneous disease, upon further analyzing the AML sub groups such as age <60 years (Supplementary Fig. S3A and Supplementary Table S6) or IA induction regimen (Supplementary Fig. S3B and Supplementary Table S7), we found that CLL-1low expression was still an independent adverse predictor.
Discussion
Molecular and cytogenetic markers can independently predict and assess AML prognosis. However, the results of these examinations are time-consuming and delaying. The speed and high sensitivity of multicolor flow cytometry make it more and more appealing to clinicians. Therefore, we used a 10-color flow cytometry (Navios) to investigate the prognostic value of CLL-1 in AML leukemia cells.
This study is the first to report the prognostic value of CLL-1 in AML. While analyzing the CLL-1 expression in de novo AML cell population, we discovered that the low expression level of CLL-1 in AML was an independent molecular predictor of poor prognosis (Fig. 4), mainly in the following aspects: first, the CLL-1low group had a higher proportion of poor cytogenetics and abnormal genes than the CLL-1high group and the differences were statistically significant (P < 0.05); second, multivariate Cox regression model showed that the CLL-1low group is still an independent prognostic value compared with other well-established factors; and third, survival analysis showed that the EFS and OS of CLL-1low group were significantly lower than the CLL-1high group.
As described before, of the 84 intermediate- and favorable-risk patients, CLL-1 could identify and differentiate 27 patients with poor prognosis, modifying their risk and prognostic stratification status, hence prompting for treatment strategy optimization, therefore improving their long-term survival. Moreover, of the 39 poor-risk patients (Supplementary Fig. S3C), CLL-1 could also identify 20 patients with worst prognosis, but this could not prompt for changes in their treatment strategy or eventual outcome, hence the significance of CLL-1 in such patients is limited. In short, as an additional adverse predictor, CLL-1 can be used in the intermediate-, favorable-, and poor-risk AML patients since it can not only determine poor-risk patients with worst outcome, but can also differentiate intermediate- and favorable-risk patients with poor prognosis.
Interestingly, from the 77 CLL-1high cases, we separated the patients into 2 groups with respect to the status of NPM1 with a gene panel consisting of mutant c-KIT and FLT3-ITD: patients with wild-type (WT) NPM1, but with mutant c-KIT/FLT3-ITD were classified as CLL-1high with unfavorable gene panel and those with mutant NPM1 with wild-type c-KIT/FLT3-ITD were classified as CLL-1high with favorable gene panel [7,24]. We also discovered that the OS and EFS of the CLL-1high with unfavorable gene panel were significantly lower than the CLL-1high with unfavorable gene panel (P < 0.01 and P < 0.05 respectively) (Supplementary Fig. S3D). After including them into the Cox analysis, we still found that the EFS and OS were lower in the CLL-1low group (Supplementary Table S8). Henceforth, the results indicate that in AML, CLL-1low can more accurately and independently indicate poor prognosis, whereas CLL-1high should be combined with other genetic markers to predict prognosis.
The mechanism underlying low CLL-1 expression in leukemia cells being a predictor of poor prognosis is not clear. Several reports consider that CLL-1 is similar to other receptors of the Dectin-1 family [25]: CLL-1 is encoded by the CLEC12A gene, located at 12p13,2 and 802 bp in full length, containing 12 exons, encoding three classic transcripts (“canonical” sequence), all located in the NK gene complex (NKC) [26]. The CLEC12A gene homology is also found in rats, mice, and dogs, suggesting that this receptor has sequence conservation in different species, stating that there is a collection of protein tyrosine phosphatase (SHP-1) and SHP-2 phosphates at the ITIM motifs at the cytoplasmic tail of CLL-1, leading to the phosphorylation of the serine in the Src homology domain (SH2) then fusion to the myeloid inhibitory C-type lectin-like receptor (MICL) stem region, transmembrane region and cytoplasmic tail, after interaction with the spleen tyrosine kinase (Syk-coupled interaction). In this way, it can effectively inhibit the production of cytokines and chemokines induced by zymosan [27]. In another study, CLL-1 could effectively inhibit the release of lipopolysaccharide (LPS)-induced IL-12p40, IL-12p70, and LPS-induced cytokines and chemokines, including TNFγ, macrophage inflammatory protein 2, IL-2, IL-10, IL-6, and IL-23 [28], simultaneously limiting inflammatory reaction, hence controlling infection spread. Konstantin Neumann reported that Clec12a-deficient mice exhibited hyperinflammatory responses after radiation-induced thymocyte killing in vivo and compared them to the WT mice by injecting dead cells into Clec12a-deficient mice and they observed that these animals had an increased number of infiltrating neutrophils [29]. There are reports that CLL-1 has the ability to identify aging and apoptotic cells [30,31]. Whether the CLL-1 expressions in AML cells can help clearing up the apoptotic cells destroyed after chemotherapy administration and inhibition of inflammatory cytokines requires further in vitro and in vivo investigations.
