Abstract
Alzheimer’s disease (AD) is a chronic, progressive and prevalent neurodegenerative disease characterized by the loss of higher cognitive functions and an associated loss of memory. The thus far “incurable” stigma for AD prevails because of variations in the success rates of different treatment protocols in animal and human studies. Among the classical hypotheses explaining AD pathogenesis, the amyloid hypothesis is currently being targeted for drug development. The underlying concept is to prevent the formation of these neurotoxic peptides which play a central role in AD pathology and trigger a multispectral cascade of neurodegenerative processes post-aggregation. This could possibly be achieved by pharmacological inhibition of β- or γ-secretase or stimulating the non-amyloidogenic α-secretase. Melatonin the pineal hormone is a multifunctioning indoleamine. Production of this amphiphilic molecule diminishes with advancing age and this decrease runs parallel with the progression of AD which itself explains the potential benefits of melatonin in line of development and devastating consequences of the disease progression. Our recent studies have revealed a novel mechanism by which melatonin stimulates the nonamyloidogenic processing and inhibits the amyloidogenic processing of β-amyloid precursor protein (βAPP) by stimulating α-secretases and consequently down regulating both β- and γ-secretases at the transcriptional level. In this review, we discuss and evaluate the neuroprotective functions of melatonin in AD pathogenesis, including its role in the classical hypotheses in cellular and animal models and clinical interventions in AD patients, and suggest that with early detection, melatonin treatment is qualified to be an anti-AD therapy.
Keywords: Alzheimer's disease, aging, amyloid-β peptide, melatonin, secretases, neuroprotection
1. INTRODUCTION
Alzheimer’s disease (AD) is the most common form of dementia and the cause of premature senility, resulting in progressive mental deterioration and neurobehavioral deficits. Histopathologically, AD is characterized by extracellular deposits of Aβ in plaques [1] and intracellular neurofibrillary
tangles mainly composed of an abnormally hyper-phosphorylated form of the microtubule-associated protein tau [2]. Additionally, cholinergic dysfunction in the central nervous system (CNS) contributes significantly to the cognitive decline associated with advanced age and AD [3, 4]. Melatonin (N-acetyl-5-methoxytryptamine) is a neurohormone produced by the pineal gland. It is highly pleiotropic, acting as a local regulatory molecule in probably all tissues [5]. This molecule was initially found to influence circadian rhythms [6, 7] and seasonal breeding [8, 9]. More recently, it was discovered to be a potent antioxidant and free radical scavenger [10, 11]. Its nuclear localization suggests its possible genomic actions [12-14].
Melatonin levels in individuals increase from birth and peak around puberty [15]. Its synthesis [16] and levels decline in middle-aged and most elderly individuals [17] along with a simultaneous reduction in the urinary excretion of its metabolites [18]. The functional versatility of this natural ubiquitous molecule enables it to regulate numerous aspects of biological and physiological functions [19], and its decline consequently alters related biological functions during aging and in diseased conditions [20]. In addition to its low level of intrinsic toxicity, studies have shown melatonin’s ability to reduce the side effects and increase the efficacy of large number of drugs [21]. Moreover, it stimulates antioxidant defense systems in the brain [22, 23]. Melatonin easily diffuses through all morphophysiological barriers [24] and reaches every subcellular organelle [25]. It has been speculated that melatonin may actually be produced in all cells, i.e., in mitochondria [26].
The decline in melatonin production in aged individuals [27] is itself a significant factor that may contribute to age-related neurodegenerative disease processes [28] and has been suggested as one of the primary reasons for the development of AD. This is possible because as the pineal gland becomes impaired, most likely due to dysfunction of the suprachiasmatic nucleus (SCN), decreased melatonin levels and altered circadian rhythms may contribute to aging. A decline in cerebrospinal fluid (CSF) melatonin levels parallels the progression of AD neuropathology [29], which makes the brain cells more vulnerable to the oxidative damage that is observed to be more severe in AD brains [30, 31]. The high melatonin concentrations in the CSF supports the likelihood that melatonin would have exceptional protective effects in the CNS [32, 33]. Of interest is that the melatonin levels are lower in patients with AD than in age-matched control subjects [34, 35].
As a powerful free radical scavenger [36, 37], melatonin also displays anti-amyloidogenic properties, which qualifies it to be a promising therapeutic candidate for AD [38, 39]. Interestingly, inhibition of melatonin biosynthesis in rats causes signs that resemble AD pathology [40]. Many studies report increased free radical production, lipid peroxidation, oxidative DNA damage, oxidative protein damage, decreased ATP production, and reduced cell viability in postmortem AD brains compared to brains from age-matched healthy subjects [41, 42]. In this context, melatonin exhibits a broad diversity of effects to reduce neurodegenerative changes in the CNS [38, 43, 44].
An interesting fact regarding the circadian pattern of Aβ production is that Aβ levels reportedly rise when awake and fall during sleep [45], which could possibly be due to the day-night melatonin rhythm. Chronobiological disturbances such as sundowning contribute to mental decline [46], agitation and confusion in AD patients, and melatonin supplementation alleviates symptoms of sundowning and improves cognition [47-51]. Experiments in animal models of AD suggest that melatonin may disrupt the production and accumulation of both plaques and tangles, which are pathological hallmarks of AD [52-54]. Melatonin supplementation also helps combat sleep disorders associated with AD [55, 56] and prevents memory and cognitive impairment in AD patients [57]. Although many therapeutic investigations are underway to provide brain protection against AD, the most challenging is the regulation of disease initiation and prevention of the progression of neuropathology. In this review, we thoroughly discuss the likely preventive role of melatonin in AD and its remarkable function in controlling the progression of this devastating disease.
2. MELATONIN IN AGING AND AD: PROSPECTS FOR LIFE EXPANSION AND DELAYING SENESCENCE
2.1. Is Melatonin the Key to Unraveling the Incomprehensible Connection between Genetically Programmed Aging and AD?
The onset of puberty initiates a slow decline in blood melatonin levels. This decrease in the secretion of melatonin continues with advancing age [58], and by middle age, the levels drop to the point where they may trigger and initiate neurodegenerative changes in the brain. A number of clinical investigations and experimental studies have been performed that may partially explain this association. The pineal gland is part of the central biological system that some believe to be the initiation site for senescence [59]. Alterations in the retina-SCN-pineal axis and disrupted receptor-mediated effects of melatonin at the level of the SCN [60] leads to desynchronized melatonin rhythms and reduced melatonin production during aging; this also occurs in patients with AD [61-64]. These changes contribute to the deterioration of the structural, physiological, molecular and biochemical functions of the nervous system. The severe degenerative changes in the SCN result in abnormal nocturnal melatonin levels in AD patients [65]. In comparison to normal aging individuals [58, 66], the nighttime melatonin concentrations are reported to be especially low in AD patients [63]. Decreased melatonin levels in the CSF [29] and in the serum [67] of AD patients suggests dysfunction of the sympathetic regulation of pineal melatonin synthesis by the SCN during the early stages of AD [34, 35]. A diminishing level of melatonin may be a predisposing factor for AD [68], with this decline paralleling disease progression in AD patients [62].
The neurological markers of AD begin to develop after puberty [69], which coincides with the onset of melatonin synthesis decline. Additionally, there is a significant loss in the melatonin precursor serotonin in preclinical AD stages [70]. Melatonin concentrations in the ventricular CSF are reduced to one-fifth of those in elderly controls [71]. This difference is even more significant in (APOE) e4/4 type patients [71]. Furthermore, neurodegeneration in AD modulates the expression and physiological actions of both melatonin receptors (MT1/MT2) [72, 73], and interestingly, a reduced number of melatonin receptors in the pineal and cortical brain areas are found in AD [74]. Studies have demonstrated that chronic melatonin treatment slows the functional decline in the aging brain [75-78].
2.2. Aging Women More Prone to AD than Men
AD affects women earlier and more severely than men [79, 80]. Genetic evaluation reveals that female ApoE-4 carriers have twice the risk compared to male carriers for eventually developing AD, and studies have implicated the ApoE-4 gene-estrogen interaction in this marked increase in the risk. Prior to menopause, estrogen enhances neuronal cell division and proliferation [81, 82] and delays the onset and progression of neurological diseases. After menopause, the degree of impaired neurogenesis increases with age and is more pronounced in females, which could possibly be due to the dysregulation of the circulating levels of estrogens in aging women [79, 80, 83]. As there is a natural decline of estrogen levels during menopause, it is hypothesized that the loss of estrogens might predispose to AD pathology by increasing the risk and advancing the onset of the disease in aging women. In this context, animal studies have demonstrated that the cessation of fertility increases the vulnerability of brain cells to oxidative damage and dysfunction and that melatonin supplementation reverses these deleterious effects by preventing inflammation and apoptotic processes in a similar manner to estrogens [84].
2.3. Melatonin and Longevity
Well-regulated circadian rhythms correlate with improved longevity of several organisms. Melatonin synchronizes circadian rhythms [85-87] and may reduce the probability of age-associated neurodegenerative disorders [27] associated to altered biological rhythms [88]. The extensive therapeutic applications of melatonin are based on its anti-aging, senescence-delaying [89], antioxidant functions [90-92] and substantial protective and regenerative properties in the CNS [93]. Experimental studies have demonstrated the life extending property of melatonin [94-96]. When administered to aging animals [97], melatonin increased their life span by almost 20%. Although results in rodent models provide evidence for a melatonin/longevity connection, these studies must be extended to other species to strengthen the presumed association.
Melatonin increases the expression of the “longevity protein” sirtuin 1(SIRT1) [98, 99] which triggers the expression of a host of self-healing genes [98]. SIRT1 attenuates the amyloidogenic processing of βAPP both in cell cultures and in transgenic mouse models of AD [100, 101]. SIRT1 RNA and protein levels are significantly reduced in AD patients [102], which implies that the over expression of SIRT1 may be protective against AD phenotypes. In Tg2576 neuron cultures, SIRT1 promoted α-secretase activity and attenuated Aβ content in the brain [103] via the activation of ADAM10 [104]. Altered DNA methylation [105, 106] and histone modifications [107] are common epigenetic changes observed in aging and AD brains. Melatonin regulates these epigenetic processes in the neurons [108] which is one of the possible mechanisms by which it delays senescence and increases lifespan in healthy and diseased states. Herein, we thoroughly review the therapeutic role of melatonin as it relates to various hypotheses of AD (Fig. 1). This summary includes data from cell culture and animal models to clinical investigations in AD patients. Collectively, the actions of melatonin as a neuroprotective agent are consistent with it having anti-AD effects.
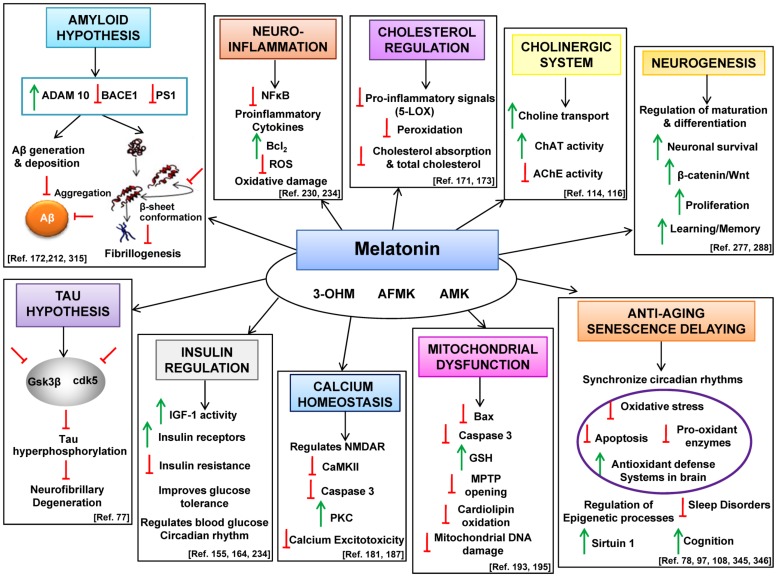
Fig. (1).
Comprehensive summarization of melatonin therapy in different classical hypotheses of AD pathogenesis. This figure summarizes the potential therapeutic targets of melatonin in AD treatment. Melatonin is metabolized into its kynuramine derivatives AFMK (N1-acetyl-N2-formyl-5-methoxykynuramine), AMK (N1-acetyl-5-methoxykynuramine) and 3-OHM (cyclic 3-hydroxymelatonin) which also possess neuroprotective biological and pharmacological properties. Melatonin levels strongly decrease with advancing age and patients with AD exhibit lower melatonin levels than age matched controls. In this context, melatonin via its action on the circadian oscillators modulate and synchronize the rhythms, regulates the epigenetic processes and stimulates the anti-oxidant defense system in the brain thereby improving cognition and sleep disorders. Melatonin also promotes neuronal survival by stimulating neurogenesis. Among the classical hypotheses of AD, melatonin has a protective effect on the cholinergic system by stimulating both choline transport and ChAT activity and down regulating AchE activity. By regulating the important kinases (GSK3β, cdk5) melatonin attenuates hyperphosphorylation of tau thereby precluding tangle formation. Melatonin stimulates the non-amyloidogenic and down regulates the amyloidogenic processing of βAPP thereby precluding the formation of Aβ peptides. Aβ-induced microglial activation is an important factor in AD pathogenesis and in this frame of reference melatonin attenuates proinflammatory cytokines, inhibits NFκB activity and reduces oxidative damage. Melatonin regulates blood glucose circadian rhythm by stimulating IGF-1 activity and modulating insulin resistance. Melatonin regulates Aβ-induced altered calcium and mitochondrial homeostasis thus protecting cells against oxidative stress and cell death and also regulates cholesterol homeostasis further preventing peroxidation of neuronal membrane lipids. Therefore, the diverse biological and physiological properties of melatonin employ it to be a neuroprotective drug in the treatment of AD. (↑ = stimulation, ⊥ = inhibition).
3. COMPREHENSIVE REVIEW OF MELATONIN THERAPY IN DIFFERENT CLASSICAL HYPOTHESES OF AD PATHOGENESIS
Among the classical hypotheses that attempts to explain AD, the “cholinergic hypothesis” [109] was developed because of the role of acetylcholine (ACh) in learning and memory; it is well established that the loss of cholinergic neurotransmission and reduced levels of ACh [110] contribute significantly to deterioration in cognitive functions as observed in AD patients [3] and, in older patients [4] due to an impairment in ACh release [111]. Melatonin and its metabolites [36, 37] are powerful free radical scavengers [112, 113], and melatonin supplementation is beneficial and protective of the cholinergic system. It promotes choline transport [114], prevents the reduction of choline acetyltrans-ferase (ChAT) activity by reducing ChAT nitrosylation and/or oxidation [115] in the frontal cortex and hippocampus [53, 115] and inhibits the increase in acetylcholinesterase (AChE) activity [116].
Neurofibrillary aggregates containing phosphorylated tau are another major hallmark of AD; their number correlates with the severity of dementia in this disease. The toxic pathological modifications [117] of tau contribute significantly to neurodegeneration and play a central role in AD pathogenesis [118]. Melatonin efficiently attenuates tau hyperphosphorylation by affecting the most relevant protein kinases involved in tau modifications in neurofibrillary degeneration; these enzymes include glycogen synthase kinase 3 beta (GSK3β) [119-122] and cyclin-dependent kinase 5 (Cdk5) [123]. These kinases are significantly elevated in aged [124] and AD brains [125]. This has been documented in a number of experimental models [126-131]. Interestingly, a comparative study between AD pathology and rat models with melatonin inhibition, which may activate cdk-5 [132], revealed similarities between the two, supporting the idea that reduced melatonin levels correlate with AD pathology [40].
The CNS is lipid-rich and therefore highly vulnerable to oxidative damage since lipids are easily oxidized. Free radicals are involved in the pathogenesis of neuronal degeneration, and in AD the brain is under increased continuous oxidative stress [133]. The oxidative damage prior to the onset of pathology in AD patients exceeds the levels observed in elderly controls, which could possibly be related to diminished melatonin levels. Melatonin is a chronobiotic-cytoprotective agent [134] that prevents oxidative stress due to its direct and indirect antioxidative actions [11, 135, 136]. Melatonin is highly lipid soluble and enters all subcellular compartments with ease [137], and along with its ability to directly scavenge highly toxic free radicals [11], it downregulates prooxidant enzymes [138] and upregulates antioxidant enzymes by transcriptionally regulating nuclear factor (erythroid-derived 2)-like 2 (Nrf2) [139, 140], thus protecting against increased oxidative damage [141] in the brain. Finally, melatonin chelates transition metals which via the Fenton/Haber-Weiss reactions, generate the highly toxic hydroxyl radical [142].
Melatonin is more potent than vitamins C and E [36] and provides remarkable protection against oxidative stress in the brain, even at physiological concentrations [19, 143, 144]. Melatonin and its metabolites function as broad-spectrum antioxidants [145-147], and under enhanced oxidative stress in aging and AD [148] the role of melatonin in providing brain protection has been extensively reviewed and critiqued [135, 149-151], making this molecule a promising therapeutic candidate with regard to AD pathology [38, 39]; in addition to the protection it affords the brain, in senescent mouse models, chronic melatonin treatment increased lifespan [152].
The age-related melatonin decline induces insulin resistance, glucose intolerance, sleep disturbance, and metabolic circadian disorganization [153], which are commonly observed in AD pathology. Melatonin reduces brain damage by enhancing IGF-1 activity [154, 155], probably due to melatonin-induced sleep improvement [156]. The activation of protein kinase B (Akt) by melatonin [157] reactivates IGF-1, thereby reducing insulin resistance [158, 159] and Aβ accumulation [160-162]. Melatonin influences the circadian blood glucose rhythm [163], improves glucose tolerance and increases insulin receptors in addition to glucose clearance from the blood [164].
Cholesterol modulates and enhances Aβ production [165-167] and negatively regulates α-secretase activity [168] with augmentation of both β- and γ -secretase activities, resulting in increased Aβ production. Exposure to cholesterol-lowering agents [169, 170] reduces the amyloid load in cultured cells and in the brain of mammalian models. Melatonin significantly reduces cholesterol absorption [171], and the most widely known effect of melatonin on lipids is to prevent their peroxidation [143, 172, 173]. The protein levels and enzyme activity of 5-lipoxygenase-activating protein (5-LOX), an active Aβ inducer [174], and 12/15-LOX are increased in AD brains [175]. 5-LOX overexpression in elderly subjects may be related to the age-associated melatonin deficiency [176] since melatonin suppresses 5-LOX gene expression through its high-affinity nuclear receptors [177], even in the nanomolar range [178, 179]. This reduces the proinflammatory response [180], which contributes to oxidative stress.
The Ca2+ overload in neurons results in excitotoxicity, thereby inducing cell death and neurodegeneration. The activation of the amyloidogenic pathway leads to a remodeling of the neuronal Ca2+ signaling pathway [181, 182], which alters the normal Ca2+-dependent mechanisms responsible for learning and memory. Ca2+/calpain activity declines during aging, which makes βAPP more vulnerable to attack by β- and γ-secretases, resulting in Aβ formation. Over activation of N-methyl-D-aspartate receptor (NMDAR) also stimulates the amyloidogenic processing of βAPP. Aβ selectively interacts with the NMDA receptor [183], leading to dysregulation of Ca2+. Melatonin controls the NMDA receptor [184], possibly by redox modulation [185], and regulates intracellular free Ca2+ by directly binding to calmodulin [186, 187], thereby decreasing the activity and autophosphorylation of Ca2+/calmodulin-dependent protein kinase II (CAMK II) and activating protein kinase C (PKC), even in the nanomolar range [188]. This protects cells from calcium-dependent caspases. Calreticulin, another calcium regulator, functions as a molecular chaperone for the Aβ precursor protein [189], and its relationship with melatonin [190] may be relevant in AD.
Mitochondrial dysfunction has an early and predominant role in AD [191, 192]. Melatonin provides mitochondrial protection by preventing cardiolipin oxidation, avoiding 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) opening and restoring Ca2+ balance [193-195]. Caspase-3 levels are higher in AD brains than in age-matched controls [196], and the activation of caspase-3 accounts for increased β-secretase activity, thereby augmenting Aβ production [197]. In contrast, melatonin downregulates caspase-3 [198], which is linked directly to cell death in AD [199] and enhances anti-apoptotic bcl-2 expression in AD transgenic mice and in ischemic brains [200].
Glucocorticoids and corticotropin-releasing hormones induce AD-associated pathologies [201], possibly related to hyperactivity of the hypothalamic-pituitary-adrenal (HPA) axis. Melatonin modulates the activity of this axis [202], and its nocturnal elevation influences the cortisol rhythm, which indicates the importance of the melatonin/corticoid relationship [203]. Furthermore, a significant rise in serum cortisol levels has been demonstrated in elderly individuals and is more pronounced in demented subjects compared to young controls during the night [204].
3.1. Melatonin in Aβ Toxicity
Aβ peptides are produced via the amyloidogenic processing of βAPP, and the imbalance between the production and clearance of Aβ leads to its accumulation; this triggers a cascade of pathological mechanisms in the brain, disturbing the overall cellular homeostasis. Relative to this, several anti-amyloid processing effects of melatonin have been demonstrated in in vitro and in vivo studies. Aβ by itself is capable of generating free radicals, resulting in protein, DNA and lipid oxidation along with diminished energy metabolism. Melatonin prevents Aβ25–35-induced circadian alterations [205] and Aβ-induced oxidative stress [150], lipid peroxidation [206], cellular death and DNA damage in neuroblastoma cell cultures [172, 207] and human platelets [47]. Melatonin also protects the APP + presenilin 1 (PS1) double-transgenic mouse model of AD from cognitive impairment [208]. Melatonin reduces Aβ generation and deposition both in vivo [209] and in vitro [210-212] and prevents Aβ aggregation via direct interaction [213, 214]. Melatonin also exhibits antifibrillogenic effects [52, 215], alleviates behavioral deficits associated with apoptosis and cholinergic system dysfunction [216, 217], inhibits amyloid pathology [218] and increases survival in transgenic mouse models [209]. ApoE4 aggravates the effects of Aβ. Melatonin reversed the profibrillogenic activity of ApoE4 and neutralized its neurotoxic combination with Aβ [52]. Aβ activates caspase 3 and induces apoptosis [219], while melatonin alleviates the Aβ-induced rise in caspase-3 activity [220, 221].
N1-acetyl-N2-formyl 5-methoxykynuramine (AFMK) [145], a melatonin metabolite, prevented Aβ-induced toxicity [222] though such effects were not observed in old transgenic mouse models. Melatonin reduced Aβ-induced AChE activity and prevented acetylcholine degradation [214]. Accumulated Aβ activated microglia [223-225], induced an inflammatory response via stimulation of the NFκB-dependent pathway leading to cytokine release [226]. NFκB stimulation was followed by neuritic injury, hyperphosphorylation of the tau protein, and the formation of neurofibrillary tangles, leading to cognitive impairment [227] and ultimately to neuronal dysfunction and cell death.
Cytokines transcriptionally upregulate beta-site amyloid precursor protein cleaving enzyme 1 (BACE1) mRNA, protein and enzymatic activity [228]. Via this means, a vicious cycle forms between Aβ-induced cytokines, which further increase Aβ production by upregulating BACE1. Melatonin possesses anti-inflammatory properties [229, 230]. By inhibiting NFκB [231, 232], melatonin suppresses the production of proinflammatory cytokines [30, 233-235] and also attenuates the Aβ-induced secretion of IL-1β and IL-6 [236] in transgenic mouse models. Melatonin prevents Aβ-induced depletion of bcl-2 [221] and enhances bcl-2 expression in AD transgenic mice and in ischemic brains [200]. Based on studies by [237]), melatonin may impair the Aβ-induced assembly of phagocytic oxidase (PHOX) by inhibiting the translocation of the p47phox and p67phox subunits of PHOX from the cytosol to the plasma membrane, thus eventually preventing the subsequent production of ROS.
Melatonin restores insulin/insulin receptor mechanisms and increases PI3-K/Akt signaling activity, resulting in the inhibition of GSK-3β and less Aβ accumulation and reduced tau hyperphosphorylation [238]. Acting through its MT2 receptor, melatonin stimulates phospholipase C (PLC) and activates PKC via diacylglycerol (DAG) [239], which in turn phosphorylates and inactivates GSK-3β. Thus, by activating PKC, melatonin might impair Aβ overproduction. It has been speculated that melatonin, which prevents JNK activation under oxidative stress conditions [240], may also employ this mechanism to prevent the activation of GSK-3β. Studies have shown that melatonin potentiates the neuroprotective properties of resveratrol against beta-amyloid-induced neuro-degeneration by modulating 5'AMP-activated protein kinase (AMPK) pathway [241]. Melatonin controls apoptosis and stress-mediated events by regulating Bcl2/Bax, glutathione and its enzymes [242] and increases the activation of the prosurvival PI3K/Akt pathway, inhibiting GSK-3β and resulting in an increase in Forkhead box protein O1 (FOXO-1) phosphorylation [243]. Overall, melatonin protects the brain from an Aβ-induced cascade of neurodegenerative processes, thus likely playing a critical role in preventing disease progression.
4. MELATONIN: AN ENDOGENOUS REGULATOR OF NEUROGENESIS IN AGING AND AD
Adult neurogenesis contributes positively to learning and memory [244]. The potential of hippocampal precursor cells to proliferate and differentiate marks cellular plasticity and cognitive health of any organism, and low proliferation and differentiation capacities of adult hippocampal stem cells correlate with memory dysfunction [245]. Physiological aging alters neurogenesis, and the accumulation of Aβ suppresses the proliferation of neural stem and progenitor cells and neuronal differentiation in cell culture and animal models [246-248]. Dysfunctional neurogenesis exacerbates neuronal vulnerability to AD and contributes to memory impairment [249]. Studies in transgenic animal models have reported reduced hippocampal neurogenesis [249] in the sub-ventricular zone (SVZ) and sub-granular zone (SGZ) [247, 250, 251] associated with Aβ peptides [252, 253] and increased amyloid precursor protein intracellular domain (AICD) expression, which consequently reduces hippocampal progenitor cell proliferation and survival [254]. Oligomeric Aβ inhibits proliferation [255] and decreases neurogenesis via the downregulation of β-catenin [256] and apoptosis, thereby leading to Wnt/β-catenin signaling impairment [257]; this further activates GSK3β, resulting in stimulation of Aβ production and tau phosphorylation [258]. Evidence from other clinical studies showed decreased expression of β-catenin [259, 260] and reduced Wnt/β-catenin signaling in AD brains [237]. Wnt/β-catenin prevents Aβ-induced toxicity and apoptosis and inhibits GSK-3β activity and tau phosphorylation [261]. Therefore, sustaining or enhancing the production of new neurons is now regarded as a potential therapeutic strategy to prevent AD-associated cognitive decline.
