Abstract
Objective
To study the effects of oral contraceptive pills (OCP), the first line treatment for PCOS, on HDL-C function (reverse cholesterol efflux capacity) and lipoprotein particles measured by NMR spectroscopy.
Design
Secondary analysis of a randomized controlled trial (OWL-PCOS) of OCP or Lifestyle (intensive lifestyle modification) or Combined (OCP+Lifestyle) treatment for 16 weeks.
Patients
87 overweight/obese women with PCOS at two academic centers
Measurements
Change in HDL-C efflux capacity and lipoprotein particles.
Results
HDL-C efflux capacity increased significantly at 16 weeks in the OCP group (0.11; 95% CI 0.03, 0.18, p=0.008) but not in the Lifestyle (p=0.39) or Combined group (p=0.18). After adjusting for HDL-C and TG levels, there was significant mean change in efflux in the Combined group (0.09; 95% CI 0.01, 0.15; p=0.01). Change in HDL-C efflux correlated inversely with change in serum testosterone (rs = −0.21; p=0.05). In contrast, OCP use induced an atherogenic LDL-C profile with increase in small (p=0.006) and large LDL-particles (p=0.002). Change in small LDL-particles correlated with change in serum testosterone (rs = −0.31, p=0.009) and insulin sensitivity index (rs = −0.31, p=0.02). Both Lifestyle and Combined groups did not show significant changes in the atherogenic LDL-particles.
Conclusions
OCP use is associated with improved HDL-C function and a concomitant atherogenic LDL-C profile. Combination of a Lifestyle program with OCP use improved HDL-C function and mitigated adverse effects of OCP on lipoproteins. Our study provides evidence for use of OCP in overweight/obese women with PCOS when combined with Lifestyle changes.
Keywords: PCOS, weight loss, OCP, obesity
Introduction
Polycystic ovary syndrome (PCOS) is a heterogeneous condition, affecting 10–15% of the reproductive age population. The common gynecologic, dermatologic and reproductive presentation is often exacerbated by obesity and associated with metabolic risk factors and mood and anxiety disorders1. Adolescents and reproductive age women with PCOS have a significantly increased risk of metabolic syndrome2 that is more pronounced in the hyperandrogenic PCOS phenotypes3. In a meta-analysis including 2256 young women with PCOS and 4130 controls, the odds ratio (OR) for metabolic syndrome after adjusting for obesity was 2.2 (95% CI 1.36-3.56)4. The most common metabolic abnormality detected in this population is dyslipidemia characterized by low high density lipoprotein cholesterol (HDL-C) and high triglyceride (TG) levels. although, LDL-C levels are also reported to be increased in PCOS5,6. Collectively, metabolic and lipid abnormalities suggest an increased risk for cardiovascular disease (CVD), although currently available risk stratification tools such as the Framingham risk score typically indicate a low 10 year risk of coronary heart disease in these women2. The longitudinal CARDIA study showed persistent risk of dyslipidemia over a 20 year period in women with PCOS compared to controls7. Although there is limited longitudinal data on the overall risk of CV events in women with PCOS8; the presence of early and persistent dyslipidemia should be recognized and treated as a modifiable CV risk factor9.
Oral contraceptives pills (OCP) are first line therapy used to treat symptoms related to PCOS. Favorable effects of OCP on lipid profile, namely increase in HDL-C levels and potentially harmful effects from increase in TG levels have been well described in the general population10,11. Also, a few studies suggest an increased risk of myocardial infarction and stroke with prolonged use of hormonal contraceptives12. In a meta-analysis including 35 studies, treatment of primarily lean women with PCOS with OCP was associated with significant increase in both TG and HDL-C levels however; there was no significant change in fasting glucose, LDL-C levels or indices of insulin resistance13. There is no data on long-term CV outcomes in women with PCOS treated with OCP. We recently published the OWL-PCOS study, a three arm randomized controlled trial, and reported that overweight/obese women with PCOS treated with low dose OCP for 4 months had an increased risk of metabolic syndrome [OR=2.47; 95% CI (1.42, 4.27)] compared to women randomized to an intensive lifestyle modification intervention with weight loss [OR=1.18; 95% CI (0.63, 2.19)] or the combination of OCP and lifestyle intervention [OR=0.72; 95% CI (0.44, 1.17)]14. Interestingly there were no significant changes in mean serum HDL-C or LDL-C levels but a significant increase in serum TG levels after 16 weeks treatment with OCP.
We have previously demonstrated that standard lipid measurements do not always capture the lipid modulation in PCOS.15 Despite similar serum HDL-C levels to controls, women with PCOS had decreased cholesterol efflux capacity (CEC); a marker of HDL-C function and also exhibited an atherogenic lipid profile measured by nuclear magnetic resonance (NMR) spectroscopy. To our knowledge advanced lipid phenotyping to assess the impact of common treatments in PCOS, such as OCP use, has not been performed. Therefore, in a secondary analysis of the OWL-PCOS study we examined the effects of OCP treatment on HDL-C efflux capacity and compared it with an intensive lifestyle modification intervention for weight loss and the combination of the two treatments. In addition, we used NMR spectroscopy to characterize the modulation of lipid particle composition and number in the three study arms.