In short, CLL-1 predictor can effectively discriminate poor survival cases from intermediate- or favorable-risk groups. Henceforth, it could supplement current AML prognostic risk stratification system, thus potentially optimizing the clinical management of AML.
Supplementary Material
Acknowledgments
The authors are grateful to Pr. Zheng-Yi Wang for his original idea of this research and hereby thank the staff working in the Department of Hematology of Rui Jin Hospital, Bei Zhan Hospital, and Central Hospital of Xu Hui district of Shanghai. This work was partly supported by the Zhabei Youth Health Research Funds (2011-QN01) and partly by the Outstanding Young Talents Training “Qing-Yun plan” of the Zhabei District Health System (2012-ZD12) and the National Natural Science Foundation of China (81570178).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Duque-Afonso J. and Cleary ML. (2014). The AML salad bowl. Cancer Cell 25:265–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cancer Genome Atlas Research N, Ley TJ, Miller C, Ding L, Raphael BJ, Mungall AJ, Robertson A, Hoadley K, Triche TJ Jr., et al. (2013). Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med 368:2059–2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pulte D, Redaniel MT, Jansen L, Brenner H. and Jeffreys M. (2013). Recent trends in survival of adult patients with acute leukemia: overall improvements, but persistent and partly increasing disparity in survival of patients from minority groups. Haematologica 98:222–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Jonge HJ, Woolthuis CM, Vos AZ, Mulder A, van den Berg E, Kluin PM, van der Weide K, de Bont ES, Huls G, Vellenga E. and Schuringa JJ. (2011). Gene expression profiling in the leukemic stem cell-enriched CD34+ fraction identifies target genes that predict prognosis in normal karyotype AML. Leukemia 25:1825–1833 [DOI] [PubMed] [Google Scholar]
- 5.Dang H, Chen Y, Kamel-Reid S, Brandwein J. and Chang H. (2013). CD34 expression predicts an adverse outcome in patients with NPM1-positive acute myeloid leukemia. Hum Pathol 44:2038–2046 [DOI] [PubMed] [Google Scholar]
- 6.Zhu HH, Liu YR, Jiang H, Lu J, Qin YZ, Jiang Q, Bao L, Ruan GR, Jiang B. and Huang X. (2013). CD34 expression on bone marrow blasts is a novel predictor of poor prognosis independent of FlT3-ITD in acute myeloid leukemia with the NPM1-mutation. Leuk Res 37:624–630 [DOI] [PubMed] [Google Scholar]
- 7.Patel JP, Gonen M, Figueroa ME, Fernandez H, Sun Z, Racevskis J, Van Vlierberghe P, Dolgalev I, Thomas S, et al. (2012). Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med 366:1079–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dohner H, Weisdorf DJ. and Bloomfield CD. (2015). Acute myeloid leukemia. N Engl J Med 373:1136–1152 [DOI] [PubMed] [Google Scholar]
- 9.Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M. and Vardiman JW. (2016). The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 127:2391–2405 [DOI] [PubMed] [Google Scholar]
- 10.Vardiman JW, Harris NL. and Brunning RD. (2002). The World Health Organization (WHO) classification of the myeloid neoplasms. Blood 100:2292–2302 [DOI] [PubMed] [Google Scholar]
- 11.Bakker AB, van den Oudenrijn S, Bakker AQ, Feller N, van Meijer M, Bia JA, Jongeneelen MA, Visser TJ, Bijl N, et al. (2004). C-type lectin-like molecule-1: a novel myeloid cell surface marker associated with acute myeloid leukemia. Cancer Res 64:8443–8450 [DOI] [PubMed] [Google Scholar]
- 12.Marshall AS, Willment JA, Lin HH, Williams DL, Gordon S. and Brown GD. (2004). Identification and characterization of a novel human myeloid inhibitory C-type lectin-like receptor (MICL) that is predominantly expressed on granulocytes and monocytes. J Biol Chem 279:14792–14802 [DOI] [PubMed] [Google Scholar]