Melatonin is a positive endogenous regulator of neurogenesis during aging [262], and experimental studies have shown that it prevents the abatement in proliferation and neurogenesis that occurs in aging and AD. The melatonin receptors MT1 [263] and MT2 [264] are expressed in neural stem cells and in the adult brain [265]. Interestingly, the MT1 and MT2 agonists agomelatine [266, 267], in the adult rat hippocampus, and buspirone potentiate neurogenesis in combination with melatonin in major depressive disorder (MDD) [268]. Melatonin enhances dendritogenesis [269], dendritic maturation of doublecortin (DCX)-positive cells [270] and, in combination with exercise, enhances neurogenesis and neuronal survival [271, 272]. Sleep deprivation, which is directly linked to reduced melatonin levels in AD, leads to depressed hippocampal neurogenesis and increased Aβ generation; this contributes to memory dysfunction. Interestingly, animal studies demonstrated that melatonin modulates the proliferation and differentiation of neural stem cells [273] and promotes the survival of new neurons [274]. Its administration also prevented the loss of pyramidal neurons in vivo [275] and induced neurogenesis in the dentate gyrus of adult pinealectomized rats [276].
A variety of clinical investigations and animal studies have revealed the possible mechanisms by which melatonin regulates neurogenesis. It increases adult hippocampal [277] and subventricular zone [278] progenitor cell proliferation and neurogenesis via melatonin receptor-mediated ERK signaling and by enhancing BDNF-like growth factors. Melatonin also prevents methamphetamine-induced [279] and dexamethasone-mediated alterations in hippocampal neurogenesis in the mouse brain [280]. A comparative clinical investigation in AD patients and healthy controls revealed that Aβ-induced interruption of β-catenin signaling may contribute to the impairment of neurogenesis of hippocampal progenitor cells in AD [257]; similar results were observed in AD transgenic mouse models. In this context, melatonin has been shown to increase β-catenin protein expression and activation in human neuroblastoma cell cultures [281], and via its receptors, melatonin stimulates the PI3K/Akt [264] and PLC/DAG pathways and activates PKC [239]; this in turn phosphorylates and inactivates GSK-3β.
Cholinesterase inhibitors promote the survival of newly generated neurons and increases neurogenesis in adult mice [282], while melatonin decreases AChE activity and exhibits an anti-amnesic effect [283]. Our recent findings demonstrate that melatonin stimulates the α-secretases [284] at the transcriptional level and increases sAPPα production, which is known to promote neural progenitor cell (NPC) proliferation [285] and advance the survival of neurons [286]. Oxidative stress impairs adult neurogenesis during aging [287]. In contrast, melatonin supplementation prevents oxidative stress-induced impairment and induces neuronal stem cell proliferation and differentiation [288] by stimulating the α-secretases. While collectively the findings support melatonin as an anti-AD agent, additional mechanistic studies would help to validate this association.
5. ROLE OF MELATONIN IN REGULATING THE NON-AMYLOIDOGENIC AND AMYLOIDOGENIC PROCESSING OF ΒAPP: PREVENTION OF Aβ PRODUCTION
The non-amyloidogenic α-secretase cleavage of βAPP is mainly performed by two enzymes (ADAM10 and ADAM17) responsible for the constitutive and PKC-regulated pathways [289-291] that thereby preclude the formation of Aβ. In contrast, the amyloidogenic cleavage of βAPP by BACE1 consequently results in the formation of Aβ peptides [292]. Many examinations of the AD brain have shown a reduction in α-secretase or an increase in β-secretase, or both [293]. Clinical investigations in AD patients have reported reduced α-secretase activity and lower levels of ADAM10 [294, 295] and sAPPα levels in the CSF [296, 297] and platelets [295]. This perturbed balance favors β-secretase cleavage, releasing proapoptotic sAPPβ [298] along with Aβ overproduction and the associated decline in cognitive functions. Interestingly, elevated BACE1 expression is associated with aging and oxidative stress [299]. The enhanced BACE1 protein and activity has also been documented in the brains of AD patients [300-303]. In this context, α-secretase-promoting therapeutic agents are neuroprotective by enhancing sAPPα formation [304-306] Enhanced α-secretase activity [307] and the overexpression of ADAM10 (Postina et al., 2004, Schroeder et al., 2009) andADAM9/ADAM17 (Kuhn et al., 2010, Weskamp et al., 2002) increased the release of sAPPα and limit sAPPβ, thereby reducing the formation of Aβ40 and Aβ42; this further reduces Aβ peptide levels [307].
A reduction in BACE1 prevents cholinergic dysfunction, neuronal loss and memory deficits, along with a reduction in Aβ40/42 levels [308, 309]. Studies in AD mouse models have shown that knockout of BACE1 reduced Aβ production [310, 311], amyloid plaque load and AD-related symptoms [308, 312]. As discussed previously, many earlier studies focused on the ability of melatonin to protect against Aβ deposition, aggregation, fibrillogenesis, pro-inflammatory effects, and oxidative and apoptotic neurotoxicity. Cell culture studies show that melatonin inhibits sAPPβ secretion [210, 313] and reduces the levels of Aβ peptides in the murine cerebral cortex [314].
We recently investigated whether melatonin directly controls the secretases responsible for βAPP processing. We documented that near physiological concentrations of melatonin positively control the α-secretases ADAM10 and ADAM17 at the transcriptional level and downregulate the BACE1 protein and mRNA levels and activity [284, 315] (Fig. 2). We also investigated the underlying signaling mechanisms by which melatonin regulates βAPP proteolytic cleavage. Activation of ERK/MAPK signaling in the CNS is critical for synaptic plasticity and memory, and PKC-dependent α-secretase competes with β-secretase for cleavage of β-APP and thus prevents its amyloidogenic processing. Interestingly, melatonin regulates PKC [316, 317], and PKC also mediates the circadian entrainment effects of melatonin in the master biological clock [318]. Via stimulation of its plasma receptors, melatonin induces ERK phosphorylation through three distinct signaling pathways (Gq/PLC/PKC, Gi/PI3K/PDK1/PKC or Gs/cAMP/PKA), which leads to the activation of transcription factors, including CREB, Oct-1, and HIF-1. This activation further stimulates ADAM10 and ADAM17 promoter transactivation and mRNA levels [284]. Moreover, the melatonin-dependent activation of α-secretase would likely suppress Aβ production, as previously discussed, and would also impair AICD production by favoring the α/γ over the β/γ pathway [319-321] and reducing AICD-dependent GSK3β expression [322]. This may explain the previously-described inhibitory effect of melatonin on this tau-phosphorylating kinase [323]. The phosphorylation of GSK-3β by PKC leads to γ-secretase inactivation. GSK-3 may be one of the common signaling pathways that increases Aβ generation and tau hyperphosphorylation; thus melatonin could regulate βAPP processing through the PKC and GSK-3 pathways. BACE1 and PS-1 are key enzymes in neuronal Aβ formation and in their absence, Aβ synthesis is either abolished or considerably reduced [324]. We also demonstrated that in the amyloidogenic pathway, melatonin attenuates BACE1 and PS1 protein expression and mRNA levels via melatonin receptors (MT1/2) [315], possibly by inhibiting NF-κB activation.
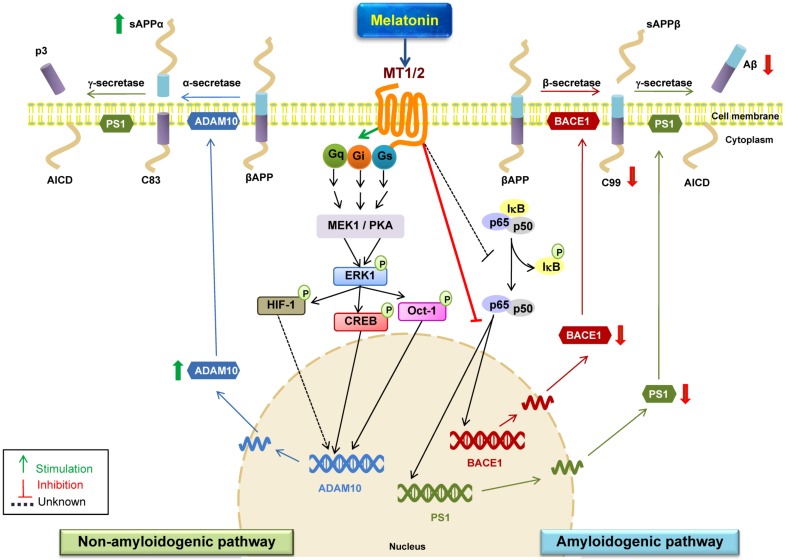
Fig. (2).
Schematic representations of melatonin-dependent regulation of βAPP processing secretases and its possible underlying mechanisms. The proteolytic processing of βAPP by the non-amyloidogenic pathway precludes Aβ formation. Cleavage by α-secretase (ADAM 10) releases sAPPα and C83 which further undergoes cleavage by γ-secretase to generate P3 and AICD fragments; thereby preventing the generation and release of Aβ peptides. In amyloidogenic pathway, βAPP is cleaved by β-secretase (BACE1) releasing sAPPβ and C99 which is subsequently cleaved by γ-secretase to produce Aβ 1-40 and Aβ 1-42 peptides. Stimulation of the melatonin receptors located at the plasma membrane induce ERK phosphorylation probably via three distinct signaling pathways (Gq/PLC/PKC, Gi/PI3K/PDK1/PKC or Gs/cAMP/PKA) thus triggering the activation of transcription factors CREB, Oct-1, and HIF-1 which further increase ADAM10 promoter transactivation and mRNA levels consequently granting more α-secretase activity and sAPPα production fomenting neuroprotection. In the amyloidogenic pathway, melatonin in a receptor-dependent manner attenuates BACE1 and PS1 protein expression and transcriptional levels and decreases levels of C99 which is further cleaved by γ-secretase to generate Aβ peptide. BACE1 promoter region has multiple transcription factor binding sites like for NF-κB. Melatonin inhibits NF-κB activation which could possibly regulate melatonin induced decrease in BACE1 and C99 expression. Thus melatonin-dependent transcriptional activation of α-secretases and down regulation of BACE1 and PSI levels strongly indicates the beneficial role of melatonin in AD pathology thereby precluding Aβ production. Aβ, beta-amyloid; ADAM10, α-secretase; BACE1, β-secretase; Mel, melatonin; MT1/2, melatonin receptor; NF-κB, nuclear factor- kappa light-chain enhancer of activated B cells; PS1, presenilin1; PKA, Protein kinase A; MEK1, mitogen-activated protein kinase kinase 1; AICD, The amyloid precursor protein intracellular domain.
Aging is the major risk factor for AD, and the phenomenon of Aβ accumulation and aggregation is similar to what is observed in the AD brain, with significant increases in the Aβ42:Aβ40 ratio [325]. Studies in aged animal models have confirmed elevated BACE1 [326] and γ-secretase [327] activities, which ultimately lead to increased Aβ accumulation. We found that in the hippocampus of aged mice melatonin upregulated the aged-induced loss in ADAM10 and downregulated the rise in BACE1 and PS1 protein levels through transcriptional regulation [328]. NF-κB upregulates BACE1 expression [329], whereas melatonin downregulates NF-κB activation in SH-SY5Y cells by inhibiting the translocation of the NF-κB subunit (p65-p50) into the nucleus [139]. This suggests that the melatonin-induced reduction in BACE1 levels may occur through the inactivation of the NF-κB pathway. Moreover, melatonin upregulates ADAM10 through the SIRT1 pathway activation [243]; this is an alternate mechanism by which melatonin may upregulate ADAM10 expression, as observed in aged mouse models [328]. Deregulation of the proteolytic processing of βAPP by secretases is an early event in AD pathology, with a melatonin deficiency having serious effects [330]. This suggests that melatonin supplementation should be started at a very early stage of AD to provide maximum brain protection and prevention. Studies in transgenic animals also support the view that melatonin should be given at an early phase to regulate Aβ metabolism, mainly by preventing formation of Aβ [331]. Moreover, enhancing α-secretase activity by increasing the expression and activity of ADAM10 and 17 and/or downregulating BACE1/PS1 is a promising means of the treatment of AD.
6. MELATONIN REGULATION OF EPIGENETIC MECHANISMS-IMPLICATIONS IN AD
The main epigenetic modifications that contribute to disease pathogenesis are bizarre patterns of DNA methylation, histone modifications and RNA-based mechanisms controlled by non-coding RNAs and microRNAs (miRNAs) which are heritable but reversible changes in gene function without causing any alteration in the DNA sequence. These mechanisms are involved in learned behavior, cognition, formation and maintenance of memory and neuro-degenerative pathology [332-334]. Epigenetic alterations like differentially DNA methylated genes and histone acetylated genes in AD have been extensively reviewed [335, 336]; these aberrant epigenetic changes trigger alterations at the transcriptional level of genes like βAPP [337], BACE1 and PS1[338] which are involved in the pathogenesis leading to dementia/AD. Neuropathology-associated with the hyper-methylation of the gene ankyrin 1 (ANK1) implicates cortical deregulation which is a primary site of AD pathogenesis [339] suggesting that epigenetic changes might contribute to AD beyond expectation [340]. Therapeutics for epigenetic alterations in AD is by itself challenging and therefore early diagnosis would aid in successful treatments. PET imaging allows noninvasive visualizations of differential HDAC activity, neuronal hypomethylation, and imaging Aβ plaque deposits and tangles [341] which are the epigenetically influenced events and would thus provide beneficial insights for preventing, early diagnosis, and treating AD. Likewise, epigenetic therapeutic approaches for AD include HDAC inhibitors, Sirtuins, HATs, DNA methylation modulation and miRNAs [342].
Interestingly, melatonin possesses all of the above required properties in being a comprehensive future epigenetic therapy for AD [108, 343]. Concerning the mechanisms of epigenetic regulation by melatonin in AD, there are many studies demonstrating various targets and signaling pathways involved, which would provide a better understanding in this particular area of research. Epigenetic mechanisms might work through the circadian clock to regulate neuronal function and influence disease states [344] which suggests a potential role of melatonin in epigenetic regulation [345, 346]. Extended sleep disturbances causes histone modifications along with significant alterations in the levels of DNA methylation [347]. Sleep and circadian disorders in AD appear early in the course of the disease [348] which might be due to decreased melatonin levels which supposedly parallels AD progression. As such melatonin has been known to regulate sleep/circadian rhythm disorders. It also regulates antioxidant and pro-inflammatory genes via epigenetic on/off mechanisms [349], reverses tumor growth through the epigenetic mechanism of global DNA methylation [350] and ameliorates prenatal dexamethasone induced epigenetic alterations in the rat hippocampus [351]. HDAC inhibitors reverse the abnormal histone acetylation and improve cognitive function and memory in transgenic mouse models of AD [352, 353]. In this context, melatonin has been shown to inhibit HDACs [354] which shows it capability of reversing the observed epigenetic changes.
MicroRNAs (miRNAs) are post-transcriptional regulators involved in numerous biological processes involved in AD pathogenesis. It has been speculated that melatonin mediates the regulation of gene expression at the post-transcriptional level through its effect on miRNA expression. The profiling of the pineal miRNAs (miR-182, miR-96, miR-183, miR-483) notified that melatonin synthesis also somehow lies under the epigenetic control [355]. Regarding AD, several miRNAs have been identified which directly regulate βAPP expression and processing along with its secretases thus regulating Aβ load. These miRNAs are also involved in regulation of BACE1 cleavage and expression which has been comprehensively reviewed [356]. One of them is miR-124 which not only controls BACE1 gene expression [357] but is also the most abundant miRNA in the brain and affects a broad spectrum of biological functions in the CNS [358]. Interestingly, melatonin by regulating miR-124 attenuates memory and synaptic disorder thereby aiding drug discovery in AD [359]. Surprisingly of interest is the fact that melatonin receptor 1 has been identified as a direct target of miR-29b [360] suggesting possible regulatory mechanisms of melatonin via miR-29b. The miRNAs (miR-103, miR-107 and miR-1306) possibly regulate ADAM10 expression [361] and as melatonin stimulates ADAM10 activity which simultaneously prevents amyloidogenic processing of βAPP, it might have an epigenetic control over this processing via the above mentioned miRNAs.
Measurements of miRNAs in the CSF has emerged as a novel diagnostic tool for various neurological conditions. Interestingly melatonin has been recently reported to be involved in controlling miR-497 expression [362]. Down regulation of miR-125b, miR-23a, miR-26b has been shown in serum from AD patients [363] out of which miR-23a has been shown to regulate ER stress and melatonin has the ability to regulate expression of miR-23a [364]. miR-34a is a potential therapeutic target for AD as its alteration is associated with dysfunction of synaptic plasticity, energy metabolism, and resting state network activity [365]. It is over expressed in specific brain regions of AD patients which correlate with the severity of the disease. Melatonin modulates miR-34a/SIRT1 pathway [366] which adds on to another regulatory function of this neurohormone in mi-RNAs involved in AD. Expression of long interspersed element-1 (LINE-1) can introduce genomic instability and its methylation is increased in AD patients [367] and melatonin regulates LINE-1 in a receptor dependent mechanism [368]. A very recent investigation [369] provided the first evidence of PIWI-interacting RNAs (piRNAs) in the human brain and its dysregulation in AD. Interestingly, piRNA effects the expression of melatonin receptor type1A [370] and as DNA methylation pattern and chromatin remodeling via histone deacetylation may be associated with changes in melatonin receptor expression therefore it becomes reasonable to suggest that melatonin might also control epigenetic mechanisms in AD via interacting with piRNAs.
Extracellular miRNAs (miR-9, miR-34c, miR-101, miR-132) in CSF and serum from AD patients correlates with the status, progression and characteristic features (neurofibrillary tangles and amyloid plaques) of AD pathology [371]. Overexpression of α-synuclein has been reported in the CSF of AD patients [372] and this increase contributes to cognitive dysfunctions and memory impairments similar to that of AD mice model [373]. Significant down-regulation in miR-132 expression has been associated with α-synuclein accumulation and neuronal malfunction in α-synuclein (A30P)-transgenic mice [374, 375]. Melatonin prevents up regulation of α-synuclein in amphetamine-treated rats [376] which might aid in regulating miR-132. miR-9 also targets SIRT1 which is reduced in AD [102, 377]. Decreased SIRT1 levels would indicate a potential increase in miR-9, or the increase of another miRNA targeting SIRT1. Interestingly, elevated miR-34c also correlates with decrease in SIRT1. As melatonin naturally enhances SIRT1, it might affect the expression patterns of both miR-9 and miR-34c.
Cyclooxydenase-2 (COX-2) and βAPP are known miR-101 targets implicated in AD [378]. It is possible that miR-101 down-regulation might contribute significantly to AD pathology by enhancing βAPP expression, tau phosphorylation and contributing to inflammation through the up regulation of COX-2 expression. Melatonin suppresses COX2 by inhibiting p52 acetylation and binding [234] and by inhibiting COX-2/iNOS and NF-κB/p300 signaling pathways [379] which implicate that melatonin might regulate these mechanisms via modulating miR-101. The proliferation, fate specification and differentiation of adult neural stem cells are regulated by epigenetic mechanisms which are relevant to AD etiology and pathogenesis [380]. In this context, melatonin has been known to promote neurogenesis [278], so it could be envisioned how melatonin by regulating epigenetic mechanisms could modulate both degenerative and regenerative processes in AD pathogenesis thus meeting the preventive and curative parameters of anti-AD regime.
7. MELATONIN CLINICAL INTERVENTIONS IN AD-FUTURE PROSPECTS IN ANTI-AD DRUG DEVELOPMENT
A number of clinical trials with melatonin supplementa-tion have been successful in terms of the prognosis of AD. Diminished levels of melatonin correlate with AD pathology; therefore, the replacement of physiological levels of melatonin in the brain could impede the progression of this disease [150, 381]. Mild cognitive impairment (MCI) is an etiologically heterogeneous syndrome that precedes dementia. It has been shown that melatonin significantly retarded the progression of MCI patients to AD over time, and melatonin is beneficial in AD and MCI patients [47, 49, 381-384] by significantly improving cognitive and emotional performance [385]. Clinical investigations of the efficacy of melatonin in treating patients with MCI and AD have been extensively reviewed and discussed by [70, 331, 383]. As early diagnosis and preventive therapeutic implementation guard against the development of neurodegenerative diseases such as AD, studies have shown that the time of melatonin supplementation may be crucial in AD pathogenesis [217]. Investigations in patients showed the efficacy of prolonged melatonin release in elderly and insomnia patients, which would consequently decrease Aβ levels [386, 387]. Human clinical interventions for AD usually begin only after the symptoms appear, but the actual degeneration is initiated much earlier before these symptoms appear; therefore, along with a preventive therapeutic strategy, the early detection of AD is equally important for the successful outcome of this devastating disorder. In this context, our previous studies suggest that melatonin is a potential therapeutic agent for preventing and thereby reducing the risk of AD. Melatonin should be advanced as an anti-AD drug for humans. Importantly, the inter-individual pharmacokinetics in relation to the concentration of melatonin [388] in the plasma and the proposed treatment doses should also be investigated, and new dosage paradigms should be explored for use in clinical trials.
CONCLUDING REMARKS
In summary, this review emphasizes on the diverse roles of the pineal hormone melatonin as an anti-AD regime. We have done a comprehensive evaluation of various experimental and clinical investigations regarding the therapeutic effects of melatonin in AD. Along with its neuroprotective roles in the three classical hypotheses (cholinergic deficits, tau integrity and β-amyloid aggregation and deposition) we also discuss the underlying molecular mechanisms of melatonin action in the multispectral degenerative cascade triggered during the progressing stages of AD. Inhibiting β-secretase and/or enhancing α-secretase processing of βAPP is a preventive strategy in combating this disorder and our recent studies demonstrated that melatonin qualifies to be a defensive and preventive anti-AD drug. The rationale of the therapeutic approach in AD must be focused on its early detection as the stage of the disease and its irreversible nature is critical in terms of the relevant clinical outcomes of any medications prescribed. Although there are ethical issues when it comes to the toxicity parameters and precautionary methods during clinical trials with different therapeutic strategies in the diseased and non-AD control groups, it should be noted that the toxicity of melatonin is almost negligible. Therefore, melatonin could be considered as a broad-spectrum strategy in AD, although a substantial amount of clinical interventional trials with melatonin should be implemented to validate its overall efficacy and be clinically accepted as an anti-AD drug.
CONSENT FOR PUBLICATION
Not applicable.