Methods
OWL-PCOS Trial
Stored fasting blood samples were obtained for subjects who completed 16 weeks of participation in the preconception intervention phase of the OWL-PCOS study14. Briefly, the trial was a randomized open-label, two-site study of three preconception treatment groups: continuous oral contraception (OCP, 20 mcg ethinyl estradiol/ 1mg norethindrone acetate), an intensive lifestyle modification program designed to produce 10% weight loss (LS), or the combination of both (OCP+LS) as described previously14. We randomized 149 women with BMI 27–42 kg/m2 and 18–40 years of age with PCOS who had no major medical disorders including contraindications to sibutramine or orlistat (weight loss medication) or oral contraceptive use such as uncontrolled hypertension ≥150/100 mmHg or an abnormal EKG, and who were not taking confounding medications (sex steroids, insulin sensitizers, and other infertility drugs). We used modified Rotterdam criteria16 to diagnose PCOS. All women had ovulatory dysfunction with either hyperandrogenism (by Ferrimen-Galwey score17 or an elevated testosterone leve18) or a polycystic ovary on transvaginal ultrasound. Our lifestyle modification (LS) program was multi-focal, and included recommendations for caloric restriction (promoted with the use of meal replacement products), increased physical activity and counseling in behavioral modification strategies. The diet was designed to create a 500 calorie deficit based on initial weight with a macronutrient profile comprising of 30% calories from protein, 45% calories from carbohydrate, and 25% calories from fat19. We followed the Diabetes Prevention Program recommendations for increasing physical activity (principally brisk walking or similar aerobic activity) 5 days per week20. To promote additional weight loss, we used a weight loss medication in those participants who were medically appropriate for usage. We began the study with sibutramine (Brand name: Meridia) at a dose of 5mg/day and titrated up to a maximum dose of 15 mg/day if tolerated. When sibutramine was removed from the market by the Food and Drug Administration secondary to health concerns, we substituted over the counter orlistat 60 mg (Brand Name: Alli) with breakfast, lunch and dinner. Only subjects that had given written informed consent for use of their stored serum samples in secondary studies were included. University of Pennsylvania IRB approval was obtained to utilize the deidentifed samples. We have also published the main outcomes for the OWL-PCOS Study14 and the protocol and case report forms can be accessed at: http://ctsi.psu.edu/owl-pcos/. The trial was registered at Clinicaltrials.gov (OWL PCOS: NCT00704912).
Serum Assays
Glucose and insulin levels were measured at the Penn State College of Medicine. Total testosterone (T) and sex hormone-binding globulin (SHBG) were performed at the Core Ligand Laboratory at the University of Virginia. These assays had interassay coefficients of variation (CV) <10%, including the T assay, which was optimized by increasing the sample volume to reproducibly measure T levels in the female range14
Advanced lipid phenotyping
Cholesterol efflux capacity
Subjects were included in the study if serum samples were available at both baseline and end of 16 weeks. Six subjects were excluded from the study for baseline triglyceride level ≥2.26mmol/Lor baseline HDL level 1.68mmol/L. HDL cholesterol efflux capacity (CEC) was measured by a validated ex vivo system involving the incubation of macrophages with apolipoprotein B-depleted serum from subjects15. 774 cells, derived from a murine macrophage cell line, were plated and radiolabeled with 74kBq of 3H-cholesterol per milliliter. ABCA1 was up-regulated by means of a 6-h incubation with 0.3 mM 8-(4-chlorophenylthio)-cyclic AMP. Subsequently, efflux mediums containing 2.8% apolipoprotein B-depleted serum were added for 4 h. To prepare Apo-B-depleted serum, samples were thawed prior to Apo-B precipitation. Briefly, 40 parts polyethylene glycol solution (PEG; 20% PEG 8000 MW in 200 mM glycine buffer, pH 7.4) was added to 100 parts serum and mixed by pipetting, then incubated at room temperature for 20 min before spinning in a microcentrifuge at 10,000 rpm for 30 min at 4 °C. Apo-B-containing lipoproteins are pelleted by this procedure, and the supernatant, which contains the HDL fraction, is recovered and diluted in 14 mM MEM-HEPES (no bicarbonate) + 0.15 mM cAMP to 2.8% (equivalent to 2% serum). All steps were performed in the presence of the acyl-coenzyme A cholesterol acyltransferase inhibitor CP113,818 (2 μg per milliliter). Liquid scintillation counting was used to quantify the efflux of radioactive cholesterol from the cells. The quantity of radioactive cholesterol incorporated into cellular lipids was calculated by means of isopropanol extraction of control wells not exposed to patient serum. Efflux was calculated by using the following formula: (μCi of 3H-cholesterol in media containing 2.8% apoB-depleted subject plasma-μCi of 3H-cholesterol in plasma-free media / μCi of 3H-cholesterol in media containing 2.8% apoB-depleted pooled control plasma-μCi of 3H-cholesterol in pooled control plasma-free media). All assays were performed in duplicate. The inter and intra assay coefficients of variation for the HDL Efflux assay was <10%.
Lipoprotein subclass particle concentrations (HDL-P, LDL-P and VLDL-P) and average diameters (in nanometers) were measured using an automated nuclear magnetic resonance spectroscopy at the NIH Clinical laboratory (Vantera, Labcorp)21. Particle concentrations of the different-sized lipoprotein subclasses were derived from the measured amplitudes of the characteristic lipid methyl group NMR signals they emit. Subclasses were summed to provide concentrations of total LDL-P, total HDL-P and total very large LDL particles (VLDL-P). Mean LDL-P and HDL-P sizes are weighted-averages (i.e., the diameter of each subclass multiplied by its relative concentration.)’
Statistical Analysis
Groups were compared at baseline using exact chi-square test, ANOVA, or Kruskal-Wallis test as appropriate. A linear mixed-effects model was used to assess changes from baseline within each group and to compare the changes between the groups. Changes from this model are reported as a mean difference and 95% confidence interval (CI), with the exception of testosterone which was log-transformed and therefore the change represents a ratio of geometric means. HDL and TG were included as time-varying covariates in the model where efflux was measured as outcome. Spearman correlation coefficients (rs) were used to assess the association between baseline lipoprotein parameters, as well as the changes in efflux and lipoproteins with the changes in weight, testosterone and insulin sensitivity index (ISI).
Results
Table 1 shows the baseline characteristics for subjects included in this study. There were no significant differences between the three groups for age and BMI and the diagnostic characteristics of PCOS such as clinical and biochemical hyperandrogenism and polycystic ovary morphology.
Table 1.