- 13.Zhao X, Singh S, Pardoux C, Zhao J, Hsi ED, Abo A. and Korver W. (2010). Targeting C-type lectin-like molecule-1 for antibody-mediated immunotherapy in acute myeloid leukemia. Haematologica 95:71–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu H, Zhou Q, Deshmukh V, Phull H, Ma J, Tardif V, Naik RR, Bouvard C, Zhang Y, et al. (2014). Targeting human C-type lectin-like molecule-1 (CLL1) with a bispecific antibody for immunotherapy of acute myeloid leukemia. Angew Chem Int Ed Engl 53:9841–9845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plato A, Willment JA. and Brown GD. (2013). C-type lectin-like receptors of the dectin-1 cluster: ligands and signaling pathways. Int Rev Immunol 32:134–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kerrigan AM. and Brown GD. (2009). C-type lectins and phagocytosis. Immunobiology 214:562–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sancho D. and Reis e Sousa C. (2012). Signaling by myeloid C-type lectin receptors in immunity and homeostasis. Annu Rev Immunol 30:491–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marshall AS, Willment JA, Pyz E, Dennehy KM, Reid DM, Dri P, Gordon S, Wong SY. and Brown GD. (2006). Human MICL (CLEC12A) is differentially glycosylated and is down-regulated following cellular activation. Eur J Immunol 36:2159–2169 [DOI] [PubMed] [Google Scholar]
- 19.Yu C, Kong QL, Zhang YX, Weng XQ, Wu J, Sheng Y, Jiang CL, Zhu YM, Cao Q, et al. (2015). Clinical significance of day 5 peripheral blast clearance rate in the evaluation of early treatment response and prognosis of patients with acute myeloid leukemia. J Hematol Oncol 8:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dohner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK, Dombret H, Fenaux P, Grimwade D, et al. ; European LeukemiaNet. (2010). Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood 115:453–474 [DOI] [PubMed] [Google Scholar]
- 21.Al-Mawali A, Gillis D. and Lewis I. (2009). The role of multiparameter flow cytometry for detection of minimal residual disease in acute myeloid leukemia. Am J Clin Pathol 131:16–26 [DOI] [PubMed] [Google Scholar]
- 22.Chen X, Xie H, Wood BL, Walter RB, Pagel JM, Becker PS, Sandhu VK, Abkowitz JL, Appelbaum FR. and Estey EH. (2015). Relation of clinical response and minimal residual disease and their prognostic impact on outcome in acute myeloid leukemia. J Clin Oncol 33:1258–1264 [DOI] [PubMed] [Google Scholar]
- 23.Terwijn M, van Putten WL, Kelder A, van der Velden VH, Brooimans RA, Pabst T, Maertens J, Boeckx N, de Greef GE, et al. (2013). High prognostic impact of flow cytometric minimal residual disease detection in acute myeloid leukemia: data from the HOVON/SAKK AML 42A study. J Clin Oncol 31:3889–3897 [DOI] [PubMed] [Google Scholar]
- 24.Rollig C, Bornhauser M, Thiede C, Taube F, Kramer M, Mohr B, Aulitzky W, Bodenstein H, Tischler HJ, et al. (2011). Long-term prognosis of acute myeloid leukemia according to the new genetic risk classification of the European LeukemiaNet recommendations: evaluation of the proposed reporting system. J Clin Oncol 29:2758–2765 [DOI] [PubMed] [Google Scholar]
- 25.Chiba S, Ikushima H, Ueki H, Yanai H, Kimura Y, Hangai S, Nishio J, Negishi H, Tamura T, et al. (2014). Recognition of tumor cells by Dectin-1 orchestrates innate immune cells for anti-tumor responses. Elife 3:e04177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sattler S, Ghadially H, Reiche D, Karas I. and Hofer E. (2010). Evolutionary development and expression pattern of the myeloid lectin-like receptor gene family encoded within the NK gene complex. Scand J Immunol 72:309–318 [DOI] [PubMed] [Google Scholar]
- 27.Hardison SE. and Brown GD. (2012). C-type lectin receptors orchestrate antifungal immunity. Nat Immunol 13:817–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen CH, Floyd H, Olson NE, Magaletti D, Li C, Draves K. and Clark EA. (2006). Dendritic-cell-associated C-type lectin 2 (DCAL-2) alters dendritic-cell maturation and cytokine production. Blood 107:1459–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neumann K, Castineiras-Vilarino M, Hockendorf U, Hannesschlager N, Lemeer S, Kupka D, Meyermann S, Lech M, Anders HJ, et al. (2014). Clec12a is an inhibitory receptor for uric acid crystals that regulates inflammation in response to cell death. Immunity 40:389–399 [DOI] [PubMed] [Google Scholar]
- 30.Sancho D. and Reis e Sousa C. (2013). Sensing of cell death by myeloid C-type lectin receptors. Curr Opin Immunol 25:46–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamasaki S, Ishikawa E, Sakuma M, Hara H, Ogata K. and Saito T. (2008). Mincle is an ITAM-coupled activating receptor that senses damaged cells. Nat Immunol 9:1179–1188 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.