ACKNOWLEDGEMENTS
This study was supported in part by the Thailand Research Fund (TRF) (DPG5780001) and a Mahidol University Research Grant to PG.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Glenner G.G., Wong C.W. Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun. 1984;120(3):885–890. doi: 10.1016/s0006-291x(84)80190-4. [http://dx.doi.org/10.1016/S0006-291X(84)80190-4]. [PMID: 6375662]. [DOI] [PubMed] [Google Scholar]
- 2.Goedert M. Tau protein and the neurofibrillary pathology of Alzheimer’s disease. Trends Neurosci. 1993;16(11):460–465. doi: 10.1016/0166-2236(93)90078-z. [http:// dx.doi.org/10.1016/0166-2236(93)90078-Z]. [PMID: 7507619]. [DOI] [PubMed] [Google Scholar]
- 3.Bartus R.T., Dean R.L., III, Beer B., Lippa A.S. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217(4558):408–414. doi: 10.1126/science.7046051. [http://dx.doi.org/10.1126/science.7046051]. [PMID: 7046051]. [DOI] [PubMed] [Google Scholar]
- 4.Bartus R.T. On neurodegenerative diseases, models, and treatment strategies: lessons learned and lessons forgotten a generation following the cholinergic hypothesis. Exp. Neurol. 2000;163(2):495–529. doi: 10.1006/exnr.2000.7397. [http://dx.doi.org/10.1006/exnr.2000.7397]. [PMID: 10833325]. [DOI] [PubMed] [Google Scholar]
- 5.Hardeland R. Melatonin: signaling mechanisms of a pleiotropic agent. Biofactors. 2009;35(2):183–192. doi: 10.1002/biof.23. [http://dx.doi.org/10.1002/ biof.23]. [PMID: 19449447]. [DOI] [PubMed] [Google Scholar]
- 6.Stehle J.H., von Gall C., Korf H.W. Melatonin: a clock-output, a clock-input. J. Neuroendocrinol. 2003;15(4):383–389. doi: 10.1046/j.1365-2826.2003.01001.x. [http:// dx.doi.org/10.1046/j.1365-2826.2003.01001.x]. [PMID: 12622838]. [DOI] [PubMed] [Google Scholar]
- 7.Vriend J., Reiter R.J. Melatonin feedback on clock genes: a theory involving the proteasome. J. Pineal Res. 2015;58(1):1–11. doi: 10.1111/jpi.12189. [http://dx.doi.org/10.1111/jpi.12189]. [PMID: 25369242]. [DOI] [PubMed] [Google Scholar]
- 8.Revel F.G., Masson-Pévet M., Pévet P., Mikkelsen J.D., Simonneaux V. Melatonin controls seasonal breeding by a network of hypothalamic targets. Neuroendocrinology. 2009;90(1):1–14. doi: 10.1159/000219588. [http://dx.doi.org/10.1159/000219588]. [PMID: 19451698]. [DOI] [PubMed] [Google Scholar]
- 9.Reiter R.J., Tan D.X., Sanchez-Barcelo E., Mediavilla M.D., Gitto E., Korkmaz A. Circadian mechanisms in the regulation of melatonin synthesis: disruption with light at night and the pathophysiological consequences. J. Exp. Integr. Med. 2011;1(1):13–22. [http://dx.doi.org/10.5455/jeim.101210.ir.001]. [Google Scholar]
- 10.Manchester L.C., Coto-Montes A., Boga J.A., Andersen L.P., Zhou Z., Galano A., Vriend J., Tan D.X., Reiter R.J. Melatonin: an ancient molecule that makes oxygen metabolically tolerable. J. Pineal Res. 2015;59(4):403–419. doi: 10.1111/jpi.12267. [http://dx.doi.org/10.1111/ jpi.12267]. [PMID: 26272235]. [DOI] [PubMed] [Google Scholar]
- 11.Reiter R.J., Mayo J.C., Tan D.X., Sainz R.M., Alatorre-Jimenez M., Qin L. Melatonin as an antioxidant: under promises but over delivers. J. Pineal Res. 2016;61(3):253–278. doi: 10.1111/jpi.12360. [http://dx.doi.org/ 10.1111/jpi.12360]. [PMID: 27500468]. [DOI] [PubMed] [Google Scholar]
- 12.Menendez-Pelaez A., Poeggeler B., Reiter R.J., Barlow-Walden L., Pablos M.I., Tan D.X. Nuclear localization of melatonin in different mammalian tissues: immunocytochemical and radio-immunoassay evidence. J. Cell. Biochem. 1993;53(4):373–382. doi: 10.1002/jcb.240530415. [http://dx.doi.org/10.1002/jcb.240530415]. [PMID: 8300754]. [DOI] [PubMed] [Google Scholar]
- 13.Menendez-Pelaez A., Reiter R.J. Distribution of melatonin in mammalian tissues: the relative importance of nuclear versus cytosolic localization. J. Pineal Res. 1993;15(2):59–69. doi: 10.1111/j.1600-079x.1993.tb00511.x. [http://dx.doi.org/ 10.1111/j.1600-079X.1993.tb00511.x]. [PMID: 8283386]. [DOI] [PubMed] [Google Scholar]
- 14.Venegas C., García J.A., Escames G., Ortiz F., López A., Doerrier C., García-Corzo L., López L.C., Reiter R.J., Acuña-Castroviejo D. Extrapineal melatonin: analysis of its subcellular distribution and daily fluctuations. J. Pineal Res. 2012;52(2):217–227. doi: 10.1111/j.1600-079X.2011.00931.x. [http://dx.doi.org/10.1111/j.1600-079X.2011.00931.x]. [PMID: 21884551]. [DOI] [PubMed] [Google Scholar]
- 15.Payne J.K. The trajectory of biomarkers in symptom management for older adults with cancer. Semin. Oncol. Nurs. 2006;22(1):31–35. doi: 10.1016/j.soncn.2005.10.005. [http://dx.doi.org/10.1016/j.soncn.2005.10.005]. [PMID: 16458180]. [DOI] [PubMed] [Google Scholar]
- 16.Karasek M., Reiter R.J. Melatonin and aging. Neuroendocrinol. Lett. 2002;23(Suppl. 1):14–16. [PMID: 12019345]. [PubMed] [Google Scholar]
- 17.Sharma M., Palacios-Bois J., Schwartz G., Iskandar H., Thakur M., Quirion R., Nair N.P. Circadian rhythms of melatonin and cortisol in aging. Biol. Psychiatry. 1989;25(3):305–319. doi: 10.1016/0006-3223(89)90178-9. [http://dx. doi.org/10.1016/0006-3223(89)90178-9]. [PMID: 2914154]. [DOI] [PubMed] [Google Scholar]
- 18.Mahlberg R., Tilmann A., Salewski L., Kunz D. Normative data on the daily profile of urinary 6-sulfatoxymelatonin in healthy subjects between the ages of 20 and 84. Psychoneuroendocrinology. 2006;31(5):634–641. doi: 10.1016/j.psyneuen.2006.01.009. [http://dx.doi.org/10.1016/j.psyneuen.2006.01.009]. [PMID: 16584848]. [DOI] [PubMed] [Google Scholar]
- 19.Reiter R.J., Tan D.X., Galano A. Melatonin: exceeding expectations. Physiology (Bethesda) 2014;29(5):325–333. doi: 10.1152/physiol.00011.2014. [http://dx.doi.org/10.1152/physiol.00011.2014]. [PMID: 25180262]. [DOI] [PubMed] [Google Scholar]
- 20.Hardeland R. Melatonin in aging and disease -multiple consequences of reduced secretion, options and limits of treatment. Aging Dis. 2012;3(2):194–225. [PMID: 22724080]. [PMC free article] [PubMed] [Google Scholar]
- 21.Reiter R.J., Tan D.X., Sainz R.M., Mayo J.C., Lopez-Burillo S. Melatonin: reducing the toxicity and increasing the efficacy of drugs. J. Pharm. Pharmacol. 2002;54(10):1299–1321. doi: 10.1211/002235702760345374. [http://dx. doi.org/10.1211/002235702760345374]. [PMID: 12396291]. [DOI] [PubMed] [Google Scholar]
- 22.Barlow-Walden L.R., Reiter R.J., Abe M., Pablos M., Menendez-Pelaez A., Chen L.D., Poeggeler B. Melatonin stimulates brain glutathione peroxidase activity. Neurochem. Int. 1995;26(5):497–502. doi: 10.1016/0197-0186(94)00154-m. [http://dx.doi.org/10.1016/0197-0186(94) 00154-M]. [PMID: 7492947]. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez C., Mayo J.C., Sainz R.M., Antolín I., Herrera F., Martín V., Reiter R.J. Regulation of antioxidant enzymes: a significant role for melatonin. J. Pineal Res. 2004;36(1):1–9. doi: 10.1046/j.1600-079x.2003.00092.x. [http://dx.doi.org/10.1046/j.1600-079X.2003.00092.x]. [PMID: 14675124]. [DOI] [PubMed] [Google Scholar]
- 24.Reiter R.J. Melatonin: lowering the high price of free radicals. News Physiol. Sci. 2000;15:246–250. doi: 10.1152/physiologyonline.2000.15.5.246. [PMID: 11390919]. [DOI] [PubMed] [Google Scholar]
- 25.Gupta Y.K., Gupta M., Kohli K. Neuroprotective role of melatonin in oxidative stress vulnerable brain. Indian J. Physiol. Pharmacol. 2003;47(4):373–386. [PMID: 15266948]. [PubMed] [Google Scholar]
- 26.Tan D.X., Manchester L.C., Liu X., Rosales-Corral S.A., Acuna-Castroviejo D., Reiter R.J. Mitochondria and chloroplasts as the original sites of melatonin synthesis: a hypothesis related to melatonin’s primary function and evolution in eukaryotes. J. Pineal Res. 2013;54(2):127–138. doi: 10.1111/jpi.12026. [http://dx.doi.org/10.1111/jpi.12026]. [PMID: 23137057]. [DOI] [PubMed] [Google Scholar]
- 27.Reiter R.J. The pineal gland and melatonin in relation to aging: a summary of the theories and of the data. Exp. Gerontol. 1995;30(3-4):199–212. doi: 10.1016/0531-5565(94)00045-5. [http://dx.doi.org/10.1016/0531-5565(94)00045-5]. [PMID: 7556503]. [DOI] [PubMed] [Google Scholar]
- 28.Reiter R.J., Tan D.X., Poeggeler B., Menendez-Pelaez A., Chen L.D., Saarela S. Melatonin as a free radical scavenger: implications for aging and age-related diseases. Ann. N. Y. Acad. Sci. 1994;719:1–12. doi: 10.1111/j.1749-6632.1994.tb56817.x. [http://dx.doi.org/10.1111/j.1749-6632.1994. tb56817.x]. [PMID: 8010585]. [DOI] [PubMed] [Google Scholar]
- 29.Zhou J.N., Liu R.Y., Kamphorst W., Hofman M.A., Swaab D.F. Early neuropathological Alzheimer’s changes in aged individuals are accompanied by decreased cerebrospinal fluid melatonin levels. J. Pineal Res. 2003;35(2):125–130. doi: 10.1034/j.1600-079x.2003.00065.x. [http://dx.doi.org/10.1034/ j.1600-079X.2003.00065.x]. [PMID: 12887656]. [DOI] [PubMed] [Google Scholar]
- 30.Rosales-Corral S., Tan D.X., Reiter R.J., Valdivia-Velázquez M., Martínez-Barboza G., Acosta-Martínez J.P., Ortiz G.G. Orally administered melatonin reduces oxidative stress and proinflammatory cytokines induced by amyloid-beta peptide in rat brain: a comparative, in vivo study versus vitamin C and E. J. Pineal Res. 2003;35(2):80–84. doi: 10.1034/j.1600-079x.2003.00057.x. [http://dx.doi.org/10.1034/j.1600-079X.2003.00057.x]. [PMID: 12887649]. [DOI] [PubMed] [Google Scholar]
- 31.Smith M.A., Hirai K., Hsiao K., Pappolla M.A., Harris P.L., Siedlak S.L., Tabaton M., Perry G. Amyloid-beta deposition in Alzheimer transgenic mice is associated with oxidative stress. J. Neurochem. 1998;70(5):2212–2215. doi: 10.1046/j.1471-4159.1998.70052212.x. [http://dx.doi.org/10.1046/ j.1471-4159.1998.70052212.x]. [PMID: 9572310]. [DOI] [PubMed] [Google Scholar]
- 32.Legros C., Chesneau D., Boutin J.A., Barc C., Malpaux B. Melatonin from cerebrospinal fluid but not from blood reaches sheep cerebral tissues under physiological conditions. J. Neuroendocrinol. 2014;26(3):151–163. doi: 10.1111/jne.12134. [http://dx.doi.org/10.1111/ jne.12134]. [PMID: 24460899]. [DOI] [PubMed] [Google Scholar]
- 33.Reiter R.J., Tan D.X., Kim S.J., Cruz M.H. Delivery of pineal melatonin to the brain and SCN: role of canaliculi, cerebrospinal fluid, tanycytes and Virchow-Robin perivascular spaces. Brain Struct. Funct. 2014;219(6):1873–1887. doi: 10.1007/s00429-014-0719-7. [http://dx.doi.org/10.1007/ s00429-014-0719-7]. [PMID: 24553808]. [DOI] [PubMed] [Google Scholar]
- 34.Wu Y.H., Feenstra M.G., Zhou J.N., Liu R.Y., Toranõ J.S., Van Kan H.J., Fischer D.F., Ravid R., Swaab D.F. Molecular changes underlying reduced pineal melatonin levels in Alzheimer disease: alterations in preclinical and clinical stages. J. Clin. Endocrinol. Metab. 2003;88(12):5898–5906. doi: 10.1210/jc.2003-030833. [http://dx.doi.org/10.1210/jc. 2003-030833]. [PMID: 14671188]. [DOI] [PubMed] [Google Scholar]
- 35.Wu Y.H., Swaab D.F. The human pineal gland and melatonin in aging and Alzheimer’s disease. J. Pineal Res. 2005;38(3):145–152. doi: 10.1111/j.1600-079X.2004.00196.x. [http://dx.doi.org/10.1111/j.1600-079X.2004.00196.x]. [PMID: 15725334]. [DOI] [PubMed] [Google Scholar]
- 36.Galano A., Tan D.X., Reiter R.J. Melatonin as a natural ally against oxidative stress: a physicochemical examination. J. Pineal Res. 2011;51(1):1–16. doi: 10.1111/j.1600-079X.2011.00916.x. [http://dx.doi.org/10.1111/j.1600-079X. 2011.00916.x]. [PMID: 21752095]. [DOI] [PubMed] [Google Scholar]
- 37.Galano A., Tan D.X., Reiter R.J. On the free radical scavenging activities of melatonin’s metabolites, AFMK and AMK. J. Pineal Res. 2013;54(3):245–257. doi: 10.1111/jpi.12010. [http://dx.doi.org/10.1111/jpi.12010]. [PMID: 22998574]. [DOI] [PubMed] [Google Scholar]
- 38.Rosales-Corral S.A., Acuña-Castroviejo D., Coto-Montes A., Boga J.A., Manchester L.C., Fuentes-Broto L., Korkmaz A., Ma S., Tan D.X., Reiter R.J. Alzheimer’s disease: pathological mechanisms and the beneficial role of melatonin. J. Pineal Res. 2012;52(2):167–202. doi: 10.1111/j.1600-079X.2011.00937.x. [http://dx.doi.org/10.1111/j.1600-079X. 2011.00937.x]. [PMID: 22107053]. [DOI] [PubMed] [Google Scholar]
- 39.Lin L., Huang Q.X., Yang S.S., Chu J., Wang J.Z., Tian Q. Melatonin in Alzheimer’s disease. Int. J. Mol. Sci. 2013;14(7):14575–14593. doi: 10.3390/ijms140714575. [http://dx.doi.org/10.3390/ijms140714575]. [PMID: 23857055]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu L.Q., Wang S.H., Ling Z.Q., Wang D.L., Wang J.Z. Effect of inhibiting melatonin biosynthesis on spatial memory retention and tau phosphorylation in rat. J. Pineal Res. 2004;37(2):71–77. doi: 10.1111/j.1600-079X.2004.00136.x. [http://dx.doi.org/10.1111/j.1600-079X.2004.00136.x]. [PMID: 15298664]. [DOI] [PubMed] [Google Scholar]
- 41.Devi L., Prabhu B.M., Galati D.F., Avadhani N.G., Anandatheerthavarada H.K. Accumulation of amyloid precursor protein in the mitochondrial import channels of human Alzheimer’s disease brain is associated with mitochondrial dysfunction. J. Neurosci. 2006;26(35):9057–9068. doi: 10.1523/JNEUROSCI.1469-06.2006. [http://dx.doi.org/10.1523/ JNEUROSCI.1469-06.2006]. [PMID: 16943564]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Butterfield D.A., Drake J., Pocernich C., Castegna A. Evidence of oxidative damage in Alzheimer’s disease brain: central role for amyloid beta-peptide. Trends Mol. Med. 2001;7(12):548–554. doi: 10.1016/s1471-4914(01)02173-6. [http://dx.doi.org/10.1016/S1471-4914(01)02173-6]. [PMID: 11733217]. [DOI] [PubMed] [Google Scholar]
- 43.Ali T., Badshah H., Kim T.H., Kim M.O. Melatonin attenuates D-galactose-induced memory impairment, neuroinflammation and neurodegeneration via RAGE/NF-K B/JNK signaling pathway in aging mouse model. J. Pineal Res. 2015;58(1):71–85. doi: 10.1111/jpi.12194. [http://dx.doi.org/10.1111/jpi.12194]. [PMID: 25401971]. [DOI] [PubMed] [Google Scholar]
- 44.Yang Y., Jiang S., Dong Y., Fan C., Zhao L., Yang X., Li J., Di S., Yue L., Liang G., Reiter R.J., Qu Y. Melatonin prevents cell death and mitochondrial dysfunction via a SIRT1-dependent mechanism during ischemic-stroke in mice. J. Pineal Res. 2015;58(1):61–70. doi: 10.1111/jpi.12193. [http://dx.doi.org/10.1111/jpi.12193]. [PMID: 25401748]. [DOI] [PubMed] [Google Scholar]
- 45.Kang J.E., Lim M.M., Bateman R.J., Lee J.J., Smyth L.P., Cirrito J.R., Fujiki N., Nishino S., Holtzman D.M. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326(5955):1005–1007. doi: 10.1126/science.1180962. [http://dx.doi.org/10.1126/ science.1180962]. [PMID: 19779148]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moe K.E., Vitiello M.V., Larsen L.H., Prinz P.N. Symposium: Cognitive processes and sleep disturbances: Sleep/wake patterns in Alzheimer’s disease: relationships with cognition and function. J. Sleep Res. 1995;4(1):15–20. doi: 10.1111/j.1365-2869.1995.tb00145.x. [http://dx.doi.org/10.1111/j.1365-2869.1995.tb00145.x]. [PMID: 10607136]. [DOI] [PubMed] [Google Scholar]
- 47.Brusco L.I., Márquez M., Cardinali D.P. Monozygotic twins with Alzheimer’s disease treated with melatonin: Case report. J. Pineal Res. 1998;25(4):260–263. doi: 10.1111/j.1600-079x.1998.tb00396.x. [http://dx.doi.org/10.1111/j.1600-079X.1998.tb00396.x]. [PMID: 9885996]. [DOI] [PubMed] [Google Scholar]
- 48.Brusco L.I., Fainstein I., Márquez M., Cardinali D.P. Effect of melatonin in selected populations of sleep-disturbed patients. Biol. Signals Recept. 1999;8(1-2):126–131. doi: 10.1159/000014580. [http://dx.doi.org/10.1159/ 000014580]. [PMID: 10085474]. [DOI] [PubMed] [Google Scholar]
- 49.Brusco L.I., Márquez M., Cardinali D.P. Melatonin treatment stabilizes chronobiologic and cognitive symptoms in Alzheimer’s disease. Neuroendocrinol. Lett. 2000;21(1):39–42. [PMID: 11455329]. [PubMed] [Google Scholar]
- 50.Cohen-Mansfield J., Garfinkel D., Lipson S. Melatonin for treatment of sundowning in elderly persons with dementia - a preliminary study. Arch. Gerontol. Geriatr. 2000;31(1):65–76. doi: 10.1016/s0167-4943(00)00068-6. [http://dx.doi.org/10.1016/S0167-4943(00)00068-6]. [PMID: 10989165]. [DOI] [PubMed] [Google Scholar]
- 51.Mahlberg R., Kunz D., Sutej I., Kühl K.P., Hellweg R. Melatonin treatment of day-night rhythm disturbances and sundowning in Alzheimer disease: an open-label pilot study using actigraphy. J. Clin. Psychopharmacol. 2004;24(4):456–459. doi: 10.1097/01.jcp.0000132443.12607.fd. [http://dx.doi.org/10.1097/01.jcp.0000132443.12607.fd]. [PMID: 15232344]. [DOI] [PubMed] [Google Scholar]
- 52.Poeggeler B., Miravalle L., Zagorski M.G., Wisniewski T., Chyan Y.J., Zhang Y., Shao H., Bryant-Thomas T., Vidal R., Frangione B., Ghiso J., Pappolla M.A. Melatonin reverses the profibrillogenic activity of apolipoprotein E4 on the Alzheimer amyloid Abeta peptide. Biochemistry. 2001;40(49):14995–15001. doi: 10.1021/bi0114269. [http://dx.doi.org/10.1021/bi0114269]. [PMID: 11732920]. [DOI] [PubMed] [Google Scholar]
- 53.Feng Z., Chang Y., Cheng Y., Zhang B.L., Qu Z.W., Qin C., Zhang J.T. Melatonin alleviates behavioral deficits associated with apoptosis and cholinergic system dysfunction in the APP 695 transgenic mouse model of Alzheimer’s disease. J. Pineal Res. 2004;37(2):129–136. doi: 10.1111/j.1600-079X.2004.00144.x. [http://dx.doi.org/10.1111/j.1600-079X. 2004.00144.x]. [PMID: 15298672]. [DOI] [PubMed] [Google Scholar]
- 54.Wang X.C., Zhang J., Yu X., Han L., Zhou Z.T., Zhang Y., Wang J.Z. Prevention of isoproterenol-induced tau hyper-phosphorylation by melatonin in the rat. Sheng Li Xue Bao. 2005;57(1):7–12. [PMID: 15719129]. [PubMed] [Google Scholar]
- 55.Cardinali D.P., Brusco L.I., Liberczuk C., Furio A.M. The use of melatonin in Alzheimer’s disease. Neuroendocrinol. Lett. 2002;23(Suppl. 1):20–23. [PMID: 12019347]. [PubMed] [Google Scholar]
- 56.Cardinali D.P., Furio A.M., Brusco L.I. The use of chronobiotics in the resynchronization of the sleep/wake cycle. Therapeutical application in the early phases of Alzheimer’s disease. Recent Pat. Endocr. Metab. Immune Drug Discov. 2011;5(2):80–90. doi: 10.2174/187221411799015354. [http:// dx.doi.org/10.2174/187221411799015354]. [PMID: 22074583]. [DOI] [PubMed] [Google Scholar]
- 57.McCurry S.M., Reynolds C.F., Ancoli-Israel S., Teri L., Vitiello M.V. Treatment of sleep disturbance in Alzheimer’s disease. Sleep Med. Rev. 2000;4(6):603–628. doi: 10.1053/smrv.2000.0127. [http://dx.doi.org/10.1053/smrv. 2000.0127]. [PMID: 12531038]. [DOI] [PubMed] [Google Scholar]
- 58.Reiter R.J. The ageing pineal gland and its physiological consequences. BioEssays. 1992;14(3):169–175. doi: 10.1002/bies.950140307. [http://dx.doi.org/ 10.1002/bies.950140307]. [PMID: 1586370]. [DOI] [PubMed] [Google Scholar]
- 59.Pierpaoli W. Neuroimmunomodulation of aging. A program in the pineal gland. Ann. N. Y. Acad. Sci. 1998;840:491–497. doi: 10.1111/j.1749-6632.1998.tb09587.x. [http://dx. doi.org/10.1111/j.1749-6632.1998.tb09587.x]. [PMID: 9629275]. [DOI] [PubMed] [Google Scholar]
- 60.Wu Y.H., Zhou J.N., Van Heerikhuize J., Jockers R., Swaab D.F. Decreased MT1 melatonin receptor expression in the suprachiasmatic nucleus in aging and Alzheimer’s disease. Neurobiol. Aging. 2007;28(8):1239–1247. doi: 10.1016/j.neurobiolaging.2006.06.002. [http://dx.doi.org/ 10.1016/j.neurobiolaging.2006.06.002]. [PMID: 16837102]. [DOI] [PubMed] [Google Scholar]
- 61.Kunz D., Schmitz S., Mahlberg R., Mohr A., Stöter C., Wolf K.J., Herrmann W.M. A new concept for melatonin deficit: on pineal calcification and melatonin excretion. Neuropsychopharmacology. 1999;21(6):765–772. doi: 10.1016/S0893-133X(99)00069-X. [http://dx.doi.org/10.1016/ S0893-133X(99)00069-X]. [PMID: 10633482]. [DOI] [PubMed] [Google Scholar]
- 62.Skene D.J., Vivien-Roels B., Sparks D.L., Hunsaker J.C., Pévet P., Ravid D., Swaab D.F. Daily variation in the concentration of melatonin and 5-methoxytryptophol in the human pineal gland: effect of age and Alzheimer’s disease. Brain Res. 1990;528(1):170–174. doi: 10.1016/0006-8993(90)90214-v. [http://dx.doi.org/10.1016/0006-8993(90)90214-V]. [PMID: 2245336]. [DOI] [PubMed] [Google Scholar]
- 63.Skene D.J., Swaab D.F. Melatonin rhythmicity: effect of age and Alzheimer’s disease. Exp. Gerontol. 2003;38(1-2):199–206. doi: 10.1016/s0531-5565(02)00198-5. [http://dx.doi.org/10.1016/S0531-5565(02)00198-5]. [PMID: 12543278]. [DOI] [PubMed] [Google Scholar]
- 64.Swaab D.F., Fliers E., Partiman T.S. The suprachiasmatic nucleus of the human brain in relation to sex, age and senile dementia. Brain Res. 1985;342(1):37–44. doi: 10.1016/0006-8993(85)91350-2. [http://dx.doi.org/ 10.1016/0006-8993(85)91350-2]. [PMID: 4041816]. [DOI] [PubMed] [Google Scholar]
- 65.Mishima K., Tozawa T., Satoh K., Matsumoto Y., Hishikawa Y., Okawa M. Melatonin secretion rhythm disorders in patients with senile dementia of Alzheimer’s type with disturbed sleep-waking. Biol. Psychiatry. 1999;45(4):417–421. doi: 10.1016/s0006-3223(97)00510-6. [http://dx.doi.org/ 10.1016/S0006-3223(97)00510-6]. [PMID: 10071710]. [DOI] [PubMed] [Google Scholar]
- 66.Pandi-Perumal S.R., Zisapel N., Srinivasan V., Cardinali D.P. Melatonin and sleep in aging population. Exp. Gerontol. 2005;40(12):911–925. doi: 10.1016/j.exger.2005.08.009. [http://dx.doi.org/10.1016/j.exger.2005.08.009]. [PMID: 16183237]. [DOI] [PubMed] [Google Scholar]
- 67.Uchida K., Okamoto N., Ohara K., Morita Y. Daily rhythm of serum melatonin in patients with dementia of the degenerate type. Brain Res. 1996;717(1-2):154–159. doi: 10.1016/0006-8993(96)00086-8. [http://dx.doi.org/10.1016/ 0006-8993(96)00086-8]. [PMID: 8738265]. [DOI] [PubMed] [Google Scholar]
- 68.Lahiri D.K., Chen D., Lahiri P., Rogers J.T., Greig N.H., Bondy S. Melatonin, metals, and gene expression: implications in aging and neurodegenerative disorders. Ann. N. Y. Acad. Sci. 2004;1035:216–230. doi: 10.1196/annals.1332.014. [http://dx.doi.org/10.1196/annals.1332.014]. [PMID: 15681810]. [DOI] [PubMed] [Google Scholar]