Baseline characteristics and laboratory data.
| OCP (N=33) |
Lifestyle (N=29) |
Combined (N=25) |
|
|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |
| Demographics | |||
| Age (years) | 29.6 (3.2) | 28.8 (3.2) | 28.6 (3.6) |
| Hispanic: N (%) | 2 (6.1%) | 1 (3.4%) | 3 (12.0%) |
| Caucasian: N (%) | 30 (90.9%) | 23 (79.3%) | 21 (84.0%) |
| Black/African-American: N (%) | 1 (3.0%) | 1 (3.4%) | 2 (8.0%) |
| Other/multi-racial: N (%) | 2 (6.1%) | 5 (17.2%) | 2 (8.0%) |
| Nulliparous: N (%) | 24 (72.7%) | 24 (82.8%) | 23 (92.0%) |
| Biometric | |||
| Weight (kg) | 93.1 (14.7) | 97.2 (15.1) | 96.1 (13.1) |
| BMI (kg/m2) | 34.7 (4.1) | 35.1 (4.7) | 35.0 (4.1) |
| Waist (Waist circumference (cm) | 105.5 (10.9) | 108.6 (13.4) | 106.6 (10.5) |
| Ferriman-Gallwey Hirsutism Score | 16.1 (7.2) | 19.9 (9.7) | 17.2 (7.6) |
| Ultrasound parameters | |||
| Total Ovarian Volume (cm3)* | 22.2 (17.5, 29.7) | 22.1 (13.5, 28.1) | 16.1 (12.8, 23.8) |
| Serum results | |||
| Testosterone (nmol/L)* | 1.8 (1.4, 2.4) | 2.0 (1.3, 2.7) | 1.7 (1.3,2.6) |
| Sex hormone binding globulin (nmol/L)* | 27.5 (20.2, 34.7) | 29.5 (22.2, 38.1) | 23.7 (17.0, 38.3) |
| Fasting Glucose (mmol/L) | 4.81 (0.52) | 4.86 (0.55) | 4.98 (0.63) |
| Fasting Insulin (uU/ml)* | 22.0 (17.0, 27.0) | 25.0 (21.0, 32.0) | 24.0 (15.0, 28.0) |
Median (25th percentile, 75th percentile). There are no significant differences between groups.
At baseline, the HDL-C efflux in the OCP group was significantly higher than the Combined group (difference of adjusted means=0.09, 95% CI 0.03, 0.16, p=0.005) (Figure 1). There was no difference between Lifestyle and either the OCP or Combined groups. At baseline, HDL-C efflux positively correlated with HDL-C (rs=0.69; 95% CI (0.54, 0.80); p<.0001) and total HDL-P (rs=0.70; 95% CI (0.56, 0.81); p<.0001). The mean change in HDL-C efflux capacity from baseline to the 16 week visit in the OCP group was 0.11; 95% CI (0.03, 0.18); p=0.008, in the Lifestyle group was 0.04; 95% CI (−0.01, 0.11); p=0.39, and in the Combined group was 0.06; 95% CI (−0.03, 0.15); p=0.18. Figure 1 shows the change in efflux on adjusting for HDL-C and TG in the OCP group (mean change=0.05; 95% CI (0.03, 0.18); p=0.13), Lifestyle group (mean change=0.03; 95% CI (−0.04, 0.08); p=0.48), and Combined group (mean change=0.09; 95% CI (0.01, 0.15); p=0.01). There was a significant decrease in mean BMI over the 16 week period in both the Lifestyle group (mean change=−2.3; 95% CI (−2.7, −1.8); p<0.001) and Combined group (mean change=−2.6; 95% CI (−3.2, −2.1); p<0.001) but not in the OCP group (mean change=−0.4; 95% CI (−0.9, 0.0); p=0.08). Correspondingly there was significant improvement in the ISI in the Lifestyle group (mean change=1.3; 95% CI (1.1, 1.5); p<.0001) and Combined group (mean change=1.2; 95% CI (1.0, 1.4); p=0.03) but not in the OCP group (mean change=0.9; 95% CI (0.8, 1.0); p=0.11). As expected, there was a significant decrease in testosterone levels in the OCP group (ratio of geometric means=0.4; 95% CI (0.3, 0.5); p<0.001) and Combined group (ratio of geometric means=0.4; 95% CI (0.4, 0.5); p<0.001) but not in the Lifestyle group (ratio of geometric means=0.9; 95% CI (0.8, 1.1); p=0.30). Change in HDL-C efflux inversely correlated with change in serum testosterone levels (rs= −0.21; 95% CI (−0.40, 0.00); p=0.05) but not change in BMI (rs=0.19; 95% CI (−0.03, 0.38); p=0.09) or change in ISI (rs=−0.02; 95% CI (−0.26, 0.23); p=0.89).
Figure 1.
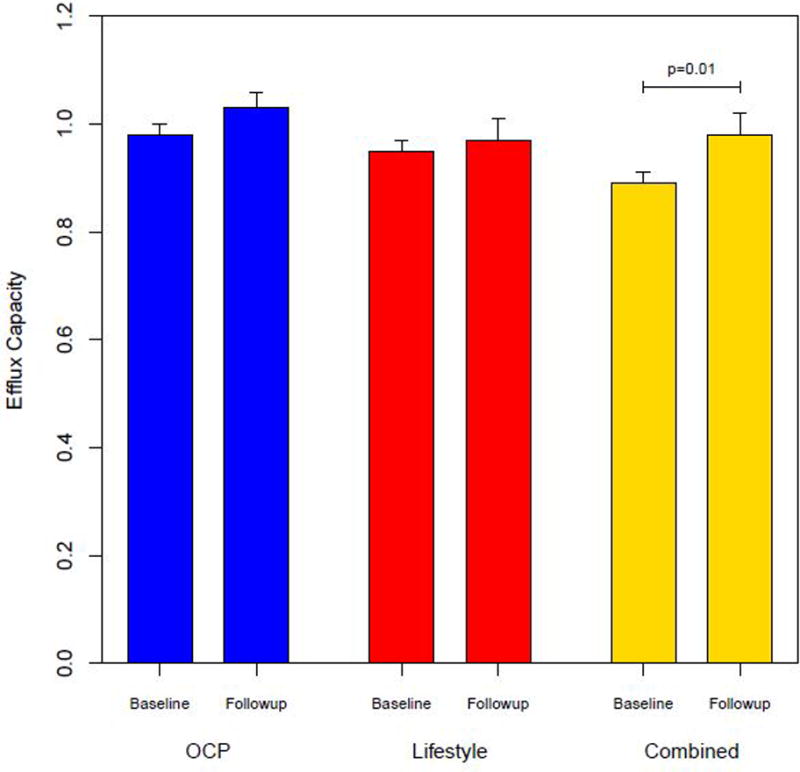
Cholesterol efflux capacity at baseline and following 16 weeks interventions with OCP, Lifestyle interventions and Combined treatment in overweight/obese women with PCOS.(mean ±SE)
Table 2 shows baseline serum lipoprotein values and there were no significant differences between groups. There was a positive correlation between baseline HDL-C and total HDL-P (rs=0.85, p<.0001), LDL-C and total LDL-P (rs=0.88, p<.0001), and TG and total VLDL-P (rs=0.70, p<.0001). Figure 2 shows change in mean levels in lipoprotein particle size and numbers in all three groups after treatment for 16 weeks. Corresponding to the increase in HDL-C efflux, OCP use for 16 weeks was associated with a significant increase in total HDL-P including small HDL-P (p=0.04) and decrease in HDL-P size (p=0.004) compared to baseline. Also, total LDL-P including small LDL-P (p=0.006) and large LDL-P (p=0.002) increased with a corresponding decrease in LDL-P size (p=0.04) with OCP use. There were no significant changes in VLDL-P with OCP treatment. The Lifestyle and Combined groups did not show significant changes in HDL-P and LDL-P or their size compared to baseline after 16 weeks intervention. The change in HDL-P size was significantly different in the OCP group versus the Lifestyle group (p=0.02) as was the change in LDL-P size (p=0.02). The OCP group had a significant increase in small LDL-P compared to the Lifestyle group (p=0.03), and large LDL-P was significantly increased in the OCP versus combined group (p=0.01). Changes in lipoprotein particle concentrations and size were not significantly different between the Lifestyle and Combined groups.
Table 2.
Baseline measurements for lipoprotein particle size and numbers (mean±SD)
| OCP | Lifestyle | Combined | |
|---|---|---|---|
| Total Cholesterol mmol/L | 3.97 (0.98) | 3.88 (1.15) | 4.20 (0.92) |
| HDL-C mmol/L | 1.02 (0.2) | 0.97 (0.3) | 1.10 (0.22) |
| HDL P Total HDL-P uM | 25.6 (6.0) | 25.8 (6.3) | 29.0 (5.6) |
| Large medium HDL-P uM | 12.1 (5.7) | 11.9 (7.0) | 13.9 (7.2) |
| Large HDL-P uM | 3.3 (2.0) | 2.8 (1.9) | 3.3 (1.7) |
| Medium HDL-P uM | 8.0 (4.9) | 8.4 (5.9) | 9.8 (6.1) |
| Small HDL-P uM | 13.0 (4.2) | 13.4 (4.2) | 14.8 (4.1) |
| HDL-P size nm | 9.1 (0.3) | 8.9 (0.4) | 8.9 (0.3) |
| IDL-P nM | 262.3 (127.1) | 195.8 (118.3) | 217.5 (112.1) |
| LDL-C mmol/L | 2.64 (0.79) | 2.58 (0.91) | 2.79 (0.82) |
| LDL-P Total LDL-P nM | 1000.9 (370.3) | 1006.1 (362.2) | 1102.9 (363.7) |
| Large LDL-P nM | 344.0 (171.9) | 375.7 (174.3) | 404.0 (198.5) |
| Small LDL-P nM | 283.0 (295.3) | 322.4 (225.4) | 357.7 (278.7) |
| LDL-P size nm | 21.3 (0.7) | 21.1 (0.7) | 21.2 (0.7) |
| Triglycerides mmol/L | 1.16 (0.38) | 1.18 (0.46) | 1.21 (0.42) |
| Very Large LDL-P nM | 1085.8 (344.7) | 1094.9 (346.1) | 1179.4 (338.2) |
| VLDL P Total VLDL / Chylomicrons nM | 53.1 (22.0) | 57.7 (28.9) | 53.9 (23.3) |
| Large/medium VLDL-p nM | 20.6 (12.5) | 21.3 (11.1) | 19.8 (10.8) |
| Large VLDL-P nM | 3.3 (2.9) | 3.7 (2.9) | 3.8 (3.6) |
| Medium VLDL-P nM | 18.0 (12.6) | 18.2 (9.9) | 16.4 (9.3) |
| Small VLDL-P nM | 32.8 (14.5) | 36.3 (20.8) | 34.2 (16.4) |
| VLDL-Psize nm | 47.6 (7.1) | 47.1 (6.5) | 48.5 (9.2) |
There are no significant differences between groups.
Figure 2.
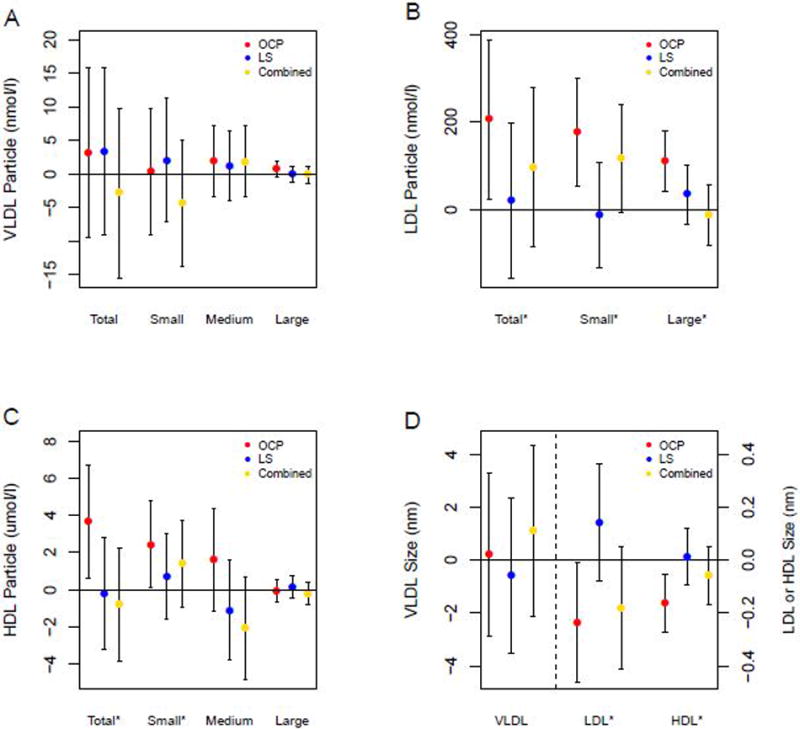
Mean change (with 95% CI) from baseline in lipoprotein particle size and number after 16 weeks intervention according to treatment group.