- 69.Hedden T., Gabrieli J.D. Insights into the ageing mind: a view from cognitive neuroscience. Nat. Rev. Neurosci. 2004;5(2):87–96. doi: 10.1038/nrn1323. [http://dx.doi.org/10.1038/nrn1323]. [PMID: 14735112]. [DOI] [PubMed] [Google Scholar]
- 70.Cardinali D.P., Furio A.M., Brusco L.I. Clinical aspects of melatonin intervention in Alzheimer’s disease progression. Curr. Neuropharmacol. 2010;8(3):218–227. doi: 10.2174/157015910792246209. [http://dx.doi.org/10.2174/ 157015910792246209]. [PMID: 21358972]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu R.Y., Zhou J.N., van Heerikhuize J., Hofman M.A., Swaab D.F. Decreased melatonin levels in postmortem cerebrospinal fluid in relation to aging, Alzheimer’s disease, and apolipoprotein E-epsilon4/4 genotype. J. Clin. Endocrinol. Metab. 1999;84(1):323–327. doi: 10.1210/jcem.84.1.5394. [PMID: 9920102]. [DOI] [PubMed] [Google Scholar]
- 72.Savaskan E., Olivieri G., Meier F., Brydon L., Jockers R., Ravid R., Wirz-Justice A., Müller-Spahn F. Increased melatonin 1a-receptor immunoreactivity in the hippocampus of Alzheimer’s disease patients. J. Pineal Res. 2002;32(1):59–62. doi: 10.1034/j.1600-079x.2002.00841.x. [http://dx. doi.org/10.1034/j.1600-079x.2002.00841.x]. [PMID: 11841602]. [DOI] [PubMed] [Google Scholar]
- 73.Savaskan E., Ayoub M.A., Ravid R., Angeloni D., Fraschini F., Meier F., Eckert A., Müller-Spahn F., Jockers R. Reduced hippocampal MT2 melatonin receptor expression in Alzheimer’s disease. J. Pineal Res. 2005;38(1):10–16. doi: 10.1111/j.1600-079X.2004.00169.x. [http://dx.doi.org/ 10.1111/j.1600-079X.2004.00169.x]. [PMID: 15617532]. [DOI] [PubMed] [Google Scholar]
- 74.Brunner P., Sözer-Topcular N., Jockers R., Ravid R., Angeloni D., Fraschini F., Eckert A., Müller-Spahn F., Savaskan E. Pineal and cortical melatonin receptors MT1 and MT2 are decreased in Alzheimer’s disease. Eur. J. Histochem. 2006;50(4):311–316. [PMID: 17213040]. [PubMed] [Google Scholar]
- 75.Bondy S.C., Lahiri D.K., Perreau V.M., Sharman K.Z., Campbell A., Zhou J., Sharman E.H. Retardation of brain aging by chronic treatment with melatonin. Ann. N. Y. Acad. Sci. 2004;1035:197–215. doi: 10.1196/annals.1332.013. [http://dx.doi.org/10.1196/annals.1332.013]. [PMID: 15681809]. [DOI] [PubMed] [Google Scholar]
- 76.Bubenik G.A., Konturek S.J. Melatonin and aging: prospects for human treatment. J. Physiol. Pharmacol. 2011;62(1):13–19. [PMID: 21451205]. [PubMed] [Google Scholar]
- 77.Ali T., Kim M.O. Melatonin ameliorates amyloid beta-induced memory deficits, tau hyperphosphorylation and neurodegeneration via PI3/Akt/GSk3β pathway in the mouse hippocampus. J. Pineal Res. 2015;59(1):47–59. doi: 10.1111/jpi.12238. [http://dx.doi.org/10.1111/jpi.12238]. [PMID: 25858697]. [DOI] [PubMed] [Google Scholar]
- 78.Stefanova N.A., Maksimova K.Y., Kiseleva E., Rudnitskaya E.A., Muraleva N.A., Kolosova N.G. Melatonin attenuates impairments of structural hippocampal neuroplasticity in OXYS rats during active progression of Alzheimer’s disease-like pathology. J. Pineal Res. 2015;59(2):163–177. doi: 10.1111/jpi.12248. [http://dx.doi.org/ 10.1111/jpi.12248]. [PMID: 25988948]. [DOI] [PubMed] [Google Scholar]
- 79.Baum L.W. Sex, hormones, and Alzheimer’s disease. J. Gerontol. A Biol. Sci. Med. Sci. 2005;60(6):736–743. doi: 10.1093/gerona/60.6.736. [http://dx.doi.org/ 10.1093/gerona/60.6.736]. [PMID: 15983176]. [DOI] [PubMed] [Google Scholar]
- 80.Webber K.M., Casadesus G., Perry G., Atwood C.S., Bowen R., Smith M.A. Gender differences in Alzheimer disease: the role of luteinizing hormone in disease pathogenesis. Alzheimer Dis. Assoc. Disord. 2005;19(2):95–99. doi: 10.1097/01.wad.0000165512.90864.3f. [http://dx.doi.org/10.1097/01.wad. 0000165512.90864.3f]. [PMID: 15942328]. [DOI] [PubMed] [Google Scholar]
- 81.Gould E., Tanapat P., Rydel T., Hastings N. Regulation of hippocampal neurogenesis in adulthood. Biol. Psychiatry. 2000;48(8):715–720. doi: 10.1016/s0006-3223(00)01021-0. [http://dx.doi.org/10.1016/S0006-3223(00)01021-0]. [PMID: 11063968]. [DOI] [PubMed] [Google Scholar]
- 82.Tanapat P., Hastings N.B., Reeves A.J., Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J. Neurosci. 1999;19(14):5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [PMID: 10407020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Manly J.J., Merchant C.A., Jacobs D.M., Small S.A., Bell K., Ferin M., Mayeux R. Endogenous estrogen levels and Alzheimer’s disease among postmenopausal women. Neurology. 2000;54(4):833–837. doi: 10.1212/wnl.54.4.833. [http://dx.doi.org/10.1212/WNL.54.4.833]. [PMID: 10690972]. [DOI] [PubMed] [Google Scholar]
- 84.Kireev R.A., Vara E., Viña J., Tresguerres J.A. Melatonin and oestrogen treatments were able to improve neuroinflammation and apoptotic processes in dentate gyrus of old ovariectomized female rats. Age (Dordr.) 2014;36(5):9707. doi: 10.1007/s11357-014-9707-3. [http://dx.doi.org/10.1007/ s11357-014-9707-3]. [PMID: 25135305]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Armstrong S.M. Melatonin: the internal Zeitgeber of mammals? Pineal. Res. Rev. 1989;7:157–202. [Google Scholar]
- 86.Armstrong S.M., Redman J.R. Melatonin: a chronobiotic with anti-aging properties? Med. Hypotheses. 1991;34(4):300–309. doi: 10.1016/0306-9877(91)90046-2. [http://dx.doi.org/10.1016/0306-9877(91)90046-2]. [PMID: 1865836]. [DOI] [PubMed] [Google Scholar]
- 87.Kunz D. Chronobiotic protocol and circadian sleep propensity index: new tools for clinical routine and research on melatonin and sleep. Pharmacopsychiatry. 2004;37(4):139–146. doi: 10.1055/s-2004-827167. [http://dx.doi. org/10.1055/s-2004-827167]. [PMID: 15467968]. [DOI] [PubMed] [Google Scholar]
- 88.Srinivasan V. Melatonin, biological rhythm disorders and phototherapy. Indian J. Physiol. Pharmacol. 1997;41(4):309–328. [PMID: 10235654]. [PubMed] [Google Scholar]
- 89.Maestroni G.J., Conti A. Beta-endorphin and dynorphin mimic the circadian immunoenhancing and anti-stress effects of melatonin. Int. J. Immunopharmacol. 1989;11(4):333–340. doi: 10.1016/0192-0561(89)90078-7. [http:// dx.doi.org/10.1016/0192-0561(89)90078-7]. [PMID: 2570759]. [DOI] [PubMed] [Google Scholar]
- 90.Benot S., Goberna R., Reiter R.J., Garcia-Mauriño S., Osuna C., Guerrero J.M. Physiological levels of melatonin contribute to the antioxidant capacity of human serum. J. Pineal Res. 1999;27(1):59–64. doi: 10.1111/j.1600-079x.1999.tb00597.x. [http://dx.doi.org/10.1111/j.1600-079X.1999.tb00597.x]. [PMID: 10451025]. [DOI] [PubMed] [Google Scholar]
- 91.Tan D.X., Chen L.D., Poeggeler B., Manchester L., Reiter R.J. Melatonin: a potent, endogenous hydroxyl radical scavenger. Endocr. J. 1993;1:57–60. [Google Scholar]
- 92.Yon J.H., Carter L.B., Reiter R.J., Jevtovic-Todorovic V. Melatonin reduces the severity of anesthesia-induced apoptotic neurodegeneration in the developing rat brain. Neurobiol. Dis. 2006;21(3):522–530. doi: 10.1016/j.nbd.2005.08.011. [http://dx.doi.org/10.1016/j.nbd.2005.08. 011]. [PMID: 16289675]. [DOI] [PubMed] [Google Scholar]
- 93.Hardeland R. Melatonin and the theories of aging: a critical appraisal of melatonin’s role in antiaging mechanisms. J. Pineal Res. 2013;55(4):325–356. doi: 10.1111/jpi.12090. [PMID: 24112071]. [DOI] [PubMed] [Google Scholar]
- 94.Thomas J.N., Smith-Sonneborn J. Supplemental melatonin increases clonal lifespan in the protozoan Paramecium tetraurelia. J. Pineal Res. 1997;23(3):123–130. doi: 10.1111/j.1600-079x.1997.tb00344.x. [http://dx.doi.org/10.1111/ j.1600-079X.1997.tb00344.x]. [PMID: 9406982]. [DOI] [PubMed] [Google Scholar]
- 95.Bonilla E., Medina-Leendertz S., Díaz S. Extension of life span and stress resistance of Drosophila melanogaster by long-term supplementation with melatonin. Exp. Gerontol. 2002;37(5):629–638. doi: 10.1016/s0531-5565(01)00229-7. [http://dx.doi.org/10.1016/S0531-5565(01)00229-7]. [PMID: 11909680]. [DOI] [PubMed] [Google Scholar]
- 96.Pierpaoli W., Dall’Ara A., Pedrinis E., Regelson W. The pineal control of aging. The effects of melatonin and pineal grafting on the survival of older mice. Ann. N. Y. Acad. Sci. 1991;621:291–313. doi: 10.1111/j.1749-6632.1991.tb16987.x. [http://dx.doi.org/10.1111/j.1749-6632.1991.tb16987.x]. [PMID: 1859093]. [DOI] [PubMed] [Google Scholar]
- 97.Masana M.I., Dubocovich M.L. Melatonin receptor signaling: finding the path through the dark. Sci. STKE. 2001;2001(107):pe39. doi: 10.1126/stke.2001.107.pe39. [PMID: 11698691]. [DOI] [PubMed] [Google Scholar]
- 98.Chang H.M., Wu U.I., Lan C.T. Melatonin preserves longevity protein (sirtuin 1) expression in the hippocampus of total sleep-deprived rats. J. Pineal Res. 2009;47(3):211–220. doi: 10.1111/j.1600-079X.2009.00704.x. [http://dx. doi.org/10.1111/j.1600-079X.2009.00704.x]. [PMID: 19627456]. [DOI] [PubMed] [Google Scholar]
- 99.Cristòfol R., Porquet D., Corpas R., Coto-Montes A., Serret J., Camins A., Pallàs M., Sanfeliu C. Neurons from senescence-accelerated SAMP8 mice are protected against frailty by the sirtuin 1 promoting agents melatonin and resveratrol. J. Pineal Res. 2012;52(3):271–281. doi: 10.1111/j.1600-079X.2011.00939.x. [http://dx.doi.org/10.1111/j.1600-079X.2011. 00939.x]. [PMID: 22085194]. [DOI] [PubMed] [Google Scholar]
- 100.Wang J., Fivecoat H., Ho L., Pan Y., Ling E., Pasinetti G.M. The role of Sirt1: at the crossroad between promotion of longevity and protection against Alzheimer’s disease neuropathology. Biochim. Biophys. Acta. 2010;1804(8):1690–1694. doi: 10.1016/j.bbapap.2009.11.015. [http://dx.doi. org/10.1016/j.bbapap.2009.11.015]. [PMID: 19945548]. [DOI] [PubMed] [Google Scholar]
- 101.Albani D., Polito L., Batelli S., De Mauro S., Fracasso C., Martelli G., Colombo L., Manzoni C., Salmona M., Caccia S., Negro A., Forloni G. The SIRT1 activator resveratrol protects SK-N-BE cells from oxidative stress and against toxicity caused by alpha-synuclein or amyloid-beta (1-42) peptide. J. Neurochem. 2009;110(5):1445–1456. doi: 10.1111/j.1471-4159.2009.06228.x. [http://dx.doi.org/10.1111/j.1471-4159. 2009.06228.x]. [PMID: 19558452]. [DOI] [PubMed] [Google Scholar]
- 102.Julien C., Tremblay C., Emond V., Lebbadi M., Salem N., Jr, Bennett D.A., Calon F. Sirtuin 1 reduction parallels the accumulation of tau in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2009;68(1):48–58. doi: 10.1097/NEN.0b013e3181922348. [http://dx.doi.org/10.1097/NEN. 0b013e3181922348]. [PMID: 19104446]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Qin W., Yang T., Ho L., Zhao Z., Wang J., Chen L., Zhao W., Thiyagarajan M., MacGrogan D., Rodgers J.T., Puigserver P., Sadoshima J., Deng H., Pedrini S., Gandy S., Sauve A.A., Pasinetti G.M. Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction. J. Biol. Chem. 2006;281(31):21745–21754. doi: 10.1074/jbc.M602909200. [http://dx.doi.org/10.1074/jbc.M602909200]. [PMID: 16751189]. [DOI] [PubMed] [Google Scholar]
- 104.Tippmann F., Hundt J., Schneider A., Endres K., Fahrenholz F. Up-regulation of the alpha-secretase ADAM10 by retinoic acid receptors and acitretin. FASEB J. 2009;23(6):1643–1654. doi: 10.1096/fj.08-121392. [http://dx.doi.org/10.1096/fj.08-121392]. [PMID: 19144697]. [DOI] [PubMed] [Google Scholar]
- 105.West R.L., Lee J.M., Maroun L.E. Hypomethylation of the amyloid precursor protein gene in the brain of an Alzheimer’s disease patient. J. Mol. Neurosci. 1995;6(2):141–146. doi: 10.1007/BF02736773. [http://dx.doi.org/10.1007/BF02736773]. [PMID: 8746452]. [DOI] [PubMed] [Google Scholar]
- 106.Tohgi H., Utsugisawa K., Nagane Y., Yoshimura M., Genda Y., Ukitsu M. Reduction with age in methylcytosine in the promoter region -224 approximately -101 of the amyloid precursor protein gene in autopsy human cortex. Brain Res. Mol. Brain Res. 1999;70(2):288–292. doi: 10.1016/s0169-328x(99)00163-1. [http://dx.doi.org/10.1016/S0169-328X(99)00163-1]. [PMID: 10407177]. [DOI] [PubMed] [Google Scholar]
- 107.Ding H., Dolan P.J., Johnson G.V. Histone deacetylase 6 interacts with the microtubule-associated protein tau. J. Neurochem. 2008;106(5):2119–2130. doi: 10.1111/j.1471-4159.2008.05564.x. [http://dx.doi.org/10.1111/j.1471-4159.2008. 05564.x]. [PMID: 18636984]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jenwitheesuk A., Nopparat C., Mukda S., Wongchitrat P., Govitrapong P. Melatonin regulates aging and neurodegeneration through energy metabolism, epigenetics, autophagy and circadian rhythm pathways. Int. J. Mol. Sci. 2014;15(9):16848–16884. doi: 10.3390/ijms150916848. [http://dx.doi.org/10.3390/ijms150916848]. [PMID: 25247581]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Drachman D.A., Leavitt J. Human memory and the cholinergic system. A relationship to aging? Arch. Neurol. 1974;30(2):113–121. doi: 10.1001/archneur.1974.00490320001001. [http://dx.doi.org/10.1001/archneur.1974.00490320001001]. [PMID: 4359364]. [DOI] [PubMed] [Google Scholar]
- 110.Terry A.V., Jr, Buccafusco J.J. The cholinergic hypothesis of age and Alzheimer’s disease-related cognitive deficits: recent challenges and their implications for novel drug development. J. Pharmacol. Exp. Ther. 2003;306(3):821–827. doi: 10.1124/jpet.102.041616. [http://dx.doi.org/ 10.1124/jpet.102.041616]. [PMID: 12805474]. [DOI] [PubMed] [Google Scholar]
- 111.Gibson G.E., Peterson C. Aging decreases oxidative metabolism and the release and synthesis of acetylcholine. J. Neurochem. 1981;37(4):978–984. doi: 10.1111/j.1471-4159.1981.tb04484.x. [http://dx.doi.org/10.1111/j.1471-4159.1981. tb04484.x]. [PMID: 7320734]. [DOI] [PubMed] [Google Scholar]
- 112.Xu S., Pi H., Zhang L., Zhang N., Li Y., Zhang H., Tang J., Li H., Feng M., Deng P., Guo P., Tian L., Xie J., He M., Lu Y., Zhong M., Zhang Y., Wang W., Reiter R.J., Yu Z., Zhou Z. Melatonin prevents abnormal mitochondrial dynamics resulting from the neurotoxicity of cadmium by blocking calcium-dependent translocation of Drp1 to the mitochondria. J. Pineal Res. 2016;60(3):291–302. doi: 10.1111/jpi.12310. [http://dx.doi.org/10.1111/jpi.12310]. [PMID: 26732476]. [DOI] [PubMed] [Google Scholar]
- 113.Pazar A., Kolgazi M., Memisoglu A., Bahadir E., Sirvanci S., Yaman A., Yeğen B.C., Ozek E. The neuroprotective and anti-apoptotic effects of melatonin on hemolytic hyperbilirubinemia-induced oxidative brain damage. J. Pineal Res. 2016;60(1):74–83. doi: 10.1111/jpi.12292. [http://dx.doi.org/10.1111/jpi.12292]. [PMID: 26511903]. [DOI] [PubMed] [Google Scholar]
- 114.Wang J.Z., Wang Z.F. Role of melatonin in Alzheimer-like neurodegeneration. Acta Pharmacol. Sin. 2006;27(1):41–49. doi: 10.1111/j.1745-7254.2006.00260.x. [http://dx.doi.org/10.1111/j.1745-7254.2006.00260.x]. [PMID: 16364209]. [DOI] [PubMed] [Google Scholar]
- 115.Guermonprez L., Ducrocq C., Gaudry-Talarmain Y.M. Inhibition of acetylcholine synthesis and tyrosine nitration induced by peroxynitrite are differentially prevented by antioxidants. Mol. Pharmacol. 2001;60(4):838–846. [PMID: 11562447]. [PubMed] [Google Scholar]
- 116.Agrawal R., Tyagi E., Shukla R., Nath C. A study of brain insulin receptors, AChE activity and oxidative stress in rat model of ICV STZ induced dementia. Neuropharmacology. 2009;56(4):779–787. doi: 10.1016/j.neuropharm.2009.01.005. [http://dx.doi.org/10.1016/j.neuropharm.2009.01.005]. [PMID: 19705549]. [DOI] [PubMed] [Google Scholar]
- 117.Brion J.P., Anderton B.H., Authelet M., Dayanandan R., Leroy K., Lovestone S., Octave J.N., Pradier L., Touchet N., Tremp G. Neurofibrillary tangles and tau phosphorylation. Biochem. Soc. Symp. 2001;(67):81–88. doi: 10.1042/bss0670081. [http://dx.doi.org/10.1042/bss0670081]. [PMID: 11447842]. [DOI] [PubMed] [Google Scholar]
- 118.Maccioni R.B., Farías G., Morales I., Navarrete L. The revitalized tau hypothesis on Alzheimer’s disease. Arch. Med. Res. 2010;41(3):226–231. doi: 10.1016/j.arcmed.2010.03.007. [http://dx.doi.org/10.1016/j.arcmed.2010. 03.007]. [PMID: 20682182]. [DOI] [PubMed] [Google Scholar]
- 119.Ishiguro K., Omori A., Takamatsu M., Sato K., Arioka M., Uchida T., Imahori K. Phosphorylation sites on tau by tau protein kinase I, a bovine derived kinase generating an epitope of paired helical filaments. Neurosci. Lett. 1992;148(1-2):202–206. doi: 10.1016/0304-3940(92)90839-y. [http://dx.doi.org/10.1016/0304-3940(92)90839-Y]. [PMID: 1284442]. [DOI] [PubMed] [Google Scholar]
- 120.Takashima A., Noguchi K., Sato K., Hoshino T., Imahori K. Tau protein kinase I is essential for amyloid beta-protein-induced neurotoxicity. Proc. Natl. Acad. Sci. USA. 1993;90(16):7789–7793. doi: 10.1073/pnas.90.16.7789. [http://dx.doi.org/10.1073/pnas.90.16.7789]. [PMID: 8356085]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Imahori K., Uchida T. Physiology and pathology of tau protein kinases in relation to Alzheimer’s disease. J. Biochem. 1997;121(2):179–188. [PMID: 9089387]. [PubMed] [Google Scholar]
- 122.Avila J., Hernández F. GSK-3 inhibitors for Alzheimer’s disease. Expert Rev. Neurother. 2007;7(11):1527–1533. doi: 10.1586/14737175.7.11.1527. [http://dx.doi.org/ 10.1586/14737175.7.11.1527]. [PMID: 17997701]. [DOI] [PubMed] [Google Scholar]
- 123.Alvarez A., Muñoz J.P., Maccioni R.B. Cdk5-p35 stable complex is involved in the beta-amyloid-induced deregulation of Cdk5 activity in hippocampal neurons. Exp. Cell Res. 2001;264(2):266–274. doi: 10.1006/excr.2001.5152. [http://dx.doi.org/10.1006/excr.2001.5152]. [PMID: 11262183]. [DOI] [PubMed] [Google Scholar]
- 124.Wen Y., Planel E., Herman M., Figueroa H.Y., Wang L., Liu L., Lau L.F., Yu W.H., Duff K.E. Interplay between cyclin-dependent kinase 5 and glycogen synthase kinase 3 beta mediated by neuregulin signaling leads to differential effects on tau phosphorylation and amyloid precursor protein processing. J. Neurosci. 2008;28(10):2624–2632. doi: 10.1523/JNEUROSCI.5245-07.2008. [http://dx.doi.org/10.1523/ JNEUROSCI.5245-07.2008]. [PMID: 18322105]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Patrick G.N., Zukerberg L., Nikolic M., de la Monte S., Dikkes P., Tsai L.H. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature. 1999;402(6762):615–622. doi: 10.1038/45159. [http://dx.doi.org/10.1038/45159]. [PMID: 10604467]. [DOI] [PubMed] [Google Scholar]
- 126.Deng Y.Q., Xu G.G., Duan P., Zhang Q., Wang J.Z. Effects of melatonin on wortmannin-induced tau hyperphosphorylation. Acta Pharmacol. Sin. 2005;26(5):519–526. doi: 10.1111/j.1745-7254.2005.00102.x. [http://dx.doi.org/10.1111/ j.1745-7254.2005.00102.x]. [PMID: 15842767]. [DOI] [PubMed] [Google Scholar]
- 127.Li S.P., Deng Y.Q., Wang X.C., Wang Y.P., Wang J.Z. Melatonin protects SH-SY5Y neuroblastoma cells from calyculin A-induced neurofilament impairment and neurotoxicity. J. Pineal Res. 2004;36(3):186–191. doi: 10.1111/j.1600-079x.2004.00116.x. [http://dx.doi.org/10.1111/j.1600-079X.2004.00116.x]. [PMID: 15009509]. [DOI] [PubMed] [Google Scholar]
- 128.Liu S.J., Wang J.Z. Alzheimer-like tau phosphorylation induced by wortmannin in vivo and its attenuation by melatonin. Acta Pharmacol. Sin. 2002;23(2):183–187. [PMID: 11866882]. [PubMed] [Google Scholar]
- 129.Li X.C., Wang Z.F., Zhang J.X., Wang Q., Wang J.Z. Effect of melatonin on calyculin A-induced tau hyperphosphorylation. Eur. J. Pharmacol. 2005;510(1-2):25–30. doi: 10.1016/j.ejphar.2005.01.023. [http://dx.doi.org/ 10.1016/j.ejphar.2005.01.023]. [PMID: 15740721]. [DOI] [PubMed] [Google Scholar]
- 130.Xiong Y.F., Chen Q., Chen J., Zhou J., Wang H.X. Melatonin reduces the impairment of axonal transport and axonopathy induced by calyculin A. J. Pineal Res. 2011;50(3):319–327. doi: 10.1111/j.1600-079X.2010.00846.x. [http://dx.doi.org/10.1111/j.1600-079X.2010.00846.x]. [PMID: 21244478]. [DOI] [PubMed] [Google Scholar]
- 131.Wang Y.P., Li X.T., Liu S.J., Zhou X.W., Wang X.C., Wang J.Z. Melatonin ameliorated okadaic-acid induced Alzheimer-like lesions. Acta Pharmacol. Sin. 2004;25(3):276–280. [PMID: 15000877]. [PubMed] [Google Scholar]
- 132.Wang S., Zhu L., Shi H., Zheng H., Tian Q., Wang Q., Liu R., Wang J.Z. Inhibition of melatonin biosynthesis induces neurofilament hyperphosphorylation with activation of cyclin-dependent kinase 5. Neurochem. Res. 2007;32(8):1329–1335. doi: 10.1007/s11064-007-9308-y. [http://dx.doi.org/10.1007/s11064-007-9308-y]. [PMID: 17401652]. [DOI] [PubMed] [Google Scholar]
- 133.Markesbery W.R. Oxidative stress hypothesis in Alzheimer’s disease. Free Radic. Biol. Med. 1997;23(1):134–147. doi: 10.1016/s0891-5849(96)00629-6. [http://dx. doi.org/10.1016/S0891-5849(96)00629-6]. [PMID: 9165306]. [DOI] [PubMed] [Google Scholar]
- 134.Cardinali D.P., Furio A.M., Reyes M.P. Clinical perspectives for the use of melatonin as a chronobiotic and cytoprotective agent. Ann. N. Y. Acad. Sci. 2005;1057:327–336. doi: 10.1196/annals.1356.025. [http://dx.doi.org/ 10.1196/annals.1356.025]. [PMID: 16399904]. [DOI] [PubMed] [Google Scholar]
- 135.Reiter R.J. Oxidative damage in the central nervous system: protection by melatonin. Prog. Neurobiol. 1998;56(3):359–384. doi: 10.1016/s0301-0082(98)00052-5. [http://dx.doi.org/10.1016/S0301-0082(98)00052-5]. [PMID: 9770244]. [DOI] [PubMed] [Google Scholar]
- 136.Reiter R.J., Tan D.X., Pappolla M.A. Melatonin relieves the neural oxidative burden that contributes to dementias. Ann. N. Y. Acad. Sci. 2004;1035:179–196. doi: 10.1196/annals.1332.012. [http://dx.doi.org/10.1196/annals. 1332.012]. [PMID: 15681808]. [DOI] [PubMed] [Google Scholar]
- 137.Reiter R.J. Pineal melatonin: cell biology of its synthesis and of its physiological interactions. Endocr. Rev. 1991;12(2):151–180. doi: 10.1210/edrv-12-2-151. [http://dx.doi.org/10.1210/edrv-12-2-151]. [PMID: 1649044]. [DOI] [PubMed] [Google Scholar]