* p<0.05 change in the OCP group compared to baseline
The changes in lipoprotein concentrations and size associated with changes in BMI, testosterone and ISI in all 3 treatment groups are shown in Table 3. Change in small LDL-P inversely correlated with change in testosterone (rS=−0.31; p=0.01) and ISI (rS=−0.31; p=0.02) while change in LDL-P size positively correlated with change in testosterone (rS=0.26; p=0.03) and inversely correlated with change in glucose area under the curve (AUC) (rS=−0.55; p<.001, data not shown in table). Change in ISI was inversely significantly correlated with change in large-medium VLDL-P (rS=−0.30; p=0.02), change in large VLDL-P (rS=−0.33; p=0.01), change in medium VLDL-P (rS=−0.29; p=0.03), and change in VLDL-P size (rS=−0.34; p=0.01). There were also significant positive associations of change in small VLDL-P with change in testosterone (rS=0.25; p=0.04) and change in VLDL-P size with change in BMI (rS=0.28; p=0.03). Additionally, change in BMI was positively correlated with change in large-medium HDL-P (rS=0.26; p=0.03) and medium HDL-P (rS=0.30; p=0.01).
Table 3.
Associations of changes in BMI, testosterone and insulin sensitivity index (ISI) with changes in lipoprotein particle concentrations and size.
| Change in BMI | Change in Testosterone | Change in Insulin Sensitivity Index | |
|---|---|---|---|
| rs (95% CI) | rs (95% CI) | rs (95% CI) | |
| HDL-P Total HDL-P uM | 0.19 (−0.05, 0.41) | −0.09 (−0.32, 0.15) | −0.19 (−0.43, 0.08) |
| Large medium HDL-P uM | 0.26 (0.02, 0.47)* | −0.01 (−0.25, 0.23) | −0.23 (−0.47, 0.04) |
| Large HDL-P uM | −0.09 (−0.32, 0.15) | 0.00 (−0.24, 0.24) | 0.05 (−0.22, 0.31) |
| Medium HDL-P uM | 0.30 (0.07, 0.51)* | −0.01 (−0.25, 0.23) | −0.26 (−0.49, 0.01) |
| Small HDL-P uM | 0.02 (−0.22, 0.26) | −0.13 (−0.36, 0.12) | −0.05 (−0.31, 0.23) |
| HDL-P size nm | −0.21 (−0.43, 0.04) | 0.07 (−0.18, 0.30) | 0.17 (−0.10, 0.42) |
| IDL-P nM | −0.08 (−0.31, 0.16) | 0.16 (−0.08, 0.39) | 0.03 (−0.24, 0.29) |
| LDL-P Total LDL-P nM | 0.15 (−0.09, 0.38) | −0.12 (−0.35, 0.12) | −0.23 (−0.47, 0.04) |
| Large LDL-P nM | 0.18 (−0.06, 0.40) | 0.09 (−0.16, 0.32) | −0.01 (−0.28, 0.25) |
| Small LDL-P nM | 0.21 (−0.03, 0.43) | −0.31 (−0.51, −0.08)* | −0.31 (−0.53, −0.05)* |
| LDL-P size nm | −0.18 (−0.40, 0.07) | 0.26 (0.02, 0.48)* | 0.23 (−0.05, 0.47) |
| Very Large LDL-P nM | 0.14 (−0.10, 0.37) | −0.10 (−0.16, 0.32) | −0.22 (−0.46, 0.05) |
| VLDL-P Total VLDL / Chylomicrons nM | −0.04 (−0.28, 0.20) | 0.22 (−0.03, 0.43) | −0.13 (−0.39, 0.14) |
| Large medium VLDL-P nM | 0.10 (−0.14, 0.34) | −0.02 (−0.26, 0.22) | −0.30 (−0.53, −0.04)* |
| Large VLDL-P nM | 0.16 (−0.09, 0.38) | −0.04 (−0.28, 0.20) | −0.33 (−0.55, −0.07)* |
| Medium VLDL_P nM | 0.08 (−0.17, 0.31) | 0.01 (−0.23, 0.25) | −0.29 (−0.52, −0.02)* |
| Small VLDL-P nM | −0.10 (−0.34, 0.14) | 0.25 (0.01, 0.46)* | −0.02 (−0.29, 0.25) |
| VLDL-P size nm | 0.28 (0.03, 0.49)* | −0.17 (−0.40, 0.09) | −0.34 (−0.57, −0.07)* |
P<0.05
Discussion
Our study demonstrates for the first time that oral combined hormonal contraceptives used at a low dose (20mcg ethinyl estradiol) for 16 weeks are associated with significant increase in cholesterol efflux capacity. Moreover, OCP use also significantly increased HDL–P levels measured by NMR spectroscopy, although HDL-C levels only showed a modest increase using standard lipid assays14. The Combined group also showed significant increase in cholesterol efflux capacity after adjusting for HDL-C and TG levels. In contrast, OCP use alone resulted in significant increase in LDL-P, especially small LDL-P, suggesting an unfavorable atherogenic effect especially in this high risk population. These effects were ameliorated with the addition of lifestyle changes as seen in the Combined group providing evidence for the use of OCP with Lifestyle changes in overweight/obese women with PCOS.
Studies in both pre and post-menopausal women have demonstrated that estrogen increases serum HDL-C levels measured using standard lipid assays13,22. Lower doses of oral estrogen as used in hormone replacement therapy regimens are also associated with an increase in HDL-C levels23. To our knowledge our study is the first to demonstrate an increase in HDL-C function as determined by cholesterol efflux capacity with use of OCP. Our findings may be primarily related to an increase in HDL-C levels. One previous study has examined the role of endogenous estradiol status (pre and post-menopausal) and gender on reverse cholesterol efflux and did not report any significant differences between the groups24. In contrast, in healthy women with normal lipid profile compared to age matched males, scavenger receptor (SR)-B1 mediated cholesterol efflux capacity was found to be significantly higher25. The authors reported that the increased cholesterol efflux in women in their study was associated with levels of serum HDL-C >1.42mmol/L. Physiological and supraphysiological doses of testosterone have also been reported to increase expression of SR-B1 in vitro using a human hepatocyte cell line and macrophages26 and ethinyl estradiol increased SR-B1 expression in rat hepatocytes27. In one study, 6 month treatment of male to female transgenders with high doses of estradiol showed a significant increase in HDL-C levels but interestingly no change in cholesterol acceptor capacity was observed28. Other potential mechanisms for the effects of estrogen include a decrease in hepatic lipase associated with an increase in Apo A1 associated HDL-C29,30. The mechanism by which exogenously administrated estrogen, via OCP, increases cholesterol efflux capacity is however unclear and could be related to the proposed actions of estrogen on hepatic lipase or secondary to a decrease in serum androgens as noted in our study.
In a large meta-analysis, use of OCP for at least 3 months in women with PCOS significantly increased TG levels but not LDL-C levels measured by standard lipid assays13. We have previously shown that women with PCOS demonstrated an atherogenic lipid profile comprising of increase VLDL-P and small LDL-P, independent of obesity, when measured by NMR spectroscopy15. It is unclear if hyperandrogenemia, insulin resistance or inflammation associated with PCOS could explain our original findings of atherogenic dyslipidemia in PCOS31,32. In the Framingham Heart Study, the number of small LDL particles was elevated in subjects with metabolic syndrome33 We reported an increase in metabolic syndrome after 16 weeks OCP use in the OWL-PCOS study14 and our current results clearly indicate atherogenic changes as measured by NMR spectroscopy in the same group. Although it has been speculated that androgens may be associated with dyslipidemia by influencing central body fat distribution, increasing hepatic lipase activity, and exacerbating insulin resistance34, in our study total testosterone levels were significantly lowered after use of OCP and cannot explain the increase in LDL-P. Longitudinal studies will better quantify the cumulative CV risk of the paradoxical effects of OCP on lipoproteins in women with PCOS.
Modest weight loss in women with PCOS is associated with improvements in reproductive function with no significant changes in lipid profiles35. In our study lifestyle modification intervention alone was associated with a 6–7% weight loss but did not demonstrate a significant impact on either cholesterol efflux capacity or lipoprotein particle concentrations and size. Our findings differ from other studies that show decrease cholesterol efflux capacity after modest weight loss in obese subjects36,37. In fact the Combined arm of our study showed a significant improvement in HDL-C efflux. Further our study suggests that combining lifestyle modifications with OCP use in obese PCOS women may be the best therapeutic intervention, as it decreased VLDL-P and did not increase LDL-P concentrations.
The strengths of our study include randomization to three commonly used interventions, measurement of lipid parameters using NMR spectroscopy, measurement of HDL-C function and no use of cholesterol lowering agents. Our results are generalizable to an obese PCOS population which represents a significant majority of women with this condition in the U.S. The limitations of our study include that we performed a secondary analysis of a randomized clinical trial that was not designed a priori to assess the impact of the selected interventions on lipid profiles and cholesterol efflux capacity. It is not clear if other doses of ethinyl estradiol and different types of progestins will have similar effects. The duration of the intervention was short and replication studies should include longer duration of OCP use.
Our study demonstrated that use of OCP in an overweight/obese PCOS population results in varying effects on lipids including atherogenic lipoprotein changes i.e. an increase in LDL-P concentrations but yet, beneficial increases in HDL-C efflux capacity. The long term cardiovascular impact of these lipid changes in this high risk population is currently unclear; however results from two recent studies indicate that HDL-C function may be a better marker of CV outcomes independent of HDL-C levels38,39. For example, HDL-C function but not HDL-C levels, has been inversely associated with incident CV events as shown in a cohort from the Dallas Heart study (n=2927. These authors showed a 67% reduction in incident CV events when comparing highest quartile of HDL-C efflux with lowest quartile at baseline. Our findings may provide insights into the controversies regarding the overall impact of OCP on cardiovascular risk in young women in the general population. However, these findings need to be replicated in studies including larger numbers of subjects using OCP for a longer duration. In the meantime as addition of caloric restriction and exercise clearly improved cholesterol efflux capacity and ameliorated the adverse lipoprotein changes associated with OCP therapy alone, this should be the preferred treatment in obese women with PCOS.
Acknowledgments
RSL reports consulting fees from Euroscreen, Astra Zeneca, Clarus 39 Therapeutics, Takeda and research funding from Ferring and Astra Zeneca. SE reports research funding from AbbVie. AK reports ownership of Merck Stock. DA reports consulting fees from BAROnova, EnteroMedics, and Ethicon. CC is on the Medical Advisory Board of NORA Therapeutics.
Funding Source: This project was supported by the Eunice Kennedy Shriver National Institutes of Child Health and Human Development (NICHD), National Center for Research Resources, and the National Center for Advancing Translational Sciences at the National Institutes of Health, through Grants R01 HD056510 (RSL), UL1 TR000127 (Penn State Clinical and Translational Institute) and U54 HD29834 (UVA Core Ligand Assay Core of the Specialized Cooperative Centers Program in Reproduction of the NICHD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
Footnotes
Trial Registration: OWL-PCOS NCT00704912
AD, NM, MP, PKE, SG,KA,CS and NW report no conflicts.