- 138.Hardeland R. Antioxidative protection by melatonin: multiplicity of mechanisms from radical detoxification to radical avoidance. Endocrine. 2005;27(2):119–130. doi: 10.1385/endo:27:2:119. [http://dx.doi.org/10.1385/ ENDO:27:2:119]. [PMID: 16217125]. [DOI] [PubMed] [Google Scholar]
- 139.Permpoonputtana K., Govitrapong P. The anti-inflammatory effect of melatonin on methamphetamine-induced proinflammatory mediators in human neuroblastoma dopamine SH-SY5Y cell lines. Neurotox. Res. 2013;23(2):189–199. doi: 10.1007/s12640-012-9350-7. [http://dx.doi.org/10.1007/ s12640-012-9350-7]. [PMID: 22903344]. [DOI] [PubMed] [Google Scholar]
- 140.Vriend J., Reiter R.J. The Keap1-Nrf2-antioxidant response element pathway: a review of its regulation by melatonin and the proteasome. Mol. Cell. Endocrinol. 2015;401:213–220. doi: 10.1016/j.mce.2014.12.013. [http://dx. doi.org/10.1016/j.mce.2014.12.013]. [PMID: 25528518]. [DOI] [PubMed] [Google Scholar]
- 141.Chan K., Han X.D., Kan Y.W. An important function of Nrf2 in combating oxidative stress: detoxification of acetaminophen. Proc. Natl. Acad. Sci. USA. 2001;98(8):4611–4616. doi: 10.1073/pnas.081082098. [http://dx.doi.org/ 10.1073/pnas.081082098]. [PMID: 11287661]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Galano A., Medina M.E., Tan D.X., Reiter R.J. Melatonin and its metabolites as copper chelating agents and their role in inhibiting oxidative stress: a physicochemical analysis. J. Pineal Res. 2015;58(1):107–116. doi: 10.1111/jpi.12196. [http://dx.doi.org/10.1111/jpi.12196]. [PMID: 25424557]. [DOI] [PubMed] [Google Scholar]
- 143.Reiter R., Tang L., Garcia J.J., Muñoz-Hoyos A. Phar-macological actions of melatonin in oxygen radical pathophysiology. Life Sci. 1997;60(25):2255–2271. doi: 10.1016/s0024-3205(97)00030-1. [http://dx.doi.org/10.1016/ S0024-3205(97)00030-1]. [PMID: 9194681]. [DOI] [PubMed] [Google Scholar]
- 144.Reiter R.J., Tan D., Kim S.J., Manchester L.C., Qi W., Garcia J.J., Cabrera J.C., El-Sokkary G., Rouvier-Garay V. Augmentation of indices of oxidative damage in life-long melatonin-deficient rats. Mech. Ageing Dev. 1999;110(3):157–173. doi: 10.1016/s0047-6374(99)00058-5. [http://dx.doi.org/10.1016/S0047-6374(99)00058-5]. [PMID: 10576246]. [DOI] [PubMed] [Google Scholar]
- 145.Tan D.X., Manchester L.C., Burkhardt S., Sainz R.M., Mayo J.C., Kohen R., Shohami E., Huo Y.S., Hardeland R., Reiter R.J. N1-acetyl-N2-formyl-5-methoxykynuramine, a biogenic amine and melatonin metabolite, functions as a potent antioxidant. FASEB J. 2001;15(12):2294–2296. doi: 10.1096/fj.01-0309fje. [PMID: 11511530]. [DOI] [PubMed] [Google Scholar]
- 146.Tan D.X., Reiter R.J., Manchester L.C., Yan M.T., El-Sawi M., Sainz R.M., Mayo J.C., Kohen R., Allegra M., Hardeland R. Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger. Curr. Top. Med. Chem. 2002;2(2):181–197. doi: 10.2174/1568026023394443. [http://dx.doi.org/10.2174/1568026023394443]. [PMID: 11899100]. [DOI] [PubMed] [Google Scholar]
- 147.Ressmeyer A.R., Mayo J.C., Zelosko V., Sáinz R.M., Tan D.X., Poeggeler B., Antolín I., Zsizsik B.K., Reiter R.J., Hardeland R. Antioxidant properties of the melatonin metabolite N1-acetyl-5-methoxykynuramine (AMK): scavenging of free radicals and prevention of protein destruction. Redox Rep. 2003;8(4):205–213. doi: 10.1179/135100003225002709. [http://dx.doi.org/10.1179/135100003225002709]. [PMID: 14599344]. [DOI] [PubMed] [Google Scholar]
- 148.Butterfield D.A., Boyd-Kimball D. Amyloid beta-peptide(1-42) contributes to the oxidative stress and neurodegeneration found in Alzheimer disease brain. Brain Pathol. 2004;14(4):426–432. doi: 10.1111/j.1750-3639.2004.tb00087.x. [http://dx.doi.org/10.1111/j.1750-3639.2004.tb00087.x]. [PMID: 15605990]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Srinivasan V. Melatonin oxidative stress and neurodegenerative diseases. Indian J. Exp. Biol. 2002;40(6):668–679. [PMID: 12587715]. [PubMed] [Google Scholar]
- 150.Srinivasan V., Pandi-Perumal S.R., Maestroni G.J., Esquifino A.I., Hardeland R., Cardinali D.P. Role of melatonin in neurodegenerative diseases. Neurotox. Res. 2005;7(4):293–318. doi: 10.1007/BF03033887. [http://dx.doi.org/10.1007/BF03033887]. [PMID: 16179266]. [DOI] [PubMed] [Google Scholar]
- 151.Srinivasan V. Melatonin, oxidative stress and ageing. Curr. Sci. 1999;76:46–54. [Google Scholar]
- 152.Rodríguez M.I., Escames G., López L.C., López A., García J.A., Ortiz F., Sánchez V., Romeu M., Acuña-Castroviejo D. Improved mitochondrial function and increased life span after chronic melatonin treatment in senescent prone mice. Exp. Gerontol. 2008;43(8):749–756. doi: 10.1016/j.exger.2008.04.003. [http://dx.doi.org/10.1016/j.exger. 2008.04.003]. [PMID: 18485648]. [DOI] [PubMed] [Google Scholar]
- 153.Cipolla-Neto J., Amaral F.G., Afeche S.C., Tan D.X., Reiter R.J. Melatonin, energy metabolism, and obesity: a review. J. Pineal Res. 2014;56(4):371–381. doi: 10.1111/jpi.12137. [http://dx.doi.org/10.1111/jpi.12137]. [PMID: 24654916]. [DOI] [PubMed] [Google Scholar]
- 154.Vriend J., Sheppard M.S., Bala R.M. Melatonin increases serum insulin-like growth factor-I in male Syrian hamsters. Endocrinology. 1988;122(6):2558–2561. doi: 10.1210/endo-122-6-2558. [http://dx.doi.org/ 10.1210/endo-122-6-2558]. [PMID: 3371258]. [DOI] [PubMed] [Google Scholar]
- 155.Sivakumar V., Ling E.A., Lu J., Kaur C. Role of glutamate and its receptors and insulin-like growth factors in hypoxia induced periventricular white matter injury. Glia. 2010;58(5):507–523. doi: 10.1002/glia.20940. [http://dx.doi.org/10.1002/glia.20940]. [PMID: 19795501]. [DOI] [PubMed] [Google Scholar]
- 156.Garfinkel D., Laudon M., Nof D., Zisapel N. Improvement of sleep quality in elderly people by controlled-release melatonin. Lancet. 1995;346(8974):541–544. doi: 10.1016/s0140-6736(95)91382-3. [http://dx.doi.org/10.1016/ S0140-6736(95)91382-3]. [PMID: 7658780]. [DOI] [PubMed] [Google Scholar]
- 157.Anhê G.F., Caperuto L.C., Pereira-Da-Silva M., Souza L.C., Hirata A.E., Velloso L.A., Cipolla-Neto J., Carvalho C.R. In vivo activation of insulin receptor tyrosine kinase by melatonin in the rat hypothalamus. J. Neurochem. 2004;90(3):559–566. doi: 10.1111/j.1471-4159.2004.02514.x. [http://dx.doi.org/10.1111/j.1471-4159.2004.02514.x]. [PMID: 15255933]. [DOI] [PubMed] [Google Scholar]
- 158.Frölich L., Blum-Degen D., Bernstein H.G., Engelsberger S., Humrich J., Laufer S., Muschner D., Thalheimer A., Türk A., Hoyer S., Zöchling R., Boissl K.W., Jellinger K., Riederer P. Brain insulin and insulin receptors in aging and sporadic Alzheimer’s disease. J. Neural Transm. (Vienna) 1998;105(4-5):423–438. doi: 10.1007/s007020050068. [http://dx.doi.org/10.1007/s007020050068]. [PMID: 9720972]. [DOI] [PubMed] [Google Scholar]
- 159.Zenobi P.D., Graf S., Ursprung H., Froesch E.R. Effects of insulin-like growth factor-I on glucose tolerance, insulin levels, and insulin secretion. J. Clin. Invest. 1992;89(6):1908–1913. doi: 10.1172/JCI115796. [http://dx.doi.org/10.1172/JCI115796]. [PMID: 1601998]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Carro E., Torres-Aleman I. Insulin-like growth factor I and Alzheimer’s disease: therapeutic prospects? Expert Rev. Neurother. 2004;4(1):79–86. doi: 10.1586/14737175.4.1.79. [http://dx.doi.org/10.1586/ 14737175.4.1.79]. [PMID: 15853618]. [DOI] [PubMed] [Google Scholar]
- 161.Carro E., Torres-Aleman I. The role of insulin and insulin-like growth factor I in the molecular and cellular mechanisms underlying the pathology of Alzheimer’s disease. Eur. J. Pharmacol. 2004;490(1-3):127–133. doi: 10.1016/j.ejphar.2004.02.050. [http://dx.doi.org/10.1016/ j.ejphar.2004.02.050]. [PMID: 15094079]. [DOI] [PubMed] [Google Scholar]
- 162.Sharma S., Prasanthi R.P.J., Schommer E., Feist G., Ghribi O. Hypercholesterolemia-induced Abeta accumulation in rabbit brain is associated with alteration in IGF-1 signaling. Neurobiol. Dis. 2008;32(3):426–432. doi: 10.1016/j.nbd.2008.08.002. [http://dx.doi.org/10.1016/j.nbd.2008. 08.002]. [PMID: 18775495]. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 163.Dhar M., Dayal S.S., Ramesh Babu C.S., Arora S.R. Effect of melatonin on glucose tolerance and blood glucose circadian rhythm in rabbits. Indian J. Physiol. Pharmacol. 1983;27(2):109–117. [PMID: 6885122]. [PubMed] [Google Scholar]
- 164.Nduhirabandi F., Du Toit E.F., Blackhurst D., Marais D., Lochner A. Chronic melatonin consumption prevents obesity-related metabolic abnormalities and protects the heart against myocardial ischemia and reperfusion injury in a prediabetic model of diet-induced obesity. J. Pineal Res. 2011;50(2):171–182. doi: 10.1111/j.1600-079X.2010.00826.x. [PMID: 21073520]. [DOI] [PubMed] [Google Scholar]
- 165.Sparks D.L., Scheff S.W., Hunsaker J.C., III, Liu H., Landers T., Gross D.R. Induction of Alzheimer-like beta-amyloid immunoreactivity in the brains of rabbits with dietary cholesterol. Exp. Neurol. 1994;126(1):88–94. doi: 10.1006/exnr.1994.1044. [http://dx.doi.org/10.1006/ exnr.1994.1044]. [PMID: 8157129]. [DOI] [PubMed] [Google Scholar]
- 166.Refolo L.M., Pappolla M.A., LaFrancois J., Malester B., Schmidt S.D., Thomas-Bryant T., Tint G.S., Wang R., Mercken M., Petanceska S.S., Duff K.E. A cholesterol-lowering drug reduces beta-amyloid pathology in a transgenic mouse model of Alzheimer’s disease. Neurobiol. Dis. 2001;8(5):890–899. doi: 10.1006/nbdi.2001.0422. [http://dx.doi.org/10.1006/nbdi.2001.0422]. [PMID: 11592856]. [DOI] [PubMed] [Google Scholar]
- 167.Ghribi O., Golovko M.Y., Larsen B., Schrag M., Murphy E.J. Deposition of iron and beta-amyloid plaques is associated with cortical cellular damage in rabbits fed with long-term cholesterol-enriched diets. J. Neurochem. 2006;99(2):438–449. doi: 10.1111/j.1471-4159.2006.04079.x. [http://dx. doi.org/10.1111/j.1471-4159.2006.04079.x]. [PMID: 17029598]. [DOI] [PubMed] [Google Scholar]
- 168.Xiu J., Nordberg A., Qi X., Guan Z.Z. Influence of cholesterol and lovastatin on alpha-form of secreted amyloid precursor protein and expression of alpha7 nicotinic receptor on astrocytes. Neurochem. Int. 2006;49(5):459–465. doi: 10.1016/j.neuint.2006.03.007. [http://dx.doi.org/10.1016/ j.neuint.2006.03.007]. [PMID: 16675062]. [DOI] [PubMed] [Google Scholar]
- 169.Fassbender K., Simons M., Bergmann C., Stroick M., Lutjohann D., Keller P., Runz H., Kuhl S., Bertsch T., von Bergmann K., Hennerici M., Beyreuther K., Hartmann T. Simvastatin strongly reduces levels of Alzheimer’s disease beta -amyloid peptides Abeta 42 and Abeta 40 in vitro and in vivo. Proc. Natl. Acad. Sci. USA. 2001;98(10):5856–5861. doi: 10.1073/pnas.081620098. [http://dx.doi. org/10.1073/pnas.081620098]. [PMID: 11296263]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Poirier J. Apolipoprotein E and cholesterol metabolism in the pathogenesis and treatment of Alzheimer’s disease. Trends Mol. Med. 2003;9(3):94–101. doi: 10.1016/s1471-4914(03)00007-8. [http://dx.doi.org/10.1016/S1471-4914(03)00007-8]. [PMID: 12657430]. [DOI] [PubMed] [Google Scholar]
- 171.Hussain S.A. Effect of melatonin on cholesterol absorption in rats. J. Pineal Res. 2007;42(3):267–271. doi: 10.1111/j.1600-079X.2006.00415.x. [http://dx.doi.org/10.1111/ j.1600-079X.2006.00415.x]. [PMID: 17349025]. [DOI] [PubMed] [Google Scholar]
- 172.Pappolla M.A., Sos M., Omar R.A., Bick R.J., Hickson-Bick D.L., Reiter R.J., Efthimiopoulos S., Robakis N.K. Melatonin prevents death of neuroblastoma cells exposed to the Alzheimer amyloid peptide. J. Neurosci. 1997;17(5):1683–1690. doi: 10.1523/JNEUROSCI.17-05-01683.1997. [PMID: 9030627]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Reiter R.J., Tan D.X., Galano A. Melatonin reduces lipid peroxidation and membrane viscosity. Front. Physiol. 2014;5:377. doi: 10.3389/fphys.2014.00377. [http://dx.doi.org/10.3389/fphys.2014.00377]. [PMID: 25339906]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Firuzi O., Zhuo J., Chinnici C.M., Wisniewski T., Praticò D. 5-Lipoxygenase gene disruption reduces amyloid-beta pathology in a mouse model of Alzheimer’s disease. FASEB J. 2008;22(4):1169–1178. doi: 10.1096/fj.07-9131.com. [http://dx.doi.org/10.1096/fj.07-9131.com]. [PMID: 17998412]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Praticò D., Zhukareva V., Yao Y., Uryu K., Funk C.D., Lawson J.A., Trojanowski J.Q., Lee V.M. 12/15-lipoxygenase is increased in Alzheimer’s disease: possible involvement in brain oxidative stress. Am. J. Pathol. 2004;164(5):1655–1662. doi: 10.1016/S0002-9440(10)63724-8. [http:// dx.doi.org/10.1016/S0002-9440(10)63724-8]. [PMID: 15111312]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Manev H., Uz T., Sugaya K., Qu T. Putative role of neuronal 5-lipoxygenase in an aging brain. FASEB J. 2000;14(10):1464–1469. doi: 10.1096/fj.14.10.1464. [http://dx.doi.org/10.1096/fj.14.10.1464]. [PMID: 10877840]. [DOI] [PubMed] [Google Scholar]
- 177.Uz T., Longone P., Manev H. Increased hippocampal 5-lipoxygenase mRNA content in melatonin-deficient, pinealectomized rats. J. Neurochem. 1997;69(5):2220–2223. doi: 10.1046/j.1471-4159.1997.69052220.x. [http://dx.doi.org/10.1046/j.1471-4159.1997.69052220.x]. [PMID: 9349570]. [DOI] [PubMed] [Google Scholar]
- 178.Becker-André M., Wiesenberg I., Schaeren-Wiemers N., André E., Missbach M., Saurat J.H., Carlberg C. Pineal gland hormone melatonin binds and activates an orphan of the nuclear receptor superfamily. J. Biol. Chem. 1994;269(46):28531–28534. [PMID: 7961794]. [PubMed] [Google Scholar]
- 179.Wiesenberg I., Missbach M., Carlberg C. The potential role of the transcription factor RZR/ROR as a mediator of nuclear melatonin signaling. Restor. Neurol. Neurosci. 1998;12(2-3):143–150. [PMID: 12671309]. [PubMed] [Google Scholar]
- 180.Steinhilber D., Brungs M., Werz O., Wiesenberg I., Danielsson C., Kahlen J.P., Nayeri S., Schräder M., Carlberg C. The nuclear receptor for melatonin represses 5-lipoxygenase gene expression in human B lymphocytes. J. Biol. Chem. 1995;270(13):7037–7040. doi: 10.1074/jbc.270.13.7037. [http://dx.doi.org/10.1074/jbc.270.13.7037]. [PMID: 7706239]. [DOI] [PubMed] [Google Scholar]
- 181.Bojarski L., Herms J., Kuznicki J. Calcium dysregulation in Alzheimer’s disease. Neurochem. Int. 2008;52(4-5):621–633. doi: 10.1016/j.neuint.2007.10.002. [http://dx.doi.org/10.1016/j.neuint.2007.10.002]. [PMID: 18035450]. [DOI] [PubMed] [Google Scholar]
- 182.Green K.N., LaFerla F.M. Linking calcium to Abeta and Alzheimer’s disease. Neuron. 2008;59(2):190–194. doi: 10.1016/j.neuron.2008.07.013. [http://dx. doi.org/10.1016/j.neuron.2008.07.013]. [PMID: 18667147]. [DOI] [PubMed] [Google Scholar]
- 183.Wu J., Anwyl R., Rowan M.J. beta-Amyloid selectively augments NMDA receptor-mediated synaptic transmission in rat hippocampus. Neuroreport. 1995;6(17):2409–2413. doi: 10.1097/00001756-199511270-00031. [http://dx. doi.org/10.1097/00001756-199511270-00031]. [PMID: 8747164]. [DOI] [PubMed] [Google Scholar]
- 184.Escames G., León J., López L.C., Acuña-Castroviejo D. Mechanisms of N-methyl-D-aspartate receptor inhibition by melatonin in the rat striatum. J. Neuroendocrinol. 2004;16(11):929–935. doi: 10.1111/j.1365-2826.2004.01250.x. [http://dx.doi.org/10.1111/j.1365-2826.2004.01250.x]. [PMID: 15584934]. [DOI] [PubMed] [Google Scholar]
- 185.Andrabi S.A., Sayeed I., Siemen D., Wolf G., Horn T.F. Direct inhibition of the mitochondrial permeability transition pore: a possible mechanism responsible for anti-apoptotic effects of melatonin. FASEB J. 2004;18(7):869–871. doi: 10.1096/fj.03-1031fje. [PMID: 15033929]. [DOI] [PubMed] [Google Scholar]
- 186.Benítez-King G., Ríos A., Martínez A., Antón-Tay F. In vitro inhibition of Ca2+/calmodulin-dependent kinase II activity by melatonin. Biochim. Biophys. Acta. 1996;1290(2):191–196. doi: 10.1016/0304-4165(96)00025-6. [http:// dx.doi.org/10.1016/0304-4165(96)00025-6]. [PMID: 8645723]. [DOI] [PubMed] [Google Scholar]
- 187.Pozo D., Reiter R.J., Calvo J.R., Guerrero J.M. Inhibition of cerebellar nitric oxide synthase and cyclic GMP production by melatonin via complex formation with calmodulin. J. Cell. Biochem. 1997;65(3):430–442. doi: 10.1002/(sici)1097-4644(19970601)65:3<430::aid-jcb12>3.0.co;2-j. [http://dx.doi.org/10.1002/ (SICI)1097-4644(19970601)65:3<430:AID-JCB12>3.0.CO;2-J]. [PMID: 9138098]. [DOI] [PubMed] [Google Scholar]
- 188.Antón-Tay F., Ramírez G., Martínez I., Benítez-King G. In vitro stimulation of protein kinase C by melatonin. Neurochem. Res. 1998;23(5):601–606. doi: 10.1023/a:1022474402458. [http://dx.doi.org/10.1023/A:1022474402458]. [PMID: 9566597]. [DOI] [PubMed] [Google Scholar]
- 189.Johnson R.J., Xiao G., Shanmugaratnam J., Fine R.E. Calreticulin functions as a molecular chaperone for the beta-amyloid precursor protein. Neurobiol. Aging. 2001;22(3):387–395. doi: 10.1016/s0197-4580(00)00247-5. [http://dx.doi.org/10.1016/S0197-4580(00)00247-5]. [PMID: 11378243]. [DOI] [PubMed] [Google Scholar]
- 190.Macías M., Escames G., Leon J., Coto A., Sbihi Y., Osuna A., Acuña-Castroviejo D. Calreticulin-melatonin. An unexpected relationship. Eur. J. Biochem. 2003;270(5):832–840. doi: 10.1046/j.1432-1033.2003.03430.x. [http://dx. doi.org/10.1046/j.1432-1033.2003.03430.x]. [PMID: 12603316]. [DOI] [PubMed] [Google Scholar]
- 191.Moreira P.I., Carvalho C., Zhu X., Smith M.A., Perry G. Mitochondrial dysfunction is a trigger of Alzheimer’s disease pathophysiology. Biochim. Biophys. Acta. 2010;1802(1):2–10. doi: 10.1016/j.bbadis.2009.10.006. [http://dx.doi.org/10.1016/j.bbadis.2009.10.006]. [PMID: 19853658]. [DOI] [PubMed] [Google Scholar]
- 192.Wang X., Wang W., Li L., Perry G., Lee H.G., Zhu X. Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim. Biophys. Acta. 2014;1842(8):1240–1247. doi: 10.1016/j.bbadis.2013.10.015. [http:// dx.doi.org/10.1016/j.bbadis.2013.10.015]. [PMID: 24189435]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Paradies G., Petrosillo G., Paradies V., Reiter R.J., Ruggiero F.M. Melatonin, cardiolipin and mitochondrial bioenergetics in health and disease. J. Pineal Res. 2010;48(4):297–310. doi: 10.1111/j.1600-079X.2010.00759.x. [http://dx. doi.org/10.1111/j.1600-079X.2010.00759.x]. [PMID: 20433638]. [DOI] [PubMed] [Google Scholar]
- 194.Jou M.J., Peng T.I., Reiter R.J., Jou S.B., Wu H.Y., Wen S.T. Visualization of the antioxidative effects of melatonin at the mitochondrial level during oxidative stress-induced apoptosis of rat brain astrocytes. J. Pineal Res. 2004;37(1):55–70. doi: 10.1111/j.1600-079X.2004.00140.x. [http:// dx.doi.org/10.1111/j.1600-079X.2004.00140.x]. [PMID: 15230869]. [DOI] [PubMed] [Google Scholar]
- 195.Jou M.J., Peng T.I., Yu P.Z., Jou S.B., Reiter R.J., Chen J.Y., Wu H.Y., Chen C.C., Hsu L.F. Melatonin protects against common deletion of mitochondrial DNA-augmented mitochondrial oxidative stress and apoptosis. J. Pineal Res. 2007;43(4):389–403. doi: 10.1111/j.1600-079X.2007.00490.x. [http://dx.doi.org/10.1111/j.1600-079X.2007.00490.x]. [PMID: 17910608]. [DOI] [PubMed] [Google Scholar]
- 196.Shimohama S., Tanino H., Fujimoto S. Changes in caspase expression in Alzheimer’s disease: comparison with development and aging. Biochem. Biophys. Res. Commun. 1999;256(2):381–384. doi: 10.1006/bbrc.1999.0344. [http://dx.doi.org/10.1006/bbrc.1999.0344]. [PMID: 10079193]. [DOI] [PubMed] [Google Scholar]
- 197.Tesco G., Koh Y.H., Kang E.L., Cameron A.N., Das S., Sena-Esteves M., Hiltunen M., Yang S.H., Zhong Z., Shen Y., Simpkins J.W., Tanzi R.E. Depletion of GGA3 stabilizes BACE and enhances beta-secretase activity. Neuron. 2007;54(5):721–737. doi: 10.1016/j.neuron.2007.05.012. [http://dx.doi.org/10.1016/j.neuron.2007.05.012]. [PMID: 17553422]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Espino J., Bejarano I., Redondo P.C., Rosado J.A., Barriga C., Reiter R.J., Pariente J.A., Rodríguez A.B. Melatonin reduces apoptosis induced by calcium signaling in human leukocytes: Evidence for the involvement of mitochondria and Bax activation. J. Membr. Biol. 2010;233(1-3):105–118. doi: 10.1007/s00232-010-9230-0. [http://dx.doi.org/ 10.1007/s00232-010-9230-0]. [PMID: 20130848]. [DOI] [PubMed] [Google Scholar]
- 199.Louneva N., Cohen J.W., Han L.Y., Talbot K., Wilson R.S., Bennett D.A., Trojanowski J.Q., Arnold S.E. Caspase-3 is enriched in postsynaptic densities and increased in Alzheimer’s disease. Am. J. Pathol. 2008;173(5):1488–1495. doi: 10.2353/ajpath.2008.080434. [http://dx.doi. org/10.2353/ajpath.2008.080434]. [PMID: 18818379]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ling X., Zhang L.M., Lu S.D., Li X.J., Sun F.Y. Protective effect of melatonin on injuried cerebral neurons is associated with bcl-2 protein over-expression. Zhongguo Yao Li Xue Bao. 1999;20(5):409–414. [PMID: 10678086]. [PubMed] [Google Scholar]
- 201.Notarianni E. Hypercortisolemia and glucocorticoid receptor-signaling insufficiency in Alzheimer’s disease initiation and development. Curr. Alzheimer Res. 2013;10(7):714–731. doi: 10.2174/15672050113109990137. [http:// dx.doi.org/10.2174/15672050113109990137]. [PMID: 23906001]. [DOI] [PubMed] [Google Scholar]
- 202.Romijn H.J. The pineal, a tranquillizing organ? Life Sci. 1978;23(23):2257–2273. doi: 10.1016/0024-3205(78)90191-1. [http://dx.doi.org/10.1016/0024-3205(78)90191-1]. [PMID: 366320]. [DOI] [PubMed] [Google Scholar]
- 203.Sorenson D. An adventitious role of cortisol in degenerative processes due to decreased opposition by insulin: implications for aging. Med. Hypotheses. 1981;7(3):315–331. doi: 10.1016/0306-9877(81)90068-2. [http://dx.doi.org/ 10.1016/0306-9877(81)90068-2]. [PMID: 6261101]. [DOI] [PubMed] [Google Scholar]
- 204.Ferrari E., Arcaini A., Gornati R., Pelanconi L., Cravello L., Fioravanti M., Solerte S.B., Magri F. Pineal and pituitary-adrenocortical function in physiological aging and in senile dementia. Exp. Gerontol. 2000;35(9-10):1239–1250. doi: 10.1016/s0531-5565(00)00160-1. [http://dx. doi.org/10.1016/S0531-5565(00)00160-1]. [PMID: 11113605]. [DOI] [PubMed] [Google Scholar]
- 205.Furio A.M., Cutrera R.A., Castillo T.V., Pérez L.S., Riccio P., Caccuri R.L., Brusco L.L., Cardinali D.P. Effect of melatonin on changes in locomotor activity rhythm of Syrian hamsters injected with beta amyloid peptide 25-35 in the suprachiasmatic nuclei. Cell. Mol. Neurobiol. 2002;22(5-6):699–709. doi: 10.1023/A:1021805023906. [http://dx. doi.org/10.1023/A:1021805023906]. [PMID: 12585689]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Daniels W.M., van Rensburg S.J., van Zyl J.M., Taljaard J.J. Melatonin prevents beta-amyloid-induced lipid peroxidation. J. Pineal Res. 1998;24(2):78–82. doi: 10.1111/j.1600-079x.1998.tb00370.x. [http://dx.doi.org/10.1111/j.1600-079X.1998.tb00370.x]. [PMID: 9510431]. [DOI] [PubMed] [Google Scholar]
- 207.Pappolla M.A., Chyan Y.J., Poeggeler B., Bozner P., Ghiso J., LeDoux S.P., Wilson G.L. Alzheimer beta protein mediated oxidative damage of mitochondrial DNA: prevention by melatonin. J. Pineal Res. 1999;27(4):226–229. doi: 10.1111/j.1600-079x.1999.tb00619.x. [http://dx.doi.org/10.1111/j. 1600-079X.1999.tb00619.x]. [PMID: 10551770]. [DOI] [PubMed] [Google Scholar]
- 208.Olcese J.M., Cao C., Mori T., Mamcarz M.B., Maxwell A., Runfeldt M.J., Wang L., Zhang C., Lin X., Zhang G., Arendash G.W. Protection against cognitive deficits and markers of neurodegeneration by long-term oral administration of melatonin in a transgenic model of Alzheimer disease. J. Pineal Res. 2009;47(1):82–96. doi: 10.1111/j.1600-079X.2009.00692.x. [http://dx.doi.org/10.1111/j.1600-079X.2009.00692.x]. [PMID: 19538338]. [DOI] [PubMed] [Google Scholar]
- 209.Matsubara E., Bryant-Thomas T., Pacheco Quinto J., Henry T.L., Poeggeler B., Herbert D., Cruz-Sanchez F., Chyan Y.J., Smith M.A., Perry G., Shoji M., Abe K., Leone A., Grundke-Ikbal I., Wilson G.L., Ghiso J., Williams C., Refolo L.M., Pappolla M.A., Chain D.G., Neria E. Melatonin increases survival and inhibits oxidative and amyloid pathology in a transgenic model of Alzheimer’s disease. J. Neurochem. 2003;85(5):1101–1108. doi: 10.1046/j.1471-4159.2003.01654.x. [http://dx.doi.org/10.1046/j.1471-4159.2003. 01654.x]. [PMID: 12753069]. [DOI] [PubMed] [Google Scholar]
- 210.Song W., Lahiri D.K. Melatonin alters the metabolism of the beta-amyloid precursor protein in the neuroendocrine cell line PC12. J. Mol. Neurosci. 1997;9(2):75–92. doi: 10.1007/BF02736852. [http://dx.doi.org/10.1007/ BF02736852]. [PMID: 9407389]. [DOI] [PubMed] [Google Scholar]
- 211.Zhang Y.C., Wang Z.F., Wang Q., Wang Y.P., Wang J.Z. Melatonin attenuates beta-amyloid-induced inhibition of neurofilament expression. Acta Pharmacol. Sin. 2004;25(4):447–451. [PMID: 15066211]. [PubMed] [Google Scholar]
- 212.Wang X.C., Zhang Y.C., Chatterjie N., Grundke-Iqbal I., Iqbal K., Wang J.Z. Effect of melatonin and melatonylvalpromide on beta-amyloid and neurofilaments in N2a cells. Neurochem. Res. 2008;33(6):1138–1144. doi: 10.1007/s11064-007-9563-y. [http://dx.doi.org/10.1007/s11064-007-9563-y]. [PMID: 18231852]. [DOI] [PubMed] [Google Scholar]
- 213.Cheng X., van Breemen R.B. Mass spectrometry-based screening for inhibitors of beta-amyloid protein aggregation. Anal. Chem. 2005;77(21):7012–7015. doi: 10.1021/ac050556a. [http://dx.doi.org/10.1021/ac050556a]. [PMID: 16255603]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Masilamoni J.G., Jesudason E.P., Dhandayuthapani S., Ashok B.S., Vignesh S., Jebaraj W.C., Paul S.F., Jayakumar R. The neuroprotective role of melatonin against amyloid beta peptide injected mice. Free Radic. Res. 2008;42(7):661–673. doi: 10.1080/10715760802277388. [http://dx. doi.org/10.1080/10715760802277388]. [PMID: 18654881]. [DOI] [PubMed] [Google Scholar]
- 215.Pappolla M., Bozner P., Soto C., Shao H., Robakis N.K., Zagorski M., Frangione B., Ghiso J. Inhibition of Alzheimer beta-fibrillogenesis by melatonin. J. Biol. Chem. 1998;273(13):7185–7188. doi: 10.1074/jbc.273.13.7185. [http://dx.doi.org/10.1074/jbc.273.13.7185]. [PMID: 9516407]. [DOI] [PubMed] [Google Scholar]
- 216.Feng Z., Zhang J.T. Protective effect of melatonin on beta-amyloid-induced apoptosis in rat astroglioma C6 cells and its mechanism. Free Radic. Biol. Med. 2004;37(11):1790–1801. doi: 10.1016/j.freeradbiomed.2004.08.023. [http://dx.doi.org/10.1016/j.freeradbiomed.2004.08.023]. [PMID: 15528038]. [DOI] [PubMed] [Google Scholar]
- 217.Feng Z., Qin C., Chang Y., Zhang J.T. Early melatonin supplementation alleviates oxidative stress in a transgenic mouse model of Alzheimer’s disease. Free Radic. Biol. Med. 2006;40(1):101–109. doi: 10.1016/j.freeradbiomed.2005.08.014. [http://dx.doi.org/10.1016/j.freeradbiomed.2005.08.014]. [PMID: 16337883]. [DOI] [PubMed] [Google Scholar]
- 218.Pappolla M.A., Chyan Y.J., Poeggeler B., Frangione B., Wilson G., Ghiso J., Reiter R.J. An assessment of the antioxidant and the antiamyloidogenic properties of melatonin: implications for Alzheimer’s disease. J. Neural Transm. (Vienna) 2000;107(2):203–231. doi: 10.1007/s007020050018. [http://dx.doi.org/10.1007/s007020050018]. [PMID: 10847561]. [DOI] [PubMed] [Google Scholar]
- 219.Harada J., Sugimoto M. Activation of caspase-3 in beta-amyloid-induced apoptosis of cultured rat cortical neurons. Brain Res. 1999;842(2):311–323. doi: 10.1016/s0006-8993(99)01808-9. [http://dx.doi.org/10.1016/S0006-8993(99) 01808-9]. [PMID: 10526127]. [DOI] [PubMed] [Google Scholar]
- 220.Feng Z., Cheng Y., Zhang J.T. Long-term effects of melatonin or 17 beta-estradiol on improving spatial memory performance in cognitively impaired, ovariectomized adult rats. J. Pineal Res. 2004;37(3):198–206. doi: 10.1111/j.1600-079X.2004.00158.x. [http://dx.doi.org/10.1111/j.1600-079X. 2004.00158.x]. [PMID: 15357665]. [DOI] [PubMed] [Google Scholar]
- 221.Jang M.H., Jung S.B., Lee M.H., Kim C.J., Oh Y.T., Kang I., Kim J., Kim E.H. Melatonin attenuates amyloid beta25-35-induced apoptosis in mouse microglial BV2 cells. Neurosci. Lett. 2005;380(1-2):26–31. doi: 10.1016/j.neulet.2005.01.003. [http://dx.doi.org/10.1016/j.neulet.2005. 01.003]. [PMID: 15854745]. [DOI] [PubMed] [Google Scholar]
- 222.Quinn J., Kulhanek D., Nowlin J., Jones R., Praticò D., Rokach J., Stackman R. Chronic melatonin therapy fails to alter amyloid burden or oxidative damage in old Tg2576 mice: implications for clinical trials. Brain Res. 2005;1037(1-2):209–213. doi: 10.1016/j.brainres.2005.01.023. [http://dx.doi. org/10.1016/j.brainres.2005.01.023]. [PMID: 15777772]. [DOI] [PubMed] [Google Scholar]
- 223.Tan J., Town T., Paris D., Mori T., Suo Z., Crawford F., Mattson M.P., Flavell R.A., Mullan M. Microglial activation resulting from CD40-CD40L interaction after beta-amyloid stimulation. Science. 1999;286(5448):2352–2355. doi: 10.1126/science.286.5448.2352. [http://dx.doi. org/10.1126/science.286.5448.2352]. [PMID: 10600748]. [DOI] [PubMed] [Google Scholar]
- 224.Park S.Y., Jin M.L., Kim Y.H., Kim Y., Lee S.J. Anti-inflammatory effects of aromatic-turmerone through blocking of NF-κB, JNK, and p38 MAPK signaling pathways in amyloid β-stimulated microglia. Int. Immunopharmacol. 2012;14(1):13–20. doi: 10.1016/j.intimp.2012.06.003. [http://dx.doi.org/10.1016/j.intimp.2012.06.003]. [PMID: 22728094]. [DOI] [PubMed] [Google Scholar]
- 225.Meraz-Ríos M.A., Toral-Rios D., Franco-Bocanegra D., Villeda-Hernández J., Campos-Peña V. Inflammatory process in Alzheimer’s Disease. Front. Integr. Nuerosci. 2013;7:59. doi: 10.3389/fnint.2013.00059. [http://dx.doi.org/10.3389/fnint.2013.00059]. [PMID: 23964211]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Combs C.K., Karlo J.C., Kao S.C., Landreth G.E. beta-Amyloid stimulation of microglia and monocytes results in TNFalpha-dependent expression of inducible nitric oxide synthase and neuronal apoptosis. J. Neurosci. 2001;21(4):1179–1188. doi: 10.1523/JNEUROSCI.21-04-01179.2001. [PMID: 11160388]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Hardy J.A., Higgins G.A. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256(5054):184–185. doi: 10.1126/science.1566067. [http://dx.doi.org/10.1126/science.1566067]. [PMID: 1566067]. [DOI] [PubMed] [Google Scholar]
- 228.Sastre M., Dewachter I., Landreth G.E., Willson T.M., Klockgether T., van Leuven F., Heneka M.T. Nonsteroidal anti-inflammatory drugs and peroxisome proliferator-activated receptor-gamma agonists modulate immunostimulated processing of amyloid precursor protein through regulation of beta-secretase. J. Neurosci. 2003;23(30):9796–9804. doi: 10.1523/JNEUROSCI.23-30-09796.2003. [PMID: 14586007]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Mauriz J.L., Collado P.S., Veneroso C., Reiter R.J., González-Gallego J. A review of the molecular aspects of melatonin’s anti-inflammatory actions: recent insights and new perspectives. J. Pineal Res. 2013;54(1):1–14. doi: 10.1111/j.1600-079X.2012.01014.x. [http://dx.doi.org/10.1111/j.1600-079X.2012.01014.x]. [PMID: 22725668]. [DOI] [PubMed] [Google Scholar]
- 230.Dong Y., Fan C., Hu W., Jiang S., Ma Z., Yan X., Deng C., Di S., Xin Z., Wu G., Yang Y., Reiter R.J., Liang G. Melatonin attenuated early brain injury induced by subarachnoid hemorrhage via regulating NLRP3 inflammasome and apoptosis signaling. J. Pineal Res. 2016;60(3):253–262. doi: 10.1111/jpi.12300. [http://dx.doi.org/10.1111/ jpi.12300]. [PMID: 26639408]. [DOI] [PubMed] [Google Scholar]
- 231.Mohan N., Sadeghi K., Reiter R.J., Meltz M.L. The neurohormone melatonin inhibits cytokine, mitogen and ionizing radiation induced NF-kappa B. Biochem. Mol. Biol. Int. 1995;37(6):1063–1070. [PMID: 8747536]. [PubMed] [Google Scholar]
- 232.Chuang J.I., Mohan N., Meltz M.L., Reiter R.J. Effect of melatonin on NF-kappa-B DNA-binding activity in the rat spleen. Cell Biol. Int. 1996;20(10):687–692. doi: 10.1006/cbir.1996.0091. [http://dx.doi.org/10.1006/ cbir.1996.0091]. [PMID: 8969462]. [DOI] [PubMed] [Google Scholar]
- 233.Cardinali D.P., Ritta M.N., Fuentes A.M., Gimeno M.F., Gimeno A.L. Prostaglandin E release by rat medial basal hypothalamus in vitro. Inhibition by melatonin at submicromolar concentrations. Eur. J. Pharmacol. 1980;67(1):151–153. doi: 10.1016/0014-2999(80)90025-4. [http:// dx.doi.org/10.1016/0014-2999(80)90025-4]. [PMID: 7418729]. [DOI] [PubMed] [Google Scholar]
- 234.Deng W.G., Tang S.T., Tseng H.P., Wu K.K. Melatonin suppresses macrophage cyclooxygenase-2 and inducible nitric oxide synthase expression by inhibiting p52 acetylation and binding. Blood. 2006;108(2):518–524. doi: 10.1182/blood-2005-09-3691. [http://dx.doi.org/10.1182/ blood-2005-09-3691]. [PMID: 16609073]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Shen Y., Zhang G., Liu L., Xu S. Suppressive effects of melatonin on amyloid-beta-induced glial activation in rat hippocampus. Arch. Med. Res. 2007;38(3):284–290. doi: 10.1016/j.arcmed.2006.10.007. [http://dx. doi.org/10.1016/j.arcmed.2006.10.007]. [PMID: 17350477]. [DOI] [PubMed] [Google Scholar]
- 236.Clapp-Lilly K.L., Smith M.A., Perry G., Duffy L.K. Melatonin reduces interleukin secretion in amyloid-beta stressed mouse brain slices. Chem. Biol. Interact. 2001;134(1):101–107. doi: 10.1016/s0009-2797(00)00319-7. [http://dx.doi. org/10.1016/S0009-2797(00)00319-7]. [PMID: 11248225]. [DOI] [PubMed] [Google Scholar]
- 237.Zhou F., Zhang L., Wang A., Song B., Gong K., Zhang L., Hu M., Zhang X., Zhao N., Gong Y. The association of GSK3 beta with E2F1 facilitates nerve growth factor-induced neural cell differentiation. J. Biol. Chem. 2008;283(21):14506–14515. doi: 10.1074/jbc.M706136200. [http://dx.doi.org/10.1074/jbc.M706136200]. [PMID: 18367454]. [DOI] [PubMed] [Google Scholar]
- 238.Hardeland R., Cardinali D.P., Srinivasan V., Spence D.W., Brown G.M., Pandi-Perumal S.R. Melatonin--a pleiotropic, orchestrating regulator molecule. Prog. Neurobiol. 2011;93(3):350–384. doi: 10.1016/j.pneurobio.2010.12.004. [http://dx.doi.org/10.1016/j.pneurobio.2010.12.004]. [PMID: 21193011]. [DOI] [PubMed] [Google Scholar]
- 239.McArthur A.J., Hunt A.E., Gillette M.U. Melatonin action and signal transduction in the rat suprachiasmatic circadian clock: activation of protein kinase C at dusk and dawn. Endocrinology. 1997;138(2):627–634. doi: 10.1210/endo.138.2.4925. [http://dx.doi.org/10.1210/endo.138.2.4925]. [PMID: 9002996]. [DOI] [PubMed] [Google Scholar]
- 240.Chetsawang B., Govitrapong P., Ebadi M. The neuroprotective effect of melatonin against the induction of c-Jun phosphorylation by 6-hydroxydopamine on SK-N-SH cells. Neurosci. Lett. 2004;371(2-3):205–208. doi: 10.1016/j.neulet.2004.08.068. [http://dx.doi.org/10.1016/j.neulet.2004.08.068]. [PMID: 15519758]. [DOI] [PubMed] [Google Scholar]
- 241.Kwon K.J., Kim H.J., Shin C.Y., Han S.H. Melatonin potentiates the neuroprotective properties of resveratrol against beta-amyloid-induced neurodegeneration by modulating AMP-activated protein kinase pathways. J. Clin. Neurol. 2010;6(3):127–137. doi: 10.3988/jcn.2010.6.3.127. [http://dx. doi.org/10.3988/jcn.2010.6.3.127]. [PMID: 20944813]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Luchetti F., Betti M., Canonico B., Arcangeletti M., Ferri P., Galli F., Papa S. ERK MAPK activation mediates the antiapoptotic signaling of melatonin in UVB-stressed U937 cells. Free Radic. Biol. Med. 2009;46(3):339–351. doi: 10.1016/j.freeradbiomed.2008.09.017. [http://dx.doi.org/10. 1016/j.freeradbiomed.2008.09.017]. [PMID: 18930812]. [DOI] [PubMed] [Google Scholar]
- 243.Tajes M., Gutierrez-Cuesta J., Ortuño-Sahagun D., Camins A., Pallàs M. Anti-aging properties of melatonin in an in vitro murine senescence model: involvement of the sirtuin 1 pathway. J. Pineal Res. 2009;47(3):228–237. doi: 10.1111/j.1600-079X.2009.00706.x. [http://dx.doi.org/10.1111/j.1600-079X.2009.00706.x]. [PMID: 19650880]. [DOI] [PubMed] [Google Scholar]
- 244.Deng W., Aimone J.B., Gage F.H. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat. Rev. Neurosci. 2010;11(5):339–350. doi: 10.1038/nrn2822. [http://dx.doi.org/10.1038/nrn2822]. [PMID: 20354534]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Coras R., Siebzehnrubl F.A., Pauli E., Huttner H.B., Njunting M., Kobow K., Villmann C., Hahnen E., Neuhuber W., Weigel D., Buchfelder M., Stefan H., Beck H., Steindler D.A., Blümcke I. Low proliferation and differentiation capacities of adult hippocampal stem cells correlate with memory dysfunction in humans. Brain. 2010;133(11):3359–3372. doi: 10.1093/brain/awq215. [http://dx.doi.org/ 10.1093/brain/awq215]. [PMID: 20719879]. [DOI] [PubMed] [Google Scholar]
- 246.Haughey N.J., Liu D., Nath A., Borchard A.C., Mattson M.P. Disruption of neurogenesis in the subventricular zone of adult mice, and in human cortical neuronal precursor cells in culture, by amyloid beta-peptide: implications for the pathogenesis of Alzheimer’s disease. Neuromolecular Med. 2002;1(2):125–135. doi: 10.1385/NMM:1:2:125. [http://dx.doi.org/10.1385/NMM:1:2:125]. [PMID: 12025858]. [DOI] [PubMed] [Google Scholar]
- 247.Donovan M.H., Yazdani U., Norris R.D., Games D., German D.C., Eisch A.J. Decreased adult hippocampal neurogenesis in the PDAPP mouse model of Alzheimer’s disease. J. Comp. Neurol. 2006;495(1):70–83. doi: 10.1002/cne.20840. [http://dx.doi.org/10.1002/cne.20840]. [PMID: 16432899]. [DOI] [PubMed] [Google Scholar]
- 248.Wen P.H., Hof P.R., Chen X., Gluck K., Austin G., Younkin S.G., Younkin L.H., DeGasperi R., Gama Sosa M.A., Robakis N.K., Haroutunian V., Elder G.A. The presenilin-1 familial Alzheimer disease mutant P117L impairs neurogenesis in the hippocampus of adult mice. Exp. Neurol. 2004;188(2):224–237. doi: 10.1016/j.expneurol.2004.04.002. [http://dx.doi.org/10.1016/j.expneurol.2004.04.002]. [PMID: 15246822]. [DOI] [PubMed] [Google Scholar]
- 249.Mu Y., Gage F.H. Adult hippocampal neurogenesis and its role in Alzheimer’s disease. Mol. Neurodegener. 2011;6:85. doi: 10.1186/1750-1326-6-85. [http://dx.doi.org/10.1186/1750-1326-6-85]. [PMID: 22192775]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Rodríguez J.J., Jones V.C., Tabuchi M., Allan S.M., Knight E.M., LaFerla F.M., Oddo S., Verkhratsky A. Impaired adult neurogenesis in the dentate gyrus of a triple transgenic mouse model of Alzheimer’s disease. PLoS One. 2008;3(8):e2935. doi: 10.1371/journal.pone.0002935. [http://dx.doi.org/10.1371/journal.pone.0002935]. [PMID: 18698410]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Hamilton A., Holscher C. The effect of ageing on neurogenesis and oxidative stress in the APP(swe)/PS1(deltaE9) mouse model of Alzheimer’s disease. Brain Res. 2012;1449:83–93. doi: 10.1016/j.brainres.2012.02.015. [http://dx. doi.org/10.1016/j.brainres.2012.02.015]. [PMID: 22418058]. [DOI] [PubMed] [Google Scholar]
- 252.Haughey N.J., Nath A., Chan S.L., Borchard A.C., Rao M.S., Mattson M.P. Disruption of neurogenesis by amyloid beta-peptide, and perturbed neural progenitor cell homeostasis, in models of Alzheimer’s disease. J. Neurochem. 2002;83(6):1509–1524. doi: 10.1046/j.1471-4159.2002.01267.x. [http://dx.doi.org/10.1046/j.1471-4159.2002.01267.x]. [PMID: 12472904]. [DOI] [PubMed] [Google Scholar]
- 253.Martinez-Canabal A. Reconsidering hippocampal neurogenesis in Alzheimer’s disease. Front. Neurosci. 2014;8:147. doi: 10.3389/fnins.2014.00147. [http://dx. doi.org/10.3389/fnins.2014.00147]. [PMID: 24966809]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Ghosal K., Stathopoulos A., Pimplikar S.W. APP intracellular domain impairs adult neurogenesis in transgenic mice by inducing neuroinflammation. PLoS One. 2010;5(7):e11866. doi: 10.1371/journal.pone.0011866. [http://dx. doi.org/10.1371/journal.pone.0011866]. [PMID: 20689579]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Porayette P., Gallego M.J., Kaltcheva M.M., Bowen R.L., Vadakkadath M.S., Atwood C.S. Differential processing of amyloid-beta precursor protein directs human embryonic stem cell proliferation and differentiation into neuronal precursor cells. J. Biol. Chem. 2009;284(35):23806–23817. doi: 10.1074/jbc.M109.026328. [http://dx.doi.org/ 10.1074/jbc.M109.026328]. [PMID: 19542221]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Chen Y., Bodles A.M. Amyloid precursor protein modulates beta-catenin degradation. J. Neuroinflammation. 2007;4:29. doi: 10.1186/1742-2094-4-29. [http://dx.doi.org/10.1186/1742-2094-4-29]. [PMID: 18070361]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.He P., Shen Y. Interruption of beta-catenin signaling reduces neurogenesis in Alzheimer’s disease. J. Neurosci. 2009;29(20):6545–6557. doi: 10.1523/JNEUROSCI.0421-09.2009. [http://dx.doi.org/10.1523/JNEUROSCI.0421-09.2009]. [PMID: 19458225]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Phiel C.J., Wilson C.A., Lee V.M., Klein P.S. GSK-3alpha regulates production of Alzheimer’s disease amyloid-beta peptides. Nature. 2003;423(6938):435–439. doi: 10.1038/nature01640. [http://dx.doi.org/10.1038/ nature01640]. [PMID: 12761548]. [DOI] [PubMed] [Google Scholar]
- 259.Anderton B.H., Dayanandan R., Killick R., Lovestone S. Does dysregulation of the Notch and wingless/Wnt pathways underlie the pathogenesis of Alzheimer’s disease? Mol. Med. Today. 2000;6(2):54–59. doi: 10.1016/s1357-4310(99)01640-8. [http://dx.doi.org/10.1016/S1357-4310(99)01640-8]. [PMID: 10652477]. [DOI] [PubMed] [Google Scholar]
- 260.Lie D.C., Colamarino S.A., Song H.J., Désiré L., Mira H., Consiglio A., Lein E.S., Jessberger S., Lansford H., Dearie A.R., Gage F.H. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437(7063):1370–1375. doi: 10.1038/nature04108. [http://dx.doi. org/10.1038/nature04108]. [PMID: 16251967]. [DOI] [PubMed] [Google Scholar]
- 261.Alvarez A.R., Godoy J.A., Mullendorff K., Olivares G.H., Bronfman M., Inestrosa N.C. Wnt-3a overcomes beta-amyloid toxicity in rat hippocampal neurons. Exp. Cell Res. 2004;297(1):186–196. doi: 10.1016/j.yexcr.2004.02.028. [http://dx.doi.org/10.1016/j.yexcr.2004.02.028]. [PMID: 15194435]. [DOI] [PubMed] [Google Scholar]
- 262.Ramírez-Rodríguez G., Vega-Rivera N.M., Benítez-King G., Castro-García M., Ortíz-López L. Melatonin supplementation delays the decline of adult hippocampal neurogenesis during normal aging of mice. Neurosci. Lett. 2012;530(1):53–58. doi: 10.1016/j.neulet.2012.09.045. [http://dx.doi.org/10.1016/j.neulet.2012.09.045]. [PMID: 23043890]. [DOI] [PubMed] [Google Scholar]
- 263.Niles L.P., Armstrong K.J., Rincón Castro L.M., Dao C.V., Sharma R., McMillan C.R., Doering L.C., Kirkham D.L. Neural stem cells express melatonin receptors and neurotrophic factors: colocalization of the MT1 receptor with neuronal and glial markers. BMC Neurosci. 2004;5:41. doi: 10.1186/1471-2202-5-41. [http://dx.doi.org/10.1186/1471-2202-5-41]. [PMID: 15511288]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Kong X., Li X., Cai Z., Yang N., Liu Y., Shu J., Pan L. ; Zuo P. Melatonin regulates the viability and differentiation of rat midbrain neural stem cells. Cell. Mol. Neurobiol. 2008;28(4):569–579. doi: 10.1007/s10571-007-9212-7. [http://dx.doi.org/10.1007/s10571-007-9212-7]. [PMID: 17912627]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Lacoste B., Angeloni D., Dominguez-Lopez S., Calderoni S., Mauro A., Fraschini F., Descarries L., Gobbi G. Anatomical and cellular localization of melatonin MT1 and MT2 receptors in the adult rat brain. J. Pineal Res. 2015;58(4):397–417. doi: 10.1111/jpi.12224. [http://dx. doi.org/10.1111/jpi.12224]. [PMID: 25726952]. [DOI] [PubMed] [Google Scholar]