References
- 1.Dunaif A. Polycystic ovary syndrome in 2011: Genes, aging and sleep apnea in polycystic ovary syndrome. Nat Rev Endocrinol. 2011 Dec 20;8(2):72–4. doi: 10.1038/nrendo.2011.227. [DOI] [PubMed] [Google Scholar]
- 2.Dokras A, Bochner M, Hollinrake E, Markham S, Vanvoorhis B, Jagasia DH. Screening women with polycystic ovary syndrome for metabolic syndrome. Obstet Gynecol. 2005 Jul;106(1):131–7. doi: 10.1097/01.AOG.0000167408.30893.6b. [DOI] [PubMed] [Google Scholar]
- 3.Panidis D, Tziomalos K, Misichronis G, Papadakis E, Betsas G, Katsikis I, Macut D. Insulin resistance and endocrine characteristics of the different phenotypes of polycystic ovary syndrome: a prospective study. Hum Reprod. 2012;27:541–549. doi: 10.1093/humrep/der418. [DOI] [PubMed] [Google Scholar]
- 4.Moran LJ, Misso ML, Wild RA, Norman RJ. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2010;16(4):347–63. doi: 10.1093/humupd/dmq001. [DOI] [PubMed] [Google Scholar]
- 5.Legro RS, Kunselman AR, Dunaif A. Prevalence and predictors of dyslipidemia in women with polycystic ovary syndrome. Am J Med. 2001 Dec 1;111(8):607–13. doi: 10.1016/s0002-9343(01)00948-2. [DOI] [PubMed] [Google Scholar]
- 6.Wild RA, Rizzo M, Clifton S, Carmina E. Lipid levels in polycystic ovary syndrome: systematic review and meta-analysis. Fertil Steril. 2011 Mar 1;95(3):1073–9. doi: 10.1016/j.fertnstert.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 7.Wang ET, Calderon-Margalit R, Cedars MI, Daviglus ML, Merkin SS, Schreiner PJ, Sternfeld B, Wellons M, Schwartz SM, Lewis C, Williams OD, Siscovick DS, Bibbins-Domingo K. Polycystic ovary syndrome and risk for long-term diabetes and dyslipidemia. Obstet Gynecol. 2011 Jan;117(1):6–13. doi: 10.1097/AOG.0b013e31820209bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Groot PC, Dekkers OM, Romijn JA, Dieben SW, Helmerhorst FM. PCOS, coronary heart disease, stroke and the influence of obesity: a systematic review and meta-analysis. Hum Reprod Update. 2011;17:495–500. doi: 10.1093/humupd/dmr001. [DOI] [PubMed] [Google Scholar]
- 9.Wild RA, Carmina E, Diamanti-Kandarakis E, Dokras A, Escobar-Morreale HF, Futterweit W, Lobo R, Norman RJ, Talbott E, Dumesic DA. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society. J Clin Endocrinol Metab. 2010 May;95(5):2038–49. doi: 10.1210/jc.2009-2724. [DOI] [PubMed] [Google Scholar]
- 10.Ball MJ, Ashwell E, Jackson M, Gillmer MD. Comparison of two triphasic contraceptives with different progestogens: effects on metabolism and coagulation proteins. Contraception. 1990 Apr;41(4):363–76. doi: 10.1016/0010-7824(90)90036-u. [DOI] [PubMed] [Google Scholar]
- 11.Van Rooijen M, Schoultz BV, Silveira A, Hamsten A, Bremme K. Different effects of oral contraceptives containing levonorgestrel or desogestrel on plasma lipoproteins and coagulation factor VII. Am J ObstetGynecol. 2002;186:44–48. doi: 10.1067/mob.2002.119179. [DOI] [PubMed] [Google Scholar]
- 12.Harvey RE, Coffman KE, Miller VM. Women-specific factors to consider in risk, diagnosis and treatment of cardiovascular disease. Womens Health (Lond Engl) 2015 Mar;11(2):239–57. doi: 10.2217/whe.14.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halperin IJ, Kumar SS, Stroup DF, Laredo SE. The association between the combined oral contraceptive pill and insulin resistance, dysglycemia and dyslipidemia in women with polycystic ovary syndrome: a systematic review and meta-analysis of observational studies. Hum Reprod. 2011 Jan;26(1):191–201. doi: 10.1093/humrep/deq301. [DOI] [PubMed] [Google Scholar]
- 14.Legro RS, Dodson WC, Kris-Etherton PM, Kunselman AR, Stetter CM, Williams NI, Gnatuk CL, Estes SJ, Fleming J, Allison KC, Sarwer DB, Coutifaris C, Dokras A. Randomized Controlled Trial of Preconception Interventions in Infertile Women With Polycystic Ovary Syndrome. J Clin Endocrinol Metab. 2015 Nov;100(11):4048–58. doi: 10.1210/jc.2015-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roe A, Hillman J, Butts S, Smith M, Rader D, Playford M, Mehta NN, Dokras A. Decreased cholesterol efflux capacity and atherogenic lipid profile in young women with PCOS. J Clin Endocrinol Metab. 2014 May;99(5):E841–7. doi: 10.1210/jc.2013-3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Legro RS, Brzyski RG, Diamond MP, et al. The Pregnancy in Polycystic Ovary Syndrome II study: Baseline characteristics and effects of obesity from a multicenter randomized clinical trial. Fertil Steril. 2014;101:258–269 e258. doi: 10.1016/j.fertnstert.2013.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Legro RS, Schlaff WD, Diamond MP, et al. Total testosterone assays in women with polycystic ovary syndrome: Precision and correlation with hirsutism. J Clin Endocrinol Metab. 2010;95:5305–5313. doi: 10.1210/jc.2010-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wadden TA, West DS, Delahanty L, et al. The Look AHEAD study: A description of the lifestyle intervention and the evidence supporting it. Obesity. 2006;14:737–752. doi: 10.1038/oby.2006.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Otvos JD, Mora S, Shalaurova I, Greenland P, Mackey RH, Goff DC., Jr Clinical implications of discordance between low-density lipoprotein cholesterol and particle number. J Clin Lipidol. 2011;5:105–113. doi: 10.1016/j.jacl.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walsh BW, Schiff I, Rosner B, Greenberg L, Ravnikar V, Sacks FM. Effects of postmenopausal estrogen replacement on the concentrations and metabolism of plasma lipoproteins. N Engl J Med. 1991;325:1196–1204. doi: 10.1056/NEJM199110243251702. [DOI] [PubMed] [Google Scholar]