- 266.Srinivasan V., Zakaria R., Othman Z., Lauterbach E.C., Acuña-Castroviejo D. Agomelatine in depressive disorders: its novel mechanisms of action. J. Neuropsychiatry Clin. Neurosci. 2012;24(3):290–308. doi: 10.1176/appi.neuropsych.11090216. [http://dx.doi.org/10.1176/appi.neuropsych. 11090216]. [PMID: 23037643]. [DOI] [PubMed] [Google Scholar]
- 267.Soumier A., Banasr M., Lortet S., Masmejean F., Bernard N., Kerkerian-Le-Goff L., Gabriel C., Millan M.J., Mocaer E., Daszuta A. Mechanisms contributing to the phase-dependent regulation of neurogenesis by the novel antidepressant, agomelatine, in the adult rat hippocampus. Neuropsychopharmacology. 2009;34(11):2390–2403. doi: 10.1038/npp.2009.72. [http://dx.doi.org/10.1038/npp.2009.72]. [PMID: 19571795]. [DOI] [PubMed] [Google Scholar]
- 268.Fava M., Targum S.D., Nierenberg A.A., Bleicher L.S., Carter T.A., Wedel P.C., Hen R., Gage F.H., Barlow C. An exploratory study of combination buspirone and melatonin SR in major depressive disorder (MDD): a possible role for neurogenesis in drug discovery. J. Psychiatr. Res. 2012;46(12):1553–1563. doi: 10.1016/j.jpsychires.2012.08.013. [http:// dx.doi.org/10.1016/j.jpsychires.2012.08.013]. [PMID: 22998742]. [DOI] [PubMed] [Google Scholar]
- 269.Domínguez-Alonso A., Ramírez-Rodríguez G., Benítez-King G. Melatonin increases dendritogenesis in the hilus of hippocampal organotypic cultures. J. Pineal Res. 2012;52(4):427–436. doi: 10.1111/j.1600-079X.2011.00957.x. [http:// dx.doi.org/10.1111/j.1600-079X.2011.00957.x]. [PMID: 22257024]. [DOI] [PubMed] [Google Scholar]
- 270.Ramirez-Rodriguez G., Ortíz-López L., Domínguez-Alonso A., Benítez-King G.A., Kempermann G. Chronic treatment with melatonin stimulates dendrite maturation and complexity in adult hippocampal neurogenesis of mice. J. Pineal Res. 2011;50(1):29–37. doi: 10.1111/j.1600-079X.2010.00802.x. [http://dx.doi.org/10.1111/j.1600-079X.2010.00802.x]. [PMID: 20880317]. [DOI] [PubMed] [Google Scholar]
- 271.Liu J., Somera-Molina K.C., Hudson R.L., Dubocovich M.L. Melatonin potentiates running wheel-induced neurogenesis in the dentate gyrus of adult C3H/HeN mice hippocampus. J. Pineal Res. 2013;54(2):222–231. doi: 10.1111/jpi.12023. [http://dx.doi.org/10.1111/jpi.12023]. [PMID: 23190173]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Hong Y., Palaksha K.J., Park K., Park S., Kim H.D., Reiter R.J., Chang K.T. Melatonin plus exercise-based neuro-rehabilitative therapy for spinal cord injury. J. Pineal Res. 2010;49(3):201–209. doi: 10.1111/j.1600-079X.2010.00786.x. [http://dx.doi.org/10.1111/j.1600-079X.2010. 00786.x]. [PMID: 20626592]. [DOI] [PubMed] [Google Scholar]
- 273.Moriya T., Horie N., Mitome M., Shinohara K. Melatonin influences the proliferative and differentiative activity of neural stem cells. J. Pineal Res. 2007;42(4):411–418. doi: 10.1111/j.1600-079X.2007.00435.x. [http://dx.doi. org/10.1111/j.1600-079X.2007.00435.x]. [PMID: 17439558]. [DOI] [PubMed] [Google Scholar]
- 274.Ramírez-Rodríguez G., Klempin F., Babu H., Benítez-King G., Kempermann G. Melatonin modulates cell survival of new neurons in the hippocampus of adult mice. Neuropsychopharmacology. 2009;34(9):2180–2191. doi: 10.1038/npp.2009.46. [http://dx.doi.org/10.1038/npp.2009.46]. [PMID: 19421166]. [DOI] [PubMed] [Google Scholar]
- 275.De Butte M., Pappas B.A. Pinealectomy causes hippocampal CA1 and CA3 cell loss: reversal by melatonin supplementation. Neurobiol. Aging. 2007;28(2):306–313. doi: 10.1016/j.neurobiolaging.2005.12.004. [http://dx.doi.org/10.1016/ j.neurobiolaging.2005.12.004]. [PMID: 16412535]. [DOI] [PubMed] [Google Scholar]
- 276.Rennie K., De Butte M., Pappas B.A. Melatonin promotes neurogenesis in dentate gyrus in the pinealectomized rat. J. Pineal Res. 2009;47(4):313–317. doi: 10.1111/j.1600-079X.2009.00716.x. [http://dx.doi.org/10.1111/j.1600-079X.2009.00716.x]. [PMID: 19796045]. [DOI] [PubMed] [Google Scholar]
- 277.Tocharus C., Puriboriboon Y., Junmanee T., Tocharus J., Ekthuwapranee K., Govitrapong P. Melatonin enhances adult rat hippocampal progenitor cell proliferation via ERK signaling pathway through melatonin receptor. Neuroscience. 2014;275:314–321. doi: 10.1016/j.neuroscience.2014.06.026. [http://dx.doi.org/10.1016/j.neuroscience.2014.06.026]. [PMID: 24956284]. [DOI] [PubMed] [Google Scholar]
- 278.Sotthibundhu A., Phansuwan-Pujito P., Govitrapong P. Melatonin increases proliferation of cultured neural stem cells obtained from adult mouse subventricular zone. J. Pineal Res. 2010;49(3):291–300. doi: 10.1111/j.1600-079X.2010.00794.x. [http://dx.doi.org/10.1111/j.1600-079X. 2010.00794.x]. [PMID: 20663047]. [DOI] [PubMed] [Google Scholar]
- 279.Singhakumar R., Boontem P., Ekthuwapranee K., Sotthibundhu A., Mukda S., Chetsawang B., Govitrapong P. Melatonin attenuates methamphetamine-induced inhibition of neurogenesis in the adult mouse hippocampus: An in vivo study. Neurosci. Lett. 2015;606:209–214. doi: 10.1016/j.neulet.2015.09.011. [http://dx.doi.org/10.1016/j.neulet.2015.09. 011]. [PMID: 26366944]. [DOI] [PubMed] [Google Scholar]
- 280.Ruksee N., Tongjaroenbuangam W., Mahanam T., Govitrapong P. Melatonin pretreatment prevented the effect of dexamethasone negative alterations on behavior and hippocampal neurogenesis in the mouse brain. J. Steroid Biochem. Mol. Biol. 2014;143:72–80. doi: 10.1016/j.jsbmb.2014.02.011. [http://dx.doi.org/10.1016/j.jsbmb.2014.02.011]. [PMID: 24589478]. [DOI] [PubMed] [Google Scholar]
- 281.Jeong J.K., Lee J.H., Moon J.H., Lee Y.J., Park S.Y. Melatonin-mediated β-catenin activation protects neuron cells against prion protein-induced neurotoxicity. J. Pineal Res. 2014;57(4):427–434. doi: 10.1111/jpi.12182. [http://dx.doi.org/10.1111/jpi.12182]. [PMID: 25251028]. [DOI] [PubMed] [Google Scholar]
- 282.Kaneko N., Okano H., Sawamoto K. Role of the cholinergic system in regulating survival of newborn neurons in the adult mouse dentate gyrus and olfactory bulb. Genes Cells. 2006;11(10):1145–1159. doi: 10.1111/j.1365-2443.2006.01010.x. [http://dx.doi.org/10.1111/j.1365-2443.2006.01010.x]. [PMID: 16999735]. [DOI] [PubMed] [Google Scholar]
- 283.Agrawal R., Tyagi E., Shukla R., Nath C. Effect of insulin and melatonin on acetylcholinesterase activity in the brain of amnesic mice. Behav. Brain Res. 2008;189(2):381–386. doi: 10.1016/j.bbr.2008.01.015. [http://dx.doi. org/10.1016/j.bbr.2008.01.015]. [PMID: 18336929]. [DOI] [PubMed] [Google Scholar]
- 284.Shukla M., Htoo H.H., Wintachai P., Hernandez J.F., Dubois C., Postina R., Xu H., Checler F., Smith D.R., Govitrapong P., Vincent B. Melatonin stimulates the nonamyloidogenic processing of βAPP through the positive transcriptional regulation of ADAM10 and ADAM17. J. Pineal Res. 2015;58(2):151–165. doi: 10.1111/jpi.12200. [http://dx.doi.org/10.1111/jpi.12200]. [PMID: 25491598]. [DOI] [PubMed] [Google Scholar]
- 285.Demars M.P., Bartholomew A., Strakova Z., Lazarov O. Soluble amyloid precursor protein: a novel proliferation factor of adult progenitor cells of ectodermal and mesodermal origin. Stem Cell Res. Ther. 2011;2(4):36. doi: 10.1186/scrt77. [http://dx.doi.org/10.1186/scrt77]. [PMID: 21878106]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Ishida A., Furukawa K., Keller J.N., Mattson M.P. Secreted form of beta-amyloid precursor protein shifts the frequency dependency for induction of LTD, and enhances LTP in hippocampal slices. Neuroreport. 1997;8(9-10):2133–2137. doi: 10.1097/00001756-199707070-00009. [http://dx.doi.org/10.1097/00001756-199707070-00009]. [PMID: 9243598]. [DOI] [PubMed] [Google Scholar]
- 287.Yuan T.F., Gu S., Shan C., Marchado S., Arias-Carrión O. Oxidative Stress and Adult Neurogenesis. Stem Cell Rev. 2015;11(5):706–709. doi: 10.1007/s12015-015-9603-y. [http://dx.doi.org/10.1007/s12015-015-9603-y]. [PMID: 26100529]. [DOI] [PubMed] [Google Scholar]
- 288.Sarlak G., Jenwitheesuk A., Chetsawang B., Govitrapong P. Effects of melatonin on nervous system aging: neurogenesis and neurodegeneration. J. Pharmacol. Sci. 2013;123(1):9–24. doi: 10.1254/jphs.13r01sr. [http://dx.doi.org/10.1254/jphs.13R01SR]. [PMID: 23985544]. [DOI] [PubMed] [Google Scholar]
- 289.Lopez-Perez E., Zhang Y., Frank S.J., Creemers J., Seidah N., Checler F. Constitutive alpha-secretase cleavage of the beta-amyloid precursor protein in the furin-deficient LoVo cell line: involvement of the pro-hormone convertase 7 and the disintegrin metalloprotease ADAM10. J. Neurochem. 2001;76(5):1532–1539. doi: 10.1046/j.1471-4159.2001.00180.x. [http://dx.doi.org/10.1046/j.1471-4159.2001.00180.x]. [PMID: 11238737]. [DOI] [PubMed] [Google Scholar]
- 290.Vincent B., Checler F. α-Secretase in Alzheimer’s disease and beyond: mechanistic, regulation and function in the shedding of membrane proteins. Curr. Alzheimer Res. 2012;9(2):140–156. doi: 10.2174/156720512799361646. [http://dx.doi.org/10.2174/156720512799361646]. [PMID: 21605031]. [DOI] [PubMed] [Google Scholar]
- 291.Bandyopadhyay S., Goldstein L.E., Lahiri D.K., Rogers J.T. Role of the APP non-amyloidogenic signaling pathway and targeting alpha-secretase as an alternative drug target for treatment of Alzheimer’s disease. Curr. Med. Chem. 2007;14(27):2848–2864. doi: 10.2174/092986707782360060. [http://dx.doi.org/10.2174/092986707782360060]. [PMID: 18045131]. [DOI] [PubMed] [Google Scholar]
- 292.Vassar R., Bennett B.D., Babu-Khan S., Kahn S., Mendiaz E.A., Denis P., Teplow D.B., Ross S., Amarante P., Loeloff R., Luo Y., Fisher S., Fuller J., Edenson S., Lile J., Jarosinski M.A., Biere A.L., Curran E., Burgess T., Louis J.C., Collins F., Treanor J., Rogers G., Citron M. Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286(5440):735–741. doi: 10.1126/science.286.5440.735. [http://dx.doi.org/10.1126/science.286.5440.735]. [PMID: 10531052]. [DOI] [PubMed] [Google Scholar]
- 293.Tyler S.J., Dawbarn D., Wilcock G.K., Allen S.J. alpha- and beta-secretase: profound changes in Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2002;299(3):373–376. doi: 10.1016/s0006-291x(02)02635-9. [http://dx.doi.org/ 10.1016/S0006-291X(02)02635-9]. [PMID: 12445809]. [DOI] [PubMed] [Google Scholar]
- 294.Bernstein H.G., Bukowska A., Krell D., Bogerts B., Ansorge S., Lendeckel U. Comparative localization of ADAMs 10 and 15 in human cerebral cortex normal aging, Alzheimer disease and Down syndrome. J. Neurocytol. 2003;32(2):153–160. doi: 10.1023/b:neur.0000005600.61844.a6. [http://dx.doi.org/ 10.1023/B:NEUR.0000005600.61844.a6]. [PMID: 14707550]. [DOI] [PubMed] [Google Scholar]
- 295.Colciaghi F., Marcello E., Borroni B., Zimmermann M., Caltagirone C., Cattabeni F., Padovani A., Di Luca M. Platelet APP, ADAM 10 and BACE alterations in the early stages of Alzheimer disease. Neurology. 2004;62(3):498–501. doi: 10.1212/01.wnl.0000106953.49802.9c. [http://dx.doi. org/10.1212/01.WNL.0000106953.49802.9C]. [PMID: 14872043]. [DOI] [PubMed] [Google Scholar]
- 296.Colciaghi F., Borroni B., Pastorino L., Marcello E., Zimmermann M., Cattabeni F., Padovani A., Di Luca M. [alpha]-Secretase ADAM10 as well as [alpha]APPs is reduced in platelets and CSF of Alzheimer disease patients. Mol. Med. 2002;8(2):67–74. [PMID: 12080182]. [PMC free article] [PubMed] [Google Scholar]
- 297.Chow V.W., Savonenko A.V., Melnikova T., Kim H., Price D.L., Li T., Wong P.C. Modeling an anti-amyloid combination therapy for Alzheimer’s disease. Sci. Transl. Med. 2010;2(13):13ra1. doi: 10.1126/scitranslmed.3000337. [http://dx.doi.org/10.1126/scitranslmed.3000337]. [PMID: 20371462]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Nikolaev A., McLaughlin T., O’Leary D.D., Tessier-Lavigne M. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature. 2009;457(7232):981–989. doi: 10.1038/nature07767. [http://dx.doi. org/10.1038/nature07767]. [PMID: 19225519]. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 299.Tamagno E., Bardini P., Obbili A., Vitali A., Borghi R., Zaccheo D., Pronzato M.A., Danni O., Smith M.A., Perry G., Tabaton M. Oxidative stress increases expression and activity of BACE in NT2 neurons. Neurobiol. Dis. 2002;10(3):279–288. doi: 10.1006/nbdi.2002.0515. [http://dx.doi.org/10.1006/nbdi.2002.0515]. [PMID: 12270690]. [DOI] [PubMed] [Google Scholar]
- 300.Yang L.B., Lindholm K., Yan R., Citron M., Xia W., Yang X.L., Beach T., Sue L., Wong P., Price D., Li R., Shen Y. Elevated beta-secretase expression and enzymatic activity detected in sporadic Alzheimer disease. Nat. Med. 2003;9(1):3–4. doi: 10.1038/nm0103-3. [http://dx.doi.org/10.1038/nm0103-3]. [PMID: 12514700]. [DOI] [PubMed] [Google Scholar]
- 301.Li R., Lindholm K., Yang L.B., Yue X., Citron M., Yan R., Beach T., Sue L., Sabbagh M., Cai H., Wong P., Price D., Shen Y. Amyloid beta peptide load is correlated with increased beta-secretase activity in sporadic Alzheimer’s disease patients. Proc. Natl. Acad. Sci. USA. 2004;101(10):3632–3637. doi: 10.1073/pnas.0205689101. [http://dx.doi.org/10.1073/pnas.0205689101]. [PMID: 14978286]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Holsinger R.M., McLean C.A., Beyreuther K., Masters C.L., Evin G. Increased expression of the amyloid precursor beta-secretase in Alzheimer’s disease. Ann. Neurol. 2002;51(6):783–786. doi: 10.1002/ana.10208. [http://dx.doi.org/10.1002/ana.10208]. [PMID: 12112088]. [DOI] [PubMed] [Google Scholar]
- 303.Johnston J.A., Liu W.W., Todd S.A., Coulson D.T., Murphy S., Irvine G.B., Passmore A.P. Expression and activity of beta-site amyloid precursor protein cleaving enzyme in Alzheimer’s disease. Biochem. Soc. Trans. 2005;33(Pt 5):1096–1100. doi: 10.1042/BST20051096. [http://dx.doi. org/10.1042/BST0331096]. [PMID: 16246054]. [DOI] [PubMed] [Google Scholar]
- 304.Stein T.D., Anders N.J., DeCarli C., Chan S.L., Mattson M.P., Johnson J.A. Neutralization of transthyretin reverses the neuroprotective effects of secreted amyloid precursor protein (APP) in APPSW mice resulting in tau phosphorylation and loss of hippocampal neurons: support for the amyloid hypothesis. J. Neurosci. 2004;24(35):7707–7717. doi: 10.1523/JNEUROSCI.2211-04.2004. [http://dx.doi.org/10.1523/ JNEUROSCI.2211-04.2004]. [PMID: 15342738]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Hooper N.M., Turner A.J. The search for alpha-secretase and its potential as a therapeutic approach to Alzheimer s disease. Curr. Med. Chem. 2002;9(11):1107–1119. doi: 10.2174/0929867023370121. [http://dx.doi.org/10.2174/ 0929867023370121]. [PMID: 12052175]. [DOI] [PubMed] [Google Scholar]
- 306.Vincent B., Govitrapong P. Activation of the α-secretase processing of AβPP as a therapeutic approach in Alzheimer’s disease. J. Alzheimers Dis. 2011;24(Suppl. 2):75–94. doi: 10.3233/JAD-2011-110218. [PMID: 21422515]. [DOI] [PubMed] [Google Scholar]
- 307.Kojro E., Gimpl G., Lammich S., Marz W., Fahrenholz F. Low cholesterol stimulates the nonamyloidogenic pathway by its effect on the alpha -secretase ADAM 10. Proc. Natl. Acad. Sci. USA. 2001;98(10):5815–5820. doi: 10.1073/pnas.081612998. [http://dx.doi.org/10.1073/pnas. 081612998]. [PMID: 11309494]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Ohno M., Sametsky E.A., Younkin L.H., Oakley H., Younkin S.G., Citron M., Vassar R., Disterhoft J.F. BACE1 deficiency rescues memory deficits and cholinergic dysfunction in a mouse model of Alzheimer’s disease. Neuron. 2004;41(1):27–33. doi: 10.1016/s0896-6273(03)00810-9. [http:// dx.doi.org/10.1016/S0896-6273(03)00810-9]. [PMID: 14715132]. [DOI] [PubMed] [Google Scholar]
- 309.Ohno M., Cole S.L., Yasvoina M., Zhao J., Citron M., Berry R., Disterhoft J.F., Vassar R. BACE1 gene deletion prevents neuron loss and memory deficits in 5XFAD APP/PS1 transgenic mice. Neurobiol. Dis. 2007;26(1):134–145. doi: 10.1016/j.nbd.2006.12.008. [http://dx.doi.org/10. 1016/j.nbd.2006.12.008]. [PMID: 17258906]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Cai H., Wang Y., McCarthy D., Wen H., Borchelt D.R., Price D.L., Wong P.C. BACE1 is the major beta-secretase for generation of Abeta peptides by neurons. Nat. Neurosci. 2001;4(3):233–234. doi: 10.1038/85064. [http://dx.doi.org/10.1038/85064]. [PMID: 11224536]. [DOI] [PubMed] [Google Scholar]
- 311.Luo Y., Bolon B., Kahn S., Bennett B.D., Babu-Khan S., Denis P., Fan W., Kha H., Zhang J., Gong Y., Martin L., Louis J.C., Yan Q., Richards W.G., Citron M., Vassar R. Mice deficient in BACE1, the Alzheimer’s beta-secretase, have normal phenotype and abolished beta-amyloid generation. Nat. Neurosci. 2001;4(3):231–232. doi: 10.1038/85059. [http://dx.doi.org/10.1038/85059]. [PMID: 11224535]. [DOI] [PubMed] [Google Scholar]
- 312.McConlogue L., Buttini M., Anderson J.P., Brigham E.F., Chen K.S., Freedman S.B., Games D., Johnson-Wood K., Lee M., Zeller M., Liu W., Motter R., Sinha S. Partial reduction of BACE1 has dramatic effects on Alzheimer plaque and synaptic pathology in APP Transgenic Mice. J. Biol. Chem. 2007;282(36):26326–26334. doi: 10.1074/jbc.M611687200. [http://dx.doi.org/10.1074/jbc.M611687200]. [PMID: 17616527]. [DOI] [PubMed] [Google Scholar]
- 313.Lahiri D.K. Melatonin affects the metabolism of the beta-amyloid precursor protein in different cell types. J. Pineal Res. 1999;26(3):137–146. doi: 10.1111/j.1600-079x.1999.tb00575.x. [http://dx.doi.org/10.1111/j.1600-079X.1999.tb00575.x]. [PMID: 10231726]. [DOI] [PubMed] [Google Scholar]
- 314.Lahiri D.K., Chen D., Ge Y.W., Bondy S.C., Sharman E.H. Dietary supplementation with melatonin reduces levels of amyloid beta-peptides in the murine cerebral cortex. J. Pineal Res. 2004;36(4):224–231. doi: 10.1111/j.1600-079X.2004.00121.x. [http://dx.doi.org/10.1111/j.1600-079X.2004. 00121.x]. [PMID: 15066046]. [DOI] [PubMed] [Google Scholar]
- 315.Panmanee J., Nopparat C., Chavanich N., Shukla M., Mukda S., Song W., Vincent B., Govitrapong P. Melatonin regulates the transcription of βAPP-cleaving secretases mediated through melatonin receptors in human neuroblastoma SH-SY5Y cells. J. Pineal Res. 2015;59(3):308–320. doi: 10.1111/jpi.12260. [http://dx.doi.org/10.1111/ jpi.12260]. [PMID: 26123100]. [DOI] [PubMed] [Google Scholar]
- 316.Schuster C., Williams L.M., Morris A., Morgan P.J., Barrett P. The human MT1 melatonin receptor stimulates cAMP production in the human neuroblastoma cell line SH-SY5Y cells via a calcium-calmodulin signal transduction pathway. J. Neuroendocrinol. 2005;17(3):170–178. doi: 10.1111/j.1365-2826.2005.01288.x. [http://dx.doi.org/10.1111/j.1365-2826.2005. 01288.x]. [PMID: 15796769]. [DOI] [PubMed] [Google Scholar]
- 317.Peschke E., Mühlbauer E., Musshoff U., Csernus V.J., Chankiewitz E., Peschke D. Receptor (MT(1)) mediated influence of melatonin on cAMP concentration and insulin secretion of rat insulinoma cells INS-1. J. Pineal Res. 2002;33(2):63–71. doi: 10.1034/j.1600-079x.2002.02919.x. [http://dx.doi.org/10.1034/j.1600-079X.2002.02919.x]. [PMID: 12153439]. [DOI] [PubMed] [Google Scholar]
- 318.Jarzynka M.J., Passey D.K., Ignatius P.F., Melan M.A., Radio N.M., Jockers R., Rasenick M.M., Brydon L., Witt-Enderby P.A. Modulation of melatonin receptors and G-protein function by microtubules. J. Pineal Res. 2006;41(4):324–336. doi: 10.1111/j.1600-079X.2006.00371.x. [http://dx.doi. org/10.1111/j.1600-079X.2006.00371.x]. [PMID: 17014689]. [DOI] [PubMed] [Google Scholar]
- 319.Goodger Z.V., Rajendran L., Trutzel A., Kohli B.M., Nitsch R.M., Konietzko U. Nuclear signaling by the APP intracellular domain occurs predominantly through the amyloidogenic processing pathway. J. Cell Sci. 2009;122(Pt 20):3703–3714. doi: 10.1242/jcs.048090. [http://dx.doi.org/10.1242/jcs.048090]. [PMID: 19773363]. [DOI] [PubMed] [Google Scholar]
- 320.Belyaev N.D., Kellett K.A., Beckett C., Makova N.Z., Revett T.J., Nalivaeva N.N., Hooper N.M., Turner A.J. The trans-criptionally active amyloid precursor protein (APP) intracellular domain is preferentially produced from the 695 isoform of APP in a beta-secretase-dependent pathway. J. Biol. Chem. 2010;285(53):41443–41454. doi: 10.1074/jbc.M110.141390. [http://dx.doi.org/10.1074/jbc.M110.141390]. [PMID: 20961856]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Flammang B., Pardossi-Piquard R., Sevalle J., Debayle D., Dabert-Gay A.S., Thévenet A., Lauritzen I., Checler F. Evidence that the amyloid-β protein precursor intracellular domain, AICD, derives from β-secretase-generated C-terminal fragment. J. Alzheimers Dis. 2012;30(1):145–153. doi: 10.3233/JAD-2012-112186. [PMID: 22406447]. [DOI] [PubMed] [Google Scholar]
- 322.Kim H.S., Kim E.M., Lee J.P., Park C.H., Kim S., Seo J.H., Chang K.A., Yu E., Jeong S.J., Chong Y.H., Suh Y.H. C-terminal fragments of amyloid precursor protein exert neurotoxicity by inducing glycogen synthase kinase-3beta expression. FASEB J. 2003;17(13):1951–1953. doi: 10.1096/fj.03-0106fje. [PMID: 12923068]. [DOI] [PubMed] [Google Scholar]
- 323.Peng C.X., Hu J., Liu D., Hong X.P., Wu Y.Y., Zhu L.Q., Wang J.Z. Disease-modified glycogen synthase kinase-3β intervention by melatonin arrests the pathology and memory deficits in an Alzheimer’s animal model. Neurobiol. Aging. 2013;34(6):1555–1563. doi: 10.1016/j.neurobiolaging.2012.12.010. [http://dx.doi.org/10.1016/j.neurobiolaging. 2012.12.010]. [PMID: 23402899]. [DOI] [PubMed] [Google Scholar]
- 324.Walter J., Haass C. Secretases as targets for beta-amyloid lowering drugs. Drug Dev. Res. 2002;56(2):201–210. [http://dx. doi.org/10.1002/ddr.10076]. [Google Scholar]
- 325.Puzzo D., Loreto C., Giunta S., Musumeci G., Frasca G., Podda M.V., Arancio O., Palmeri A. Effect of phosphodiesterase-5 inhibition on apoptosis and beta amyloid load in aged mice. Neurobiol. Aging. 2014;35(3):520–531. doi: 10.1016/j.neurobiolaging.2013.09.002. [http://dx.doi.org/10.1016/ j.neurobiolaging.2013.09.002]. [PMID: 24112792]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Fukumoto H., Rosene D.L., Moss M.B., Raju S., Hyman B.T., Irizarry M.C. Beta-secretase activity increases with aging in human, monkey, and mouse brain. Am. J. Pathol. 2004;164(2):719–725. doi: 10.1016/s0002-9440(10)63159-8. [http://dx.doi.org/10.1016/S0002-9440(10)63159-8]. [PMID: 14742275]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Placanica L., Zhu L., Li Y.M. Gender- and age-dependent gamma-secretase activity in mouse brain and its implication in sporadic Alzheimer disease. PLoS One. 2009;4(4):e5088. doi: 10.1371/journal.pone.0005088. [http:// dx.doi.org/10.1371/journal.pone.0005088]. [PMID: 19352431]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Mukda S., Panmanee J., Boontem P., Govitrapong P. Melatonin administration reverses the alteration of amyloid precursor protein-cleaving secretases expression in aged mouse hippocampus. Neurosci. Lett. 2016;621:39–46. doi: 10.1016/j.neulet.2016.04.013. [http://dx.doi.org/10.1016/ j.neulet.2016.04.013]. [PMID: 27068758]. [DOI] [PubMed] [Google Scholar]
- 329.Chami L., Buggia-Prévot V., Duplan E., Del Prete D., Chami M., Peyron J.F., Checler F. Nuclear factor-κB regulates βAPP and β- and γ-secretases differently at physiological and supraphysiological Aβ concentrations. J. Biol. Chem. 2012;287(29):24573–24584. doi: 10.1074/jbc.M111.333054. [http://dx.doi.org/10.1074/jbc.M111.333054]. [PMID: 22654105]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Pandi-Perumal S.R., BaHammam A.S., Brown G.M., Spence D.W., Bharti V.K., Kaur C., Hardeland R., Cardinali D.P. Melatonin antioxidative defense: therapeutical implications for aging and neurodegenerative processes. Neurotox. Res. 2013;23(3):267–300. doi: 10.1007/s12640-012-9337-4. [http://dx.doi.org/10.1007/s12640-012-9337-4]. [PMID: 22739839]. [DOI] [PubMed] [Google Scholar]
- 331.Cardinali D.P., Vigo D.E., Olivar N., Vidal M.F., Brusco L.I. Melatonin therapy in patients with alzheimer’s disease. Antioxidants. 2014;3(2):245–277. doi: 10.3390/antiox3020245. [http://dx.doi.org/10.3390/antiox3020245]. [PMID: 26784870]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Sweatt J.D. The emerging field of neuroepigenetics. Neuron. 2013;80(3):624–632. doi: 10.1016/j.neuron.2013.10.023. [http://dx.doi.org/10.1016/j.neuron.2013.10.023]. [PMID: 24183015]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Zovkic I.B., Guzman-Karlsson M.C., Sweatt J.D. Epigenetic regulation of memory formation and maintenance. Learn. Mem. 2013;20(2):61–74. doi: 10.1101/lm.026575.112. [http://dx.doi.org/10.1101/lm.026575.112]. [PMID: 23322554]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Jarome T.J., Thomas J.S., Lubin F.D. The epigenetic basis of memory formation and storage. Prog. Mol. Biol. Transl. Sci. 2014;128:1–27. doi: 10.1016/B978-0-12-800977-2.00001-2. [http://dx.doi.org/10.1016/B978-0-12-800977-2.00001-2]. [PMID: 25410539]. [DOI] [PubMed] [Google Scholar]
- 335.Sanchez-Mut J.V., Gräff J. Epigenetic Alterations in Alzheimer’s Disease. Front. Behav. Neurosci. 2015;9:347. doi: 10.3389/fnbeh.2015.00347. [http://dx.doi.org/ 10.3389/fnbeh.2015.00347]. [PMID: 26734709]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Bennett D.A., Yu L., Yang J., Srivastava G.P., Aubin C., De Jager P.L. Epigenomics of Alzheimer’s disease. Transl. Res. 2015;165(1):200–220. doi: 10.1016/j.trsl.2014.05.006. [http://dx.doi.org/10.1016/j.trsl.2014.05. 006]. [PMID: 24905038]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Nguyen K.V. The human β-amyloid precursor protein: biomolecular and epigenetic aspects. Biomol. Concepts. 2015;6(1):11–32. doi: 10.1515/bmc-2014-0041. [http://dx.doi.org/10.1515/bmc-2014-0041]. [PMID: 25719338]. [DOI] [PubMed] [Google Scholar]
- 338.Fuso A., Seminara L., Cavallaro R.A., D’Anselmi F., Scarpa S. S-adenosylmethionine/homocysteine cycle alterations modify DNA methylation status with consequent deregulation of PS1 and BACE and beta-amyloid production. Mol. Cell. Neurosci. 2005;28(1):195–204. doi: 10.1016/j.mcn.2004.09.007. [http://dx.doi.org/10.1016/j.mcn.2004.09.007]. [PMID: 15607954]. [DOI] [PubMed] [Google Scholar]
- 339.Lunnon K., Smith R., Hannon E., De Jager P.L., Srivastava G., Volta M., Troakes C., Al-Sarraj S., Burrage J., Macdonald R., Condliffe D., Harries L.W., Katsel P., Haroutunian V., Kaminsky Z., Joachim C., Powell J., Lovestone S., Bennett D.A., Schalkwyk L.C., Mill J. Methylomic profiling implicates cortical deregulation of ANK1 in Alzheimer’s disease. Nat. Neurosci. 2014;17(9):1164–1170. doi: 10.1038/nn.3782. [http://dx.doi.org/10.1038/ nn.3782]. [PMID: 25129077]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Lord J., Cruchaga C. The epigenetic landscape of Alzheimer’s disease. Nat. Neurosci. 2014;17(9):1138–1140. doi: 10.1038/nn.3792. [http://dx.doi.org/ 10.1038/nn.3792]. [PMID: 25157507]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Couto P.J., Millis R.M. Imaging of epigenetic influences on alzheimer's disease. Int. J. Alzheimers Dis. 2015:575078. doi: 10.1155/2015/575078. [http://dx.doi.org/10.1155/2015/575078]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Adwan L., Zawia N.H. Epigenetics: a novel therapeutic approach for the treatment of Alzheimer’s disease. Pharmacol. Ther. 2013;139(1):41–50. doi: 10.1016/j.pharmthera.2013.03.010. [http://dx.doi.org/10.1016/j.pharmthera.2013.03.010]. [PMID: 23562602]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Hardeland R. Melatonin, noncoding RNAs, messenger RNA stability and epigenetics--evidence, hints, gaps and perspectives. Int. J. Mol. Sci. 2014;15(10):18221–18252. doi: 10.3390/ijms151018221. [http://dx.doi.org/ 10.3390/ijms151018221]. [PMID: 25310649]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Masri S., Sassone-Corsi P. The circadian clock: a framework linking metabolism, epigenetics and neuronal function. Nat. Rev. Neurosci. 2013;14(1):69–75. doi: 10.1038/nrn3393. [http://dx.doi.org/10.1038/nrn3393]. [PMID: 23187814]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Reiter R.J., Tan D.X., Gitto E., Sainz R.M., Mayo J.C., Leon J., Manchester L.C., Vijayalaxm i, Kilic E., Kilic U. Pharmacological utility of melatonin in reducing oxidative cellular and molecular damage. Pol. J. Pharmacol. 2004;56(2):159–170. [PMID: 15156066]. [PubMed] [Google Scholar]
- 346.Korkmaz A., Reiter R.J. Epigenetic regulation: a new research area for melatonin? J. Pineal Res. 2008;44(1):41–44. doi: 10.1111/j.1600-079X.2007.00509.x. [PMID: 18078446]. [DOI] [PubMed] [Google Scholar]
- 347.Qureshi I.A., Mehler M.F. Epigenetics of sleep and chronobiology. Curr. Neurol. Neurosci. Rep. 2014;14(3):432. doi: 10.1007/s11910-013-0432-6. [http://dx.doi.org/10.1007/s11910-013-0432-6]. [PMID: 24477387]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Urrestarazu E., Iriarte J. Clinical management of sleep disturbances in Alzheimer’s disease: current and emerging strategies. Nat. Sci. Sleep. 2016;8:21–33. doi: 10.2147/NSS.S76706. [http://dx.doi.org/ 10.2147/NSS.S76706]. [PMID: 26834500]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Korkmaz A., Rosales-Corral S., Reiter R.J. Gene regulation by melatonin linked to epigenetic phenomena. Gene. 2012;503(1):1–11. doi: 10.1016/j.gene.2012.04.040. [http://dx.doi.org/10.1016/j.gene.2012.04.040]. [PMID: 22569208]. [DOI] [PubMed] [Google Scholar]
- 350.Schwimmer H., Metzer A., Pilosof Y., Szyf M., Machnes Z.M., Fares F., Harel O., Haim A. Light at night and melatonin have opposite effects on breast cancer tumors in mice assessed by growth rates and global DNA methylation. Chronobiol. Int. 2014;31(1):144–150. doi: 10.3109/07420528.2013.842925. [http://dx.doi.org/10.3109/07420528.2013.842925]. [PMID: 24131150]. [DOI] [PubMed] [Google Scholar]
- 351.Lui C.C., Hsu M.H., Kuo H.C., Chen C.C., Sheen J.M., Yu H.R., Tiao M.M., Tain Y.L., Chang K.A., Huang L.T. Effects of melatonin on prenatal dexamethasone-induced epigenetic alterations in hippocampal morphology and reelin and glutamic acid decarboxylase 67 levels. Dev. Neurosci. 2015;37(2):105–114. doi: 10.1159/000368768. [http://dx.doi.org/10.1159/000368768]. [PMID: 25720733]. [DOI] [PubMed] [Google Scholar]
- 352.Gräff J., Rei D., Guan J.S., Wang W.Y., Seo J., Hennig K.M., Nieland T.J., Fass D.M., Kao P.F., Kahn M., Su S.C., Samiei A., Joseph N., Haggarty S.J., Delalle I., Tsai L.H. An epigenetic blockade of cognitive functions in the neurodegenerating brain. Nature. 2012;483(7388):222–226. doi: 10.1038/nature10849. [http://dx.doi.org/10.1038/ nature10849]. [PMID: 22388814]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Kilgore M., Miller C.A., Fass D.M., Hennig K.M., Haggarty S.J., Sweatt J.D., Rumbaugh G. Inhibitors of class 1 histone deacetylases reverse contextual memory deficits in a mouse model of Alzheimer’s disease. Neuropsychopharmacology. 2010;35(4):870–880. doi: 10.1038/npp.2009.197. [http://dx.doi.org/10.1038/npp.2009.197]. [PMID: 20010553]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Wu T.H., Kuo H.C., Lin I.C., Chien S.J., Huang L.T., Tain Y.L. Melatonin prevents neonatal dexamethasone induced programmed hypertension: histone deacetylase inhibition. J.Steroid Biochem. Mol. Biol. 2014;144 Pt B:253–259. doi: 10.1016/j.jsbmb.2014.07.008. [http://dx.doi.org/10.1016/j.jsbmb.2014.07.008] [DOI] [PubMed] [Google Scholar]
- 355.Clokie S.J., Lau P., Kim H.H., Coon S.L., Klein D.C. MicroRNAs in the pineal gland: miR-483 regulates melatonin synthesis by targeting arylalkylamine N-acetyltransferase. J. Biol. Chem. 2012;287(30):25312–25324. doi: 10.1074/jbc.M112.356733. [http://dx.doi.org/10.1074/ jbc.M112.356733]. [PMID: 22908386]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Cacabelos R., Torrellas C. Epigenetics of aging and alzheimer’s disease: implications for pharmacogenomics and drug response. Int. J. Mol. Sci. 2015;16(12):30483–30543. doi: 10.3390/ijms161226236. [http://dx.doi.org/ 10.3390/ijms161226236]. [PMID: 26703582]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Fang M., Wang J., Zhang X., Geng Y., Hu Z., Rudd J.A., Ling S., Chen W., Han S. The miR-124 regulates the expression of BACE1/β-secretase correlated with cell death in Alzheimer’s disease. Toxicol. Lett. 2012;209(1):94–105. doi: 10.1016/j.toxlet.2011.11.032. [http://dx.doi.org/ 10.1016/j.toxlet.2011.11.032]. [PMID: 22178568]. [DOI] [PubMed] [Google Scholar]
- 358.Sun Y., Luo Z.M., Guo X.M., Su D.F., Liu X. An updated role of microRNA-124 in central nervous system disorders: a review. Front. Cell. Neurosci. 2015;9:193. doi: 10.3389/fncel.2015.00193. [http://dx.doi.org/10.3389/ fncel.2015.00193]. [PMID: 26041995]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Wang X., Wang Z.H., Wu Y.Y., Tang H., Tan L., Wang X., Gao X.Y., Xiong Y.S., Liu D., Wang J.Z., Zhu L.Q. Melatonin attenuates scopolamine-induced memory/synaptic disorder by rescuing EPACs/miR-124/Egr1 pathway. Mol. Neurobiol. 2013;47(1):373–381. doi: 10.1007/s12035-012-8355-9. [http://dx.doi.org/10.1007/s12035-012-8355-9]. [PMID: 23054680]. [DOI] [PubMed] [Google Scholar]
- 360.Zhu H.Q., li Q., Dong L.Y., Zhou Q., Wang H., Wang Y. MicroRNA-29b promotes high-fat diet-stimulated endothelial permeability and apoptosis in apoE knock-out mice by down-regulating MT1 expression. Int. J. Cardiol. 2014;176(3):764–770. doi: 10.1016/j.ijcard.2014.07.095. [http://dx.doi.org/10.1016/j.ijcard.2014.07.095]. [PMID: 25131924]. [DOI] [PubMed] [Google Scholar]
- 361.Augustin R., Endres K., Reinhardt S., Kuhn P.H., Lichtenthaler S.F., Hansen J., Wurst W., Trümbach D. Computational identification and experimental validation of microRNAs binding to the Alzheimer-related gene ADAM10. BMC Med. Genet. 2012;13:35. doi: 10.1186/1471-2350-13-35. [http://dx.doi.org/10.1186/1471-2350-13-35]. [PMID: 22594617]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Kim Y.D., Hwang S.L., Lee E.J., Kim H.M., Chung M.J., Elfadl A.K., Lee S.E., Nedumaran B., Harris R.A., Jeong K.S. Melatonin ameliorates alcohol-induced bile acid synthesis by enhancing miR-497 expression. J. Pineal Res. 2017;62(2) doi: 10.1111/jpi.12386. [http://dx.doi.org/10.1111/jpi.12386]. [PMID: 28095641]. [DOI] [PubMed] [Google Scholar]
- 363.Galimberti D., Villa C., Fenoglio C., Serpente M., Ghezzi L., Cioffi S.M., Arighi A., Fumagalli G., Scarpini E. Circulating miRNAs as potential biomarkers in Alzheimer’s disease. J. Alzheimers Dis. 2014;42(4):1261–1267. doi: 10.3233/JAD-140756. [PMID: 25024331]. [DOI] [PubMed] [Google Scholar]
- 364.Kim S.J., Kang H.S., Lee J.H., Park J.H., Jung C.H., Bae J.H., Oh B.C., Song D.K., Baek W.K. Im, S.S. Melatonin ameliorates ER stress-mediated hepatic steatosis through miR-23a in the liver. Biochem. Biophys. Res. Commun. 2015;458(3):462–469. doi: 10.1016/j.bbrc.2015.01.117. [http://dx.doi.org/10.1016/j.bbrc.2015.01.117]. [PMID: 25660457]. [DOI] [PubMed] [Google Scholar]
- 365.Sarkar S., Jun S., Rellick S., Quintana D.D., Cavendish J.Z., Simpkins J.W. Expression of microRNA-34a in Alzheimer’s disease brain targets genes linked to synaptic plasticity, energy metabolism, and resting state network activity. Brain Res. 2016;1646:139–151. doi: 10.1016/j.brainres.2016.05.026. [http://dx.doi.org/10.1016/j.brainres.2016.05.026]. [PMID: 27235866]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Carloni S., Favrais G., Saliba E., Albertini M.C., Chalon S., Longini M., Gressens P., Buonocore G., Balduini W. Melatonin modulates neonatal brain inflammation through endoplasmic reticulum stress, autophagy, and miR-34a/silent information regulator 1 pathway. J. Pineal Res. 2016;61(3):370–380. doi: 10.1111/jpi.12354. [http:// dx.doi.org/10.1111/jpi.12354]. [PMID: 27441728]. [DOI] [PubMed] [Google Scholar]
- 367.Bollati V., Galimberti D., Pergoli L., Dalla Valle E., Barretta F., Cortini F., Scarpini E., Bertazzi P.A., Baccarelli A. DNA methylation in repetitive elements and Alzheimer disease. Brain Behav. Immun. 2011;25(6):1078–1083. doi: 10.1016/j.bbi.2011.01.017. [http://dx.doi.org/10.1016/ j.bbi.2011.01.017]. [PMID: 21296655]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.deHaro D., Kines K.J., Sokolowski M., Dauchy R.T., Streva V.A., Hill S.M., Hanifin J.P., Brainard G.C., Blask D.E., Belancio V.P. Regulation of L1 expression and retrotransposition by melatonin and its receptor: implications for cancer risk associated with light exposure at night. Nucleic Acids Res. 2014;42(12):7694–7707. doi: 10.1093/nar/gku503. [http://dx.doi.org/10.1093/nar/gku503]. [PMID: 24914052]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Roy J., Sarkar A., Parida S., Ghosh Z., Mallick B. Small RNA sequencing revealed dysregulated piRNAs in Alzheimer’s disease and their probable role in pathogenesis. Mol. Biosyst. 2017;13(3):565–576. doi: 10.1039/c6mb00699j. [http://dx.doi.org/10.1039/C6MB00699J]. [PMID: 28127595]. [DOI] [PubMed] [Google Scholar]
- 370.Esposito T., Magliocca S., Formicola D., Gianfrancesco F. piR_015520 belongs to Piwi-associated RNAs regulates expression of the human melatonin receptor 1A gene. PLoS One. 2011;6(7):e22727. doi: 10.1371/journal.pone.0022727. [http://dx.doi.org/10.1371/journal.pone.0022727]. [PMID: 21818375]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Burgos K., Malenica I., Metpally R., Courtright A., Rakela B., Beach T., Shill H., Adler C., Sabbagh M., Villa S., Tembe W., Craig D., Van Keuren-Jensen K. Profiles of extracellular miRNA in cerebrospinal fluid and serum from patients with Alzheimer’s and Parkinson’s diseases correlate with disease status and features of pathology. PLoS One. 2014;9(5):e94839. doi: 10.1371/journal.pone.0094839. [http://dx.doi.org/ 10.1371/journal.pone.0094839]. [PMID: 24797360]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Tateno F., Sakakibara R., Kawai T., Kishi M., Murano T. Alpha-synuclein in the cerebrospinal fluid differentiates synucleinopathies (Parkinson Disease, dementia with Lewy bodies, multiple system atrophy) from Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2012;26(3):213–216. doi: 10.1097/WAD.0b013e31823899cc. [http://dx.doi.org/10.1097/ WAD.0b013e31823899cc]. [PMID: 22037599]. [DOI] [PubMed] [Google Scholar]
- 373.Lee M.K., Stirling W., Xu Y., Xu X., Qui D., Mandir A.S., Dawson T.M., Copeland N.G., Jenkins N.A., Price D.L. Human alpha-synuclein-harboring familial Parkinson’s disease-linked Ala-53 --> Thr mutation causes neurodegenerative disease with alpha-synuclein aggregation in transgenic mice. Proc. Natl. Acad. Sci. USA. 2002;99(13):8968–8973. doi: 10.1073/pnas.132197599. [http://dx.doi.org/10.1073/pnas. 132197599]. [PMID: 12084935]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Gillardon F., Mack M., Rist W., Schnack C., Lenter M., Hildebrandt T., Hengerer B. MicroRNA and proteome expression profiling in early-symptomatic α-synuclein(A30P)-transgenic mice. Proteomics Clin. Appl. 2008;2(5):697–705. doi: 10.1002/prca.200780025. [http://dx.doi.org/ 10.1002/prca.200780025]. [PMID: 21136867]. [DOI] [PubMed] [Google Scholar]
- 375.Wanet A., Tacheny A., Arnould T., Renard P. miR-212/132 expression and functions: within and beyond the neuronal compartment. Nucleic Acids Res. 2012;40(11):4742–4753. doi: 10.1093/nar/gks151. [http://dx.doi.org/10.1093/nar/gks151]. [PMID: 22362752]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Sae-Ung K., Uéda K., Govitrapong P., Phansuwan-Pujito P. Melatonin reduces the expression of alpha-synuclein in the dopamine containing neuronal regions of amphetamine-treated postnatal rats. J. Pineal Res. 2012;52(1):128–137. doi: 10.1111/j.1600-079X.2011.00927.x. [http://dx. doi.org/10.1111/j.1600-079X.2011.00927.x]. [PMID: 21851386]. [DOI] [PubMed] [Google Scholar]
- 377.Saunders L.R., Sharma A.D., Tawney J., Nakagawa M., Okita K., Yamanaka S., Willenbring H., Verdin E. miRNAs regulate SIRT1 expression during mouse embryonic stem cell differentiation and in adult mouse tissues. Aging (Albany NY) 2010;2(7):415–431. doi: 10.18632/aging.100176. [http://dx.doi.org/10.18632/aging.100176]. [PMID: 20634564]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Delay C., Mandemakers W., Hébert S.S. MicroRNAs in Alzheimer’s disease. Neurobiol. Dis. 2012;46(2):285–290. doi: 10.1016/j.nbd.2012.01.003. [http://dx.doi.org/10.1016/j.nbd.2012.01.003]. [PMID: 22285895]. [DOI] [PubMed] [Google Scholar]
- 379.Yi C., Zhang Y., Yu Z., Xiao Y., Wang J., Qiu H., Yu W., Tang R., Yuan Y., Guo W., Deng W. Melatonin enhances the anti-tumor effect of fisetin by inhibiting COX-2/iNOS and NF-κB/p300 signaling pathways. PLoS One. 2014;9(7):e99943. doi: 10.1371/journal.pone.0099943. [http://dx.doi.org/10.1371/journal.pone.0099943]. [PMID: 25000190]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Fitzsimons C.P., van Bodegraven E., Schouten M., Lardenoije R., Kompotis K., Kenis G., van den Hurk M., Boks M.P., Biojone C., Joca S., Steinbusch H.W., Lunnon K., Mastroeni D.F., Mill J., Lucassen P.J., Coleman P.D., van den Hove D.L., Rutten B.P. Epigenetic regulation of adult neural stem cells: implications for Alzheimer’s disease. Mol. Neurodegener. 2014;9:25. doi: 10.1186/1750-1326-9-25. [http://dx.doi.org/10.1186/1750-1326-9-25]. [PMID: 24964731]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Asayama K., Yamadera H., Ito T., Suzuki H., Kudo Y., Endo S. Double blind study of melatonin effects on the sleep-wake rhythm, cognitive and non-cognitive functions in Alzheimer type dementia. J. Nippon Med. Sch. 2003;70(4):334–341. doi: 10.1272/jnms.70.334. [http://dx.doi.org/10.1272/jnms.70.334]. [PMID: 12928714]. [DOI] [PubMed] [Google Scholar]
- 382.Srinivasan V., Kaur C., Pandi-Perumal S., Brown G.M., Cardinali D.P. Melatonin and its agonist ramelteon in Alzheimer's disease: possible therapeutic value. Int. J. Alzheimers Dis. 2010. 2011, 741974. [DOI] [PMC free article] [PubMed]
- 383.Cardinali D.P., Vigo D.E., Olivar N., Vidal M.F., Furio A.M., Brusco L.I. Therapeutic application of melatonin in mild cognitive impairment. Am. J. Neurodegener. Dis. 2012;1(3):280–291. [PMID: 23383398]. [PMC free article] [PubMed] [Google Scholar]
- 384.Rondanelli M., Opizzi A., Faliva M., Mozzoni M., Antoniello N., Cazzola R., Savarè R., Cerutti R., Grossi E., Cestaro B. Effects of a diet integration with an oily emulsion of DHA-phospholipids containing melatonin and tryptophan in elderly patients suffering from mild cognitive impairment. Nutr. Neurosci. 2012;15(2):46–54. doi: 10.1179/1476830511Y.0000000032. [http://dx.doi.org/10.1179/1476830511Y. 0000000032]. [PMID: 22334085]. [DOI] [PubMed] [Google Scholar]
- 385.Furio A.M., Brusco L.I., Cardinali D.P. Possible therapeutic value of melatonin in mild cognitive impairment: a retrospective study. J. Pineal Res. 2007;43(4):404–409. doi: 10.1111/j.1600-079X.2007.00491.x. [http://dx.doi.org/ 10.1111/j.1600-079X.2007.00491.x]. [PMID: 17910609]. [DOI] [PubMed] [Google Scholar]
- 386.Wade A.G., Farmer M., Harari G., Fund N., Laudon M., Nir T., Frydman-Marom A., Zisapel N. Add-on prolonged-release melatonin for cognitive function and sleep in mild to moderate Alzheimer’s disease: a 6-month, randomized, placebo-controlled, multicenter trial. Clin. Interv. Aging. 2014;9:947–961. doi: 10.2147/CIA.S65625. [PMID: 24971004]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Peck J.S., LeGoff D.B., Ahmed I., Goebert D. Cognitive effects of exogenous melatonin administration in elderly persons: a pilot study. Am. J. Geriatr. Psychiatry. 2004;12(4):432–436. doi: 10.1176/appi.ajgp.12.4.432. [PMID: 15249281]. [DOI] [PubMed] [Google Scholar]
- 388.Yeleswaram K., McLaughlin L.G., Knipe J.O., Schabdach D. Pharmacokinetics and oral bioavailability of exogenous melatonin in preclinical animal models and clinical implications. J. Pineal Res. 1997;22(1):45–51. doi: 10.1111/j.1600-079x.1997.tb00302.x. [http://dx.doi.org/10.1111/j.1600-079X. 1997.tb00302.x]. [PMID: 9062870]. [DOI] [PubMed] [Google Scholar]