- 23.Godsland IF. Effects of postmenopausal hormone replacement therapy on lipid, lipoprotein, and apolipoprotein (a) concentrations: analysis of studies published from 1974–2000. Fertil Steril. 2001 May;75(5):898–915. doi: 10.1016/s0015-0282(01)01699-5. [DOI] [PubMed] [Google Scholar]
- 24.Badeau RM, Metso J, Kovanen PT, Lee-Rueckert M, Tikkanen MJ, Jauhiainen M. The impact of gender and serum estradiol levels on HDL-mediated reverse cholesterol transport. Eur J Clin Invest. 2013 Apr;43(4):317–23. doi: 10.1111/eci.12044. [DOI] [PubMed] [Google Scholar]
- 25.Catalano G, Duchene E, Julia Z, Le Goff W, Bruckert E, Chapman MJ, Guerin M. J Lipid Res. 2008 Mar;49(3):635–43. doi: 10.1194/jlr.M700510-JLR200. Epub 2007 Dec 5. Cellular SR-BI and ABCA1-mediated cholesterol efflux are gender-specific in healthy subjects. [DOI] [PubMed] [Google Scholar]
- 26.Langer C, Gansz B, Goepfert C, Engel T, Uehara Y, von Dehn G, Jansen H, Assmann G, von Eckardstein A. Biochem Biophys Res Commun. 2002 Sep 6;296(5):1051–7. doi: 10.1016/s0006-291x(02)02038-7. Testosterone up-regulates scavenger receptor BI and stimulates cholesterol efflux from macrophages. [DOI] [PubMed] [Google Scholar]
- 27.Fluiter K, Sattler W, De Beer MC, Connell PM, van der Westhuyzen DR, van Berkel TJ. Scavenger receptor BI mediates the selective uptake of oxidized cholesterol esters by rat liver. J Biol Chem. 1999 Mar 26;274(13):8893–9. doi: 10.1074/jbc.274.13.8893. [DOI] [PubMed] [Google Scholar]
- 28.Wultsch A, Kaufmann U, Ott J, Stojakovic T, Scharnagl H, Stangl H, Strobl WM. Profound Changes in Sex Hormone Levels during Cross-Sex Hormone Therapy of Transsexuals do not Alter Serum Cholesterol Acceptor Capacity. J Sex Med. 2015 Jun;12(6):1436–9. doi: 10.1111/jsm.12878. [DOI] [PubMed] [Google Scholar]
- 29.Sérougne C, Feurgard C, Hajri T, Champarnaud G, Férézou J, Mathé D, Lutton C. Catabolism of HDL1 cholesteryl ester in the rat. Effect of ethinyl estradiol treatment. C R Acad Sci III. 1999 Jul;322(7):591–6. doi: 10.1016/s0764-4469(00)88529-7. [DOI] [PubMed] [Google Scholar]
- 30.Tikkanen MJ, Nikkila EA, Kussi T, Sipinens S. High density lipoprotein-2 and hepatic lipase: reciprocal changes produced by estrogen and norgestrel. J Clin Endocrinol Metab. 1982;54:1113–1117. doi: 10.1210/jcem-54-6-1113. [DOI] [PubMed] [Google Scholar]
- 31.McGillicuddy FC, de la Llera Moya M, Hinkle CC, Joshi MR, Chiquoine EH, Billheimer JT, Rothblat GH. Reilly MInflammation impairs reverse cholesterol transport in vivo. Circulation. 2009 Mar 3;119(8):1135–45. doi: 10.1161/CIRCULATIONAHA.108.810721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsuki K, Tamasawa N, Yamashita M, Tanabe J, Murakami H, Matsui J, Imaizumi T, Satoh K, Suda T. Metformin restores impaired HDL-mediated cholesterol efflux due to glycation. Atherosclerosis. 2009 Oct;206(2):434–8. doi: 10.1016/j.atherosclerosis.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 33.Kathiresan S, Otvos JD, Sullivan LM, Keyes MJ, Schaefer EJ, Wilson PW, D’Agostino RB, Vasan RS, Robins SJ. Increased small low-density lipoprotein particle number: A prominent feature of the metabolic syndrome in the framingham heart study. Circulation. 2006;113:20–29. doi: 10.1161/CIRCULATIONAHA.105.567107. [DOI] [PubMed] [Google Scholar]
- 34.van de Woestijne AP, Monajemi H, Kalkhoven E, Visseren FL. Adipose tissue dysfunction and hypertriglyceridemia: mechanisms and management. Obes Rev. 2011 Oct;12(10):829–40. doi: 10.1111/j.1467-789X.2011.00900.x. [DOI] [PubMed] [Google Scholar]
- 35.Moran LJ, Hutchison SK, Norman RJ, Teede HJ. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst Rev. 2011 Jul 6;(7) doi: 10.1002/14651858.CD007506.pub3. [DOI] [PubMed] [Google Scholar]
- 36.Aicher BO, Haser EK, Freeman LA, Carnie AV, Stonik JA, Wang X, Remaley AT, Kato GJ, Cannon RO., 3rd Diet-induced weight loss in overweight or obese women and changes in high-density lipoprotein levels and function. Obesity (Silver Spring) 2012 Oct;20(10):2057–62. doi: 10.1038/oby.2012.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vasudevan M, Tchoua U, Gillard BK, Jones PH, Ballantyne CM, Pownall HJ. Modest diet-induced weight loss reduces macrophage cholesterol efflux to plasma of patients with metabolic syndrome. J Clin Lipidol. 2013 Nov-Dec;7(6):661–70. doi: 10.1016/j.jacl.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khera AV, Cuchel M, laLleraMoya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, Neeland IJ, Yuhanna IS, Rader DR, de Lemos JA, Shaul PW. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med. 2014 Dec 18;371(25):2383–93. doi: 10.1056/NEJMoa1409065. [DOI] [PMC free article] [PubMed] [Google Scholar]


